Abstract
Water salinity is a critical cause of the decrease in quality of fodder plants. The use of saline water as an alternative to fresh water requires the production of elite plant varieties that can tolerate excess amounts of sodium chloride. In this study, we evaluated six genotypes of barley (Hordeum vulgare L.) used as sprout fodder for their tolerance to saline water conditions. The six genotypes were tested for their germination vigor, α-amylase activity, root system architecture (RSA) phenotyping, relative water content (RWC), chlorophyll content (ChC), reactive oxygen species accumulation (ROS), and total antioxidant capacity. Increasing the salt concentration caused a significant decrease in the germination time, α-amylase activity, germination percentage, ChC, and RWC of all the genotypes, but significant differences in the RSA and ChC were detected. In addition, the plasticity of these characteristics at the seedling stage increased their potential to select varieties that could produce high amounts of green fodder when fresh water alternatives are used. Strong and positive correlations were detected between the green carpet formed under the salt treatment and ChC at the seedling stage for a local genotype and G134.
1. Introduction
The world’s growing population has triggered an increase in the demand for food and feed production, and the use of different water types and alternative irrigation systems is considered an important and sustainable solution due to the shortage and limitations of fresh water as well as the salinity of water in many regions, especially in the arid and semi-arid ones. Livestock rearing in arid and semi-arid regions, including the study area in Saudi Arabia, faces great challenges, the most important of which is securing fodder. This is due to the scarcity of irrigation water as well as climatic fluctuations, which have caused the low productivity of natural pastures. The climate of the Kingdom of Saudi Arabia is characterized by very low rainfall and high temperatures in most regions of the country [1], and along with climatic fluctuations, it affects the cultivation and production of forage. The preservation of groundwater resources is also strategically important in arid regions. Hence, it has become necessary to search for suitable alternative means of securing fodder for livestock. The Saudi government is currently developing and supporting alternative projects devoted to the production of fodder, which uses large amounts of irrigation water, in order to reduce field cultivation as much as possible. One of the most important of these alternatives is the production of green fodder using hydroponics, a technique whose use has recently been expanded and developed in Saudi Arabia. This forage production system, which is not affected by weather conditions nor by the nature of the soil, can reduce the gap in the supply of forage in arid regions [2]. Feeding livestock with hydroponic fodder is also considered safe and healthy as no pesticides or fertilizers are added to it [3]. Hydroponic fodder can be produced from many grains such as barley, maize, wheat, oat, and rye [4].
Hydroponic green fodder (GF) is produced from forage grains that are germinated and grown inside special growing rooms with appropriate growing conditions for a short period of time [5]. Barley (Hordeum vulgare L.) is the most widely used species in the production of green fodder with hydroponics [6]. It is worth noting that sprouting one kg of barley grain in a hydroponic system has the advantage of producing up to 10 kg of fresh green fodder in 8 days, at any time of year [7], in addition to its shorter growth cycle from seed to green forage, which can be extended to 7–8 days [8]. The production of hydroponically sprouted barley has been recommended for arid and semi-arid regions due to its high efficiency in water use [9]. The sprouting of grains increases total protein, sugars, certain vitamins, crude fiber, and minerals [10]. Moreover, when animals feed on sprouted barley fodder, the benefits from it are higher compared to when they feed on dry barley grains. This is because the efficiency of digesting sprouted barley grains is high compared to the digestion of dry grains due to the activation of hydrolytic enzymes during the grain sprouting process [11]. In addition to the aforementioned nutritional benefits, green hydroponic fodder is highly palatable to animals, and its production process is economical and inexpensive [12]. Salo [13] indicated that feeding with hydroponic fodder increases the productivity of dairy cows due to the increase in the proportion of digested materials. Agius et al. [14] also studied the effect of feeding with hydroponic fodder on the quality of milk and concluded that the quality of milk components improved when the animals fed on hydroponic barley fodder.
The salinity of the irrigation water leads to a decrease in crop yield and affects the germination of crop seeds in varying proportions [15]. The effect of irrigation water salinity on cereal crops differs according to the species, as barley is more tolerant to water salinity than wheat. It also varies according to the barley cultivar [16].
Glycophytes are greatly affected by salinity; this results in the uptake and accumulation of salt ions in plant cells, which leads to a decrease in seed germination due to the inhibition of α-amylase activity [17,18]. It also leads to a reduction in plant growth, either by creating osmotic stress or by specific ion toxicity, thus resulting in cell division and elongation inhibition due to disturbances in plant metabolism [19,20]. In general, the uptake of most of the important macro and micro elements is inhibited by salinity treatments through the apoplastic route [21,22]. The significance of this apoplastic flow varies across species and environmental conditions [23], wherein salt tolerance involves the prevention of salt leakage along the apoplast, through the endodermal barrier of root cells and subsequently to the stele and xylem [24,25,26].
The extension of the root system of sprouted barley plays an essential role in the increase in ash content from day 4 due to the enhanced mineral uptake [27]. Moreover, roots represent an unneglectable part of the dry matter of the sprouted carpet. Functionally, plant roots are characterized by the presence of a meristematic and elongation zone, in which the rate of cell division is high and reactive oxygen species (ROS) serve as signaling molecules, which is the same as the functional role of hormones [28]. The overproduction of ROS is potentially harmful as they are regarded as the regulators of cellular component damage and irreparable metabolic dysfunction, which consequently results in mitochondrial dysfunction and programed cell death [29]. The enzymatic activity of superoxide dismutase in wheat plants results in primary root elongation under osmotic stress [30]. Selection according to RSA plasticity under stressful conditions has become a point of interest in the production of elite varieties and has led to improved water and nutrient consumption and adaptation to abiotic stresses [31]. As it is an image-based system, RSA phenotyping has become a high-throughput technique that can shorten the time required to evaluate a high number of genotype sets [32,33].
