Lack of Significant Effects of Glyphosate on Glyphosate-Resistant Maize in Different Field Locations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Growing Conditions
2.2. Materials
2.3. Experimental Design, Herbicide Application, and Weed Removal
2.4. Growth Variables
2.5. Foliar Mineral Content
2.6. Crop Yield Variables
2.7. Grain Quality Variables
2.8. Glyphosate and Aminomethylphosphonic Acid (AMPA) Residues in Grain
2.9. Statistical Analysis
3. Results
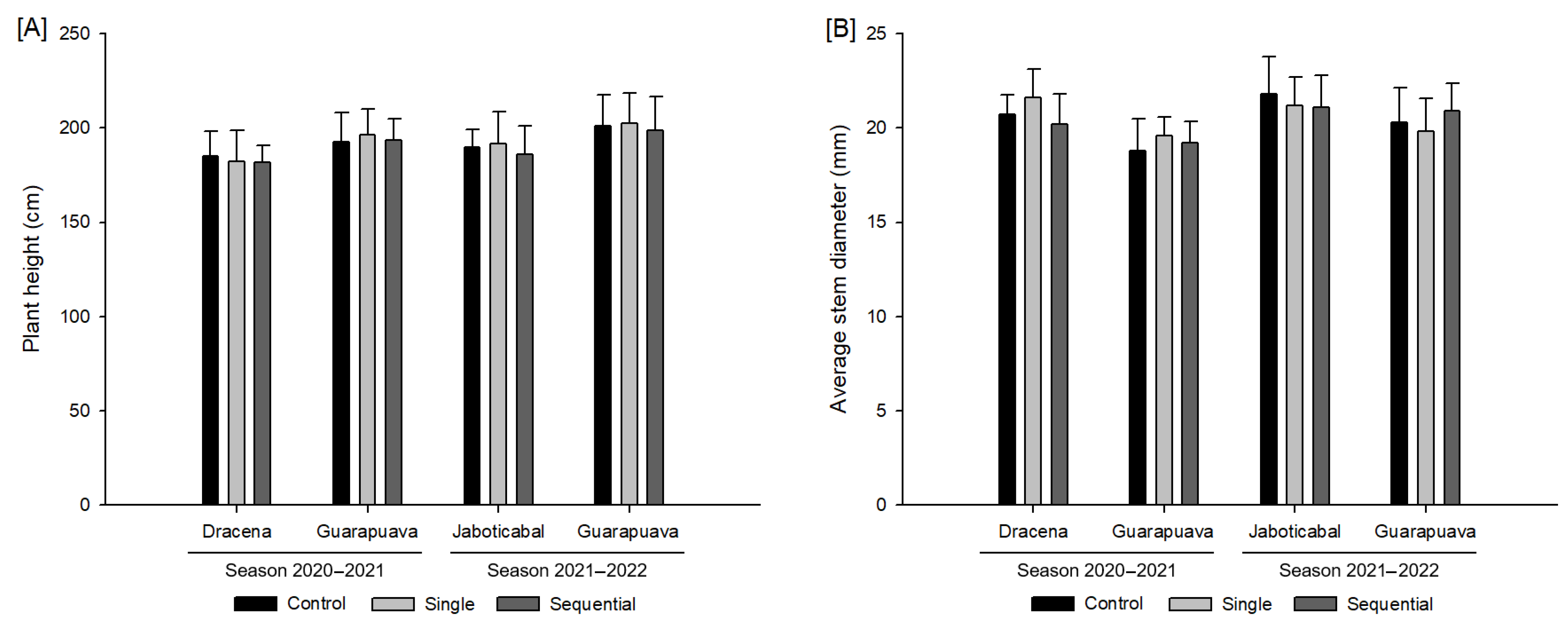
3.1. Growth Variables
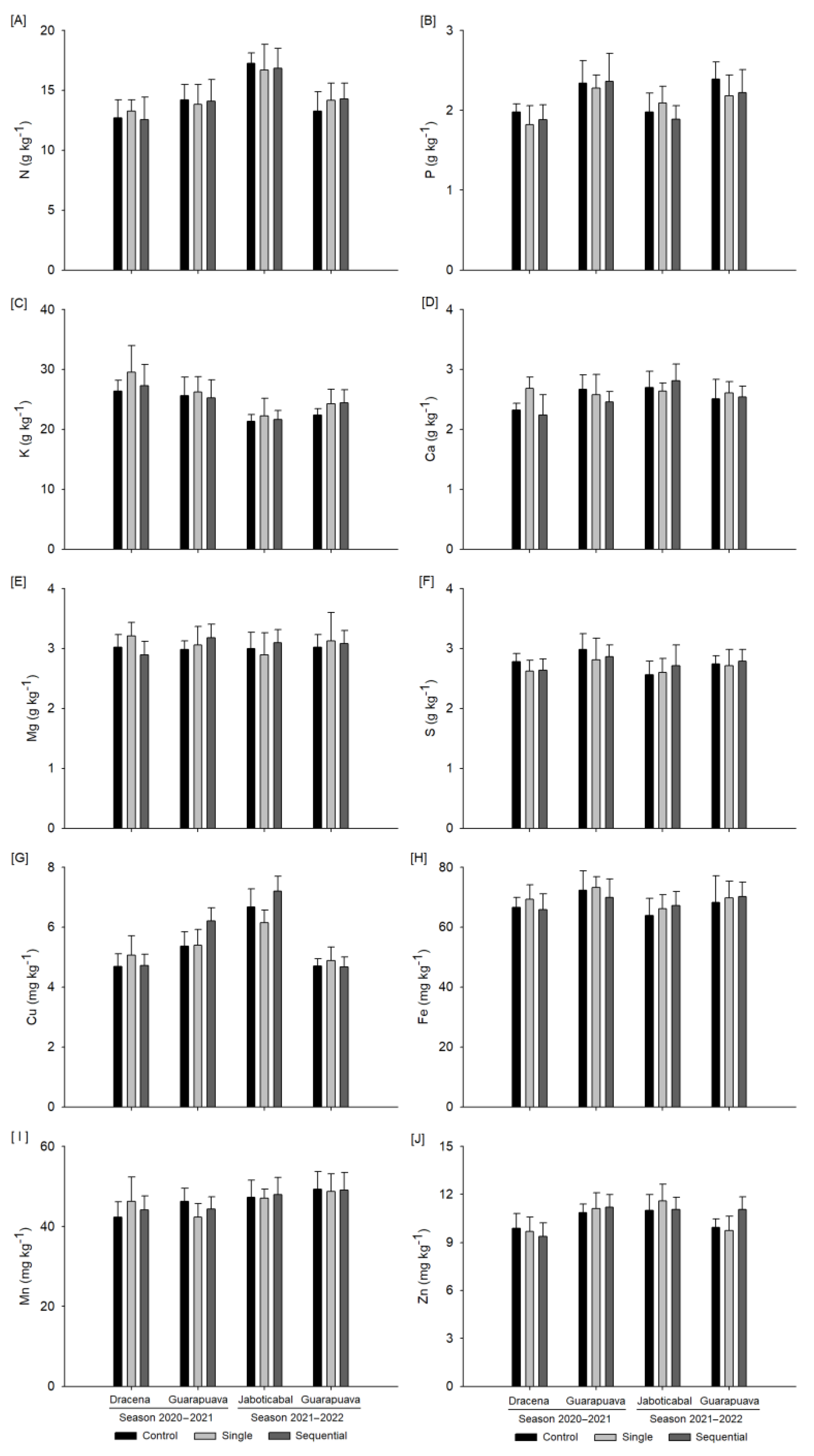
3.2. Foliar Mineral Content
3.3. Crop Yield Variables
3.4. Grain Quality Variables
3.5. Glyphosate and AMPA Residues in Grain
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bott, S.; Tesfamariam, T.; Candan, H.; Cakmak, I.; Römheld, V.; Newmann, G. Glyphosate-induced impairment of plant growth and micronutrient status in glyphosate-resistant soybean (Glycine max L.). Plant Soil 2008, 68, 185–194. [Google Scholar] [CrossRef]
- Zobiole, L.H.S.; Oliveira, R.S., Jr.; Huber, D.M.; Constantin, J.; de Castro, C.; Oliviera, F.A.; Oliviera, A., Jr. Glyphosate reduces shoot concentration of mineral nutrients in glyphosate-resistant soybeans. Plant Soil 2010, 328, 57–69. [Google Scholar] [CrossRef]
- Zobiole, L.H.S.; Oliveira, R.S., Jr.; Kremer, R.J.; Constantin, J.; Yamada, T.; de Castro, C.; Oliviera, F.A.; Oliviera, A., Jr. Effect of glyphosate on symbiotic N2 fixation and nickel concentration in glyphosate-resistant soybeans. Appl. Soil Ecol. 2010, 44, 176–180. [Google Scholar] [CrossRef]
- Zobiole, L.H.S.; Oliveira, R.S., Jr.; Kremer, R.J.; Muniz, A.S.; Oliviera, A., Jr. Nutrient accumulation and photosynthesis in glyphosate-resistant soybeans is reduced under glyphosate use. J. Plant Nutrit. 2010, 33, 1860–1873. [Google Scholar] [CrossRef]
- Zobiole, L.H.S.; Kremer, R.J.; Oliveira, R.S., Jr.; Constantine, J. Glyphosate affects chlorophyll, nodulation and nutrient accumulation of “second generations” glyphosate-resistant soybean. Pestic. Biochem. Physiol. 2011, 99, 53–60. [Google Scholar] [CrossRef]
- Zobiole, L.H.S.; Kremer, R.J.; de Oliveira, R.S., Jr.; Constantin, J. Glyphosate effects on photosynthesis, nutrient accumulation, and nodulation in glyphosate-resistant soybean. J. Plant Nutrit. Soil Sci. 2012, 175, 318–330. [Google Scholar] [CrossRef]
- Yamada, T.; Kremer, R.J.; Castro, P.R.C.; Wood, B.W. Glyphosate interactions with physiology, nutrition, and disease of plants: Threat to agricultural sustainability. Eur. J. Agron. 2009, 31, 111–113. [Google Scholar] [CrossRef]
