1. Introduction
The Great Hungarian Plain is experiencing mounting water stress due to shifting climatic conditions, such as uneven precipitation, falling groundwater tables, and persistent drought events. Although the Körös River is a crucial component of the region’s irrigation infrastructure, its unpredictable flow patterns frequently result in insufficient water availability during periods critical to crop productivity.
Globally, farming activities represent the most significant demand for freshwater resources, with estimates suggesting that nearly 70% of total withdrawals are linked to agricultural use [
1]. This sector remains fundamental to food production systems and is indispensable for maintaining global food supply chains. Currently, the total area of land suitable for agricultural use is estimated at approximately 1.5 billion hectares, of which about 23%—approximately 355 million hectares—is irrigated. Despite its relatively small share, irrigated land contributes to around 40% of global food production, whereas the remaining 83% (approximately 1.25 billion hectares), which relies solely on rainfall, produces only about 60% [
2]. Across the globe, irrigation infrastructure has been established on an estimated 355 million hectares of land, serving various sectors, with agriculture being the predominant user. However, about 20% of these areas—around 62 million hectares—are already affected by significant salt and sodium accumulation, which negatively impacts soil fertility, water retention, and plant nutrient uptake. The degradation of soil through salinization is linked to significant reductions in crop yields worldwide, resulting in an estimated yearly economic loss of USD 27.3 billion [
3]. This challenge is compounded by the intensifying global water crisis, which places added pressure on the long-term sustainability of agricultural water use—especially in dryland areas. In this context, alternative water sources—such as effluents from intensive aquaculture operations—are gaining attention for meeting crop irrigation demands. The practice of repurposing aquaculture wastewater supports both water conservation and nutrient management, as it allows for the reintegration of nitrogen and phosphorus into agricultural systems—elements frequently present in such effluent streams [
4].
Sorghum (
Sorghum bicolor L. Moench) is a heat-tolerant, annual C4 plant species that is extensively grown in dry and semi-dry climates for purposes ranging from grain and forage production to bioenergy applications [
5,
6]. It is recognized as a promising forage crop in these environments due to its exceptional tolerance to drought and salinity. Under saline irrigation regimes, sorghum hybrids often exhibit diverse physiological and agronomic responses, including differences in growth vigor, nutrient absorption (notably nitrogen and phosphorus), chlorophyll content, and potential impacts on soil conditions [
7,
8]. Several studies have demonstrated that certain sorghum hybrids exhibit tolerance to mild salinity under specific soil conditions—for instance, at salt concentrations around 0.32%—a trait that supports their suitability for cultivation in salt-affected environments [
7].
Plant responses to saline irrigation are highly dependent on salt concentration. While modest salinity may provide essential nutrients that support growth, higher levels typically disrupt water balance and lead to osmotic stress, limiting overall plant performance. Ghalkhani et al. (2023) demonstrated that sorghum hybrids respond differently to water scarcity and irrigation methods, with drip irrigation promoting greater biomass production, especially under drought stress [
4]. Similarly, Gao et al. (2021) evaluated 66 sorghum germplasm lines and found that Sudan grass-type cultivars exhibited greater salinity tolerance compared to other varietal groups that were more sensitive to elevated salt levels [
9].
Despite its general tolerance, sorghum remains vulnerable to and developmental constraints under salt stress. In sweet sorghum genotypes, high salt concentrations may interfere with key early growth stages, including seed germination and seedling establishment, ultimately leading to lower biomass production [
8]. At the cellular level, salinity stress can damage the plasma membrane, reduce photosynthetic efficiency, and disrupt ion homeostasis, leading to both osmotic and oxidative stress [
7,
10,
11].
Chlorophyll levels serve as a reliable marker of physiological stress in plants. Under saline irrigation conditions, these concentrations often decline as a result of salt-induced stress mechanisms. However, this negative effect may be partially offset by the presence of beneficial nutrients, such as nitrates and phosphates, in the effluent. Additionally, trace elements commonly found in aquaculture effluent, such as Fe and Zn, can influence chlorophyll synthesis and photosynthetic function [
12,
13,
14].
When irrigating with saline aquaculture effluent, plants absorb not only water but also substantial amounts of dissolved salts and nutrients. The accumulation of sodium (Na
+) and chloride (Cl
−) ions may interfere with the uptake of essential elements such as potassium (K
+), calcium (Ca
2+), and magnesium (Mg
2+), leading to ionic imbalances. However, organic compounds and microbial activity present in the effluent may enhance nutrient mobilization and uptake [
15,
16,
17].
In addition, the long-term application of irrigation water with elevated salinity can lead to increased soil salt concentrations, particularly in soils with poor drainage. However, certain soil amendments—such as the application of biochar or organic manures—may improve soil structure, reduce salt accumulation, and enhance soil organic matter content [
18]. Wu et al. (2025) demonstrated that treatments combining organic matter, improved sorghum growth and soil carbon sequestration in saline soils [
7].
In our previous research [
5], we irrigated various grain sorghum cultivars using effluent from an intensive African catfish aquaculture system. We found that yield and nutrient uptake were generally favorable under effluent irrigation; however, significant differences were observed among genotypes. While salt tolerance and water use efficiency are critical traits in grain sorghum, they are equally relevant for forage sorghum hybrids—especially in terms of biomass production, chlorophyll content, and long-term soil responses.
While previous studies have explored the use of saline aquaculture effluent for crop irrigation, most have focused on short-term effects, greenhouse conditions, or grain sorghum genotypes. In contrast, our study provides a multi-year field evaluation of forage sorghum hybrids under five distinct irrigation regimes, including both freshwater and effluent sources. The novelty of this research lies in its hybrid-specific analysis of biomass yield, nutrient uptake, and soil responses, with particular emphasis on nitrogen dynamics and water use efficiency. By comparing effluent doses and irrigation volumes across four growing seasons, we offer practical insights into optimizing crop water use under real-world conditions. This approach distinguishes our work from previous reports and contributes to the development of sustainable irrigation strategies in water-limited environments.
The aim of the present study is to assess the growth, chlorophyll concentration, nutrient and ion uptake, and changes in soil physical and chemical properties of different forage sorghum hybrids irrigated with intensive aquaculture effluent under field conditions. Additionally, we aim to the extent to which these hybrids exhibit adaptive responses to elevated salt loads, and how such irrigation practices influence soil functioning as a plant growth medium. Our hypothes that saline aquaculture effluent irrigation will enhance forage sorghum growth and nutrient uptake without causing detrimental soil salinity buildup under Hungarian field conditions.
2. Materials and Methods
2.1. Description of the Experimental Site and Plant Material
The experiment was conducted at the Lysimeter Research Station of the Irrigation and Water Management Research Centre, Hungarian University of Agriculture and Life Sciences, located in Szarvas, Hungary (46°51′49″ N, 20°31′39″ E). The field trial was established in 2016 and extended over four growing seasons (2016, 2017, 2019, and 2020), using a 160 m
2 open-field microplot area (
Figure 1). The study focused on evaluating the irrigation response of three forage sorghum hybrids (‘
GK Áron’, ‘
GK Balázs’, ‘
GK Erik’), developed by the Cereal Research Non-Profit Ltd., located in Szeged, Hungary.
