Abstract
Trace element malnutrition causes the development of chronic degenerative diseases. The consumption of minerals and other compounds of biochemical origin through the intake of vegetables can attenuate these deficiencies to a great extent. Because the content in the plant depends on the conditions where it develops, there are still deficiencies that should be taken into consideration. For example, in Mexico, the intake of selenium does not cover the recommended daily requirement. The objective of this study was to use selenium nanoparticles (nSe) as a selenium (Se) source and to determine the effects on agronomic indices, antioxidant compounds, enzymatic activity, and accumulation of Se in fruits of a jalapeño pepper crop. Different concentrations of nSe (1, 15, 30, and 45 mg L−1) were supplied via drench to jalapeño pepper plants at 15, 30, 45, and 60 days after transplanting. The results indicate that applying nSe via drench with 45 mg L−1 increased crop yield and antioxidant compounds. Moreover, all doses evaluated modified the activity of the enzymes ascorbate peroxidase (APX), glutathione peroxidase (GSH-Px), and phenylalanine ammonium lyase (PAL), as well as improved the concentration of Se in fruits. The nSe incorporation via drench is an alternative to increase the content of Se and other nutraceutical compounds in jalapeño pepper fruits, possibly positively influencing human nutrition when consumed.
1. Introduction
Selenium (Se) was recognized in 1957 as an essential element for mammals that prevents necrotic degeneration of the liver. Additionally, it is the only trace element with a specific codon to selenoproteins with antioxidant and anti-inflammatory effects [1,2]. Plants can uptake Se through the uptake mechanism it shares with sulfur (S) [3], which allows it to be absorbed as selenate (SeO42−), selenite (SeO32−), and nano-Se; or in its organic forms of selenomethionine (SeMet) or selenocysteine (SeCys) [4]. Se is rapidly metabolized to SeCys [5] and can also be incorporated into primary and secondary metabolites derived from SeMet and SeCys, such as selenoglutathione, aromatic compounds derived from phenylalanine, and tyrosine [3]. Biochemical changes of plants in response to the incorporation of Se in plant metabolism are aimed at improving the defense system by detoxification of free radicals through the increase in non-enzymatic compounds such as proline, flavonoids, polyphenols, alkaloids, and glutathione, and antioxidant enzymes such as catalase (CAT, EC: 1.11.1.6) and ascorbate peroxidase (APX, EC: 1.11.1.11) [6], which can mitigate oxidative stress generated by biotic and abiotic factors. Despite its importance in human nutrition, in Mexico, Seintake is well below the recommended requirements (80–221 μg/day) [7], since the average intake for adults is 37.6 to 51.8 µg/day, mainly obtained from the consumption of beans, corn tortillas, and milk, covering 32%, 24%, and 19% respectively [8]. In general, the content of Se in food is low, mainly due to its low concentration in most agricultural soils [9]. Therefore, it offers the opportunity to develop crop biofortification programs to increase the intake of Se at least to the minimum amount of the recommended range [10]. The most common way to introduce Se into the food chain is through biofortification [11]. On the other hand, using nanotechnology in agriculture represents an innovation in research to generate effective products that can be applied to crops and transform the agricultural sector [12]. In this respect, nanoparticles of Se (nSe) show high bioactivity, high antioxidant capacity, and beneficial impacts on primary and secondary plant metabolism [6]. The size of these nanomaterials is an essential factor determining their uptake and mobility in plant tissues and cells [13]. The use of nSe in agriculture is due to its biostimulant effect [14]. This effect is carried out in two phases: (1) First, there is the modification of the physicochemical properties of the cell membrane, by inducing changes in the activity of receptors, transporters, and proteins, in addition to modifying the transport of metabolites. (2) It occurs through changes corresponding to the release of the complex that makes up the nanoparticle, chemical element, or carbon compounds [15]. This interaction results from the induction of antioxidant metabolism in crops and the edible part [6], for example, protection against lipid peroxidation by antioxidant compounds [6].
The primary diet of the Mexican population is corn, beans, and pepper [16]. Pepper (Capsicum spp) is a crop native to South America [17]. It is primarily consumed for its spicy flavor, generated by the presence of capsaicinoids [18]. Although the main attraction of Capsicum fruits is their piquancy to the palate, it is also a food with great nutritional value [19]. It is a source of ascorbic acid (vitamin C), vitamins A, E, and B, secondary metabolites such as carotenoids, phenolic compounds, some minerals, and other compounds with antioxidant capacity, whose content varies according to the species, degree of maturity, and the crop development conditions (fertilization, stress, post-harvest management, etc.) [20,21]. Several clinical studies have demonstrated the benefits of bioactive compounds contained in the fruits of Capsicum to improving quality of life, attributed mainly to flavonoids, alkaloids, vitamins, carotenoids, and capsaicin [22]. They are also attributed with anti-obesity effects, antioxidant activity and cardiovascular protection, antimicrobial, antifungal, and antiviral activity, protection against disorders in the urinary system and renal failure, and anticancer activity [23].
These qualities, together with the effects attributed to nSe, make to pepper an appopiate crop for biofortification; being one of the main essential foods of the Mexican diet positions it as a vital source of Se and other compounds, a high-quality food. Based on the above, the objective of this work is to evaluate supplementation with nSe as a source of Se in the cultivation of jalapeño pepper (Capsicum annuum L.) hybrid Durango F-1, and to determine the effects on agronomic indices, antioxidant capacity, enzymatic activity, and Se accumulation in fruits.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
The field experimental phase was developed in a tunnel-type greenhouse with a semi-transparent polyethylene cover and natural ventilation with side vents, belonging to the Universidad Autónoma Agraria Antonio Narro. It used Jalapeño pepper (Capsicum annuum L.) hybrid Durango F-1 seeds (Starseeds International Inc., Pue, MX), which were germinated in 200-cavity polystyrene trays containing a peat moss–perlite growth medium (70/30; v/v). At 65 days of age, they were transplanted into 10 L polyethylene pots, with 8 L of growth medium made up of peat moss–perlite in a 1:1 ratio (v/v). Nutrition was supplied through the Steiner nutrient solution [24] and an automated irrigation system. The greenhouse conditions during the crop cycle were 21 °C temperature and 51% relative humidity. The average maximum conditions were 565 µmol m−2 s−1 of photosynthetically active radiation, 1095 W m−2 of incident solar radiation (outside the greenhouse), and 730 W m−2 inside the greenhouse. The pots were ordered with a separation of 0.90 × 0.35 m, obtaining a total experimental area of 31.5 m2. Thirty days after transplanting (DAT), the plants were tutored using white agricultural raffia.
2.2. Experimental Design and Treatment Application
The experimental design was completely randomized. Spherical Se nanoparticles (nSe) of an average size of 20 nm [25] were used, at concentrations of 0, 1, 15, 30, and 45 mg L−1, supplied via drench at the base of the stem at 15, 30, 45 and 60 DAT. Each treatment consisted of ten plants, each one represented an experimental unit (EU). An amount of 100 mL of solution was supplied, receiving 1, 15, 30 and 45 mg L−1. The EU of the control treatment (0 mg L−1) was provided with 100 mL of deionized water. The nSe were sonicated (BRANSON 1510R-DHT, Bransonic, CT, USA) to ensure the homogeneity of the solution.
2.3. Agronomic Indices
The agronomic parameters considered were stem diameter, root and aerial part fresh weights, root and aerial part dry weights, height gain, and yield. The procedures are described below. The vertical growth of the plants was measured on four occasions, one day before each application of the treatments, using a magnetic flexometer (FCN-55M, TRUPER, EdoMex, MX), and the results were reported in cm. Stem diameter was evaluated at 90 DAT, using a digital vernier (500-192-30 Mitutoyo Co., YOK, JPN); results were reported in mm. Root and aerial part fresh weights were determined at 90 DAT, employing a granatary balance (SPX2202, OHAUS, Inc., NJ, USA); the results were expressed in g. To determine the dry weights of the root and aerial part, the samples were introduced into a drying oven at 60 °C for 72 h (Arsa, AR-290AD, Jal, MX) or until constant weight; the results were reported in g. Yield was obtained from the fruits harvested at 80 DAT; a granatary balance (SPX2202, OHAUS, Inc., NJ, USA) was used; the results were expressed in g plant−1. Five EUs of each treatment were considered for evaluation.
2.4. Photosynthetic Pigments
Photosynthetic pigments were estimated using the method described by Wellburn [26], with some modifications. 60 mg of lyophilized leaf tissue and 5 mL of pure methanol were added to a test tube. The mixture was shaken vigorously and allowed to stand 24 h at room temperature without illumination. The absorbance of the samples was read in a UV–Vis spectrophotometer (GENESYS 10S UV-Vis, Thermo Fisher Scientific, Inc., MA, USA) at 666, 653, and 470 nm. The absorbances obtained were entered into the following equations:
where Chl a represents chlorophyll a, and Chl b represents chlorophyll b. Results were expressed in mg g−1 dry weight.
2.5. Physicochemical Analysis of the Fruit
Total soluble solids (TSS), pH, and titratable acidity (TA) were determined at harvest time. For this purpose, the juice of the fruits was extracted using a porcelain mortar and pestle. TSS were measured using a digital refractometer (MASTER-PM, ATAGO, Inc., WA, USA), and the results were expressed in °Brix. The pH was measured using a previously calibrated benchtop pH meter (LAQUA PH1100, HORIBA Advanced Techno, Co., KP, JPN). TA was determined by titration with 0.1 N NaOH [27], and the results expressed as a percentage of citric acid. Fruit length and equatorial diameter were measured using a digital vernier (500-192-30 Mitutoyo Co., YOK, JPN); results were reported in mm. Firmness was determined using a hand-held texturometer (Force Dial FDK 20, WAGNER Instruments, CT, USA); results were expressed in kg cm−2. Five fruits from each selected EU were considered for the measurements.
