QTL Mapping by Chromosome Segment Substitution Lines (CSSLs) Reveals Candidate Gene Controlling Leaf Sucrose Content in Soybean (Glycine max (L.) Merr.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Measurement of Leaf Sucrose Content
2.3. Method of QTL Mapping
2.4. Candidate Gene Mining and Sequence Alignment
2.5. Real-Time Quantitative PCR Analysis of Candidate Genes
2.6. Haplotype Analysis
3. Results
3.1. Distribution of Sucrose Content in CSSLs Population
3.2. Identification of QTLs Associated with Leaf Sucrose Content
3.3. Candidate Genes Identification
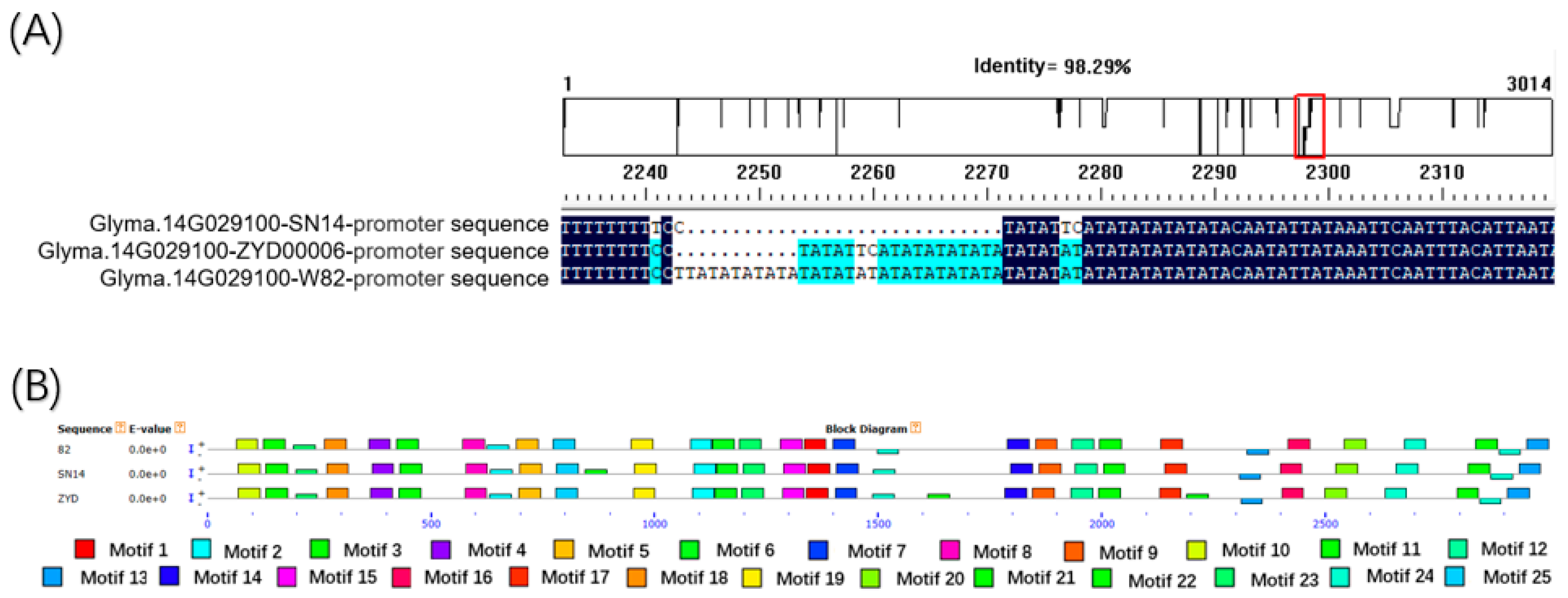
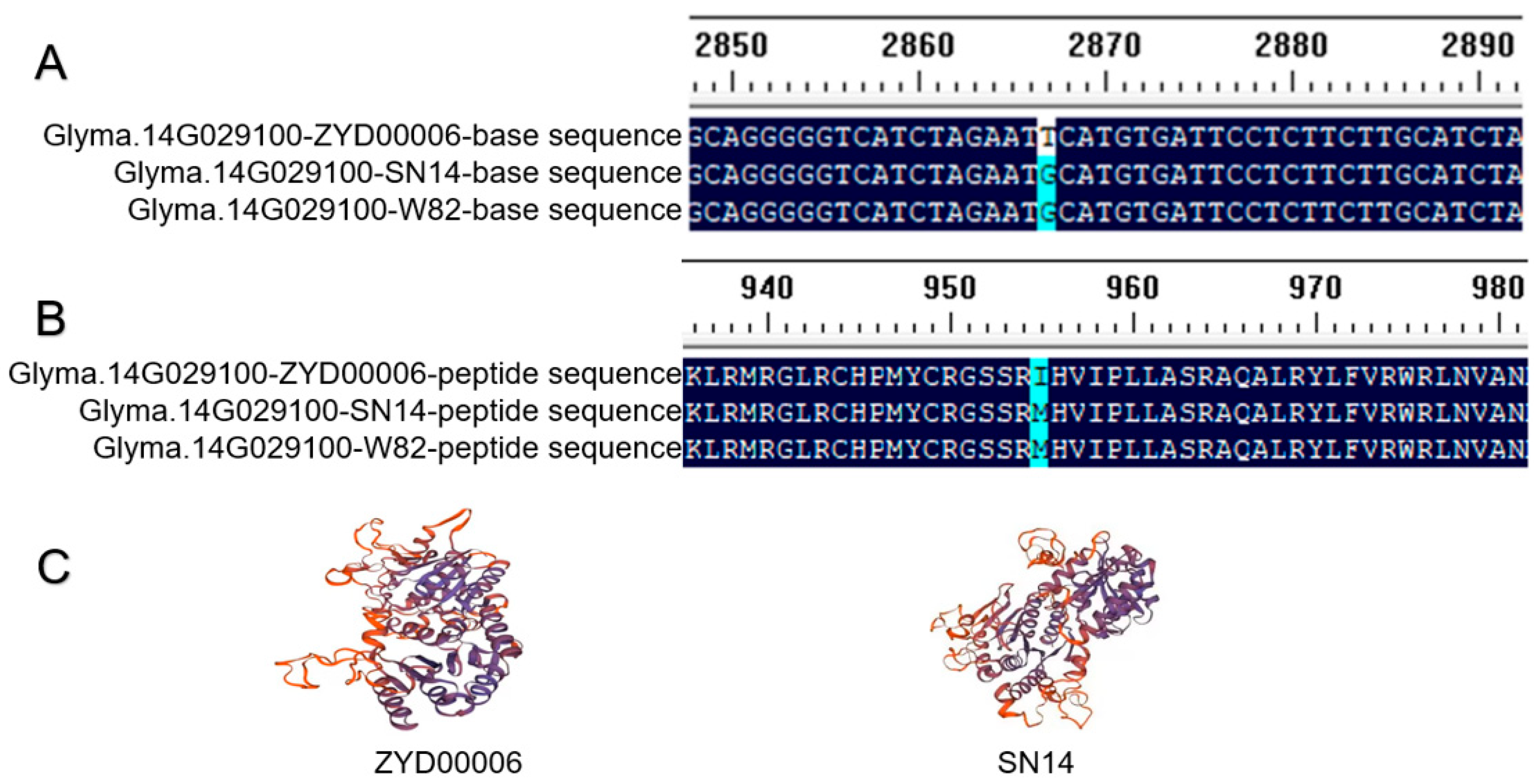
3.4. Candidate Gene Sequence Alignment of SN14 and ZYD00006
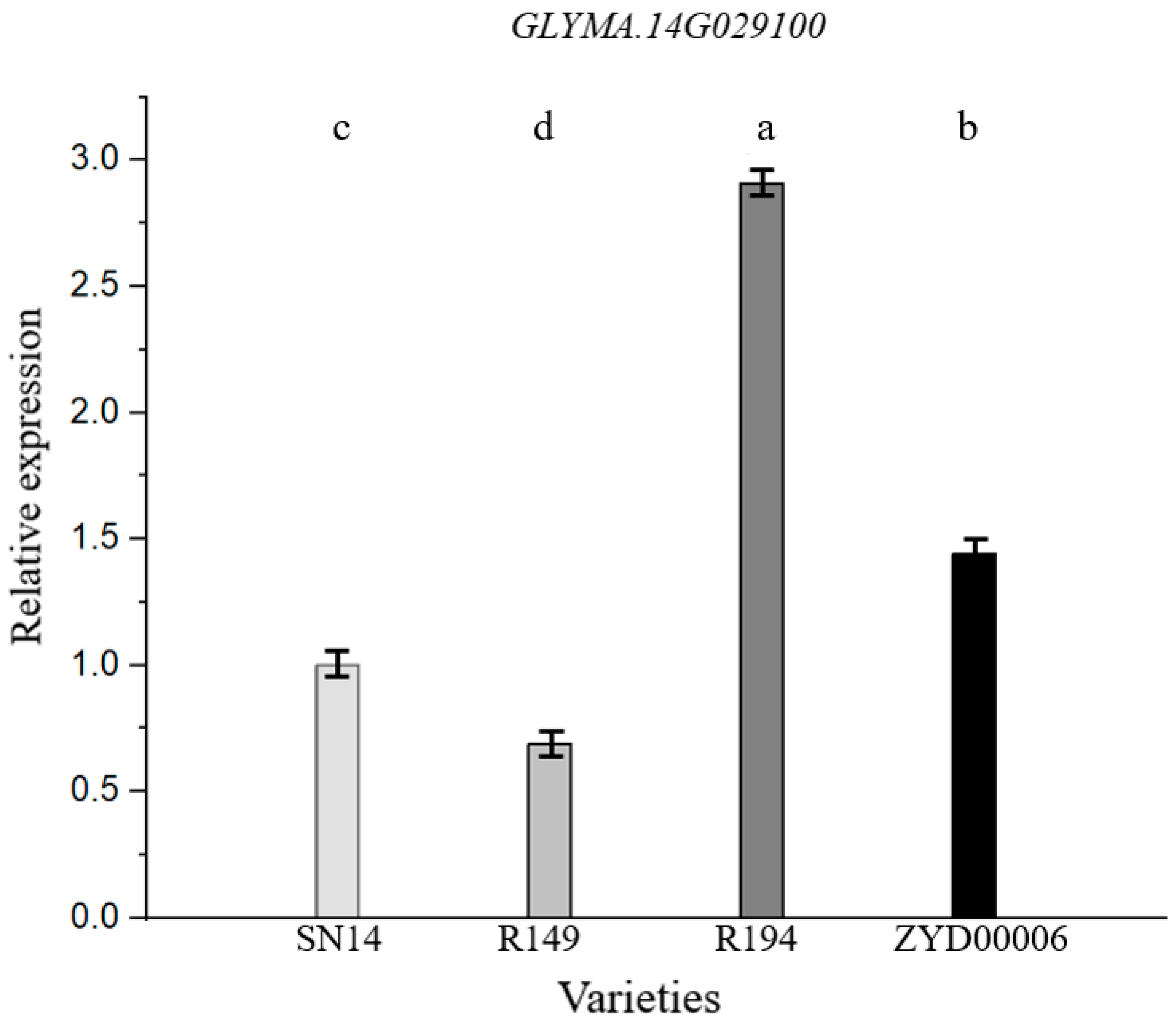
3.5. qRT-PCR Analysis of Candidate Genes
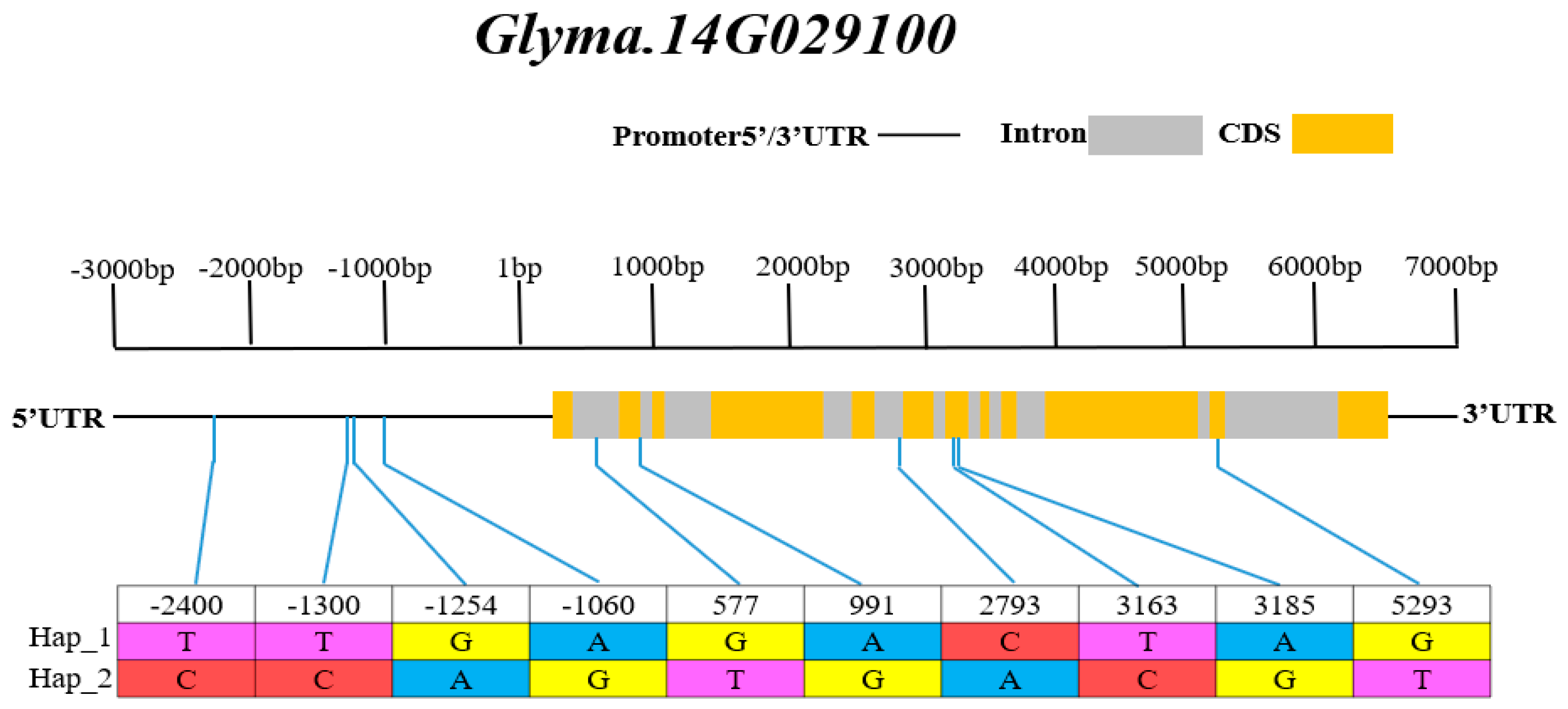
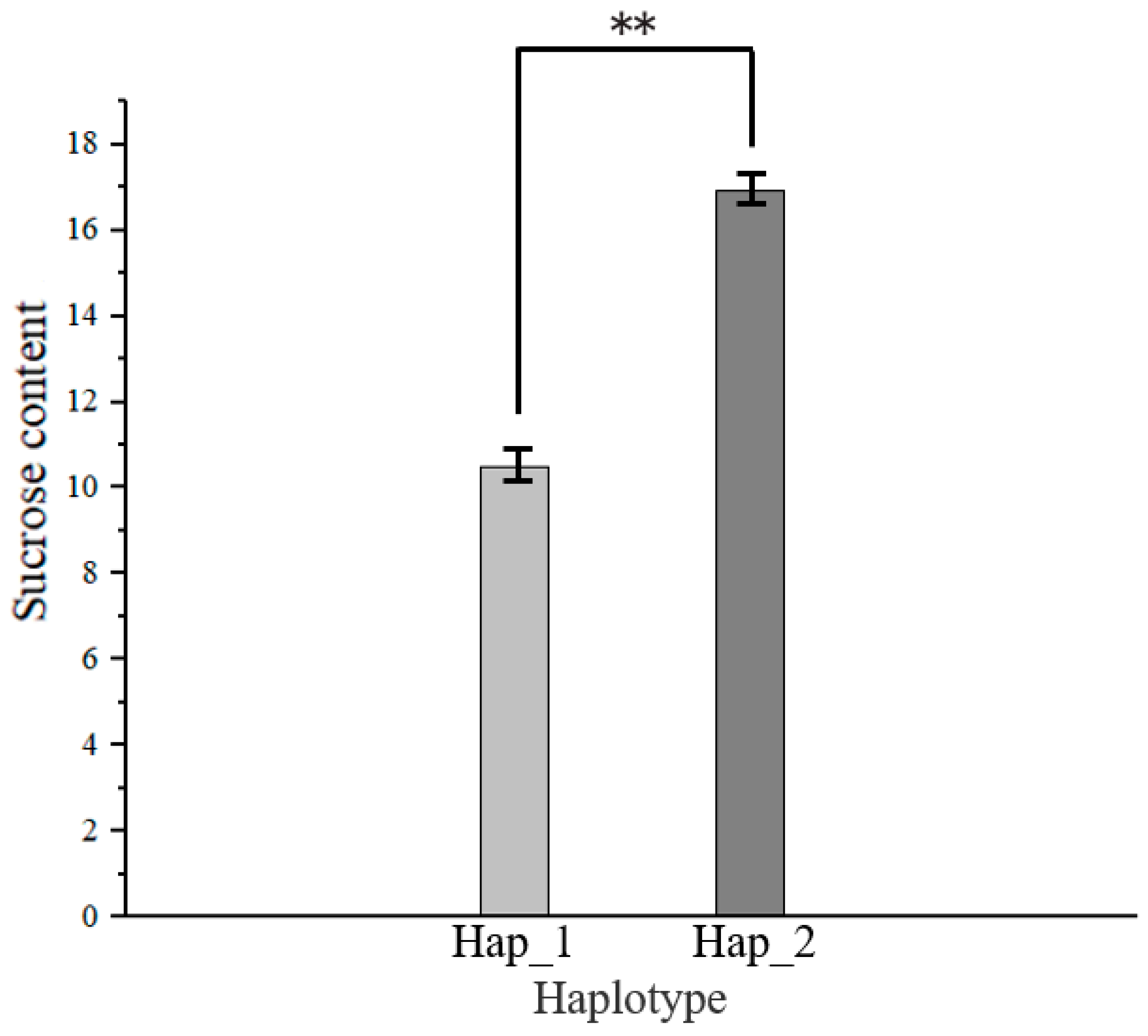
3.6. Haplotype Analysis of Candidate Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anderson, E.J.; Ali, M.L.; Beavis, W.D.; Chen, P.; Clemente, T.E.; Diers, B.W.; Graef, G.L.; Grassini, P.; Hyten, D.L.; McHale, L.K.; et al. Soybean [Glycine max (L.) Merr.] Breeding: History, Improvement, Production and Future Opportunities. In Advances in Plant Breeding Strategies: Legumes; Springer: Cham, Switzerland, 2019; pp. 431–516. [Google Scholar]