These sorghum hybrids are distinguished by their strong stalk structure and high yield capacity. Their remarkable drought resilience and ability to thrive in low-fertility soils make them well-suited for cultivation under challenging environmental conditions. Due to their excellent standability, they can be sown in monoculture or in combination with silage maize of FAO maturity group 400. The hybrids belong to the medium-maturity group, reaching plant heights between 200 and 300 cm, and are known for their juicy stalks and high sugar content (14–16 °Brix).
2.2. Experimental Design and Irrigation Treatments
In each growing season, sowing was carried out in the last week of April or the first week of May, once the top 10 cm of soil had reached a temperature of 10–12 °C. The experimental plots were established with a row spacing of 70 cm, and planting densities ranged between 200,000 and 240,000 plants per hectare, corresponding to 160–178 individuals per plot. Each plot consisted of four rows (1 m wide), and measurements were consistently taken from the two central rows, with three (height, SPAD values, mineral content) and six (biomass product) replications per treatment. The resulting sampling area for each replicate measured 3 m in length and 2.1 m in width. Throughout the four experimental years, nutrient supply and plant protection treatments were applied according to seasonal needs and hybrid requirements. In 2016 and 2020, foliar fertilization was performed using Wuxal Super at a dose of 4 L/ha. In 2017 and 2019, sodium supplementation was applied via Dosatron at a rate of 40 kg/ha Na active ingredient. Plant protection measures included insecticide treatments such as Fedona 10 EC in 2016, and a combination of Decis Mega, Mospilan 20 SG, and Mister in 2020. No chemical plant protection was applied in 2017 and 2019.
Due to the technical constraints of the drip irrigation system, the experimental layout followed a non-randomized fixed-effect design. Each forage sorghum hybrid was assigned to a separate band, and the five irrigation treatments (C, K30, K45, E30, E45) were applied in a fixed order within each row. Within each hybrid-treatment combination, three or six subsamples were treated as true replicates and were used directly in the statistical analysis as individual observations. Thus, the analysis was based on three or six replicates per treatment, and no averaging was performed at the plot level. Statistical evaluations were performed separately for each hybrid and year, using one-way ANOVA to compare irrigation treatments within each hybrid. This approach allowed for hybrid-specific insights under the environmental conditions of each growing season. Consequently, each treatment was assigned to three large plots (totaling 15 plots), and each plot contained one forage sorghum hybrid (‘GK Áron’, ‘GK Balázs’, or ‘GK Erik’). This layout ensured consistent replication and allowed for statistical analysis of treatment effects.
All three hybrids were irrigated weekly using two water sources: freshwater (Körös River oxbow lake: K30, K45) and aquaculture effluent (E30, E45). Irrigation amounts, based on the breeder’s recommendations, were either 30 or 45 mm per week, delivered through a drip irrigation system [
19]. The number of irrigation events varied between four and eight per year, resulting in a total irrigation input of 120–360 mm. Natural precipitation during the growing season contributed an additional 144–296 mm, while 40–120 mm of supplementary water was applied during germination. Overall, the combined water input across the experimental years ranged from 404 to 641 mm, ensuring adequate crop water supply under the prevailing conditions.
For plant height, SPAD values, and chemical analyses, three replicates per treatment were used. These replicates were taken from distinct plants within each plot. Plant growth was monitored on a weekly basis throughout the vegetation period, with measurements including plant height and relative chlorophyll content. Relative chlorophyll levels were assessed using a handheld SPAD-502 chlorophyll meter (Konica Minolta, Osaka, Japan), which provides non-destructive estimates of leaf greenness. SPAD values were determined as the average of three measurements per plant, taken from the lower, middle, and upper leaves. For biomass determination, six individual sampling points were collected per treatment plot. These measurements were treated as independent replicates in the statistical analysis, without averaging. This approach allowed for capturing within-plot variability and provided sufficient statistical power for comparing irrigation treatments.
At the end of the growing season, plant part samples were collected for mineral nutrient analysis. During sampling, the leaves and stems of selected sorghum plants were cut into 5 cm pieces, 1 kg per hybrid per treatment was weighed into a sampling bag and finally sent for analysis. All analyses followed national and international standard protocols, including the quantification of nitrogen (N), phosphorus (P), potassium (K), and sodium (Na) content in plant parts. Nitrogen content was determined using the Kjeldahl method, based on the Hungarian standard MSZ 08 1783 28-30:1985 (Feed analysis–Determination of nitrogen content by the Kjeldahl method, published by the Hungarian Standards Institution, Budapest, Hungary, 1985), while the analytical procedures also complied with ISO 5983-2:2009 (Animal feeding stuffs—Determination of nitrogen content and calculation of crude protein content—Part 2: Block digestion and steam distillation method, published by the International Organization for Standardization, Geneva, Switzerland, 2009). The experimental protocol was based on methodologies previously described in our earlier publications (Kolozsvári et al., 2021; 2022) [
5,
20].
Harvesting was timed to occur when the sorghum reached the soft dough stage, as this phenological phase ensures optimal dry matter (d.m.) content (30–33%) for successful silage fermentation, particularly in promoting favorable lactic acid production.
The field experiments were conducted over four growing seasons (2016, 2017, 2019, and 2020), using a consistent open-field microplot setup and standardized irrigation protocols. Despite the temporal spread, the experimental conditions—including soil type, irrigation volumes, water sources, and planting densities—remained uniform, allowing for meaningful comparisons across years. Statistical evaluations were performed separately for each season and hybrid, focusing on treatment-specific effects within each year. This approach enabled hybrid-specific insights while capturing interannual variability in climate and crop response. From a spatiotemporal perspective enhances the robustness and referential value of the findings, offering practical relevance for forage sorghum production under water-limited field conditions.
2.3. Characterization of Irrigation Water
This investigation forms part of a larger experimental framework aimed at assessing the performance of various sorghum genotypes under irrigation with aquaculture effluent. The experimental setup—including water sources, irrigation volumes, soil conditions, and agronomic practices—was identical to that described in our previous publication Kolozsvári et al. [
5], which focused on grain sorghum cultivars. In contrast, the present paper investigates forage sorghum hybrids, providing complementary insights into biomass production, nutrient uptake, and soil responses. Detailed water quality parameters of the effluent are available in the referenced article. As the control (freshwater) source, we used surface water from the Körös oxbow lake located near the experimental site (46°51′38.6″ N, 20°31′28.0″ E, Szarvas, Hungary). The alternative irrigation water source was untreated effluent from an intensive African catfish (
Clarias gariepinus) aquaculture facility.
This effluent is characterized by elevated sodium (Na) levels and a high sodium adsorption ratio (SAR), attributed to the use of thermal water in the aquaculture system. After mechanical filtration, the water is pumped into sedimentation basins and subsequently discharged into the Körös oxbow. Previous studies by Kerepeczki et al. [
21] and Tavares et al. [
22] have shown that this discharge contributes to nutrient accumulation and eutrophication in the natural water body.
