Exogenous Application of Salicylic Acid Modulates Oxidative Stress during the Seed Development of Rice (Oryza sativa L.) Grain
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Plant Samples and Crop Management
2.3. Samples Preparation
2.4. Phytoprostane and Phytofuran Extracts
2.5. UHPLC-ESI-QqQ-MS/MS Analysis
2.6. Statistical Analysis
3. Results and Discussion
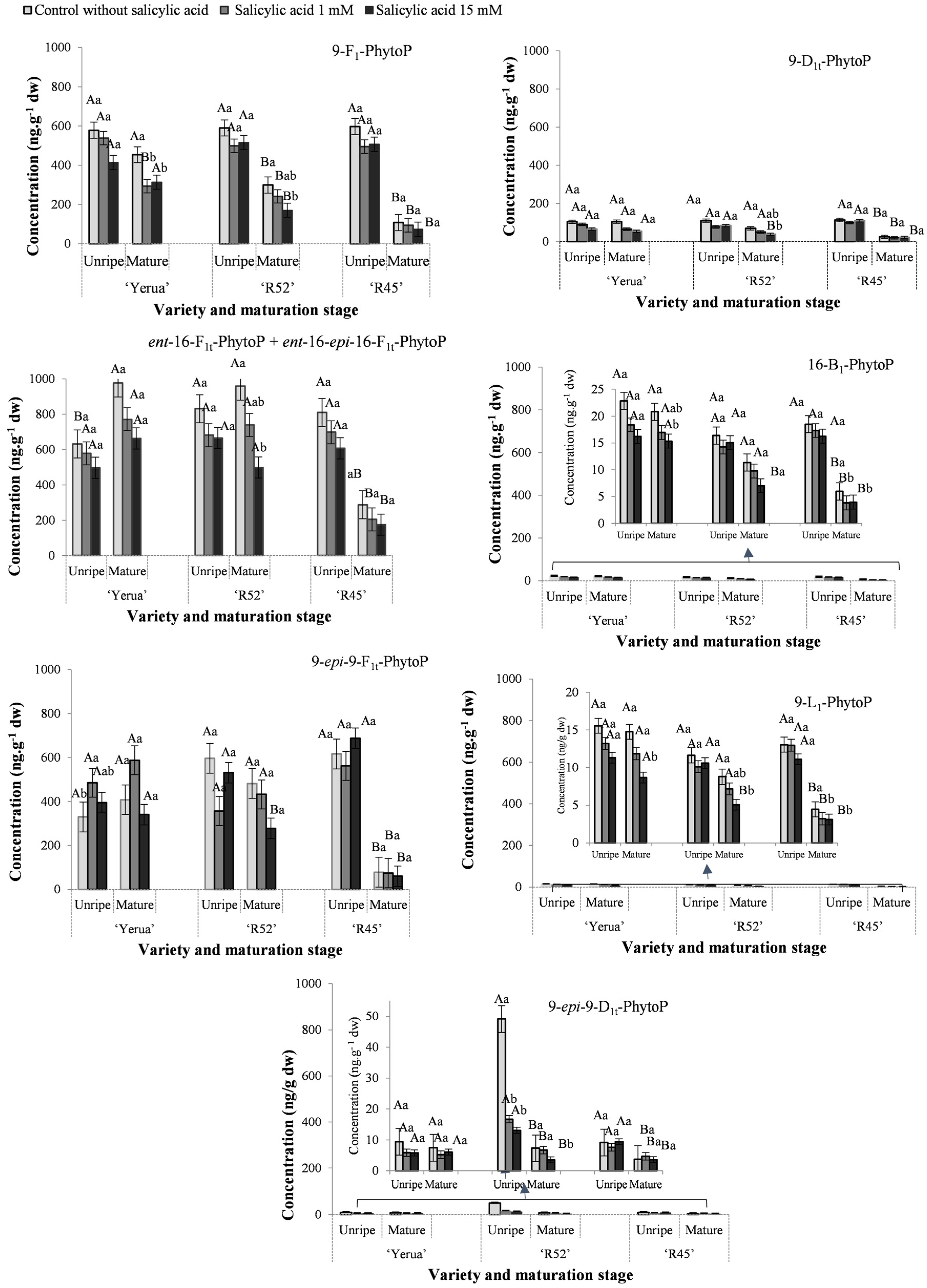
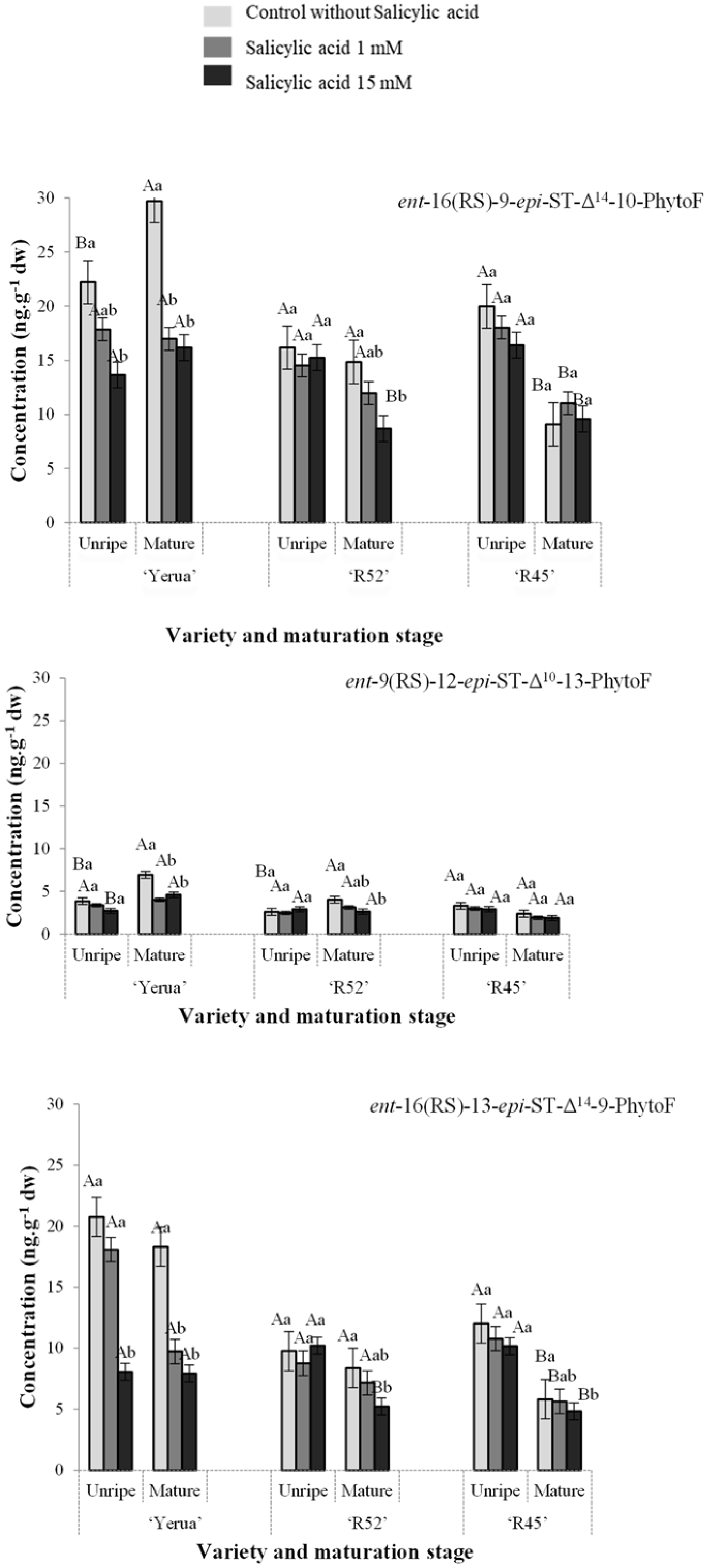
3.1. Total Content of Phytoprostanes and Phytofurans in Unripe and Ripe Rice Grains
3.2. Effect of Exogenous Salicylic Acid on the Quantitative Profile of Phytoprostanes and Phytofurans in Unripe and Mature Rice Grain
3.3. Oxidative Response of Unripe and Mature Grains after the Application of Exogenous Salicylic Acid Depending on Rice Cultivars
3.4. Effect of Exogenous Salicylic Acid on Yield and Quality of Rice Grain
3.5. Strengths and Limitations of the Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bailly, C. Research Review: Active oxygen species and antioxidants in seed biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- Lipan, L.; Collado-González, J.; Domínguez-Perles, R.; Corell, M.; Bultel-Poncé, V.; Galano, J.M.; Durand, T.; Medina, S.; Gil-Izquierdo, A.; Carbonell-Barrachina, A. Phytoprostanes and phytofurans—Oxidative stress and bioactive compounds—In almonds are affected by deficit irrigation in almond trees. J. Agric. Food Chem. 2020, 68, 7214–7225. [Google Scholar] [CrossRef] [PubMed]
- Poór, P. Review: Effects of Salicylic Acid on the Metabolism of Mitochondrial Reactive Oxygen Species in Plants. Biomolecules 2020, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Xie, Y.; Hu, L.; Si, J.; Wang, Z. Excessive nitrogen application dampens antioxidant capacity and grain filling in wheat as revealed by metabolic and physiological analyses. Sci. Rep. 2017, 7, 43363. [Google Scholar] [CrossRef]
- Yamakawa, H.; Hakata, M. Atlas of rice grain filling-related metabolism under high temperature: Joint analysis of metabolome and transcriptome demonstrated inhibition of starch accumulation and induction of amino acid accumulation. Plant Cell Physiol. 2010, 51, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Collado-González, J.; Durand, T.; Ferreres, F.; Medina, S.; Torrecillas, A.; Gil-Izquierdo, A. Phytoprostanes. Lipid Technol. 2015, 27, 127–130. [Google Scholar] [CrossRef]
