Effects of Nitrogen Application in Recovery Period after Different High Temperature Stress on Plant Growth of Greenhouse Tomato at Flowering and Fruiting Stages
Abstract
1. Introduction
2. Materials and Methods
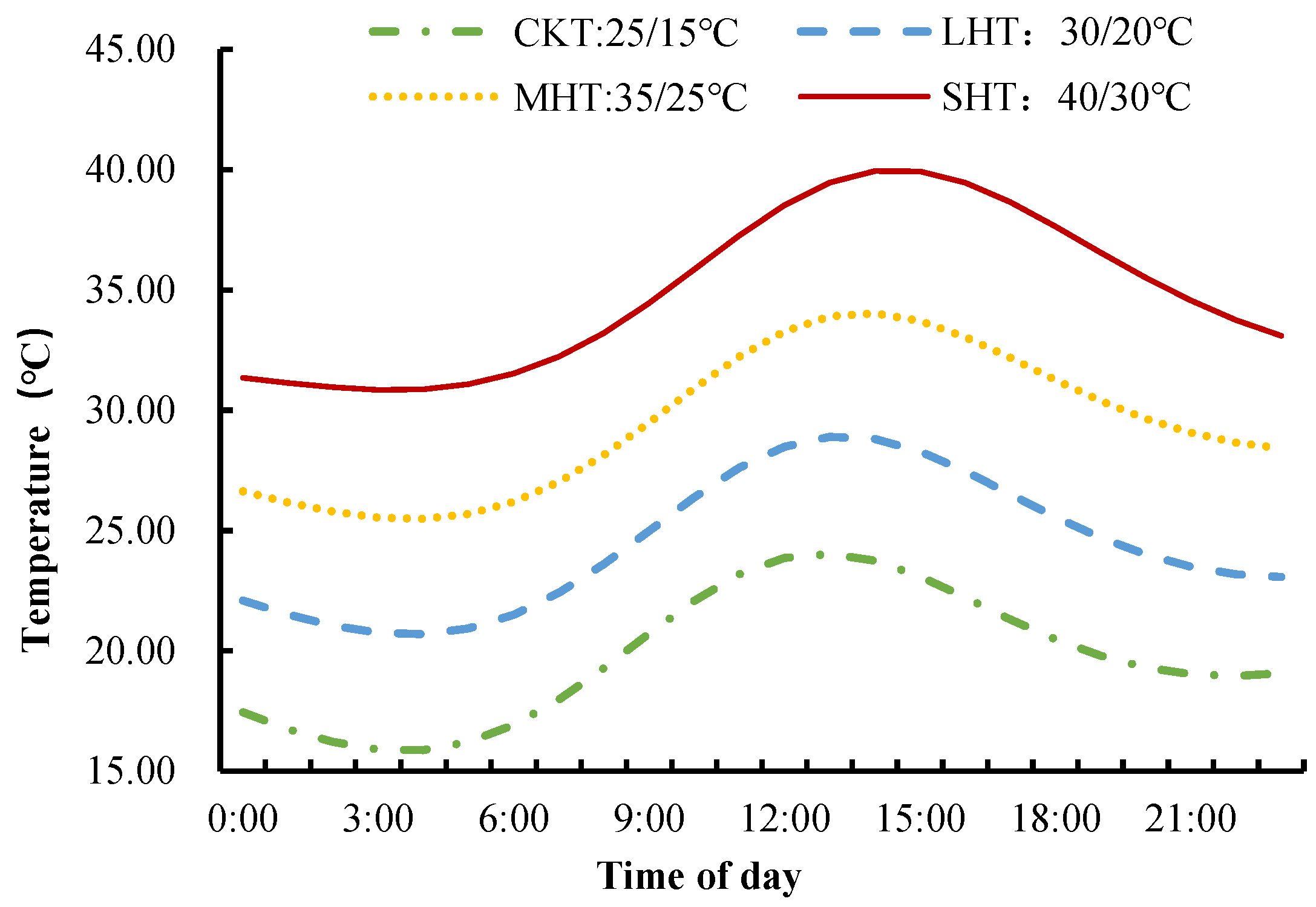
2.1. The Plant Materials and Growth Conditions
2.2. Measurement of Tomato Plant Growth Parameters
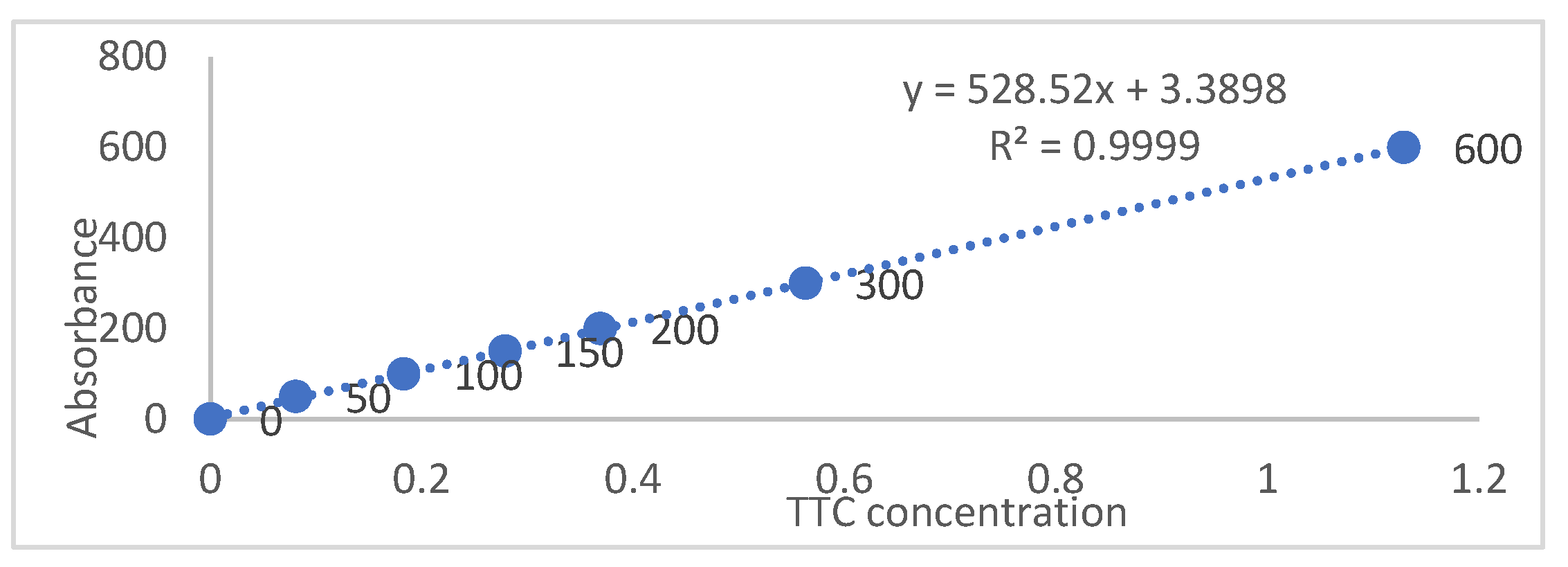
2.3. Measurement of Tomato Root Vitality
2.4. Statistical Analysis
2.5. Logistic Model
3. Results
3.1. Tomato Growth Parameters
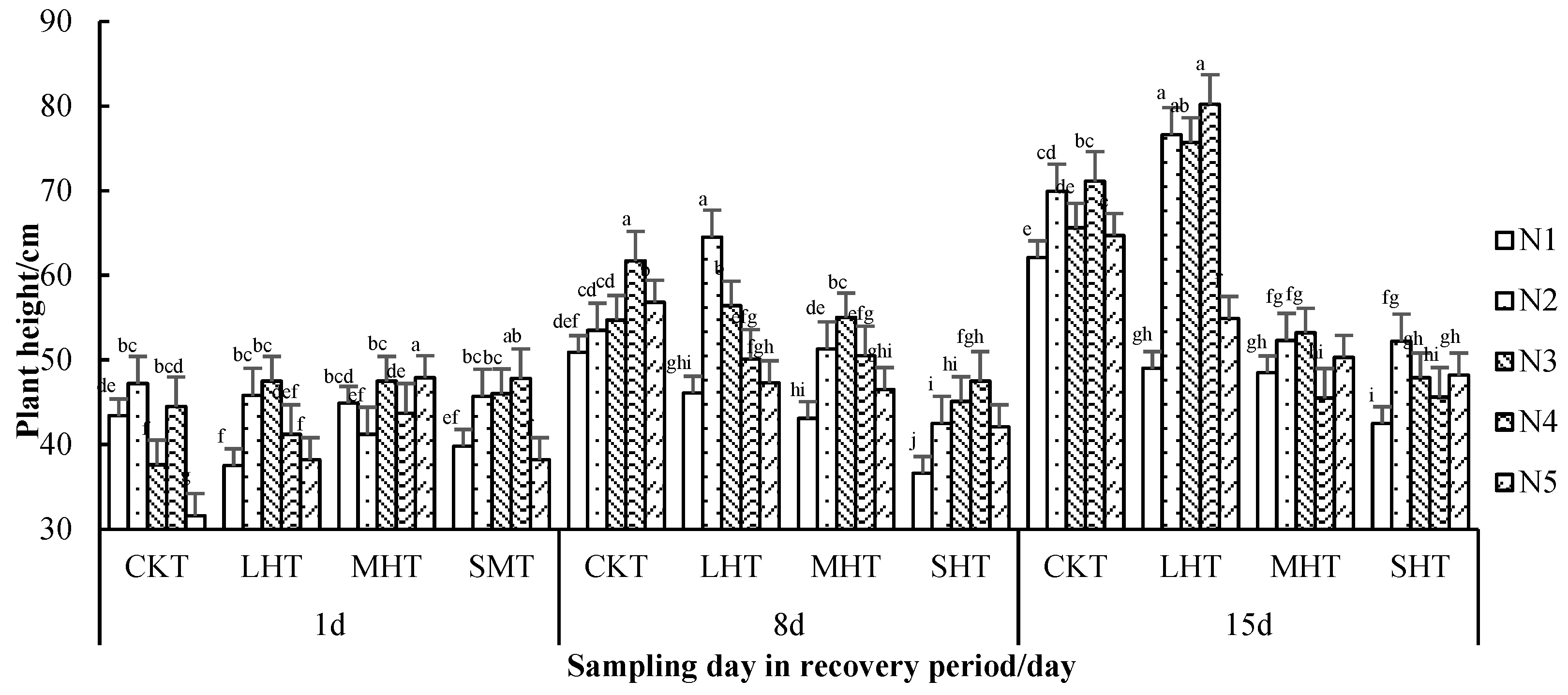
3.1.1. The Tomato Plant Height
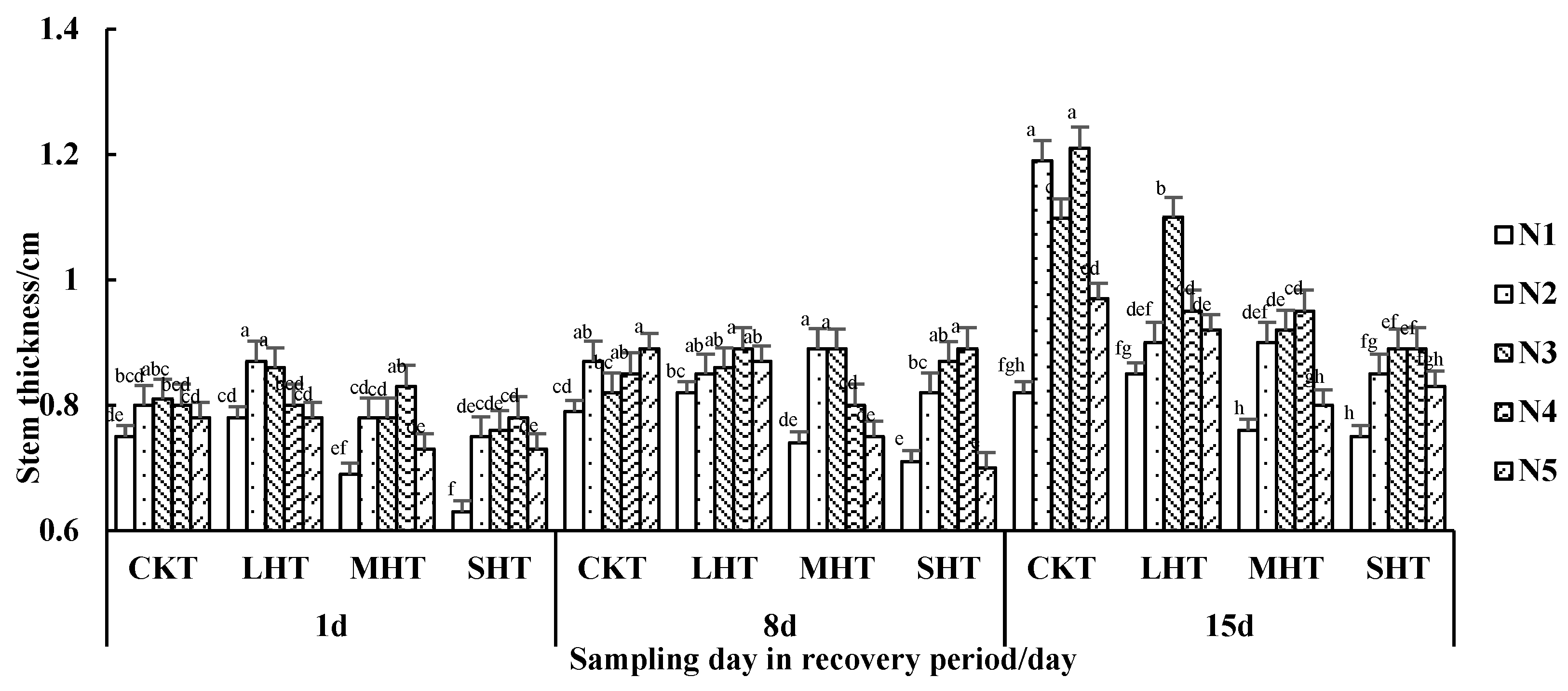
3.1.2. The Tomato Plant Stem Thickness
3.2. Dry Matter Accumulation and Distribution of Tomato Plants
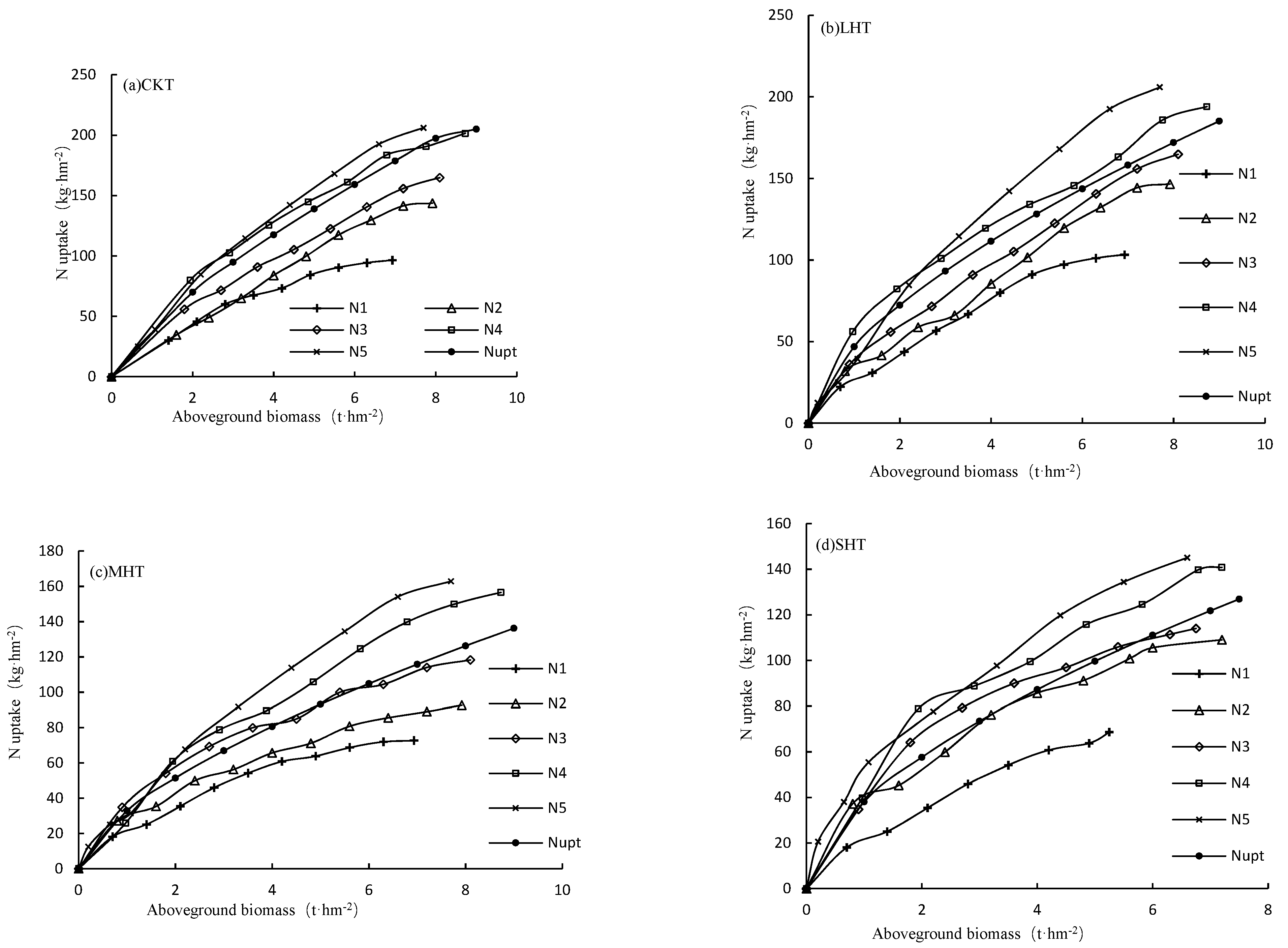
3.2.1. Dry Matter Accumulation of Tomato Plants
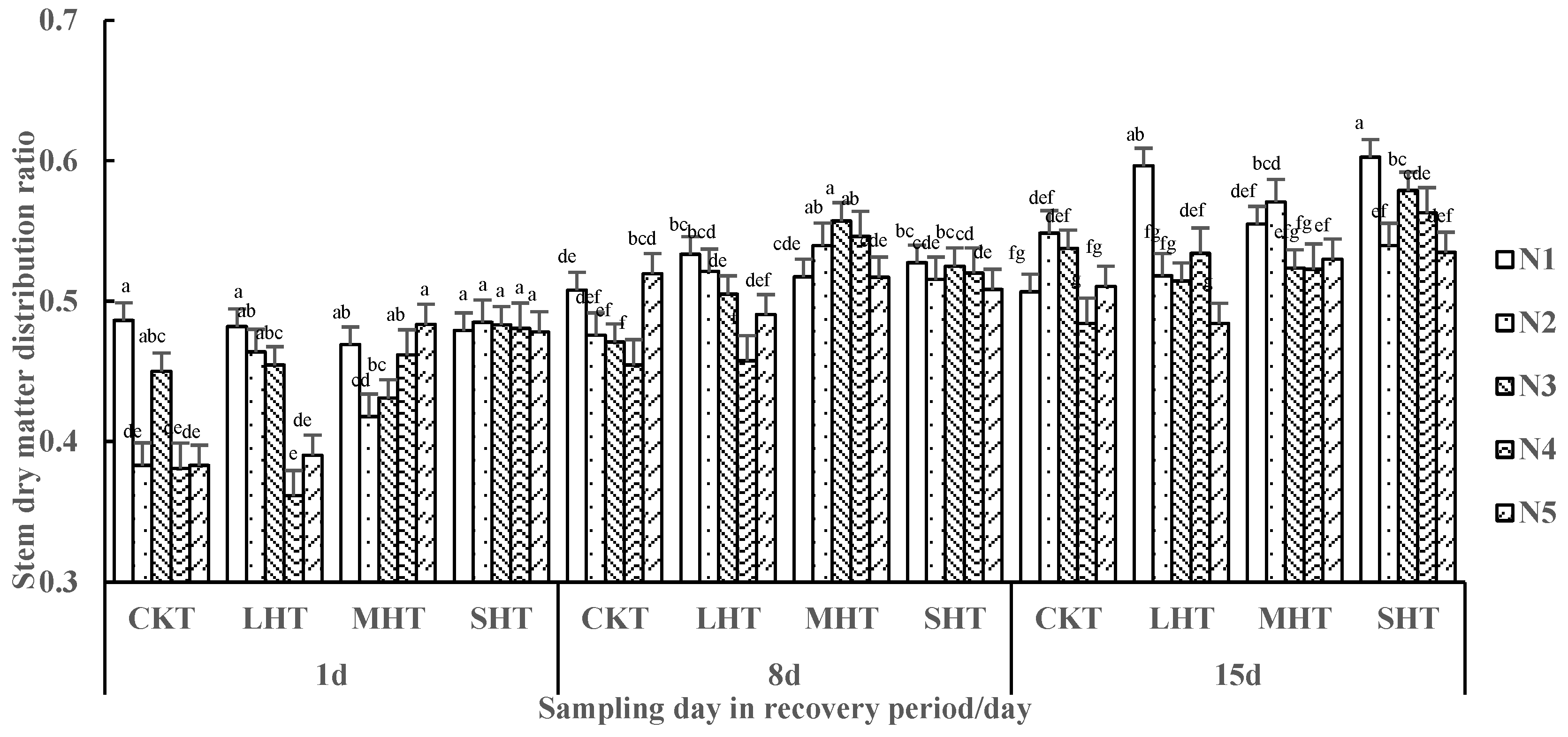
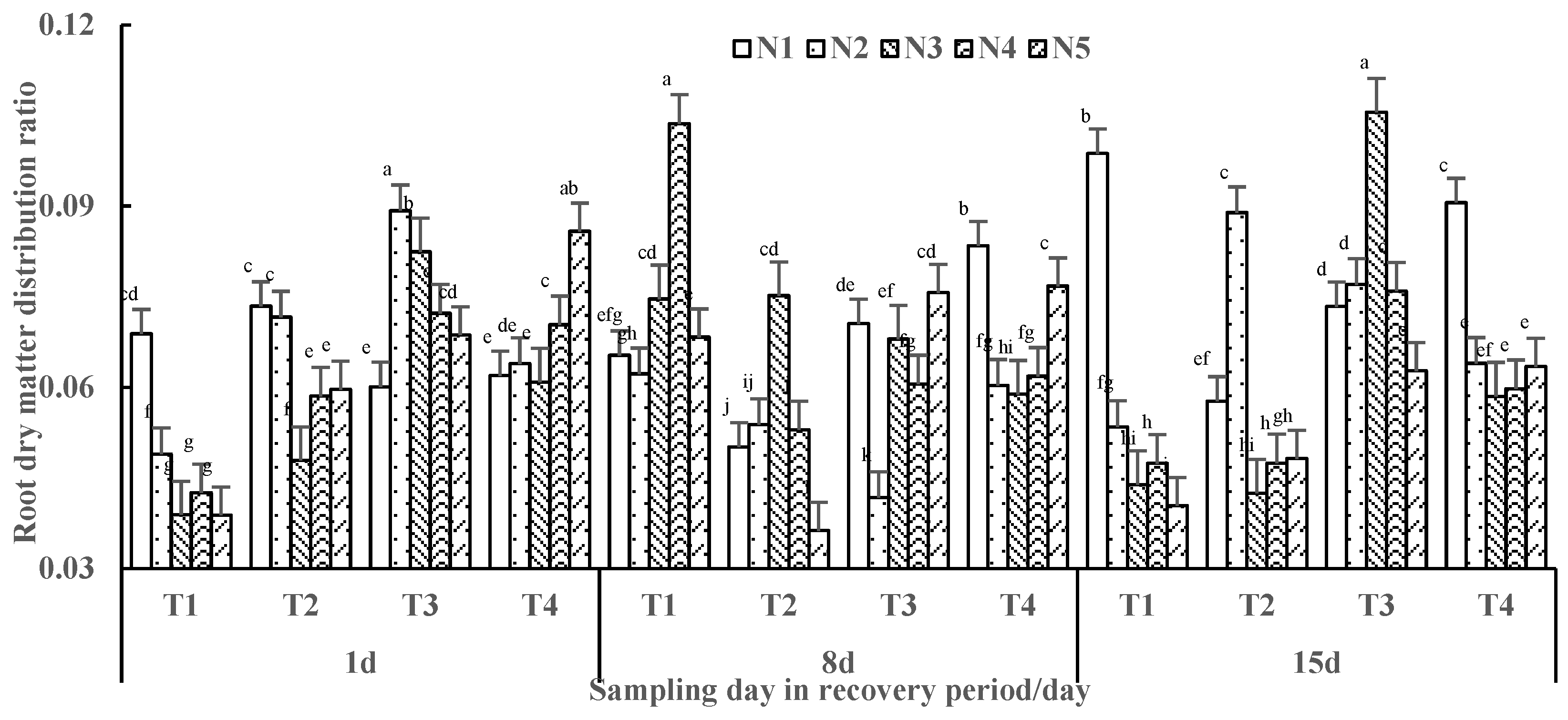
3.2.2. Dry Matter Ratio of Tomato Plants
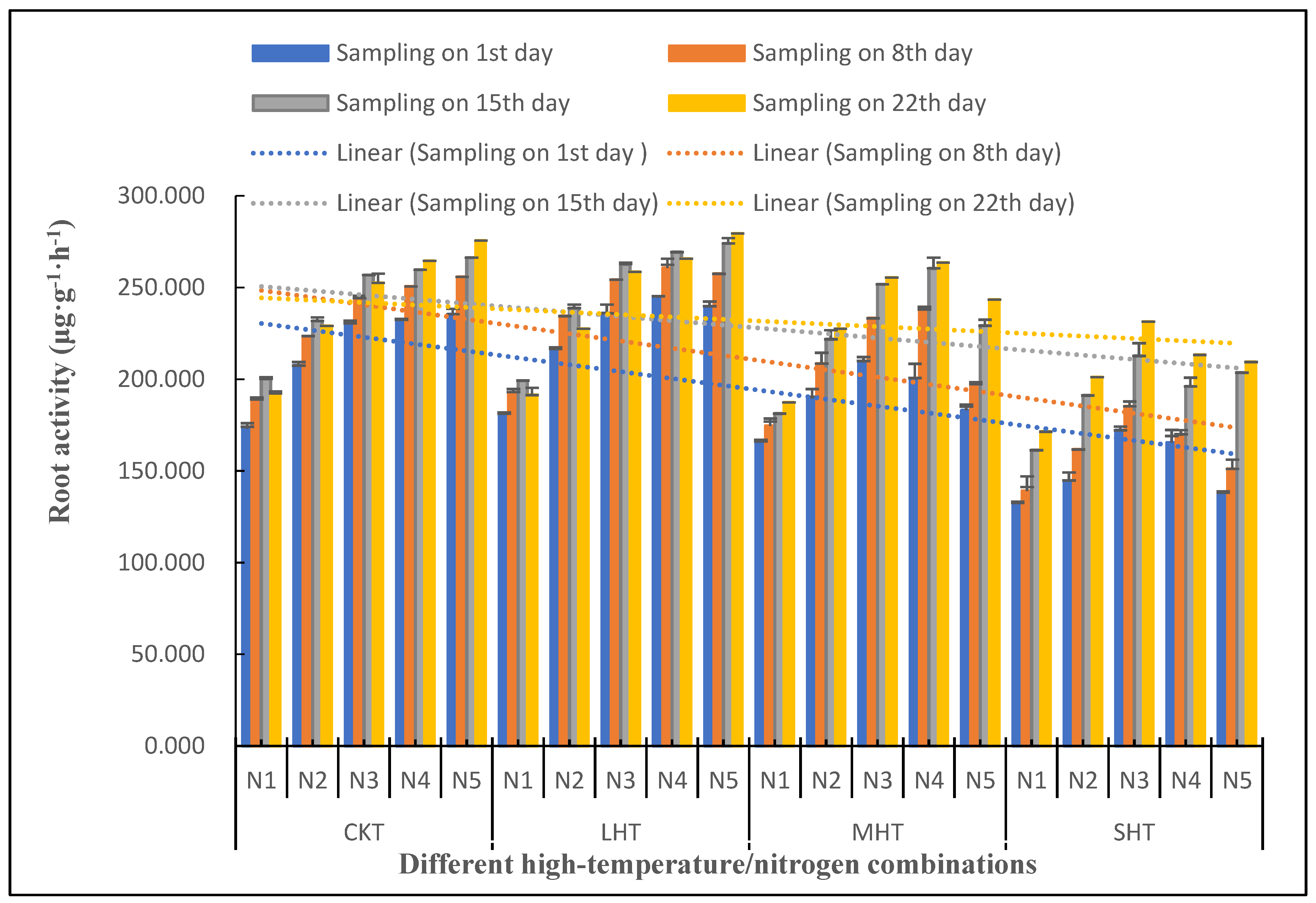
3.3. Effects of Different High-Temperature/Nitrogen Combinations on the Vitality of Tomato Plant Roots
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tubiello, F.N.; Soussana, J.F.; Howden, S.M. Crop and pasture response to climate change. Proc. Natl. Acad. Sci. USA 2007, 104, 19686–19690. [Google Scholar] [CrossRef] [PubMed]
- Kotak, S.; Larkindale, J.; Lee, U.; von Koskull-Döring, P.; Vierling, E.; Scharf, K.-D. Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 2007, 10, 310–316. [Google Scholar] [CrossRef]
- El-Gizawy, A.; Gomaa, H.; El-Habbasha, K.; Mohamed, S. Effect of different shading levels on tomato plants 1. Growth, flowering and chemical composition. Acta Hortic. 1993, 323, 341–348. [Google Scholar] [CrossRef]
- Johnson, R.C.; Kanemasu, E.T. Yield and Development of Winter Wheat at Elevated Temperatures. Agron. J. 1983, 75, 561. [Google Scholar] [CrossRef]
- De Koning, A. Development and Dry Matter Distribution in Glasshouse Tomato: A Quantitative Approach; Wageningen Agricultural University: Wageningen, The Netherlands, 1994; pp. 102–240. [Google Scholar]
- Bertin, N.; Guichard, S.; Leonardi, C.M.; Longuenesse, J.J.; Langlois, D.; Navez, B. Seasonal Evolution of the Quality of Fresh Glasshouse Tomatoes under Mediterranean Conditions, as Affected by Air Vapour Pressure Deficit and Plant Fruit Load. Ann. Bot. 2000, 85, 741–750. [Google Scholar] [CrossRef]
