Cytosolic Fructose-1,6-bisphosphate Aldolases Modulate Primary Metabolism and Phytohormone Homeostasis in Soybean
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Genome-Wide Identification of FBA Gene Family and Phylogenetic Analysis
2.3. Analysis of Expression Pattern and Subcellular Localization
2.4. Vector Construction and Plant Transformation
2.5. Determination of FBA Enzyme Activity and Observation of Epidermal Cells of Leaves
2.6. Metabolomic Analysis and Determination of Phytohormone Content
2.7. Transcriptomic Analysis
3. Results
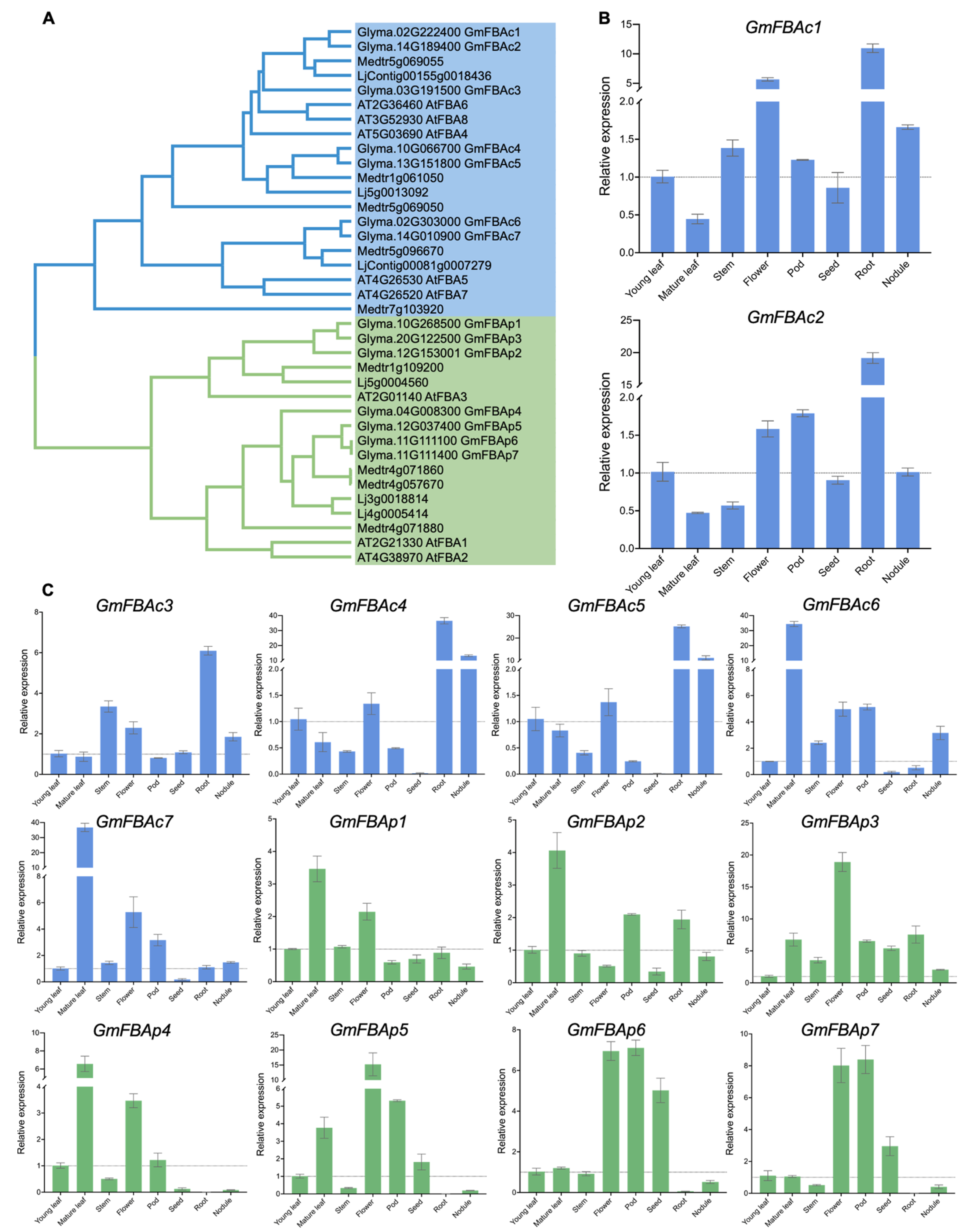
3.1. Identification and Phylogenetic Analysis of GmFBAs Gene Family
3.2. Differential Expression Profiles of GmFBAs Genes
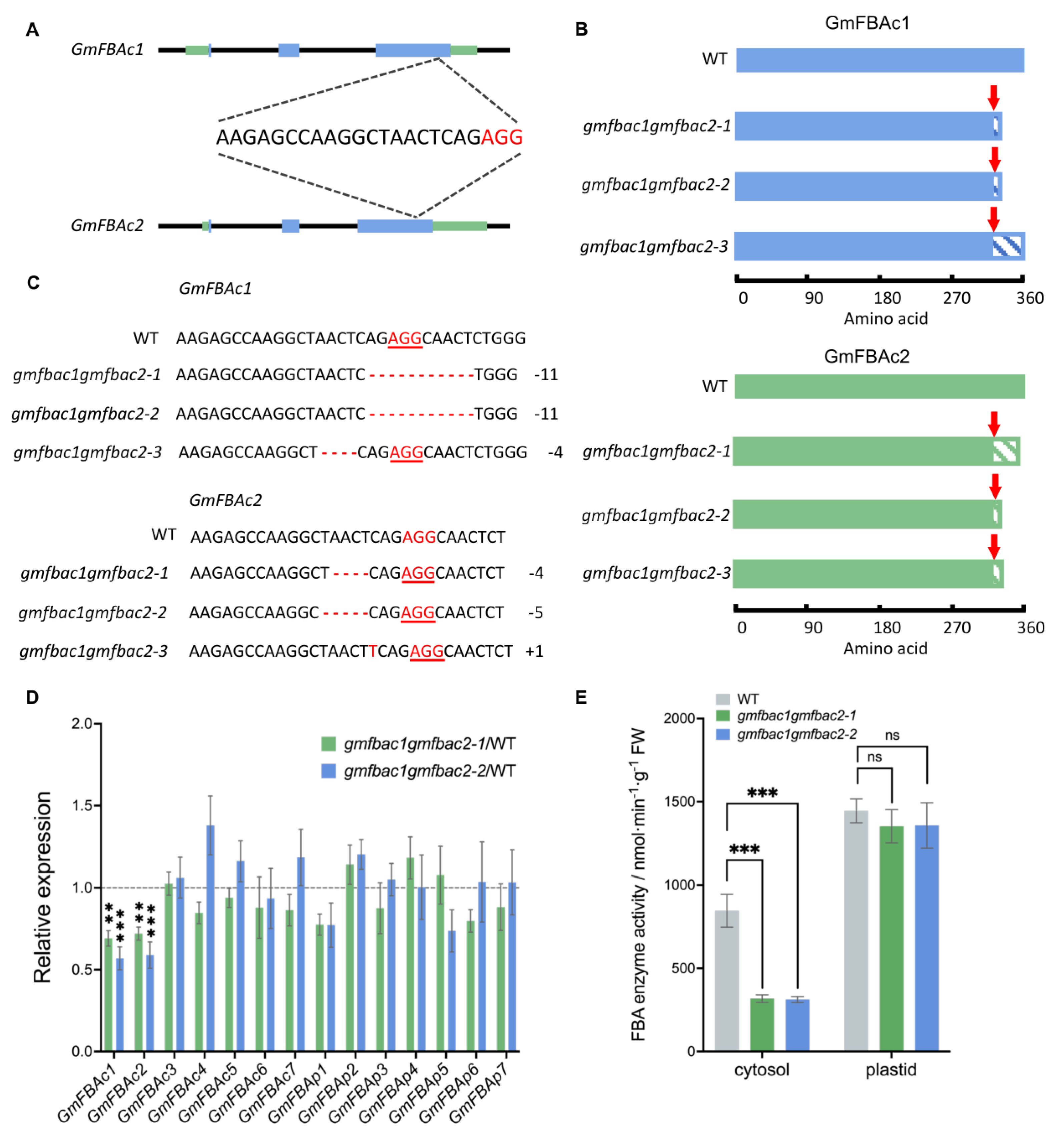
3.3. Generation of gmfbac1gmfbac2 Double Mutants
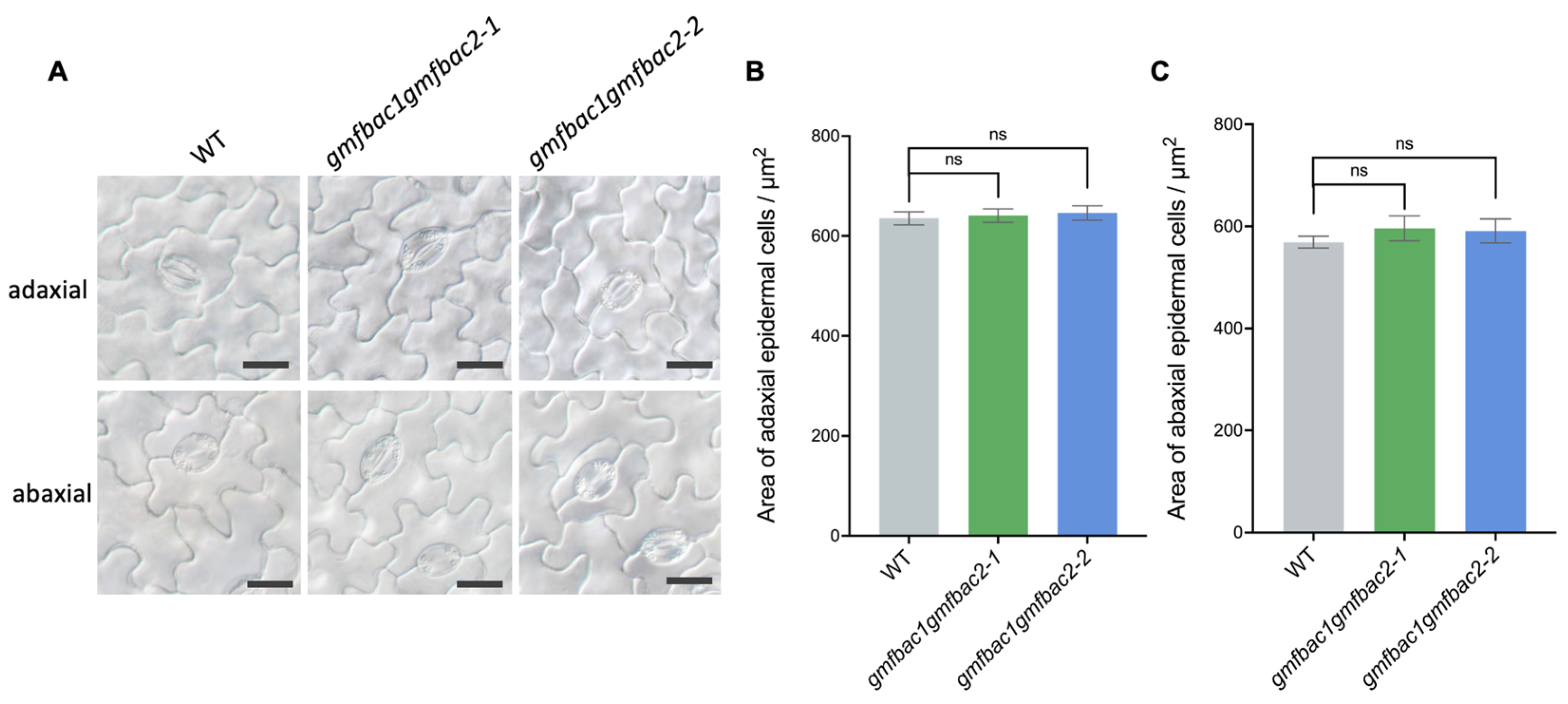
3.4. Phenotypes of Retarded Vegetative Growth in gmfbac1gmfbac2
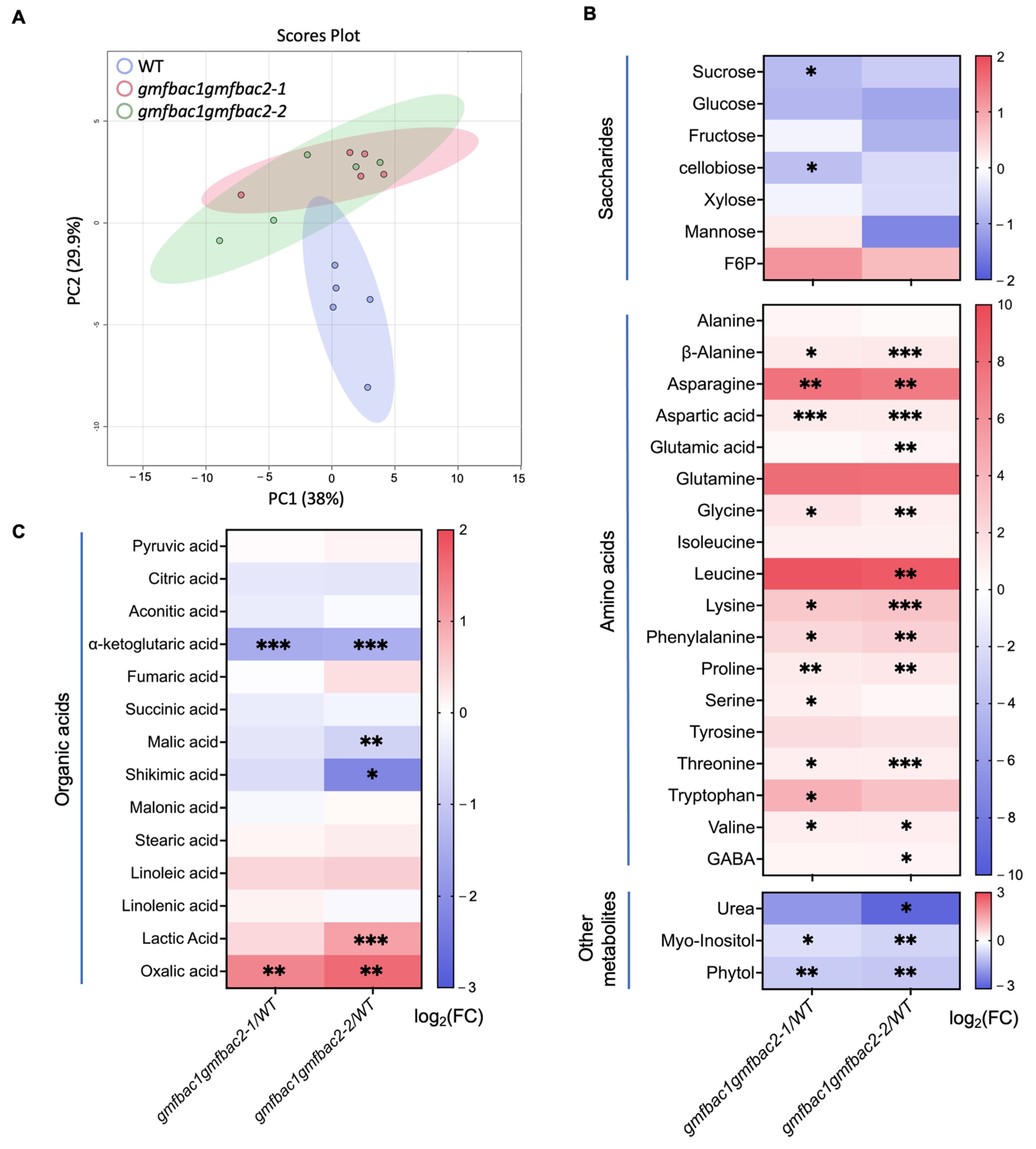
3.5. Metabolomic and Phytochemical Analysis Reveal Altered Primary Metabolism and Phytohormones Contents in gmfbac1gmfbac2 Mutants
3.6. The gmfbac1gmfbac2 Mutation Affects Auxin and Jasmonic Acid Content and Signaling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joyard, J.; Ferro, M.; Masselon, C.; Seigneurin-Berny, D.; Salvi, D.; Garin, J.; Rolland, N. Chloroplast proteomics highlights the subcellular compartmentation of lipid metabolism. Prog. Lipid Res. 2010, 49, 128–158. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Qiu, Z.; Wang, X.; Gong, P.; Xu, Q.; Yu, Q.; Guan, Y. Pooled CRISPR/Cas9 reveals redundant roles of plastidial phosphoglycerate kinases in carbon fixation and metabolism. Plant J. Cell. Mol. Biol. 2019, 98, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Z.; Zhang, A.; Bhawal, R.; Li, C.; Zhang, S.; Cheng, L.; Hua, J. Reduction of the canonical function of a glycolytic enzyme enolase triggers immune responses that further affect metabolism and growth in Arabidopsis. Plant Cell 2022, 34, 1745–1767. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Alvarado, G.P.; Sánchez-Nieto, S. Plant Hexokinases are Multifaceted Proteins. Plant Cell Physiol. 2017, 58, 1151–1160. [Google Scholar] [CrossRef]
- Cai, B.; Qiang, Y.; Yang, L.; Bi, H.; Ai, X. Genome-wide analysis of the fructose 1,6-bisphosphate aldolase (FBA) gene family and functional characterization of FBA7 in tomato. Plant Physiol. Biochem. 2016, 108, 251–265. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiao, F.; Tang, H.; Xu, H.; Zhang, L.; Wu, H. Genome-wide characterization, evolution, and expression profiling of FBA gene family in response to light treatments and abiotic stress in Nicotiana tabacum. Plant Signal. Behav. 2021, 16, 1938442. [Google Scholar] [CrossRef]
- Lu, W.; Tang, X.; Huo, Y.; Xu, R.; Qi, S.; Huang, J.; Zheng, C.; Wu, C. Identification and characterization of fructose 1,6-bisphosphate aldolase genes in Arabidopsis reveal a gene family with diverse responses to abiotic stresses. Gene 2012, 503, 65–74. [Google Scholar] [CrossRef]
- Carrera, D.; George, G.M.; Fischer-Stettler, M.; Galbier, F.; Eicke, S.; Truernit, E.; Streb, S.; Zeeman, S.C. Distinct plastid fructose bisphosphate aldolases function in photosynthetic and non-photosynthetic metabolism in Arabidopsis. J. Exp. Bot. 2021, 72, 3739–3755. [Google Scholar] [CrossRef]
- Simkin, A.J.; Lopez-Calcagno, P.E.; Davey, P.A.; Headland, L.R.; Lawson, T.; Timm, S.; Bauwe, H.; Raines, C.A. Simultaneous stimulation of sedoheptulose 1,7-bisphosphatase, fructose 1,6-bisphophate aldolase and the photorespiratory glycine decarboxylase-H protein increases CO2 assimilation, vegetative biomass and seed yield in Arabidopsis. Plant Biotechnol. J. 2017, 15, 805–816. [Google Scholar] [CrossRef]