- Staniek, K.; Nohl, H. Are mitochondria a permanent source of reactive oxygen species? Biochim. Biophys. Acta 2000, 1460, 268–275. [Google Scholar] [CrossRef]
- Mateo, A.; Funck, D.; Muhlenbock, P.; Kular, B.; Mullineaux, P.M.; Karpinski, S. Oxygen Metabolism, ROS and Redox Signalling in Plants Special Issue Controlled levels of salicylic acid are required for optimal photosynthesis and redox homeostasis. J. Exp. Bot. 2006, 57, 1795–1807. [Google Scholar] [CrossRef]
- Dey, P.M.; Harborne, J.B. The plant, the cell and its molecular components. Plant Biochem. 1997, 554. [Google Scholar] [CrossRef]
- Leung, K.S.; Oger, C.; Gu, A.; Bultel-Poncé, V.; Vigor, C.; Durand, T.; Gil-Izquierdo, A.; Medina, S.; Galano, J.M.; Lee, J.C.Y. Alpha-linolenic acid, phytoprostanes and phytofurans in plant, algae and food. In Lipids in Plants and Algae; From Fundamental Science to Industrial, Applications; Fabrice, R., Eric, M., Eds.; Elsevier: London, UK, 2022; pp. 437–468. [Google Scholar] [CrossRef]
- Medina, S.; Collado-González, J.; Ferreres, F.; Londoño-Londoño, J.; Jiménez-Cartagena, C.; Guy, A.; Durand, T.; Galano, J.-M.; Gil-Izquierdo, A. Valorization strategy of banana passion fruit shell wastes as rich source of phytoprostanes and phenolic compounds. J. Food Nutr. Res. 2017, 5, 801–808. [Google Scholar] [CrossRef]
- Mueller, M.J. Archetype signals in plants: The phytoprostanes. Curr. Opinion Plant. Biol. 2004, 7, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Cuyamendous, C.; Leung, K.S.; Durand, T.; Lee, J.C.Y.; Oger, C.; Galano, J.M. Synthesis and discovery of phytofurans: Metabolites of α-linolenic acid peroxidation. Chem. Comm. 2015, 51, 15696–15699. [Google Scholar] [CrossRef] [PubMed]
- Pinciroli, M.; Domínguez-Perles, R.; Abellán, A.; Guy, A.; Durand, T.; Oger, C.; Galano, J.M.; Ferreres, F.; Gil-Izquierdo, A. Comparative Study of the Phytoprostane and Phytofuran Content of indica and japonica Rice (Oryza sativa L.) Flours. J. Agric. Food Chem. 2017, 65, 8938–8947. [Google Scholar] [CrossRef] [PubMed]
- Pinciroli, M.; Domínguez-Perles, R.; Abellán, A.; Bultel-Ponce, V.T.; Durand, T.; Galano, J.M.; Ferreres, F.; Gil-Izquierdo, A. Statement of Foliar Fertilization Impact on Yield, Composition, and Oxidative Biomarkers in Rice. J. Agric. Food Chem. 2019, 67, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Collado-González, J.; Cano-Lamadrid, M.; Pérez-López, D.; Carbonell-Barrachina, A.A.; Centeno, A.; Medina, S.; Griñán, I.; Guy, A.; Galano, J.M.; Durand, T.; et al. Effects of deficit irrigation, rootstock and roasting on the contents of fatty acids, phytoprostanes, and phytofurans in pistachio kernels. J. Agric. Food Chem. 2020, 68, 8915–8924. [Google Scholar] [CrossRef]
