Utilization of Prickly Pear Peels Flour as a Natural Source of Minerals, Dietary Fiber and Antioxidants: Effect on Cakes Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Cake Ingredients
2.3. Preparation of Prickly Pear Peel Powders
2.4. Processing of Cake
2.5. Weight, Volume and Specific Volume of Cake Samples Determination
2.6. Color of Cake Samples Determination
2.7. Descriptive Sensory Analysis of Cake Samples
2.8. Chemical Composition
2.9. Determination of P and K
2.10. Determination of Trace Element Levels
2.10.1. Microwave Digestion
2.10.2. Trace Element Levels Determination by ICP-OES
2.11. Total Phenolic Content Determination
2.12. Total Flavonoid Content Determination
2.13. Total Anthocyanin Content Determination
2.14. Total Carotenoids Content Determination
2.15. Antioxidant Activity
2.16. Ultra-High-Performance Liquid-Chromatography–Mass Spectrometry (UPLC-MS/MS) Identification of Phytochemicals Compounds
2.17. MDA Determination by TBARS
2.18. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition of Prickly Pear Peel Flour
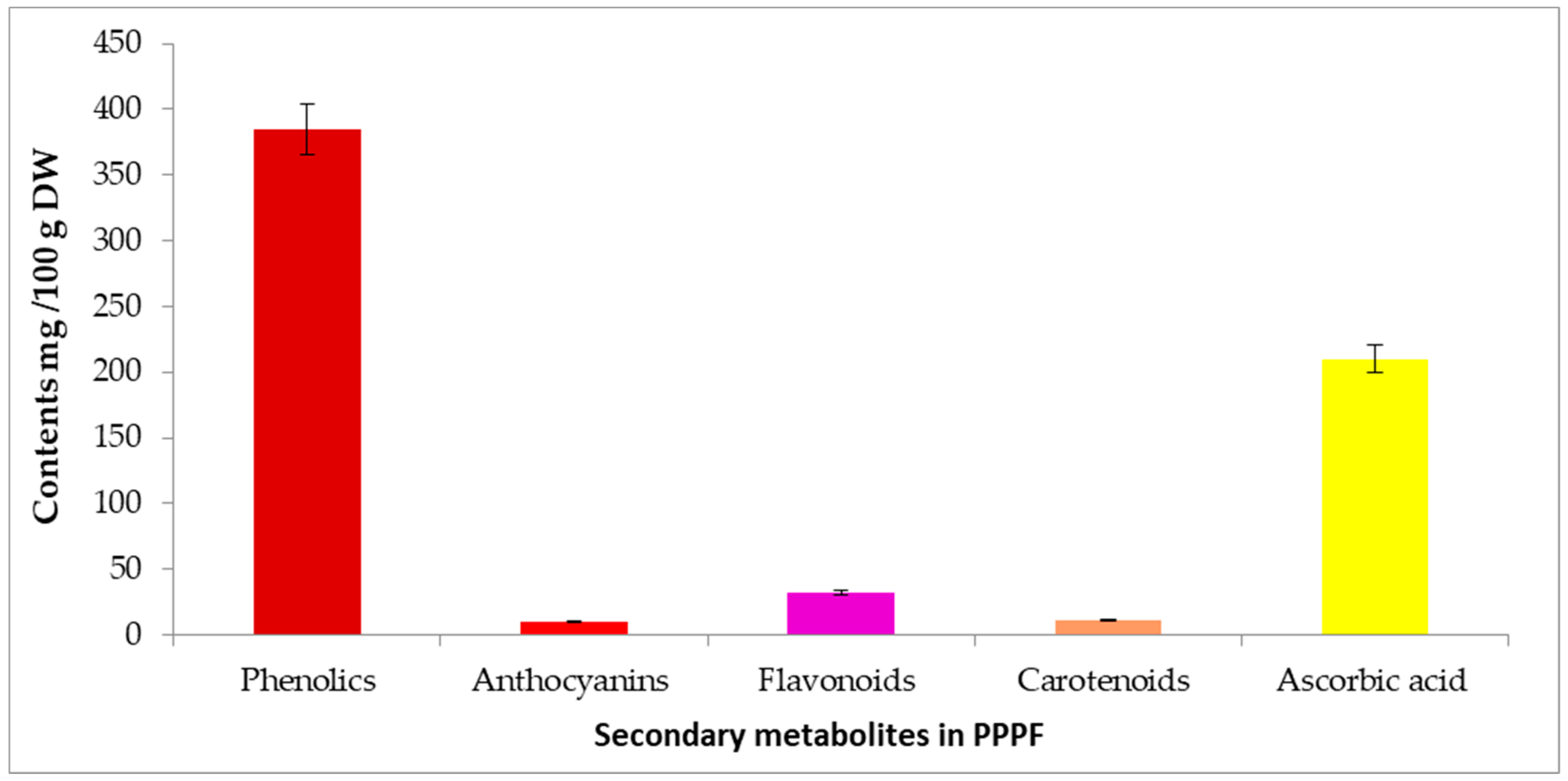
3.2. Secondary Metabolites Content of Prickly Pear Peel Flour
3.3. Antioxidants Activity of Prickly Pear Peel Flour
3.4. Mineral Content of Prickly Pear Peel Flour
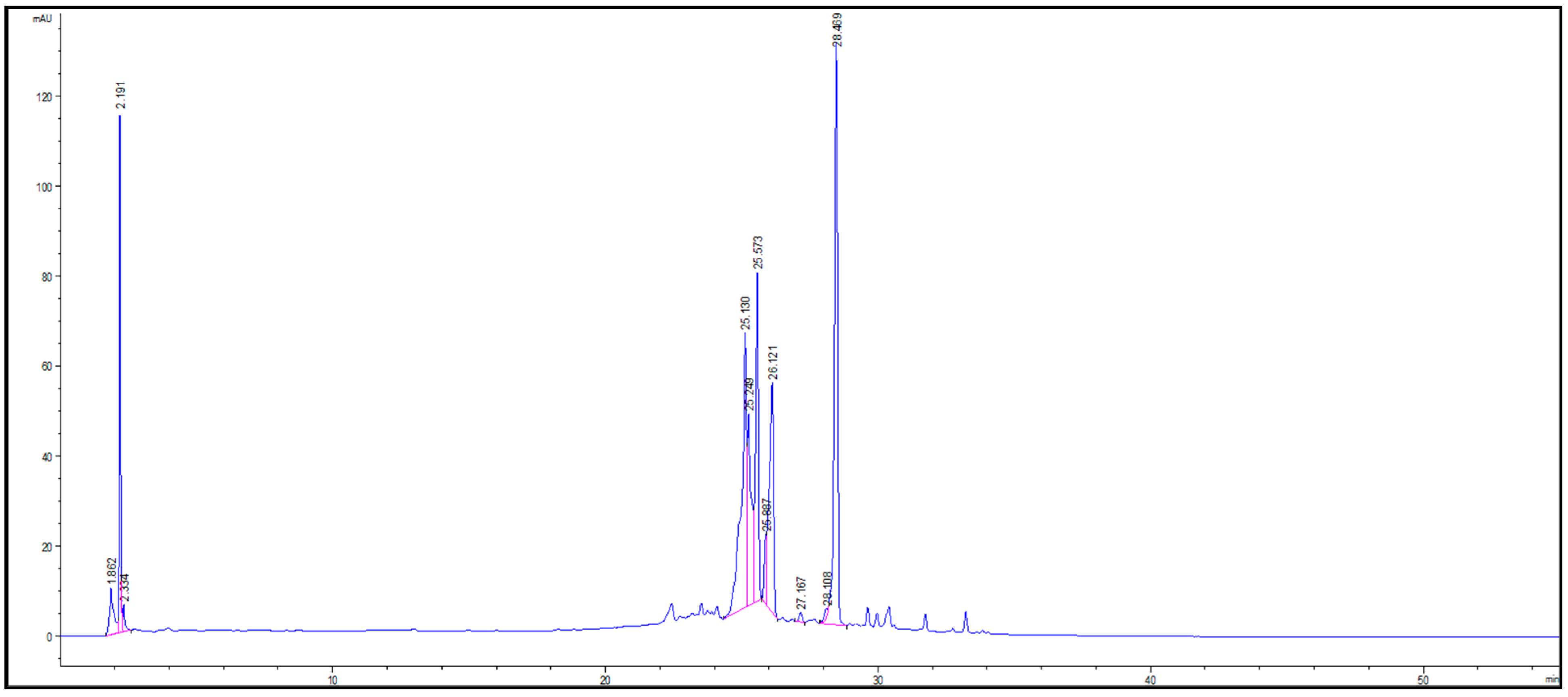
3.5. Polyphenolic Compounds of Prickly Pear Peels
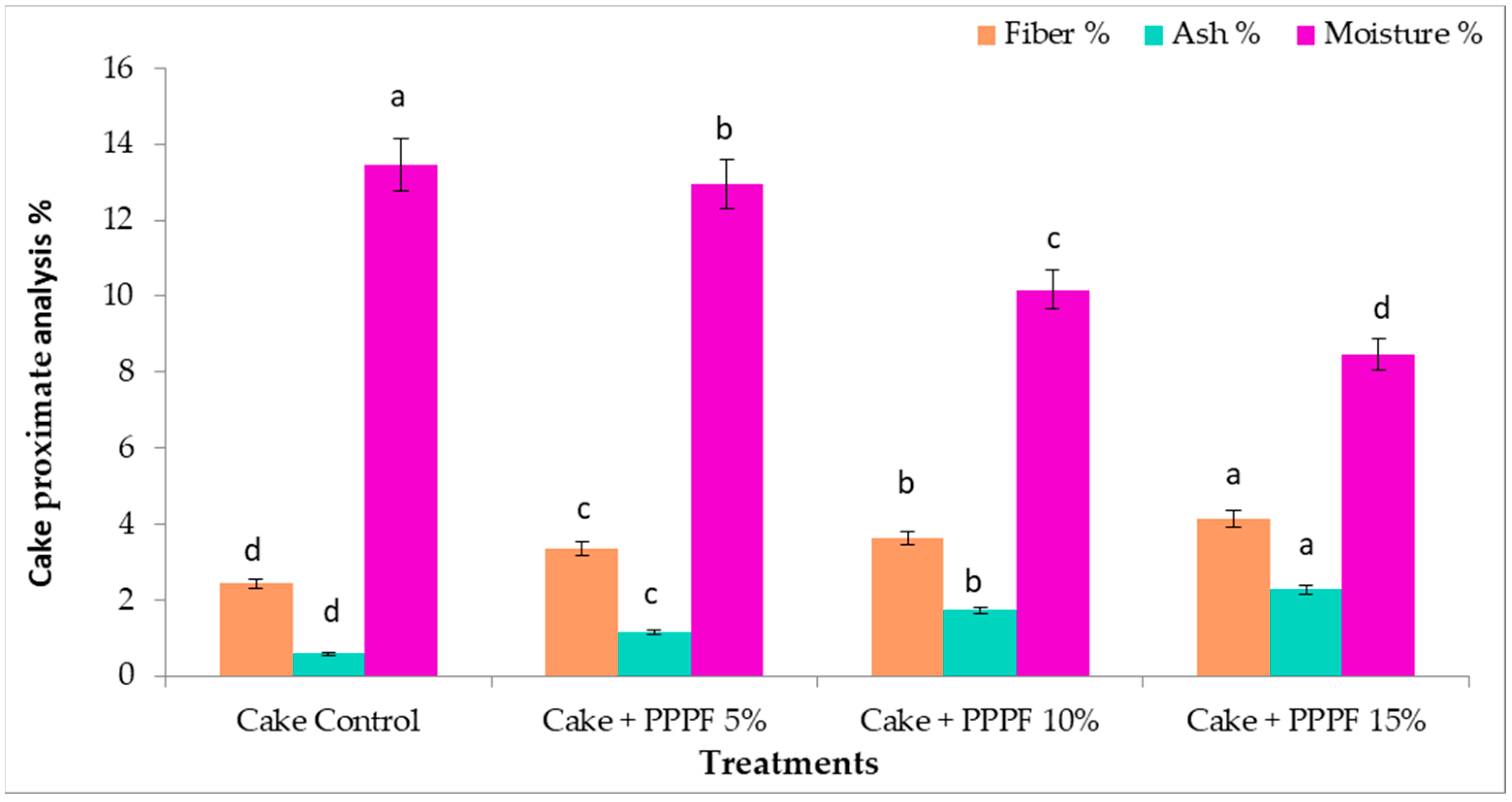
3.6. Proximate Analysis of Cakes Prepared by Replacing Flour with Various Levels of Prickly Pear Peel Flour
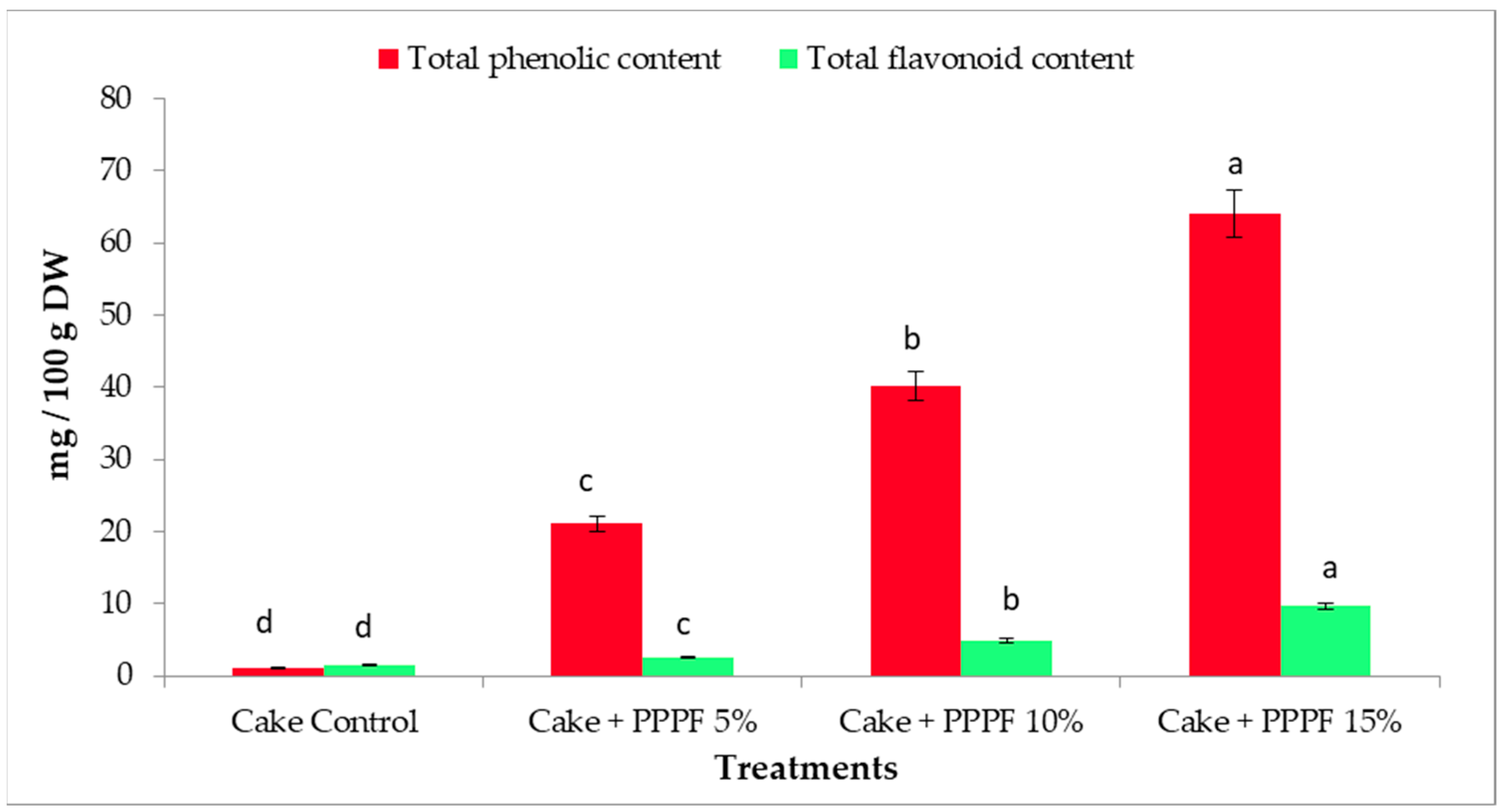
3.7. Phenolic Compounds of Cakes Prepared by Replacing Flour with Various Levels of Prickly Pear Peel Flour
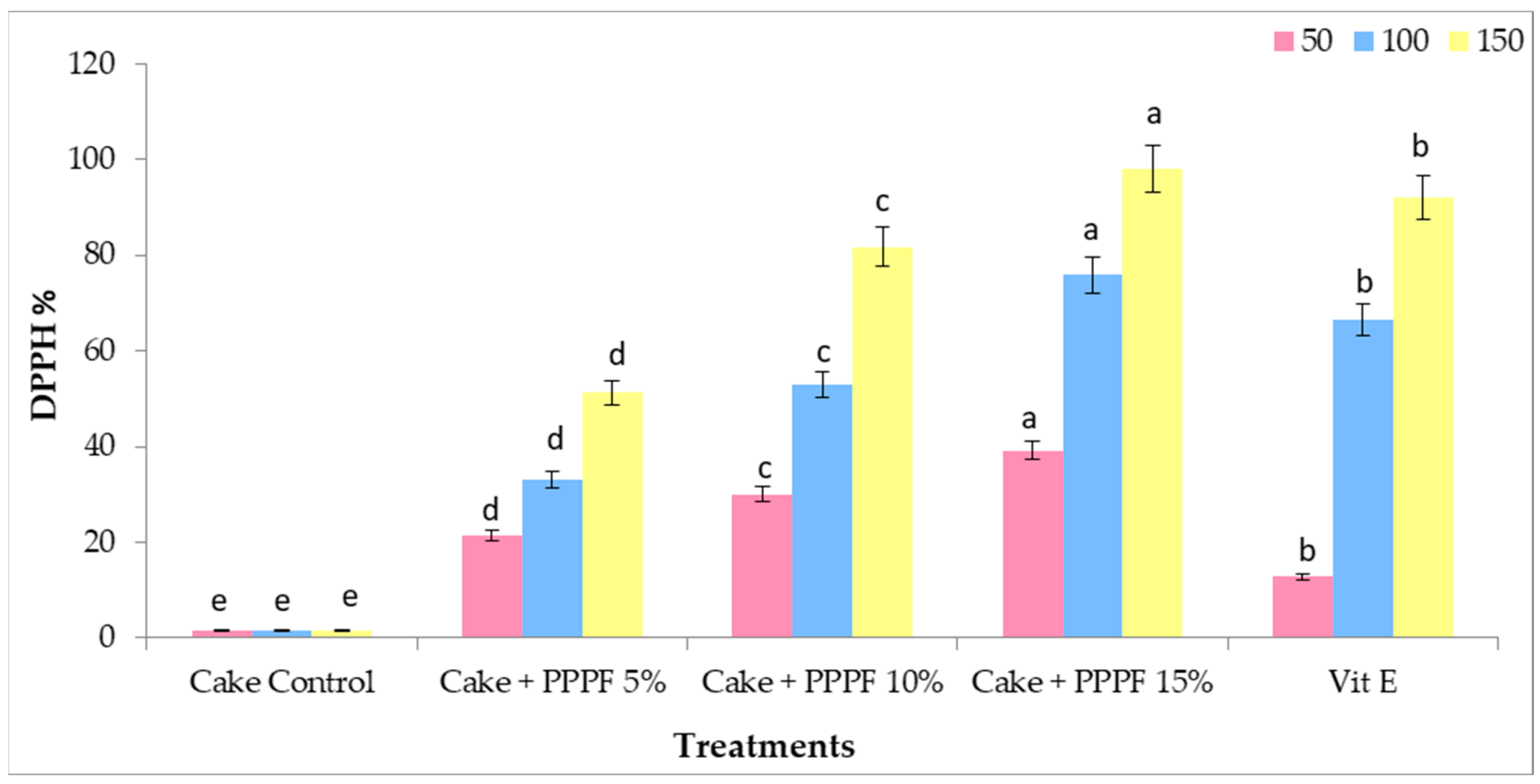
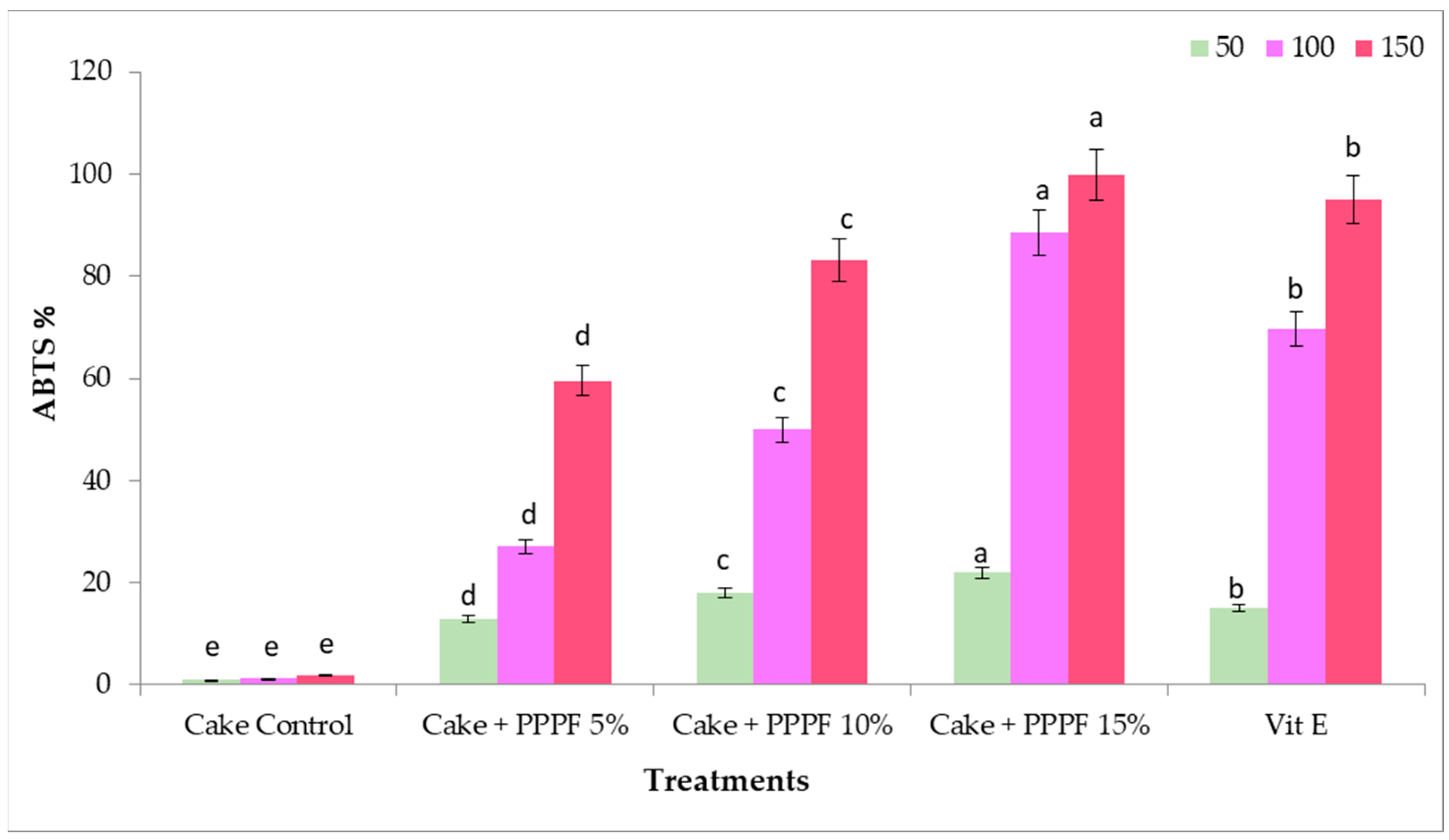
3.8. Antioxidants Activity of Cakes Prepared by Replacing Flour with Various Levels of Prickly Pear Peel Flour
3.9. Minerals Content of Cakes Prepared by Replacing Flour with Different Levels of PPPF
3.10. Evaluation of Cakes Prepared by Replacing Flour with Different Levels of PPPF
3.10.1. Physicochemical Characterization
3.10.2. Color
3.11. Sensory Evaluation
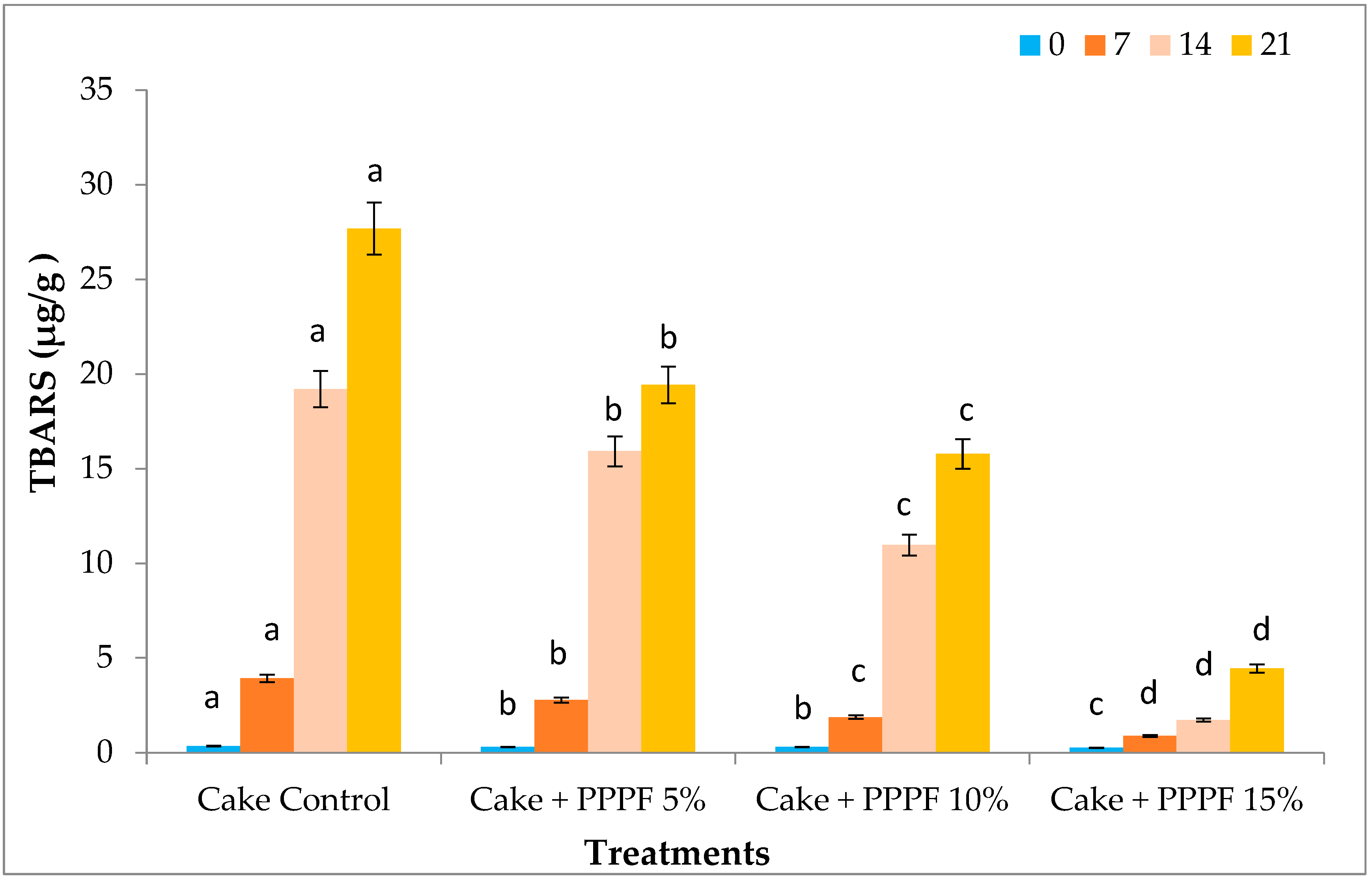
3.12. Thiobarbituric Acid Reactive Substances (TBARS) Cakes Prepared by Replacing Flour with Various Levels of PPPF during Storage (Days)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oniszczuk, A.; W’ojtowicz, A.; Oniszczuk, T.; Matwijczuk, A.; Dib, A.; Markut-Miotła, E. Opuntia fruits as food enriching ingredient, the first step towards new functional food products. Molecules 2020, 25, 916. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Garcia, C.; Fessard, A.; Munekata, P.E.S.; Lorenzo, J.M.; Aboudia, A.; Ouadia, A.; Remize, F. Opuntia ficus indica edible parts: A food and nutritional security perspective. Food Rev. Int. 2020, 38, 930–952. [Google Scholar] [CrossRef]
- Marquez, B.; Ayala, G.X.; Wing, R.R. Acculturation and weight loss strategies among latinas. J. Immigr. Minor. Health 2015, 17, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Reda, T.H.; Atsbha, M.K. Nutritional composition, antinutritional factors, antioxidant activities, functional properties, and sensory evaluation of cactus pear (Opuntia ficus-indica) seeds grown in tigray region, Ethiopia. Int. J. Food Sci. 2019, 2019, 5697052. [Google Scholar] [CrossRef] [PubMed]
- Tahir, H.E.; Xiaobo, Z.; KomLa, M.G.; Adam, M.A. Nopal cactus (Opuntia ficus-indica (L.) Mill) as a source of bioactive compounds. In Wild Fruits: Composition, Nutritional Value and Products; Mariod, A.A., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 333–358. [Google Scholar] [CrossRef]
- Bouazizia, S.; Montevecchib, G.; Antonellib, A.; Hamdia, M. Effects of prickly pear (Opuntia ficus-indica L.) peel flour as an innovative ingredient in biscuits formulation. LWT—Food Sci. Technol. 2020, 124, 109155. [Google Scholar] [CrossRef]
