Isolation, Characterization and Phylogenetic Analysis of Stagonospora tainanensis, the Pathogen Causing Sugarcane Leaf Blight in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Survey, Pathogen Isolation and Verification
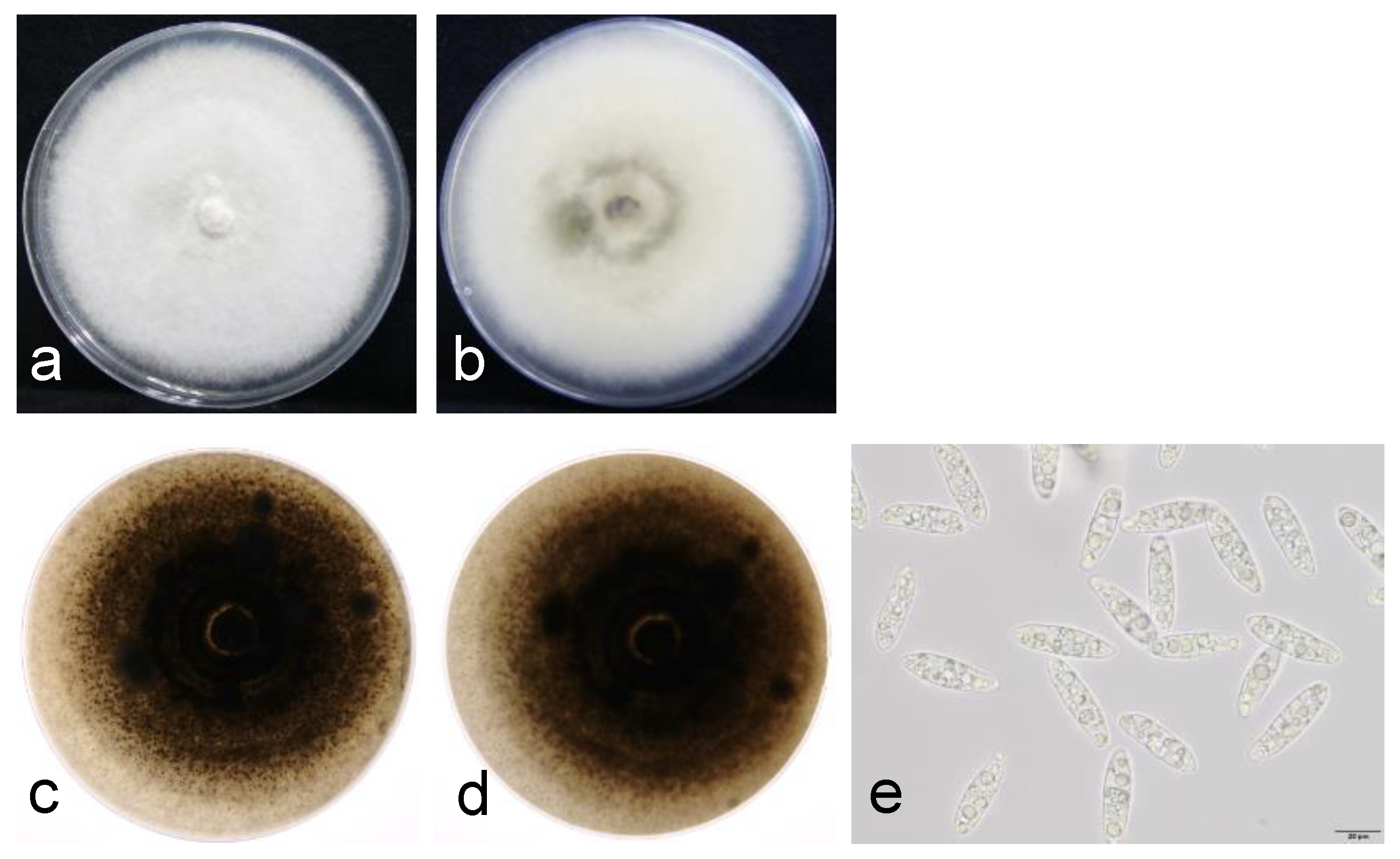
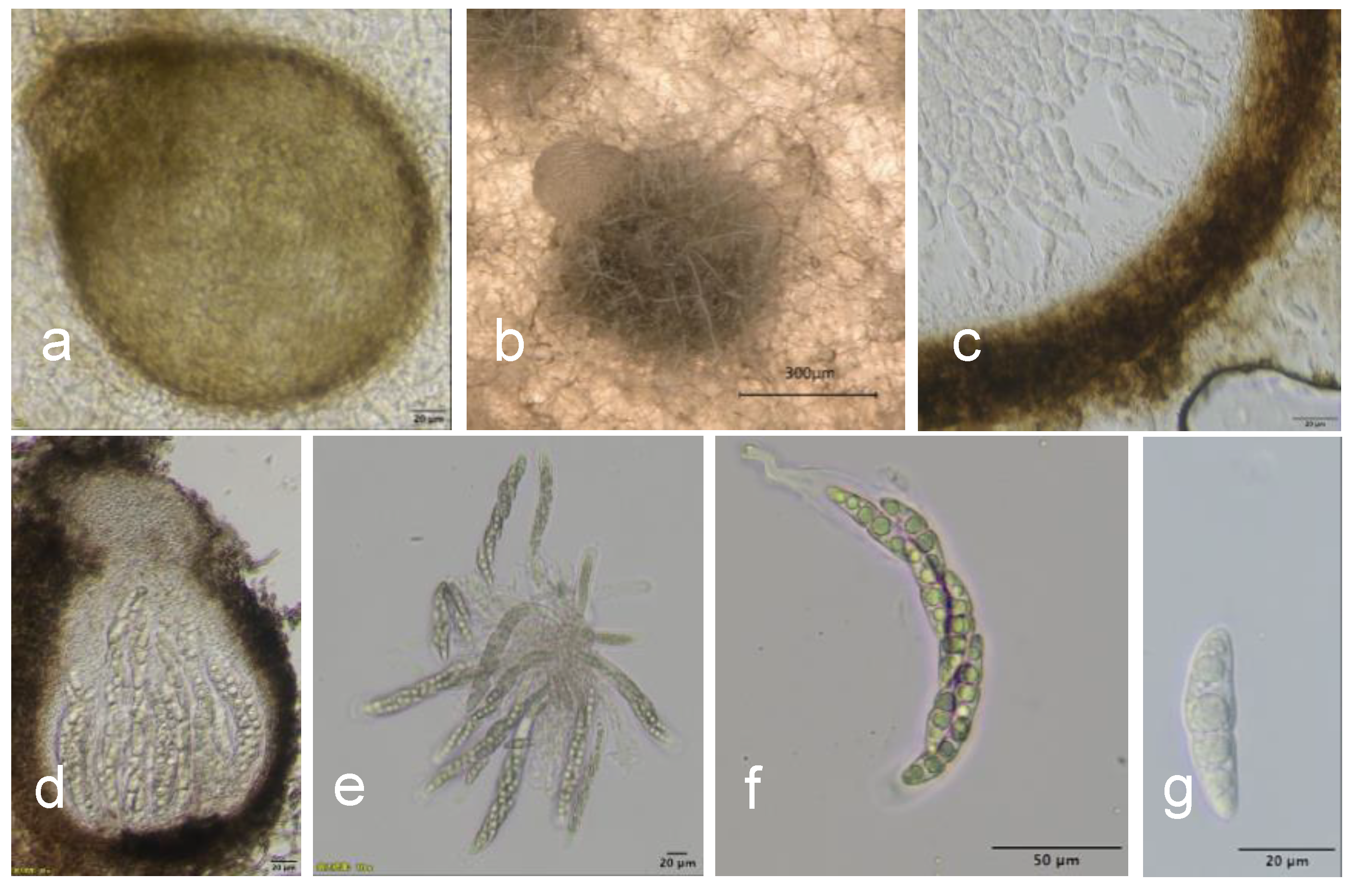
2.2. Morphological Identification
2.3. Molecular Studies
2.4. Characterization of the Fungus In Vitro Cultural Conditions
2.5. Statistical Analysis
3. Results
3.1. Field Survey and Pathogen Isolation
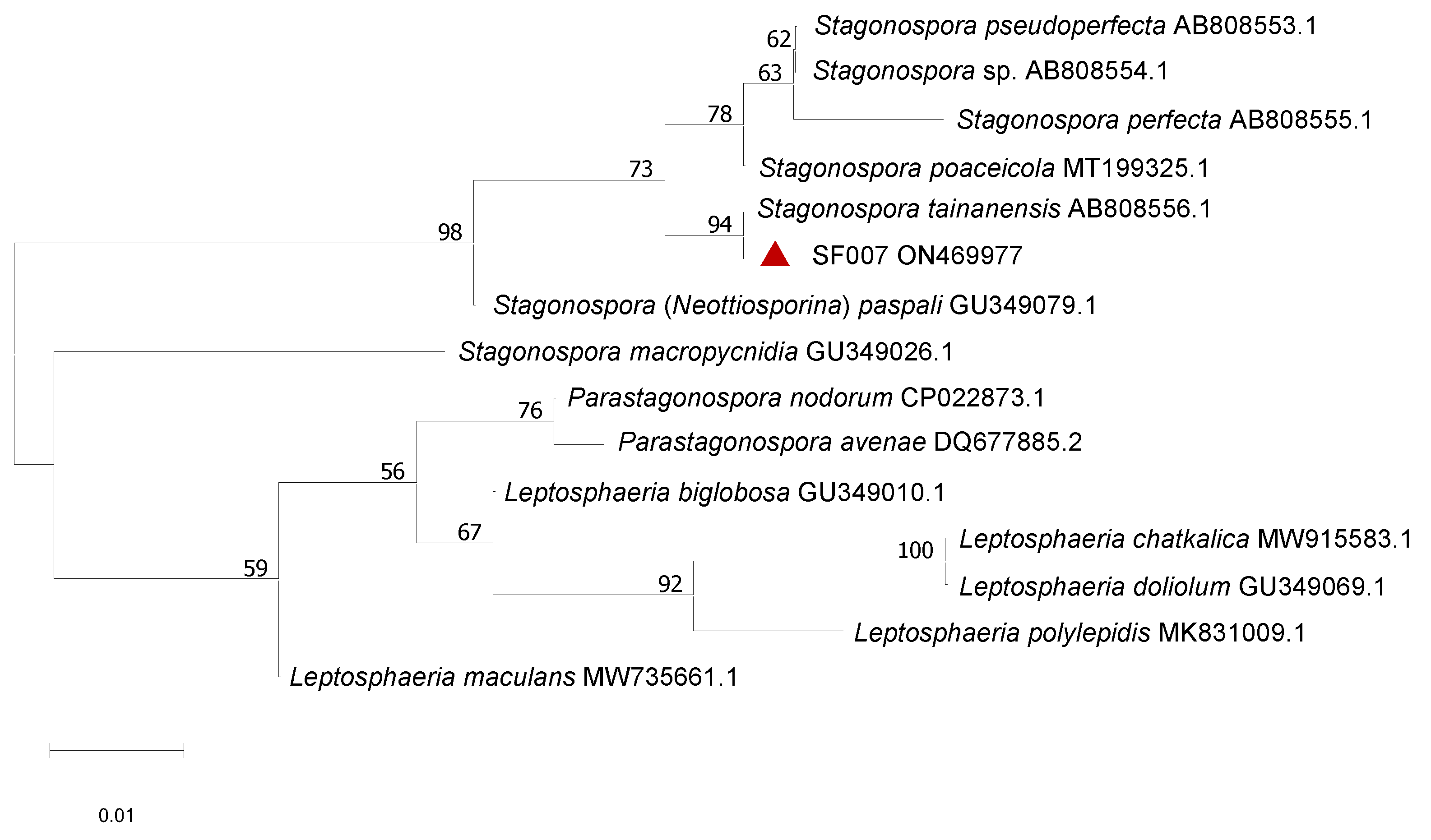
3.2. Morphological and Phylogenetic Analysis of the Pathogen
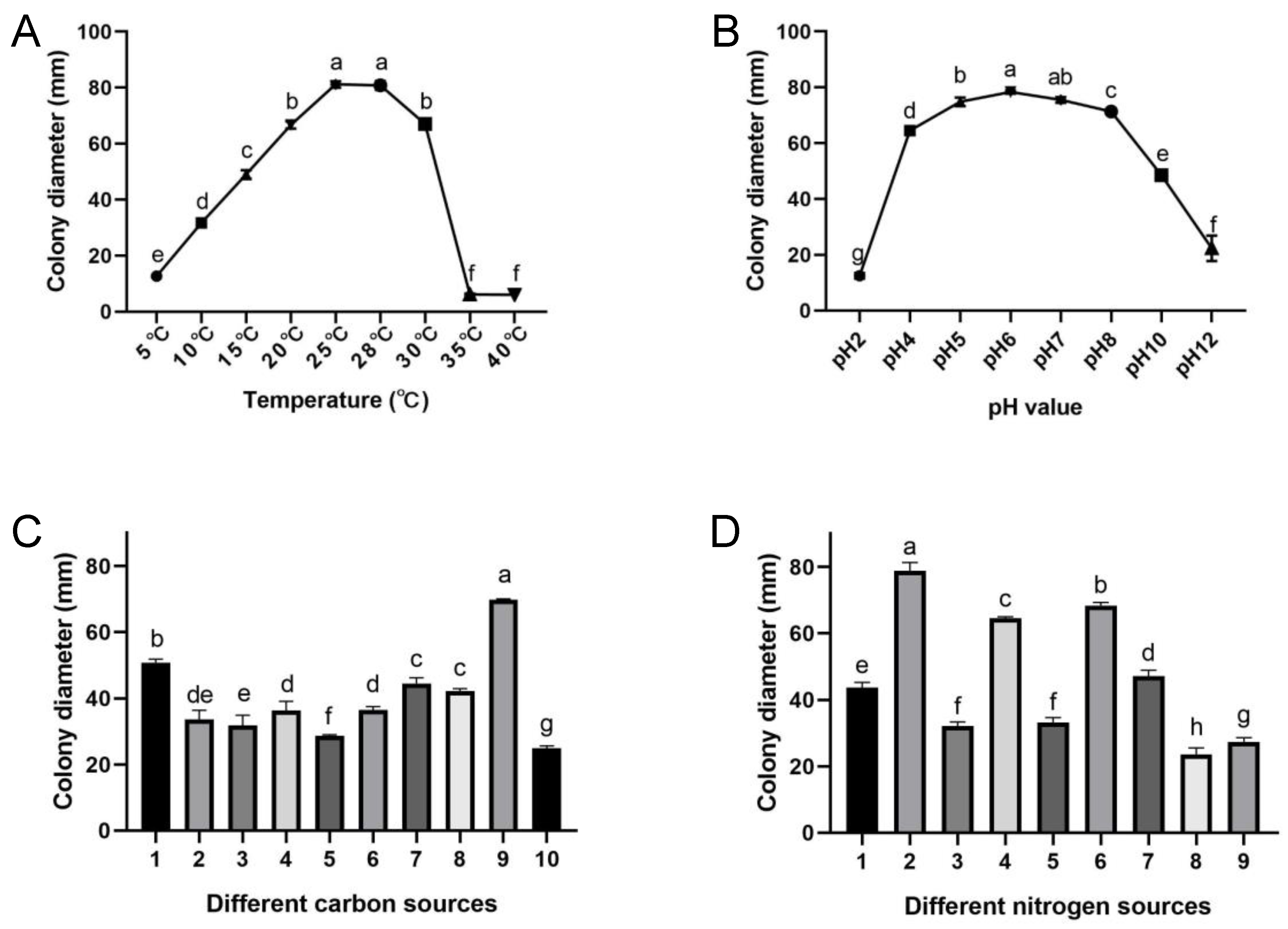
3.3. Optimized Culture Conditions for the Pathogen
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cardona, C.A.; Quintero, J.A.; Paz, I.C. Production of bioethanol from sugarcane bagasse: Status and perspectives. Bioresour. Technol. 2010, 101, 4754–4766. [Google Scholar] [CrossRef] [PubMed]
- Waclawovsky, A.J.; Sato, P.M.; Lembke, C.G.; Moore, P.H.; Souza, G.M. Sugarcane for bioenergy production: An assessment of yield and regulation of sucrose content. Plant Biotechnol. J. 2010, 8, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Khan, M.; Sharif, R.; Mujtaba, M.; Gao, S. Sugarcane Omics: An Update on the Current Status of Research and Crop Improvement. Plants 2019, 8, 344. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tian, H.C.; Huang, Z.G. Research on the Development Status of Sugarcane Industry in China. Sugar Crops China 2017, 39, 67–70. (In Chinese) [Google Scholar]
- Liu, Y.; Li, Y.; Liang, W.; Song, Q.; Qin, X.; Ye, L. Development status of foreign sugarcane industry. World Agric. 2015, 436, 147–152. (In Chinese) [Google Scholar] [CrossRef]
- Yadav, R.L.; Solomon, S. Potential of developing sugarcane by-product based industries in India. Sugar Tech 2006, 8, 104–111. [Google Scholar] [CrossRef]
- Viswanathan, R. Varietal Degeneration in Sugarcane and its Management in India. Sugar Tech 2016, 18, 1–7. [Google Scholar] [CrossRef]
- Rao, G.P.; Viswanathan, R.; Singh, S.B. Current situation of sugarcane diseases in India. Sugarcane Crop Manag. 2002, 734, 1–9. [Google Scholar]
- Viswanathan, R.; Rao, G.P. Disease Scenario and Management of Major Sugarcane Diseases in India. Sugar Tech 2011, 13, 336–353. [Google Scholar] [CrossRef]
- Wang, Z.T.; Lu, G.L.; Wu, Q.B.; Li, A.T.; Que, Y.X.; Xu, L.P. Isolating QTL controlling sugarcane leaf blight resistance using a two-way pseudo-testcross strategy. Crop J. 2021, 10, 1131–1140. [Google Scholar] [CrossRef]
