Abstract
Inadequately managed agricultural waste significantly impacts the environment, health, and economy. This pollution stems from the underutilization, inadequate awareness, and insufficient treatment of agricultural waste. Fruit and vegetable wastes are valuable sources of bioactive compounds. This study aimed to revalorize discarded waste from red habanero chili peppers (Capsicum chinense Jacq.) by extracting bioactive compounds through different extraction processes: maceration (ME), maceration assisted by ultrasound (US), Soxhlet extraction (SE), supercritical fluid extraction (SFE), and supercritical fluid extraction with a co-solvent (SFEC). The extraction processes had significant effects on extraction efficiency and phytochemical profile (capsaicinoids and carotenoids recovery). The results indicated that the highest-efficiency process was SFEC, in addition to its high phytochemicals recovery (14.9 mg of total capsaicinoids and total carotenoids 292.09 µg per gram of sample). Concerning the phytochemical profile of the extract, the maceration process yielded the highest concentration of compounds, followed by US and SFEC. These data reveal that the use of the SFE and SFEC processes is recommended for extracting phytochemicals with biological activity from red habanero chili pepper waste for diverse industrial applications.
1. Introduction
Red habanero chili pepper (Capsicum chinense Jacq.) is a highly aromatic chili pepper and is an important crop in Mexico. The main edible part of the red habanero chili peppers is the pericarp which contains high amounts of ascorbic acid, vitamins A and E, carotenoids, and phenolic compounds. Therefore, it has significant potential health benefits [1,2,3]. In fact, around 70% of the production is consumed fresh, and the rest is processed and largely dedicated to export. The quality of peppers is defined by consumer perception, mainly for sensorial characteristics, such as, size, color, or texture. In the case of habanero peppers, a significant factor impacting their quality is the variation in fruit size and weight [4]. Smaller habanero peppers (classified as second or third grade) tend to command lower prices in the market, thereby leading to financial losses for farmers. Furthermore, approximately 20–30% of the yearly production incurs postharvest losses due to physiological damages resulting from improper postharvest handling practices [5,6,7].
Habanero peppers graded as second or third grade, including chili peppers that may have defects of up to 25% on the surface of the fruit, present an opportunity as potential sources for deriving nutraceuticals and other biomolecules [4,7]. This is due to their inherent content of compounds exhibiting antioxidant activity, thereby facilitating the valorization of these byproducts (Figure 1). The implementation of various unitary operations (such as drying, extraction, or milling) stands out as a pivotal stage in the process of obtaining bioactive compounds from this residual material [8,9,10,11].
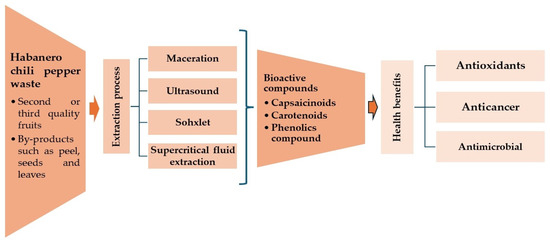
Figure 1.
Schematic representation of different extraction processes for bioactive compounds recovery from habanero chili pepper waste and their health benefits; adapted from Kumar et al. [12].
Different authors have shown that Capsicum extract presents high biological effects such as antioxidant, anti-inflammatory, antifungal, and anti-carcinogenic properties. Therefore, it is possible to use it in many applications in the pharmaceutical, chemical, and food industries [13,14,15,16] due to its bioactive compounds content (mainly capsaicinoids and carotenoids). Among capsaicinoids, capsaicin (C) and dihydrocapsaicin (DHC) are the major compounds, representing 89–98% of this variety [17]. Capsanthin is the major carotenoid present in capsicum extracts and lacks provitamin A activity, but it has demonstrated efficacy as a free radical scavenger and has been shown to raise HDL cholesterol levels in plasma [18]. Nevertheless, the bioactivity of capsicum oleoresin is influenced by factors such as the maturity stage, specific Capsicum spp. variety, and extraction process [19].
To efficiently recover bioactive compounds for downstream applications in the industry, it is advisable to apply a selective extraction process for the target compound, thus providing final extracts of higher value.
Among the industrial products obtained from habanero chili peppers, Capsicum oleoresin, an oily nature extract of intense color and fragrance, characteristic of chili peppers, has a diverse industrial application. The traditional Capsicum extraction process consists of three stages: dehydration of fresh red peppers, milling and extraction by organic solvents (n-hexane, ethanol, and others), using techniques such as maceration or Soxhlet [20]. However, the process time, the need for large quantities of solvent, and the requirement to treat the waste generated due to the dangerous effects on human health and the environment are some disadvantages associated with the conventional process. The affinity between solvent and solute, the mass transfer, the use of a co-solvent, and the properties of the solvents (environmental safety, non-human toxicity, and financial feasibility) should also be considered in the selection of the suitable solvent for an extraction process [21]. Carbon dioxide (CO2), supercritical water, organic salt-based solvents such as ionic liquids, and deep eutectic solvents are recognized as environmentally friendly solvents. On the other hand, ethanol, terpenes, glycerol, and methyl esters of fatty acids from vegetable oils are identified as agricultural solvents [22]. Ethanol is widely used as an organic solvent in the extractions of Capsicum due to its low toxicity and good extraction capacity [23,24,25]. The authors also observed that, sometimes, the addition of water to the extraction solvent helps to increase the effectiveness of the extraction in terms of extractable solids and capsaicinoids extraction [23,25].
Recently, different technologies were developed for Capsicum extraction, such as microwave-assisted extraction, ultrasound-assisted extraction, and supercritical fluid extraction [19,26,27,28,29]. Supercritical fluid extraction has been investigated extensively as an alternative to traditional methods that use organic solvent for extraction and fractionation of bioactive compounds from natural matrices. Supercritical fluid extraction utilizes a fluid that behaves both as a gas and a liquid, exploiting properties like diffusion, viscosity, and surface tension, offering the tunability of thermodynamic and transport properties [27,30]. CO2 is the most common supercritical fluid used due to its low cost, non-toxicity, non-flammability, inertness, and relatively low supercritical conditions (31.35 °C and 7.39 MPa) [27]. Supercritical CO2 is limited to dissolving compounds with a medium–high molecular weight and low polarity such as carotenoids [30]. An alternative for the extraction of organic compounds with a greater polarity is the addition of CO2 modifiers such as co-solvents (ethanol, methanol, water, etc.), increasing the extraction yield [27]. In addition, this alternative extraction process has attracted the attention of bioactive compounds’ recovery from habanero chili pepper’s by-products (Table 1).