2.4. Characterization of the Soil
Soil parameters were assessed before and after the experiment. Initial soil sampling was performed on 24 April 2016 in the designated sorghum cultivation area. Samples were collected from two depth intervals (0–30 cm and 30–60 cm) using a composite sampling method, with each replicate consisting of subsamples from three distinct points within each main plot. The study was conducted using six separate replicates, allowing for consistent evaluation across treatments. The detailed methodologies for soil and plant sampling, as well as the analytical procedures—including nutrient extraction and quantification—follow those described in our previous publications (Kolozsvári et al., 2021 [
20]) Kolozsvári et al., 2022 [
5]), where the full protocols are available.
The field trial was established on a Vertisol, the predominant soil type in this region. The average values of the main agrochemical parameters are summarized in
Table 1. At the beginning of the long-term experiment, the soil exhibited a near-neutral to slightly alkaline pH. The carbonate content indicated a mildly calcareous nature, while the organic carbon level was low, with organic matter content remaining below 2%, classifying the soil as poorly supplied in this regard.
The measured levels of nitrate, extracted using KCl, indicated insufficient nitrogen in the soil, highlighting the importance of applying additional nitrogen fertilizer. In contrast, phosphorus and potassium concentrations were considerably high. The sodium content, determined by AL extraction, remained within acceptable limits. This parameter was closely monitored throughout the study due to the application of runoff water with elevated sodium levels for irrigation purposes.
2.5. Meteorological Conditions
Meteorological data were recorded using Agromet solar automatic weather station located in Szarvas, Hungary. For the years 2016, 2017, 2019, and 2020, the dataset includes monthly values of key climatic parameters: precipitation (mm), along with average, minimum, and maximum temperatures (°C). These parameters provide a comprehensive overview of the climatic conditions during the experimental periods.
Table 2 presents the annual precipitation totals varied considerably across the studied years, with the highest cumulative rainfall observed in 2016 (633.6 mm) and the lowest in 2017 (530.6 mm). Monthly precipitation patterns showed notable variability, with June and July typically being the wettest months, particularly in 2016 and 2019, when June precipitation reached 124.4 mm and 162.4 mm, respectively.
Temperature records reflected the characteristics of a temperate continental climate, marked by cold winter periods and warm summer conditions. The mean monthly temperatures ranged from −6.7 °C (January 2017) to 24.1 °C (August 2019). The lowest recorded minimum temperature was −19.6 °C in January 2017, while the highest maximum temperature reached 39.2 °C in August 2017.
Spring and autumn months exhibited transitional thermal conditions, with April and October showing moderate temperatures and variable precipitation. Notably, April 2019 was exceptionally dry (0.6 mm), while October 2020 was markedly wet (89.7 mm).
These climatic data are essential for interpreting the environmental context of the field experiment and assessing the potential influence of weather variability on the observed agronomic outcomes.
2.6. Statistical Analysis
One-way ANOVA was performed separately for each hybrid and growing season, comparing irrigation treatments within each year. This approach was chosen to evaluate hybrid-specific responses under the environmental conditions of each season. In the statistical analysis, subsamples (e.g., six biomass measurements per treatment) were treated as replicates. These were spatially distributed within the central rows of each plot to reflect internal heterogeneity. No plot-level averaging was performed. This design was considered appropriate given the fixed plot layout and the aim to assess treatment effects within each hybrid and year. To evaluate differences among groups, Tukey’s HSD test was applied post hoc, considering results significant at p ≤ 0.05. Two-way ANOVA was used to determine the WUE value. Soil sample values were tested using the Kruskal–Wallis non-parametric test. Statistical analyses of plant height were performed using the data collected at the final measurement date for each growing season.
3. Results
3.1. Characterization of Phenological Parameters
Plant Height Data in Relation to Different Irrigation Methods
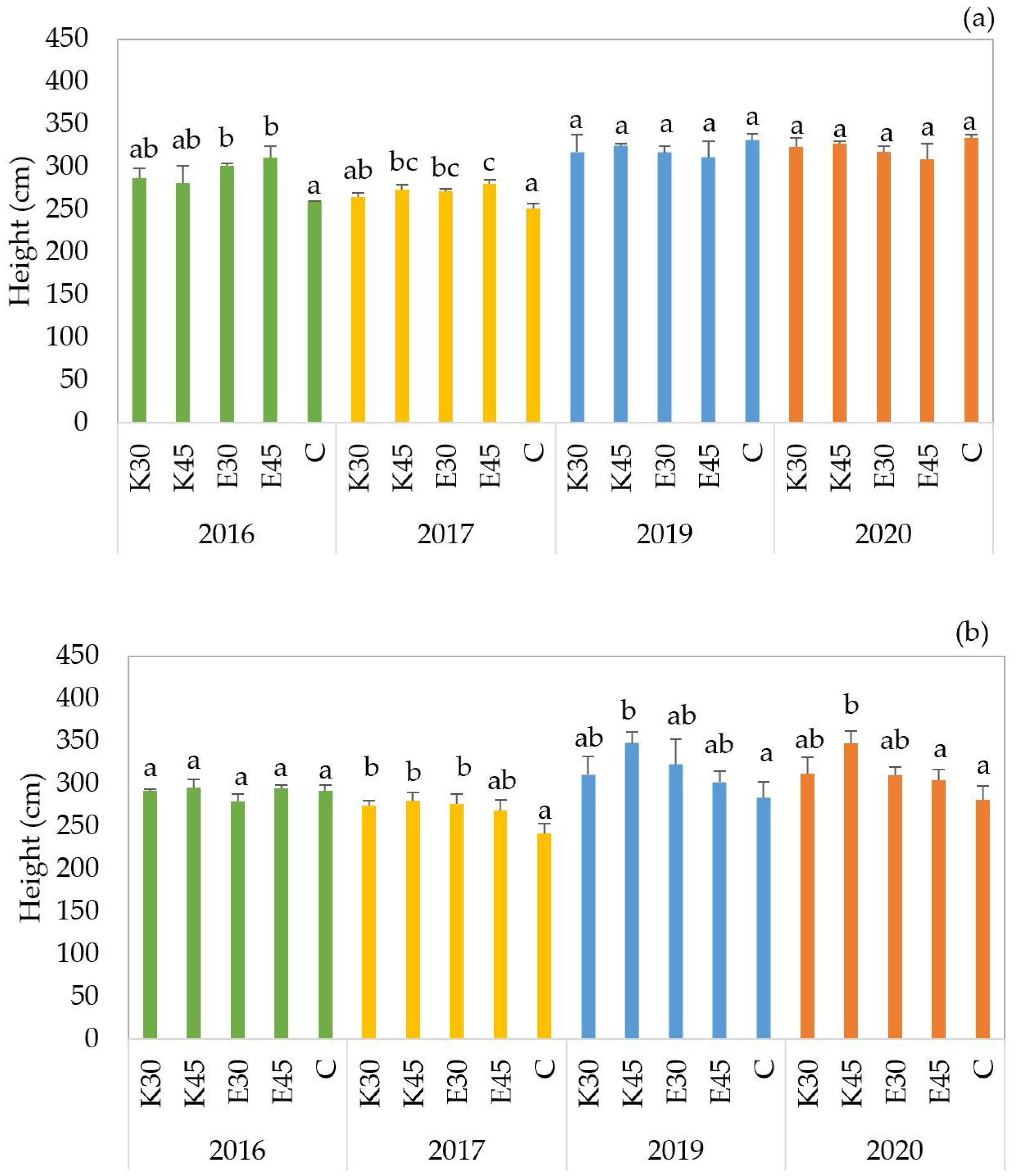
In the first growing season (2016), the heights of ‘
GK Áron’ hybrid plants ranged from between 260 cm and 312 cm (
Figure 2a). The lowest plant heights were recorded under the control (C) treatment, whereas the E45 treatment resulted in the tallest specimens. A similar pattern was noted in the second season (2017), with the C treatment resulting in the lowest heights and the E45 treatment the highest. In the last two seasons (2019 and 2020), plant heights exceeded 300 cm across all treatments. Where we found that the lowest values were measured in treatment E45 and the highest in treatment C. Significant differences among treatments were detected only in the first two years. In 2016, plants under E45 (
p = 0.002) and E30 (
p = 0.010) treatments were significantly taller than those in the control. In 2017, significantly higher plant heights were observed in E30 (
p = 0.004), K45 (
p = 0.002), and E45 (
p < 0.001) treatments compared to the control. In contrast, no statistically significant differences were found in 2019 and 2020, suggesting that more favorable environmental conditions may have minimized the effect of irrigation treatments on plant height during those years.