- Valero-Galv’an, J.; Gonz’alez-Fern’andez, R.; Sigala-Hern’andez, A.; Núnez-Gast’elum, J.A.; Ruiz-May, E.; Rodrigo-García, J.; Larqu’e-Saavedra, A.; del Rocío Martínez-Ruiz, N. Sensory attributes, physicochemical and antioxidant characteristics, and protein profile of wild prickly pear fruits (O. macrocentra Engelm., O. phaeacantha Engelm., and O. engelmannii Salm-Dyck ex Engelmann.) and commercial prickly pear fruits (O. ficus-indica (L.) Mill.). Food Res. Int. 2021, 140, 109909. [Google Scholar] [CrossRef]
- Patil, K.; Dagadkhair, A. Physicochemical characteristics and antioxidant potential of opuntia fruit: A review. Pharma Innov. 2019, 8, 376–380. [Google Scholar]
- De Santiago, E.; Dominguez-Fernandez, M.; Cid, C.; De Pena, M.P. Impact of cooking process on nutritional composition and antioxidants of cactus cladodes (Opuntia ficus-indica). Food Chem. 2018, 240, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Gouws, C.A.; Georgouopoulou, E.; Mellor, D.D.; Naumovski, N. The effect of juicing methods on the phytochemical and antioxidant characteristics of the purple prickly pear (Opuntia ficus indica)—Preliminary findings on juice and pomace. Beverages 2019, 5, 28. [Google Scholar] [CrossRef]
- Msaddak, L.; Siala, R.; Fakhfakh, N.; Ayadi, M.A.; Nasri, M.; Zouari, N. Cladodes from prickly pear as a functional ingredient: Effect on fat retention, oxidative stability, nutritional and sensory properties of cookies. Int. J. Food Sci. Nutr. 2015, 66, 851–857. [Google Scholar] [CrossRef]
- Bakari, S.; Daoud, A.; Felhi, S.; Slim, S.; Gharsallah, N.; Kadri, A. Proximate analysis, mineral composition, phytochemical contents, antioxidant and antimicrobial activities and GC-MS investigation of various solvent extracts of cactus cladode. Food Sci. Technol. 2016, 37, 286–293. [Google Scholar] [CrossRef]
- Liguori, G.; Gentile, C.; Gaglio, R.; Perrone, A.; Guarcello, R.; Francesca, N.; Fretto, S.; Inglese, P.; Settanni, L. Effect of addition of Opuntia ficus-indica mucilage on the biological leavening, physical, nutritional, antioxidant and sensory aspects of bread. J. Biosci. Bioeng. 2020, 129, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, R.; Parafati, L.; Restuccia, C.; Fallico, B. Application of prickly pear fruit extract to improve domestic shelf life, quality and microbial safety of sliced beef. Food Chem. Toxicol. 2018, 118, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Albuquerque, T.; Pereira, P.; Ramalho, R.; Vicente, F.; Oliveira, M.; Costa, H. Opuntia ficus-indica (L.) Mill.: A multi-benefit potential to be exploited. Molecules 2021, 26, 951. [Google Scholar] [CrossRef] [PubMed]
- Parafati, L.; Restuccia, C.; Palmeri, R.; Fallico, B.; Arena, E. Characterization of prickly pear peel flour as a bioactive and functional ingredient in bread preparation. Foods 2020, 9, 1189. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Elmelegy, A.A.; Eldesoky, S.E.; Safwat, G. Phytochemical screening, antimicrobial, antioxidant, anticancer activities and nutritional values of cactus (Opuntia ficus indicia) pulp and peel. Fresenius Environ. Bull. 2019, 28, 1534–1551. [Google Scholar]
- El-Beltagi, H.S.; El-Senousi, N.A.; Ali, Z.A.; Omran, A.A. The impact of using chickpea flour and dried carp fish powder on pizza quality. PLoS ONE 2017, 12, e0183657. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; El-Mogy, M.M.; Parmar, A.; Mansour, A.T.; Shalaby, T.A.; Ali, M.R. Phytochemical characterization and utilization of dried red beetroot (Beta vulgaris) peel extract in maintaining the quality of Nile Tilapia Fish Fillet. Antioxidants 2022, 11, 906. [Google Scholar] [CrossRef]
- Abdel-Rahim, E.A.; El-Beltagi, H.S.; Romela, R.M. White Bean seeds and Pomegranate peel and fruit seeds as hypercholesterolemic and hypolipidemic agents in albino rats. Grasas Aceites 2013, 64, 50–58. [Google Scholar] [CrossRef]