Table 1.
Bioactive compounds recovery from different chili habanero pepper wastes.
Therefore, the main aim of this study was to explore the influence of extraction processes on bioactive compounds (capsaicinoids and carotenoids) in red habanero chili pepper waste (second or third grade). The extraction processes evaluated included conventional methods such as Soxhlet (SE) and maceration (ME), as well as non-conventional methods such as ultrasound-assisted maceration (US) and high-pressure processes using supercritical CO2 (SFE) and co-solvent (SFEC). Some aspects of the process efficiency are discussed in terms of the chemical profile of the extract and the extraction efficiency. This study is expected to contribute to the development of sustainable and economically viable processes for bioactive compounds’ recovery from habanero peppers waste, thereby mitigating post-harvest losses and maximizing the utilization of agricultural byproducts.
2. Materials and Methods
2.1. Chemicals
The reference standards of capsaicinoids (capsaicin (97%) and dihydrocapsaicin (90%)) and carotenoids (capsanthin (95%), zeaxanthin (97%), and β-carotene (93%)) were purchased from Sigma–Aldrich Chemical Co., Ltd. (St. Louis, MO, USA) and stored at 8 °C. The water was obtained from a Milli-Q water deionization system (Millipore, Bedford, MA, USA). The methanol, glacial acetic acid, and methyl tert-butyl ether (MTBE) for chromatographic separation were HPLC grade and were purchased from Merck (Darmstadt, Germany). Carbon dioxide (99.995%) was provided by Abello-Linde S.A. (Barcelona, Spain). The ethanol (95%) used for the extractions process was provided by Golden Bell Reagents (Zapopan, Mexico).
2.2. Sample Preparation
Second- or third-grade fruits (length ˂ 2 cm) of red habanero chili pepper were collected from a local market (Veracruz, Mexico). Prior to the extraction process, the fruits were dried as reported by Olguín-Rojas et al. [36]. Slices of 5 ± 1 mm were dried at 70 °C and 1.5 m s−1 of temperature and air velocity, respectively, until they reached 10% of moisture in a wet base. This process was performed using a tray drying oven model A39854-14 (Apex Engineering, Calvert City, KY, USA). The dried product was stored in laminated bags that were perfectly sealed under vacuum and stored at −20 °C prior to the extraction process.
2.3. Extractions Experiments
2.3.1. Maceration Extraction (ME)
Maceration extraction is a common and simple method, which is also suitable for extracting compounds from plant matrices, and it requires minimal equipment. The maceration process was carried out using a sample (dried base, db)–solvent ratio of 1:5 (w:w), and a mixture of ethanol–water (70:30, w:w) was used as a solvent. The process was performed on a Benchtop Orbital Shaker, model MaxQ 4450 (Thermo Fisher Scientific, Waltham, MA, USA). The operating conditions used were as follows: temperature 50 °C, 150 rpm (orbital radius: 9.5 cm), 150 min of extraction time.
2.3.2. Soxhlet Extraction (SE)
Soxhlet extraction is a common and conventional process, which is effective for extracting compounds that are soluble in the extraction solvent. Soxhlet extraction was conducted with 15.0 ± 0.5 g of dried, sliced habanero and 250 g of solvent (ethanol–water 70:30, w:w). The samples were packed in a filter paper and inserted in the Soxhlet extractor. The heating power was set to reach 2 cycles per hour in such a way that 5 extraction cycles were achieved within 150 min.
2.3.3. Maceration Extraction Assisted by Ultrasound (US)
Ultrasound-assisted extraction enhances the mass transfer rate by applying ultrasonic waves, enhancing the penetration of the solvent into the plant matrix. The maceration assisted by ultrasound was carried out using a sample (db)–solvent ratio of 1:5 (w:w); a mixture of ethanol–water (70:30, w:w) was used as the solvent. The process was performed in an ultrasonic bath (Westprime Systems, Cat. No. B90-055H; Chino, CA, USA) that was operated at 45 kHz and 100 W in a continuous mode for 30 min. A cooling bath with recirculation was coupled to maintain an extraction temperature of 50 °C.
2.3.4. Supercritical Fluid Extraction (SFE and SFEC)
Supercritical CO2 extraction is an environmentally friendly alternative, leveraging the unique properties of CO2 in its supercritical state, such as a high diffusion and low viscosity, to enhance the extraction of plant matrices. Furthermore, the incorporation of a cosolvent, such as ethanol, alters the polarity of the solvent, thereby facilitating the extraction of a broader range of compounds.
Supercritical fluid extraction (SFE) and supercritical fluid extraction with co-solvent (SFEC) were conducted using a high-pressure apparatus supplied by Thar Technology, model SF100 (Pittsburgh, PA, USA). The apparatus comprised an extraction vessel with a capacity of 100 mL, equipped with a thermostatic jacket for controlling the extraction temperature; two pumps with a maximum flow rate of 50 g min−1 (one for CO2 and the other for the co-solvent); a back pressure valve regulator to control the system’s pressure; and a cyclonic separator. For SFE and SFEC, the extraction vessel was loaded with 15.0 ± 0.5 g of the sample. The conditions of supercritical extraction were set according to Aguiar et al. [37]: 15 MPa, 40 °C, 90 min, and a CO2 flow rate of 1.2 kg h−1. For SFEC, ethanol was added at a flow rate of 0.3 kg h−1. The extracts were recovered in a cyclonic separator.