- Cai, B.; Ning, Y.; Li, Q.; Li, Q.; Ai, X. Effects of the Chloroplast Fructose-1,6-Bisphosphate Aldolase Gene on Growth and Low-Temperature Tolerance of Tomato. Int. J. Mol. Sci. 2022, 23, 728. [Google Scholar] [CrossRef]
- Zeng, Y.; Tan, X.; Zhang, L.; Jiang, N.; Cao, H. Identification and expression of fructose-1,6-bisphosphate aldolase genes and their relations to oil content in developing seeds of tea oil tree (Camellia oleifera). PLoS ONE 2014, 9, e107422. [Google Scholar] [CrossRef] [PubMed]
- Haake, V.; Zrenner, R.; Sonnewald, U.; Stitt, M. A moderate decrease of plastid aldolase activity inhibits photosynthesis, alters the levels of sugars and starch, and inhibits growth of potato plants. Plant J. 1998, 14, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-S.; Koo, K.M.; Ryu, J.; Hong, M.J.; Kim, S.H.; Kwon, S.-J.; Kim, J.-B.; Choi, J.-i.; Ahn, J.-W. Overexpression of fructose-1,6-bisphosphate aldolase 1 enhances accumulation of fatty acids in Chlamydomonas reinhardtii. Algal Res. 2020, 47, 101825. [Google Scholar] [CrossRef]
- Koo, K.M.; Jung, S.; Lee, B.S.; Kim, J.-B.; Jo, Y.D.; Choi, H.-I.; Kang, S.-Y.; Chung, G.-H.; Jeong, W.-J.; Ahn, J.-W. The Mechanism of Starch Over-Accumulation in Chlamydomonas reinhardtii High-Starch Mutants Identified by Comparative Transcriptome Analysis. Front. Microbiol. 2017, 8, 858. [Google Scholar] [CrossRef] [PubMed]
- Garagounis, C.; Kostaki, K.I.; Hawkins, T.J.; Cummins, I.; Fricker, M.D.; Hussey, P.J.; Hetherington, A.M.; Sweetlove, L.J. Microcompartmentation of cytosolic aldolase by interaction with the actin cytoskeleton in Arabidopsis. J. Exp. Bot. 2017, 68, 885–898. [Google Scholar] [CrossRef]
- van der Linde, K.; Gutsche, N.; Leffers, H.M.; Lindermayr, C.; Müller, B.; Holtgrefe, S.; Scheibe, R. Regulation of plant cytosolic aldolase functions by redox-modifications. Plant Physiol. Biochem. 2011, 49, 946–957. [Google Scholar] [CrossRef]