- Abdel-Rahim, E.A.; El-Beltagi, H.S. Constituents of apple, parsley and lentil edible plants and their therapy treatments for blood picture as well as liver and kidney functions against lipidemic disease. Electron. J. Environ. Agric. Food Chem. 2010, 9, 1117–1127. [Google Scholar]
- Afify, A.E.-M.M.R.; El-Beltagi, H.S.; Aly, A.A.; El-Ansary, A.E. Antioxidant enzyme activities and lipid peroxidation as biomarker for potato tuber stored by two essential oils from Caraway and Clove and its main component carvone and eugenol. Asian Pac. J. Trop. Biomed. 2012, 2, S772–S780. [Google Scholar] [CrossRef]
- Elkatry, H.O.; Ahmed, A.R.; El-Beltagi, H.S.; Mohamed, H.I.; Eshak, N.S. Biological activities of grape seed by-products and their potential use as natural sources of food additives in the production of Balady bread. Foods 2022, 11, 1948. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; Eshak, N.S.; Mohamed, H.I.; Bendary, E.S.A.; Danial, A.W. Physical characteristics, minerals content, antioxidants and antibacterial activities of Punica granatum or Citrus sinensis peel extracts and their applications to improve cake quality. Plants 2022, 11, 1740. [Google Scholar] [CrossRef]
- Ahmed, A.S. Nutritional and Technological Studies on the Effect of Phytochemicals on Obesity Injuries and Their Related Diseases by Using Experimental Animals. Ph.D. Thesis, Faculty of Specific Education, Port Saied University, Port Saied, Egypt, 2016. [Google Scholar]
- Bensadón, S.; Hervert-Hernández, D.; Sáyago-Ayeri, S.G.; Goñi, I. By-products of Opuntia ficus-indica as a source of antioxidant dietary fiber. Plant Foods Hum. Nutr. 2010, 65, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, J.A.; Almela, L.; Obón, J.M.; Castellar, R. Determination of antioxidant constituents in cactus pear fruits. Plant Foods Hum. Nutr. 2010, 65, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Carranza, P.; Jattar-Santiago, K.Y.; Avila-Sosa, R.; Pérez-Xochipa, I.; Guerrero-Beltrán, J.A.; Ochoa-Velasco, C.E.; Ruiz-López, I.I. Antioxidant fortification of yogurt with red cactus pear peel and its mucilage. CyTA J. Food 2019, 17, 824–833. [Google Scholar] [CrossRef]
- Cai, W.; Gu, X.; Tang, J. Extraction, purification and characterization of the flavonoids from Opuntia milpa alta skin. Czech. J. Food Sci. 2010, 28, 108–116. [Google Scholar] [CrossRef]
- Diaz-Vela, J.; Totosaus, A.; Perez-Chabela, M.L. Integration of agroindustrial co-products as functional food ingredients: Cactus pear (Opuntia ficus indica) flour and pineapple (Ananas comosus) peel flour as fiber source in cooked sausages inoculated with lactic acid bacteria. J. Food Process. Preserv. 2015, 39, 2630–2638. [Google Scholar] [CrossRef]
- Mahfouz, M.Z.; Abd-Elnoor, A.V. Housewives’ knowledges and practices of utilizing prickly pear peels and its use in fortifying some bakery products (cake & biscuits): An applied study. Home Econ. J. 2020, 36, 163–199. [Google Scholar] [CrossRef]
- Chougui, N.; Djerroud, N.; Naraoui, F.; Hadjal, S.; Aliane, K.; Zeroual, B.; Larbat, R. Physicochemical properties and storage stability of margarine containing Opuntia ficus-indica peel extract as antioxidant. Food Chem. 2015, 173, 382–390. [Google Scholar] [CrossRef]
- Miranda, D.V.; Rojas, M.L.; Pagador, S.; Lescano, L.; Sanchez-Gonzalez, J.; Linares, G. Gluten-free snacks based on brown rice and amaranth flour with incorporation of Cactus pear peel powder: Physical, nutritional, and sensorial properties. Int. J. Food Sci. 2018, 2018, 7120327. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.M.; Sallam, E.M. Utilization of prickly pear peels to improve quality of pan bread. Arab. J. Nucl. Sci. Appl. 2016, 49, 151–163. [Google Scholar]
- Hedayati, S.; Ansari, S.; Javaheri, Z.; Golmakani, M.T.; Ansarifar, E. Multi-objective optimization of cakes formulated with fig or date syrup and different hydrocolloids based on TOPSIS. LWT 2022, 171, 114088. [Google Scholar] [CrossRef]