Hydroponically produced green forage and its use can be a solution to the lack of fresh forage and fresh water, which are common problems in many parts of Saudi Arabia. Since hydroponic green sprout production requires germination and growth vigor varieties with highly branched roots in order to form the root layer, which is very important in retaining water and supporting seedling growth, this study assessed the suitability of some barley genotypes to tolerate salinity stress resulting from the use of alternative water sources. In the study area, the alternative water source is usually well water, which is often salty to varying degrees. This objective is achieved through the assessment of the α-amylase activity, RSA plasticity after salinity treatment, the ability of the adapted roots to scavenge accumulated cellular ROS, and finally genotype suitability to sprout in salt water.
2. Materials and Methods
2.1. Germination Test
The genotypes of the barley (Hordeum vulgare L.) seeds used in this study were the following: the local cultivar Qasimi and the five cultivars Giza 123 (G123), Giza 124 (G124), Giza 132 (G132), Giza 134 (G134), and Giza 2000 (G2000). The Giza cultivars were obtained from the Barley Research Department, Field Crops Research Institute, Agriculture Research Centre, Egypt. Grains were sterilized with a sodium hypochlorite solution (5%) for 10 min and then washed 3 times with sterilized distilled water. Fifty seeds were then germinated in 9 cm-diameter Petri dishes and supplemented with 25% MS solution; the pH was adjusted to 5.8–6.0 with three treatments: 0 mM (control), 50 mM (S1), and 100 mM (S2) NaCl; three replicates were performed for each treatment. The pH was adjusted to 5.8 –6.0 using potassium hydroxide (KOH). The beginning of germination was considered as the emergence of the radicle after 4 days; it was performed in a growth chamber with a light cycle of 15 h light and 9 h dark, and temperatures of 20 °C for day and 16 °C for night. The germination percentage was recorded after a period of 8 days.
2.2. α-Amylase Activity Assay
To assess the α-amylase (EC 3.2.1.1) activity, the seeds of the six genotypes were germinated in three salinity treatments (MS medium 0, MS supplemented with 50 mM NaCl, MS supplemented with 100 mM NaCl). Forty-eight hours after sowing, fifteen seeds from each Petri dish were removed, washed, and rubbed between paper towels to remove excess water from the surface. The seeds were ground in 100 mL pre-cooled distilled water and then homogenized. The mixture was cooled in a 5 °C bath for 12 min, filtered, and then centrifuged at 10,000× g at 3 °C for 15 min. The clear supernatant was separated to assay the α-amylase activity using the dinitrosalicylic acid method established in [34]. To inactivate β-amylase, the supernatant was preheated at 70 °C for 15 min. Then, a 1% starch solution was prepared by dissolving 1 gm of soluble starch in a sodium acetate buffer. The pH was subsequently adjusted to 5.6 before incubating the solution at 40 °C for 15 min and pre-cooling it to 25 °C. After that, a 1:1 volume mixture of the supernatant and the 1% starch solution were incubated in a test tube for 3 min. Then, 0.5 mL of 3 5-dinitrosalicylic acid in water was added to the mixture, and the test tube was incubated in boiling water for 5 min. Finally, the tube was allowed to cool for 45 min. The absorbance was measured using Ultrospec 2100 pro UV/Visible 153 spectrophotometer (Amercham Biosciences, Piscataway, NJ, USA) at 540 nm, with maltose as the reducing sugar standard. The activity of one unit of α-amylase can be defined as the produced μ-moles of maltose per minute.
2.3. Barley Sprouts Materials and Experimental Design
A kilogram of seed for each genotype was sprouted in trays and subjected to three treatments—0 (control), 50 (S1), and 100 (S2) mM NaCl—in an automated hydroponic chamber with sprayer irrigation, a LED lighting system, and a capacity of 18 trays with the size of 70 × 30 × 5 cm (Figure 1). The growth chamber was set to a light cycle of 16 h light and 8 h dark, and day/night temperatures of 21–22/17–19 °C for 8 days. Sterilized seeds (as mentioned above) were soaked in tap water overnight (nearly 10 h), spread in the trays, and then manually irrigated with a salt solution every 8 h/day. Green fodder carpets were then removed from the chamber and weighed to measure the fresh yield. The fresh green leaves were subsequently dried in an oven at 60 °C for 48 h. The dry weight of each carpet was measured.

Figure 1.
The six barley genotypes after germination in 70 × 30 × 5 cm trays: grown in growth chambers for 8 days, irrigated with 0 mM (control), 50 mM (S1), and 100 mM (S2) NaCl.
2.4. Chlorophyll Content (ChC)
Total chlorophyll content (ChC) was measured following the extraction. Fresh leaves (in three replicates for each treatment) were ground in liquid nitrogen at about 10 mg FW−1 each, which were then placed in 15 mL falcon tubes, homogenized with 80% (v/v) ethanol using a rocking shaker, incubated at 4 °C for 72 h, and then centrifuged at 7000× g for 5 min. The supernatant was transferred into new tubes and measured using an Ultrospec 2100 pro UV/Visible spectrophotometer (Amercham Biosciences). Following the method described by [35], the total chlorophyll content was calculated as follows:
TC = [20.2 × (A645) + 8.02 × (A663)]. The ChC was expressed as mg/g weight.