- Olas, J.; Apelt, F.; Annunziata, M.; John, S.; Richard, S.; Gupta, S.; Kragler, F.; Balazadeh, S.; Mueller-Roeber, B. Primary carbohydrate metabolism genes participate in heat-stress memory at the shoot apical meristem of Arabidopsis thaliana. Mol. Plant 2021, 14, 1508–1524. [Google Scholar] [CrossRef]
- Simkin, A.J.; McAusland, L.; Headland, L.R.; Lawson, T.; Raines, C.A. Multigene manipulation of photosynthetic carbon assimilation increases CO2 fixation and biomass yield in tobacco. J. Exp. Bot. 2015, 66, 4075–4090. [Google Scholar] [CrossRef]
- Uematsu, K.; Suzuki, N.; Iwamae, T.; Inui, M.; Yukawa, H. Increased fructose 1,6-bisphosphate aldolase in plastids enhances growth and photosynthesis of tobacco plants. J. Exp. Bot. 2012, 63, 3001–3009. [Google Scholar] [CrossRef]
- Philippe, L.; Berardini, T.Z.; Li, D.; David, S.; Christopher, W.; Rajkumar, S.; Robert, M.; Kate, D.; Alexander, D.L.; Margarita, G.H. The Arabidopsis Information Resource (TAIR): Improved gene annotation and new tools. Nucleic Acids Res. 2012, 40, 1202–1210. [Google Scholar]
- Goodstein, D.M.; Shu, S.; Russell, H.; Rochak, N.; Hayes, R.D.; Joni, F.; Therese, M.; William, D.; Uffe, H.; Nicholas, P. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Yuan, J.; Kuang, H.; Gong, P.; Li, S.; Zhang, Z.; Liu, B.; Sun, J.; Yang, M.; Yang, L. Generation of a multiplex mutagenesis population via pooled CRISPR-Cas9 in soya bean. Plant Biotechnol. J. 2020, 18, 721–731. [Google Scholar] [CrossRef]
- Chen, L.; Cai, Y.; Liu, X.; Yao, W.; Guo, C.; Sun, S.; Wu, C.; Jiang, B.; Han, T.; Hou, W. Improvement of Soybean Agrobacterium-Mediated Transformation Efficiency by Adding Glutamine and Asparagine into the Culture Media. Int. J. Mol. Sci. 2018, 19, 3039. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Chen, F.; Qi, S.; Zhang, L.; Wu, S. Loss or duplication of key regulatory genes coincides with environmental adaptation of the stomatal complex in Nymphaea colorata and Kalanchoe laxiflora. Hortic. Res. 2018, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Jiang, J.; Xie, H.; Bai, M.; Xu, Q.; Wang, X.; Yu, X.; Chen, Z.; Guan, Y. Metabolomics Reveals Distinct Carbon and Nitrogen Metabolic Responses to Magnesium Deficiency in Leaves and Roots of Soybean [Glycine max (Linn.) Merr.]. Front. Plant Sci. 2017, 8, 2091. [Google Scholar] [CrossRef]
- López-Ibáñez, J.; Pazos, F.; Chagoyen, M. MBROLE 2.0-functional enrichment of chemical compounds. Nucleic Acids Res. 2016, 44, W201–W204. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, Z.; Cui, Y.; Ke, M.; Xu, H.; Xu, Q.; Chen, J.; Li, Y.; Huang, L.; Zhao, H.; et al. GmPIN-dependent polar auxin transport is involved in soybean nodule development. Plant Cell 2021, 33, 2981–3003. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Valliyodan, B.; Cannon, S.B.; Bayer, P.E.; Shu, S.; Brown, A.V.; Ren, L.; Jenkins, J.; Chung, C.Y.; Chan, T.F.; Daum, C.G.; et al. Construction and comparison of three reference-quality genome assemblies for soybean. Plant J. 2019, 100, 1066–1082. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef] [PubMed]
- Koeck, T.; Levison, B.; Hazen, S.L.; Crabb, J.W.; Stuehr, D.J.; Aulak, K.S. Tyrosine nitration impairs mammalian aldolase A activity. Mol. Cell. Proteom. 2004, 3, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Chen, X.; Ni, Q.; Lu, Y.; Wu, B.; Wang, H.; Yin, Z.; Zhou, W.; Dong, X. Estimation of hereditary fructose intolerance prevalence in the Chinese population. Orphanet J. Rare Dis. 2022, 17, 326. [Google Scholar] [CrossRef]