- Santino, A.; Taurino, M.; De Domenico, S.; Bonsegna, S.; Poltronieri, P.; Pastor, V.; Flors, V. Jasmonate signaling in plant development and defense response to multiple (a) biotic stresses. Plant. Cell Rep. 2013, 32, 1085–1098. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Matos, A.R.; Mendes, A.T.; Scotti-Campos, P.; Arrabaça, J.D. Study of the effects of salicylic acid on soybean mitochondrial lipids and respiratory properties using the alternative oxidase as a stress-reporter protein. Physiol. Plant. 2009, 137, 485–497. [Google Scholar] [CrossRef]
- Wang, J.; Mengting, L.; Islam, F.; Gill, R.A.; Yang, C.; Ali, B.; Yan, G.; Zhou, W. Salicylic acid mediates antioxidant defense system and ABA pathway related gene expression in Oryza sativa against quinclorac toxicity. Ecotoxicol. Environ. Saf. 2016, 133, 146–156. [Google Scholar] [CrossRef]
- Wang, J.; Shi, C.Z.Y.; Long, M.; Islam, F.; Yang, C.; Yang, S.; He, Y.; Zhou, W. Evaluation of quinclorac toxicity and alleviation by salicylic acid in rice seedlings using ground-based visible/near-infrared hyperspectral imaging. Plant Met. 2020, 16, 30. [Google Scholar] [CrossRef]
- Wang, J.; Islam, F.; Li, L.; Long, M.; Yang, C.; Jin, X.; Mao, B.; Zhou, W. Complementary RNA-Sequencing Based Transcriptomics and iTRAQ Proteomics Reveal the Mechanism of the Alleviation of Quinclorac Stress by Salicylic Acid in Oryza sativa ssp. Japonica. Int. J. Mol. Sci. 2017, 18, 1975. [Google Scholar] [CrossRef]
- Yu, L.; Li, Y.; Ding, T.; Wang, R.; Peng, C.; Xiao, L. Reduced grain chalkiness and its possible physiological mechanism in transgenic rice. Crop J. 2015, 3, 125–134. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, X.; Wang, Z.; Jiang, Y.; Liu, Z.; Alexander, D.; Ding, Y. Metabolomic analysis of pathways related to rice grain chalkiness by a notched-belly mutant with high occurrence of white-belly grains. BMC Plant Biol. 2017, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, T.; Shiraya, T.; Kaneko, K.; Wada, K. Proteomics of rice grain under high temperature stress. Front Plant Sci. 2013, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.; Borisjuk, L.; Wobus, U. Molecular Physiology of Legume Seed Development. Annu. Rev. Plant Biol. 2005, 56, 253–279. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Liang, Y.C.; Zhu, Y.G.; Zhao, F.J. Role of salicylic acid in alleviating oxidative damage in rice roots (Oryza sativa) subjected to cadmium stress. Environ. Pollut. 2007, 147, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Cuyamendous, C.; Leung, K.S.; Bultel-Poncé, V.; Guy, A.; Durand, T.; Galano, J.M.; Lee, J.C.; Oger, C. Total Synthesis and in Vivo Quantitation of Phytofurans Derived from α- Linolenic Acid. Eur. J. Org. Chem. 2017, 17, 2486–2490. [Google Scholar] [CrossRef]
- El Fangour, S.; Guy, A.; Despres, V.; Vidal, J.P.; Rossi, J.C.; Durand, T. Total syntheses of the eight diastereoisomers of the syn-anti-synphytoprostanes F1 types II and I. J. Org. Chem. 2004, 69, 2498–2503. [Google Scholar] [CrossRef]
- El Fangour, S.; Guy, A.; Vidal, J.P.; Rossi, J.C.; Durand, T. A flexible synthesis of the phytoprostanes B1 Type I and II. J. Org. Chem. 2005, 70, 989–997. [Google Scholar] [CrossRef]
- Guy, A.; Flanagan, S.; Durand, T.; Oger, C.; Galano, J.M. Facile synthesis of cyclopentenone B1- and L1- type phytoprostanes. Front. Chem. 2015, 3, 41. [Google Scholar] [CrossRef]
- Oger, C.; Brinkmann, Y.; Bouazzaoui, S.; Durand, T.; Galano, J.M. Stereocontrolled access to isoprostanes via a bicyclo [3.3.0] octene framework. Org. Lett. 2008, 10, 5087–5090. [Google Scholar] [CrossRef] [PubMed]
- Pinot, E.; Guy, A.; Fournial, A.; Balas, L.; Rossi, J.C.; Durand, T. Total synthesis of the four enantiomerically pure diasteroisomers of the phytoprostanes E1Type II and of the 15-E2t-isoprostanes. J. Org. Chem. 2008, 73, 3063–3069. [Google Scholar] [CrossRef] [PubMed]
- Collado-González, J.; Medina, S.; Durand, T.; Guy, A.; Galano, J.M.; Torrecillas, A.; Ferreres, F.; Gil-Izquierdo, A. New UHPLC–QqQ-MS/MS method for quantitative and qualitative determination of free phytoprostanes in foodstuffs of commercial olive and sunflower oils. Food Chem. 2015, 178, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Perles, R.; Abellán, A.; León-Pérez, D.; Ferreres, F.; Guy, A.; Oger, C.; Galano, J.M.; Durand, T.; Gil-Izquierdo, A. Sorting out the phytoprostane and phytofuran profile in vegetable oils. Food Res. Int. 2018, 107, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Medina, S.; Gil-Izquierdo, A.; Abu-Reidah, I.; Durand, T.; Guy, A.; Galano, J.