Synergy of Biochar and Organic Fertilizer Reduces Phosphorus Leaching
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Materials
2.2. Preparation of Sample
2.3. Determination of Phosphorus Fractionations
2.4. Kinetic Leaching of Phosphorus
2.5. Sequential Extraction of Phosphorus
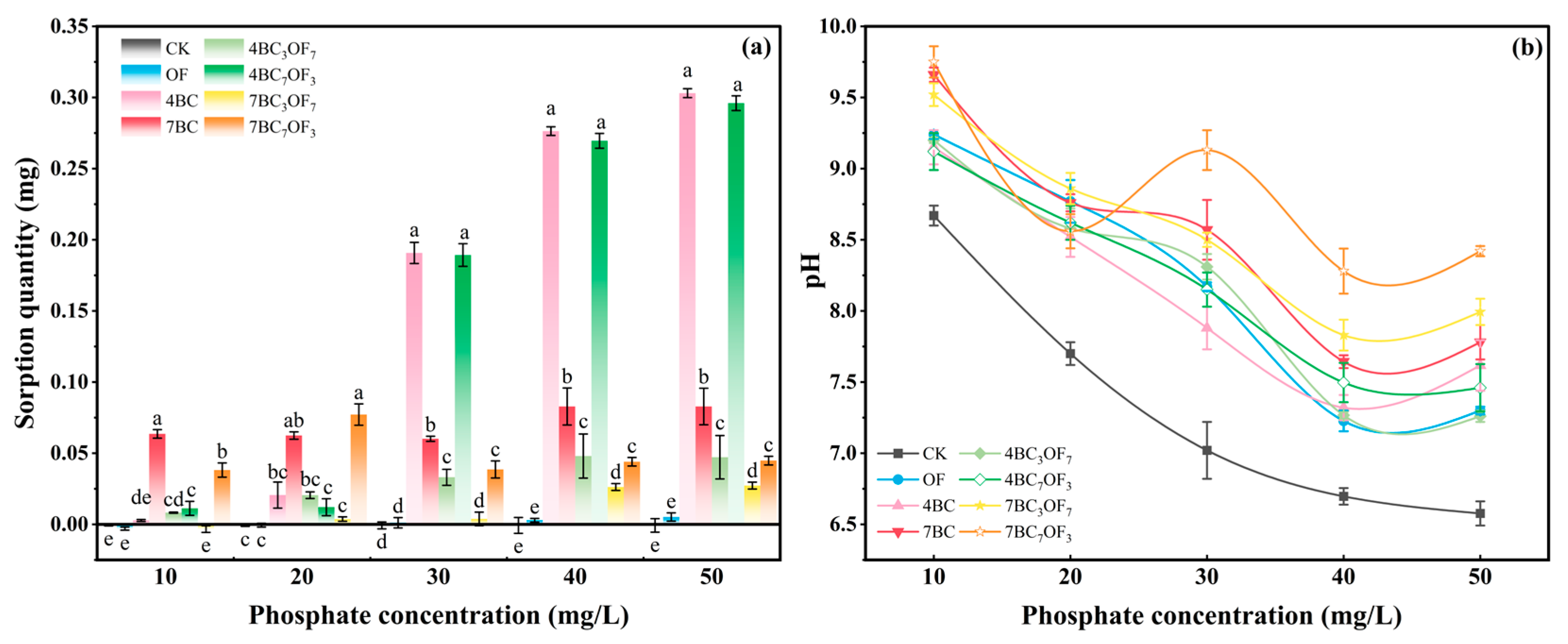
2.6. P Sorption Experiment
2.7. Statistics Analysis
3. Results
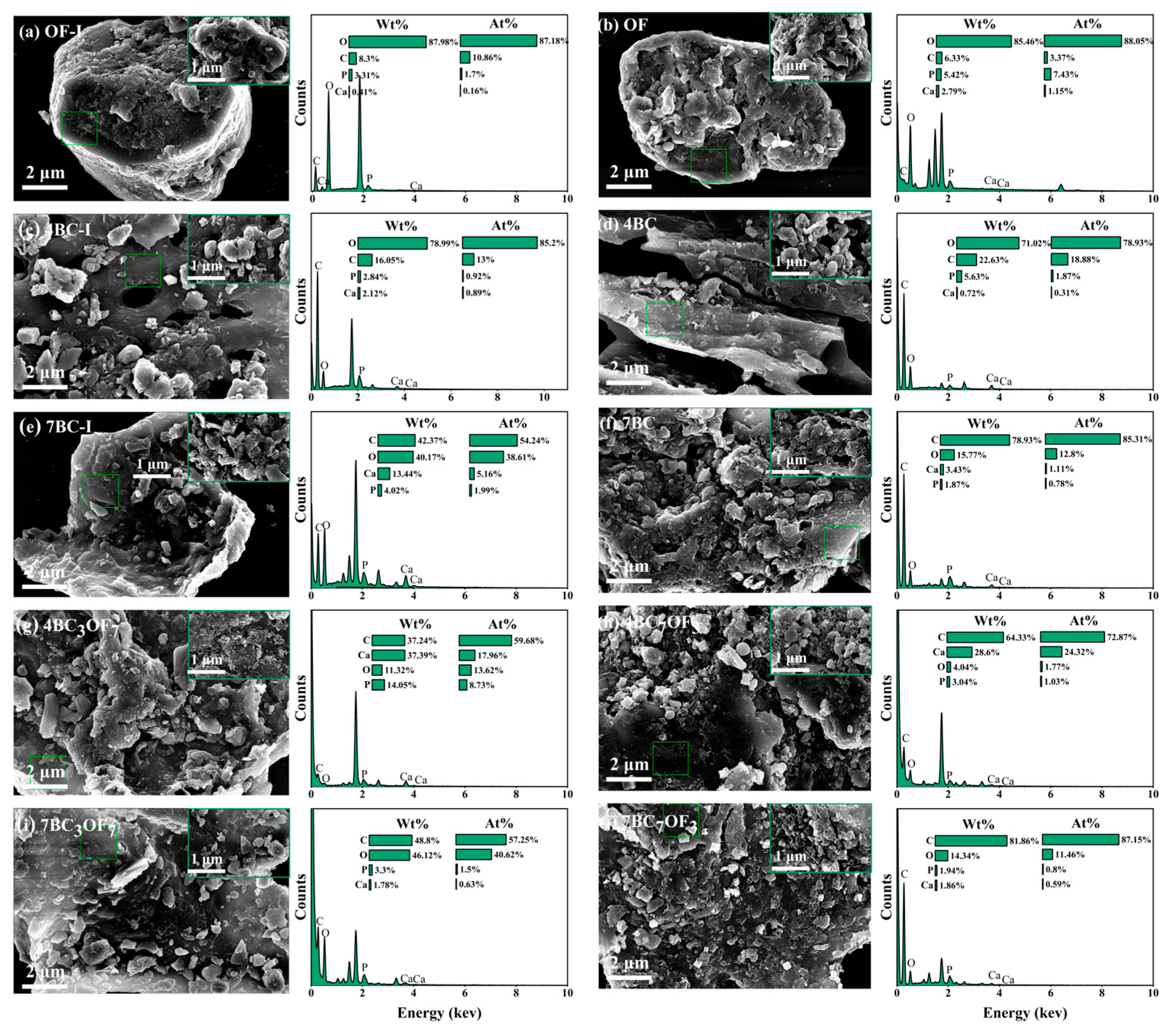
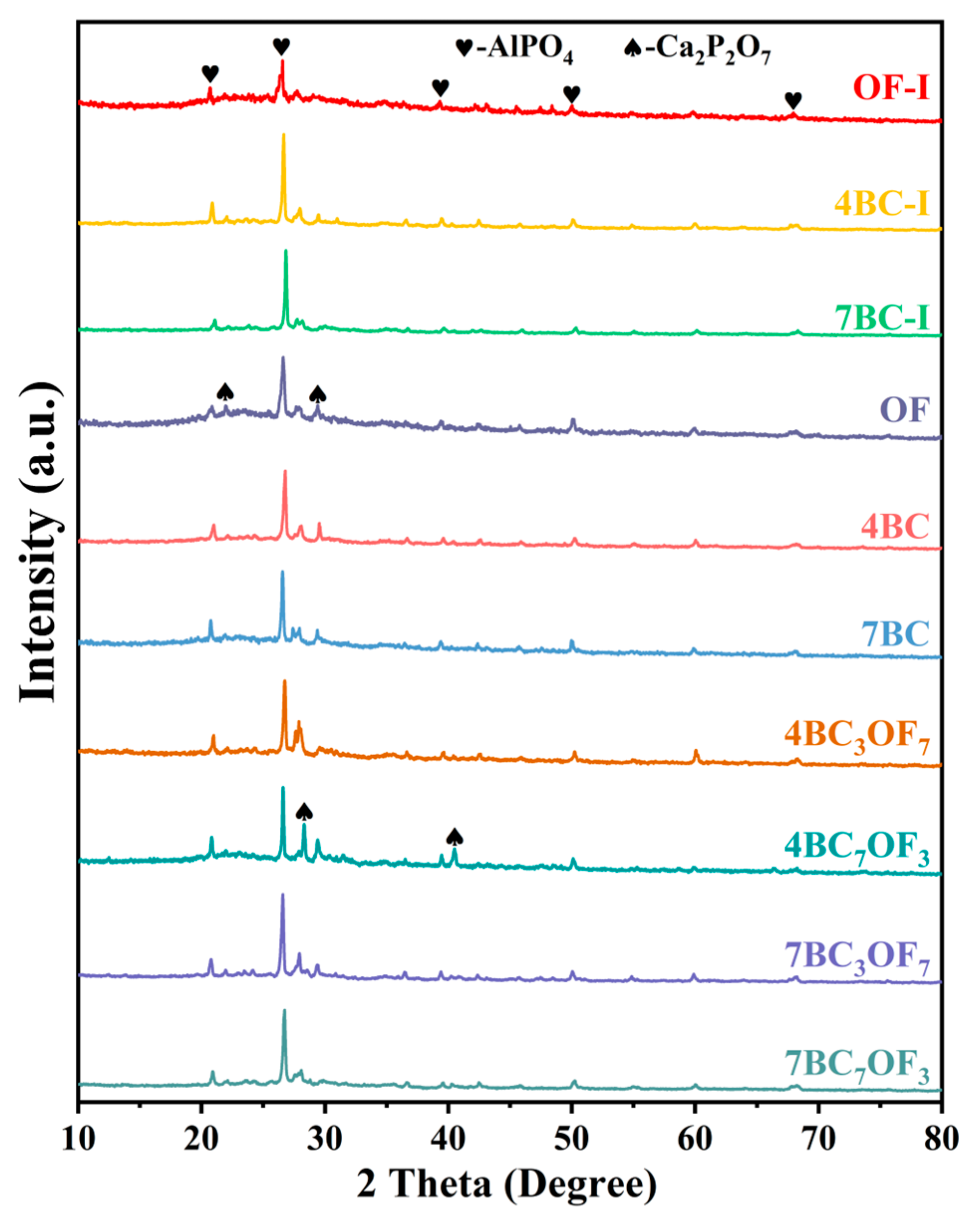
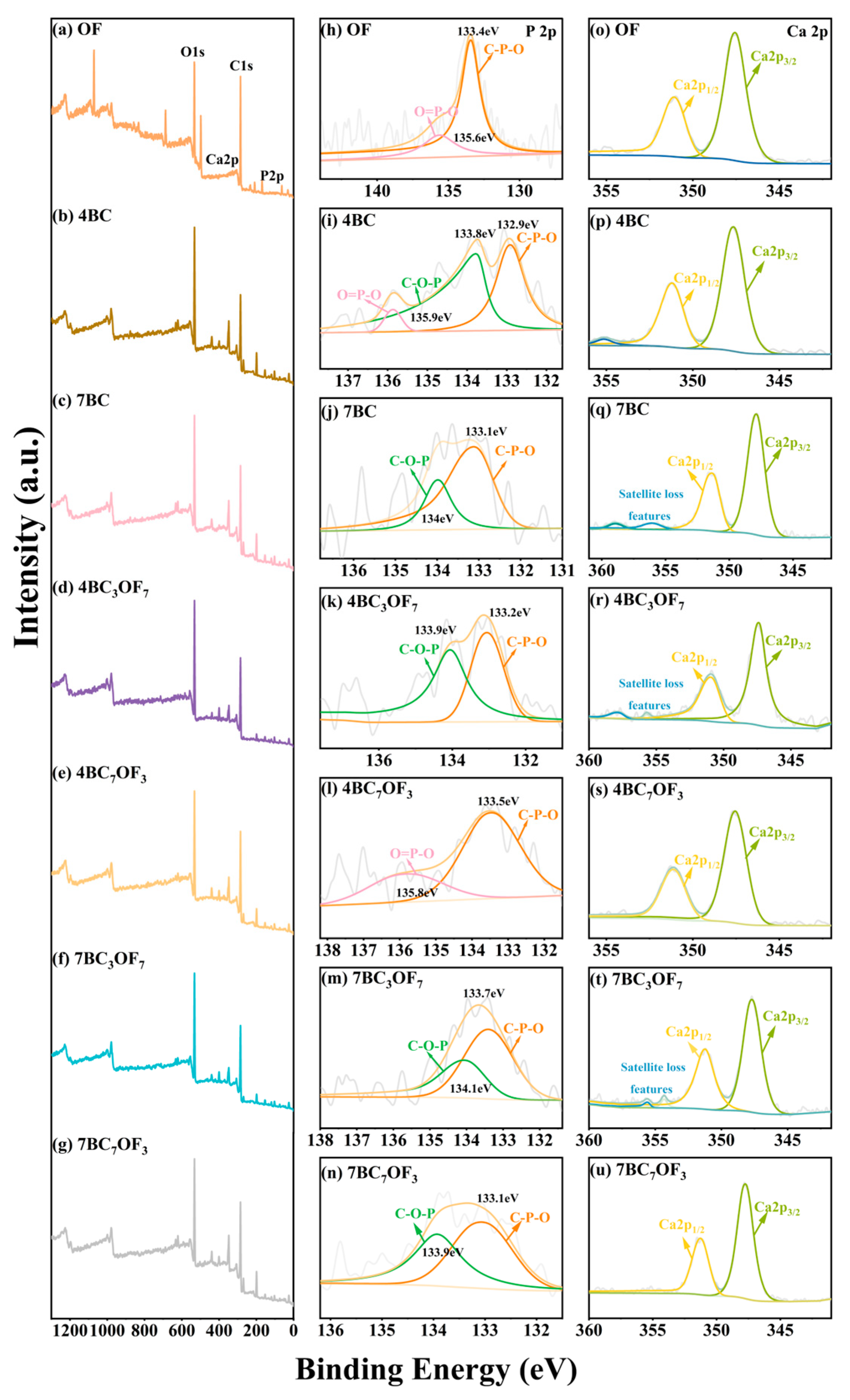
3.1. Sample Analysis
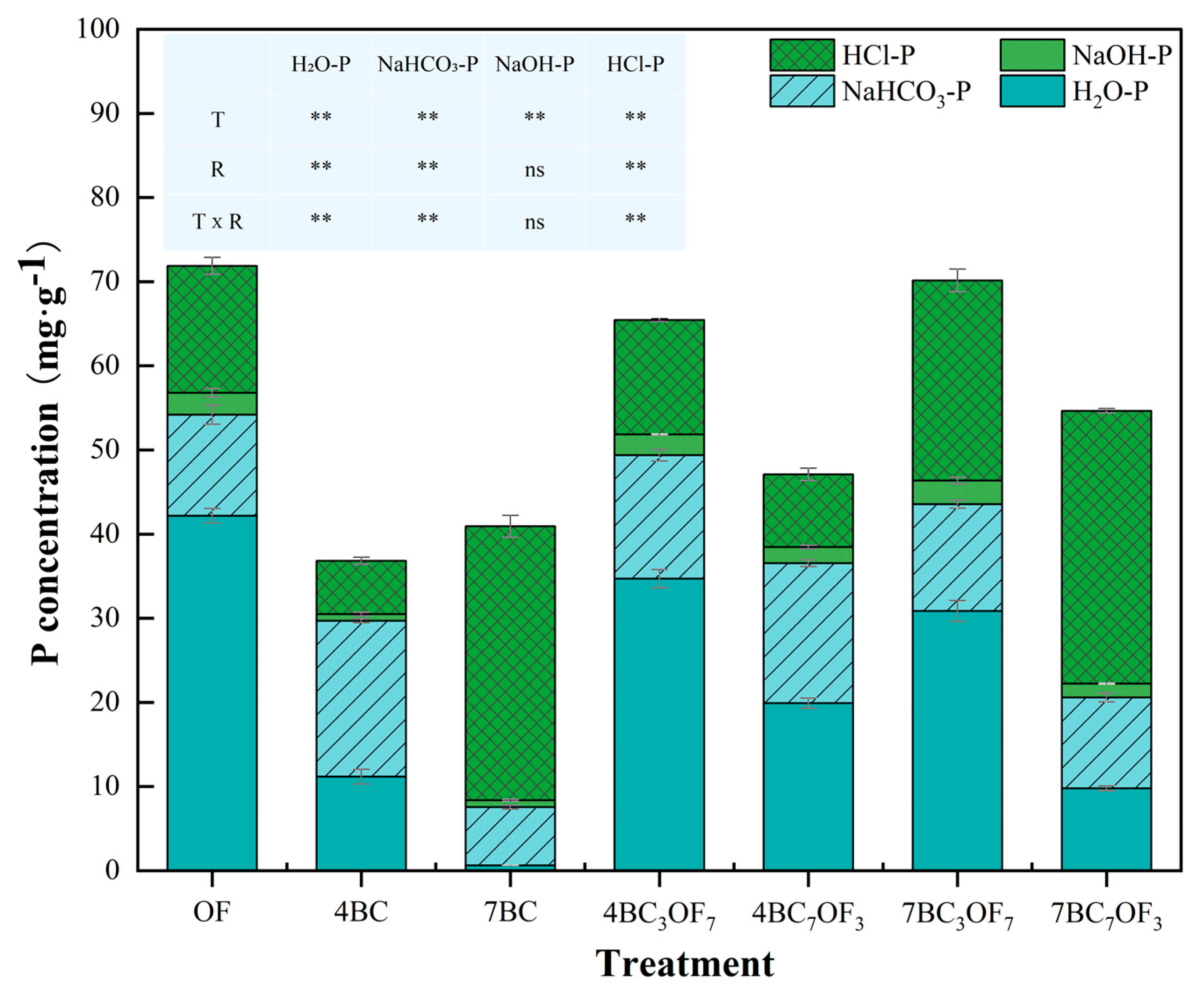
3.2. Effect of Combined Application of Biochar and Organic Fertilizer on Phosphorus Fractionations
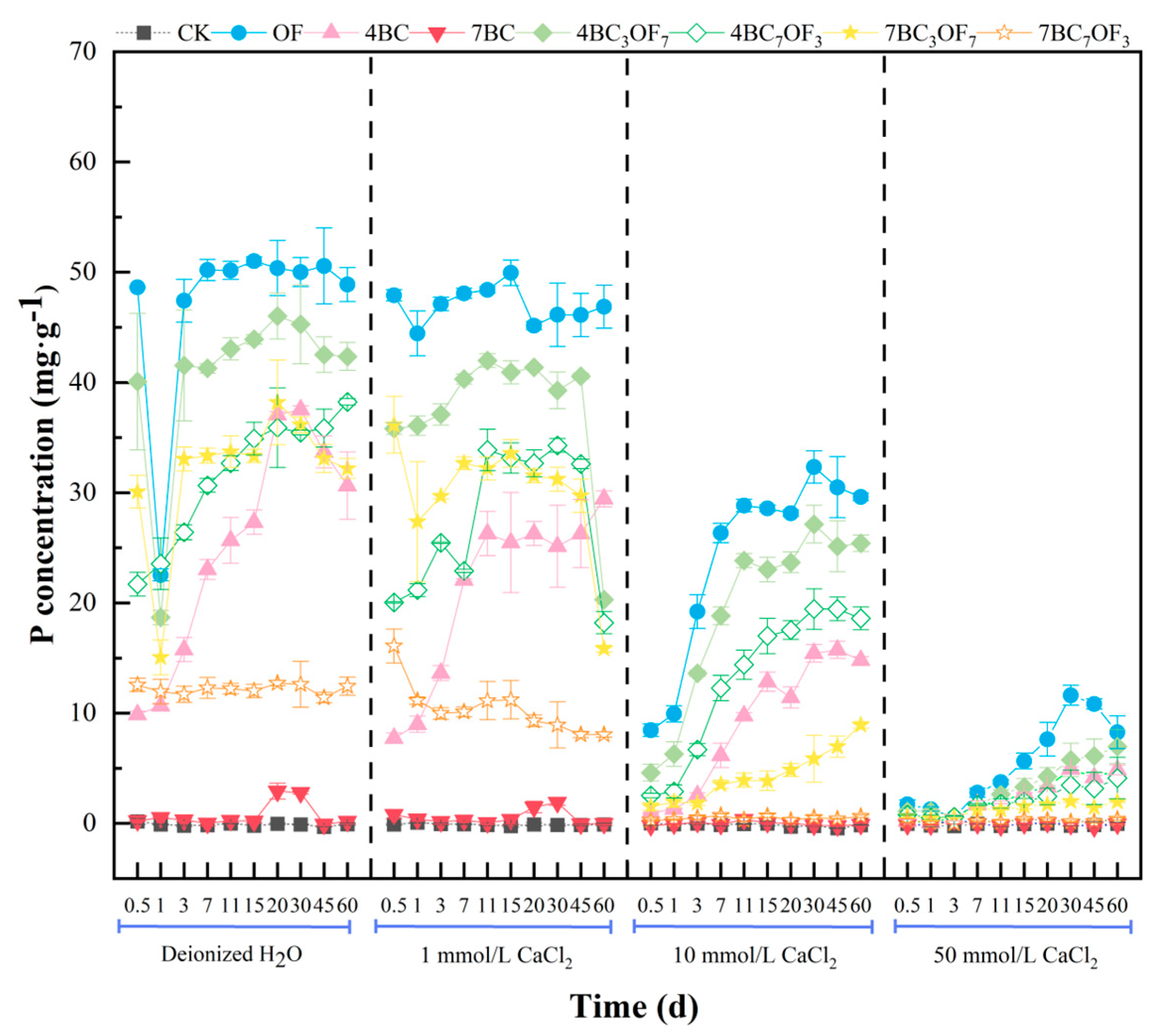
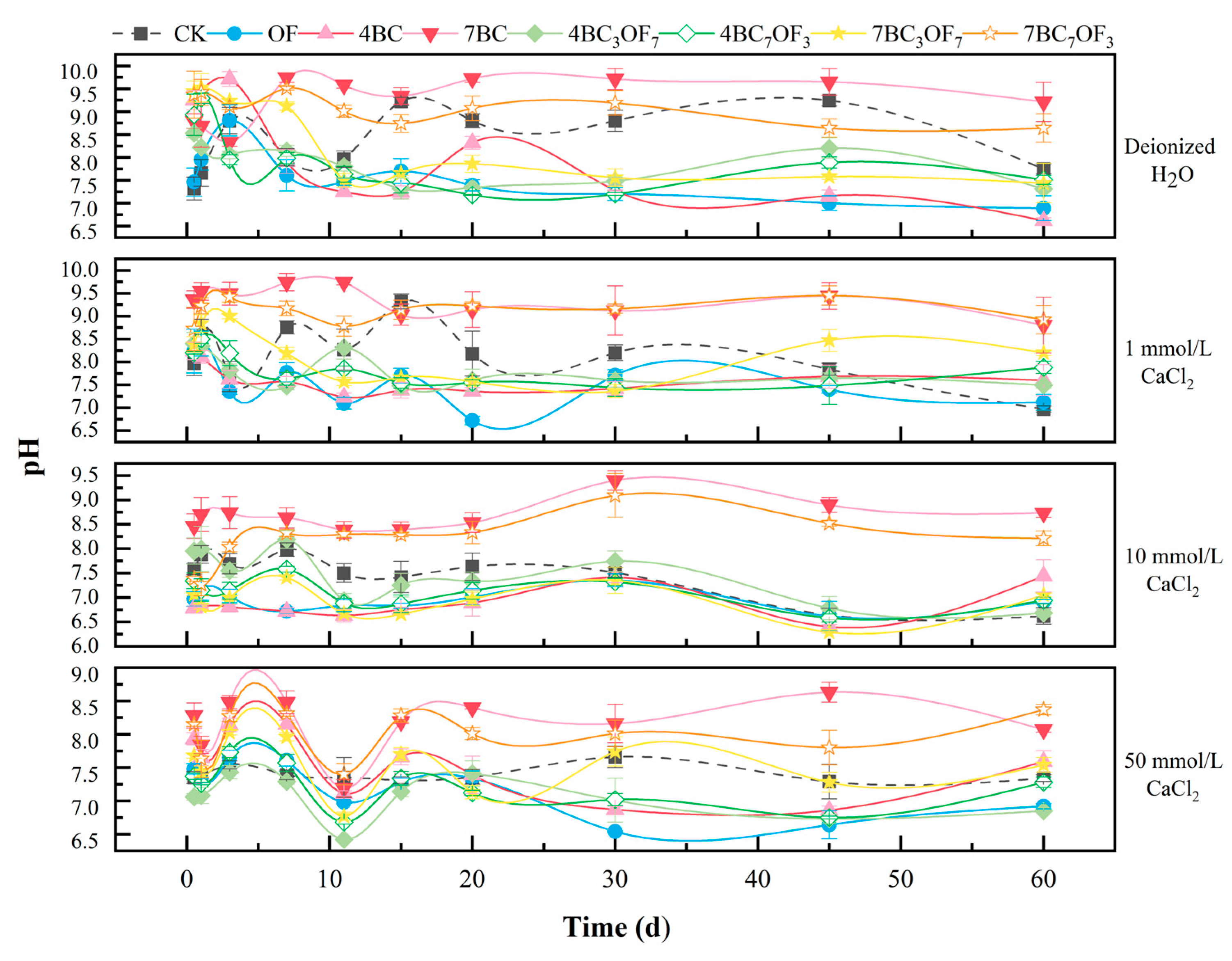
3.3. Effect of Ca2+ on Available Phosphorus Leaching from Biochar and Organic Fertilizer
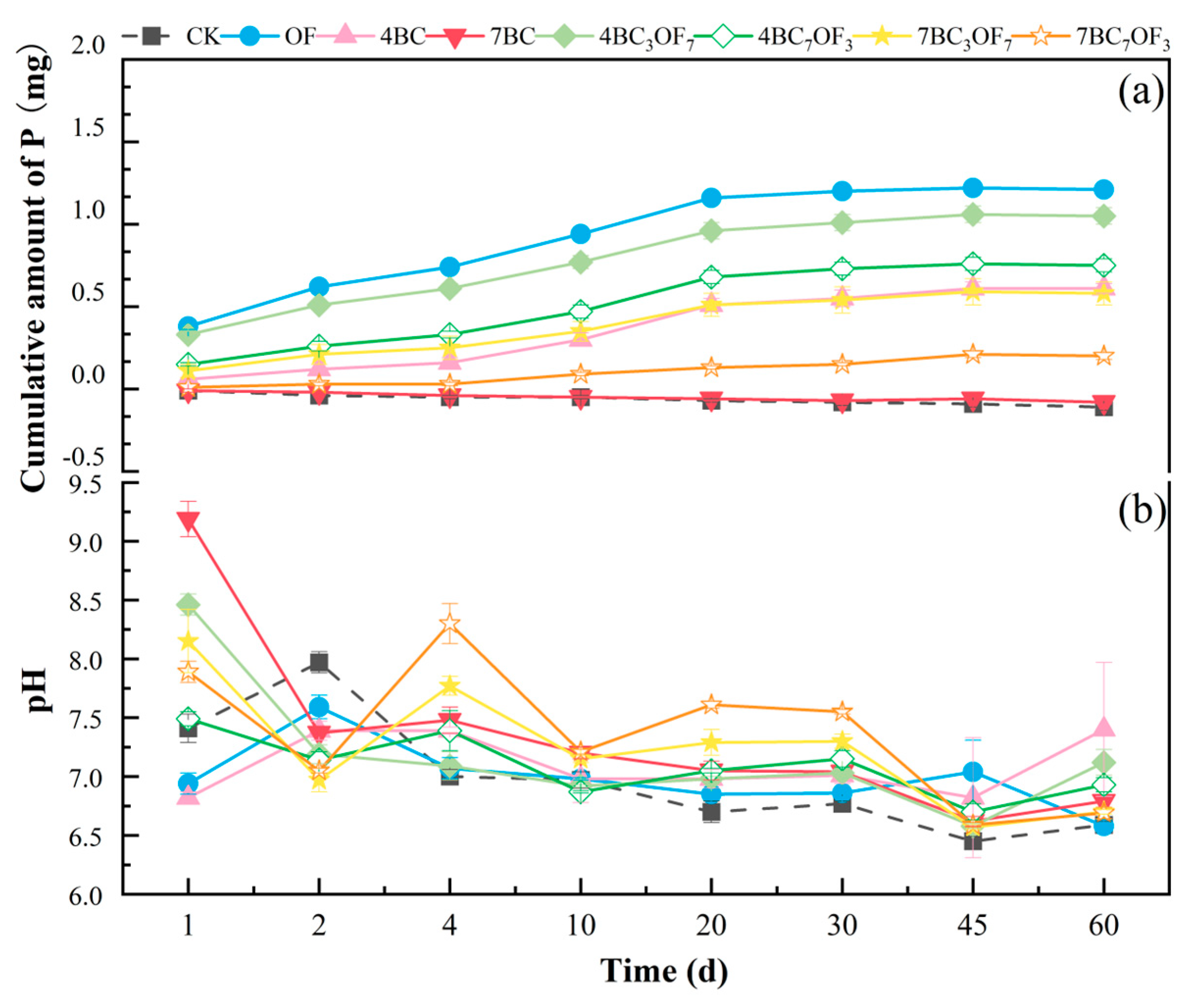
3.4. The Effects of Combined Application of Biochar and Organic Fertilizer on Cumulative Phosphorus Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogwu, M.C.; Patterson, M.E.; Senchak, P.A. Phosphorus Mining and Bioavailability for Plant Acquisition: Environmental Sustainability Perspectives. Environ. Monit. Assess. 2025, 197, 572. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, P.; Li, Y.; Ma, L.; Alva, A.; Dou, Z.; Chen, Q.; Zhang, F. Phosphorus in China’s Intensive Vegetable Production Systems: Overfertilization, Soil Enrichment, and Environmental Implications. J. Environ. Qual. 2013, 42, 982–989. [Google Scholar] [CrossRef]