- Wilderjans, E.; Luyts, A.; Brijs, K.; Delcour, J.A. Ingredient functionality in batter type cake making. Trends Food Sci. Technol. 2013, 30, 6–15. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, F.; Huang, W.; Tang, X.; Zou, Q.; Li, Z.; Ogawa, A. Sucrose substitution by polyols in sponge cake and their effects on the foaming and thermal properties of egg protein. Food Hydrocoll. 2016, 57, 153–159. [Google Scholar] [CrossRef]
- El-Gendi, H.; Salama, A.; El-Fakharany, E.M.; Saleh, A.K. Optimization of bacterial cellulose production from prickly pear peels and its ex situ impregnation with fruit byproducts for antimicrobial and strawberry packaging applications. Carbohydrate Polym. 2023, 302, 120383. [Google Scholar] [CrossRef]
- Ayadi, M.A.; Abdelmaksoud, W.; Ennouri, M.; Attia, H. Cladodes from Opuntia ficus indica as a source of dietary fiber: Effect on dough characteristics and cake making. Ind. Crops Prod. 2009, 30, 40–47. [Google Scholar] [CrossRef]
- Jeddou, K.B.; Bouaziz, F.; Zouari-Ellouzi, S.; Chaari, F.; Ellouz-Chaabouni, S.; Ellouz-Ghorbel, R.; Nouri-Ellouz, O. Improvement of texture and sensory properties of cakes by addition of potato peel powder with high level of dietary fiber and protein. Food Chem. 2017, 217, 668–677. [Google Scholar] [CrossRef]
- Segundo, C.; Román, L.; Gómez, M.; Martínez, M.M. Mechanically fractionated flour isolated from green bananas (M. cavendishii var. nanica) as a tool to increase the dietary fiber and phytochemical bioactivity of layer and sponge cakes. Food Chem. 2017, 219, 240–248. [Google Scholar] [CrossRef]
- Ahmed, A.R. Influence of chemical properties of wheat-lupine flour blends on cake quality. Am. J. Food Sci. Technol. 2014, 2, 67–75. [Google Scholar] [CrossRef]
- Randez-Gil, F.; Prieto, J.A.; Murcia, A.; Sanz, P. Construction of baker٫s yeast strains that secret Aspergilus oryzae alph-amylase and their use in bread making. J. Cereal Sci. 1995, 21, 185–193. [Google Scholar] [CrossRef]
- Francis, F.J. Colorimetry of foods. In Physical Properties of Foods; Peleg, M., Bagly, E.B., Eds.; The AVI Publishing Company Inc.: Westort, CT, USA, 1983; pp. 105–123. [Google Scholar]
- A.O.C.C. American of Cereal Association Chemists Approved Method of the AOAC, 10th ed.; American Association of Cereal Chemists: St. Paul, MD, USA, 1996; pp. 4, 13, 61. [Google Scholar]
- A.O.A.C. Association of Official Analytical Chemists. In Official Methods of Analysis, 18th ed.; AOAC International: Arlington, VA, USA, 2010. [Google Scholar]
- Asker, A.; Treptow, H. Quality Assurance in Tropical Fruits Processing; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1993. [Google Scholar]
- Tobaruela, E.D.C.; Santos, A.D.O.; de Almeida-Muradian, L.B.; Araujo, E.D.S.; Lajolo, F.M.; Menezes, E.W. Application of dietary fiber method AOAC 2011.25 in fruit and comparison with AOAC 991.43 method. Food Chem. 2018, 238, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, J. Postharvest Biological Control; Wiely Eastern Limited.: New Delhi, India, 1985. [Google Scholar]
- Marin, B.; Chopin, E.I.B.; Jupinet, B.; Gauthier, D. Comparison of microwave-assisted digestion procedures for total trace element content determination in calcareous soils. Talanta 2008, 77, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Goffman, F.D.; Bergman, C.J. Rice kernel phenolic content and its relationship with antiradical efficiency. J. Sci. Food Agric. 2004, 84, 1235–1240. [Google Scholar] [CrossRef]
- Chang, C.; Yang, M.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Young, J.C.; Rabalski, I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef]
- Wang, Y.C.; Chuang, Y.C.; Hsu, H.W. The flavonoid, carotenoid and pectin content in peels of citrus cultivated in Taiwan. Food Chem. 2008, 106, 277–284. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Lourenço, R.V.; Bittante, A.M.Q.B.; Moraes, I.C.F.; Sobral, P.J.d.A. Gelatin-based films reinforced with montmorillonite and activated with nanoemulsion of ginger essential oil for food packaging applications. Food Packag. Shelf Life 2016, 10, 87–96. [Google Scholar] [CrossRef]
