Abstract
Light intensity and spectrum have a direct effect on the growth and development of plants and on the qualitative characteristics of their productions. LED technology seems to offer interesting prospects for its possible use in confined environments (growth chambers, bioreactors, greenhouses, etc). On the basis of these considerations, we tested the effects on the in vitro growth and development of micropropagated pineapple (Ananas comosus (L.) Merr.) plantlets using four different light spectra: (1) fluorescence light (FL—control); (2) white LED (WL—blue 20%, green 36%, red 38%, far red 6%, and UV 1%); (3) predominantly red LED (RL—blue 12%, green 19%, red 61%, and far red 8%); (4) Red/Blue LED (RL/BL—67% red and 33% blue) and two light intensities: 80 and 100 μmol m−2s−1 (16/8 h light/dark photoperiod). A. comosus showed a different morphogenetic response to the light spectra and their intensity and to their interaction. Among the LED lights to date tested, at 80 μmol m−2s−1 the best results on shoot multiplication were obtained under the RL light and to a lesser extent under the WL. This last treatment resulted also in a significant improvement in shoot quality in terms of secondary shoot dry weight and total chlorophyll concentration when compared to all the other light spectra tested.
1. Introduction
The first applications of light-emitting diodes (LEDs) in the mid-1980s were addressed to support the enhancement of novel lighting systems specifically designed for the National Aeronautics and Space Administration (NASA) to carry out researches on plant growth in a space station [1]. In recent years, LEDs have shown promise in the domain of plant lighting for controlled agricultural environments, such as greenhouses, as well as for in vitro cultures in growth chambers.
These last depend entirely upon artificial lighting for all their needs. When compared to the traditional light sources used for in vitro propagation (fluorescent lamps-FLs), LEDs possess advantages, such as the possibility to control spectral composition, wavelength specificity, reduced heat radiation (the heat generated is negligible when compared to fluorescent lamps), greater durability, and a much lower power consumption [2].
LEDs for in vitro cultures are able to supply light within the spectral range crucial for photosynthesis [3,4,5,6] and in the photo-morphogenic responses [7] without wasting energy on nonproductive wavelengths [1]. Metabolite productions may be also enhanced by choosing the appropriate LED illumination [8].
LED illumination has been observed to induce a variety of desired morphological and/or physiological changes in in vitro plants, such as the inflorescence differentiation [9]. Shoot proliferation is the most crucial stage of the micropropagation process and the success of a protocol largely depends on the rate and modality of this multiplication [10]. In the recent past, several studies concerned with the effects of the spectral quality of LED systems on in vitro plantlets have been carried out. The positive effects of LED lamps on in vitro proliferation have been reported for several different species, such as apple rootstocks [11,12], Euphorbia mili and Spathiphyllum cannifolium [13], Anthurium andreanum Lind [14], Banana [15], Brassica napus [16], Populus euroamericana [17], Saccharum spp. [18], Chrysanthemum × grandiflorum, Gerbera jamesonii, Heuchera × hybrida, Ficus benjamina, and Lamprocapnos spectabilis [19] among others. The most used LED colors or combinations for in vitro culture are white, red, blue, and mixture rates of both blue and red.
However, contrasting results are often evidenced when fluorescent white lamps and LED with different light spectra are compared. These results could be attributed to the variability of light requirements among each genus and species [20], to the interaction with the different light intensities, and to the plant growth regulators in the medium [7]. For these reasons, even if the LEDs lamps require about 32% less energy than the fluorescent tubes per μmol m−2 s−1 delivered to the plants [21], many tissue culture laboratories hesitate to replace conventional lighting systems with LEDs out of the apprehension of an unpredictable and aberrant in vitro morphology [22]. In a recent review [23] the authors suggested that the significant variability regarding the effects of light in in vitro research could be attributed to the lack of clear experimental protocols, unlike in in vivo studies [21]. Among other concerns, the following issues are particularly noteworthy: (i) The experiments on light spectra, which typically cover only one propagation cycle, have a short timescale that severely restricts the understanding of the effects of light quality on the stability of in vitro shoot proliferation (especially with regard to the red light effects); (ii) The quality and quantity of the applied exogenous growth regulators (especially cytokinins) since they interfere with the effects of some light spectra. Hence, further studies need to determine the optimal LED illumination for the optimal in vitro propagation rate for each plant species, using a protocol which implies at least three growing cycles and a very low cytokinin content in the substrate.
Pineapple (Ananas comosus (L.) Merr.) is a very important fruit in tropical and subtropical regions, largely consumed all over the world. Its propagation is mainly carried out asexually using vegetative material. Micropropagation has been recognized to offer interesting prospects for pineapple genetic improvement, or for the commercial production of plantlets [24]. In spite of obtaining high in vitro multiplication rates, pineapple plantlets production by micropropagation is rather expensive and the reduction in production cost is still a major goal [25]. With this in mind, the present research investigated the combined effects of four light spectra and two light intensities on in vitro shoot proliferation of pineapple throughout three proliferation cycles.