For the ‘
GK Balázs’ hybrid, the lowest plant height values were also recorded during the first and the second year (
Figure 2b). In 2016, plant heights ranged from 279 cm (E30) to 296 cm (E45). In 2017, the lowest value was recorded under the C treatment (242 cm), while the highest occurred under K45 (280 cm). During the 2019 and 2020 seasons, plant height exceeded 300 cm in all irrigated treatments. In 2020, only the control treatment remained below this value. One-way ANOVA showed no significant differences among treatments in 2016. However, in 2017, significantly higher plant heights compared to the control were observed under K30 (
p = 0.020), K45 (
p = 0.008), and E30 (
p = 0.015). In both 2019 and 2020, plants in K45 treatment exhibited significantly greater heights than the control (
p < 0.01). These results indicate a positive response of the ‘
GK Balázs’ hybrid to irrigation, especially under treatments using fishpond effluent.
In 2016, plant height for the ‘
GK Erik’ hybrid ranged between 307 cm and 320 cm. In 2017, the shortest plants were recorded under the C treatment (250 cm), while the tallest were found in the K30 treatment (292 cm) (
Figure 2c). In the last two seasons, plant height ranged from 322 cm to 382 cm. As with the previous hybrids, the shortest plants were consistently found in the control plots, whereas the tallest were observed under the E45 treatment. No statistically significant differences were found among treatments in 2016. However, in 2017, plants in several irrigated treatments showed significantly greater height than the control.
Across all sorghum hybrids, irrigation treatments consistently led to increased plant height compared to the non-irrigated control group. The highest values were typically observed under effluent irrigation, especially during the early vegetative growth stages. Control plots consistently showed the lowest plant height, underscoring the importance of supplemental water in promoting vegetative development.
3.2. Physiological Measurement of the Experimental Plants
Evaluation of Leaf Chlorophyll Content (SPAD) in Forage Sorghum Hybrids
Table 3 summarizes the relative chlorophyll content of forage sorghum leaves measured over four growing seasons (2016, 2017, 2019, and 2020).
Chlorophyll content in ‘GK Áron’ exhibited significant variability between years and treatments. In 2016, the highest SPAD readings were recorded under the K45 and control (C) treatments (50.2 and 50.4, respectively), while the E45 treatment resulted significantly lower values (44.3). In contrast, no statistically significant differences among treatments were found in 2017. However, E45 treatment resulted in the highest SPAD values (51.5-2019 and 52.4-2020), which were significantly higher than the other treatments. These findings suggest that irrigation using effluent water (E45) had a beneficial effect on leaf chlorophyll concentration in this hybrid, particularly under favorable environmental conditions.
The chlorophyll content of ‘GK Balázs’ was more uniform across treatments in 2016 and 2017, with no significant differences. In 2019, the E45 and control treatments produced significantly higher SPAD values (49.9 and 45.8, p < 0.01, respectively), while K45 and E30 treatments showed reduced values. A similar observation was found in 2020, where E45 again resulted in the highest chlorophyll content (50.3, p < 0.001).