- Gillaspy, G.E. The cellular language of myo-inositol signaling. New Phytol. 2011, 192, 823–839. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.; Zhang, Y.; Yan, J.; Ahammed, G.J.; Bu, X.; Sun, X.; Liu, Y.; Xu, T.; Qi, H.; et al. SlFHY3 and SlHY5 act compliantly to enhance cold tolerance through the integration of myo-inositol and light signaling in tomato. New Phytol. 2022, 233, 2127–2143. [Google Scholar] [CrossRef]
- Gutbrod, K.; Romer, J.; Dörmann, P. Phytol metabolism in plants. Prog. Lipid Res. 2019, 74, 1–17. [Google Scholar] [CrossRef]
- Stortenbeker, N.; Bemer, M. The SAUR gene family: The plant’s toolbox for adaptation of growth and development. J. Exp. Bot. 2019, 70, 17–27. [Google Scholar] [CrossRef]
- Zhang, G.Z.; Jin, S.H.; Jiang, X.Y.; Dong, R.R.; Li, P.; Li, Y.J.; Hou, B.K. Ectopic expression of UGT75D1, a glycosyltransferase preferring indole-3-butyric acid, modulates cotyledon development and stress tolerance in seed germination of Arabidopsis thaliana. Plant Mol. Biol. 2016, 90, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Kozuka, T.; Kobayashi, J.; Horiguchi, G.; Demura, T.; Sakakibara, H.; Tsukaya, H.; Nagatani, A. Involvement of auxin and brassinosteroid in the regulation of petiole elongation under the shade. Plant Physiol. 2010, 153, 1608–1618. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yuan, L.; Song, X.; Yu, X.; Sundaresan, V. AHP2, AHP3, and AHP5 act downstream of CKI1 in Arabidopsis female gametophyte development. J. Exp. Bot. 2017, 68, 3365–3373. [Google Scholar] [CrossRef] [PubMed]
- Chini, A.; Fonseca, S.; Fernández, G.; Adie, B.; Chico, J.M.; Lorenzo, O.; García-Casado, G.; López-Vidriero, I.; Lozano, F.M.; Ponce, M.R.; et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature 2007, 448, 666–671. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, X.; Wang, D.; Wu, D.; Fu, Y.; Bian, C.; Jin, L.; Zhang, Y. Leaf cytokinin accumulation promotes potato growth in mixed nitrogen supply by coordination of nitrogen and carbon metabolism. Plant Sci. 2022, 324, 111416. [Google Scholar] [CrossRef]