2.5. Relative Water Content (RWC)
After the 8th day of barley growth, the relative water content (RWC) of the barley leaves was measured using following the formula: RWC = [(FW − DW)/(SW − DW)] × 100%. Fresh weight (FW) was measured first, and then the leaves were saturated for 48 h in double distilled water. Saturated weight (SW) was subsequently measured, and then the leaves were dried. Finally, dry weight was measured.
2.6. RSA Trait Measurements
Twenty seedlings of each genotype were sprouted in a magenta box as an experimental unit. The boxes were incubated in the growth chamber under the same sprouting growth conditions and salt concentrations. The experiment consisted of three treatments (control, S1 and S2) with three replicates for each genotype. The sampling of roots was carried out by randomly selecting one seedling from each genotype from each box; toluidine red was used to stain the roots, as described by [29]. Then, the roots were spread on a glass sheet, and the excess staining solution was removed carefully using tissue paper. Finally, the roots were scanned using a flatbed scanner and read with the WinRHIZO software (V5.0, Regent Instruments, Quebec City, QC, Canada) The RSA functional traits, including total root length (TRL), lateral root number (LRN), and root volume (RV), were determined.
2.7. Histochemical Staining
Three replicates of root tips from each carpet were selected for the measurement of the generated ROS, as described by Saquib et al. [36]. A staining mixture was prepared using 0.25 μM DCFH-DA in 1 × PBS buffer. The root tips were incubated in the staining mixture for 20 min and washed twice using the PBS buffer. Finally, the root tips were imaged using a fluorescence microscope (Nikon 136 Eclipse 80i, Tokyo, Japan).
2.8. Antioxidant Activity Determination
The antioxidant activity was determined by calculating the IC50 value, defined as the amount of sample (weight) necessary to reduce the stable radical DPPH (Sigma–Aldrich, Merck KGaA, Darmstadt, Germany) by 50%. The IC50 value was calculated as described by Sharma and Bhat [37]; a 1:1 reaction mix of 0.15 mM DPPH and the root crude extract (from the control and different treatments) at various dilutions in methanol (150, 250, 500, 750, and 1000 mg mL−1) were prepared. The tubes were kept in the dark at room temperature for 1 h. At 517 nm wave length, the absorbance of the reaction mixture was measured using an Ultrospec 2100 pro UV/Visible 153 spectrophotometer (Amercham Biosciences). The control sample was prepared using only methanol, without the tissue extract, and the same steps were followed. The antioxidant activity was calculated using the following equation:
Radical scavenging activity (%) = [1 − (Absorbance treatment/Absorbance control)] × 100.
2.9. Statistical Analysis
All the experiments were designed with three replicates for each genotype. The data of all morphological and physiological traits and antioxidant activity were analyzed with two-way ANOVA tests using the XLSTAT statistical package (Version 2018, Excel Add-ins soft SARL, New York, NY, USA). The mean values were compared using Duncan’s multiple range test at a probability level of 0.05.
3. Results
3.1. Germination Test and the α-Amylase Activity Assay
An in vitro experiment was conducted to investigate the effect of different concentrations of salt (0, 50 mM, and 100 mM NaCl) on the germination of six barley genotypes. All the genotypes were significantly affected after exposure to saline water, but under the S100 treatment, G134 showed the highest germination rate, while G123 showed the lowest germination rate under S100 (Figure 2A).
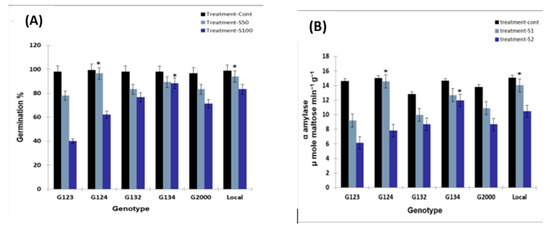
Figure 2.
(A) Germination rate and (B) the reduction in α-amylase activity caused by NaCl stress. Barley seeds were germinated in 0 (control), 50 mM, and 100 mM NaCl. After treatment for 48 h, α-amylase activity was detected in 15 grains. * Means significant difference between the genotypes at probability level of 0.05.
As expected, after the genotypes were exposed to NaCl stress for 72 h, a marked decrease in α-amylase activity was observed in the salinity treatments compared to the control with the exception of G124 and the local genotypes, which presented stable α-amylase activity under the S1 treatment.
Meanwhile, under the S2 treatment, the G134 genotype had the highest α-amylase activity, followed by the local genotype. In general, the activity of the enzyme was reduced by increasing the salinity level. However, this reduction was not equal and was dependent on the genetic background. G123 had the maximum reduction under both treatments, and the minimum reduction for the high treatment was recorded for G134 (Figure 2B).
3.2. Chlorophyll Content (Chl Cnt) and Relative Water Content (RWC)
The different NaCl concentrations had a significant effect on the chlorophyll content of some barley genotypes tested, such as G123 and G132 (Figure 3A). However, the other genotypes were not affected and presented enhanced chlorophyll content under a different salinity level compared to the previous two genotypes (Figure 3A), which may be due to the maintenance of the high chlorophyll content despite the reduction in the green part of the sprouts. The results presented in Figure 3B show that spraying barley using water with different salinity levels affected the RWC negatively, and that a gradual decrease was noticed for all genotypes due to the increase in the salinity level. Meanwhile, the genotypes G123 and G132 showed lower values for RWC under the S2 treatment.
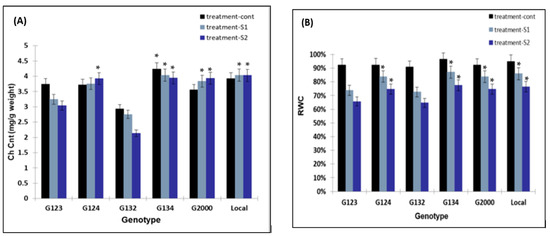
Figure 3.