- Leu, L.; Wang, Z.; Lin, K.; Kwo, C.; Shang, K. Leaf blight of sugarcane in Taiwan. VI. preliminary studies of the mode of inheritance of resistance in sugarcane against the disease. Res. Rep. Taiwan Sugar Res. Inst. 1976, 71, 45–53. [Google Scholar]
- Wang, Z.T.; Ren, H.; Xu, F.; Lu, G.L.; Cheng, W.; Que, Y.X.; Xu, L.P. Genome-Wide Characterization of Lectin Receptor Kinases in Saccharum spontaneum L. and Their Responses to Stagonospora tainanensis Infection. Plants 2021, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.H. The causal organism of sugarcane leaf blight. Mycologia 1979, 71, 892–898. [Google Scholar] [CrossRef]
- Tanaka, K.; Hirayama, K.; Yonezawa, H.; Sato, G.; Toriyabe, A.; Kudo, H.; Hashimoto, A.; Matsumura, M.; Harada, Y.; Kurihara, Y.; et al. Revision of the Massarineae (Pleosporales, Dothideomycetes). Stud. Mycol. 2015, 82, 75–136. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, X.; Ren, H.; Zeng, R.; Wang, Z.; Hu, H.; Bao, J.; Que, Y. The First Telomere-to-Telomere Chromosome-Level Genome Assembly of Stagonospora tainanensis Causing Sugarcane Leaf Blight. J. Fungi 2022, 8, 1088. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. 1990, 18, 315–322. [Google Scholar]
- Rehner, S.A.; Buckley, E. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef]
- Sun, R.H.; Xu, Y.; Wang, Z.X.; Lu, G.L.; Xu, N.C.; Huang, P.G. ldentification of pathogen causing dieback on Ternstroemia gymnanthera. Plant Prot. 2020, 46, 185–188. [Google Scholar] [CrossRef]