2.4. Extraction Efficiency
The extraction efficiency is defined as the relationship between the concentration of solids that are recovered in the extract and the initial concentration of extractable solids in dried habanero chili pepper. The extraction efficiency was determined based on the calculated yield (Equation (1)), , which is the ratio of the number of solids in the extract () to the extractable solids in the sample () (Equation (2)).
where is the mass of the extract obtained in each process, is the extractable solids mass fraction in the extract, is the mass of habanero chili pepper in the extraction process, and is the extractable solids mass fraction. Both and were measured in each extraction process; and were determined experimentally as mentioned in the next section.
2.5. Analysis
2.5.1. Determination of Extractable Solids
The extractable solids () in dried, red habanero chili pepper waste were determined through a sequential extraction with an ethanolic solution of 70% (w:w) at a ratio of 1 g of sample with 100 g of solvent. was determined in 0.44 ± 0.01 g per g of raw material. The extractable solids in the extracts () were determined as follows: an aliquot (5 mL) of each extract was concentrated and dried with a vacuum rotary evaporator, model R-205 (Büchi, Flawil, Switzerland), at 60 °C and 7.2 × 103 Pa. After that, the samples were put in a vacuum oven (Lab Line Instrument, Mod. 3818-1, Tripunithura Kochi, India) at 60 °C and 6 × 104 Pa until reaching a constant weight. The extractable solids’ mass fraction in the extract () was calculated by the weight difference according to Equation (3).
where is the initial weight, and is the final weight. The analyses were performed in triplicate.
2.5.2. Determination of Total Capsaicinoids
The total capsaicinoids was obtained as the sum of capsaicin and dihydrocapsaicin (Figure 2B). The total capsaicinoids was evaluated in the different extracts obtained as follows: 3 mL of each extract (except for the SFEC process) was filtered by a syringe filter and analyzed by UHPLC-Fl. The extracts obtained by the SFEC process were dissolved in a known volume of ethanol, and 3 mL was filtered by a syringe filter and analyzed by UHPLC-Fl. The chromatographic analyses were carried out according to Vazquez-Espinosa et al. [38]. The separation and quantification of individual capsaicinoids were conducted using a UHPLC (ACQUITY UPLC H-Class, Waters, Milford, MA, USA) system equipped with an ACQUITY UPLC Quaternary Pump System, an ACQUITY UPLC Auto Sampler with temperature control adjusted at 15 °C, a column oven set to 50 °C for the chromatographic separation, an ACQUITY UPLC® Photodiode Array (PDA) Detector, and an ACQUITY UPLC® Fluorescence (FLR) Detector. Empower 3 software (Ver. Feature Release 5, Waters) was used to control the equipment and for data acquisition. Capsaicinoids were analyzed on a Waters ACQUITY UPLC BEH C18 column (50 mm × 2.1 mm I.D., particle size 1.7 µm). A gradient method was used for the chromatographic separation with acidified water (0.1% acetic acid, solvent A) and acidified acetonitrile (0.1% acetic acid, solvent B) working at a flow rate of 0.8 mL min−1. The gradients used were as follows: 0 min, 0%B; 0.50 min, 45%B; 1.60 min, 45%B; 1.95 min, 50% B; 2.45 min, 55% B; 2.80 min, 63% B; 3.00 min, 63% B; 4.00 min, 100% B; 6.00 min, 100% B. The temperature of the column was kept constant at 50 °C. The wavelengths used for fluorescence detection were 278 nm (excitation) and 305 nm (emission). For the capsaicinoids quantification, calibration curves for capsaicin (C) and dihydrocapsaicin (DHC) were used (y = 2046731.37x + 55924.05 for capsaicin and y = 2194087.16x + 36229.73 for dihydrocapsaicin), which are the two capsaicinoids standards that are commercially available. Correlation coefficients (r2) (0.9997 for C and 0.9999 for DHC) were calculated. Limits of detection (0.074 mg L−1 and 0.062 mg L−1 for C and DHC, respectively) and limits of quantification (0.247 mg L−1 and 0.207 mg L−1 for C and DHC, respectively) were determined as the analyte concentration corresponding to the standard deviation of the signal of the blank values (n = 10) plus 3 or 10 times, respectively, divided by the slope of the linear regression.
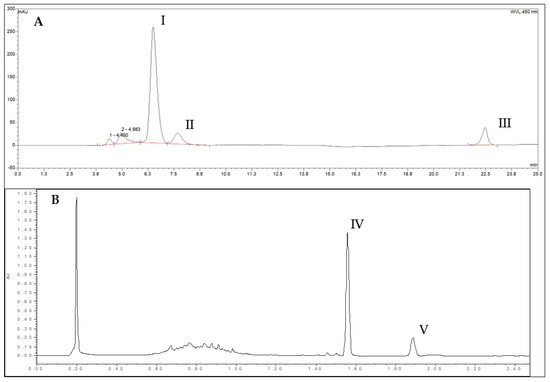
Figure 2.
Chromatograms of carotenoids (λ = 450 nm) (A) and capsaicinoids (λ = 278 nm (excitation) and λ = 305 nm (emission)) (B) present in the capsicum extracts: I, Capsanthin; II, Zeaxanthin; III, β-carotene; IV, Capsaicin; V, Dihydrocapsaicin.
2.5.3. Determination of Total Carotenoids
Total carotenoids was obtained as the sum of principal carotenoids (capsanthin, zeaxanthin, and β-carotene (Figure 2A)). The determination of individual carotenoids was performed according to Minguez-Mosquera and Hornero-Mendez [15], which is based on the quantification of insaponificable carotenoids. The sample in contact with a nonpolar solvent mixture (20 mL of petroleum ether–ethyl ether, 1:1) was placed in a separating funnel. The organic and inorganic phases were separated using distilled water. The organic phase was incubated with a solution of 20% KOH in methanol for 12 h, and then it was concentrated in vacuum to dry and suspended in methanol. The total carotenoids was evaluated in the different extracts obtained as follows: 3 mL of each extract (except for the SFEC process) was filtered by a syringe filter and analyzed by HPLC following the analysis method reported by Giuffrida et al. [17] with modifications. The analyses were carried out using a Dionex chromatographic system (Sunnyvale, CA, USA). This system included an automated sample injector (ASI-100), a liquid pump (P680), and a thermostatic column compartment (TCC-100). For the identification of carotenoid compounds, a photodiode array detector (PDA-100) and a universal chromatography interface (UCI-50) were utilized. The wavelength used for UV–Vis detection was 450 nm. The software used for data analysis was Chromeleon Ver. 6.60. The carotenoids were separated using a C30 reverse phase column Develosil® (Phenomenex Inc., Torrance, CA, USA, 5 μm, 150 × 4.6 mm). For the chromatographic method, we utilized a gradient of two solvents’ ratios MeOH:MTBE:H2O (82:16:02, solvent A) and (10:88:02, solvent B), working at a flowrate of 0.5 mL min−1. The gradient method used was as follows: 0 min, 0% B; 8 min, 0% B; 26 min, 100% B; 36 min, 100% B. The temperature of the column was held constant at 30 °C.