- Adams, S.R.; Cockshull, K.E.; Cave, C. Effect of temperature on the growth and development of tomato fruits. Ann. Bot. 2001, 5, 869–877. [Google Scholar] [CrossRef]
- Amarjeet, B.; Mohini, S.; Sangeeta, S. Allelopathic effects of Parthenium hysteron phorus on the chlorophyll nitrogen, protein and ascorbic acid content of Lantana camara. Alleopathy J. 2005, 15, 305–310. [Google Scholar]
- Rohn, S.; Rawel, H.M.; Kroll, J. Inhibitory Effects of Plant Phenols on the Activity of Selected Enzymes. J. Agric. Food Chem. 2002, 50, 3566–3571. [Google Scholar] [CrossRef]
- Cruz, R.; Ayala, C.G.; Anaya, A.L. Allelochemical stress pro-duced by the aqueous leachates of Callicarpa acuminata: Effection roots of beans, maize and tomato. Physiol. Piantarum 2002, 116, 20–27. [Google Scholar] [CrossRef]
- Mary, M.; Peet, D.H.; Willits, R. Response of ovule development and post-pollen production processes in male-sterile tomatoes to chronic, sub-acute high-temperature stress. J. Exp. Bot. 1997, 48, 67–74. [Google Scholar]
- Holder, R.; Cockshull, K.E. Effects of humidity on the growth and yield of glasshouse tomatoes. J. Hortic. Sci. 1990, 65, 31–39. [Google Scholar] [CrossRef]
- Lingling, X.; Fangying, T. The impact of climate on agricultural production in the spring of 2015. Chin. Agric. Meteorol. 2015, 36, 372–373. [Google Scholar]
- El Ahmadi, A.B.; Stevens, M.A. Reproductive Responses of Heat-tolerant Tomatoes to High Temperatures1. J. Am. Soc. Hortic. Sci. 1979, 104, 686–691. [Google Scholar] [CrossRef]
- James, D.; Borphukan, B.; Fartyal, D.; Ram, B. Concurrent overexpression of OsGS1; 1 and OsGS2 Genes in transgenic rice (Oryza sativa L.): Impact on tolerance to abiotic stresses. Front. Plant Sci. 2018, 9, 786–804. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Mu, Y.; Kang, J.; Zhao, J.; Wu, H. Effects of different nitrogen application rates on chlorophyll content and fluorescence characteristics of spring wheat with high temperature after anthesis. South J. Agric. Sci. 2017, 48, 609–615. [Google Scholar]
- Jingjing, Z.D. Effect Mechanism of Different Nitrogen Application Rates on Yield Formation of Spring Wheat under High Temperature Stress after Anthesis. Ph.D. Thesis, Ningxia University, Yinchuan, China, 2015. [Google Scholar]
- Dupont, F.M.; Hurkman, W.J.; Vensel, W.H.; Tanaka, C.; Kothari, K.M.; Chung, O.K.; Altenbach, S.B. Protein accumulation and composition in wheat grains: Effects of mineral nutrients and high temperature. Eur. J. Agron. 2006, 25, 96–107. [Google Scholar] [CrossRef]