-M.; Domínguez-Perles, R. Evaluation of Phoenixdactylifera edible parts and by-products as a source of phytoprostanes and phytofurans. J. Agric. Food Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Pinciroli, M.; Dominguez-Perles, R.; Garbi, M.; Abellan, A.; Oger, C.; Durand, T.; Galano, J.M.; Ferreres, F.; Gil-Izquierdo, A. Impact of salicylic acid content and growing environment on phytoprostane and phytofuran (stress biomarkers) in Oryza sativa L. J. Agric. Food Chem. 2018, 66, 12561–12570. [Google Scholar] [CrossRef]
- Carrasco-Del Amor, A.M.; Collado-González, J.; Aguayo, E.; Guy, A.; Galano, J.M.; Durand, T.; Gil-Izquierdo, A. Phytoprostanes in almonds: Identification, quantification, and impact of cultivar and type of cultivation. RSC Adv. 2015, 5, 51233–51241. [Google Scholar] [CrossRef]
- Yonny, M.E.; Rodríguez-Torresi, A.; Cuyamendous, C.; Reversat, G.; Oger, C.; Galano, J.M.; Durand, T.; Vigor, C.; Nazareno, M.A. Thermal stress in melon plants: Phytoprostanes and phytofurans as oxidative stress biomarkers and the effect of antioxidant supplementation. J. Agric. Food Chem. 2016, 64, 8296–8304. [Google Scholar] [CrossRef]
- León-Pérez, D.; Collado-González, J.; Cano-Lamadrid, M.; Durand, T.; Guy, A.; Galano, J.M.; Carbonell-Barrachina, A.; Londoño-Londoño, J.; Ferreres, F.; Gil-Izquierdo, A.; et al. Bioactive plant oxylipins-based lipidomics in eighty worldwide commercial dark chocolates: Effect of cocoa and fatty acid composition on their dietary burden. Microchem. J. 2020, 157, 105803. [Google Scholar] [CrossRef]
- Carrasco-Del Amor, M.; Aguayo, E.; Collado-Gonzalez, J.; Guy, A.; Galano, J.M.; Durand, T.; Gil-Izquierdo, A. Impact of processing conditions on the phytoprostanes profile of three types of nut kernels. Free Radic. Res. 2017, 51, 141–147. [Google Scholar] [CrossRef]
- Collado-Gonzalez, J.; Perez-Lopez, D.; Memmi, H.; Gijon, M.C.; Medina, S.; Durand, T.; Guy, A.; Galano, J.M.; Ferreres, F.; Torrecillas, A.; et al. Water deficit during pit hardening enhances phytoprostanes content, a plant biomarker of oxidative stress in extra virgin olive oil. J. Agric. Food Chem. 2015, 63, 3784–3792. [Google Scholar] [CrossRef] [PubMed]