The effect of different NaCl concentrations on the chlorophyll content of the leaves (A) after 8 days of sprouting and (B) the RWC. * Means significant difference between the genotypes at probability level of 0.05.
3.3. RSA Trait Measurements
RSA is known to be strongly affected by stressful conditions. The ANOVA test showed the significant differences in the RSA traits caused by different levels of salt stress that the studied genotypes had undergone compared to the control conditions (Figure 4). The ability of the genotypes to maintain better RSA traits under the salt stress conditions was assessed. Upon increasing the salinity level, a gradual decrease in all the traits was observed. However, with the progressive increase in the salinity potential in the magenta box, the G123 showed the maximum reduction in all measured traits, while the local genotype maintained a steep reduction in both the TRL and RV. Overall, the G134 showed the maximum values for all traits under all treatments, while a significant decrease occurred in the LRN.
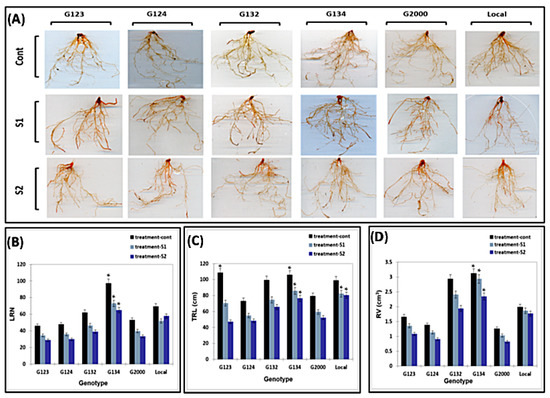
Figure 4.
Selected stained root for photo scanning and analyzing of control and salinity levels of all genotypes, respectively (A). Effect of different salinity levels on the RSA of six barley genotypes (B–D). The 8-day-old barley seedlings that were grown in the magenta box and stained with toluidine red. Means significant difference between the genotypes at probability level of 0.05.
3.4. Histochemical Staining
The visualization of H2O2 in roots subjected to the highest salinity level with H2DCFDA fluorescence showed a differential distribution of the stain in the roots of the six genotypes. However, the fluorescence of the G134 and local genotypes (Figure 5D,F) was lighter than that of the other four genotypes. Under salt stress, both the G134 and local genotypes were able to scavenge the overproduction ROS in the root zones, while the other four genotypes revealed a non-typical pattern for the DCF fluorescence.
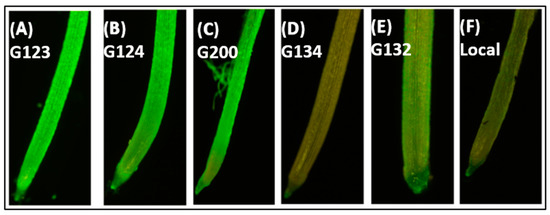
Figure 5.
Effect of different salinity levels on ROS accumulation in the apical meristem of 8-day-old barley genotypes. The seedlings grown in the magenta box and roots were stained using H2DCFDA.
3.5. Antioxidant Activity Determination
The salinity treatment considerably induced the plant redox system. Our results showed that the root extracts of all the genotypes exhibited a significant rise in their total antioxidant capacity under high salt treatment compared to the control. Based on the results, G134, G123, and the local genotypes showed lower IC50 values under the salt stress treatment (Figure 6). G2000, G124, and G132 showed higher IC50 values under the same conditions (Figure 6). In addition, the genotypes G134 and G123 maintained the highest scavenging activity among all the genotypes under the high salt treatment, unlike G2000, which showed the lowest scavenging capacity under salt stress. Table 1 shows percentage of decrease in calculated IC50 for barley root extract value between control and salt treatment (S2) of each genotype, where genotype G123 displayed the highest percentage while genotype G2000 displayed the lowest.
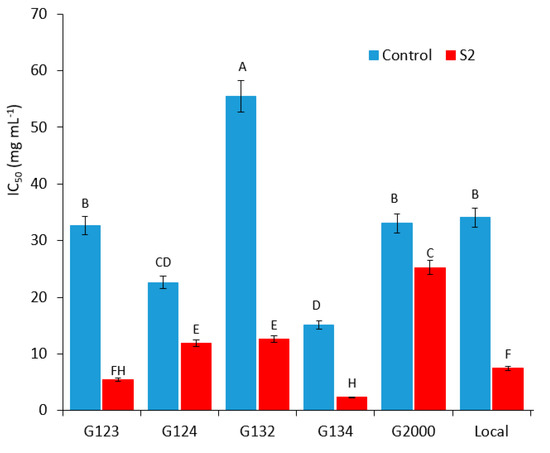
Figure 6.
Calculated IC50 for barley root extracts, demonstrating a genotype × treatment interaction (p = 0.0001). Data points within genotypes and treatments that have the same letter are not significantly different at a 95% level of confidence.

Table 1.
Percentage of decrease in calculated IC50 for barley root extract value between control and salt treatment (S2) of each genotype.