2.6. Total Proteins
Protein quantification was performed with the Bradford method [28], using 100 µL of biomolecule extract mixed with 1 mL of Bradford reagent (Bradford Assay, ThermoFisher Scientific, MA, USA). The absorbance of the samples was read with a UV–Vis spectrophotometer (GENESYS 10S UV-Vis, Thermo Fisher Scientific, Inc., MA, USA) at 595 nm. The calibration curve was performed using bovine serum albumin as a standard. The results were expressed in g 100 g−1 dry weight (DW).
2.7. Antioxidant Status of Fruit
2.7.1. Sample Processing
At 70 DAT, fruits of homogeneous size were selected from each treatment and immediately frozen at −80 °C. Once frozen, they were subjected to a lyophilization process under the following conditions: temperature −84 °C, vacuum pressure 0.080 mBar (FreeZone 2.5, Labconco, MO, USA). The samples were macerated in a mortar and pestle until a fine powder was obtained and stored until the determinations in lyophilized tissue were performed. The same procedure was performed on leaf tissue to determine photosynthetic pigments at the flowering and fructification stage.
2.7.2. Biomolecule Extraction
To extract biomolecules, 10 mg of Polyvinylpyrrolidone (PVP) and 100 mg of lyophilized fruit tissue were weighed. Subsequently, 2 mL of phosphate buffer (pH 7.0–7.2; 100 mM) was added, and the mixture was shaken for 5 s in a vortex and then extracted by ultrasound for 5 min (BRANSON 1510R-DHT, Bransonic, CT, USA). The mixture was centrifuged at 12,500 rpm for 10 min at 4 °C (PrismTMR, Labnet International, Inc., NJ, USA). The supernatant was filtered through a 0.45 µm pore size nylon membrane. A 1:15 dilution with phosphate buffer was made and stored at −80 °C. The extract was used for enzymatic determinations, total protein, and antioxidant activity [29].
2.7.3. Non-Enzymatic Antioxidants
The Vitamin C content in the fruit was determined with the 2,6-dichloroindophenol method, with some modifications [30], using UV–Vis spectrophotometer. First, metaphosphoric acid (HPO3; 0.36 M) was used as the extracting solution. Then, 2 mL of solution was added to 10 mg of lyophilized fruit tissue and extracted by ultrasound for 5 min. The mixture was centrifuged at 5000 rpm for 10 min at 4 °C (PrismTMR, Labnet International, Inc, NJ, USA). Next, 200 µL of the supernatant was mixed with 1.8 mL of 2,6-dichloroindophenol (0.09 M), and the absorbance of the sample was read at 515 nm (GENESYS 10S UV-Vis, Thermo Fisher Scientific, Inc., MA, USA). Vitamin C was estimated by interpolating the results against a previously established calibration curve with ascorbic acid (0–120 ppm). The results were expressed as milligrams of ascorbic acid equivalents per gram of dry weight (mg AAE g−1 DW).
The total phenolic compounds were estimated using the Folin–Ciocalteu method (2 M, Folin & Ciocalteu’s phenol reagent, Sigma Aldrich, Darmstadt, GER) at 765 nm [31], for which 100 mg of lyophilized fruit tissue was added to 2 mL of 95% methanol and subjected to ultrasound extraction. The calibration curve was performed using gallic acid (97.5–102.5%, Sigma Aldrich, Darmstadt, GER). The results were expressed in milligrams of equivalent gallic acid per 100 g dry weight (mg GAE 100 g−1 DW).
The total flavonoid content was determined with the colorimetric method described by Shraim et al. [32], with some modifications, at 510 nm. To quantify total flavonoids, 2 mL of 95% methanol was added to 100 mg of lyophilized fruit and subjected to ultrasound extraction. The calibration curve was performed using catechin as a standard (≥96% Sigma Aldrich, Darmstadt, GER). The results were expressed in milligrams of equivalent catechin per 100 g dry weight (mg CE 100 g−1 DW).
The antioxidant activity was determined by the inhibitory capacity of the 2,2′azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+) radical [33]. The radical was obtained from the reaction of ABTS at 7 mM with potassium persulfate (K2S2O8, 2.45 mM) for 16 h at room temperature in the dark. The absorbance was adjusted to 0.700 ± 0.020 at 734 nm at an approximate temperature of 30 °C. Then, 1.8 mL of the diluted ABTS+ solution and 0.2 mL of biomolecule extract were taken, homogenized, and kept in darkness. After 6 min, the absorbance of the samples was read at 734 nm in a UV–Vis spectrophotometer (GENESYS 10S UV-Vis, Thermo Fisher Scientific, Inc., MA, USA), and the percentage of inhibition was calculated based on the concentration of ascorbic acid of the standard reference curve.
The quantification of glutathione was carried out according to the procedure described by Xue et al. [34], which is based on the reaction of 5,5-dithiobis-2-nitrobenzoic acid (DNTB). An amount of 480 µL of biomolecule extract was mixed with 2.2 mL of 0.32 M Na2HPO4 and 320 µL of 1 mM DNTB. It was vigorously shaken for 5 s, and the absorbance was read at 412 nm in a UV–Vis spectrophotometer (GENESYS 10S UV-Vis, Thermo Fisher Scientific, Inc., MA, USA). The estimation was performed using the regression equation of the curve, and the results were expressed in µM GSH equivalent g−1 DW.
2.7.4. Enzymatic Activity
The activity of the catalase enzyme (CAT 1.11.1.6) and ascorbate peroxidase (APX 1.11.1.11) was estimated following the protocol described by Elavarthin & Martin [35]. The activity of the glutathione peroxidase enzyme (GSH-Px 1.11.1.9) was estimated with the method described by Xue et al. [34]. The estimation of phenylalanine ammonia lyase enzyme activity (PAL 4.3.1.5) was carried out according to the procedure of Chen et al. [36]. The results were expressed as U g−1 of total protein. The enzyme units (U) for each enzyme are defined as follows:
- CAT: a U is equivalent to the amount of mM of H2O2 consumed per milliliter per minute.
- APX: a U is equivalent to µM of oxidized ascorbate per milliliter per minute.
- GSH-Px: a U is equivalent to the amount of GSH in µM per milliliter per minute.
- PAL: a U is defined as the amount in mM of trans-cinnamic acid produced per milliliter per minute.
2.8. Se Content in Fruit
Se content was determined in fruit according to the procedure described by Pedrero et al. [37], for which 25 mg of the sample was digested in 2.5 mL of concentrated HNO3 and 1 mL of H2O2 in an analytical microwave oven. The resulting solution was diluted to 25 mL with deionized water. ICP-MS determined the concentration of Se. The results were expressed in µg g−1 of dry weight.
2.9. Statistical Analysis
Five replicates per treatment were randomly selected for the agronomic and fruit quality evaluations. The photosynthetic pigment analyses were performed on three replicates per treatment. The information obtained from the assessments was subjected to a one-way analysis of variance (ANOVA) and a Tukey’s test (p ≤ 0.05) using the INFOSTAT 2016 program (http://www.infostat.com.ar (accessed on 20 February 2023)). Additionally, an Anderson–Darling test was performed to the first plant-height evaluation to confirm equality in the data distribution.
3. Results
3.1. Agronomic Indices
3.1.1. Growth Dynamics
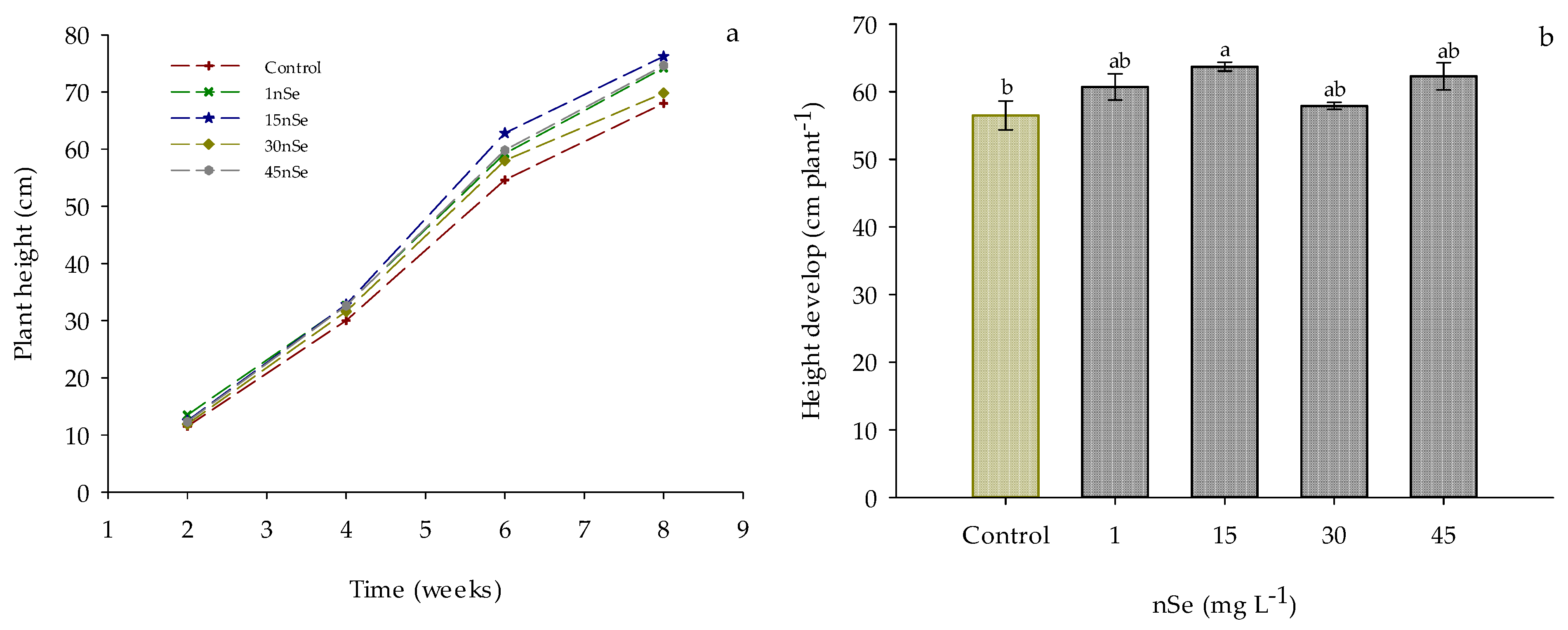
Supplementation with nSe influenced the growth dynamics of the plants. In Figure 1a, a distinction in growth is observed starting from the fourth week, with the plants in the 15 mg L−1 nSe treatment standing out from the beginning. This trend continues during the following weeks of evaluation. Additionally, slower growth is observed in the control and 30 mg L−1 nSe treatments. This difference in growth dynamics was confirmed when evaluating the total height gain of the plant. As shown in Figure 1b, a significant increase of 12.74% in plant growth is observed when supplementing with 15 mg L−1 nSe through drenching, with 62.3 cm for this treatment as compared to 56.5 cm for the control treatment. Furthermore, no significant difference is observed between treatments 1, 30, and 45 mg L−1 and the reference treatment (control).
