Exogenous Melatonin Effects on Drought-Stressed Longan Plants: Physiology and Transcriptome Insights
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Plant Materials
2.2. Water Treatments and Exogenous MT Application
2.3. Sampling and Determination
2.4. RNA Extraction
2.5. Library Preparation, and Illumina Hiseq X Ten/NovaSeq 6000 Sequencing
2.6. Statistics Analysis
3. Results
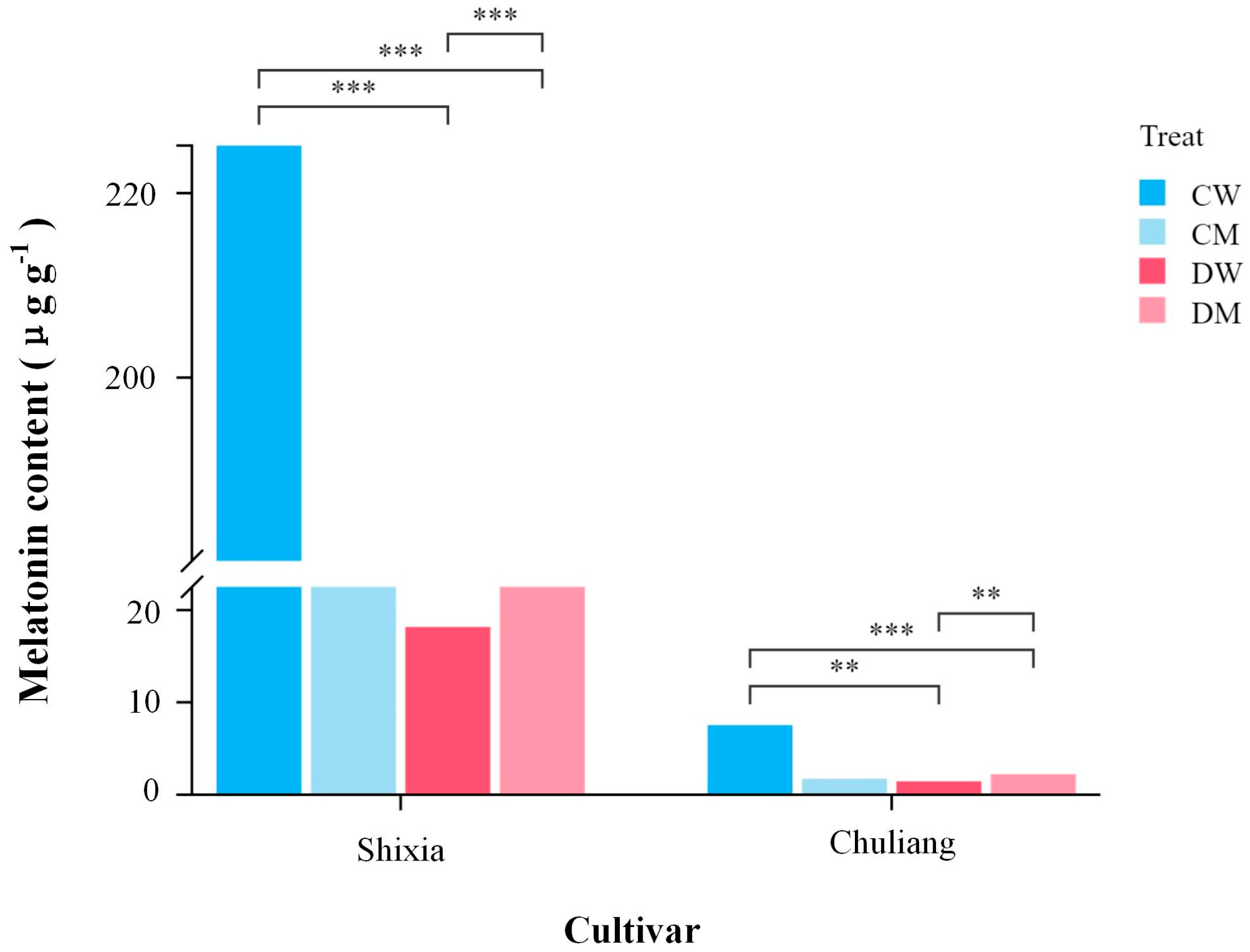
3.1. Efffects of Exogenous MT on Endogenous MT Content of Longan Cultivars Under Drought Stress
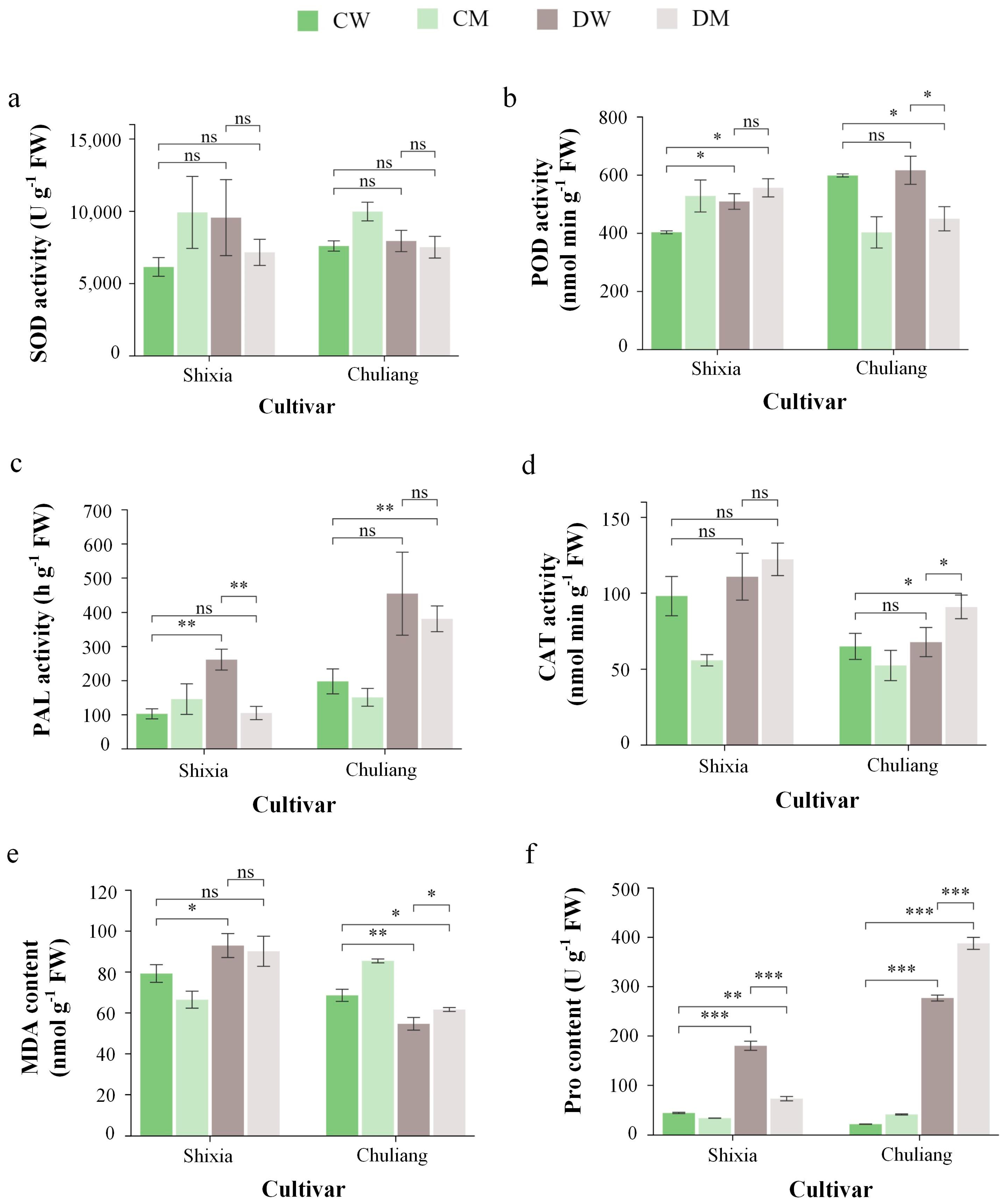
3.2. Effects of Exogenous MT on Plant Defense Enzymes and Related Compound Under Drought Stress
3.3. Transcriptional Responses to Exogenous MT Application Under Drought Stress
3.3.1. Principal Component Analysis (PCA) and Differential Gene Expression Analysis
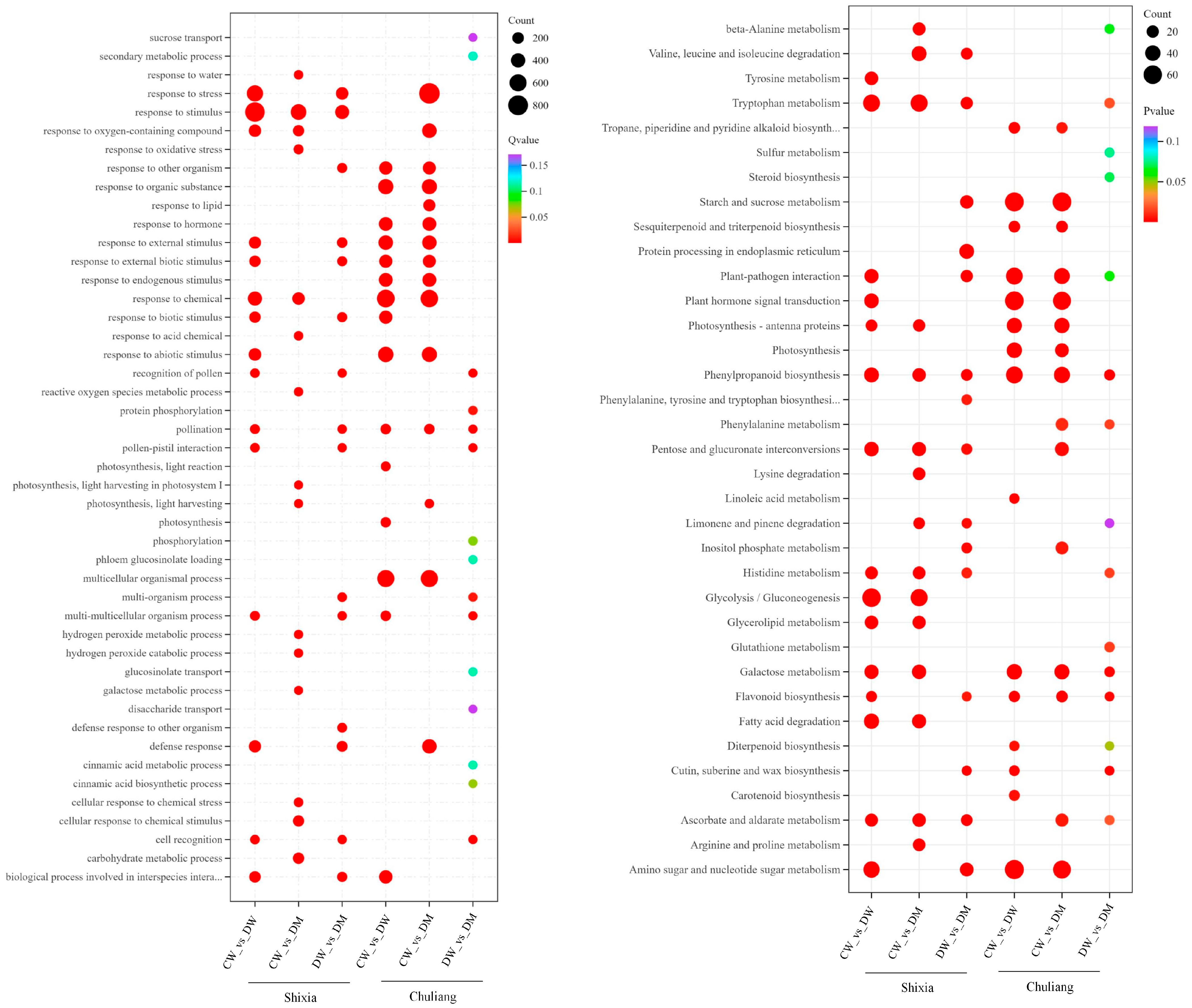
3.3.2. GO and KEGG Functional Classification
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, J.; Pan, L.; Qin, S.; Peng, H.; Wang, Y.; Han, Z. Analysis on genetic relations in different ecotypes of longan (Dimocarpus longan Lour.) germplasm resources by ISSR markers. J. Plant Genet. Resour. 2013, 14, 65–69. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Modern pharmacological actions of Longan fruits and their usages in traditional herbal remedies. J. Med. Plants Stud. 2019, 7, 179–185. [Google Scholar]
- Zeng, S.; Wang, K.; Liu, X.; Hu, Z.; Zhao, L. Potential of longan (Dimocarpus longan Lour.) in functional food: A review of molecular mechanism-directing health benefit properties. Food Chem. 2024, 437, 137812. [Google Scholar] [CrossRef]
- Jue, D.; Sang, X.; Liu, L.; Shu, B.; Wang, Y.; Liu, C.; Xie, J.; Shi, S. Identification of WRKY gene family from dimocarpus longan and its expression analysis during flower induction and abiotic stress responses. Int. J. Mol. Sci. 2018, 19, 2169. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Li, L.; Lin, J.; Yang, K.; Ma, Z.; Xu, Z. Integrated risk evaluation of multiple disasters affecting longyan yield in Fujian Province, East China. Chin. J. Appl. Ecol. 2012, 23, 819–826. [Google Scholar] [CrossRef]