- Mansouri, H.; El Amine, B.; Ait Said, H.; Noukrati, H.; Perreault, F.; Oukarroum, A.; Ben Youcef, H. Assessing Brushite-Polyphosphate Nanocomposites as Nanofertilizers for Improved Phosphorus Use Efficiency in Maize. J. Agric. Food Res. 2025, 22, 102131. [Google Scholar] [CrossRef]
- Nahidan, S.; Ghasemzadeh, M. Biochemical Phosphorus Transformations in a Calcareous Soil as Affected by Earthworm, Cow Manure and Its Biochar Additions. Appl. Soil. Ecol. 2022, 170, 104310. [Google Scholar] [CrossRef]
- Liu, S.; Yang, D.; Wang, F.; Xu, J. Meta-analysis of the effect of fertilizer application on phosphorus forms in agricultural soils in China. Soil. Fertil. Sci. China 2024, 8, 43–51. [Google Scholar]
- Zhang, T.; Xu, H.; Ru, S.; Su, D. Distribution of Phosphorus in Soil Profiles after Continuous Application of Different Fertilizers. Environ. Sci. 2017, 38, 5247–5255. [Google Scholar]
- Ali, A.; Jabeen, N.; Chachar, Z.; Chachar, S.; Ahmed, S.; Ahmed, N.; Laghari, A.A.; Sahito, Z.A.; Farruhbek, R.; Yang, Z. The Role of Biochar in Enhancing Soil Health & Interactions with Rhizosphere Properties and Enzyme Activities in Organic Fertilizer Substitution. Front. Plant Sci. 2025, 16, 1595208. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Zhang, H.; Zhang, W.; Yu, X.; Lei, Q. Research Progress on Cropland Phosphorus Balance in China. Chin. J. Eco-Agric. 2015, 23, 1–8. [Google Scholar]
- Kalkhajeh, Y.K.; Huang, B.; Sørensen, H.; Holm, P.E.; Hansen, H.C.B. Phosphorus Accumulation and Leaching Risk of Greenhouse Vegetable Soils in Southeast China. Pedosphere 2021, 31, 683–693. [Google Scholar] [CrossRef]