- Guan, C.; Wu, B.; Yu, T.; Wang, Q.; Krogan, N.T.; Liu, X.; Jiao, Y. Spatial Auxin Signaling Controls Leaf Flattening in Arabidopsis. Curr. Biol. 2017, 27, 2940–2950.e4. [Google Scholar] [CrossRef]
- Tadege, M.; Lin, H.; Bedair, M.; Berbel, A.; Wen, J.; Rojas, C.M.; Niu, L.; Tang, Y.; Sumner, L.; Ratet, P. STENOFOLIA regulates blade outgrowth and leaf vascular patterning in Medicago truncatula and Nicotiana sylvestris. Plant Cell 2011, 23, 2125–2142. [Google Scholar] [CrossRef]
- Wang, B.; Chu, J.; Yu, T.; Xu, Q.; Sun, X.; Yuan, J.; Xiong, G.; Wang, G.; Wang, Y.; Li, J. Tryptophan-independent auxin biosynthesis contributes to early embryogenesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2015, 112, 4821–4826. [Google Scholar] [CrossRef]
- Baldi, B.G.; Maher, B.R.; Cohen, S. Stable Isotope Labeling, in Vivo, of d- and l-Tryptophan Pools in Lemna gibba and the Low Incorporation of Label into Indole-3-Acetic Acid. Plant. Physiol. 1991, 95, 1203–1208. [Google Scholar] [CrossRef]
- Last, R.L. Tryptophan Mutants in Arabidopsis: The Consequences of Duplicated Tryptophan Synthase b Genes. Plant Cell 1991, 3, 345–358. [Google Scholar]
- Yokoyama, R.; de Oliveira, M.; Kleven, B.; Maeda, H. The entry reaction of the plant shikimate pathway is subjected to highly complex metabolite-mediated regulation. Plant Cell 2021, 33, 671–696. [Google Scholar] [CrossRef]
- Scalabrin, E.; Radaelli, M.; Capodaglio, G. Simultaneous determination of shikimic acid, salicylic acid and jasmonic acid in wild and transgenic Nicotiana langsdorffii plants exposed to abiotic stresses. Plant Physiol. Biochem. 2016, 103, 53–60. [Google Scholar] [CrossRef]