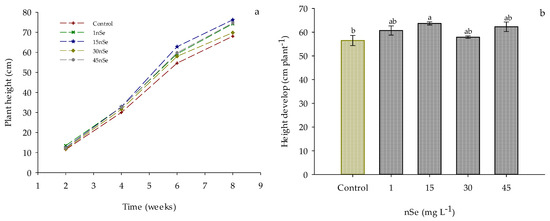
Figure 1.
Growth dynamics of Jalapeño pepper plants with nSe application. (a) weekly growth; (b) total height gain. Different letters indicate a difference between treatments (Tukey test p ≤ 0.05). n = 5 ± standard error.
3.1.2. Crop Attributes
The agronomic determinations of the crop were influenced by supplementation with nSe. The results obtained from the evaluations are shown in Table 1, and it can be observed that supplementation with nSe at different concentrations did not significantly modify these parameters (p > 0.05).

Table 1.
Agronomic attributes of Jalapeño pepper crop with nSe application.
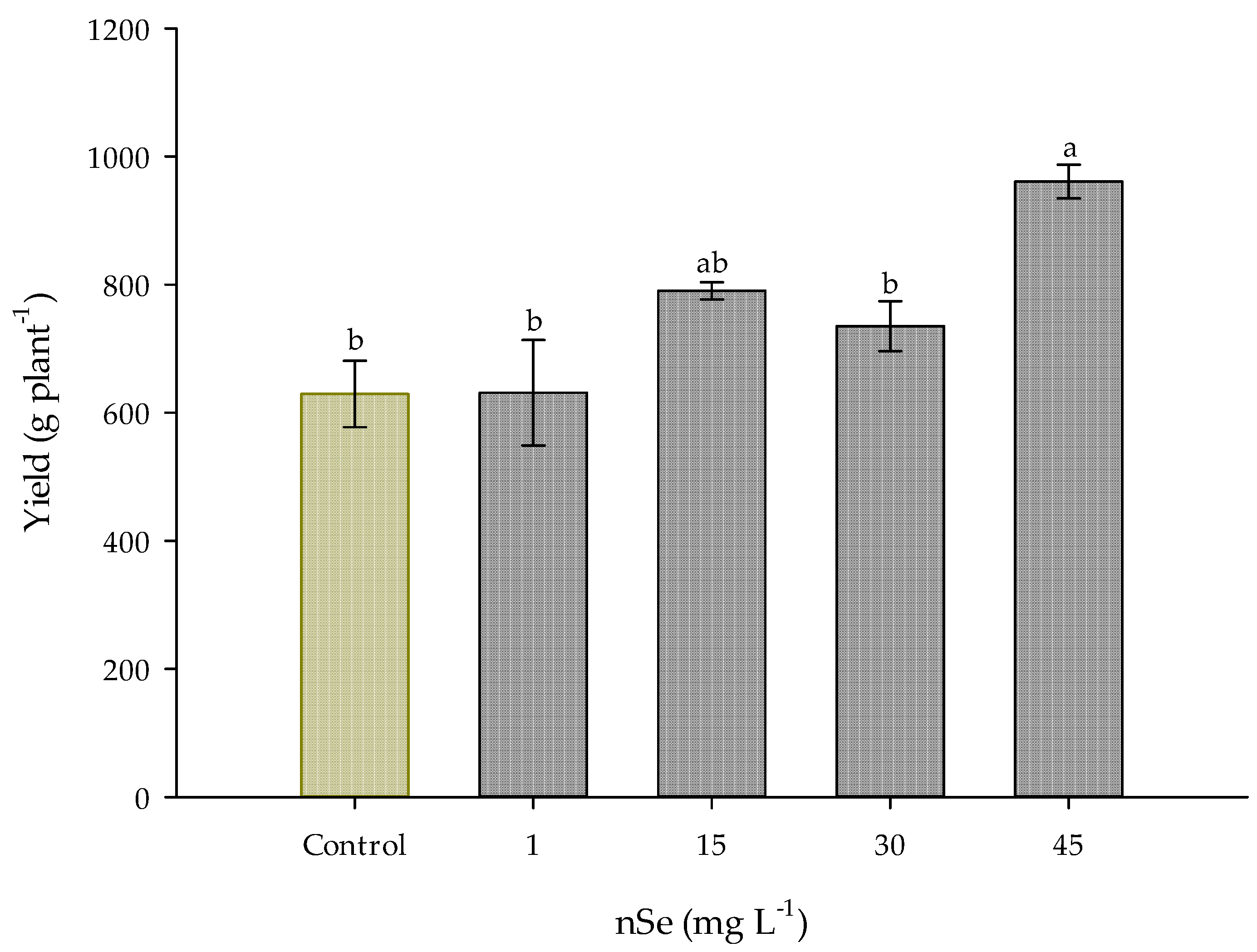
On the other hand, crop yield had a significant increase (p ≤ 0.05) due to the effect of nSe supplementation at the dose of 45 mg L−1; compared to the control treatment (629 g plant−1), it increased by 52.75%, corresponding to 960.8 g plant−1. The treatments of 1, 15, and 30 mg L−1 of nSe did not show a significant difference in this parameter, taking the control treatment as a reference (Figure 2).
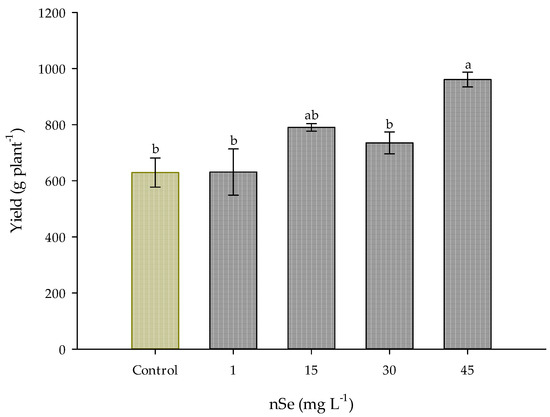
Figure 2.
The yield of Jalapeño pepper crop with the application of nSe. Different letters indicate significant differences between treatments (Tukey test p ≤ 0.05). n = 5 ± standard error.