- Sagara, T.; Bhandari, D.R.; Spengler, B.; Vollmann, J. Spermidine and other functional phytochemicals in soybean seeds: Spatial distribution as visualized by mass spectrometry imaging. Food Sci. Nutr. 2020, 8, 675–682. [Google Scholar] [CrossRef]
- Yun, D.-Y.; Kang, Y.-G.; Yun, B.; Kim, E.-H.; Kim, M.; Park, J.S.; Lee, J.H.; Hong, Y.-S. Distinctive Metabolism of Flavonoid between Cultivated and Semiwild Soybean Unveiled through Metabolomics Approach. J. Agric. Food Chem. 2016, 64, 5773–5783. [Google Scholar] [CrossRef]
- Stitt, M.; Zeeman, S.C. Starch turnover: Pathways, regulation and role in growth. Curr. Opin. Plant Biol. 2012, 15, 282–292. [Google Scholar] [CrossRef]
- Comparot-Moss, S.; Denyer, K. The evolution of the starch biosynthetic pathway in cereals and other grasses. J. Exp. Bot. 2009, 60, 2481–2492. [Google Scholar] [CrossRef]
- Wind, J.; Smeekens, S.; Hanson, J. Sucrose: Metabolite and signaling molecule. Phytochemistry 2010, 71, 1610–1614. [Google Scholar] [CrossRef]
- Tarpley, L.; Vietor, D.M. Compartmentation of sucrose during radial transfer in mature sorghum culm. BMC Plant Biol. 2007, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Chia, T.; Thorneycroft, D.; Chapple, A.; Messerli, G.; Chen, J.; Zeeman, S.C.; Smith, S.M.; Smith, A.M. A cytosolic glucosyltransferase is required for conversion of starch to sucrose in Arabidopsis leaves at night. Plant J. 2004, 37, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, W.E.; Rohwer, J.M.; Botha, F.C. Partial purification and characterisation of sucrose synthase in sugarcane. J. Plant Physiol. 2005, 162, 11–20. [Google Scholar] [CrossRef]