In the ‘GK Erik’ hybrid, differences among treatments were moderate. SPAD values did not significantly vary across treatments in 2016 and 2017. In 2019, the highest SPAD value was measured under E45 (47.6, p < 0.01), whereas E30 and C treatments showed lower readings. In 2020, E45 reached the highest chlorophyll content (48.6, p = 0.011), while K45 and E30 treatments were lower. These results suggest that ‘GK Erik’ maintained relatively stable chlorophyll levels across varying water conditions, indicating a degree of drought tolerance and adaptive capacity.
3.3. Mineral Element Content of Plant Parts
3.3.1. Nitrogen Content
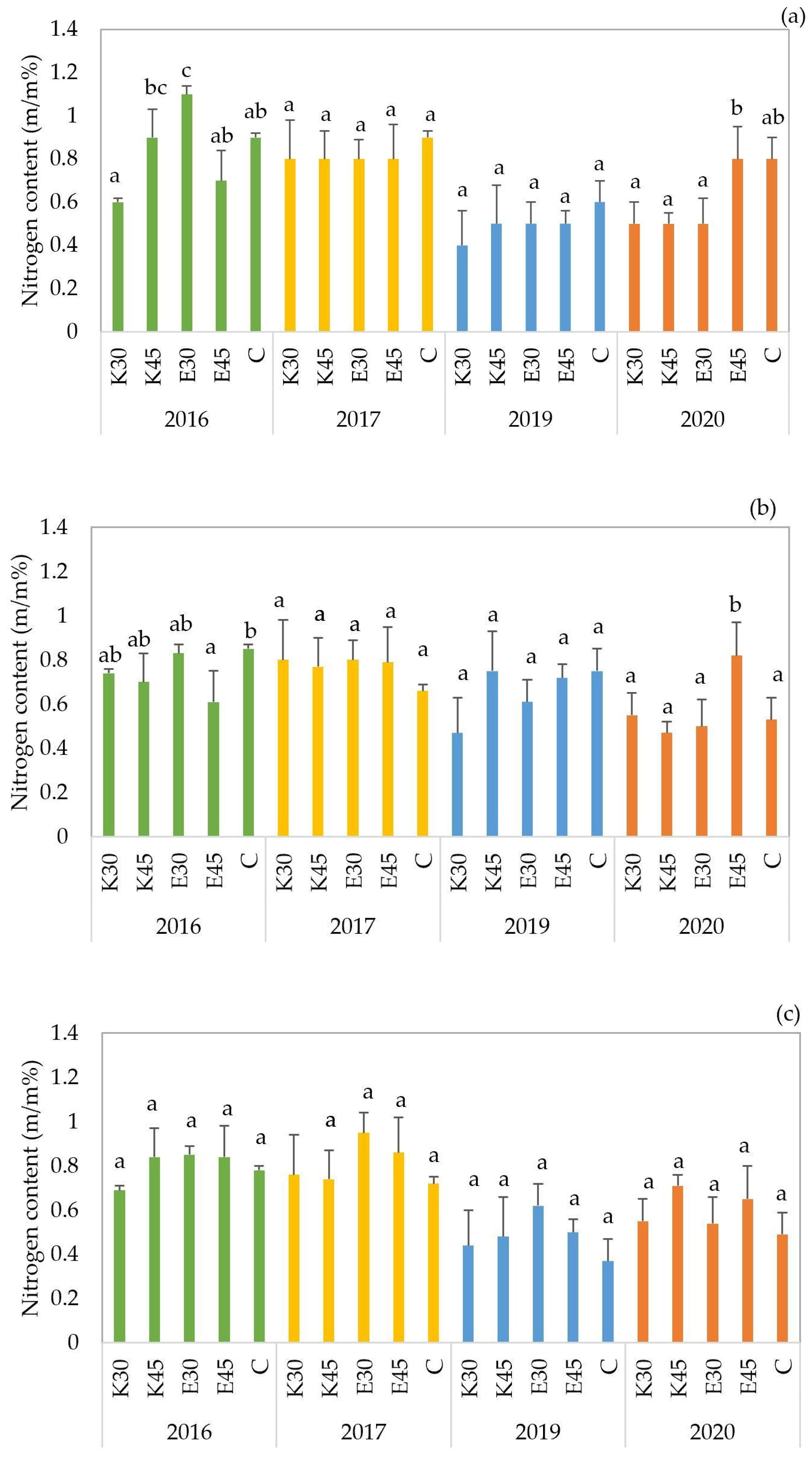
The nitrogen content in the plant parts of the ‘
GK Áron’ hybrid showed notable variation across irrigation treatments and years (
Figure 3a). The highest nitrogen concentration was recorded under the E30 treatment in 2016 (1.1 m/m%), which was significantly higher than in other treatments and years. In contrast, the lowest nitrogen content was observed under K30 in 2019 (0.4 m/m%). Across the four years, the control treatment (C) maintained relatively high nitrogen levels (0.9 m/m% in 2016 and 2017, 0.6 m/m% in 2019, and 0.8 m/m% in 2020), comparable to or exceeding some irrigated treatments. Statistical analysis revealed a significant difference among treatments in 2016, where E30 (
p < 0.001) higher. In other years, differences were less pronounced, but E45 in 2020 (0.8 m/m%,
p = 0.01) also showed a relatively high value, indicating potential benefits of higher effluent doses under dry conditions.
The nitrogen content in the plant parts of the ‘
GK Balázs’ hybrid showed moderate variation across irrigation treatments and years (
Figure 3b). The highest value was recorded under the control treatment (C) in 2016 (0.85 m/m%), followed closely by, E30 in 2016 (0.83 m/m%), and E45 in 2020 (0.82 m/m%). In contrast, the lowest nitrogen concentration was observed under K30 in 2020 (0.47 m/m%), indicating that lower effluent doses may not consistently supply sufficient nitrogen. Statistical analysis revealed significant differences in 2016 and 2020, where C (
p = 0.035) and E45 (
p= 0.002) treatments were consistently assigned to higher significance groups compared to other treatments.
The nitrogen content of the ‘
GK Erik’ forage sorghum hybrid ranged between 0.37 m/m% (C treatment in 2019) and 0.95 m/m% (E30 treatment in 2017) across the four growing seasons (2016, 2017, 2019, 2020) and irrigation treatments (
Figure 3c). Although effluent irrigation (E30, E45) generally resulted in slightly higher nitrogen concentrations compared to freshwater (K30, K45) and control (C) treatments, no statistically significant differences were observed among treatments in any of the examined years. In the case of ‘
GK Erik’, nitrogen content values remained relatively consistent across treatments and years, without statistically significant differences. This indicates that the hybrid maintained stable nitrogen uptake under varying irrigation regimes and water qualities.
3.3.2. Phosphorus Content
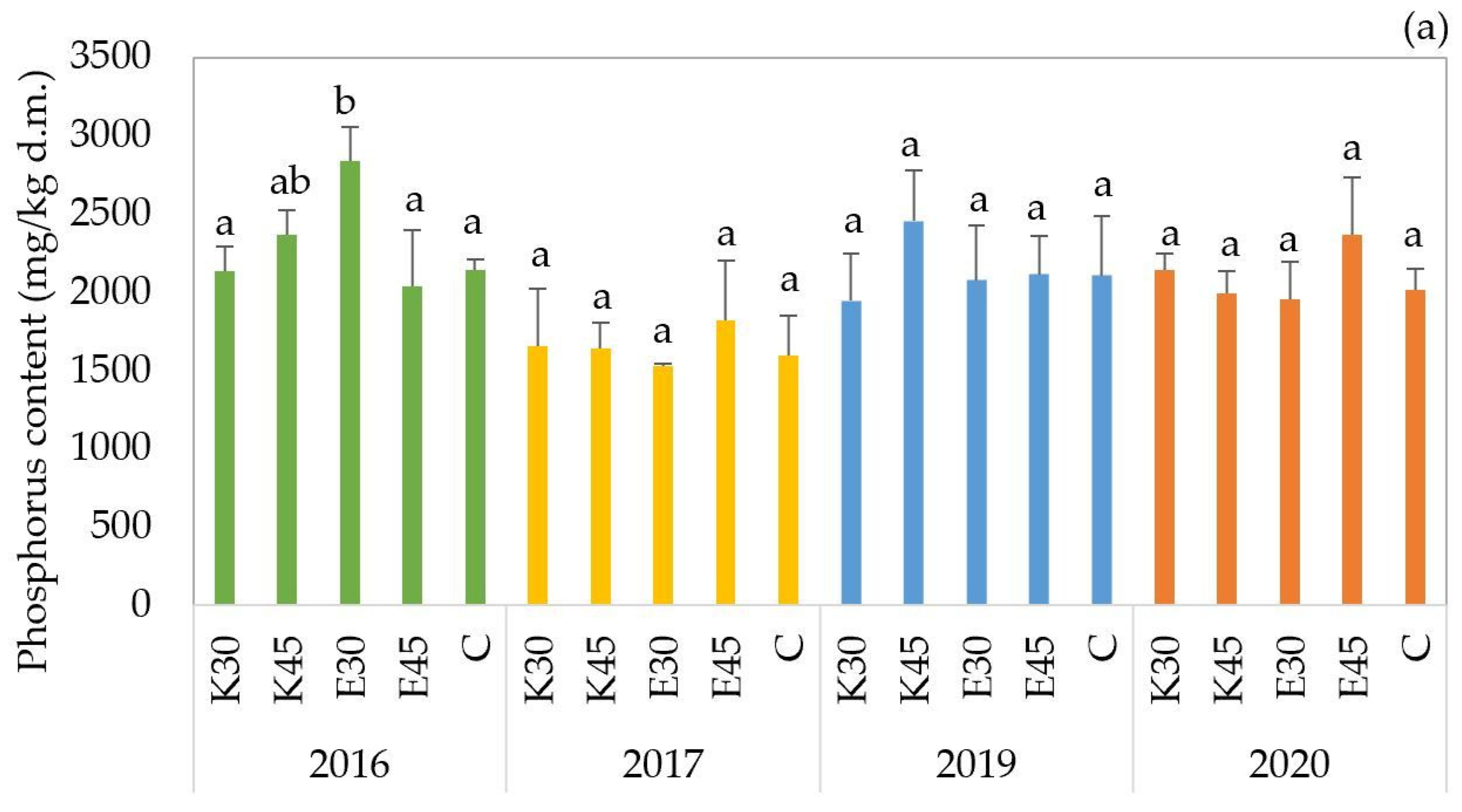
The phosphorus (P) content of the ‘
GK Áron’ forage sorghum hybrid varied across treatments and growing seasons, ranging from 1533 to 2840 mg/kg d.m. (
Figure 4a). In 2016, a statistically significant difference was observed among treatments (
p < 0.01), with the E30 treatment resulting in the highest phosphorus accumulation (2840 mg/kg d.m.), significantly exceeding the values measured in other treatments. In the subsequent years (2017–2020), phosphorus concentrations fluctuated between 1533 and 2457 mg/kg d.m., but no significant differences were detected among treatments. The control plots (C) remained relatively stable across years (1597–2147 mg/kg d.m.), while freshwater treatments (K30, K45) and effluent treatments (E30, E45) showed moderate variation.