- Su, Y.; Ding, M.; Li, Z.; Sun, H. Study on effect of climate condition on the longan yield and longan climate index in Guangxi. J. Trop. Meteorol. 2006, 22, 308–312. [Google Scholar] [CrossRef]
- Mahouachi, J.; Fernández-Galván, D.; Gómez-Cadenas, A. Abscisic acid, indole-3-acetic acid and mineral–nutrient changes induced by drought and salinity in longan (Dimocarpus longan Lour.) plants. Acta Physiol. Plant. 2013, 35, 3137–3146. [Google Scholar] [CrossRef]
- Girona, J.; Mata, M.; Campo, J.d.; Biru, A.; Paris, C.; Blanco, V. Apple trees’ behavior to a single-season megadrought stress. Irrig. Sci. 2025, 43, 871–886. [Google Scholar] [CrossRef]
- Blanco, V.; Kalcsits, L. Interannual carry-over effects of severe drought on field-grown young pear trees. Agr. Forest Meteorol. 2025, 367, 110502. [Google Scholar] [CrossRef]
- Yang, L.; Chen, L. Stress physiology and molecular biology of fruit crops. Int. J. Mol. Sci. 2024, 25, 706. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, L.; Li, L. Meteorological condition analysis for the Qinzhou city litchi and longan output in 2005. J. Guangxi Meteorol. 2005, 26, 27–28. [Google Scholar] [CrossRef]
- Wang, X.; Luo, Z.; Li, X.; Li, Y.; Li, J.; Ding, X. Influences of high temperature and drought stress on the longan industry of Luzhou area. China Fruit Veg. 2024, 44, 71–75. [Google Scholar] [CrossRef]
- Shil, S.; Das, S.; Tani, M.; Rime, J.; Sow, S.; Haokip, S.W.; Sheikh, K.A.; Bhargava, M.S.; Pertin, O.; Singh, S. Unlocking nature’s stress reliever: The role of melatonin in enhancing the resilience of fruit crops against abiotic stress. Appl. Fruit Sci. 2024, 66, 2469–2479. [Google Scholar] [CrossRef]
- Himanshu; Sharma, S.; Rana, V.S.; Ankit; Thakur, V.; Kumar, A.; Prachi; Thakur, S.; Sharma, N. Unlocking the sustainable role of melatonin in fruit production and stress tolerance: A review. CABI Agric. Biosci. 2024, 5, 103. [Google Scholar] [CrossRef]