- Zahedi, M.; McDonald, G.; Jenner, C.F. Nitrogen supply to the grain modifies the effects of temperature on starch and protein accumulation during grain filling in wheat. Aust. J. Agric. Res. 2004, 55, 551–564. [Google Scholar] [CrossRef]
- Pressman, E.; Peet, M.M.; Pharr, D.M. The Effect of Heat Stress on Tomato Pollen Characteristics is Associated with Changes in Carbohydrate Concentration in the Developing Anthers. Ann. Bot. 2002, 90, 631–636. [Google Scholar] [CrossRef]
- Xu, J.; Wolters-Arts, M.; Mariani, C.; Huber, H.; Rieu, I. Heat stress affects vegetative and reproductive performance and trait correlations in tomato (Solanum lycopersicum). Euphytica 2017, 213, 156. [Google Scholar] [CrossRef]
- Camejo, D.; Rodríguez, P.; Morales, M.A.; Dell’Amico, J.M.; Torrecillas, A. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 2005, 162, 281–289. [Google Scholar] [CrossRef]
- Francesca, S.; Arena, C.; Hay Mele, B.; Schettini, C.; Ambrosino, P.; Barone, A.; Rigano, M.M. The Use of a Plant-Based Biostimulant Improves Plant Performances and Fruit Quality in Tomato Plants Grown at Elevated Temperatures. Agronomy 2020, 10, 363. [Google Scholar] [CrossRef]
- Changfu, R.; Anhe, C.; Baoguo, L. Physiological and biochemical basis of the effect of high temperature on flowering and fruiting of hybrid rice. J. Southwest Univ. (Nat. Sci. Ed.) 1990, 5, 440–443. [Google Scholar]
- Naiyao, M.D. Mitigation Effect and Physiological Mechanism of Nitrogen Fertilizer on High Temperature Stress in the Early Stage of Grain Filling in Rice. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2016. [Google Scholar]
- Xuejun, Z.; Ying, Z.; Xiaoqun, C.; Lishu, W.; Chengxiao, H. Effects of nitrogen application rate in drip irrigation on vegetable yield, nitrogen balance and soil nitrate accumulation in two years. China Agric. Sci. 2007, 11, 2535–2545. [Google Scholar]
- Huimin, J.; Jianfeng, Z.; Juncheng, Y.; Xiaozong, S.; Zhaohui, L.; Lihua, J. Effects of different nitrogen fertilizer dosages on the yield, quality and soil nitrate nitrogen accumulation of facility tomato. J. Agric. Environ. Sci. 2010, 29, 2338–2345. [Google Scholar]
- Xin, W.; Fuyu, M.; Ming, D.; Huam, F. Dynamic simulation of plant growth, nitrogen accumulation and utilization of processed tomato under different nitrogen application levels. Chin. J. Appl. Ecol. 2014, 25, 1043–1050. [Google Scholar]