- Pitaksaringkarn, W.; Matsuoka, K.; Asahina, M.; Miura, K.; Sage-Ono, K.; Ono, M.; Yokoyama, R.; Nishitani, K.; Ishii, T.; Iwai, H.; et al. XTH20 and XTH19 regulated by ANAC071 under auxin flow are involved in cell proliferation in incised Arabidopsis inflorescence stems. Plant J. 2014, 80, 604–614. [Google Scholar] [CrossRef]
- Noir, S.; Bömer, M.; Takahashi, N.; Ishida, T.; Tsui, T.L.; Balbi, V.; Shanahan, H.; Sugimoto, K.; Devoto, A. Jasmonate controls leaf growth by repressing cell proliferation and the onset of endoreduplication while maintaining a potential stand-by mode. Plant Physiol. 2013, 161, 1930–1951. [Google Scholar] [CrossRef]
- Guan, Y.; Ding, L.; Jiang, J.; Shentu, Y.; Zhao, W.; Zhao, K.; Zhang, X.; Song, A.; Chen, S.; Chen, F. Overexpression of the CmJAZ1-like gene delays flowering in Chrysanthemum morifolium. Hortic. Res. 2021, 8, 87. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Z.; Bai, M.; Kuang, H.; Wang, X.; Yu, X.; Zhong, X.; Guan, Y. Cytosolic Fructose-1,6-bisphosphate Aldolases Modulate Primary Metabolism and Phytohormone Homeostasis in Soybean. Agronomy 2023, 13, 1383. https://doi.org/10.3390/agronomy13051383
Qiu Z, Bai M, Kuang H, Wang X, Yu X, Zhong X, Guan Y. Cytosolic Fructose-1,6-bisphosphate Aldolases Modulate Primary Metabolism and Phytohormone Homeostasis in Soybean. Agronomy. 2023; 13(5):1383. https://doi.org/10.3390/agronomy13051383
Chicago/Turabian StyleQiu, Zhimin, Mengyan Bai, Huaqin Kuang, Xin Wang, Xiaomin Yu, Xiangbin Zhong, and Yuefeng Guan. 2023. "Cytosolic Fructose-1,6-bisphosphate Aldolases Modulate Primary Metabolism and Phytohormone Homeostasis in Soybean" Agronomy 13, no. 5: 1383. https://doi.org/10.3390/agronomy13051383
APA StyleQiu, Z., Bai, M., Kuang, H., Wang, X., Yu, X., Zhong, X., & Guan, Y. (2023). Cytosolic Fructose-1,6-bisphosphate Aldolases Modulate Primary Metabolism and Phytohormone Homeostasis in Soybean. Agronomy, 13(5), 1383. https://doi.org/10.3390/agronomy13051383