- Zhou, K.; Li, H.; Li, X.; Zhou, B.; Wei, X.; Wang, Y.; Liu, N.; Li, X.; Zhan, X.; Han, X. Long-Term Combined Organic and Inorganic Fertilization Alters Soil Phosphorus Fractions and Peanut Uptake. Agronomy 2025, 15, 2104. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, W. Adsorptive Removal of Phosphate from Water Using Corn Straw Derived Biochar. Environ. Prog. Sustain. Energy 2025, e70125. [Google Scholar] [CrossRef]
- Huang, Q.; Maidinur, A.; Hu, Y.; He, X.; Jia, H. Effects of Biochar and Nitrogen Additionon Plant Growth and Soil Characteristics in Desertified Alpine Grasslands. J. Xinjiang Agric. Univ. 2024, 47, 216–223. [Google Scholar]
- Jiang, R.; Gao, R.; Li, X.; Xing, Z.; Yu, G. Exogenous Calcium-Induced Phosphorus Transformation in Pig Manure Pyrolysis: Insights from Pot Experiment and Material Flow Analysis for Phosphorus Speciation. J. Environ. Chem. Eng. 2025, 13, 11747. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Peng, Y.; Xiang, P.; Zhou, Y.; Yao, B.; Zhou, Y.; Sun, C. Co-Application of Biochar and Organic Fertilizer Promotes the Yield and Quality of Red Pitaya (Hylocereus polyrhizus) by Improving Soil Properties. Chemosphere 2022, 294, 133619. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Lan, Y.; Liu, Z.; Yang, X.; Liu, S.; He, T.; Wang, D.; Meng, J.; Chen, W. Responses of Organic and Inorganic Phosphorus Fractions in Brown Earth to Successive Maize Stover and Biochar Application: A 5-Year Field Experiment in Northeast China. J. Soils Sediments 2020, 20, 2367–2376. [Google Scholar] [CrossRef]