- Jiqing, W.; Sheping, L. Effects of nitrogen application rate on tomato growth and nitrogen utilization efficiency. Henan Agric. Sci. 2015, 44, 94–97. [Google Scholar]
- Zhongliang, L.; Junjie, G.; Duanyin, G.; Yanyan, Z.; Juan, J.; Shiqi, L.; Xiaofei, T. Effects of nitrogen application rate on quality, yield and nutrient uptake of tomato cultivated in facility substrates. Resour. Environ. Arid. Reg. 2019, 33, 163–167. [Google Scholar]
- Bo, J.; Ning, N.; Wenlong, Z.; Ming, D. Effects of different nitrogen application rates on the growth and soil nitrogen balance of processed tomato. Xinjiang Agric. Sci. 2020, 57, 1830–1838. [Google Scholar]
- Jin, Z.; Yanghuan, W.; Fang, C.; Xuejie, M. Effects of nitrogen application on the growth and development, quality and yield of tomato under drip irrigation. Xinjiang Agric. Sci. 2020, 57, 498–506. [Google Scholar]
- Liang, L.; Ridoutt, B.; Lal, R.; Wang, D.; Wu, W.; Peng, P.; Hang, S.; Wang, L.; Zhao, G. Nitrogen footprint and nitrogen use efficiency of greenhouse tomato production in North China. J. Clean. Prod. 2018, 208, 285–296. [Google Scholar] [CrossRef]
- Yi, Z.; Yu, S.; Xiaohui, H.; Zhirong, Z.; Kai, C.; Hao, Z. Effects of exogenous Spd on nitrogen metabolism and content of main mineral elements in tomato seedlings under saline-alkali stress. Chin. J. Appl. Ecol. 2013, 24, 1401–1408. [Google Scholar]
- Lam, H.-M.; Coschigano, K.T.; Oliveira, I.C.; Melo-Oliveira, R.; Coruzzi, G.M. The molecular-genetics of nitrogen assimilation into amino acids in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 569–593. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y. Effects of Light-Nitrogen Interaction on Tomato Carbon and Nitrogen Metabolism. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2008. [Google Scholar]
- Jaynes, D.B.; Colvin, T.S. Corn Yield and Nitrate Loss in Subsurface Drainage from Midseason Nitrogen Fertilizer Application. Agron. J. 2006, 98, 1479–1487. [Google Scholar] [CrossRef]
- Li, J.S.; Yang, Z.Q.; Li, Y.X. Establishment of critical nitrogen dilution curve model and nitrogen nutrition diagnosis of grapes under different water conditions. Chin. J. Agric. Meteorol. 2019, 40, 523–533. [Google Scholar]
- Lin, W.; Zaiqiang, Y.; Mingtian, W.; Shiqiong, Y.; Xia, C.; Jie, Z. The effect of relative air humidity on the nutrient content and dry matter distribution of tomato seedlings under high temperature. Chin. J. Agric. Meteorol. 2018, 39, 304–313. [Google Scholar]