2. Materials and Methods
2.1. Plant Material and Culture Initiation
Pineapple apices were excised and sterilized by the immersion in 70% ethanol for 5 min, then in sodium hypochlorite (2.3% (v/v) active chlorine) for 20 min. The explants were rinsed in sterilized water under a sterile laminar airflow. The shoots were grown on MS culture medium [26], vitamins of Morel, 3% (w/v) sucrose, and solidified with 0.7% (w/v) agar (Lickson agar, Palermo, Italy). The only plant growth regulator in the medium was 0.5 mg L−1 6-benzylaminopurine (BA, Micropoli, Cesano Boscone, Italy). The pH was adjusted to 5.8.
Plantlets were then in vitro propagated in round Microbox containers (Micropoli, Cesano Boscone, Italy) under controlled environmental conditions at 23 ± 1 °C and a 16/8 h light/dark photoperiod provided by cool white-fluorescent lamps (80 μmol m−2 s−1) until the desired number of plantlets, to start the experiments, were obtained (generally from 10 to 12 weeks).
2.2. Light Treatments
The plantlets, previously propagated, were subjected to four different light spectra: (1) fluorescence light (FL—MASTER-TLD Super 80 36W/865, Philips, Eindhoven, The Nehterlands); (2) white LED (WL—blue 20%, green 36%, red 38%, far red 6%,and UV 1%—(Grow Light C65 NS12—Valoya Oy, Helsinki, Finland); (3) predominantly red LED (RL—blue 10%, green 19%, red 63%,and far red 8%—(Grow Light C65 AP673L-Valoya Oy, Helsinki, Finland); (4) Red/Blue LED (RL/BL, 67% red and 33% blue—(BS Biosystem, Catania, Italy). Two photosynthetic photon flux densities (PPFD) were also tested: 80 (I80) and 100 (I100) μmol m−2s−1. The light intensity from the various LED lamps was verified using a calibrated spectroradiometer LI-190R (LICOR Biosciences, LiCor, Inc., Lincoln, NE, USA) placed horizontally in the cabinets used for the experiments.
To test the stability of the light treatments, the shoots propagated under each light treatment were subjected to three consecutive proliferation cycles, each were of about 60 days duration, according to the species growth dynamics.
The growth conditions were 23 ± 1 °C temperature and a 16/8 h light/dark photoperiod using the same culture medium described above.
2.3. Data Collection
At the end of each cycle, the number, dry weight, chlorophyll concentration of the secondary shoots, and projected canopy index (PCI), were recorded for each light treatment.
The shoots were weighed for the determination of fresh weight, then, the sample was oven-dried to a constant weight at 105 °C to determine the dry weight.
The leaf chlorophyll was extracted and determined using the method described by [27]. The chlorophylls were overnight extracted in 5 mL of pure methanol at 4 °C in the dark from 0.2 g plant tissue. After the extraction, the total chlorophyll (a+b) concentration (CC, mg g−1 on a fresh weight basis) was measured using the absorbance UV-VIS spectrophotometer (ONDA UV-30 SCAN) in the wavelength of 652.4 and 665.2 nm, and then calculated using the following equation:
This parameter was determined only throughout two out of the three growing cycles.
A non-destructive method was developed to evaluate the projected canopy area covered by the growth of the in vitro plantlets. This method is based on the 2D image acquisition and the following elaboration by the open source Java image processing program ImageJ software (Research Services Branch, Bethesda, Md—Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, MD, USA, https://imagej.nih.gov/ij/, 1997–2018—accessed on 6 April 2023). The plant canopy measurement main flows include a 2D image acquisition, plant pixels separation from the background in a binary output. A two mega pixel CMOS camera (iDS uEye—IDS Imaging Development Systems GmbH, Obersulm, Germany) with a 12 mm focal length Pentax Tv lens, was mounted above the jar to take images from the top view in the RGB spectral range. A hue and saturation threshold were applied to refine the background and then converted to a binary B/W image to count the area covered by the white color (plantlet) and the black color (background).
Based on the abovementioned process, a projected canopy index (PCI) was calculated according to the following formula:
where FCi is the covered area at the end of each cycle and IC0 is the covered area at the beginning of the growth cycle.
2.4. Statistical Analysis
Using a factorial experimental design with four replications, the effects of both the light spectra and light intensity were evaluated on the shoot proliferation and plantlet growth. To this end, we applied a mixed model ANOVA with light spectra (WL, RL, RL/BL, and FL) and the light intensities (I80 and I100 μmol m−2s−1) as fixed factors and the growing cycle (G_Cycle) as a random factor, running the statistical analyses in R 4.2.2 (R Development Core Team 2016) and using the libraries car, lme4, and nlme. The random effects were included for each light spectra and intensity to control for the possible dependence due to the cycle effects. The Tukey post-hoc pairwise comparison tests was performed by means of the glht function from the multcomp package. The ranova function from the lmerTest package tested the random effects in the model, computing an ANOVA-like table with tests of the random-effect term in the model. The random-effect term is removed when the chi-squared test resulted non-significant.