- Maughan, P.J.; Maroof, M.S.; Buss, G.R. Identification of quantitative trait loci controlling sucrose content in soybean (Glycine max). Mol. Breed. 2000, 6, 105–111. [Google Scholar] [CrossRef]
- Kim, H.-K.; Kang, S.-T.; Cho, J.-H.; Choung, M.-G.; Suh, D.-Y. Quantitative trait loci associated with oligosaccharide and sucrose contents in soybean (Glycine max L.). J. Plant Biol. 2005, 48, 106–112. [Google Scholar] [CrossRef]
- Skoneczka, J.A.; Maroof, M.A.S.; Shang, C.; Buss, G.R. Identification of Candidate Gene Mutation Associated with Low Stachyose Phenotype in Soybean Line PI200508. Crop Sci. 2009, 49, 247–255. [Google Scholar] [CrossRef]
- Zeng, A.; Chen, P.; Shi, A.; Wang, D.; Zhang, B.; Orazaly, M.; Florez-Palacios, L.; Brye, K.; Song, Q.; Cregan, P. Identification of Quantitative Trait Loci for Sucrose Content in Soybean Seed. Crop Sci. 2014, 54, 554–564. [Google Scholar] [CrossRef]
- Sui, M.; Wang, Y.; Bao, Y.; Wang, X.; Li, R.; Lv, Y.; Yan, M.; Quan, C.; Li, C.; Teng, W.; et al. Genome-wide association analysis of sucrose concentration in soybean (Glycine max L.) seed based on high-throughput sequencing. Plant Genome 2020, 13, e20059. [Google Scholar] [CrossRef] [PubMed]