- Ruch, R.J.; Cheng, S.J.; Klaunig, J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant Caechins isolated from Chinese Green tea. Carcinogenesis 1989, 10, 1003–1008. [Google Scholar] [CrossRef]
- Vyncke, W. Evaluation of direct thiobarbituric acid extraction method for determining oxidative rancidity in mackerel (Scomber scombrus L.). Fette Seifen Anstrichm. 1975, 77, 239–240. [Google Scholar] [CrossRef]
- PSS, I. SPSS Version 16.0; SPSS Incorporated: Chicago, IL, USA, 2007. [Google Scholar]
- Duncan, D.B. Multiple range and multiple F tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Ndangui, C.B.; Petit, J.; Gaiani, C.; Nzikou, J.M.; Scher, J. Impact of thermal and chemical pretreatments on physicochemical, rheological and functional properties of sweet potato (Ipomea batatas Lam) Flour. Food Bioprocess Technol. 2014, 7, 3618–3628. [Google Scholar] [CrossRef]
- Mahloko, L.M.; Silungwe, H.; Mashau, M.E.; Kgatla, T.E. Bioactive compounds, antioxidant activity and physical characteristics of wheat-prickly pear and banana biscuits. Heliyon 2019, 5, e02479. [Google Scholar] [CrossRef] [PubMed]
- Sharoba, A. Improve the Nutritive Value of Produced Cake by Replacement Wheat Flour with Pearl Millet Flour. Ann. Agric. Sci. Moshtohor. 2021, 59, 445–454. [Google Scholar] [CrossRef]
- Abou-Bakr, T.M.; Youssef, M.M.; Moharram, H.A. Analysis, health benefits and applications of prebiotic: A Review. Alex. J. Food Sci. Technol. 2014, 11, 25–37. [Google Scholar]
- García-Cayuela, T.; Gómez-Maqueo, A.; Guajardo-Flores, D.; Welti-Chanes, J.; Cano, M.P. Characterization and quantification of individual betalain and phenolic compounds in Mexican and Spanish prickly pear (Opuntia ficus-indica L. Mill) tissues: A comparative study. J. Food Comp. Anal. 2019, 76, 1–13. [Google Scholar] [CrossRef]
- Hegazy, E.M.; Ibrahim, N.M.; Saleh, N.S.M. Determination of antioxidant and antifungal activities in cookies fortified with solar dried prickly pear peels powder. Pak. J. Biol. Sci. 2020, 23, 590–601. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; El-Mahdy, O.M.; Mohamed, H.I.; El-Ansary, A.E. Antioxidants, antimicrobial, and anticancer activities of purified chitinase of Talaromyces funiculosus Strain CBS 129594 biosynthesized using crustacean bio-wastes. Agronomy 2022, 12, 2818. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Abdelazeem, A.S.; Youssef, R.; Safwat, G. GC-MS analysis, antioxidant, antimicrobial and anticancer activities of extracts from Ficus sycomorus fruits and leaves. Not. Bot. Horti. Agrobot. Cluj-Napoca 2019, 47, 493–505. [Google Scholar] [CrossRef]
- Afify, A.M.R.; Rashed, M.M.; Ebtesam, A.M.; El-Beltagi, H.S. Effect of gamma radiation on the lipid profiles of soybean, peanut and sesame seed oils. Grasas Aceites 2013, 64, 356–368. [Google Scholar] [CrossRef]
- Naeem, M.; Basit, A.; Ahmad, I.; Mohamed, H.I.; Wasila, H. Effect of salicylic acid and salinity stress on the performance of tomato. Gesunde Pflanzen 2020, 72, 393–402. [Google Scholar] [CrossRef]
- Afify, A.E.M.M.; El-Beltagi, H.S. Effect of insecticide cyanophos on liver function in adult male rats. Fresenius Environ. Bull. 2011, 20, 1084–1088. [Google Scholar]
- Abou-Elella, F.; Ali, R. Antioxidant and Anticancer Activities of Different Constituents Extracted from Egyptian Prickly Pear Cactus (Opuntia ficus Indica) Peel. Biochem. Anal. Biochem. 2014, 3, 158. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Dawi, F.; Ashoush, I.S.; Ramadan, K.M.A. Antioxidant, anticancer and ameliorative activities of Spirulina platensis and pomegranate juice against hepatic damage induced by CCl4. Not. Bot. Hort. Agrobot. Cluj-Napoca 2020, 48, 1941–1956. [Google Scholar] [CrossRef]