4. Discussion
The extenuation of the effects of salinity through the use of elite varieties is in high demand in food and feed production for sustainable agriculture. The exposure of crop plants to salinity has been observed to decrease seed germination [37], barley seedling growth, final biomass, and chlorophyll content as well as induce oxidative stress [38]. However, it was evident that using tolerant genotypes helped to overcome salinity stress and improved barley growth [39]. The consequential progress of the germination process was achieved through three main phases: (1) water uptake, (2) lipid and starch catabolism, (3) and radicle emergence [20]. The α-amylase enzyme promoted starch hydrolysis, which is considered to be the main source of ATP [40]. At this stage, salinity caused metabolic processes to be unbalanced and restricted cellular growth and tissue expansion. Thus, we hypothesized that α-amylase activity could provide useful information about the tolerant genotypes at the early growth stage when early and vigorous sprouting occurs. The α-amylase activity and total seed germination percentage significantly decreased with an increase in the salinity level. However, the G134 and local genotypes presented a lesser reduction in the α-amylase activity compared with the other genotypes (Figure 2A,B). Furthermore, we observed that the genotypes (as G134) that showed high α-amylase activity presented highest germination rate compared to those that demonstrated decreased α-amylase activity. This indicated that the adapted genotypes (G134 and Local) show a tolerance to salt at the early growth stage due to sufficient water uptake by the seeds and the increasing occurrence of starch hydrolysis into soluble sugars. The increased amount of soluble sugars provides osmotic protection to the germinated seeds in addition to maintaining better turgidity in the emerged radical [41,42]. Consequently, it seems that the α-amylase activity in the adapted genotypes stimulated the hydrolysis of the mobilized starch into soluble sugars, which can provide energy during germination and counter the deleterious effects of osmotic stress during cell expansion after the radicle’s emergence.
Salinity stress induces an increase in Na + ion accumulation in the plant leaves, which provokes a considerable reduction in chlorophyll synthesis and leaf water content [43]. Barley is being used as a green fodder because of the high values of its grass juice factor and chlorophyll content, both of which improve livestock performance [44,45,46]. Thus, chlorophyll content is highly interesting under stresses such as salinity, as it was determined that salinity stress triggers a decline in stomatal conductance and photosynthesis [47] in addition to the ionic effect of excess amounts of Na+ and Cl−, which reduces the amount of photosynthetic pigments and enhances the role of oxidative burst as a secondary stress of salinity [48]. Usually, stomatal closure occurs with the onset of harsh conditions in order to save more water [49]. Our results showed that G123 and G132 were the genotypes that experienced the highest reduction in chlorophyll content, while the other genotypes did not present any significant effects on the chlorophyll content (Figure 3A). Moreover, our results showed that the genotypes G123 and G132 had significantly lower RWC percentages under the salinity treatment compared to the other genotypes (Figure 3B). There may be several reasons for the decreased chlorophyll content and RWC of the sensitive genotypes, including ion toxicity and osmotic imbalance between the stroma and chloroplast, which alter the electron transport activity and subsequently reduce the photosynthetic efficiency [50,51,52]. Our results for the sensitive genotypes suggest that stomatal closure possibly led to a reduction in the water absorption, which was accompanied by a decrease in the RSA traits (Figure 4B–D). It has been reported that salinity reduces the RWC due to the stomatal closure, which alters the photosynthesis and leads to low water uptake [53]; this could explain our results.
A well-developed RSA is correlated with a healthy and vigorous shoot system [54]. The RV, LRN, and TRL are some of the most important RSA traits for water and nutrient absorption [55] in addition to being responsible for a significant part of the dry matter in the sprout carpet. However, salinity results in a reduction in RSA traits, such as TRL, LRN, and RV, in sensitive genotypes [55,56,57]. RSA plasticity is considered to be a good morphological indicator that can help to differentiate between tolerant and sensitive genotypes under most stresses. This study showed that G134 presented the highest values for all measured traits under the control and all salinity levels, followed by the local genotype and G2000, G123, and G124. Thus, a vigorous RSA leads to high root and shoot biomass, and, in turn, a high carpet biomass (Figure 4B–D).
Salinity triggers the overproduction of ROS, which is first produced as a signal molecule. Then, the increased accumulation causes oxidative damage, which interrupts normal cell functioning and robustly reduces plant growth and productivity [58]. Extended salt stress causes an oxidative burst, which leads to the overproduction of ROS and causes damage to the membranes. Membrane integrity plays an essential role in the salt stress [48]. Under such conditions, the plant’s existence relies on certain key factors, including changes in the growth conditions, stress severity and duration, and, more importantly, the innate potential of plants to adapt to changing growth and environmental conditions. It has been well established that crop plants growing under stressful environmental conditions exhibit an up-regulated antioxidant defense system for the protection of major cellular pathways from free radical-triggered oxidative damage [43,59]. ROS plays different roles in root development, such as root gravitropism, cell elongation, differentiation, and lateral root formation [60,61], and its overproduction can lead to a decrease in the RSA traits [62]. In this context, the innate antioxidant defense system of plants has been the main target of present research on the sustainable production of forage and crops. In accordance with previous results, the visualization of ROS using DCF-DA staining showed normal fluorescence with the adapted genotype G134 and the local (Figure 5D,F) genotypes under severe stress, while sensitive genotypes showed high fluorescence intensity under the same conditions (Figure 5A–C,E). These results are in agreement with the DPPH quantification assay of the root crude extract in response to the total antioxidant capacity (Figure 6), which can explain the RSA reduction in the sensitive genotypes under saline conditions. The total antioxidant capacity of the roots of sensitive genotypes (G123) showed high IC50 values, which reflect a lower antioxidant capacity. On the other hand, the tolerant genotypes (G134) exhibited a low IC50, reflecting the high scavenging potential of the excess amount of ROS, which could cause damage to the root cell components. It has been reported that the overproduction of free radicals in the primary roots of maize (Zea mays) causes the inhibition of elongation [63]. In this study, a high scavenging potential was observed in the tolerant genotypes under the high salinity stress treatment, which presented a low fluorescence intensity for DCF-DA. Meanwhile, the sensitive genotypes presented the accumulation of high amounts of ROS with a high reduction in RSA traits and a lower scavenging potential under the high salinity stress treatment, in accordance with the results of [64].