- Xin, D.; Qi, Z.; Jiang, H.; Hu, Z.; Zhu, R.; Hu, J.; Han, H.; Hu, G.; Liu, C.; Chen, Q. QTL Location and Epistatic Effect Analysis of 100-Seed Weight Using Wild Soybean (Glycine soja Sieb. & Zucc.) Chromosome Segment Substitution Lines. PLoS ONE 2016, 11, e0149380. [Google Scholar] [CrossRef]
- Zheng, H.; Hou, L.; Xie, J.; Cao, F.; Wei, R.; Yang, M.; Qi, Z.; Zhu, R.; Zhang, Z.; Xin, D.; et al. Construction of Chromosome Segment Substitution Lines and Inheritance of Seed-Pod Characteristics in Wild Soybean. Front. Plant Sci. 2022, 13, 869455. [Google Scholar] [CrossRef]
- Mulsanti, I.W.; Yamamoto, T.; Ueda, T.; Samadi, A.F.; Kamahora, E.; Rumanti, I.A.; Thanh, V.C.; Adachi, S.; Suzuki, S.; Kanekatsu, M.; et al. Finding the superior allele of japonica-type for increasing stem lodging resistance in indica rice varieties using chromosome segment substitution lines. Rice 2018, 11, 25. [Google Scholar] [CrossRef]
- Ookawa, T.; Aoba, R.; Yamamoto, T.; Ueda, T.; Takai, T.; Fukuoka, S.; Ando, T.; Adachi, S.; Matsuoka, M.; Ebitani, T.; et al. Precise estimation of genomic regions controlling lodging resistance using a set of reciprocal chromosome segment substitution lines in rice. Sci. Rep. 2016, 6, 30572. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Yang, H.; Liu, X.; Li, H.; Yuan, L.; Li, W.; Fu, Z.; Tang, J.; Kang, D. Identification of heterotic loci associated with grain yield and its components using two CSSL test populations in maize. Sci. Rep. 2016, 6, 38205. [Google Scholar] [CrossRef]