Carotenoids quantification was performed by comparing the retention times and absorption spectra with principal carotenoids (capsanthin, zeaxanthin, and β-carotene). Standard calibration curves for these three carotenoids were plotted at a concentration range between 0.5 and 10 mg/L. The correlation coefficients obtained (R2) were 0.9997, 0.9996, and 0.9997 for capsanthin, zeaxanthin, and β-carotene, respectively.
2.6. Statistical Analysis
The experimental results were reported as mean ± standard deviation, and statistical analyses were conducted using one-way ANOVA followed by Tukey’s pairwise test with Minitab statistical software Ver. 16 (State College, PA, USA). Statistically significant differences were considered at a probability value of less than 5% (p < 0.05).
3. Results and Discussion
3.1. Physical Characteristics of Red Habanero Chili Pepper Extract
In recent years, the valorization of agrifood by-products through extraction processes has gained significant attention in the agrifood industry. In this context, selective extraction processes are a critical stage, not only in the recovery of bioactive compounds, but also in downstream industrial applications. The extensive use of solvents and post-extraction processes such as evaporation or concentration must be considered in workflow design and cost processes. According to our results, as expected, the extraction process had an influence on the physical attributes of red habanero chili pepper waste extracts. Typical liquid-phase extracts were obtained using maceration (ME), Soxhlet extraction (SE), maceration assisted by ultrasound (US), and supercritical fluid extraction with co-solvent (SFEC). The conventional extraction process offers easy handling, low operating pressures (ambient conditions), and relatively low temperatures. In alignment with the valorization of agrifood by-products, there is a shift away from traditional, time-consuming methods towards cleaner, more efficient, and environmentally friendly alternatives [39]. In this sense, US is an alternative for reducing the process time. With supercritical fluid extraction (SFE), solid as a powder was obtained as an extract due to the absence of any liquid solvents in the SFE process, where the diffusion of solutes was facilitated by supercritical CO2 in its gaseous phase under atmospheric conditions.
The commercial viability of capsicum extracts relies significantly on their red coloring capacity, regardless of whether they possess a pungent flavor or not. The coloring potential of the extracted oleoresins has an important role in determining their quality and, consequently, their ultimate market price. All the obtained extracts exhibited the high pungency characteristic of habanero chili pepper. However, the extraction process employed had a substantial impact on the color of the extracts. The ME, US, and SFEC processes produced extracts with red hues, whereas the SE and SFE processes yielded extracts with yellow tones. These color variations can be attributed to differences in the composition and profile of bioactive compounds present in the extracts. Furthermore, the coloring capacity of an oleoresin serves as an indicator of its carotenoid content, a topic that is explored in the subsequent sections of this discussion.
3.2. Extraction Efficiency from Different Extraction Processes
To design and select an extraction process, in addition to taking into account the efficiency of the extraction and the quality of the extract obtained, the extraction time, operability, difficulty, and cost of carrying out the extraction must also be considered. Table 2 presents the principal composition of extracts obtained by different extraction processes from red habanero chili pepper wastes. The conventional ME process at 50 °C and 150 min obtained the highest concentration of extractable solids, exceeding the SE and SFEC processes by an order of magnitude. In comparison with the US process (50 °C, 45 kHz, and 30 min), no significant differences were found; however, the total extraction time was shorter. These results are similar to those reported by Fernández-Ronco et al. [40] for carotenoids extracted from paprika. The ultrasonic-assisted maceration process encompassed cavitation, thermal, and mechanical impacts. The improved procedure could be linked to the liberation of extra bioactive compounds resulting from the breakdown of the cell walls and the disintegration of chromoplasts induced by cavitation, which is influenced by both thermal and mechanical forces [41].

Table 2.
Principal composition, extraction efficiency, and recovery of the capsaicinoids and carotenoids from red habanero chili pepper by different extraction processes.
In the SE process, a sample–solvent ratio of approximately 1:16 (w:w) was employed, and this ratio resulted in a lower concentration of extractable solids due to the excess solvent used. However, a notable drawback of the SE process lies in its high solvent consumption, which is a consequence of the system configuration requirements. These requirements include maintaining sufficient solvent for reflux while ensuring an adequate amount for the recovery vessel. In this sense, the large quantity of liquid solvent used in SFEC significantly reduced the concentration of the extractable solids.
In terms of extraction efficiency, the highest values were achieved by ME, US, and SFEC, followed by SFE (15 MPa, 40 °C, and 90 min). These results are similar to those reported by Aguiar et al. [42] for Malagueta chili pepper (C. frutescens) using supercritical CO2 with yields ranging between 11.8 and 13.6% using temperatures and pressures of 40 °C and 15 MPa.
The recovery of compounds from habanero chili peppers is dependent of the process and the solvent employed. The SCFE process achieved the highest recovery of bioactive compounds (capsaicinoids and carotenoids). The total yield of capsaicinoids (C + DHC) varied from 8.56 ± 1.27 g kg−1 of the sample (SFE) to 14.91 ± 0.38 g kg−1 of the sample (SFEC) in the different extraction processes studied. These high-concentration values of capsaicinoids were expected in habanero pepper since it is popularly known in Mexico as a very pungent pepper [17].