- Rajendrasozhan, S.; Moll, H.E.; Snoussi, M.; Romeilah, R.M.; El-Beltagi, H.S.; Shalaby, E.A.; Younes, K.M.; El-Beltagi, H.S. Phytochemical screening and antimicrobial activity of various extracts of aerial parts of Rhanterium epapposum. Processes 2021, 9, 1351. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Aldaej, M.I.; Al-Khayri, J.M.; Rezk, A.A.; Al-Mssallem, M.Q.; Sattar, M.N.; Ramadan, K.M.A. Production and antioxidant activity of secondary metabolites in Hassawi rice (Oryza sativa L.) cell suspension under salicylic acid, yeast extract, and pectin elicitation. Vitr. Cell Dev. Biol. Plant 2022, 58, 615–629. [Google Scholar] [CrossRef]
- Ramadan, K.M.A.; El-Beltagi, H.S.; Shanab, S.M.M.; El-fayoumy, E.A.; Shalaby, E.A.; Bendary, E.S.A. Potential antioxidant and anticancer activities of secondary metabolites of Nostoc linckia cultivated under Zn and Cu stress conditions. Processes 2021, 9, 1972. [Google Scholar] [CrossRef]
- Shallan, M.A.; El-Beltagi, H.S.; Mona, A.M.; Amera, T.M.; Sohir, N.A. Effect of amylose content and pre-germinated brown rice on serum blood glucose and lipids in experimental animal. Aust. J. Basic Appl. Sci. 2010, 4, 114–121. [Google Scholar]
- Dewit, M.; Bothma, C.; Hugo, A.; Sithole, T.; Absalom, C.; van Den, B.C. Physico-chemical and sensory evaluation of cactus pear (Opuntia ficus-indica L. Mill and Opuntia robusta Wendl) cladode flour in different baked products. JPACD 2015, 17, 89–106. [Google Scholar]
- Jiménez-Aguilar, D.M.; López-Martínez, J.M.; Hernández-Brenes, C.; Gutiérrez-Uribe, J.A.; Welti-Chanes, J. Dietary fiber, phytochemical composition and antioxidant activity of Mexican commercial varieties of cactus pear. J. Food Comp. Anal. 2015, 41, 66–73. [Google Scholar] [CrossRef]
- De Wit, M.; Du Toit, A.; Osthoff, G.; Hugo, A. Antioxidant content, capacity and retention in fresh and processed Cactus pear (Opuntia ficus-indica and O. robusta) fruit peels from different fruit-colored cultivars. Front. Sustain. Food Syst. 2020, 4, 133. [Google Scholar] [CrossRef]
- Yeddes, N.; Chérif, J.K.; Guyot, S.; Sotin, H.; Ayadi, M.T. Comparative study of antioxidant power, polyphenols, flavonoids and betacyanins of the peel and pulp of three Tunisian Opuntia forms. Antioxidants 2013, 2, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, E.M.C.; Coelho, M.C.; Ozcan, K.; Pinto, C.A.; Teixeira, J.A.; Saraiva, J.A.; Pintado, M. Emergent technologies for the extraction of antioxidants from Prickly pear peel and their antimicrobial activity. Foods 2021, 10, 570. [Google Scholar] [CrossRef] [PubMed]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Ornelas-Paz, J.d.J.; López-Mata, M.A.; Del-Toro-Sánchez, C.L.; Ayala-Zavala, J.F.; Márquez-Ríos, E. Total phenolic, flavonoid, tomatine, and tomatidine contents and antioxidant and antimicrobial activities of extracts of tomato plant. Int. J. Anal. Chem. 2015, 2015, 284071. [Google Scholar] [CrossRef] [PubMed]
- Kuti, J.O. Antioxidant compounds from four Opuntia cactus pear fruit varieties. Food Chem. 2004, 85, 527–533. [Google Scholar] [CrossRef]
- Slimen, J.B.; Hidouri, M.; Ghouma, M.; Salem, E.B.; Dorozhkin, S.V. Sintering of potassium doped hydroxy-fluorapatite bioceramics. Coatings 2021, 11, 858. [Google Scholar] [CrossRef]
- Agu, H.O.; Okoli, N.A. Physico-chemical, sensory, and microbiological assessments of wheat-based biscuit improved with beniseed and unripe plantain. Food Sci. Nutr. 2014, 2, 464–469. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Bilbao, A.; Vilches, P.; Angulo, I.; LLuis, J.; Fité, B.; Paseiro-Losada, P.; Manuel, J. Brewery waste as a potential source of phenolic compounds: Optimisation of the extraction process and evaluation of antioxidant and antimicrobial activities. Food Chem. 2014, 145, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, K.M.A.; El-Beltagi, H.S.; Mohamed, H.I.; Shalaby, T.A.; Galal, A.; Mansour, A.T.; Aboul Fotouh, A.A.; Bendary, E.S.A. Antioxidant, anti-cancer activity and phytochemicals profiling of Kigelia pinnata fruits. Separation 2022, 9, 379. [Google Scholar] [CrossRef]