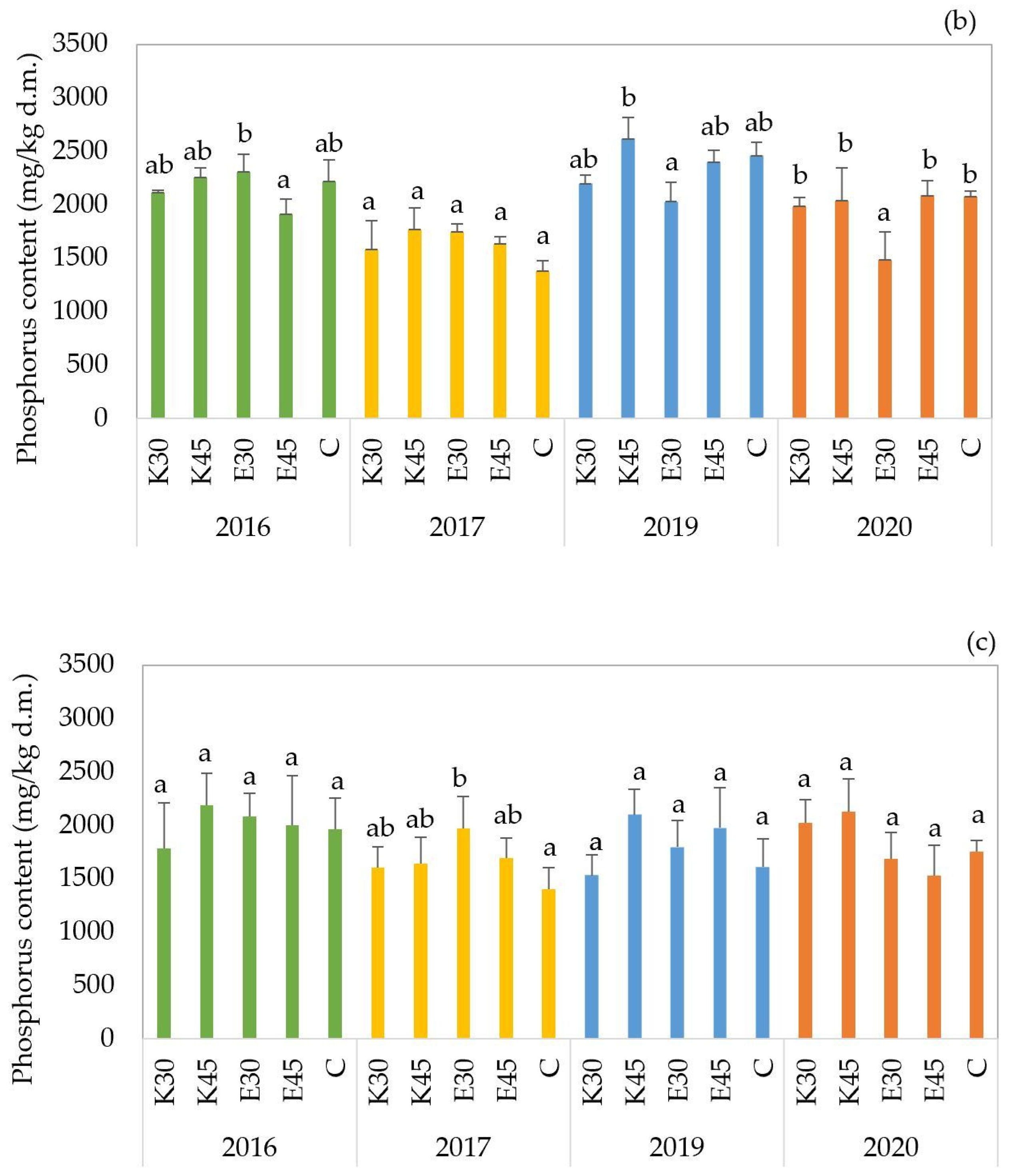
The phosphorus content of the ‘
GK Balázs’ forage sorghum hybrid showed variation across treatments and years, with values ranging from 1380 to 2617 mg/kg d.m. (
Figure 4b). In the 2016 growing season, treatment effects were found to be statistically significant, with
p-values below 0.01. The E30 treatment resulted in the highest phosphorus accumulation (2307 mg/kg d.m.) and was grouped separately, indicating significantly greater uptake compared to all other treatments. In the next year, no significant differences were detected among treatments. In 2019, the K45 treatment showed significantly higher phosphorus content (2617 mg/kg d.m.,
p = 0.033) than all other treatments, which were grouped separately. This indicates that higher-volume freshwater irrigation promoted phosphorus accumulation in that year. In the last year, the E30 treatment exhibited the lowest phosphorus concentration (1487 mg/kg d.m.
p < 0.01) and was statistically separated from all other treatments, which showed significantly higher values. This suggests that low-volume effluent irrigation may have limited phosphorus uptake under the environmental conditions of that year.
The phosphorus content of the ‘
GK Erik’ forage sorghum hybrid showed moderate variation across treatments and years, with values ranging from 1403 to 2190 mg/kg d.m. (
Figure 4c). The 2016 results showed uniformity across treatments, with statistical testing yielding a
p-value of 0.846, indicating no significant differences. In 2017, a statistically significant difference was detected in the E30 treatment (
p = 0.040) compared to the lowest C value. The E30 treatment resulted in the highest phosphorus accumulation (1970 mg/kg d.m.) and was grouped separately, indicating significantly greater uptake compared to all other treatments. In 2019, no significant differences were observed among treatments (
p = 0.467), despite numerical variation. Similarly, in 2020, no statistically significant differences were detected (
p = 0.058), although the E45 treatment showed the lowest phosphorus concentration (1530 mg/kg d.m.) compared to the other treatments. These suggest that ‘
GK Erik’ exhibits treatment-dependent phosphorus accumulation only in specific years, particularly in 2017, when low-volume effluent irrigation (E30) significantly enhanced phosphorus uptake. In other years, phosphorus content remained consistent across all treatments, which may indicate a stable nutrient response even under variable irrigation systems.