The difference in the extraction yield of total capsaicinoids in the ME (9.3 g kg−1 of sample) and SE (10.4 g kg−1 of sample) processes could be explained by the presence of water in the extraction solvent and the contact between the solvent and the sample. Barbero et al. [23] found that water, being a highly polar solvent, reduced the extraction of less polar capsaicinoids such as dihydrocapsaicin compared to capsaicin. SE showed the lowest extraction yield; however, this process provided extracts with high concentrations of capsaicinoids, similar to SFE. Therefore, it was possible to obtain extracts with higher purity. As Aguiar et al. [27] reported, the solubility of capsaicinoids in CO2 at 15 MPa and 50 °C is probably higher than the solubility of other compounds presents in second-grade red habanero chili pepper, such as triacylglycerols and carotenoids.
Comparable findings were noted by Chel-Guerrero et al. [31] for bioactive compounds from stems of habanero pepper plants. The SFE process showed promising results for obtaining compounds with anti-inflammatory activity. Conversely, the ME and SE processes demonstrated superior performance in extracting antioxidant compounds and polyphenols.
As is known, the advantage of supercritical CO2 processes is that the fluid exhibits both gas-like properties, including diffusion, viscosity, and surface tension, and liquid-like properties, demonstrating density and solvation characteristics typical of liquids. Moreover, the incorporation of a co-solvent such as ethanol enhances the specificity and speed of the target molecule, attributed to the physicochemical properties of the solvent [27]. The primary solvent in SFE is predominantly the supercritical CO2 due to its high diffusivity, low mass transfer limitations, and high surface tension. These supercritical conditions could break cell walls of the raw material and so allow for a greater penetration of the extraction solvent into the small pores of the matrix and, therefore, the extraction efficiency of the co-solvents used can be increased [43]. The solvent employed in the extraction processes (EtOH) exhibited the capability to extract both polar and non-polar substances. Consequently, the selectivity of solute recovery was diminished, resulting in an increase in the solid content in the final product. This differs from SFE, where only non-polar components were extracted due to the distinct properties, as previously mentioned regarding supercritical CO2.
These results demonstrate that the selection of the extraction process significantly influences the composition, yield, and recovery of capsaicinoids and carotenoids from red habanero chili peppers, with ME and SFEC generally yielding higher concentrations and recoveries compared to SE and SFE. In the following section, the profile of the extract obtained from red habanero chili pepper waste with respect to the main bioactive compounds (capsaicinoids and carotenoids) is discussed.
3.3. Bioactive Compound of Red Habanero Chili Pepper Extract from Different Extraction Processes
When examining the extraction process, it is essential to consider various factors, including their impact on yield, extraction time, and the quality of the extract. In this instance, the quality of chili extracts is assessed based on attributes such as color and pungency capacity. Due to their bright color and spicy taste, chilies are frequently used in the preparation of condiments and food items. In addition, chilies’ extracts are recognized for their health benefits, medicinal and phytopharmacy potential, and health-promoting functional attributes [44]. Most commercially valued oleoresins are those that exhibit a high red coloring capacity, primarily attributed to carotenoid pigments, either with or without a pungent flavor. Among these carotenoids, capsorubin, capsanthin, zeaxanthin, and β-cryptoxanthin are the primary compounds responsible for the red coloration, while β-carotene primarily serves as an antioxidant.
Table 2 presents the total capsaicinoids and carotenoids content in the extract obtained by different extraction processes. It is observed that when using SFE, the highest concentration of capsaicinoids (3.34 ± 0.37g kg−1 of extract) was obtained, which was statistically similar to the result obtained with US and ME. However, when EtOH was used as a co-solvent, the concentration of capsaicinoids (0.48 ± 0.01 g kg−1 of extract) was one order of magnitude lower. Although the yield of total capsaicinoids was improved by using a co-solvent (SFEC), the use of more solvent reduced the final concentration of capsaicinoids.
Regarding the bioactive compound profile in the extract (Table 3), as expected, in all cases, the extract obtained showed a higher concentration of capsaicin than dihydrocapsaicin; this corresponds to what was reported for the genus C. chinense [17]. Aviles-Betanzos et al. [45] reported the highest concentration of capsaicin (37.9 ± 0.84 g kg−1 extract) and dihydrocapsaicin (10.17 ± 0.18 g kg−1 extract) using SFEC at 45 °C, ≈100 bar, and 60 min. The results of capsaicinoids and carotenoids for the evaluated materials are similar to or higher than those found in the literature, which can be explained by the differences in capsicum varieties, maturate stage, or cultivation regions [19].

Table 3.
Principal bioactive compound composition of red habanero chili pepper extract by different extraction processes.
On the other hand, the highest concentration of total carotenoids was found in the ME and US extracts (25.99–30.27 g kg−1 of the extract). With respect to the carotenoids profile, the dominant carotenoid found in the extracts obtained was capsanthin (57.2–77.4%). The red color of chili fruits is due to capsanthin, capsorubin, and cryptocapsin. In C. chinense fruits, the capsanthin and capsorubin constitute > 60% of the total carotenoids. Yellow pigmentation in fruits is due to α-, β-carotene, zeaxanthin, lutein, and β-cryptoxanthin.
CO2 is a non-polar solvent, which preferentially extracts non-polar components. β-carotene, being a relatively nonvolatile compound with no functional groups, represents a hydrophobic natural product [43]. The results indicated a significant extraction of β-carotene when CO2 was used as the solvent (33.7–36.8%), in contrast to the ME process (4.0–5.1%). Moreover, any modifications in the SFE conditions influenced the polarity of the extraction fluid, resulting in the extraction of pigment fractions within specific polarity ranges. Additionally, the inclusion of EtOH in the SFE process enhanced the extraction of red pigments [46,47]. When employing SFEC, a higher concentration of carotenoids per gram of sample was extracted (Table 2). The properties of the high diffusivity of pressurized CO2 fluid, coupled with few limitations in mass transfer and surface tension, enable SFE to disrupt cell structures, facilitating the penetration of the organic solvent into the small pores of the matrix. As carotenoids are typically found within chromoplasts, these effects enhance extraction efficiency [43]. However, the concentration in the extract is reduced due to the presence of other soluble compounds, such as free fatty acids [48].