- Yang, L.; Wu, Y.; Wang, Y.; An, W.; Jin, J.; Sun, K.; Wang, X. Effects of Biochar Addition on the Abundance, Speciation, Availability, and Leaching Loss of Soil Phosphorus. Sci. Total Environ. 2021, 758, 143657. [Google Scholar] [CrossRef]
- Wilson, K.; Iqbal, J.; Obaid Abdalla Obaid Hableel, A.; Naji Khalaf Beyaha Alzaabi, Z.; Nazzal, Y. Camel Dung-Derived Biochar for the Removal of Copper (Ii) and Chromium (Iii) Ions from Aqueous Solutions: Adsorption and Kinetics Studies. ACS Omega 2024, 9, 11500–11509. [Google Scholar] [CrossRef]
- Zhang, P.; Bing, X.; Jiao, L.; Xiao, H.; Li, B.; Sun, H. Amelioration Effects of Coastal Saline-Alkali Soil by Ball-Milled Red Phosphorus-Loaded Biochar. Chem. Eng. J. 2022, 431, 133904. [Google Scholar] [CrossRef]
- Ge, Q.; Tian, Q.; Feng, K.; Wang, S.; Hou, R. Effect of Co-Application of Phosphorus-Modified Hydrochar and Zeolite on Release of Soil Available Phosphorus. Res. Environ. Sci. 2022, 35, 219–229. [Google Scholar]
- Wang, Y.; Xiao, X.; Chen, B. Biochar Impacts on Soil Silicon Dissolution Kinetics and Their Interaction Mechanisms. Sci. Rep. 2018, 8, 804. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Adeyanju, C.A.; Iwuozor, K.O.; Emenike, E.C.; Adeniyi, A.G. Retort Co-Carbonization of Daniellia oliveri Leaves: Effect of Cow Dung Co-Feed on Biochar Properties. Waste Biomass Valorization 2024, 15, 4235–4246. [Google Scholar] [CrossRef]