- Wei, T.T.; Yang, Z.Q.; Wang, L. Hourly temperature simulation model in glass and plastic greenhouses. Chin. J. Agric. Meteorol. 2018, 39, 26–37. [Google Scholar]
- Lin, M.-Y.; Chai, K.-H.; Ko, S.-S.; Kuang, L.-Y.; Lur, H.-S.; Charng, Y.-Y. A Positive Feedback Loop between HEAT SHOCK PROTEIN101 and HEAT STRESS-ASSOCIATED 32-KD PROTEIN Modulates Long-Term Acquired Thermotolerance Illustrating Diverse Heat Stress Responses in Rice Varieties. Plant Physiol. 2014, 164, 2045–2053. [Google Scholar] [CrossRef]
- Heuvelink, E. Effect of temperature on biomass allocation in tomato (Lycopersicon esculentum). Physiol. Plant. 1995, 94, 447–452. [Google Scholar] [CrossRef]
- Heuvelink, E. Growth, development and yield of a tomato crop: Periodic destructive measurements in a greenhouse. Sci. Hortic. 1995, 61, 77–99. [Google Scholar] [CrossRef]
- Heuvelink, E. Effect of fruit load on dry matter partitioning in tomato. Sci. Hortic. 1997, 69, 51–59. [Google Scholar] [CrossRef]
- Heuvelink, E.; Marcelis, L. Dry matter distribution in tomato and cucumber. Acta Hortic. 1989, 260, 149–180. [Google Scholar] [CrossRef]
- Heuvelink, E.; Buiskool, R.P. Influence of Sink-Source Interaction on Dry Matter Production in Tomato. Ann. Bot. 1995, 75, 381–389. [Google Scholar] [CrossRef]
- Heuvelink, E. Dry Matter Production in a Tomato Crop: Measurements and Simulation. Ann. Bot. 1995, 75, 369–379. [Google Scholar] [CrossRef]
- Heuvelink, E. Dry Matter Partitioning in Tomato: Validation of a Dynamic Simulation Model. Ann. Bot. 1996, 77, 71–80. [Google Scholar] [CrossRef]
- Dong, Q.; Louarn, G.; Wang, Y.; Barczi, J.F.; de Reffye, P. Does the structure–function model greenlab deal with crop phenotypic plasticity induced by plant spacing? A case study on tomato. Ann. Bot. 2008, 101, 1195–1206. [Google Scholar] [CrossRef]
- Magaña, R.H.; Adamowicz, S.; Pagès, L. Diel changes in nitrogen and carbon resource status and use for growth in young plants of tomato (Solanum lycopersicum). Ann. Bot. 2009, 103, 1025–1037. [Google Scholar] [CrossRef]
- Wiechers, D.; Kahlen, K.; Stützel, H. Dry matter partitioning models for the simulation of individual fruit growth in greenhouse cucumber canopies. Ann. Bot. 2011, 108, 1075–1084. [Google Scholar] [CrossRef]