These data underscore the importance of extraction process selection in obtaining habanero chili pepper’s second-grade extracts with specific bioactive compound profiles for various applications in both the food and pharmaceutical industries.
4. Conclusions
Different extraction processes were employed for bioactive compounds’ recovery from second- or third-grade red habanero chili peppers. The results revealed varying profiles of bioactive compounds in the extracts, depending on the extraction process used. The supercritical fluid extraction process using CO2 resulted in lower extraction yields when compared to the low-pressure methods. However, the quality of the SFE extract was significantly superior in terms of total capsaicinoids compared to other extraction methods. In this context, the revalorization of food waste emerges as a technological and innovative research area with beneficial effects for the population, the economy, and the environment. The bioactive compounds present in these extracts could find further applications in the food and pharmaceutical industries. The results obtained from this study could be used for evaluating and applying different extraction techniques in industrial processes, offering the best alternatives for obtaining high-quality commercial chili pepper extracts.
Author Contributions
Conceptualization, J.A.O.-R., L.A.V.-L. and G.d.C.R.-J.; methodology, J.A.O.-R., L.A.V.-L., G.F.B. and M.T.F.-P.; software, J.A.O.-R. and L.A.V.-L.; validation, G.F.B. and G.d.C.R.-J.; formal analysis, J.A.O.-R., L.A.V.-L., G.F.B. and G.d.C.R.-J.; investigation, J.A.O.-R. and L.A.V.-L.; resources, G.F.B., M.P., L.C. and G.d.C.R.-J.; data curation, G.F.B. and G.d.C.R.-J.; writing—original draft preparation, J.A.O.-R. and L.A.V.-L.; writing—review and editing, J.A.O.-R., L.A.V.-L., G.F.B. and G.d.C.R.-J.; visualization, G.F.B. and G.d.C.R.-J.; supervision, G.F.B. and G.d.C.R.-J.; project administration, G.F.B. and G.d.C.R.-J.; funding acquisition, G.F.B., M.P., L.C. and G.d.C.R.-J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors express their acknowledgments to Consejo Nacional de Humanidades Ciencia y Tecnología (CONAHCyT). Special acknowledgment is extended to the Mass Spectrometry Division of the Central Research Services for Science and Technology (SC-ICYT) of the University of Cadiz for collaboration throughout the analysis of the samples.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Castro-Concha, L.A.; Tuyub-Che, J.; Moo-Mukul, A.; Vazquez-Flota, F.A.; Miranda-Ham, M.L.; Bekatorou, A.; Tariq, A.; Tripathi, N.K. Antioxidant Capacity and Total Phenolic Content in Fruit Tissues from Accessions of Capsicum chinense Jacq. (Habanero Pepper) at Different Stages of Ripening. Sci. World J. 2014, 2014, 809073. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.D.; Changkija, S.; Sujata, W.; Somkuwar, B.G.; Rana, V.S.; Talukdar, N.C. Nutraceutical from Capsicum chinense Fruits in Shelf-Stable Herbal Matrix. Innov. Food. Sci. Emerg. Technol. 2017, 42, 130–137. [Google Scholar] [CrossRef]
- Menichini, F.; Tundis, R.; Bonesi, M.; Loizzo, M.R.; Conforti, F.; Statti, G.; De Cindio, B.; Houghton, P.J.; Menichini, F. The Influence of Fruit Ripening on the Phytochemical Content and Biological Activity of Capsicum chinense Jacq. Cv Habanero. Food Chem. 2009, 114, 553–560. [Google Scholar] [CrossRef]
- Diario Oficial de la Federación NORMA Oficial Mexicana NOM-189-SCFI-2012, Chile Habanero de La Península de Yucatán (Capsicum chinense Jacq.)-Especificaciones y Métodos de Prueba. 2012. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5280834&fecha=30/11/2012#gsc.tab=0 (accessed on 14 March 2024).
- Borges-Gómez, L.; Cervantes Cárdenas, L.; Ruiz Novelo, J.; Soria Fregoso, M.; Reyes Oregel, V.; Villanueva Couoh, E. Capsaicinoides En Chile Habanero (Capsicum chinense Jacq.) Bajo Diferentes Condiciones de Humedad y Nutrición. Terra Latinoamericana 2010, 28, 35–41. [Google Scholar]
- Maldonado Astudillo, Y.I.; Jimenez Hernandez, J.; Salazar Lopez, R. Fisiología y Tecnología Postcosecha Del Chile Habanero (Capsicum chinense Jacq.). 2020. Available online: http://ri.uagro.mx/handle/uagro/1655 (accessed on 14 March 2024).