5. Conclusions
Using saline water as an alternative to fresh water for the production of hydroponic barley could lead to salinity stress and result in oxidative imbalance, followed by a significant decrease in RSA traits, chlorophyll content, and RWC. Our results showed that the overproduction of ROS can trigger a high depression in the fodder carpet via the decrease in RSA traits such as RV, TRL, and LRN, which are considered to be important components of the root carpet. The tolerant genotypes showed high antioxidant activity, which reflects the importance of the redox system in controlling ROS overproduction. Future studies will focus on the quality and nutrient contents of the barley carpet irrigated with saline water.
Author Contributions
Conceptualization, methodology, data curation, writing—original draft preparation, O.A. and W.S.; software, formal analysis, O.A.; validation, investigation, N.A.-S., K.F.A., W.S. and M.S.; resources, O.A., N.A.-S. and W.S.; writing—review and editing, O.A., W.S. and M.S.; visualization, supervision, project administration, O.A. and W.S.; funding acquisition, W.S. and K.F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia-project number IFKSURG-2-41.
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number IFKSURG-2-41.
Conflicts of Interest
The authors declare no competing interests.
References
- The National Center for Meteorology—Saudi Arabia. Available online: https://ncm.gov.sa/ar/Pages/default.aspx (accessed on 15 November 2022).
- Fazaeli, H.; Golmohammadi, H.A.; Tabatatbaei, S.N. Effect of Replacing dietary corn silage with hydroponic barley green fodder on Holstein Dairy Cows Performance. Iran. J. Appl. Anim. Sci. 2021, 11, 47–57. [Google Scholar]
- Al-Karaki, G.N.; Al-Hashimi, M. Green fodder production and water use efficiency of some forage crops under hydroponic conditions. Int. Sch. Res. Not. 2012, 2012, 924672. [Google Scholar] [CrossRef]
- Muela, C.R.; Rodriguez, H.E.; Ruiz, O.; Flores, A.; Grado, J.A.; Arzola, C. Use of green fodder produced in hydroponic systems as supplement for Salers lactating cows during the dry season. In Proceedings of the American Society of Animal Science, Cincinnati, OH, USA, 24–28 July 2005. [Google Scholar]
- Sneath, R.; McIntosh, F. Review of Hydroponic Fodder Production for Beef Cattle; Department of Primary Industries: Queensland, Australia, 2003; Volume 84, p. 54. [Google Scholar]
- Fazaeli, H.; Solaymani, S.; Rouzbehan, Y. Nutritive value and performance of cereal green fodder yield in hydroponic system. Res. Anim. Prod. 2017, 8, 96–104. [Google Scholar] [CrossRef]
- Kruglyakov, Y.A. Construction of equipment for growing green fodder by a hydroponic technique. Traktory-I Sel’skokhozyaistvennye Mashiny 1989, 6, 24–27. [Google Scholar]
- Pandey, H.; Pathak, N.N. Nutritional Evaluation of Artificially Grown Barley Fodder in Lactating Corssbred Cows. Indian J. Anim. Nutr. 1991, 8, 77–78. [Google Scholar]
- Bustos, C.D.E.; Gonzalez, E.L.; Aguilera, B.A.; Esptnoza, G.J.A. Forraje hidropónico, una alternativa para la suplementación caprina en el semidesierto queretano. XXXVIII; Reunión Nacional de Investigación Pecuaria: Puebla, México, 2000; 388p. [Google Scholar]
- Lorenz, K.; D’Appolonia, B. Cereal sprouts: Composition, nutritive value, food applications. Crit. Rev. Food Sci. Nutr. 1980, 13, 353–385. [Google Scholar] [CrossRef]
- Lemmens, E.; Moroni, A.V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Lê, K.; Broeck, H.C.V.D.; Brouns, F.J.; De Brier, N.; et al. Impact of Cereal Seed Sprouting on Its Nutritional and Technological Properties: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 305–328. [Google Scholar] [CrossRef]
- Abdula, A.H. Contribution of Hydroponic Feed for Livestock Production and Productivity. Sci. Front. 2022, 3, 1–7. [Google Scholar]
- Salo, S. Effect of Hydroponic Fodder Feeding on Milk Yield and Compostion of Dairy Cow. J. Nat. Sci. Res. 2019, 9, 1–2. [Google Scholar]
- Agius, A.; Pastorelli, G.; Attard, E. Cows fed hydroponic fodder and conventional diet: Effects on milk quality. Arch. Anim. Breed. 2019, 62, 517–525. [Google Scholar] [CrossRef]
- Uçarlı, C. Effects of salinity on seed germination and early seedling stage. In Abiotic Stress Plants; Intechopen: London, UK, 2020; chapter 11; pp. 211–232. [Google Scholar] [CrossRef]
- Bagwasi, G.; Agenbag, G.A.; Swanepoel, P.A. Effect of salinity on the germination of wheat and barley in South Africa. Crop Forage Turfgrass Manag. 2020, 6, e20069. [Google Scholar] [CrossRef]
- Liu, L.; Xia, W.; Li, H.; Zeng, H.; Wei, B.; Han, S.; Yin, C. Salinity Inhibits Rice Seed Germination by Reducing α-Amylase Activity via Decreased Bioactive Gibberellin Content. Front. Plant Sci. 2018, 9, 275. [Google Scholar] [CrossRef] [PubMed]