- Xu, Q.; Shi, Y.; Yu, T.; Xu, X.; Yan, Y.; Qi, X.; Chen, X. Whole-Genome Resequencing of a Cucumber Chromosome Segment Substitution Line and Its Recurrent Parent to Identify Candidate Genes Governing Powdery Mildew Resistance. PLoS ONE 2016, 11, e0164469. [Google Scholar] [CrossRef]
- Zhai, H.; Gong, W.; Tan, Y.; Liu, A.; Song, W.; Li, J.; Deng, Z.; Kong, L.; Gong, J.; Shang, H.; et al. Identification of Chromosome Segment Substitution Lines of Gossypium barbadense Introgressed in G. hirsutum and Quantitative Trait Locus Mapping for Fiber Quality and Yield Traits. PLoS ONE 2016, 11, e0159101. [Google Scholar] [CrossRef]
- Yang, H.; Wang, W.; He, Q.; Xiang, S.; Tian, D.; Zhao, T.; Gai, J. Chromosome segment detection for seed size and shape traits using an improved population of wild soybean chromosome segment substitution lines. Physiol. Mol. Biol. Plants 2017, 23, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Jiang, Y.; Wang, Z.; Gou, Z.; Lyu, J.; Li, W.; Yu, Y.; Shu, L.; Zhao, Y.; Ma, Y.; et al. Resequencing 302 wild and cultivated accessions identifies genes related to domestication and improvement in soybean. Nat. Biotechnol. 2015, 33, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Drag, D.W.; Slattery, R.; Siebers, M.; DeLucia, E.H.; Ort, D.R.; Bernacchi, C.J. Soybean photosynthetic and biomass responses to carbon dioxide concentrations ranging from pre-industrial to the distant future. J. Exp. Bot. 2020, 71, 3690–3700. [Google Scholar] [CrossRef] [PubMed]