- Sehn, G.A.R.; Nogueira, A.D.C.; Almeida, E.L.; Chang, Y.K.; Steel, C.J. Fortification of wheat dough with calcium and magnesium ions affects empirical rheological properties. Cereal Chem. 2015, 92, 405–410. [Google Scholar] [CrossRef]
- Chinma, C.E.; Gernah, D.I. Physicochemical and sensory properties of cookies produced from cassava/soyabean/mango composite flours. J. Food Technol. 2007, 5, 256–260. [Google Scholar]
- Djeghim, F.; Bourekoua, H.; Różyło, R.; Bieńczak, A.; Tanaś, W.; Zidoune, M.N. Effect of By-Products from Selected Fruits and Vegetables on Gluten-Free Dough Rheology and Bread Properties. Appl. Sci. 2021, 11, 4605. [Google Scholar] [CrossRef]
- Martins, Z.; Pinho, O.; Ferreira, I. Fortification of Wheat Bread with Agroindustry By-Products: Statistical Methods for Sensory Preference Evaluation and Correlation with Color and Crumb Structure. J. Food Sci. 2017, 82, 2183–2191. [Google Scholar] [CrossRef]
- El-Shahat, M.S.; Rabie, M.A.; Ragab, M.; Siliha, H. Changes on physicochemical and rheological properties of biscuits substituted with the peel and alcohol-insoluble solids (AIS) from cactus pear (Opuntia ficus-indica). J. Food Sci. Technol. 2019, 56, 3635–3645. [Google Scholar] [CrossRef]
- Collar, C.; Bollain, C.; Rosell, C.M. Rheological behaviour of formulated bread doughs during mixing and heating. Food Sci. Technol. Int. 2007, 13, 99–107. [Google Scholar] [CrossRef]
- El-Shahat, M.S.; Ragab, M.; Siliha, H.A.; Rabie, M.A. Physicochemical characteristics of biscuits fortified with cactus pear peel powder. Zagazig J. Agric. Res. 2017, 44, 1073–1084. [Google Scholar] [CrossRef]

| Ingredients | Flour (g) | Sugar (g/100 g Flour) | Shortening (g/100 g Flour) | Fresh Egg (g/100 g Flour) | Milk (mL/100 g Flour) (1.5% Fat) | Baking Powder (g/100 g Flour) | Vanillin (g/100 g Flour) |
|---|---|---|---|---|---|---|---|
| Cake control | 100 | 60 | 50 | 65 | 50 | 4 | 2 |
| Cake + PPPF 5% | 95 | 60 | 50 | 65 | 50 | 4 | 2 |
| Cake + PPPF 10% | 90 | 60 | 50 | 65 | 50 | 4 | 2 |
| Cake + PPPF 15% | 85 | 60 | 50 | 65 | 50 | 4 | 2 |
| Parameters | Prickly Pear Peels |
|---|---|
| Moisture% | 6.58 ± 1.53 |
| Ash% | 10.81 ± 0.35 |
| Lipid% | 5.17 ± 0.40 |
| Crude Protein% | 3.69 ± 0.19 |
| Carbohydrates% | 49.93 ± 1.21 |
| Crude Fiber% | 25.79 ± 0.23 |
| Reducing Sugar% | 24.43 ± 0.99 |
| Total Nitrogen% | 0.59 ± 0.03 |
| Free Amino Acids% | 0.37 ± 0.01 |
| Treatment | Concentration (µg/mL) | % Inhibition of DPPH | IC50 (μg/mL) | % Inhibition of ABTS | IC50 (μg/mL) | % Inhibition of H2O2 | IC50 (μg/mL) |
|---|---|---|---|---|---|---|---|
| PPPF | 25 | 45.54 ± 0.43 | 23.41 | 40.78 ± 1.66 | 26.37 | 15.37 ± 1.57 | 41.38 |
| 50 | 76.52 ± 1.0 | 67.54 ± 2.26 | 25.16 ± 1.87 | ||||
| 100 | 98.73 ± 0.52 | 87.98 ± 1.71 | 65.36 ± 0.77 | ||||
| 150 | 100.00 ± 0.5 | 97.81 ± 0.50 | 97.21 ± 1.26 | ||||
| Vitamin E | 25 | 12.84 ± 0.16 | 2.49 | 19.96 ± 0.07 | 1.86 | 16.79 ± 1.04 | 6.45 |
| 50 | 27.04 ± 0.36 | 54.73 ± 0.64 | 35.13 ± 0.69 | ||||
| 100 | 66.46 ± 0.96 | 87.93 ± 0.97 | 60.14 ± 1.37 | ||||
| 150 | 92.03 ± 1.32 | 100.00 ± 0.00 | 99.90 ± 0.14 |
| Minerals Contents mg/100 g DW | PPPF |
|---|---|
| Mg | 963.0 ± 2.0 |
| Ca | 929.0 ± 1.0 |
| Na | 911.5 ± 0.5 |
| K | 304.5 ± 0.41 |
| Fe | 117.5 ± 0.32 |
| Mn | 99.5 ± 0.24 |
| Zn | 100.5 ± 0.27 |
| Cu | 53.0 ± 0.15 |
| Ni | 0.07 ± 0.01 |
| Cr | 0.03 ± 0.002 |
| P | 0.01 ± 0.001 |
| Se | 0.00 ± 0.0 |
| Phenolic Compound | Base Beak m/z | [M + H]− | Ret. Time [min] | Relative % | References |
|---|---|---|---|---|---|
| Gallic acid | 125 | 169 | 1.862 | 2.72 ± 0.15 | Alexandre, et al. [83] |
| Piscidic acid | 193 | 255 | 2.191 | 8.98 ± 0.25 | Alexandre, et al. [83] |
| Caffeic acid | 135 | 179 | 2.334 | 0.83 ± 0.08 | Alexandre, et al. [83] |