- Mertins, M.; Hoss, S.; Neumann, G.; Afzal, J.; Reichenbecher, W. Glyphosate, a chelating agent-relevant for ecological assessment? Environ. Sci. Pollut. Res. Internat. 2018, 25, 5298–5317. [Google Scholar] [CrossRef]
- Martinez, K.J.; Loening, U.E.; Graham, M.C. Impacts of glyphosate-based herbicides on disease resistance and health of crops: A review. Environ. Sci. Eur. 2018, 30, 2. [Google Scholar] [CrossRef]
- Duke, S.O. Glyphosate. In Herbicides: Chemistry, Degradation, and Mode of Action; Kearney, P.C., Kaufman, D.D., Eds.; Dekker: New York, NY, USA, 1988; Volume 3, pp. 1–70. [Google Scholar]
- Duke, S.O.; Reddy, K.N.; Bu, K.; Cizdziel, J.V. Effects of glyphosate on mineral content of glyphosate-resistant soybeans (Glycine max). J. Agric. Food Chem. 2012, 60, 6764–6771. [Google Scholar] [CrossRef]
- Duke, S.O.; Rimando, A.M.; Reddy, K.N.; Cizdziel, J.V.; Bellalui, N.; Shaw, D.R.; Williams, M.M.; Maul, J.E. Lack of transgene and glyphosate effects on yield and mineral and amino acid content of glyphosate-resistant soybean. Pest Manag. Sci. 2018, 74, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Henry, R.S.; Wise, K.A.; Johnson, W.G. Glyphosate’s effect upon mineral accumulation in soybean. Crop Manag. 2011, 10, 1–8. [Google Scholar] [CrossRef]
- Kandel, Y.R.; Bradley, C.A.; Wise, K.A.; Chivers, M.I.; Tenuta, A.U.; Davis, V.M.; Esker, P.D.; Smith, D.L.; Licht, M.A.; Mueller, D.S. Effect of glyphosate application on sudden death syndrome of glyphosate-resistant soybean under field conditions. Plant Dis. 2015, 99, 347–354. [Google Scholar] [CrossRef]
- Reddy, K.N.; Cizdziel, J.V.; Williams, M.M.; Maul, J.E.; Rimando, A.M.; Duke, S.O. Glyphosate resistance technology has minimal or no effect on maize mineral content and yield. J. Agric. Food Chem. 2018, 66, 10139–10146. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.R.; Rech, R.; Duke, S.O.; Carvalho, L.B. Lack of effects of glyphosate and glufosinate on growth, mineral content, and yield of glyphosate- and glufosinate-resistant maize. GM Crops Food 2018, 9, 189–198. [Google Scholar] [CrossRef]
- Albrecht, A.J.P.; Albrecht, L.P.; Barroso, A.A.M.; Cesco, V.J.S.; Krenchinski, F.H.; Silva, A.F.M.; Placido, H.F.; Rodrigues, D.M.; Filho, R.V. Glyphosate tolerant soybean response to different management systems. J. Agric. Sci. 2018, 10, 204–216. [Google Scholar] [CrossRef]