3.2. Photosynthetic Pigments
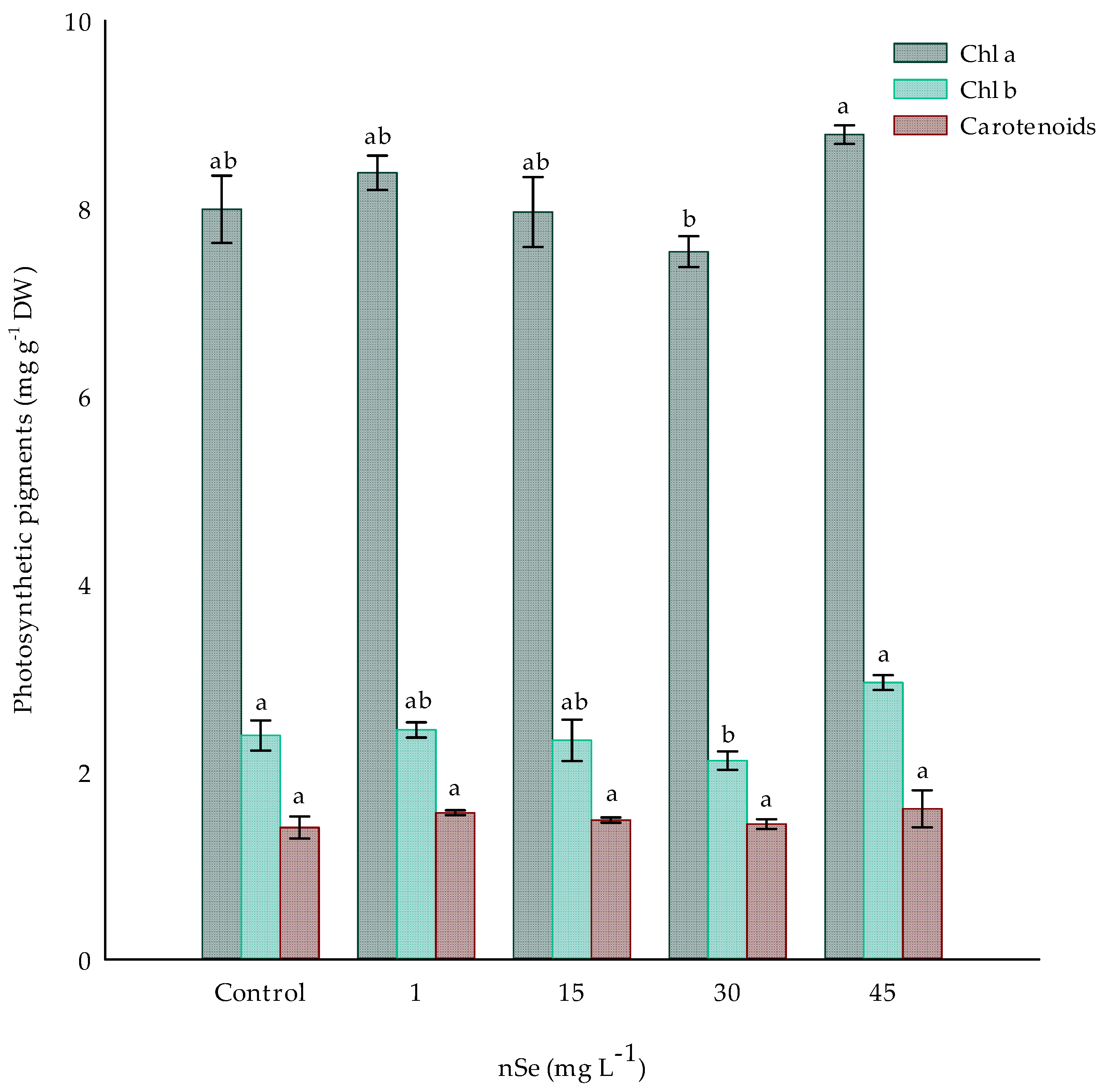
Photosynthetic pigment content in plants treated with nSe did not significantly differ between the treatments with 1, 15, 30, and 45 mg L−1 and the control treatment (Figure 3). However, there was an increase of 4.8 and 9.97% of Chl a in 1 mg L−1 and 45 mg L−1 with 8.37 and 8.78 mg g−1, respectively. For the case of Chl b, the same trend as for Chl a is observed, with increases in treatments 1 and 45 of 2.47 and 23.6%, respectively, compared to the control treatment. However, the analysis shows a significant difference between treatments 30 and 45 mg L−1 nSe, both for Chl a and Chl b, with a difference of 15.62 and 34.84 mg g−1 DW between the pigments for both treatments, respectively (Figure 3). Total carotenoids were not significantly modified by nSe supplementation.
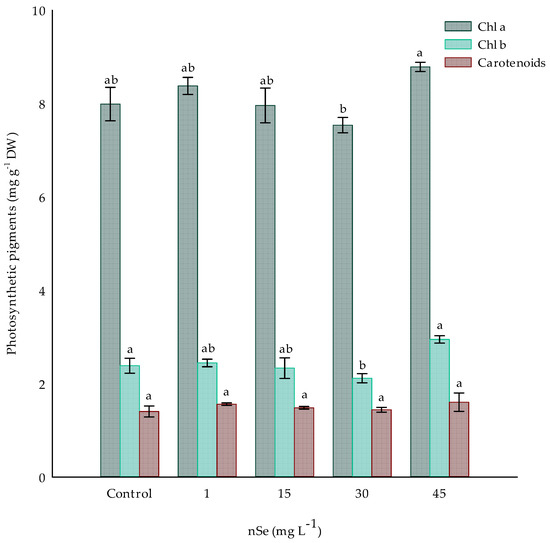
Figure 3.
Photosynthetic pigments in Jalapeño pepper plants with nSe in a drench. Different letters indicate differences between treatments (Tukey test p ≤ 0.05). Chl a: chlorophyll a; Chl b: chlorophyll b; DW: dry weight. n = 3 ± standard error.
3.3. Fruit Quality
TSS, firmness, and TA were significantly affected by nSe treatments. The changes observed are mainly between the doses of nSe applied. The highest TSS content in jalapeño pepper fruits was obtained with the application of 15 and 30 mg L−1 of nSe, as each increased by 12.5 and 9.61%, respectively, compared to the control; on the other hand, the highest dose (45 mg L−1) decreased this variable by 8.6% (Table 2).

Table 2.
Components of the fruit quality of Jalapeño pepper fruits.
The application of nSe at 30 mg L−1 increased the firmness of the fruits by 59.34% (7.76 kg cm−2) compared with the control (4.87 kg cm−2).
No significant differences were observed in the length and equatorial diameter of the fruits with the application of nSe.
The change obtained in TA was shown again among the treatments with nSe, showing an increase in TA with the supplemental application of 30 mg L−1 of nSe. Along this parameter, the control treatment was similar to the evaluated doses.
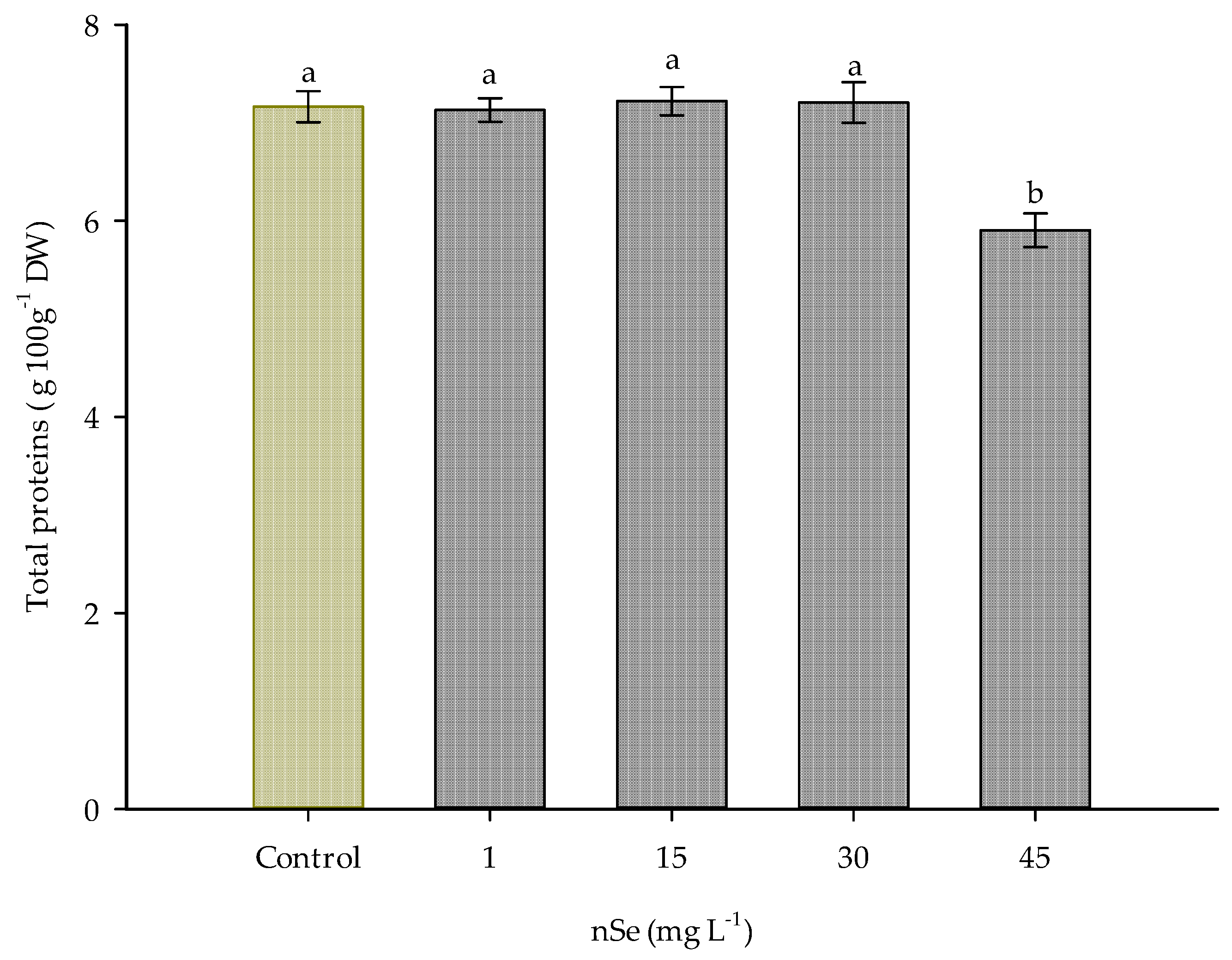
Additionally, supplementation with nSe at 45 mg L−1 induced a significant change in the amount of total proteins, reducing the amount of this molecule by 17.58% compared to the control (Figure 4).
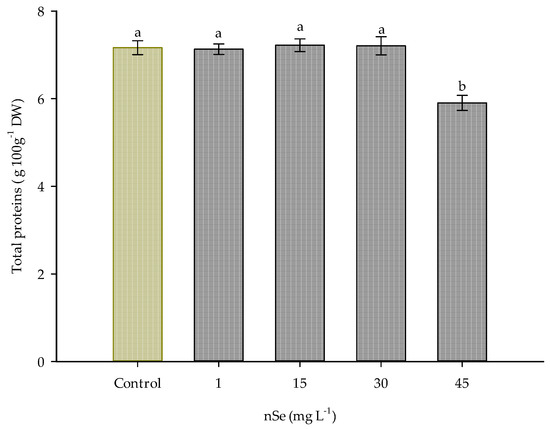
Figure 4.