3. Results
3.1. Secondary Shoot Number
The significant differences in this parameter were detected in relation to the light spectrum, intensity, and their interaction (Table 1).

Table 1.
Mixed model ANOVA (F-statistics) and means of four light spectra (L), two light intensities (I), and propagation cycle for number of secondary shoots (NSS), weight of secondary shoots (WSS), projected canopy index (PCI), and leaflets’ chlorophyll concentration (Chla+b) of pineapple.
The lower light intensity (80 μmol m−2s−1) was significantly more effective (1.63 secondary shoot plant−1 on average) than the higher one (100 μmol m−2s−1−0.94 secondary shoot plant−1 on average) in determining a higher number of secondary shoots. At the lower intensity, the Tukey post-hoc test revealed that the RL determined a significant increase in the secondary shoot number per plant (2.9) when compared to the other light treatments (Figure 1) except for the white one. In both cases, the number of shoots significantly exceeded the fluorescence one. At the higher light intensity, no significant differences were detected among the different light treatments.
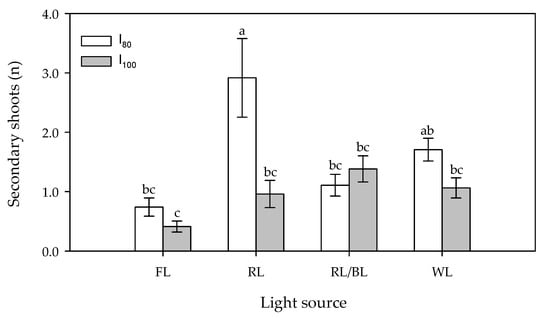
Figure 1.
Effect of light source (FL = Fluorescence, RL = predominantly Red, RL/BL = Red/Blue and WL = White light) and light intensity (I80 and I100 = 80 and 100 μmol m−2s−1, respectively) on in vitro secondary shoot number per plant. Different letters indicate significant differences for p < 0.05, error bars indicate standard error of the means.
No effect on the proliferation cycles of this character was ascertained.
3.2. Secondary Shoots Dry Weight
The ANOVA revealed that the dry weight of the in vitro secondary shoots was significantly influenced only by the light spectrum (F3,64 = 5.32, p < 0.001—Figure 2), whereas neither the Light Intensity nor the interaction affected this parameter. In the average of the light intensities, the RL determined a significantly lower secondary shoot dry weight (7.5 mg) when compared to the other lamps, which gave undifferentiated values (19.6 mg on average) (Table 1). The secondary shoot dry weight, determined by each treatment, was stable along the three proliferation cycles.
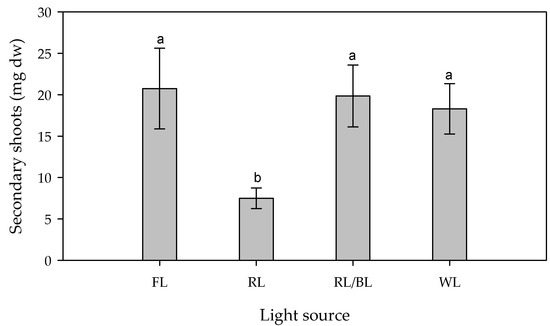
Figure 2.
Effect of different light source (FL = Fluorescence, RL = predominantly Red, RL/BL = Red/Blue, and WL = White lights) on the dry weight (mg) of in vitro secondary shoots. Different letters indicate significant differences for p < 0.05, error bars indicate standard error of the means.
3.3. Projected Canopy Index (PCI)
The significant differences were detected in relation to the LED spectra, light intensity and their interaction.
The RL/BL light treatment determined the highest coverage at both light intensities, followed by the RL treatment at 80 μmol m−2s−1 (Figure 3). The lowest surface area was determined by the RL treatment at 100 μmol m−2s−1.
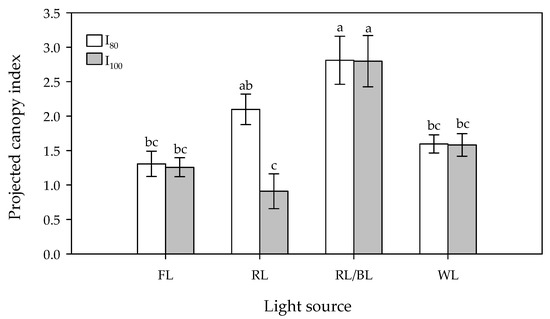
Figure 3.
Effect of light source (FL = Fluorescence, RL = predominantly Red, RL/BL = Red/Blue, and WL = White lights) and light intensity (I80 and I100 = 80 and 100 μmol m−2s−1, respectively) on in vitro projected canopy index. Different letters indicate significant differences for p < 0.05, error bars indicate standard error of the means.