- Moreira, A.; Moraes, L.C.C.; Futlan, T.; Heinrichs, R. Effect of glyphosate and zinc application on yield, soil fertility, yield components and nutritional status of soybean. Commun. Soil Sci. Plant Anal. 2016, 47, 1033–1047. [Google Scholar] [CrossRef]
- Duke, S.O.; Lydon, J.; Koskinen, W.C.; Moorman, T.B.; Chaney, R.L.; Hammerschmidt, R. Glyphosate effects on mineral nutrition, crop rhizosphere microbiota, and plant disease in glyphosate-resistant crops. J. Agric. Food Chem. 2012, 60, 10375–10397. [Google Scholar] [CrossRef]
- Duke, S.O.; Reddy, K.N. Is mineral nutrition of glyphosate-resistant crops altered by glyphosate treatment? Outlooks Pest Manag. 2018, 29, 206–209. [Google Scholar] [CrossRef]
- Kremer, R.J.; Means, N.E. Glyphosate and glyphosate-resistant crop interactions with rhizosphere microorganisms. Eur. J. Agron. 2009, 31, 153–161. [Google Scholar] [CrossRef]
- Johal, G.S.; Huber, D.M. Glyphosate effects on diseases of plants. Eur. J. Agron. 2009, 31, 144–152. [Google Scholar] [CrossRef]
- Williams, M.M.; Bradley, C.A.; Duke, S.O.; Maul, J.E.; Reddy, K.N. Goss’s wilt in sweet corn is independent of transgenic traits and glyphosate. HortSci. 2015, 50, 1791–1794. [Google Scholar] [CrossRef]
- Duke, S.O. Interaction of chemical pesticides and their formulation ingredients with microbes associated with plants and plant pests. J. Agric. Food Chem. 2018, 66, 7753–7761. [Google Scholar] [CrossRef]
- Duke, S.O. Glyphosate: Uses other than in glyphosate-resistant crops, mode of action, degradation in plants, and effects on non-target plants and agricultural microbes. Rev. Environ. Contamin. Toxicol. 2021, 255, 1–65. [Google Scholar] [CrossRef]
- Sidhu, R.S.; Hammond, B.G.; Fuchs, R.L.; Mutz, J.N.; Holden, L.R.; George, B.; Olson, T. Glyphosate-tolerant corn: The composition and feeding value from glyphosate-tolerant corn is equivalent to that of conventional corn (Zea mays L.). J. Agric. Food Chem. 2010, 48, 2305–2312. [Google Scholar] [CrossRef]
- Cerveira Junior, W.R.; Costa, Y.K.S.; Carbonari, C.A.; Duke, S.O.; Alves, P.L.C.A.; Carvalho, L.B. Growth, morphological, metabolic and photosynthetic responses of clones of eucalyptus to glyphosate. Forest Ecol. Manag. 2020, 470, 118218. [Google Scholar] [CrossRef]
- Nandula, V.K.; Reddy, K.N.; Rimando, A.M.; Duke, S.O.; Poston, D.H. Glyphosate-resistant and susceptible soybean (Glycine max) and canola (Brassica napus) dose response and metabolism relationships with glyphosate. J. Agric. Food Chem. 2007, 55, 3540–3545. [Google Scholar] [CrossRef]