- Norastehnia, A.; Sajedi, R.H.; Nojavan-Asghari, M. Inhibitory effects of methyl jasmonate on seed germination in maize (Zea mays): Effect on α-amylase activity and ethylene production. Gen. Appl. Plant Physiol. 2007, 33, 13–23. [Google Scholar]
- Bernstein, N.; Kafkafi, U. Root growth under salinity stress. In Plant Roots; CRC Press: Boca Raton, FL, USA, 2002; pp. 1222–1250. [Google Scholar]
- Zhang, N.; Zhang, H.-J.; Sun, Q.-Q.; Cao, Y.-Y.; Li, X.; Zhao, B.; Wu, P.; Guo, Y.-D. Proteomic analysis reveals a role of melatonin in promoting cucumber seed germination under high salinity by regulating energy production. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Grattan, S.R.; Grieve, C.M. Salinity–mineral nutrient relations in horticultural crops. Sci. Hortic. 1998, 78, 127–157. [Google Scholar] [CrossRef]
- Rengasamy, P. Soil chemistry factors confounding crop salinity tolerance—A review. Agronomy 2016, 6, 53. [Google Scholar] [CrossRef]
- Bramley, H.; Turner, N.; Turner, D.; Tyerman, S. Roles of Morphology, Anatomy, and Aquaporins in Determining Contrasting Hydraulic Behavior of Roots. Plant Physiol. 2009, 150, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Craig Plett, D.; Møller, I.S. Na+ transport in glycophytic plants: What we know and would like to know. Plant Cell Environ. 2010, 33, 612–626. [Google Scholar] [CrossRef]
- Flam-Shepherd, R.; Huynh, W.Q.; Coskun, D.; Hamam, A.M.; Britto, D.T.; Kronzucker, H.J. Membrane fluxes, bypass flows, and sodium stress in rice: The influence of silicon. J. Exp. Bot. 2018, 69, 1679–1692. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Fazaeli, H.; Golmohammadi, H.A.; Tabatabayee, S.N.; Asghari-Tabrizi, M. Productivity and nutritive value of barley green fodder yield in hydroponic system. World Appl. Sci. J. 2012, 16, 531–539. [Google Scholar]
- Tsukagoshi, H. Control of root growth and development by reactive oxygen species. Curr. Opin. Plant Biol. 2016, 29, 57–63. [Google Scholar] [CrossRef]
- Azab, O.; Al-Doss, A.; Alshahrani, T.; El-Hendawy, S.; Zakri, A.; Abd-ElGawad, A. Root System Architecture Plasticity of Bread Wheat in Response to Oxidative Burst under Extended Osmotic Stress. Plants 2021, 10, 939. [Google Scholar] [CrossRef]
- Terletskaya, N.V.; Lee, T.E.; Altayeva, N.A.; Kudrina, N.O.; Blavachinskaya, I.V.; Erezhetova, U. Some Mechanisms Modulating the Root Growth of Various Wheat Species under Osmotic-Stress Conditions. Plants 2020, 9, 1545. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P.; Brown, K.M. New roots for agriculture: Exploiting the root phenome. Philos. Trans. R. Soc. B 2012, 367, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- Bucksch, A.; Burridge, J.; York, L.; Das, A.; Nord, E.; Weitz, J.S.; Lynch, J.P. Image-Based High-Throughput Field Phenotyping of Crop Roots. Plant Physiol. 2014, 166, 470–486. [Google Scholar] [CrossRef] [PubMed]
- Le Bot, J.; Serra, V.; Fabre, J.; Draye, X.; Adamowicz, S.; Pagès, L. DART: A software to analyse root system architecture and development from captured images. Plant Soil 2010, 326, 261–273. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Saquib, Q.; Musarrat, J.; Siddiqui, M.A.; Dutta, S.; Dasgupta, S.; Giesy, J.P.; Al-Khedhairy, A.A. Cytotoxic and necrotic responses in human amniotic epithelial (WISH) cells exposed to organophosphate insecticide phorate. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2012, 744, 125–134. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Soufan, W.; Dewir, Y.H.; Al-Suhaibani, N.A. In vitro Evaluation of Seed Germination in Twelve Alfalfa Cultivars under Salt Stress. Phyton 2023, 92, 111–120. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Shelden, M.C.; Roessner, U.; Sharp, R.E.; Tester, M.; Bacic, A. Genetic variation in the root growth response of barley genotypes to salinity stress. Funct. Plant Biol. 2013, 40, 516–530. [Google Scholar] [CrossRef]
- He, D.; Yang, P. Proteomics of rice seed germination. Front. Plant Sci. 2013, 4, 246. [Google Scholar] [CrossRef] [PubMed]
- Ben Dkhil, B.; Denden, M. Effects of heat stress on the germination, protein degradation and mineral content in seeds of okra (Abelmoschus esculentus L.). Cont. J. Biol. Sci. 2010, 3, 51–62. [Google Scholar]
- Huangfu, L.; Zhang, Z.; Zhou, Y.; Zhang, E.; Chen, R.; Fang, H.; Li, P.; Xu, Y.; Yao, Y.; Zhu, M.; et al. Integrated physiological, metabolomic and transcriptomic analyses provide insights into the roles of exogenous melatonin in promoting rice seed germination under salt stress. Plant Growth Regul. 2021, 95, 19–31. [Google Scholar] [CrossRef]
- Mahmood, U.; Hussain, S.; Hussain, S.; Ali, B.; Ashraf, U.; Zamir, S.; Al-Robai, S.; Alzahrani, F.; Hano, C.; El-Esawi, M. Morpho-physio-biochemical and molecular responses of maize hybrids to salinity and waterlogging during stress and recovery phase. Plants 2021, 10, 1345. [Google Scholar] [CrossRef]