However, with the only exception of the RL treatment, no differences were registered in relation to the two light intensities at each light spectrum. It is noticeable that almost all the LED lights treatments gave similar (WL) or higher (RLxI80 and RL/BL at both light intensity) coverages than the FL.
Even for the PCI, no significant effect on theproliferation cycles was ascertained.
3.4. Chlorophyll Concentration
Regarding the propagation cycle, the results of the ANOVA-like table for the random effects, showed significant differences between one cycle and the next one (Figure 4). The second propagation cycle results in a higher Chl concentration in the pineapple leaves, except for the WL, where a decrease was recorded. However, even in this case, a significantly higher chlorophyll concentration was observed when compared to the other lamps. The significant interaction between both the light spectrum and intensity (Table 1, Figure 5) showed that the highest chlorophyll concentrations (97 mg g−1) were registered under the WL at the lower intensity (I80) and to a lower extent (69 mg g−1) at the I100.
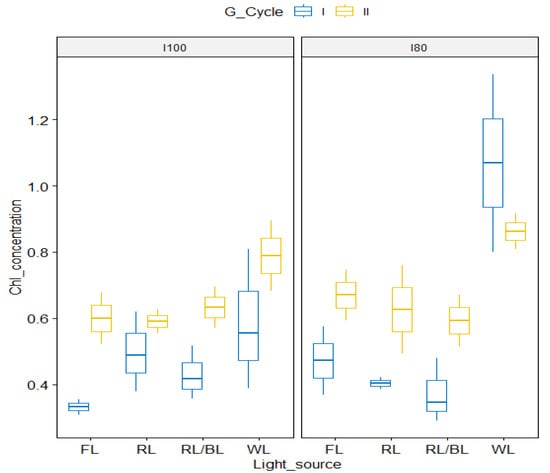
Figure 4.
Box plot of chlorophyll concentration in the two growing cycles as influenced by light spectra in relation to 100 μmol m−2s−1 (left panel) and the 80 μmol m−2s−1 (right panel) lower light intensity.
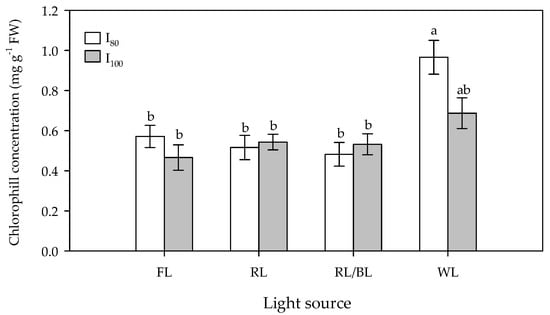
Figure 5.
Effect of light source (FL = Fluorescence, RL = predominantly Red, RL/BL = Red/Blue, and WL = White lights) and light intensity (I80 and I100 = 80 and 100 μmol m−2s−1, respectively) on total chlorophyll concentration (mg g−1) of in vitro pineapple leaves. Different letters indicate significant differences for p < 0.05, error bars indicate standard error of the means.
The lower and undifferentiated chlorophyll concentrations were registered under fluorescence and the other tested light spectra at both the light intensities (Figure 5).
4. Discussion
The selection of the optimal light intensity and spectra are a key feature to support both the effective proliferation and growth in vitro. Light intensity is involved in pigment formation and plays a crucial role in regulating the size of the leaves and stems, as well as their morphogenic pathways [28]. In vitro plants are very susceptible to high light conditions [29] and are prone to photoinhibition [30].
In pineapple, a significant interaction in light spectrum × intensity, was ascertained in the present research and showed the decisive influence of light intensity in modulating the effects of the different spectra on in vitro secondary shoot proliferation. The RL and to a lesser extend the WL determined a significant increase in the secondary shoots but only at the lower light intensity (I80). This last intensity also determined a positive effect on the chlorophyll content under the WL. The optimal light intensity for in vitro plantlets is species-specific. A hypothesis [31] suggests that the light conditions in the natural habitats of various species could provide insight into the light requirements for the optimal in vitro growth conditions, albeit on a significantly different scale. An extensive review of the literature [23] revealed that the most used light intensities ranged from 20 to 80 μmoles m−2 s−1 and only few species showed a better growth at intensities equal or exceeding 100 μmoles m−2 s−1 (eg. Actinidia deliciosa [32] and Momordica grosvenorii [33]). Our data showed that, even if pineapple is a species that grows under intense illumination in vivo, a light intensity of 100 μmoles m−2 s−1 results in a decrease in the proliferation rates. Similar adverse effects were observed on the total chlorophyll under the WL. Similarly, in Plectranthus amboinicus [34] and in sugar maple (Acer saccharum Marsh.) [35], high irradiation (>94 and 40 μmoles m−2 s−1, respectively) led to a reduced concentrations of a, b, and the total chlorophyll. The reduction, determined by the light excess, could be attributed to the production of free radicals, which degrade the photosynthetic active pigments [36].