- Sun, D. Effects of Long-Term Application of Organic Fertilizer on Soil Phosphorus Fraction Contents and Leaching Risk in Greenhouse Cultivation. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2023. [Google Scholar]
- Li, G.; Li, H.; Leffelaar, P.A.; Shen, J.; Zhang, F. Characterization of Phosphorus in Animal Manures Collected from Three (Dairy, Swine, and Broiler) Farms in China. PLoS ONE 2014, 9, e102698. [Google Scholar] [CrossRef]
- Qian, T.; Yang, Q.; Jun, D.C.F.; Dong, F.; Zhou, Y. Transformation of Phosphorus in Sewage Sludge Biochar Mediated by a Phosphate-Solubilizing Microorganism. Chem. Eng. J. 2019, 359, 1573–1580. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.J.; Park, J.-H.; Wang, J.; Wang, X.; Zhao, Z.; Song, F.; Tang, B. Pyrolysis Temperature Affects Dissolved Phosphorus and Carbon Levels in Alkali-Enhanced Biochar and Its Soil Applications. Agronomy 2022, 12, 1923. [Google Scholar] [CrossRef]
- Kumari, K.G.I.D.; Moldrup, P.; Paradelo, M.; Elsgaard, L.; Hauggaard-Nielsen, H.; De Jonge, L.W. Effects of Biochar on Air and Water Permeability and Colloid and Phosphorus Leaching in Soils from a Natural Calcium Carbonate Gradient. J. Environ. Qual. 2014, 43, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.; Ong, W.S.; Lu, D.; Zhou, Y. A Potential Phosphorus Fertilizer to Alleviate the Coming “Phosphorus Crisis”-Biochar Derived from Enhanced Biological Phosphorus Removal Sludge. Sci. Total Environ. 2022, 838, 156559. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Cheng, K.; Yang, F. Effects of Biochar on Transport and Retention of Phosphorus in Porous Media: Laboratory Test and Modeling. Environ. Pollut. 2022, 297, 118788. [Google Scholar] [CrossRef]