- Al-Baadani, H.H.; Alowaimer, A.N.; Al-Badwi, M.A.; Abdelrahman, M.M.; Soufan, W.H.; Alhidary, I.A. Evaluation of the Nutritive Value and Digestibility of Sprouted Barley as Feed for Growing Lambs: In Vivo and In Vitro Studies. Animals 2022, 12, 1206. [Google Scholar] [CrossRef] [PubMed]
- Hassen, A.; Dawid, I. Contribution of Hydroponic Feed for Livestock Production and Productivity: A Review. Int. J. Ground Sediment Water 2022, 15, 899–916. [Google Scholar]
- Kim, K.C.; Kim, J.-S. Comparative analysis of hydroponically cultivated barley sprouts yield, polyphenol and mineral content by nutrient solution treatment. J. Plant Biotechnol. 2021, 48, 193–200. [Google Scholar] [CrossRef]
- Mahlooji, M.; Seyed Sharifi, R.; Razmjoo, J.; Sabzalian, M.R.; Sedghi, M. Effect of salt stress on photosynthesis and physiological parameters of three contrasting barley genotypes. Photosynthetica 2018, 56, 549–556. [Google Scholar] [CrossRef]
- Fatima, A.; Hussain, S.; Hussain, S.; Ali, B.; Ashraf, U.; Zulfiqar, U.; Aslam, Z.; Al-Robai, S.; Alzahrani, F.; Hano, C.; et al. Differential morphophysiological, biochemical, and molecular responses of maize hybrids to salinity and alkalinity stresses. Agronomy 2021, 11, 1150. [Google Scholar] [CrossRef]
- Netondo, G.W.; Onyango, J.C.; Beck, E. Sorghum and salinity: II. Gas exchange and chlorophyll fluorescence of sorghum under salt stress. Crop Sci. 2004, 44, 806–811. [Google Scholar] [CrossRef]
- Naeem, M.S.; Warusawitharana, H.; Liu, H.; Liu, D.; Ahmad, R.; Waraich, E.A.; Xu, L.; Zhou, W. 5-Aminolevulinic acid alleviates the salinity-induced changes in Brassica napus as revealed by the ultrastructural study of chloroplast. Plant Physiol. Biochem. 2012, 57, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Yamane, K.; Kawasaki, M.; Taniguchi, M.; Miyake, H. Differential effect of NaCl and polyethylene glycol on the ultrastructure of chloroplasts in rice seedlings. J. Plant Physiol. 2003, 160, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Allel, D.; Ben-Amar, A.; Abdelly, C. Leaf photosynthesis, chlorophyll fluorescence and ion content of barley (Hordeum vulgare) in response to salinity. J. Plant Nutr. 2018, 41, 497–508. [Google Scholar] [CrossRef]
- Khan, I.; Muhammad, A.; Chattha, M.U.; Skalicky, M.; Ayub, M.A.; Anwar, M.R.; Soufan, W.; Hassan, M.U.; Rahman, A.; Brestic, M.; et al. Mitigation of Salinity-Induced Oxidative Damage, Growth, and Yield Reduction in Fine Rice by Sugarcane Press Mud Application. Front. Plant Sci. 2022, 13, 865. [Google Scholar] [CrossRef]
- Palta, J.A.; Chen, X.; Milroy, S.P.; Rebetzke, G.J.; Dreccer, M.F.; Watt, M. Large root systems: Are they useful in adapting wheat to dry environments? Funct. Plant Biol. 2011, 38, 347–354. [Google Scholar] [CrossRef]
- Manschadi, A.M.; Hammer, G.; Christopher, J.T.; Devoil, P. Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.). Plant Soil 2008, 303, 115–129. [Google Scholar] [CrossRef]
- Colombi, T.; Herrmann, A.M.; Vallenback, P.; Keller, T. Cortical Cell Diameter Is Key To Energy Costs of Root Growth in Wheat. Plant Physiol. 2019, 180, 2049–2060. [Google Scholar] [CrossRef]
- Colombi, T.; Torres, L.C.; Walter, A.; Keller, T. Feedbacks between soil penetration resistance, root architecture and water uptake limit water accessibility and crop growth—A vicious circle. Sci. Total Environ. 2018, 626, 1026–1035. [Google Scholar] [CrossRef]
- Sultan, I.; Khan, I.; Chattha, M.U.; Hassan, M.U.; Barbanti, L.; Calone, R.; Ali, M.; Majid, S.; Ghani, M.A.; Batool, M.; et al. Improved salinity tolerance in early growth stage of maize through salicylic acid foliar application. Ital. J. Agron. 2021, 16, 1–11. [Google Scholar] [CrossRef]
- Parveen, A.; Liu, W.; Hussain, S.; Asghar, J.; Perveen, S.; Xiong, Y. Silicon Priming Regulates Morpho-Physiological Growth and Oxidative Metabolism in Maize under Drought Stress. Plants 2019, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Dunand, C.; Crèvecoeur, M.; Penel, C. Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: Possible interaction with peroxidases. New Phytol. 2007, 174, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.H.; Bae, Y.S.; Lee, J.S. Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol. 2001, 126, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Liszkay, A.; van der Zalm, E.; Schopfer, P. Production of reactive oxygen intermediates (O2−, H2O2, and OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol. 2004, 136, 3114–3123. [Google Scholar] [CrossRef]
- Zamljen, T.; Medic, A.; Hudina, M.; Veberic, R.; Slatnar, A. Salt stress differentially affects the primary and secondary metabolism of peppers (Capsicum annuum L.) according to the genotype, fruit part, and salinity Level. Plants 2022, 11, 853. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).