- Chinnagounder, C.; Chandrasekaran, B.; Duraisamy, R.; Chinnasamy, N.; Kannan, S. Agronomic efficiency of herbicide tolerant crops in peninsular India—A review. J. Agric. Sci. Technol. 2020, 10, 317–326. [Google Scholar] [CrossRef]
- Hetherington, P.R.; Reynolds, T.L.; Marshall, G.; Kirkwood, R.C. The absorption, translocation and distribution of the herbicide glyphosate in maize expressing the CP-4 transgene. J. Exp. Bot. 1999, 50, 1567–1576. [Google Scholar] [CrossRef]
- Mahoney, K.; Nurse, R.E.; Everman, W.J.; Sprague, C.L.; Sikkema, P.H. Tolerance of corn (Zea mays L.) to early and late glyphosate applications. Amer. J. Plant Sci. 2014, 4, 49148. [Google Scholar] [CrossRef]
- De Araújo, G.V.; Albrecht, A.J.P.; Albrecht, L.P.; Wallace, H.; de Carvalho, P.; Migliavacca, R.A.; Silva, A.F.M. Effect of glyphosate and glufosinate on nutritional content and agronomic performance of maize possessing cp4epsps and pat transgenes. Austral. J. Crop Sci. 2021, 15, 773–779. [Google Scholar] [CrossRef]
- Yu, X.; Sun, Y.; Lin, C.; Wang, P.; Shen, Z.; Zhao, Y. Development of transgenic maize tolerant to both glyphosate and glufosinate. Agronomy 2023, 13, 226. [Google Scholar] [CrossRef]
- Correia, N.M.; Santos, E.S. Foliar levels of macro and micronutrients in glyphosate-tolerant corn submitted herbicides. Semina Ciên. Agrárias Londrina 2013, 34, 3165–3172. [Google Scholar] [CrossRef]
- De Oliveira Neto, S.S.; Bossolani, J.W.; de Freitas, S.E.; Gazola, B.; Gonçalves, A.S.F.; Zoz, T.; Calonego, J.C. Impact of glyphosate on morphophysiological traits of RR corn plants under drought stress. Acta Physiol. Plant 2023, 45, 28. [Google Scholar] [CrossRef]
- Tao, M.; Bai, X.; Zhang, J.; Wei, Y.; He, Y. Times series monitoring of transgenic maize seedlings phenotyping exhibiting glyphosate tolerance. Processes 2022, 10, 2206. [Google Scholar] [CrossRef]
- Belz, R.G.; Duke, S.O. Herbicide-mediated hormesis. Amer. Chem. Soc. Symp. Ser. 2017, 1249, 135–148. [Google Scholar]
- Brito, I.P.F.S.; Tropaldi, L.; Carbonari, C.A.; Velini, E.D. Hormetic effects of glyphosate on plants. Pest Manag. Sci. 2018, 74, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Jalal, A.; de Oliveira Junior, J.C.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.R.; dos Reis, A.R. Hormesis in plants: Physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef]