- Coleman, H.D.; Beamish, L.; Reid, A.; Park, J.-Y.; Mansfield, S.D. Altered sucrose metabolism impacts plant biomass production and flower development. Transgenic Res. 2010, 19, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Schmutz, J.; Cannon, S.B.; Schlueter, J.; Ma, J.; Mitros, T.; Nelson, W.; Hyten, D.L.; Song, Q.; Thelen, J.J.; Cheng, J.; et al. Genome sequence of the palaeopolyploid soybean. Nature 2010, 463, 178–183. [Google Scholar] [CrossRef]

| Population | Parent Population | CSSL Population | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Female (mg/g) | Male (mg/g) | Minimum (mg/g) | Maximum (mg/g) | Mean (mg/g) | SD (mg/g) | CV (%) | Skewness | Kurtosis | |
| 21-CSSL | 12.37 | 17.58 | 4.53 | 22.06 | 13.69 | 3.95 | 28.85 | 0.39 | −0.06 |
| 22-CSSL | 11.68 | 18.66 | 3.8 | 20.02 | 11.26 | 3.52 | 31.26 | 0.32 | −0.36 |
| Trait | Year | QTL | Chr. | Start Position (Mb) | End Position (Mb) | Size (Mb) | LOD | PVE (%) | Additive Effect |
|---|---|---|---|---|---|---|---|---|---|
| Sucrose Content | 21 | qSuC-D1a-1 | 1 | 0.71 | 0.77 | 0.06 | 2.69 | 4.60 | 1.10 |
| Sucrose Content | 21 | qSuC-B2-1 | 14 | 2.12 | 2.24 | 0.13 | 17.25 | 35.89 | −3.63 |
| Sucrose Content | 22 | qSuC-B2-1 | 14 | 2.12 | 2.24 | 0.13 | 14.09 | 29.77 | −3.05 |
| Chr | Start (bp) | End (bp) | Name |
|---|---|---|---|