- Gulyás, M.; Fuchs, M.; Kocsis, I.; Füleky, G. Effect of the Soil Treated with Biochar on the Rye-Grass in Laboratory Experiment. Acta Univ. Sapientiae Agric. Environ. 2014, 6, 24–32. [Google Scholar] [CrossRef]
- Feng, Y.; Luo, Y.; He, Q.; Zhao, D.; Zhang, K.; Shen, S.; Wang, F. Performance and Mechanism of a Biochar-Based Ca-La Composite for the Adsorption of Phosphate from Water. J. Environ. Chem. Eng. 2021, 9, 105267. [Google Scholar] [CrossRef]
- Xu, G.; Sun, J.; Shao, H.; Chang, S.X. Biochar Had Effects on Phosphorus Sorption and Desorption in Three Soils with Differing Acidity. Ecol. Eng. 2014, 62, 54–60. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, X.; Hua, L.; Zhou, S.; Jiang, W.; Tang, Y.; Qian, J. Effects of Tea Residue Biochar on Phosphorus Adsorption-Desorption in Soil. Pol. J. Environ. Stud. 2022, 31, 2461–2471. [Google Scholar] [CrossRef]
- Li, R.; Zhang, S.; Zhang, M.; Fei, C.; Ding, X. Phosphorus Fractions and Adsorption–Desorption in Aggregates in Coastal Saline-Alkaline Paddy Soil with Organic Fertilizer Application. J. Soils Sediments 2021, 21, 3084–3097. [Google Scholar] [CrossRef]
- Sun, X.; Gao, W.; Hao, X.; Xu, M.; Zhang, J.; Meng, H. Effects of Different Manures on Phosphorus Adsorption and Desorption of a Reclaimed Mining Soil. J. Plant Nutr. Fertil. 2023, 29, 677–689. [Google Scholar]