Concerning the effects of the light spectra, in this study the best results for secondary shoot propagation were obtained under Red enriched Light (RL) conditions and to a lesser extent under WL. These outcomes agrees with the results of different authors [19,23] reporting the enhancing role of Red and the Red/Far-Red ratio (RL:FRL) on shoot proliferation in many plant species. The primary effects of red light are associated with the activating role of phytochrome in the production of cytokinins in tissues. This counters the actions of auxins and promotes the development of secondary shoots. The RL also regulates the production of carotenoids and strigolactones, which appear to control apical dominance by altering the flow of auxins [37]. The stimulating effects of RL appear to occur during the early stages of the multiplication phases.
For these reasons, the effects of the exposure to higher quantities of red light, on the formation of new shoots and lateral buds, have been evidenced with low cytokinin concentrations in culture media [38]. The latter outcome seems to confirm the opportunity of maintaining a low quantity of these plant growth regulators in the medium to assess the effects of the different light spectra and especially of the red one [23].
Red enriched light (RL), applied in this research, determined an increase in shoot multiplication but also a significant reduction in shoot weight when compared to the plants grown under WL and RL/BL LEDs. The scanning electron microscope studies in lettuce have revealed that cultures, when continuously incubated under red light, resulted in the development of numerous minute shoot primordia, which were characterized by slower growth rates than those incubated under white light [39]. Moreover, among the tested treatments, the RL/BL determined the highest coverage at both the light intensities followed by the RL at the lower intensity. Many authors agree that RL, when compared to other light spectra, promotes leaf growth [17,40]. However, different reports indicated that RL has a low ability to activate the pathway of chlorophyll and carotenoids biosynthesis [41] and may result in leaf disorders, weak and abnormal plantlets in strawberry [42], elongated but fragile stems in chrysanthemums [43]; the so-called Red Light Syndrome [44].
In this study, WL determined the highest chlorophyll concentrations at both light intensities. Similarly, previous studies have reported a higher chlorophyll content in treatments involving white light [23]. Moreover, it was observed in Cattleya hybrid orchids grown under white light had higher levels of chlorophyll and carotenoid pigments than those obtained in plants grown under other types of light.
The positive effects of WL treatment on chlorophyll concentration may be attributed to the higher quantity of blue and green light present in these lamps when compared to the RL. Blue light is reported to enhance chlorophyll synthesis and chloroplast development. Hence, a minimum threshold of blue light is necessary for normal in vitro plant growth [3,45]. In addition, recent evidence has suggested the significant role of green light. The incorporation of green light to the combination of red and blue light has been shown to contribute to the proliferation, growth, and development of certain in vitro cultures [46]. Green light has been found to regulate various biological, morphological, and physiological processes in both in vitro and in vivo trials [2]. For these reasons, some species showed improved performances when exposed to white lights (WLs), containing all the aforementioned wavelengths [47,48]. Therefore, it is recommended to use white lamps with an optimal spectral composition in the most beneficial wavelengths, including blue, red, and green light.
Although white light (WL) may not be as efficient as red light (RL) in promoting secondary shoot induction and overcoming apical dominance, adding cytokinins to the growth medium can result in high and consistent proliferation rates.
In some instances, it may be recommended to expose the plants to at least two weeks of red light irradiation, followed by growth under WL [49]. In previous studies, when at 80 μmol m−2s−1 plants were transferred from the red light to the white one, a significantly higher secondary shoot number resulted when compared to all the other treatments (unpublished data). In species that are challenging to regenerate in vitro and/or highly sensitive to higher concentrations of cytokinins in the medium, such as Euphorbia milii and Ceratonia siliqua L., an initial application of stimulating red light (RL) or enriched RL, followed by white light (WL), may enhance the proliferation and somatogenesis [50]. Furthermore, exposing plants to a period of RL:FRL followed by WL could potentially decrease the need for exogenous plant growth regulators, particularly cytokinins. This reduction could be beneficial for promoting the subsequent phases of in vitro cultivation, such as rooting and acclimatization [23].
Moreover, since micropropagation is considered an effective large scale propagation method, the effectiveness of a micropropagation protocol depends on the proliferation rate and stability over the different growth cycles. For these reasons, we hypothesized [23] that the effects of light treatments must be verified at least for three different growing cycles, to avoid the possible progressive adverse effects, mainly under prevalent red light ratios. The present research demonstrated a substantial stability over the cycles, in three out of the four characters analyzed (number, weight of secondary shoots, and projected canopy index). The only changes concerned the almost uniform rise of the chlorophyll content during the second growing cycle along all the light treatments which, however, does not substantially modify the light treatment behaviors observed during the first cycle.
The LED lamps determined similar or improved effects on secondary shoot proliferation and quality when compared to the fluorescent ones.
5. Conclusions
Light plays a fundamental but often underestimated role on the in vitro proliferation of micropropagated plant species. The results obtained in this research stressed the importance of choosing the right light intensity and spectra to enhance the impacts of light on in vitro micropropagation of pineapple plantlets.