- Campbell, R.K.; Perring, T.M.; Barnes, G.L.; Eikenbary, R.D.; Gentry, C.R. Growth and sporulation of Beauveria bassiana and Metarrhizium anisopliae on media containing various amino acids. J. Invertebr. Pathol. 1978, 31, 289–295. [Google Scholar] [CrossRef]
- Singh, J.; Shahdeo, S.; Lal, B.; Prasad, M. Effect of vitamins on growth and sporulation of Alternaria alternata (Fr.) Keissler. Zentralblatt fur Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene. Zweite Naturwissenschaftliche Abteilung Mikrobiologie Der Landwirtschaft Der Technologie Und Des Umweltschutzes 1980, 135, 234–239. [Google Scholar] [CrossRef]
- Costa, T.P.C.; Rodrigues, E.M.; Dias, L.P.; Pupin, B.; Ferreira, P.C.; Rangel, D.E.N. Different wavelengths of visible light influence the conidial production and tolerance to ultra-violet radiation of the plant pathogens Colletotrichum acutatum and Fusarium fujikuroi. Eur. J. Plant Pathol. 2021, 159, 105–115. [Google Scholar] [CrossRef]


| Species | Isolate No. | Host | Location | GenBank Accession Number | |
|---|---|---|---|---|---|
| ITS | TEF-1α | ||||
| S. tainanensis | SF001 | sugarcane | Chongzuo, Guangxi | ON428231 | ON469971 |
| S. tainanensis | SF002 | sugarcane | Chongzuo, Guangxi | ON428232 | ON469972 |