- Fan, Y.; Lv, Z.; Li, Y.; Qin, B.; Song, Q.; Ma, L.; Wu, Q.; Zhang, W.; Ma, S.; Ma, C.; et al. Salicylic Acid Reduces Wheat Yield Loss Caused by High Temperature Stress by Enhancing the Photosynthetic Performance of the Flag Leaves. Agronomy 2022, 12, 1386. [Google Scholar] [CrossRef]
- Stafne, E.T.; Carroll, B.L. Simulated Abiotic Injury Alters Yields of Southern Interspecific Hybrid Grape Cultivars. Horticulturae 2019, 5, 44. [Google Scholar] [CrossRef]
- Ru, C.; Hu, X.; Chen, D.; Song, T.; Wang, W.; Lv, M.; Hansen, N.C. Nitrogen Modulates the Effects of Short-Term Heat, Drought and Combined Stresses after Anthesis on Photosynthesis, Nitrogen Metabolism, Yield, and Water and Nitrogen Use Efficiency of Wheat. Water 2022, 14, 1407. [Google Scholar] [CrossRef]
- Tian, Q.; Chen, F.; Zhang, F.; Mi, G. Possible Involvement of Cytokinin in Nitrate-mediated Root Growth in Maize. Plant Soil 2005, 277, 185–196. [Google Scholar] [CrossRef]
- Wang, Y.; Mi, G.; Chen, F.; Zhang, J.; Zhang, F. Response of root morphology to nitrate supply and its contribution to nitrogen accumulation in maize. J. Plant Nutr. 2005, 27, 2189–2202. [Google Scholar] [CrossRef]
- Tian, H.; De Smet, I.; Ding, Z. Shaping a root system: Regulating lateral versus primary root growth. Trends Plant Sci. 2014, 19, 426–431. [Google Scholar] [CrossRef] [PubMed]


| Nitrogen Treatment (g/plant) | High-Temperature Treatment (Day/Night) | |||
|---|---|---|---|---|
| CKT (25 °C/15 °C) | LHT (30 °C/20 °C) | MHT (35 °C/25 °C) | SHT (40/°C 30 °C) | |
| N1: 0 N (0 g/plant) | T1N1 | T2N1 | T3N1 | T4N1 |
| N2: 0.5 N (1.3 g/plant) | T1N2 | T2N2 | T3N2 | T4N2 |
| N3: 0.75 N (1.95 g/plant) | T1N3 | T2N3 | T3N3 | T4N3 |
| N4: 1 N (2.6 g/plant,CKN4) | T1N4 | T2N4 | T3N4 | T4N4 |
| N5: 1.25 N (3.25 g/plant) | T1N5 | T2N5 | T3N5 | T4N5 |
| Source | df | Plant Morphology | Dry Matter Accumulation | Dry Matter Distribution of Various Organs | Root Vitality | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant Height | Stem Diameter | Leaf | Stem | Root | |||||||||||||||||||
| Sample Times | 1d | 8d | 15d | 1d | 8d | 15d | 1d | 8d | 15d | 1d | 8d | 15d | 1d | 8d | 15d | 1d | 8d | 15d | 1d | 8d | 15d | 22d | |
| High Temperature (HT) | 4 | NS | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
| Nitrogen (N) | 5 | NS | ** | ** | ** | ** | ** | ** | 0.023 * | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
| HT × Nitrogen | 20 | * | ** | ** | * | ** | ** | ** | NS | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