- Zapata-Aguilar, J.A.; Pérez-Akaki, P.; Moo-Novelo, C.A. Análisis de La Cadena de Comercialización Del Chile Habanero de Yucatán y Su Denominación de Origen. Revista CEA 2020, 6, 109–125. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; de la Mora, Z.V.; Vázquez-Paulino, O.; Ascencio, F.; Villarruel-López, A. Bell Peppers (Capsicum annum L.) Losses and Wastes: Source for Food and Pharmaceutical Applications. Molecules 2021, 26, 5341. [Google Scholar] [CrossRef]
- Capanoglu, E.; Nemli, E.; Tomas-Barberan, F. Novel Approaches in the Valorization of Agricultural Wastes and Their Applications. J. Agric. Food. Chem. 2022, 70, 6787–6804. [Google Scholar] [CrossRef]
- Cortés-Ferré, H.E.; Guajardo-Flores, D.; Romero-De La Vega, G.; Gutierrez-Uribe, J.A. Recovery of Capsaicinoids and Other Phytochemicals Involved with TRPV-1 Receptor to Re-Valorize Chili Pepper Waste and Produce Nutraceuticals. Front. Sustain. Food Syst. 2021, 4, 588534. [Google Scholar] [CrossRef]
- Oğuzkan, S.B. Extraction of Capsinoid and Its Analogs from Pepper Waste of Different Genotypes. Nat. Prod. Commun. 2019, 14, 1. [Google Scholar] [CrossRef]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food Waste: A Potential Bioresource for Extraction of Nutraceuticals and Bioactive Compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef]
- Campos, M.R.S.; Gómez, K.R.; Ordoñez, Y.M.; Ancona, D.B. Polyphenols, Ascorbic Acid and Carotenoids Contents and Antioxidant Properties of Habanero Pepper (Capsicum chinense) Fruit. Food Nutr. Sci. 2013, 4, 47–54. [Google Scholar] [CrossRef]
- Reyes-Escogido, M.L.; Gonzalez-Mondragon, E.G.; Vazquez-Tzompantzi, E. Chemical and Pharmacological Aspects of Capsaicin. Molecules 2011, 16, 1253–1270. [Google Scholar] [CrossRef]
- Mínguez-Mosquera, M.I.; Hornero-Méndez, D. Separation and Quantification of the Carotenoid Pigments in Red Peppers (Capsicum annuum L.), Paprika, and Oleoresin by Reversed-Phase HPLC. J. Agric. Food Chem. 1993, 41, 1616–1620. [Google Scholar] [CrossRef]
- Wahyuni, Y.; Ballester, A.R.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Secondary Metabolites of Capsicum Species and Their Importance in the Human Diet. J. Nat. Prod. 2013, 76, 783–793. [Google Scholar] [CrossRef]
- Giuffrida, D.; Dugo, P.; Torre, G.; Bignardi, C.; Cavazza, A.; Corradini, C.; Dugo, G. Characterization of 12 Capsicum Varieties by Evaluation of Their Carotenoid Profile and Pungency Determination. Food Chem. 2013, 140, 794–802. [Google Scholar] [CrossRef]
- Aizawa, K.; Inakuma, T. Dietary Capsanthin, the Main Carotenoid in Paprika (Capsicum annuum), Alters Plasma High-Density Lipoprotein-Cholesterol Levels and Hepatic Gene Expression in Rats. Br. J. Nutr. 2009, 102, 1760–1766. [Google Scholar] [CrossRef]
- Melgar-Lalanne, G.; Hernández-Álvarez, A.J.; Jiménez-Fernández, M.; Azuara, E. Oleoresins from Capsicum Spp.: Extraction Methods and Bioactivity. Food Bioproc. Technol. 2017, 10, 51–76. [Google Scholar] [CrossRef]
- Fernández-Trujillo, J.P. Extracción Convencional de Oleorresina de Pimentón Dulce y Picante II. Peligros y Puntos de Control Crítico y Requerimientos Comerciales. Grasas y Aceites 2007, 58, 327–333. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Amran, M.A.; Palaniveloo, K.; Fauzi, R.; Satar, N.M.; Mohidin, T.B.M.; Mohan, G.; Razak, S.A.; Arunasalam, M.; Nagappan, T.; Sathiya Seelan, J.S. Value-Added Metabolites from Agricultural Waste and Application of Green Extraction Techniques. Sustainability 2021, 13, 11432. [Google Scholar] [CrossRef]
- Barbero, G.F.; Liazid, A.; Palma, M.; Barroso, C.G. Ultrasound-Assisted Extraction of Capsaicinoids from Peppers. Talanta 2008, 75, 1332–1337. [Google Scholar] [CrossRef]
- Chinn, M.S.; Sharma-Shivappa, R.R.; Cotter, J.L. Solvent Extraction and Quantification of Capsaicinoids from Capsicum chinense. Food Bioprod. Process. 2011, 89, 340–345. [Google Scholar] [CrossRef]
- Dong, X.; Li, X.; Ding, L.; Cui, F.; Tang, Z.; Liu, Z. Stage Extraction of Capsaicinoids and Red Pigments from Fresh Red Pepper (Capsicum) Fruits with Ethanol as Solvent. LWT-Food Sci. Technol. 2014, 59, 396–402. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Gontarek-Castro, E.; Jafari, S.M. Up-to-Date Strategies and Future Trends towards the Extraction and Purification of Capsaicin: A Comprehensive Review. Trends Food Sci. Technol. 2022, 123, 161–171. [Google Scholar] [CrossRef]
- De Aguiar, A.C.; Viganó, J.; da Silva Anthero, A.G.; Dias, A.L.B.; Hubinger, M.D.; Martínez, J. Supercritical Fluids and Fluid Mixtures to Obtain High-Value Compounds from Capsicum Peppers. Food Chem. X 2022, 13, 100228. [Google Scholar] [CrossRef]
- Fabela-Morón, M.F.; Cuevas-Bernardino, J.C.; Ayora-Talavera, T.; Pacheco, N. Trends in Capsaicinoids Extraction from Habanero Chili Pepper (Capsicum Chinense Jacq.): Recent Advanced Techniques. Food Rev. Int. 2020, 36, 105–134. [Google Scholar] [CrossRef]
- Weinhold, T.D.; Bresciani, L.F.V.; Tridapalli, C.W.; Yunes, R.A.; Hense, H.; Ferreira, S.R.S. Polygala cyparissias Oleoresin: Comparing CO2 and Classical Organic Solvent Extractions. Chem. Eng. Process. Process Intensif. 2008, 47, 109–117. [Google Scholar] [CrossRef]
- Zoccali, M.; Giuffrida, D.; Dugo, P.; Mondello, L. Direct Online Extraction and Determination by Supercritical Fluid Extraction with Chromatography and Mass Spectrometry of Targeted Carotenoids from Red Habanero Peppers (Capsicum chinense Jacq.). J. Sep. Sci. 2017, 40, 3905–3913. [Google Scholar] [CrossRef] [PubMed]