| S. tainanensis | SF003 | sugarcane | Chongzuo, Guangxi | ON428233 | ON469973 |
| S. tainanensis | SF004 | sugarcane | Chongzuo, Guangxi | ON428234 | ON469974 |
| S. tainanensis | SF005 | sugarcane | Chongzuo, Guangxi | ON428235 | ON469975 |
| S. tainanensis | SF006 | sugarcane | Chongzuo, Guangxi | ON428236 | ON469976 |
| S. tainanensis | SF007 | sugarcane | Chongzuo, Guangxi | ON428237 | ON469977 |
| S. tainanensis | SF008 | sugarcane | Chongzuo, Guangxi | ON428238 | ON469978 |
| S. tainanensis | SF009 | sugarcane | Chongzuo, Guangxi | ON428239 | ON469979 |
| S. tainanensis | SF010 | sugarcane | Chongzuo, Guangxi | ON428240 | ON469980 |
| S. tainanensis | SF011 | sugarcane | Chongzuo, Guangxi | ON428241 | ON469981 |
| Isolate | Colony Diameter (mm) | Conidial Spore (μm) | |
|---|---|---|---|
| Length | Width | ||
| SF001 | 77.00 ± 0.41 | 38.13 ± 4.64 | 12.14 ± 1.06 |
| SF002 | 79.67 ± 0.47 | 40.09 ± 2.42 | 11.89 ± 0.73 |
| SF003 | 81.83 ± 0.24 | 40.25 ± 3.72 | 11.94 ± 1.03 |
| SF004 | 73.17 ± 0.23 | 39.98 ± 4.21 | 11.61 ± 1.22 |
| SF005 | 80.50 ± 0.24 | 39.17 ± 3.34 | 11.75 ± 1.11 |
| SF006 | 73.33 ± 0.24 | 41.44 ± 2.84 | 12.07 ± 1.11 |
| SF007 | 82.33 ± 0.47 | 41.40 ± 3.91 | 11.83 ± 1.15 |
| SF008 | 71.00 ± 1.08 | 38.31 ± 3.58 | 11.52 ± 0.96 |
| SF009 | 79.17 ± 0.24 | 41.12 ± 3.26 | 11.40 ± 0.92 |