- García-García, M.C.; del Río Celestino, M.; Gil-Izquierdo, A.; Egea-Gilabert, C.; Galano, J.M.; Durand, T.; Oger, C.; Fernández, J.A.; Ferreres, F.; Domínguez-Perles, R. The Value of Legume Foods as a Dietary Source of Phytoprostanes and Phytofurans Is Dependent on Species, Genotype, and Growing Conditions. Eur. J. Lipid Sci. Technol. 2019, 121, 1800484. [Google Scholar] [CrossRef]
- Carrasco-Del Amor, A.M.; Aguayo, E.; Collado-González, J.; Guy, A.; Galano, J.M.; Durand, T.; Gil-Izquierdo, A. Impact of packaging atmosphere, storage and processing conditions on the generation of phytoprostanes as quality processing compounds in almond kernels. Food Chem. 2016, 211, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Cuyamendous, C.; de la Torre, A.; Lee, Y.Y.; Leung, K.S.; Guy, A.; Bultel-Ponce, V.; Galano, J.M.; Lee, J.C.; Oger, C.; Durand, T. The novelty of phytofurans, isofurans, dihomo-isofurans and neurofurans: Discovery, synthesis and potential application. Bochimica 2016, 130, 49–62. [Google Scholar] [CrossRef]
- Pino Ramos, L.L.; Jimenez-Aspee, F.; Theoduloz, C.; Burgos-Edwards, A.; Domínguez-Perles, R.; Oger, C.; Durand, T.; Gil-Izquierdo, A.; Bustamante, L.; Mardones, C.; et al. Phenolic, oxylipin and fatty acid profiles of the Chilean hazelnut (Gevuina avellana): Antioxidant activity and inhibition of pro-inflammatory and metabolic syndrome-associated enzymes. Food Chem. 2019, 298, 125026. [Google Scholar] [CrossRef]
- Fessel, J.P.; Porter, N.A.; Moore, K.P.; Sheller, J.R. Discovery of lipid peroxidation products formed in vivo with a substituted tetrahydrofuran ring (isofurans) that are favored by increased oxygen tension. Proc. Natl. Acad. Sci. USA 2002, 99, 16713–16718. [Google Scholar] [CrossRef]
- Panda, D.; Sarkar, R.K. Natural leaf senescence: Probed by chlorophyll fluorescence, CO2 photosynthetic rate and antioxidant enzyme activities during grain filling in different rice cultivars. Physiol. Mol. Biol. Plant. 2013, 19, 43–51. [Google Scholar] [CrossRef]
- Zhao, H.; Dai, T.; Jing, Q.; Jiang, D.; Cao, W. Leaf senescence and grain filling affected by post-anthesis high temperatures in two different wheat cultivars. J. Plant Growth Regul. 2007, 51, 149–158. [Google Scholar] [CrossRef]
- Sahu, P.K.; Mondal, S.; Sharma, D.; Sao, R.; Kumar, V.; Das, B.K. Genetic insights into fatty acid components of traditional Indian rice (Oryza sativa L.) landraces from Chhattisgarh. Indian J. Genet. Plant Breed. 2019, 79, 651–657. [Google Scholar] [CrossRef]