- Hao, X.; Sun, B.; Song, Y.; Zhang, J.; Wu, J.; Zhang, N.; Zhang, X.; Yao, W.; Xu, W. Melatonin-mediated physiological and molecular responses to abiotic stress in horticultural crops. Hortic. Plant J. 2025, 11, 1381–1396. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, D.; Tang, W.; Delaplace, P.; Chen, M.; Ma, Y. Recent advances in melatonin regulation of drought tolerance in plants. Trop. Plants 2025, 4, e005. [Google Scholar] [CrossRef]
- Durak, I.; Yurtarslanl, Z.; Canbolat, O.; Akyol, O. A methodological approach to superoxide dismutase (SOD) activity assay based on inhibition of nitroblue tetrazolium (NBT) reduction. Clin. Chim. Acta. 1993, 214, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Y.; Gao, X. A new method for accurate determination of peroxidase activity based on fluorescence decrease of guaiacol. Chin. J. Anal. Chem. 2015, 43, 1040–1046. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, C.; Xu, W. Ultraviolet spectrophotometry measurement of catalase activity in maize. Chin. Agr. Sci. Bull. 2016, 32, 159–165. [Google Scholar] [CrossRef]
- Li, J.; Fang, L.; Zhang, Y.; Yang, W.; Guo, Q.; Li, L.; Bi, C.; Yang, R. The relationship between the resistance of cotton against cotton aphid, Aphis gossypii, and the activity of phenylalanine ammonia-lyase. Chin. Bull. Entomol. 2008, 45, 422–425. [Google Scholar]
- Zhi, M.; Li, X. Improvement on the method for measuring proline content. Plant Physiol. J. 2005, 41, 355–357. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A. Estimation of malondialdehyde (MDA) by thiobarbituric acid (TBA) assay. In Plant-Microbe Interactions: Laboratory Techniques; Springer: New York, NY, USA, 2021; pp. 103–105. [Google Scholar]
- Qi, B.; Wu, C.; Liang, H.; Cui, K.; Fahad, S.; Wang, M.; Liu, B.; Nie, L.; Huang, J.; Tang, H. Optimized high-performance liquid chromatography method for determining nine cytokinins, indole-3-acetic acid and abscisic acid. Sustainability 2021, 13, 6998. [Google Scholar] [CrossRef]