| Treatment | Days after Treatment | |||||
|---|---|---|---|---|---|---|
| 1 d | 8 d | 15 d | 22 d | 29 d | 36 d | |
| CKTN1 | 2.44 ± 0.3231 fg | 3.51 ± 0.309 ij | 5.37 ± 0.546 ij | 6.98 ± 0.398 ij | 8.11 ± 0.687 h | 9.95 ± 1.051 h |
| CKTN2 | 4.00 ± 0.475 a | 5.38 ± 0.554 cd | 7.48 ± 0.481 f | 10.11 ± 1.011 cd | 11.15 ± 0.704 d | 14.39 ± 1.537 d |
| CKTN3 | 4.06 ± 0.379 a | 5.87 ± 0.615 b | 6.42 ± 0.331 gh | 9.75 ± 0.717 cd | 13.28 ± 1.021 c | 16.70 ± 1.842 c |
| CKTN4 | 3.33 ± 0.112 bc | 5.20 ± 0.628 d | 7.63 ± 0.789 f | 12.02 ± 0.948 a | 15.84 ± 1.117 a | 18.29 ± 2.002 a |
| CKTN5 | 3.23 ± 0.153 c | 3.70 ± 0.794 hij | 9.16 ± 0.511 ab | 12.63 ± 0.889 a | 16.16 ± 0.925 a | 18.96 ± 1.918 a |
| LHTN1 | 2.52 ± 0.398 ef | 4.01 ± 0.177 fgh | 8.15 ± 0.478 de | 10.47 ± 0.812 bc | 13.37 ± 1.089 c | 15.22 ± 1.261 c |
| LHTN2 | 2.22 ± 0.099 gh | 4.59 ± 0.225 e | 8.97 ± 0.630 bc | 11.15 ± 0.952 b | 14.35 ± 1.225 b | 16.79 ± 1.731 b |
| LHTN3 | 2.93 ± 0.108 d | 5.59 ± 0.487 bcd | 9.66 ± 0.622 a | 12.21 ± 1.011 a | 15.27 ± 0.889 ab | 16.98 ± 1.453 ab |
| LHTN4 | 2.90 ± 0.178 d | 4.17 ± 0.457 efg | 5.90 ± 0.323 hi | 6.67 ± 0.695 j | 11.03 ± 1.151 de | 14.78 ± 1.231 de |
| LHTN5 | 2.24 ± 0.121 gh | 3.40 ± 0.209 j | 4.52 ± 0.481 k | 6.44 ± 0.446 j | 10.00 ± 0.797 efg | 12.35 ± 1.007 efg |
| MHTN1 | 2.67 ± 0.046 def | 5.32 ± 0.225 cd | 6.72 ± 0.665 g | 7.83 ± 0.709 gh | 9.56 ± 1.019 fg | 11.30 ± 1.271 fg |
| MHTN2 | 2.71 ± 0.452 def | 6.36 ± 0.287 a | 8.44 ± 0.583 cd | 8.93 ± 0.818 ef | 10.44 ± 1.218 def | 13.20 ± 1.447 def |
| MHTN3 | 3.53 ± 0.409 b | 5.46 ± 0.877 bcd | 8.50 ± 0.908 cd | 8.89 ± 0.786 ef | 10.22 ± 0.998 defg | 12.96 ± 1.114 defg |
| MHTN4 | 3.56 ± 0.438 b | 6.55 ± 0.704 a | 7.45 ± 0.823 f | 8.89 ± 0.690 f | 9.33 ± 0.753 g | 12.65 ± 0.969 g |
| MHTN5 | 2.77 ± 0.164 de | 5.72 ± 0.526 bc | 8.44 ± 0.692 cd | 9.4 ± 0.986 de | 10.02 ± 0.996 efg | 12.40 ± 1.043 efg |
| SHTN1 | 2.12 ± 0.067 h | 3.85 ± 0.321 hi | 4.80 ± 0.342 jk | 6.63 ± 0.623 j | 7.37 ± 0.762 h | 9.12 ± 0.958 h |
| SHTN2 | 2.44 ± 0.021 fg | 4.10 ± 0.442 fgh | 6.13 ± 0.644 gh | 8.55 ± 0.776 fg | 10.15 ± 0.901 def | 12.05 ± 1.221 def |
| SHTN3 | 2.67 ± 0.146 def | 3.53 ± 0.225 ij | 6.30 ± 0.522 gh | 7.90 ± 0.487 gh | 9.23 ± 0.692 g | 11.14 ± 1.491 g |
| SHTN4 | 2.75 ± 0.125 de | 4.17 ± 0.304 efg | 6.00 ± 0.659 h | 7.54 ± 0.745 hi | 9.25 ± 0.683 g | 11.29 ± 0.948 g |