Total protein content in Jalapeño pepper fruits with nSe. DW: dry weight. Different letters indicate differences between treatments (Tukey test p ≤ 0.05). n = 5 ± standard error.
3.4. Antioxidant Status of Fruit
3.4.1. Non Enzymatic Antioxidants
Supplementary application of nSe significantly influenced the antioxidant status of fruits. Vitamin C was higher in all treatments with nSe, compared to the control treatment (Table 3). The lowest dose nSe, 1 mg L−1, increased it by 30.48%; similarly, the doses of 15 and 30 mg L−1 increased it by 25.35 and 23.50%, respectively. The most significant increase was noticed with the application of 45 mg L−1 of nSe, surpassing the control by 42.59%.

Table 3.
Antioxidant compounds in Jalapeño pepper fruits with nSe.
Doses 1 and 15 mg L−1 increased the content of total phenols by 75.17 and 74.75% more than the control, respectively. Furthermore, the concentrations of 30 and 45 mg L−1 increase these compounds by 36.34 and 18.88%, respectively.
Significant changes were observed in the concentration of flavonoids with the application of the treatments. The most distinctive difference was in the treatment with 30 mg L−1, which increased the concentration by 67.2%, compared to the control. For its part, 1 mg L−1 of nSe behaved statistically the same as the control treatment.
The antioxidant capacity increased significantly with nSe treatments. The nSe treatments inhibited the radical at an index higher than 45%, considerably surpassing the control treatment. The values obtained are shown in Table 3, highlighting the treatments with 30 and 45 mg L−1 of nSe, with 59.01 and 58.82% inhibition, respectively, which is a good index of the antioxidant status of fruits, since the higher the inhibition, the higher the antioxidant capacity.
Additionally, supplementation with nSe at a concentration of 45 mg L−1 decreased the content of GSH in jalapeño pepper fruits, decreasing the content of this compound by 30.54%, compared to the control. The treatments of 1, 15, and 30 mg L−1 of nSe were statistically the same as the control (Table 3).
3.4.2. Enzymatic Antioxidants
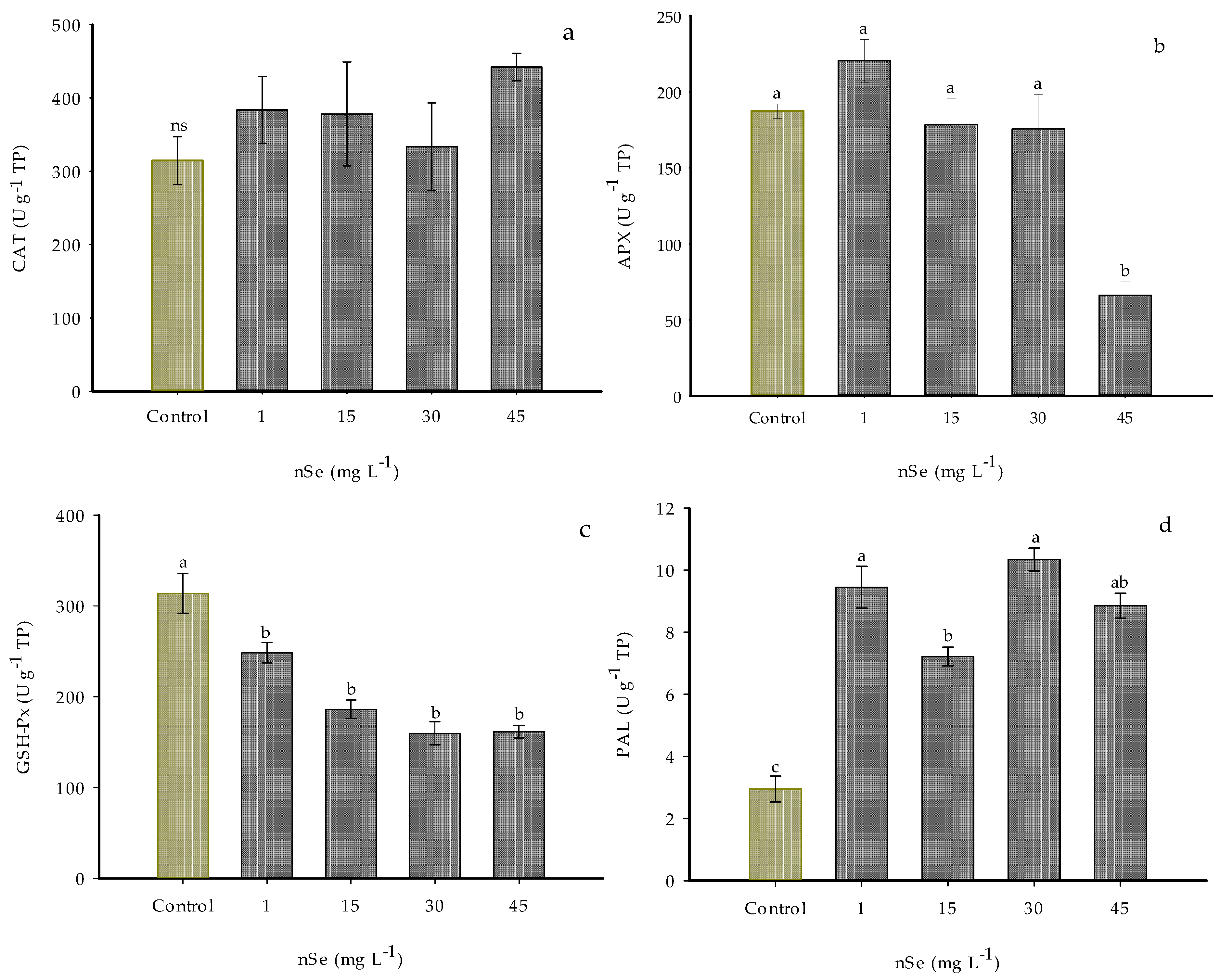
The addition of nSe modified the activity of antioxidant enzymes APX, GSH-Px, and PAL.
The CAT enzymatic activity did not show significant differences with the application of the treatments; however, significant increases were noticed in all the evaluated doses of nSe, increasing this activity between 6 and 40% (Figure 5a).
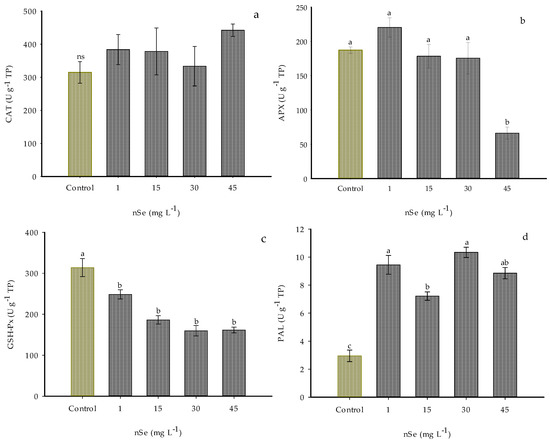
Figure 5.
Enzyme activity in Jalapeño pepper fruits with nSe. (a) CAT; (b) APX; (c) GSH-Px; (d) PAL; ns: not significant between treatments; TP: total proteins. Different letters indicate differences between treatments (Tukey test p ≤ 0.05). n = 5 ± standard error.
The activity of the enzyme APX was statistically the same as the control when 1, 15, and 30 mg L−1 of nSe were applied. In the plants subjected to the highest dose of 45 mg L−1 of nSe, more than a 60% reduction in the activity of this enzyme was observed (Figure 5b).
On the other hand, adding nSe did not favor the GSH-Px enzymatic activity. On the contrary, activity decreased by 20.9, 40.7, 49.09, and 48.49% in all the evaluated doses (1, 15, 30, and 45 mg L−1, respectively) compared to the control (Figure 5c).
For PAL, supplementation with nSe significantly increased the activity of this enzyme in all the evaluated treatments (Figure 5d). Compared to the control, the treatments of 1 and 30 mg L−1 nSe increased the PAL activity by 219.84 and 250.03%, respectively. With the addition of 15 and 45 mg L−1 nSe, the enzyme activity over the phenylpropanoid pathway was exceeded (144.25 and 199.73%, respectively) compared to the control.