| Chr14 | 2126633 | 2133000 | Glyma.14G029100 |
| Chr14 | 2136060 | 2141071 | Glyma.14G029200 |
| Chr14 | 2142803 | 2143006 | Glyma.14G029300 |
| Chr14 | 2148899 | 2149084 | Glyma.14G029400 |
| Chr14 | 2150450 | 2155697 | Glyma.14G029500 |
| Chr14 | 2158771 | 2160914 | Glyma.14G029600 |
| Chr14 | 2162666 | 2164954 | Glyma.14G029700 |
| Chr14 | 2165005 | 2165953 | Glyma.14G029800 |
| Chr14 | 2166342 | 2167231 | Glyma.14G029900 |
| Chr14 | 2169235 | 2175640 | Glyma.14G030000 |
| Chr14 | 2181345 | 2187798 | Glyma.14G030100 |
| Chr14 | 2189097 | 2189530 | Glyma.14G030200 |
| Chr14 | 2198027 | 2198251 | Glyma.14G030300 |
| Chr14 | 2204142 | 2206726 | Glyma.14G030400 |
| Chr14 | 2207318 | 2218028 | Glyma.14G030500 |
| Chr14 | 2220379 | 2225086 | Glyma.14G030600 |
| Chr14 | 2226406 | 2230206 | Glyma.14G030700 |
| Chr14 | 2233569 | 2237977 | Glyma.14G030800 |
| Gene | GO Annotation | Gene Description |
|---|---|---|
| Glyma.14G029000 | GO:0004713 | protein tyrosine kinase activity |
| Glyma.14G029100 | GO:0016157 | sucrose synthase activity |
| Glyma.14G029500 | GO:0017119 | Golgi transport complex |
| Glyma.14G030100 | GO:0003723 | RNA binding |
| Glyma.14G030000 | GO:0005840 | ribosome |
| Glyma.14G030300 | GO:0009055 | electron carrier activity |
| Glyma.14G030500 | GO:0005515 | protein binding |
| Glyma.14G030400 | GO:0009072 | aromatic amino acid family metabolic process |