- Du, P.; Wang, Q.; He, Y.; Yu, H.; Lin, L.; Zhang, Z. Lipidomic profiling and storage-induced changes in cassava flour using LC-MS/MS. Foods 2024, 13, 3039. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Bio. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Farmer, E.E.; Mueller, M.J. ROS-mediated lipid peroxidation and RES-activated signaling. Annu. Rev. Plant Biol. 2013, 64, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Sood, M. Reactive oxygen species (ROS): Plant perspectives on oxidative signalling and biotic stress response. Discov. Plants 2025, 2, 187. [Google Scholar] [CrossRef]
- Cannea, F.B.; Padiglia, A. Antioxidant defense systems in plants: Mechanisms, regulation, and biotechnological strategies for enhanced oxidative stress tolerance. Life 2025, 15, 1293. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Tian, Z.; He, J.; Wang, Z.; Zhang, Z.; Quinet, M.; Meng, Y. Exogenous melatonin enhances drought tolerance and germination in common buckwheat seeds through the coordinated effects of antioxidant and osmotic regulation. BMC Plant Biol. 2025, 25, 613. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, W.; Liu, Z.; Ren, X.; Li, Y.; Li, G.; Wang, J.; Zhu, X.; Shi, Y.; Wang, C.; et al. Transcriptomic analysis of melatonin-mediated drought stress response genes in alfalfa during germination period. BMC Plant Biol. 2025, 25, 637. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Feng, Z.; Xu, W.; Vetukuri, R.R.; Xu, X. Exogenous melatonin-stimulated transcriptomic alterations of Davidia involucrata seedlings under drought stress. Trees 2021, 35, 1025–1038. [Google Scholar] [CrossRef]
- Rochaix, J.D. Regulation of photosynthetic electron transport. BBA-Bioenergetics 2011, 1807, 375–383. [Google Scholar] [CrossRef]
- Berry, J.O.; Yerramsetty, P.; Zielinski, A.M.; Mure, C.M. Photosynthetic gene expression in higher plants. Photosynth. Res. 2013, 117, 91–120. [Google Scholar] [CrossRef]
- Hu, F.; Zhang, Y.; Guo, J. Effects of drought stress on photosynthetic physiological characteristics, leaf microstructure, and related gene expression of yellow horn. Plant Signal. Behav. 2023, 18, 2215025. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Y.; Li, M.; Ma, J.; Wang, C.; Su, J.; Yang, D. Abscisic acid associated with key enzymes and genes involving in dynamic flux of water soluble carbohydrates in wheat peduncle under terminal drought stress. Plant Physiol. Bioch. 