3.3.3. Potassium Content
The potassium content of the ‘
GK Áron’ forage sorghum hybrid showed considerable variation across treatments and years, with values ranging from 12,473 to 25,330 mg/kg d.m. (
Figure 5a). In 2016, the highest potassium accumulation was observed under the E45 treatment (25,330 mg/kg d.m.), followed closely by E30 (24,690 mg/kg d.m.), while the control treatment showed the lowest value (18,097 mg/kg d.m.). In 2017, potassium content remained relatively high across all treatments, with E30 again showing the highest value (19,423 mg/kg d.m.), although the differences among treatments were less pronounced. In 2019, potassium concentrations declined overall, with E30 still maintaining the highest value (18,930 mg/kg d.m.) and K45 the lowest (16,130 mg/kg d.m.). In 2020, the lowest potassium content was recorded under the E30 treatment (12,473 mg/kg d.m.), while the control treatment showed the highest value (14,883 mg/kg d.m.), indicating a reversal in treatment effects compared to previous years. These results suggest that ‘
GK Áron’ exhibits treatment-dependent potassium accumulation, particularly in 2016 and 2017, when effluent irrigation (E30 and E45) enhanced potassium uptake. However, in later years, the differences among treatments diminished, and in 2020, the control treatment showed the highest potassium content, possibly due to environmental or soil-related factors influencing nutrient availability.
The potassium content of the ‘
GK Balázs’ forage sorghum hybrid showed moderate variation across treatments and years, with values ranging from 13,173 to 21,297 mg/kg d.m. (
Figure 5b). In 2016, the highest potassium accumulation was observed under the K45 treatment (21,297 mg/kg d.m.), while the lowest value was recorded under the K30 treatment (17,577 mg/kg d.m.). In 2017, potassium content declined across all treatments, with values ranging from 13,927 to 15,747 mg/kg d.m., and no statistically significant differences were detected (
p > 0.05). In 2019, potassium concentrations remained relatively uniform, ranging from 14,300 to 16,450 mg/kg d.m., with no significant treatment effects. In 2020, potassium content increased slightly in the control and E45 treatments (15,330 and 15,313 mg/kg d.m., respectively), while the lowest value was observed under E30 (13,173 mg/kg d.m.). However, all treatments were statistically grouped together in each year, indicating no significant differences among them (
p > 0.05).
The potassium content of the ‘
GK Erik’ forage sorghum hybrid showed moderate variation across treatments and years, with values ranging from 13,960 to 20,323 mg/kg d.m. (
Figure 5c). In 2016, the highest potassium accumulation was observed under the E30 treatment (20,323 mg/kg d.m.), while the lowest value was recorded under the E45 treatment (17,550 mg/kg d.m.). In 2017, potassium content remained relatively high across all treatments, ranging from 15,947 to 19,727 mg/kg d.m., with E30 again showing the highest value. In 2019, potassium concentrations declined slightly, with values between 13,960 and 17,637 mg/kg d.m., and the highest value was observed under E45. In 2020, potassium content remained relatively uniform across treatments, ranging from 14,130 to 15,827 mg/kg d.m. No statistically significant variation among the treatments was observed throughout the entire duration of the four-year study (
p > 0.05).
3.3.4. Sodium Content
The sodium concentration in the plant parts of the ‘
GK Áron’ forage sorghum hybrid exhibited distinct variations across irrigation treatments and growing seasons (
Figure 6a). In 2016, the highest sodium content was recorded under the K30 treatment (70 mg/kg d.m.), followed closely by E30 (69 mg/kg d.m.), whereas the C treatment showed the lowest value (52 mg/kg d.m.). In 2017, sodium accumulation increased under the C treatment (84 mg/kg d.m.,
p = 0.002), which was significantly higher than most other treatments. Effluent irrigation treatments (E30 and E45) also resulted in elevated sodium levels (65 and 73 mg/kg d.m., respectively), suggesting enhanced sodium uptake under saline irrigation conditions. In 2019, an overall decline in sodium content in all treatments compared to the values measured in 2017. The lowest value was associated with E45, while the C (
p = 0.011) and E30 (
p = 0.045) treatments significantly higher concentration. In 2020, sodium accumulation peaked under the E45 treatment (96 mg/kg d.m.), followed by E30 (86 mg/kg d.m.), whereas K45 showed the lowest value (62 mg/kg d.m.). These findings indicate that sodium uptake in ‘
GK Áron’ is influenced by both the source and volume of irrigation water, with effluent treatments generally promoting higher sodium accumulation, particularly in the final year of the study.
The sodium content of the ‘
GK Balázs’ forage sorghum hybrid showed moderate variation across treatments and years (
Figure 6b). In 2016, the highest sodium concentration was observed under the E45 treatment (64 mg/kg d.m.), followed closely by E30 (63 mg/kg d.m.), while the lowest value was recorded under K45 (50 mg/kg d.m.). In 2017, sodium levels decreased in all treatments compared to the previous year, ranging between 45 and 43 mg/kg d.m., with minimal differences among treatments. In 2019, sodium accumulation increased again compared to the 2017, particularly under E30 and the C treatment (63 mg/kg d.m. each), suggesting enhanced uptake under effluent and non-irrigated conditions. The lowest value was observed under K30 (51 mg/kg d.m.). In 2020, sodium content varied more distinctly, with E30 showing the highest concentration (71 mg/kg d.m.), followed by E45 (70 mg/kg d.m.), while K30 recorded the lowest value (28 mg/kg d.m.). Statistical analysis confirmed significant differences in sodium accumulation, especially in 2020 (
p < 0.05).
The sodium content in the plant parts of the ‘
GK Erik’ forage sorghum hybrid showed considerable variation across treatments and years (
Figure 6c). In 2016, the highest sodium concentration was recorded under the E45 treatment (53 mg/kg d.m.), followed by E30 (45 mg/kg d.m.), while the control and K30 treatments showed the lowest values (38 mg/kg d.m.). In the next year, sodium levels increased under E30 (59 mg/kg d.m.
p = 0.001) and K30 (51 mg/kg d.m.
p = 0.002), with the control treatment remaining low (37 mg/kg d.m.). In 2019, sodium accumulation was highest under E45 (60 mg/kg d.m.), followed closely by the C treatment (57 mg/kg d.m.), while K30 showed the lowest value (43 mg/kg d.m.). In 2020, the highest sodium content was observed under K45 (74 mg/kg d.m.), followed by K30 (68 mg/kg d.m.), whereas E45 and the control treatment showed lower values (46 and 40 mg/kg d.m., respectively). Statistical analysis did not indicate significant differences among treatments, particularly in 2020.
3.4. Changes in Soil Parameters
Soil parameters were evaluated at two depths (0–30 cm and 30–60 cm) in 2020, following four years of irrigation. According to statistical tests, significant differences among treatments were detected in the 0–30 cm layer for electrical conductivity (EC), total organic carbon (TOC), available phosphorous and potassium (AL-K
2O and AL-P
2O
5). In the 30–60 cm layer, only available phosphorous (AL-P
2O
5) differed significantly among treatments, while the other measured parameters did not show statistically significant differences (
Table 4).