| SHTN5 | 2.42 ± 0.294 fg | 4.33 ± 0.263 ef | 5.31 ± 0.569 ij | 6.50 ± 0.691 j | 7.67 ± 0.730 h | 9.38 ± 0.795 h |
| Treatment | Fitted Equation | R2 | Treatment | Fitted Equation | R2 |
|---|---|---|---|---|---|
| T1N1 | Y = 12.14/(1 + 7.12e−0.080t) | 0.992 ** | T3N1 | Y = 13.65/(1 + 5.41e−0.075t) | 0.979 ** |
| T1N2 | Y = 16.20/(1 + 10.55e−0.099t) | 0.982 ** | T3N2 | Y = 14.42/(1 + 5.22e−0.084t) | 0.935 ** |
| T1N3 | Y = 23.91/(1 + 17.42e−0.088t) | 0.998 ** | T3N3 | Y = 15.69/(1 + 4.84e−0.070t) | 0.956 ** |
| T1N4 | Y = 28.71/(1 + 21.23e−0.065t) | 0.971 ** | T3N4 | Y = 21.39/(1 + 5.28e−0.046t) | 0.933 ** |
| T1N5 | Y = 30.72(1 + 22.59e−0.064t) | 0.972 ** | T3N5 | Y = 12.09/(1 + 6.28e−0.118t) | 0.960 ** |
| T2N1 | Y = 16.88/(1 + 13.36e−0.113t) | 0.977 ** | T4N1 | Y = 12.19/(1 + 6.70e−0.069t) | 0.992 ** |
| T2N2 | Y = 18.56/(1 + 13.68e−0.112t) | 0.981 ** | T4N2 | Y = 14.74/(1 + 7.21e−0.046t) | 0.993 ** |
| T2N3 | Y = 35.06/(1 + 16.39e−0.054t) | 0.976 ** | T4N3 | Y = 19.28/(1 + 8.94e−0.034t) | 0.986 ** |
| T2N4 | Y = 37.69/(1 + 19.10e−0.058t) | 0.966 ** | T4N4 | Y = 15.05/(1 + 7.99e−0.045t) | 0.992 ** |
| T2N5 | Y = 27.81/(1 + 19.27e−0.066t) | 0.993 ** | T4N5 | Y = 14.44/(1 + 7.23e−0.036t) | 0.993 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Yang, Z.; Zhang, C.; Luo, J.; Zhang, F.; Qiu, R. Effects of Nitrogen Application in Recovery Period after Different High Temperature Stress on Plant Growth of Greenhouse Tomato at Flowering and Fruiting Stages. Agronomy 2023, 13, 1439. https://doi.org/10.3390/agronomy13061439
Li C, Yang Z, Zhang C, Luo J, Zhang F, Qiu R. Effects of Nitrogen Application in Recovery Period after Different High Temperature Stress on Plant Growth of Greenhouse Tomato at Flowering and Fruiting Stages. Agronomy. 2023; 13(6):1439. https://doi.org/10.3390/agronomy13061439
Chicago/Turabian StyleLi, Chunying, Zaiqiang Yang, Chunlong Zhang, Jing Luo, Fengyin Zhang, and Rangjian Qiu. 2023. "Effects of Nitrogen Application in Recovery Period after Different High Temperature Stress on Plant Growth of Greenhouse Tomato at Flowering and Fruiting Stages" Agronomy 13, no. 6: 1439. https://doi.org/10.3390/agronomy13061439
APA StyleLi, C., Yang, Z., Zhang, C., Luo, J., Zhang, F., & Qiu, R. (2023). Effects of Nitrogen Application in Recovery Period after Different High Temperature Stress on Plant Growth of Greenhouse Tomato at Flowering and Fruiting Stages. Agronomy, 13(6), 1439. https://doi.org/10.3390/agronomy13061439