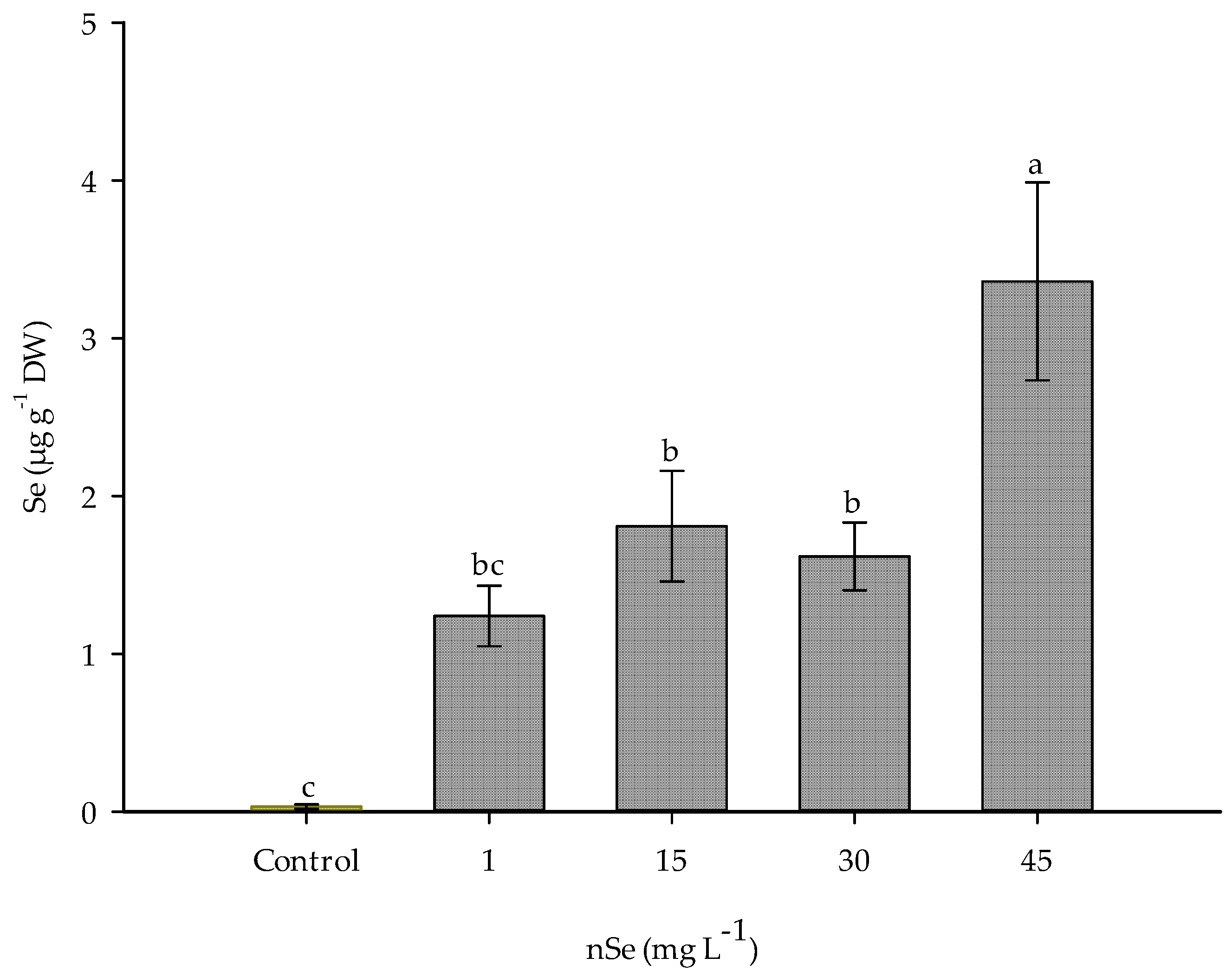
3.5. Se Content in Fruit
Plants exposed to nSe showed an accumulation of this element in the edible part (Figure 6). Compared to lowest dose of nSe, 1 mg L−1, supplementation with 45 mg L−1 increased the concentration of Se in fruits by 67.73 times. Similarly, significant increases were observed with doses of 15 and 30 mg L−1, accumulating 17.89 and 11.82 times more Se content compared to the lowest dose. mg L−1
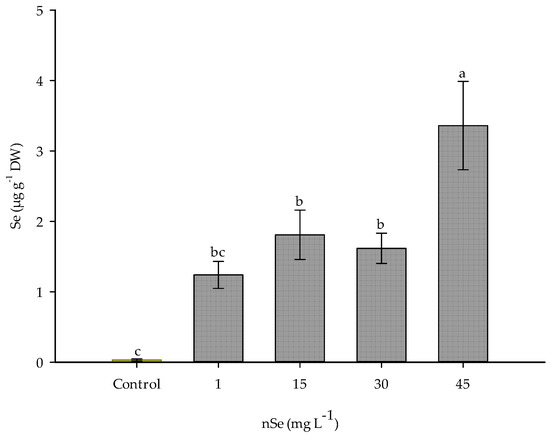
Figure 6.
Se concentration in fruits of jalapeño pepper. DW: dry weigth. Different letters indicate differences between treatments (Tukey test p ≤ 0.05). n = 5 ± standard error.
4. Discussion
Nanotechnology is a developing science with huge potential in agriculture and promotion of desirable crop characteristics. In this study, jalapeño pepper plants that received nSe showed superior growth behavior as well as a significant increase in yield and slight increases in fresh and dry weight of the aerial and root parts, compared to plants that did not receive nSe. These changes have been shown in other types of vegetables. However, the response of the plants to Se largely depends on the plant species and the concentration of Se used [38]. In this regard, with the addition of Se, different changes in plants have been observed, which include functions within the maintenance of cell structure and function and improvement of photosynthesis. It is an auxiliary in the homeostasis of nutrients [38,39], which contributes to improving the growth and development of crops [40], which could explain the changes observed in this research. Kang et al. [41] reported the application of nSe in Cucumis melo L. lead the development of thylakoidal membranes in the cells of the mesophyll of the leaves, promoting a higher photosynthetic rate and high content of chlorophylls. In the present research, changes in Chl a and Chl b in the leaves suggest a difference in the internal structure of the cells in response to supplementation with nSe. The application of nSe in various crops has provided valuable information among vegetable species, fruit trees, cereals, and even some aromatic species studied [11]. For example, in Punica granatum [42], supplementation with nSe at 2 μM led to an increase in leaf area, higher chlorophyll content, and the yield obtained per tree, the same trend observed in the present study. Similarly, in Solanum lycopersicum [43], incorporating nSe at 20 mg L−1 into the growth medium of the plants modified the total chlorophyll content and significantly increased the crop yield. This trend was also reported in Fragaria x ananassa [44] cultivation by adding nSe at 20 mg L−1, showing improvements in the concentration of Chl a, Chl b, and carotenoids, and in the agronomic attributes of the crop.
In addition to the changes observed in yield and other attributes of crop growth, variations were also recorded in the commercial quality of the fruit, highlighting TSS, firmness, TA, and total protein. It could be due to the effect generated by nanomaterials, in this case, nSe, when these are involved in the growth environment of crops. Garza-García et al. [12] point out, among others, the relevant effect of nSe on plant primary metabolism, which begins with the modification of the chlorophyll content, the precursor of photosynthesis [45]. Through this change, a higher rate of photoassimilates would be expected, and consequently, a higher range of soluble sugars, proteins, and other components in general, increasing the total TSS content [12]. The biostimulant effect of nSe is attributed to its nanometric size, because the contact with the wall and the cellular membrane generates a cascade of signaling that will cause changes in the development of the crop, highlighting the expression of genes and a change in enzymatic activity [15]. One of the enzymes that become important is PAL, a precursor of a great diversity of phenolic compounds, and, therefore, a more significant deposition of lignin in the cell wall [46], which could explain the increase in fruit firmness with nSe. A change in these variables has also been observed in other vegetables because of the application of nSe. An increase in firmness in fruits of Cucumis sativus was observed when nSe was applied at 25 mg L−1, compared to untreated fruits [47]. In Solanum lycopersicum [48], the application of nSe at 100 mg L−1 showed an increase in TSS. However, with this same treatment, there was a lower firmness of the fruits with nSe than those not treated. In fruit species such as Punica granatum, a trend of increase in TSS and a low percentage of TA wasnoticed when 2 µM of nSe was applied, compared to the fruits of the control treatment [42].
Since its discovery in 1817, the roles of Se in human metabolism and the effects of the narrow line between deficiency and toxicity for this element have gradually been reflected upon [49]. It was not until 2015 that this element was classified as a plant biostimulant [14] due to the beneficial effects that it generated in a specific concentration in the plant species that had been studied up to now. In addition, the metabolism of Se within plants comprises a vast network of complex processes developed in the chloroplast and the cytosol of the cells [40]. After Se uptake either by the roots or through the cuticle of the leaf area, Se can participate in the metabolism due to itschemical characteristics similar to sulfur (S). It can replace it in molecules containing S and induce changes in plant primary and secondary metabolism [50]. The changes evaluated with greater emphasis are the metabolism of phenylpropanoids, the activity of antioxidant enzymes, and the resistance generated in plants against different types of stress [51]. In this study, the application of nSe in drench induced an increase in the concentration of FT, FLV, and the antioxidant capacity ABTS+, which could be related to the high enzymatic activity of PAL (Figure 6), which is crucial in the initiation of the phenylpropanoid metabolism [52].
On the other hand, the activity of GSH-Px is closely related to the amount of GSH present, since it is the primary cofactor of this enzyme. This principle could indicate that the Se incorporated through the nSe replaces S in polypeptide structures, interfering in glutathione production [53]; as presented in this study, low concentration of GSH, and, therefore, little GSH-Px activity. It could also indicate interference in the ascorbate–glutathione cycle in the fruits, since the enzymes APX and GSH-Px, as well as the cofactors ascorbate and glutathione, are an essential part of this process [54]. Therefore, the response was found in the content of vitamin C in Jalapeño pepper fruits. Changes in gene expression related to antioxidant metabolism and the phenylpropanoid pathway have also been studied in response to Se supplementation. It is documented by Kang et al. [41], highlighting the overexpression of PAL, APX, CAT, and SOD genes, as well as a change in PAL, APX, CAT, and POD enzymes when supplementing with nSe. This study agrees with the PAL enzyme’s activity and the slight increase in CAT, which could be related to the gene expression that codes these enzymes [55]. Similarly, nSe have dual action within the metabolism, first by the protection they generate against reactive oxygen species (ROS), and at the same time, by acting as inducers of oxidative stress, inducing the antioxidant defense system of plants [56]
Plants can absorb Se in different chemical forms and organic compounds; it is taken up by root cells and rapidly catalyzed by the high chemical affinity it shares with S [57]. In this study, the amount of Se in the fruits increased with the evaluated nSe doses. The results suggest that the accumulated concentration in the tissue depends on the supplemented Se concentration, since the highest amount of Se was found in the fruits of the 45 mg L−1 nSe treatment. Although the results obtained are based on dry weight, it is a good indicator of the mobility of Se within the metabolism of Jalapeño pepper plants, as it translocates to the demand organs, such as fruits, which, when consumed, will provide a contribution of Se in daily intake. The ability to accumulate Se in several vegetables and fruit trees has been reported in different investigations. In Vitis vinifera [58], with the addition of Se in various concentrations, it was possible to increase the amount of this element in berries, showing a tendency of accumulation related to the dose applied. In Solanum lycopersicon L. [59], supplementation with Se in doses of 2 and 5 mg L−1 increased Se in fruit.