- Chel-Guerrero, L.D.; Oney-Montalvo, J.E.; Rodríguez-Buenfil, I.M. Phytochemical Characterization of By-Products of Habanero Pepper Grown in Two Different Types of Soils from Yucatán, Mexico. Plants 2021, 10, 779. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Pool, E.; Ramos-Díaz, A.L.; Lizardi-Jiménez, M.A.; Pech-Cohuo, S.; Ayora-Talavera, T.; Cuevas-Bernardino, J.C.; García-Cruz, U.; Pacheco, N. Effect of Solvent Polarity on the Ultrasound Assisted Extraction and Antioxidant Activity of Phenolic Compounds from Habanero Pepper Leaves (Capsicum chinense) and Its Identification by UPLC-PDA-ESI-MS/MS. Ultrason. Sonochem. 2021, 76, 105658. [Google Scholar] [CrossRef] [PubMed]
- Avilés-Betanzos, K.A.; Oney-Montalvo, J.E.; Cauich-Rodríguez, J.V.; González-Ávila, M.; Scampicchio, M.; Morozova, K.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Antioxidant Capacity, Vitamin C and Polyphenol Profile Evaluation of a Capsicum chinense By-Product Extract Obtained by Ultrasound Using Eutectic Solvent. Plants 2022, 11, 2060. [Google Scholar] [CrossRef]
- Chel-Guerrero, L.D.; Castañeda-Corral, G.; López-Castillo, M.; Scampicchio, M.; Morozova, K.; Oney-Montalvo, J.E.; Ferrentino, G.; Acevedo-Fernández, J.J.; Rodríguez-Buenfil, I.M. In Vivo Anti-Inflammatory Effect, Antioxidant Activity, and Polyphenolic Content of Extracts from Capsicum chinense by-Products. Molecules 2022, 27, 1323. [Google Scholar] [CrossRef]
- Cortes-Ferre, H.E.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A. Enzyme-Assisted Extraction of Anti-Inflammatory Compounds from Habanero Chili Pepper (Capsicum chinense) Seeds. Front. Nutr. 2022, 9, 942805. [Google Scholar] [CrossRef]
- Olguín Rojas, J.A.; Vázquez-León, L.A.; Salgado-Cervantes, M.A.; Fernandez-Barbero, G.; Díaz-Pacheco, A.; García-Alvarado, M.A.; Rodriguez-Jimenes, G.C. Water and Phytochemicals Dynamic during Drying of Red Habanero Chili Habanero Pepper (Capsicum chinense) Slices. Rev. Mex. Ing. Quim. 2019, 18, 851–864. [Google Scholar] [CrossRef]
- De Aguiar, A.C.; Sales, L.P.; Coutinho, J.P.; Barbero, G.F.; Godoy, H.T.; Martínez, J. Supercritical Carbon Dioxide Extraction of Capsicum Peppers: Global Yield and Capsaicinoid Content. J. Supercrit. Fluids 2013, 81, 210–216. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; Olguín-Rojas, J.A.; Fayos, O.; González-de-Peredo, A.V.; Espada-Bellido, E.; Ferreiro-González, M.; Barroso, C.G.; Barbero, G.F.; Garcés-Claver, A.; Palma, M. Influence of Fruit Ripening on the Total and Individual Capsaicinoids and Capsiate Content in Naga Jolokia Peppers (Capsicum chinense Jacq.). Agronomy 2020, 10, 252. [Google Scholar] [CrossRef]
- Carciochi, R.A.; D’Alessandro, L.G.; Vauchel, P.; Rodriguez, M.M.; Nolasco, S.M.; Dimitrov, K. Valorization of Agrifood By-Products by Extracting Valuable Bioactive Compounds Using Green Processes. In Ingredients Extraction by Physicochemical Methods in Food; Elsevier: Amsterdam, The Netherlands, 2017; pp. 191–228. [Google Scholar] [CrossRef]
- Fernández-Ronco, M.P.; Gracia, I.; De Lucas, A.; Rodríguez, J.F. Extraction of Capsicum annuum Oleoresin by Maceration and Ultrasound-Assisted Extraction: Influence of Parameters and Process Modeling. J. Food Process. Eng. 2013, 36, 343–352. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, G.; Yang, B.; Wu, Y.; Du, M.; Kan, J. Insights into the Stability of Carotenoids and Capsaicinoids in Water-Based or Oil-Based Chili Systems at Different Processing Treatments. Food Chem. 2021, 342, 128308. [Google Scholar] [CrossRef] [PubMed]
- De Aguiar, A.C.; Osorio-Tobón, J.F.; Silva, L.P.S.; Barbero, G.F.; Martínez, J. Economic Analysis of Oleoresin Production from Malagueta Peppers (Capsicum frutescens) by Supercritical Fluid Extraction. J. Supercrit. Fluids 2018, 133, 86–93. [Google Scholar] [CrossRef]
- Shi, J.; Yi, C.; Ye, X.; Xue, S.; Jiang, Y.; Ma, Y.; Liu, D. Effects of Supercritical CO2 Fluid Parameters on Chemical Composition and Yield of Carotenoids Extracted from Pumpkin. LWT-Food Sci. Technol. 2010, 43, 39–44. [Google Scholar] [CrossRef]
- Duranova, H.; Valkova, V.; Gabriny, L. Chili Peppers (Capsicum spp.): The Spice Not Only for Cuisine Purposes: An Update on Current Knowledge. Phytochem. Rev. 2022, 21, 1379–1413. [Google Scholar] [CrossRef]
- Avilés-Betanzos, K.A.; Scampicchio, M.; Ferrentino, G.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Evaluation of the Capsaicinoid Extraction Conditions from Mexican Capsicum chinense Var. Mayapan with Supercritical Fluid Extraction (SFE). Processes 2023, 11, 2272. [Google Scholar] [CrossRef]
- Jarén-Galán, M.; Nienaber, U.; Schwartz, S.J. Paprika (Capsicum annuum) Oleoresin Extraction with Supercritical Carbon Dioxide. J. Agric. Food Chem. 1999, 47, 3558–3564. [Google Scholar] [CrossRef]
- Richins, R.D.; Hernandez, L.; Dungan, B.; Hambly, S.; Holguin, F.O.; O’Connell, M.A. A “Green” Extraction Protocol to Recover Red Pigments from Hot Capsicum Fruit. HortScience 2010, 45, 1084–1087. [Google Scholar] [CrossRef]
- Li, G.; Song, C.; You, J.; Sun, Z.; Xia, L.; Suo, Y. Optimisation of Red Pepper Seed Oil Extraction Using Supercritical CO2 and Analysis of the Composition by Reversed-Phase HPLC-FLD-MS/MS. Int. J. Food Sci. Technol. 2011, 46, 44–51. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).