- Corrêa, E.L.; Dayan, F.E.; Owen, D.L.; Rimando, A.M.; Duke, S.O. Glyphosate-resistant and conventional canola (Brassica napus L.) responses to glyphosate and aminomethylphosphonic acid (AMPA) treatment. J. Agric. Food Chem. 2016, 64, 3508–3513. [Google Scholar] [CrossRef]
- Kepler, R.M.; Epp-Schmidt, D.; Yarwood, S.A.; Cavigelli, M.; Reddy, K.N.; Duke, S.O.; Bradley, C.; Williams, M.; Buyer, J.; Maul, J.E. Soil microbial communities in diverse agroecosystems exposed to the herbicide glyphosate. Appl. Environ. Microbiol. 2020, 86, e01744-19. [Google Scholar] [CrossRef]
- Barriuso, J.; Marín, S.; Mellado, R.P. Potential accumulative effect of the herbicide glyphosate of glyphosate-tolerant maize rhizobaterial communities over a three year cultivation period. PLoS ONE 2011, 6, e27558. [Google Scholar] [CrossRef]
- Ridley, W.P.; Sidhu, R.S.; Pyla, P.D.; Nemeth, M.A.; Breeze, M.L.; Astwood, J.D. Comparison of the nutritional profile of glyphosate-tolerant corn event NK603 with that of conventional corn (Zea mays L.). J. Agric. Food Chem. 2002, 50, 7235–7243. [Google Scholar] [CrossRef]
- Harrigan, G.G.; Ridley, W.P.; Riordan, S.G.; Nemeth, M.A.; Sorbet, R.; Trujillo, W.A.; Breeze, M.L.; Schneider, R.W. Chemical composition of glyphosate-tolerant soybean 40-3-2 grown in Europe remains equivalent with that of conventional soybean (Glycine max L.). J. Agric. Food Chem. 2007, 55, 6160–6168. [Google Scholar] [CrossRef] [PubMed]
- Cuhra, M. Review of GMO safety assessment studies: Glyphosate residues in Roundup Ready crops is an ignored issue. Environ. Sci. Eur. 2015, 27, 20. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G., Jr. Environmental and health effects of the herbicide glyphosate. Sci. Total Environ. 2018, 616, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.R.; de Souza, A.P.F.; Morais, P.P.P.; Braga, D.P.V.; Crivellari, A.C.; Favoretto, L.R.G.; Berger, G.U. Residues of glyphosate and aminomethylphosphonic acid (AMPA) in genetically modified glyphosate tolerant soybean, corn and cotton crops. Ciência Rural Santa Maria 2021, 51, 1. [Google Scholar] [CrossRef]
- Healy, C.; Hammond, B.; Kirkpatrick, J. Results of a 13-weed safety assurance study with rats fed grain from corn rootworm-protected glyphosate-tolerant MON 88017 corn. Food Chem. Toxicol. 2008, 46, 2517–2524. [Google Scholar] [CrossRef]
- Duke, S.O.; Rimando, A.M.; Pace, P.F.; Reddy, K.N.; Smeda, R.U. Isoflavone, glyphosate, and aminomethylphosphonic acid levels in seeds of glyphosate-treated, glyphosate-resistant soybean. J. Agric. Food Chem. 2003, 51, 340–344. [Google Scholar] [CrossRef]