The best results on in vitro shoot proliferation were obtained at 80 μmol m−2s−1, under the RL and to a lesser extent the WL. In both cases, the number of shoots significantly exceeded the FL one. At the same intensity, the WL also induced a better plantlet development (higher dry shoot weight and chlorophyll concentration when compared to the red enriched lamps). Thus, the use of the WL LED spectrum, at the appropriate intensity, seems to contribute to the substantial improvements in the micropropagation of pineapple when compared to fluorescence. However, in species that are particularly difficult to regenerate in vitro and/or show a high sensibility to a higher concentration of citokinins, a few days exposure to the red enriched light may exert a useful initial stimulatory effect on shoot proliferation, whereas the subsequent exposure to WL may ensure the subsequent plantlet growth.
Due to the species-specific response, it is crucial to identify the optimal wavelength and intensity of light to promote growth and development of in vitro plantlets. Therefore, detailed studies on the interaction between LEDs, their degrading-promoting effects and the stability of plant proliferation are required for each species. A careful management of these factors can ultimately lead to a more successful in vitro propagation.
Author Contributions
Conceptualization, V.C.; methodology, G.A., V.C. and A.I.; writing—original draft preparation, V.C. and A.I.; writing—review and editing, V.C., A.I., G.A., A.P. and G.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data will be made available at request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morrow, R.C. LED Lighting in Horticulture. Hortscience 2008, 43, 1947–1950. [Google Scholar] [CrossRef]
- Dutta Gupta, S.; Agarwal, A. Influence of LED lighting on in vitro plant regeneration and associated cellular redox balance. In Light Emitting Diodes for Agriculture; Dutta Gupta, S., Ed.; Springer: Singapore, 2017; pp. 273–303. [Google Scholar]
- Hahn, E.J.; Kozai, T.; Paek, K.Y. Blue and red light-emitting diodes with or without sucrose and ventilation affect in vitro growth of Rehmannia glutinosa plantlets. J. Plant Biol. 2000, 43, 247–250. [Google Scholar] [CrossRef]
- Kim, H.H.; Goins, G.D.; Wheeler, R.M.; Sager, J.C. Green-light supplementation for enhanced lettuce growth under red-and blue-light-emitting diodes. HortScience 2004, 39, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.W.; Shin, K.S.; Kim, S.K.; Paek, K.Y. Light quality affects in vitro growth of grape Teleki 5BB. J. Plant Biol. 2006, 49, 276–280. [Google Scholar] [CrossRef]
- Bello-Bello, J.J.; Perez-Sato, J.A.; Cruz-Cruz, C.A.; Martinez-Estrada, E. Light-emitting diodes: Progress in plant micropropagation. In Chlorophyll; Jacob-Lopes, E., Zepka, L.Q., Queiroz, M.I., Eds.; InTech Open: London, UK, 2017; pp. 93–103. [Google Scholar]
- Kurilčik, A.; Miklušytė-Čanova, R.; Dapkūnienė, S.; Žilinskaitė, S.; Kurilčik, G.; Tamulaitis, G.; Duchovskis, P.; Žukauskas, A. In vitro culture of Chrysanthemum plantlets using light-emitting diodes. Open Life Sci. 2008, 3, 161–167. [Google Scholar] [CrossRef]
- Hashim, M.; Ahmad, B.; Drouet, S.; Hano, C.; Abbasi, B.H.; Anjum, S. Comparative effects of different light sources on the production of key secondary metabolites in plants in vitro cultures. Plants 2021, 10, 1521. [Google Scholar] [CrossRef]
- Dewir, Y.; Chakrabarty, D.; Hahn, E.; Paek, K. The effects of paclobutrazol, light emitting diodes (LEDs) and sucrose on flowering of Euphorbia millii plantlets in vitro. Eur. J. Hortic. Sci. 2006, 71, 240. [Google Scholar]
- Cavallaro, V.; Patane, C.; Cosentino, S.L.; Di Silvestro, I.; Copani, V. Optimizing in vitro large scale production of giant reed (Arundo donax L.) by liquid medium culture. Biomass Bioenergy 2014, 69, 21–27. [Google Scholar] [CrossRef]
- Muleo, R.; Morini, S. Light quality regulates shoot cluster growth and development of MM106 apple genotype in vitro culture. Sci. Hortic. 2006, 108, 364–370. [Google Scholar] [CrossRef]
- Muleo, R.; Morini, S. Physiological dissection of blue and red light regulation of apical dominance and branching in M9 apple rootstock growing in vitro. J. Plant Physiol. 2008, 165, 1838–1846. [Google Scholar] [CrossRef] [PubMed]