The highest EC value was detected in the non-irrigated control (475.7 ± 8.5 µS/cm), whereas all irrigated treatments—despite their varied water quality—had lower EC values, ranging between 400.3 and 453.3 µS/cm. However, the only significant difference was between the EC values of the E45 and control treatments.
In the case of total organic carbon, the control plots showed significantly higher values (1.33 ± 0.01 m/m%), while the lowest values were recorded in effluent-treated soils (E30: 1.16 ± 0.08%, E45: 1.13 ± 0.06%, the latter treatment was significantly different).
The same trend was also observed for phosphorus, with the unirrigated control treatments having higher available soil phosphorus than the irrigated treatments, the difference being significant for treatments E45, E30, K45. This is the only soil parameter for which a statistically verifiable difference between the values measured in the 30–60 cm soil layer was detected. The trend is the same as in the topsoil layer, the available phosphorus content of the soil is higher in the non-irrigated treatment than in the irrigated treatments.
Soil available potassium content in the 0–30 cm layer was also highest in the non-irrigated treatment, but a significant difference was only observed between E45 and the control.
Although there was no demonstrable difference between treatments for the AL-Na parameter, irrigation water quality clearly indicates the potential for expected sodium accumulation based on elevated soil sodium content in treatments E30 and E45.
3.5. Biomass
The green biomass yield of the ‘
GK Áron’ forage sorghum hybrid showed distinct variation across irrigation treatments and growing seasons (
Figure 7a). In 2016, the significantly highest biomass yield was recorded under the K30 treatment (579 g/plant,
p = 0.026), followed by K45 (514 g/plant), while E45 resulted in the lowest value (363 g/plant). In 2017, biomass production increased across all treatments, with K45 and E30 reaching 563 and 531 g/plant, respectively. The control treatment showed the lowest yield (432 g/plant). Statistically significant differences were not detected in the dataset (
p > 0.05). In 2019, the highest yield was again recorded under K30 (562 g/plant), while E30 and E45 showed moderate values, and the C treatment remained low. As the
p-values remained above the 0.05 threshold, no significant differences could be confirmed. In the last year, E30 produced the significantly highest biomass yield (653 g/plant), followed by E45 (539 g/plant), while the control treatment showed the lowest value (405 g/plant).
The green biomass yield of the ‘
GK Balázs’ hybrid varied across irrigation treatments and growing seasons (
Figure 7b). In 2016, the significantly higher yield was recorded under the K45 treatment (498 g/plant,
p = 0.056), while the lowest value was observed under E45 (342 g/plant). In 2017, biomass production increased across all treatments, and statistically significant differences were detected among treatments, with K45 reaching the highest value (568 g/plant,
p = 0.039) and the control treatment showing the lowest (474 g/plant). In 2019, the highest biomass yield was observed under E45 (580 g/plant), followed by E30 (545 g/plant) and the control (554 g/plant), while K30 showed the lowest value (471 g/plant). The differences were not statistically significant (
p > 0.05) although effluent treatments tended to promote higher biomass accumulation. In 2020, statistical analysis confirmed significant differences, where the E30 treatment produced the highest biomass yield (596 g/plant
p = 0.087), while the control treatment showed a marked decline (363 g/plant).
The green biomass yield of the ‘
GK Erik’ forage sorghum hybrid exhibited substantial variation across irrigation treatments and growing seasons (
Figure 7c). In 2016, the highest yield was recorded under the E45 treatment (527 g/plant), followed by the control (543 g/plant), while the lowest value was observed under K30 (414 g/plant). The differences among treatments were not statistically significant (
p > 0.05). In the second growing year, biomass production remained relatively balanced, with K45 and E45 reaching 487 and 418 g/plant, respectively. The lowest yield was recorded under E30 (380 g/plant). No statistically significant differences were detected (
p > 0.05). In 2019, the significantly highest biomass yield was observed under E45 (513 g/plant,
p = 0.078), followed by E30 (454 g/plant), while K45 showed the lowest value (340 g/plant). In 2020, biomass production increased markedly across all treatments. The highest yield was recorded under K45 (809 g/plant), followed closely by E45 (798 g/plant) and E30 (756 g/plant).
Following the evaluation of fresh biomass production, water use efficiency (WUE) was assessed to determine the impact of irrigation treatments and seasonal variation. The analysis was based on fresh biomass per plant and total water input (precipitation and irrigation), calculated for each treatment across four growing seasons.
Table 5 illustrates the distribution of WUE values across treatments and years.
Water use efficiency (WUE) was analyzed using a two-way ANOVA to determine the individual and combined effects of irrigation treatment and year, without considering hybrid genotype. The results revealed statistically significant effects for all examined factors. The results revealed a strong and statistically significant influence of irrigation treatment on WUE, as reflected by the ANOVA outcome (F(4, 40) = 73.70, p < 0.0001). The year effect was also significant (F(3, 40) = 19.52, p < 0.0001), reflecting the influence of climatic variability across seasons. Furthermore, the interaction between treatment and year was statistically significant (F(12, 40) = 5.68, p < 0.0001), suggesting that the effectiveness of a given irrigation regime varied depending on the environmental conditions of the respective year.
Descriptive analysis showed that the control (non-irrigated) treatment consistently resulted in the highest WUE values across all years, likely due to the absence of irrigation-induced dilution effects. Among the irrigated treatments, moderate water input levels (K30 and E30) generally led to higher WUE values compared to higher input treatments (K45 and E45), particularly in drier years. This trend supports the hypothesis that moderate irrigation under saline conditions can enhance water productivity.
5. Conclusions
This four-year field study confirms that saline aquaculture effluent, when applied via drip irrigation at moderate volumes (30 mm/week), can effectively support forage sorghum production under water-limited conditions. Across phenological, physiological, and chemical parameters, effluent irrigation enhanced plant growth, chlorophyll content, and nutrient uptake, particularly nitrogen. Biomass yield increased by up to 61% compared to the non-irrigated control, with maximum values reaching 653 g/plant in ‘GK Áron’ and 596 g/plant in ‘GK Balázs’. ‘GK Erik’ showed the most consistent yield response, with statistically significant improvements under both effluent and surface water irrigation in 2020. Nitrogen uptake improved by 22%, and phosphorus accumulation exceeded 2800 mg/kg dry matter in some treatments.
Nutrient-rich effluent water, containing dissolved forms of nitrogen and phosphorus, contributes to improved nutrient utilization and may diminish the necessity for conventional fertilizer inputs. Soil analyses revealed no salinity buildup, with electrical conductivity remaining below 475 µS/cm. However, long-term effluent irrigation influenced soil nutrient dynamics, including a reduction in available phosphorus. Whether this reflects enhanced plant uptake or reduced availability due to fixation or leaching requires further investigation.
Moderate effluent irrigation has proven to be a practical strategy for balancing efficient water and nutrient use with the maintenance of soil health under field conditions. Hybrid selection, irrigation volume, and seasonal conditions are key factors in maximizing yield and ensuring sustainable production. Continued research is recommended to monitor long-term soil–plant interactions and to refine effluent reuse practices in environmentally responsible agriculture.