5. Conclusions
Applying nSe increases Se yield, bioactive compounds, and concentration in jalapeño pepper fruits. Doses of 45 mg L−1 of nSe increased crop yield and antioxidant compounds. Moreover, all doses evaluated modified the activity of the enzymes ascorbate peroxidase (APX), glutathione peroxidase (GSH-Px), and phenylalanine ammonium lyase (PAL), as well as improved the concentration of Se in fruits. Incorporating nSe via drench is an alternative to enhance the content of Se and other bioactive compounds in jalapeño pepper fruits, possibly positively influencing human nutrition when consumed.
Author Contributions
Conceptualization, Á.M.-M. and M.d.l.Á.S.-N.; methodology, M.d.l.Á.S.-N.; formal analysis, P.P.-R.; investigation, M.d.l.Á.S.-N.; resources, Á.M.-M., A.B.-M. and G.C.-P.; data curation, M.d.l.Á.S.-N.; writing—original draft preparation, M.d.l.Á.S.-N.; writing—review and editing, P.P.-R. and E.S.; visualization, Á.M.-M.; supervision, P.P.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data will be made available on request to the correspondent author’s email with appropriate justification.
Acknowledgments
María de los Ángeles Sariñana-Navarrete gives thanks for the financial support provided by the National Council of Science and Technology of Mexico (CONACYT) for doctoral studies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rayman, M.P. Selenium and Human Health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Duntas, L.H.; Rayman, M.P. The Role of Selenium in Type-2 Diabetes Mellitus and Its Metabolic Comorbidities. Redox. Biol. 2022, 50, 102236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chu, C. Selenium Uptake, Transport, Metabolism, Reutilization, and Biofortification in Rice. Rice 2022, 15, 1–15. [Google Scholar] [CrossRef]
- El-Ramady, H.; El-Sakhawy, T.; Omara, A.E.-D.; Prokisch, J.; Brevik, E.C. Selenium and Nano-Selenium for Plant Nutrition and Crop Quality. In Selenium and Nano-Selenium in Environmental Stress Management and Crop Quality Improvement; Hossain, M.A., Ahammed, G.J., Kolbert, Z., El-Ramady, H., Islam, T., Schiavon, M., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 55–78. ISBN 978-3-031-07063-1. [Google Scholar]
- Pilon-Smits, E.A.H.; Quinn, C.; Quinn, C.F. Selenium Metabolism in Plants. Cell Biol. Met. Nutr. 2010, 17, 225–241. [Google Scholar] [CrossRef]
- Lanza, M.G.D.B.; dos Reis, A.R. Roles of Selenium in Mineral Plant Nutrition: ROS Scavenging Responses against Abiotic Stresses. Plant Physiol. Biochem. 2021, 164, 27–43. [Google Scholar] [CrossRef]
- López-Bellido, F.J.; Sanchez, V.; Rivas, I.; López-Bellido, R.J.; López-Bellido, L. Wheat Grain Selenium Content as Affected by Year and Tillage System in a Rainfed Mediterranean Vertisol. Field Crops Res. 2019, 233, 41–48. [Google Scholar] [CrossRef]
- Rios-Lugo, M.J.; Palos-Lucio, A.G.; Victoria-Campos, C.I.; Lugo-Trampe, A.; Trujillo-Murillo, K.D.C.; López-García, M.A.; Espinoza-Ruiz, M.; Romero-Guzmán, E.T.; Hernández-Mendoza, H.; Chang-Rueda, C. Sex-Specific Association between Fasting Plasma Glucose and Serum Selenium Levels in Adults from Southern Mexico. Healthcare 2022, 10, 1665. [Google Scholar] [CrossRef]
- Díaz-Zarco, S.; Montes-de-Oca-Jiménez, R.; Rodríguez-Domínguez, M.C. Selenium Levels in Soil, Pasture and Sheep: Influence of Selenium Supplementation on IgG Concentration in Pregnant Sheep and Lambs. Terra Latinoam. 2022, 40, 1–14. [Google Scholar]
- Schiavon, M.; Nardi, S.; Dalla Vecchia, F.; Ertani, A. Selenium Biofortification in the 21st Century: Status and Challenges for Healthy Human Nutrition. Plant Soil 2020, 453, 245–270. [Google Scholar] [CrossRef] [PubMed]
- El-Ramady, H.; Faizy, S.E.-D.; Abdalla, N.; Taha, H.; Domokos-Szabolcsy, É.; Fari, M.; Elsakhawy, T.; Omara, A.E.-D.; Shalaby, T.; Bayoumi, Y. Selenium and Nano-Selenium Biofortification for Human Health: Opportunities and Challenges. Soil Syst. 2020, 4, 57. [Google Scholar] [CrossRef]
- Garza-García, J.J.O.; Hernández-Díaz, J.A.; Zamudio-Ojeda, A.; León-Morales, J.M.; Guerrero-Guzmán, A.; Sánchez-Chiprés, D.R.; López-Velázquez, J.C.; García-Morales, S. The Role of Selenium Nanoparticles in Agriculture and Food Technology. Biol. Trace. Elem. Res. 2022, 200, 2528–2548. [Google Scholar] [CrossRef] [PubMed]
- El-Bialy, S.M.; El-Mahrouk, M.E.; Elesawy, T.; Omara, A.E.-D.; Elbehiry, F.; El-Ramady, H.; Áron, B.; Prokisch, J.; Brevik, E.C.; Solberg, S.Ø. Biological Nanofertilizers to Enhance Growth Potential of Strawberry Seedlings by Boosting Photosynthetic Pigments, Plant Enzymatic Antioxidants, and Nutritional Status. Plants 2023, 12, 302. [Google Scholar] [CrossRef] [PubMed]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Juárez-Maldonado, A.; Ortega-Ortíz, H.; Morales-Díaz, A.B.; González-Morales, S.; Morelos-Moreno, Á.; Cabrera-De la Fuente, M.; Sandoval-Rangel, A.; Cadenas-Pliego, G.; Benavides-Mendoza, A. Nanoparticles and Nanomaterials as Plant Biostimulants. Int. J. Mol. Sci. 2019, 20, 162. [Google Scholar] [CrossRef] [PubMed]
- Valerino-Perea, S.; Lara-Castor, L.; Armstrong, M.E.G.; Papadaki, A. Definition of the Traditional Mexican Diet and Its Role in Health: A Systematic Review. Nutrients 2019, 11, 2803. [Google Scholar] [CrossRef] [PubMed]
- Morales-Fernández, S.D.; Moreno-Velázquez, D.; Jesús, T.-D.; Vázquez-Cruz, F.; Ibáñez-Martínez, A.; Tobar-Reyes, J.R. Phenology and Content of Capsaicinoids in Chili Produced under Greenhouse Conditions. Rev. Mex. De Cienc. Agric. 2020, 11, 663–675. [Google Scholar]
- Arce-Rodríguez, M.L.; Ochoa-Alejo, N. Biochemistry and Molecular Biology of Capsaicinoid Biosynthesis: Recent Advances and Perspectives. Plant Cell Rep. 2019, 38, 1017–1030. [Google Scholar] [CrossRef]
- Añibarro-Ortega, M.; Pinela, J.; Alexopoulos, A.; Petropoulos, S.A.; Ferreira, I.; Barros, L. The Powerful Solanaceae: Food and Nutraceutical Applications in a Sustainable World. In Advances in Food and Nutrition Research; Toldra, F., Ed.; Elsevier: London, UK, 2022; Volume 100, pp. 131–172. ISBN 978-0-323-99082-0. [Google Scholar]
- Campos-Hernández, N.; Jaramillo-Flores, M.E.; Téllez-Medina, D.I.; Alamilla-Beltrán, L. Effect of Traditional Dehydration Processing of Pepper Jalapeno Rayado (Capsicum annuum) on Secondary Metabolites with Antioxidant Activity. CyTA-J. Food 2018, 16, 316–324. [Google Scholar] [CrossRef]
- de la Cruz-Ricardez, D.D.; Ortiz-García, C.F.C.F.; del Lagunes-Espinoza, L.C.; Torres-de la Cruz, M.; Hernández-Nataren, E. Phenolic Compounds, Carotenoids and Capsaicinoids in Fruits of Capsicum Spp. from Tabasco, Mexico. Agrociencia 2020, 54, 505–519. [Google Scholar] [CrossRef]
- Antonio, A.S.; Wiedemann, L.S.M.; Veiga Junior, V.F. The Genus Capsicum: A Phytochemical Review of Bioactive Secondary Metabolites. RSC Adv. 2018, 8, 25767–25784. [Google Scholar] [CrossRef]
- Hernández-Pérez, T.; del Gómez-García, M.R.; Valverde, M.E.; Paredes-López, O. Capsicum annuum (Hot Pepper): An Ancient Latin-American Crop with Outstanding Bioactive Compounds and Nutraceutical Potential. A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2972–2993. [Google Scholar] [CrossRef]
- Steiner, A.A. A Universal Method for Preparing Nutrient Solutions of a Certain Desired Composition. Plant Soil. 1961, 15, 134–154. [Google Scholar] [CrossRef]