- del Río, L.A.; Pastori, G.M.; Palma, J.M.; Sandalio, L.M.; Sevilla, F.; Corpas, F.J.; Jiménez, A.; López-Huertas, E.; Hernández, J.A. The activated oxygen role of peroxisomes in senescence. Plant Physiol. 1998, 116, 1195–1200. [Google Scholar] [CrossRef]
- Barbosa, M.; Collado-Gonzalez, J.; Andrade, P.B.; Ferreres, F.; Valentafi, P.; Galano, J.M.; Durand, T.; Gil-Izquierdo, A. Nonenzymatic α-linolenic acid derivatives from the sea: Macroalgae as novel sources of phytoprostanes. J. Agric. Food Chem. 2015, 63, 6466–6474. [Google Scholar] [CrossRef] [PubMed]
- Marhuenda, J.; Medina, S.; Diaz-Castro, A.; Martínez-Hernández, P.; Arina, S.; Zafrilla, P.; Mulero, J.; Oger, C.; Galano, J.M.; Durand, T.; et al. Dependency of Phytoprostane Fingerprints of Must and Wine on Viticulture and Enological Processes. J. Agric. Food Chem. 2015, 63, 9022–9028. [Google Scholar] [CrossRef] [PubMed]
- Collado-González, J.; Grosso, C.; Valentão, P.; Andrade, P.B.; Ferreres, F.; Durand, T.; Guy, A.; Galano, J.M.; Torrecillas, A.; Gil-Izquierdo, A. Inhibition of α-glucosidase and α-amylase by Spanish extra virgin olive oils: The involvement of bioactive compounds other than oleuropein and hydroxytyrosol. Food Chem. 2017, 235, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sánchez, S.; Domínguez-Perles, R.; Montoro-García, S.; Gabaldón, J.A.; Guy, A.; Durand, T.; Oger, C.; Ferreres, F.; Gil-Izquierdo, A. Bioavailable phytoprostanes and phytofurans from Gracilaria longissima have anti-inflammatory effects in endothelial cells. Food Funct. 2020, 1, 5166–5178. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Duan, H.; Yang, L.; Wang, Z.Q.; Zhou, S.C.; Yang, J.C. Effect of High Temperature During Heading and Early Filling on Grain Yield and Physiological Characteristics in Indica Rice. Acta Agron. Sin. 2009, 35, 512–521. [Google Scholar] [CrossRef]
- Enyedi, A.J.; Yalpani, N.; Silverman, P.; Raskin, I. Localization, conjugation, and function of salicylic acid in tobacco during the hypersensitive reaction to tobacco mosaic virus. Proc. Natl. Acad. Sci. USA 1992, 89, 2480–2484. [Google Scholar] [CrossRef]
- Pal, M.; Kovacs, V.; Szalai, G.; Soos, V.; Ma, X.; Liu, H.; Mei, H.; Janda, T. Salicylic Acid and Abiotic Stress Responses in Rice. J. Agron. Crop Sci. 2013, 200, 1–11. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, G.; Wang, Z.; Yang, J.; Zhang, J. Ethylene and ACC levels in developing grains are related to the poor appearance and milling quality of rice. Plant Growth Regul. 2009, 58, 85–96. [Google Scholar] [CrossRef]