2020, 151, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.H.; Topping, J.F.; Liu, J.; Lindsey, K. Abscisic acid regulates root growth under osmotic stress conditions via an interacting hormonal network with cytokinin, ethylene and auxin. New Phytol. 2016, 211, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.; Dubeaux, G.; Takahashi, Y.; Schroeder, J.I. Signaling mechanisms in abscisic acid-mediated stomatal closure. Plant J. 2021, 105, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Tao, J.; Yin, C.; Chen, S.; Zhang, J.; Zhang, W. Melatonin regulates gene expressions through activating auxin synthesis and signaling pathways. Front. Plant Sci. 2022, 13, 1057993. [Google Scholar] [CrossRef]
- Gu, H.; Wang, Y.; Xie, H.; Qiu, C.; Zhang, S.; Xiao, J.; Li, H.; Chen, L.; Li, X.; Ding, Z. Drought stress triggers proteomic changes involving lignin, flavonoids and fatty acids in tea plants. Sci. Rep. 2020, 10, 15504. [Google Scholar] [CrossRef]
- Li, B.; Fan, R.; Fan, Y.; Liu, R.; Zhang, H.; Chen, T.; Liu, J.; Li, H.; Zhao, X.; Song, C. The flavonoid biosynthesis regulator PFG3 confers drought stress tolerance in plants by promoting flavonoid accumulation. Environ. Exp. Bot. 2022, 196, 104792. [Google Scholar] [CrossRef]
- Geng, D.; Shen, X.; Xie, Y.; Yang, Y.; Bian, R.; Gao, Y.; Li, P.; Sun, L.; Feng, H.; Ma, F.; et al. Regulation of phenylpropanoid biosynthesis by MdMYB88 and MdMYB124 contributes to pathogen and drought resistance in apple. Hortic. Res. 2020, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Lin, H. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Du, M.; Zang, D.; Men, Q.; Su, P.; Guo, S. Exogenous melatonin improves drought stress tolerance via regulating tryptophan metabolism and flavonoid biosynthesis pathways in wheat. Physiol. Plant. 2024, 176, e70006. [Google Scholar] [CrossRef]