- Szara, E.; Sosulski, T.; Szymańska, M. Soil Phosphorus Sorption Properties in Different Fertilization Systems. Plant Soil. Environ. 2019, 65, 78–82. [Google Scholar] [CrossRef]
- Ma, F.; Zhao, B.; Zhong, J.; Diao, J.; Zhang, Y. Characteristics Phosphate Adsorption onto Biochars Derived from Dairy Manure and Its Influencing Factors. China Environ. Sci. 2015, 35, 1156–1163. [Google Scholar]
- Kang, J.; Amoozegar, A.; Hesterberg, D.; Osmond, D.L. Phosphorus Leaching in a Sandy Soil as Affected by Organic and Inorganic Fertilizer Sources. Geoderma 2011, 161, 194–201. [Google Scholar] [CrossRef]
- Jin, J.; Fang, Y.; He, S.; Liu, Y.; Liu, C.; Li, F.; Khan, S.; Eltohamy, K.M.; Liu, B.; Liang, X. Improved Phosphorus Availability and Reduced Degree of Phosphorus Saturation by Biochar-Blended Organic Fertilizer Addition to Agricultural Field Soils. Chemosphere 2023, 317, 137809. [Google Scholar] [CrossRef]
- Serrano, M.F.; López, J.E.; Henao, N.; Saldarriaga, J.F. Phosphorus-Loaded Biochar-Assisted Phytoremediation to Immobilize Cadmium, Chromium, and Lead in Soils. ACS Omega 2024, 9, 3574–3587. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Xiang, J.; Xiong, J.; Wang, Y.; Wang, Z.; Zhang, Y. Effect of Rice-Straw Biochar Application on the Acquisition of Rhizosphere Phosphorus in Acidified Paddy Soil. Agronomy 2022, 12, 1556. [Google Scholar] [CrossRef]
| Treatment | Organic Fertilizer | 400 °C Biochar | 700 °C Biochar |
|---|---|---|---|
| CK | 0 | 0 | 0 |