- Dewir, Y.H.; Chakrabarty, D.; Kim, S.J.; Hahn, E.J.; Paek, K.Y. Effect of light-emitting diode on growth and shoot proliferation of Euphorbia millii and Spathiphyllum cannifolium. Hortic. Environ. Biotechnol. 2005, 46, 375–379. [Google Scholar]
- Martínez-Estrada, E.; Caamal-Velázquez, J.H.; Morales-Ramos, V.; Bello-Bello, J. Light emitting diodes improve in vitro shoot multiplication and growth of Anthurium andreanum Lind. Propag. Ornam. Plants 2016, 16, 3–8. [Google Scholar]
- Waman, A.A.; Bohra, P.; Sathyanarayana, B.; Umesha, K.; Gowda, B.; Ashok, T.H. In vitro shoot multiplication and root induction in silk banana variety nanjanagud rasabale as influenced by monochromatic light spectra. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2016, 86, 577–584. [Google Scholar] [CrossRef]
- Li, H.; Tang, C.; Xu, Z. The effects of different light qualities on rapeseed (Brassica napus L.) plantlet growth and morphogenesis in vitro. Sci. Hortic. 2013, 150, 117–124. [Google Scholar] [CrossRef]
- Kwon, A.; Cui, H.Y.; Lee, H.; Shin, H.; Kang, K.S.; Park, S.Y. Light quality affects shoot regeneration, cell division, and wood formation in elite clones of Populus euramericana. Acta Physiol. Plant 2015, 37, 65. [Google Scholar] [CrossRef]
- Ferreira, L.T.; de Araújo Silva, M.M.; Ulisses, C.; Camara, T.R.; Willadino, L. Using LED lighting in somatic embryogenesis and micropropagation of an elite sugarcane variety and its effect on redox metabolism during acclimatization. Plant Cell Tissue Organ Cult. 2017, 128, 211–221. [Google Scholar] [CrossRef]
- Miler, N.; Kulus, D.; Woźny, A.; Rymarz, D.; Hajzer, M.; Wierzbowski, K.; Nelke, R.; Szeffs, L. Application of wide-spectrum light-emitting diodes in micropropagation of popular ornamental plant species: A study on plant quality and cost reduction. Vitr. Cell. Dev. Biol. Plant 2019, 55, 99–108. [Google Scholar] [CrossRef]
- Dutta Gupta, S.; Jatothu, B. Fundamentals and applications of light-emitting diodes (LEDs) in vitro plant growth and morphogenesis. Plant Biotechnol. Rep. 2013, 7, 211–220. [Google Scholar] [CrossRef]
- Shukla, M.R.; Singh, A.S.; Piunno, K.; Saxena, P.K.; Jones, A.M.P. Application of 3D printing to prototype and develop novel plant tissue culture systems. Plant Methods 2017, 13, 6. [Google Scholar] [CrossRef]
- Lotfi, M.; Mars, M.; Werbrouck, S. Optimizing pear micropropagation and rooting with light emitting diodes and trans-cinnamic acid. Plant Growth Regul. 2019, 88, 173–180. [Google Scholar] [CrossRef]
- Cavallaro, V.; Pellegrino, A.; Muleo, R.; Forgione, I. Light and Plant Growth Regulators on In Vitro Proliferation. Plants 2022, 11, 844. [Google Scholar] [CrossRef]
- Reinhardt, D.H.R.C.; Bartholomew, D.P.; Souza, F.V.D.; Carvalho, A.C.P.P.D.; Pádua, T.R.P.D.; Junghans, D.T.; Matos, A.P.D. Advances in pineapple plant propagation. Rev. Bras. Frutic. 2018, 40, e302. [Google Scholar] [CrossRef]
- Usman, I.S.; Abdulmalik, M.M.; Sani, A.L.A.; Muhammad, A.S. Development of an efficient protocol for micropropagation of pineapple (Ananas comosus L. var. Smooth Cayenne). Afr. J. Agric. Res. 2013, 8, 2053–2056. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bioassay with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Debergh, P.; Aitken-Christie, J.; Cohen, D.; Grout, B.; Von Arnold, S.; Zimmerman, R.; Ziv, M. Reconsideration of the term ‘vitrification’ as used in micropropagation. Plant Cell Tissue Organ Cult. 1992, 30, 135–140. [Google Scholar] [CrossRef]
- Semorádová, Š.; Synková, H.; Pospíšilová, J. Responses of tobacco plantlets to change of irradiance during transfer from in vitro to ex vitro conditions. Photosynthetica 2002, 40, 605–614. [Google Scholar] [CrossRef]
- Kadlecek, P.; Rank, B.; Tichá, I. Photosynthesis and photoprotection in Nicotiana tabacum L. in vitro grown plantlets. J. Plant Physiol. 2003, 160, 1017–1024. [Google Scholar] [CrossRef]
- Marks, T.; Simpson, S. Effect of irradiance on shoot development in vitro. Plant Growth Regul. 1999, 28, 133–142. [Google Scholar] [CrossRef]