| Glyma.14G030600 | GO:0005515 | protein binding |
| Glyma.14G030700 | GO:0003677 | DNA binding |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; He, C.; Sun, C.; Wang, X.; Qi, Z.; Chen, Q.; Zhao, M.; Yao, X.; Zhang, D. QTL Mapping by Chromosome Segment Substitution Lines (CSSLs) Reveals Candidate Gene Controlling Leaf Sucrose Content in Soybean (Glycine max (L.) Merr.). Agronomy 2023, 13, 1592. https://doi.org/10.3390/agronomy13061592
Wu Y, He C, Sun C, Wang X, Qi Z, Chen Q, Zhao M, Yao X, Zhang D. QTL Mapping by Chromosome Segment Substitution Lines (CSSLs) Reveals Candidate Gene Controlling Leaf Sucrose Content in Soybean (Glycine max (L.) Merr.). Agronomy. 2023; 13(6):1592. https://doi.org/10.3390/agronomy13061592
Chicago/Turabian StyleWu, Yuheng, Chenyu He, Changheng Sun, Xiangran Wang, Zhaoming Qi, Qingshan Chen, Mingzhe Zhao, Xindong Yao, and Dayong Zhang. 2023. "QTL Mapping by Chromosome Segment Substitution Lines (CSSLs) Reveals Candidate Gene Controlling Leaf Sucrose Content in Soybean (Glycine max (L.) Merr.)" Agronomy 13, no. 6: 1592. https://doi.org/10.3390/agronomy13061592
APA StyleWu, Y., He, C., Sun, C., Wang, X., Qi, Z., Chen, Q., Zhao, M., Yao, X., & Zhang, D. (2023). QTL Mapping by Chromosome Segment Substitution Lines (CSSLs) Reveals Candidate Gene Controlling Leaf Sucrose Content in Soybean (Glycine max (L.) Merr.). Agronomy, 13(6), 1592. https://doi.org/10.3390/agronomy13061592