| SF010 | 79.67 ± 0.47 | 40.42 ± 3.07 | 11.08 ± 0.77 |
| SF011 | 80.33 ± 0.47 | 41.84 ± 2.54 | 12.04 ± 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Shi, Q.; Zeng, Q.; Liang, H.; Yu, Q.; Meng, J.; Chen, B. Isolation, Characterization and Phylogenetic Analysis of Stagonospora tainanensis, the Pathogen Causing Sugarcane Leaf Blight in China. Agronomy 2023, 13, 1136. https://doi.org/10.3390/agronomy13041136
Huang Z, Shi Q, Zeng Q, Liang H, Yu Q, Meng J, Chen B. Isolation, Characterization and Phylogenetic Analysis of Stagonospora tainanensis, the Pathogen Causing Sugarcane Leaf Blight in China. Agronomy. 2023; 13(4):1136. https://doi.org/10.3390/agronomy13041136
Chicago/Turabian StyleHuang, Zhenxin, Qian Shi, Quan Zeng, Haoming Liang, Quan Yu, Jiaorong Meng, and Baoshan Chen. 2023. "Isolation, Characterization and Phylogenetic Analysis of Stagonospora tainanensis, the Pathogen Causing Sugarcane Leaf Blight in China" Agronomy 13, no. 4: 1136. https://doi.org/10.3390/agronomy13041136
APA StyleHuang, Z., Shi, Q., Zeng, Q., Liang, H., Yu, Q., Meng, J., & Chen, B. (2023). Isolation, Characterization and Phylogenetic Analysis of Stagonospora tainanensis, the Pathogen Causing Sugarcane Leaf Blight in China. Agronomy, 13(4), 1136. https://doi.org/10.3390/agronomy13041136