- Kocher, M.F.; Siebenmorgen, T.J.; Norman, R.J.; Wells, B.R. Rice kernel moisture content and size distributions at harvest. Am. Soc. Agric. Eng. 1990, 33, 541–547. [Google Scholar] [CrossRef]

| Parameter | Genotype Z | Treatment Y | Interaction SA by G | ||
|---|---|---|---|---|---|
| Control | SA 1 | SA 15 | |||
| Spikelets per panicle | ‘Yerua’ | 119.8 ± 1.7 a X | 115.2 ± 6.3 a | 120.4 ± 4.5 a | N.s. |
| ‘R52’ | 112.1 ± 5.0 b | 110.3 ± 1.3 b | 132.2 ± 9.9 a | ||
| ‘R45’ | 106.2 ± 5.5 a | 107.8 ± 1.1 a | 119.5 ± 5.4 a | ||
| Chalkiness (%) | ‘Yerua’ | 18.6 ± 1.9 a | 17.8 ± 1.9 a | 15.6 ± 0.7 a | N.s. |
| ‘R52’ | 22.6 ± 2.1 a | 15.3 ± 2.0 ab | 13.8 ± 0.7 b | ||
| ‘R45’ | 15.4 ± 1.5 a | 14.0 ± 1.7 a | 11.7 ± 1.2 a | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinciroli, M.; Domínguez-Perles, R.; Medina, S.; Oger, C.; Guy, A.; Durand, T.; Cascant-Vilaplana, M.M.; Gabaldón-Hernández, J.A.; Ferreres, F.; Gil-Izquierdo, Á. Exogenous Application of Salicylic Acid Modulates Oxidative Stress during the Seed Development of Rice (Oryza sativa L.) Grain. Agronomy 2023, 13, 636. https://doi.org/10.3390/agronomy13030636
Pinciroli M, Domínguez-Perles R, Medina S, Oger C, Guy A, Durand T, Cascant-Vilaplana MM, Gabaldón-Hernández JA, Ferreres F, Gil-Izquierdo Á. Exogenous Application of Salicylic Acid Modulates Oxidative Stress during the Seed Development of Rice (Oryza sativa L.) Grain. Agronomy. 2023; 13(3):636. https://doi.org/10.3390/agronomy13030636
Chicago/Turabian StylePinciroli, María, Raúl Domínguez-Perles, Sonia Medina, Camille Oger, Alexandre Guy, Thierry Durand, Mari Merce Cascant-Vilaplana, José Antonio Gabaldón-Hernández, Federico Ferreres, and Ángel Gil-Izquierdo. 2023. "Exogenous Application of Salicylic Acid Modulates Oxidative Stress during the Seed Development of Rice (Oryza sativa L.) Grain" Agronomy 13, no. 3: 636. https://doi.org/10.3390/agronomy13030636
APA StylePinciroli, M., Domínguez-Perles, R., Medina, S., Oger, C., Guy, A., Durand, T., Cascant-Vilaplana, M. M., Gabaldón-Hernández, J. A., Ferreres, F., & Gil-Izquierdo, Á. (2023). Exogenous Application of Salicylic Acid Modulates Oxidative Stress during the Seed Development of Rice (Oryza sativa L.) Grain. Agronomy, 13(3), 636. https://doi.org/10.3390/agronomy13030636