- Dorion, S.; Ouellet, J.C.; Rivoal, J. Glutathione metabolism in plants under stress: Beyond reactive oxygen species detoxification. Metabolites 2021, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Georgiou-Siafis, S.K.; Tsiftsoglou, A.S. The key role of GSH in keeping the redox balance in mammalian cells: Mechanisms and significance of GSH in detoxification via formation of conjugates. Antioxidants 2023, 12, 1953. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N. Melatonin regulates mitochondrial enzymes and ascorbate–glutathione system during plant responses to drought stress through involving endogenous calcium. S. Afr. J. Bot. 2023, 162, 622–632. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Lal, M.K.; Kumar, R.; Chourasia, K.N.; Naga, K.C.; Kumar, D.; Das, S.K.; Zinta, G. Mechanistic insights on melatonin-mediated drought stress mitigation in plants. Physiol. Plant. 2021, 172, 1212–1226. [Google Scholar] [CrossRef]
- Dzinyela, R.; Hwarari, D.; Opoku, K.N.; Yang, L.; Movahedi, A. Enhancing drought stress tolerance in horticultural plants through melatonin-mediated phytohormonal crosstalk. Plant Cell Rep. 2024, 43, 272. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, B.; Huang, R.; Qin, X.; Xu, N.; Li, L.; Cao, K.; Qiu, H.; Chen, J. Exogenous Melatonin Effects on Drought-Stressed Longan Plants: Physiology and Transcriptome Insights. Agronomy 2025, 15, 2530. https://doi.org/10.3390/agronomy15112530
Qi B, Huang R, Qin X, Xu N, Li L, Cao K, Qiu H, Chen J. Exogenous Melatonin Effects on Drought-Stressed Longan Plants: Physiology and Transcriptome Insights. Agronomy. 2025; 15(11):2530. https://doi.org/10.3390/agronomy15112530
Chicago/Turabian StyleQi, Beibei, Rongshao Huang, Xianquan Qin, Ning Xu, Liangbo Li, Kexin Cao, Hongye Qiu, and Jianhua Chen. 2025. "Exogenous Melatonin Effects on Drought-Stressed Longan Plants: Physiology and Transcriptome Insights" Agronomy 15, no. 11: 2530. https://doi.org/10.3390/agronomy15112530
APA StyleQi, B., Huang, R., Qin, X., Xu, N., Li, L., Cao, K., Qiu, H., & Chen, J. (2025). Exogenous Melatonin Effects on Drought-Stressed Longan Plants: Physiology and Transcriptome Insights. Agronomy, 15(11), 2530. https://doi.org/10.3390/agronomy15112530