- Quiterio-Gutiérrez, T.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Hernández-Fuentes, A.D.; Sandoval-Rangel, A.; Benavides-Mendoza, A.; Cabrera-de la Fuente, M.; Juárez-Maldonado, A. The Application of Selenium and Copper Nanoparticles Modifies the Biochemical Responses of Tomato Plants under Stress by Alternaria solani. Int. J. Mol. Sci. 2019, 20, 1950. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; AOAC International: Gaithersburg, MD, USA, 1995. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hernández, H.; González-Morales, S.; Benavides-Mendoza, A.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Juárez-Maldonado, A. Effects of Chitosan–PVA and Cu Nanoparticles on the Growth and Antioxidant Capacity of Tomato under Saline Stress. Molecules 2018, 23, 178. [Google Scholar] [CrossRef]
- Garza-Alonso, C.A.; Gonzalez-Garcia, Y.; Cadenas-Pliego, G.; Olivares-Saenz, E.; Trejo-TéLlez, L.I.; Benavides-Mendoza, A. Seed Priming with ZnO Nanoparticles Promotes Early Growth and Bioactive Compounds of Moringa oleifera. Not. Bot. Horti. Agrobot. Cluj. Napoca. 2021, 49, 12546. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin–Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of Total Flavonoid Content by Aluminum Chloride Assay: A Critical Evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Xue, T.; Hartikainen, H.; Piironen, V. Antioxidative and Growth-Promoting Effect of Selenium on Senescing Lettuce. Plant Soil 2001, 237, 55–61. [Google Scholar] [CrossRef]
- Elavarthi, S.; Martin, B. Spectrophotometric Assays for Antioxidant Enzymes in Plants. In Plant Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2010; pp. 273–280. [Google Scholar]
- Chen, J.; Zou, X.; Liu, Q.; Wang, F.; Feng, W.; Wan, N. Combination Effect of Chitosan and Methyl Jasmonate on Controlling Alternaria alternata and Enhancing Activity of Cherry Tomato Fruit Defense Mechanisms. Crop. Prot. 2014, 56, 31–36. [Google Scholar] [CrossRef]
- Pedrero, Z.; Madrid, Y.; Cámara, C. Selenium Species Bioaccessibility in Enriched Radish (Raphanus sativus): A Potential Dietary Source of Selenium. J. Agric Food Chem. 2006, 54, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. Selenium Accumulation by Plants. Ann. Bot. 2016, 117, 217–235. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Pezzarossa, B. Selenium Enrichment of Horticultural Crops. Molecules 2017, 22, 933. [Google Scholar] [CrossRef]
- Chauhan, R.; Awasthi, S.; Srivastava, S.; Dwivedi, S.; Pilon-Smits, E.A.H.; Dhankher, O.P.; Tripathi, R.D. Understanding Selenium Metabolism in Plants and Its Role as a Beneficial Element. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1937–1958. [Google Scholar] [CrossRef]
- Kang, L.; Wu, Y.; Zhang, J.; An, Q.; Zhou, C.; Li, D.; Pan, C. Nano-Selenium Enhances the Antioxidant Capacity, Organic Acids and Cucurbitacin B in Melon (Cucumis melo L.) Plants. Ecotoxicol. Environ. Saf. 2022, 241, 113777. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Meybodi, N.D.H.; da Silva, J.A.T. Foliar Application of Selenium and Nano-Selenium Affects Pomegranate (Punica granatum Cv. Malase Saveh) Fruit Yield and Quality. South Afr. J. Bot. 2019, 124, 350–358. [Google Scholar] [CrossRef]
- Hernández-Hernández, H.; Quiterio-Gutiérrez, T.; Cadenas-Pliego, G.; Ortega-Ortiz, H.; Hernández-Fuentes, A.D.; Cabrera de la Fuente, M.; Valdés-Reyna, J.; Juárez-Maldonado, A. Impact of Selenium and Copper Nanoparticles on Yield, Antioxidant System, and Fruit Quality of Tomato Plants. Plants 2019, 8, 355. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Abdelrahman, M.; Hosseini, M.S.; Hoveizeh, N.F.; Tran, L.-S.P. Alleviation of the Effect of Salinity on Growth and Yield of Strawberry by Foliar Spray of Selenium-Nanoparticles. Environ. Pollut. 2019, 253, 246–258. [Google Scholar] [CrossRef]
- Azcón-Bieto, J.; Talón, M. Fundamentals of Plant Physiology; Edicions Universitat de Barcelona: Barcelona, Spain, 2000; ISBN 8483381826. [Google Scholar]
- Medrano-Macías, J.; Narvaéz-Ortiz, W.A. Selenium and Nano-Selenium as a New Frontier of Plant Biostimulant. In Selenium and Nano-Selenium in Environmental Stress Management and Crop Quality Improvement; Springer: Berlin/Heidelberg, Germany, 2022; pp. 41–54. [Google Scholar]
- Shalaby, T.A.; Abd-Alkarim, E.; El-Aidy, F.; Hamed, E.S.; Sharaf-Eldin, M.; Taha, N.; El-Ramady, H.; Bayoumi, Y.; dos Reis, A.R. Nano-Selenium, Silicon and H2O2 Boost Growth and Productivity of Cucumber under Combined Salinity and Heat Stress. Ecotoxicol. Environ. Saf. 2021, 212, 111962. [Google Scholar] [CrossRef] [PubMed]
- López-Vicente, M.; Saffan, M.M.; Koriem, M.A.; El-Henawy, A.; El-Mahdy, S.; El-Ramady, H.; Elbehiry, F.; El-Dein Omara, A.; Bayoumi, Y.; Badgar, K. Sustainable Production of Tomato Plants (Solanum lycopersicum L.) under Low-Quality Irrigation Water as Affected by Bio-Nanofertilizers of Selenium and Copper. Sustainability 2022, 14, 3236. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium Intake, Status, and Health: A Complex Relationship. Hormones 2020, 19, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Wen, D. Selenium in Horticultural Crops. Sci. Hortic. 2021, 289, 110441. [Google Scholar] [CrossRef]
- Zhang, X.; He, H.; Xiang, J.; Yin, H.; Hou, T. Selenium-Containing Proteins/Peptides from Plants: A Review on the Structures and Functions. J. Agric Food Chem. 2020, 68, 15061–15073. [Google Scholar] [CrossRef]
- Yadav, V.; Wang, Z.; Wei, C.; Amo, A.; Ahmed, B.; Yang, X.; Zhang, X. Phenylpropanoid Pathway Engineering: An Emerging Approach towards Plant Defense. Pathogens 2020, 9, 312. [Google Scholar] [CrossRef]
- El-Ramady, H.; Abdalla, N.; Alshaal, T.; El-Henawy, A.; Faizy, S.E.-D.A.; Shams, M.S.; Shalaby, T.; Bayoumi, Y.; Elhawat, N.; Shehata, S.; et al. Selenium and Its Role in Higher Plants. In Pollutants in Buildings, Water and Living Organisms; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 235–296. ISBN 978-3-319-19276-5. [Google Scholar]
- Pang, C.-H.; Wang, B.-S. Role of Ascorbate Peroxidase and Glutathione Reductase in Ascorbate–Glutathione Cycle and Stress Tolerance in Plants. In Ascorbate-Glutathione Pathway and Stress Tolerance in Plants; Anjum, N.A., Chan, M.-T., Umar, S., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 91–113. ISBN 978-90-481-9404-9. [Google Scholar]
- Zhang, F.; Li, X.; Wu, Q.; Lu, P.; Kang, Q.; Zhao, M.; Wang, A.; Dong, Q.; Sun, M.; Yang, Z.; et al. Selenium Application Enhances the Accumulation of Flavones and Anthocyanins in Bread Wheat (Triticum aestivum L.) Grains. J. Agric. Food Chem. 2022, 70, 13431–13444. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Rashmi, R.; Ulhas, R.S.; Sudheer, W.N.; Banadka, A.; Nagella, P.; Aldaej, M.I.; Rezk, A.A.-S.; Shehata, W.F.; Almaghasla, M.I. The Role of Nanoparticles in Response of Plants to Abiotic Stress at Physiological, Biochemical, and Molecular Levels. Plants 2023, 12, 292. [Google Scholar] [CrossRef]
- White, P.J. Selenium Metabolism in Plants. Biochim. Et Biophys. Acta BBA—Gen. Subj. 2018, 1862, 2333–2342. [Google Scholar] [CrossRef]
- Sariñana-Navarrete, M.Á.; Hernández-Montiel, L.G.; Sánchez-Chavez, E.; Reyes-Perez, J.J.; Murillo-Amador, B.; Reyes-González, A.; Preciado-Rangel, P. Foliar Fertilization of Sodium Selenite and Its Effects on Yield and Nutraceutical Quality in Grapevine. Rev. De La Fac. De Agron. De La Univ. Del Zulia 2021, 38, 806–824. [Google Scholar] [CrossRef]
- Rahim, F.P.; Rocio, C.G.; Adalberto, B.M.; Lidia Rosaura, S.C.; Maginot, N.H. Agronomic Biofortification with Selenium in Tomato Crops (Solanum lycopersicon L. Mill). Agriculture 2020, 10, 486. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).