- Infante, R.; Magnanini, E.; Righetti, B. The role of light and CO2 in optimising the conditions for shoot proliferation of Actinidia deliciosa in vitro. Physiol. Plant 1989, 77, 191–195. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, D.; Ma, Z.; Li, X.; Xiao, Y. Growth and photosynthetic capability of Momordica grosvenori plantlets grown photoautotrophically in response to light intensity. HortScience 2009, 44, 757–763. [Google Scholar] [CrossRef]
- Silva, S.T.; Bertolucci, S.K.V.; da Cunha, S.H.B.; Lazzarini, L.E.S.; Tavares, M.C.; Pinto, J.E.B.P. Effect of light and natural ventilation systems on the growth parameters and carvacrol content in the in vitro cultures of Plectranthus amboinicus (Lour.) Spreng. Plant Cell Tissue Organ Cult. 2017, 129, 501–510. [Google Scholar] [CrossRef]
- Singh, A.S.; Jones, A.M.P.; Shukla, M.R.; Saxena, P.K. High Light intensity stress as the limiting factor in micropropagation of sugar maple (Acer saccharum Marsh.). Plant Cell Tissue Organ Cult. 2017, 129, 209–221. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Fisiologia Vegetal; Universitat Jaume I: Castellón, Spain, 2006; Volume 10. [Google Scholar]
- Teichmann, T.; Muhr, M. Shaping plant architecture. Front. Plant Sci. 2015, 6, 233. [Google Scholar] [CrossRef] [PubMed]
- Herrington, E.; McPherson, J.C. Light quality growth promotion of Spiraea nipponica: The influence of a low photon fluence rate and transfer time to a higher fluence rate. Plant Cell Tissue Organ Cult. 1993, 32, 161–167. [Google Scholar] [CrossRef]
- Hunter, D.C.; Burritt, D.J. Light quality influences adventitious shoot production from cotyledon explants of Lettuce (Lactuca sativa L.). Vitr. Cell. Dev. Biol. Plant 2004, 40, 215–220. [Google Scholar] [CrossRef]
- Wu, H.C.; Lin, C.C. Red light-emitting diode light irradiation improves root and leaf formation in difficult to propagate Protea cynaroides L. plantlets in vitro. HortScience 2012, 47, 1490–1494. [Google Scholar] [CrossRef]
- Da Silva, M.; Debergh, P. The effect of light quality on the morphogenesis of in vitro cultures of Azorina vidalii (Wats.) Feer. Plant Cell Tissue Organ Cult. 1997, 51, 187–193. [Google Scholar] [CrossRef]
- Nhut, D.T.; Takamura, N.T.; Watanabe, H.; Tanaka, M. Light emitting diodes (LEDs) as a radiation source for micropropagation of strawberry. In Transplant Production in the 21st Century; Kubota, C., Chun, C., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 114–118. [Google Scholar]
- Kim, S.J.; Hahn, E.J.; Heo, J.W.; Paek, K. Effects of LEDs on net photosynthetic rate, growth and leaf stomata of chrysanthemum plantlets in vitro. Sci. Hortic. 2004, 101, 143–151. [Google Scholar] [CrossRef]
- Trouwborst, G.; Hogewoning, S.W.; van Kooten, O.; Harbinson, J.; van Ieperen, W. Plasticity of photosynthesis after the ‘red light syndrome’in cucumber. Environ. Exp. Bot. 2016, 121, 75–82. [Google Scholar] [CrossRef]
- Cybularz-Urban, T.; Hanus-Fajerska, E.; Swiderski, A. Effect of light wavelength on in vitro organogenesis of a Cattleya hybrid. Acta Biol. Crac. Ser. Bot. 2007, 49, 113–118. [Google Scholar]
- Golovatskaya, I.; Karnachuk, R.A. Role of green light in physiological activity of plants. Russ. J. Plant Physiol. 2015, 62, 727–740. [Google Scholar] [CrossRef]
- Karataş, M.; Aasim, M.; Dazkirli, M. Influence of light-emitting diodes and benzylaminopurin on adventitious shoot regeneration of water hyssop (Bacopa monnieri (L.) Pennell) in vitro. Arch. Biol. Sci. 2016, 68, 501–508. [Google Scholar] [CrossRef]
- Wilken, D.; Jiménez Gonzalez, E.; Gerth, A.; Gómez-Kosky, R.; Schumann, A.; Claus, D. Effect of immersion systems, lighting, and TIS designs on biomass increase in micropropagating banana (Musa Spp. Cv.’Grande Naine’AAA). Vitr. Cell. Dev. Biol. Plant 2014, 50, 582–589. [Google Scholar] [CrossRef]
- Kadkade, P.; Jopson, H. Influence of light quality on organogenesis from the embryo-derived callus of Douglas fir (Pseudotsuga menziesii). Plant Sci. Lett. 1978, 13, 67–73. [Google Scholar] [CrossRef]
- D’Onofrio, C.; Morini, S.; Bellocchi, G. Effect of light quality on somatic embryogenesis of quince leaves. Plant Cell Tissue Organ Cult. 1998, 53, 91–98. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).