- Bohm, G.M.B.; Rombaldi, C.V.; Genovise, M.I.; Castilhos, D.; Alves, B.J.R.; Rumjanek, N.G. Glyphosate effects on yield, nitrogen fixation, and seed quality in glyphosate-resistant soybean. Crop Sci. 2014, 54, 1737–7143. [Google Scholar] [CrossRef]
- Bøhn, T.; Cuhra, M.; Traavik, T.; Sanden, M.; Fagan, J.; Primicerio, R. Compositional differences in soybeans on the market: Glyphosate accumulates in Roundup Ready GM soybeans. Food Chem. 2014, 153, 207–215. [Google Scholar] [CrossRef]
- Bernal, L.; Martin, M.T.; Soto, M.E.; Nozal, M.J.; Marotti, I.; Dinelli, G.; Bernal, J.L. Development and application of liquid chromatography-mass spectrometry method to evaluate the glyphosate and aminomethylphosphonic acid dissipation in maize plants after foliar treatment. J. Agric. Food Chem. 2012, 60, 4017–4025. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.N.; Rimando, A.M.; Duke, S.O.; Nandula, V.K. Aminomethylphosphonic acid accumulation in plant species treated with glyphosate. J. Agric. Food Chem. 2008, 56, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Hearon, S.E.; Wang, M.; McDonald, T.J.; Phillips, T.D. Decreased bioavailability of aminomethylphosphonic acid (AMPA) in genetically modified corn with activated carbon or calcium montmorillonite clay inclusion in soil. J. Environ. Sci. 2021, 100, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Komoßa, D.; Gennity, I.; Sandermann, H. Plant metabolism of herbicides with C-P bonds: Glyphosate. Pestic. Biochem. Physiol. 1992, 43, 85–94. [Google Scholar] [CrossRef]
- Duke, S.O. Glyphosate degradation in glyphosate-resistant and -susceptible crops and weeds. J. Agric. Food Chem. 2011, 59, 5835–5841. [Google Scholar] [CrossRef]
- Zhou, F.-Y.; Han, H.; Han, Y.-J.; Nyporko, A.; Yu, Q.; Beckie, H.J.; Powles, S.B. Aldo-keto reductase may contribute to glyphosate resistance in Lolium rigidum. Pest Manag. Sci. 2023, 79, 1528–1537. [Google Scholar] [CrossRef]
- Pan, L.; Yu, Q.; Han, H.; Mao, L.; Nyporko, A.; Fan, L.J.; Bai, L.; Powles, S.B. Aldo-keto reductase metabolized glyphosate and confers glyphosate resistance in Echinochloa colona. Plant Physiol. 2019, 181, 1519–1534. [Google Scholar] [CrossRef]
- Reddy, K.N.; Duke, S.O.; Rimando, A.M. Aminomethylphosphonic acid, a metabolite of glyphosate, causes injury in glyphosate-treated, glyphosate-resistant soybean. J. Agric. Food Chem. 2004, 52, 5139–5143. [Google Scholar] [CrossRef]