| OF | 100% (50 mg) | 0 | 0 |
| 4BC | 0 | 100% (50 mg) | 0 |
| 7BC | 0 | 0 | 100% (50 mg) |
| 4BC7OF3 | 30% (15 mg) | 70% (35 mg) | 0 |
| 4BC3OF7 | 70% (35 mg) | 30% (15 mg) | 0 |
| 7BC7OF3 | 30% (15 mg) | 0 | 70% (35 mg) |
| 7BC3OF7 | 70% (35 mg) | 0 | 30% (15 mg) |
| Samples | Available Phosphorus (mg·g−1) | pH | EC (1:5) (μS·cm−1) | Ash (%) | Productivity (%) |
|---|---|---|---|---|---|
| Organic fertilizer (OF) | 52.03 ± 0.48 | 9.47 ± 0.03 | 10.99 ± 0.67 | / | / |
| 400 °C Biochar (4BC) | 21.77 ± 0.29 | 9.59 ± 0.01 | 6.22 ± 0.07 | 74.45 ± 0.38 | 66.25 ± 2.93 |
| 700 °C Biochar (7BC) | 3.62 ± 0.15 | 11.07 ± 0.06 | 6.87 ± 0.18 | 82.04 ± 0.58 | 51.75 ± 1.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, D.; Wang, Y.; Zheng, T.; Zhou, Q.; Sheng, J. Synergy of Biochar and Organic Fertilizer Reduces Phosphorus Leaching. Agronomy 2025, 15, 2528. https://doi.org/10.3390/agronomy15112528
Ma D, Wang Y, Zheng T, Zhou Q, Sheng J. Synergy of Biochar and Organic Fertilizer Reduces Phosphorus Leaching. Agronomy. 2025; 15(11):2528. https://doi.org/10.3390/agronomy15112528
Chicago/Turabian StyleMa, Danni, Yaofeng Wang, Tong Zheng, Qixing Zhou, and Jiandong Sheng. 2025. "Synergy of Biochar and Organic Fertilizer Reduces Phosphorus Leaching" Agronomy 15, no. 11: 2528. https://doi.org/10.3390/agronomy15112528
APA StyleMa, D., Wang, Y., Zheng, T., Zhou, Q., & Sheng, J. (2025). Synergy of Biochar and Organic Fertilizer Reduces Phosphorus Leaching. Agronomy, 15(11), 2528. https://doi.org/10.3390/agronomy15112528