- Hoagland, R.E. Effects of glyphosate on metabolism of phenolic compounds. VI. Effects of glyphosine and glyphosate metabolites on phenylalanine ammonia-lyase activity, growth, and protein, chlorophyll, and anthocyanin levels in soybean (Glycine max) seedlings. Weed Sci. 1980, 28, 393–400. [Google Scholar] [CrossRef]
- FAO/WHO. Codex Alimentarius. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/pesticide-detail/en/?p_id=158 (accessed on 23 February 2023).
- European Food Safety Authority. Commission Regulation (EU) No 293/2013 of 20 March 2013 Amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Emamectin Benzoate, Etofenprox, Etoxazole, Flutriafol, Glyphosate, Phosmet, Pyraclostrobin, Spinosad and Spirotetramat in or on Certain Products Text with EEA Relevance. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32013R0293 (accessed on 24 March 2023).
- Bromilow, R.H.; Chamberlain, K. The herbicide glyphosate and related molecules: Physicochemical and structural factors determining their phloem mobility. Pest Manag. Sci. 2000, 56, 368–373. [Google Scholar] [CrossRef]
- Ghanizadeh, H.; Harrington, K.C. Perspective: Root exudation of herbicides as a novel mode of herbicide resistance in weeds. Pest Manag. Sci. 2020, 76, 2543–2547. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bidóia, V.S.; Neto, J.C.d.S.; Maciel, C.D.d.G.; Tropaldi, L.; Carbonari, C.A.; Duke, S.O.; Carvalho, L.B.d. Lack of Significant Effects of Glyphosate on Glyphosate-Resistant Maize in Different Field Locations. Agronomy 2023, 13, 1071. https://doi.org/10.3390/agronomy13041071
Bidóia VS, Neto JCdS, Maciel CDdG, Tropaldi L, Carbonari CA, Duke SO, Carvalho LBd. Lack of Significant Effects of Glyphosate on Glyphosate-Resistant Maize in Different Field Locations. Agronomy. 2023; 13(4):1071. https://doi.org/10.3390/agronomy13041071
Chicago/Turabian StyleBidóia, Vitor Simionato, José Cristimiano dos Santos Neto, Cleber Daniel de Goes Maciel, Leandro Tropaldi, Caio Antonio Carbonari, Stephen Oscar Duke, and Leonardo Bianco de Carvalho. 2023. "Lack of Significant Effects of Glyphosate on Glyphosate-Resistant Maize in Different Field Locations" Agronomy 13, no. 4: 1071. https://doi.org/10.3390/agronomy13041071
APA StyleBidóia, V. S., Neto, J. C. d. S., Maciel, C. D. d. G., Tropaldi, L., Carbonari, C. A., Duke, S. O., & Carvalho, L. B. d. (2023). Lack of Significant Effects of Glyphosate on Glyphosate-Resistant Maize in Different Field Locations. Agronomy, 13(4), 1071. https://doi.org/10.3390/agronomy13041071