| Eucomic acid | 179 | 239 | 25.130 | 19.59 ± 0.35 | Procházková et al. [84] |
| Feruloyl-D-glucose | 193 | 355 | 25.249 | 10.10 ± 0.16 | Silva-Beltrán, et al. [85] |
| 3-O-Methylquercetin | 300 | 315 | 25.573 | 13.73 ± 0.23 | Silva-Beltrán, et al. [85] |
| Catechin | 289 | 289 | 25.887 | 2.00 ± 0.17 | Kuti [86] |
| Kaempferol | 185 | 285 | 26.121 | 14.07 ± 0.19 | Kuti [86] |
| Cinnamic | 129 | 147 | 27.167 | 0.39 ± 0.04 | Slimen, et al. [87] |
| Coumarin | 145 | 145 | 28.108 | 0.46 ± 0.01 | Procházková et al. [84] |
| Isorhamnetin | 311 | 315 | 28.469 | 27.10 ± 0.58 | Alexandre, et al. [83] |
| Minerals Content mg/100 g DW | Cake Control | Cake + PPPF 5% | Cake + PPPF 10% | Cake + PPPF 15% |
|---|---|---|---|---|
| Mg | 0.043 ± 0.003 d | 41.97 ± 0.52 c | 93.61 ± 1.10 b | 136.94 ± 0.78 a |
| Ca | 16.00 ± 0.12 d | 56.65 ± 0.69 c | 105.9 ± 0.63 b | 148.5 ± 0.85 a |
| Na | 0.045 ± 0.004 d | 39.83 ± 0.58 c | 88.17 ± 0.53 b | 129.6 ± 0.74 a |
| K | 0.260 ± 0.01 d | 14.17 ± 0.21 c | 29.70 ± 0.18 b | 43.6 ± 0.25 a |
| Fe | 3.60 ± 0.14 d | 9.02 ± 0.13 c | 14.98 ± 0.09 b | 20.4 ± 0.12 a |
| Mn | 0.57 ± 0.02 d | 5.12 ± 0.08 c | 10.19 ± 0.21 b | 14.7 ± 0.1 a |
| Zn | 0.79 ± 0.013 d | 5.39 ± 0.7 c | 10.59 ± 0.42 b | 15.1 ± 0.09 a |
| Cu | 0.001 ± 0.0 d | 2.15 ± 0.04 c | 5.18 ± 0.3 b | 7.54 ± 0.04 a |
| Ni | 0.001 ± 0.0 b | 0.001 ± 0.0 b | 0.003 ± 0.0 a | 0.003 ± 0.001 a |
| Cr | 0.00 ± 0.0 b | 0.000 ± 0.0 b | 0.002 ± 0.0 a | 0.002 ± 0.0 a |
| P | 0.130 ± 0.02 b | 0.132 ± 0.001 b | 0.132 ± 0.002 b | 0.136 ± 0.001 a |
| Se | 0.072 ± 0.001 a | 0.073 ± 0.002 a | 0.073 ± 0.001 a | 0.074 ± 0.000 a |
| Substitution | Weight (g) | Height (cm) | Volume (cm3) | Specific Volume (cm3/g) |
|---|---|---|---|---|
| Cake Control | 230± 3.06 a | 6.70 ± 0.20 b | 685 ± 5.00 b | 2.98 ± 0.02 b |
| Cake + PPPF 5% | 230 ± 2.52 a | 6.33 ± 0.21 c | 658 ± 7.64 c | 2.82 ± 0.02 c |
| Cake + PPPF 10% | 233 ± 1.53 a | 7.60 ± 0.26 a | 715 ± 5.00 a | 3.24 ± 0.03 a |
| Cake + PPPF 15% | 233 ± 1.00 a | 6.30 ± 0.15 c | 633 ± 2.52 d | 2.72 ± 0.01 d |
| Substitution | Crust | Crumb | ||||||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ∆E | L* | a* | b* | ∆E | |
| Flour | 94.68 ± 0.43 a | 23.33 ± 3.21 a | 9.51 ± 0.13 e | - | 94.68 ± 0.43 a | 23.33 ± 3.21 a | 9.51 ± 0.13 d | - |
| PPPF | 60.89 ± 1.72 c | 9.41 ± 1.07 c | 30.52 ± 1.44 d | - | 60.89 ± 1.72 d | 9.41 ± 1.07 bc | 30.52 ± 1.44 c | - |
| Cake Control | 68.81 ± 1.14 b | 11.58 ± 1.54 c | 42.97 ± 1.13 a | 0.00 c | 77.09 ± 1.13 b | 2.39 ± 0.28 c | 29.93 ± 0.47 c | 0.00 d |
| Cake + PPPF 5% | 54.23 ± 2.27 d | 12.58 ± 1.08 c | 36.75 ± 0.33 bc | 17.18 ± 2.74 a | 64.20 ± 0.81 c | 6.81 ± 2.39 d | 36.54 ± 0.60 b | 15.27 c ± 0.26 |
| Cake + PPPF 10% | 58.18 ± 3.08 c | 16.75 ± 1.60 b | 37.63 ± 0.49 b | 12.50 ± 1.95 b | 59.98 ± 0.99 d | 7.84 ± 1.82 bc | 37.14 ± 0.73 b | 19.42 b ± 0.18 |
| Cake + PPPF 15% | 53.40 ± 2.10 d | 12.2 ± 2.73 c | 35.73 ± 1.12 c | 16.36 ± 2.03 a | 54.51 ± 1.05 e | 11.19 ± 0.39 b | 38.47 ± 0.20 a | 25.7 ± 0.49 a |
| Substitution | Appearance | Crust Color | Crumb Color | Texture | Taste | Odor |
|---|---|---|---|---|---|---|
| Cake Control | 8.3 ± 0.48 c | 8.7 ± 0.95 a | 8.8 ± 0.63 a | 8.3 ± 0.95 a | 8.5 ± 0.71 c | 8.4 ± 1.07 a |
| Cake + PPPF 5% | 8.7 ± 0.95 b | 8.0 ± 0.97 c | 8.0 ± 1.06 c | 7.9 ± 1.29 b | 8.9 ± 1.17 b | 8.3 ± 1.06 a |
| Cake + PPPF 10% | 9.0 ± 0.95 a | 8.3 ± 0.95 b | 8.5 ± 0.67 b | 7.8 ± 2.20 b | 9.2 ± 1.07 a | 8.2 ± 0.92 a |
| Cake + PPPF 15% | 7.6 ± 0.88 d | 7.7 ± 0.95 d | 7.3 ± 0.82 d | 7.4 ± 2.27 c | 8.0 ± 0.82 d | 8.0 ± 0.82 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Beltagi, H.S.; Ahmed, A.R.; Mohamed, H.I.; Al-Otaibi, H.H.; Ramadan, K.M.A.; Elkatry, H.O. Utilization of Prickly Pear Peels Flour as a Natural Source of Minerals, Dietary Fiber and Antioxidants: Effect on Cakes Production. Agronomy 2023, 13, 439. https://doi.org/10.3390/agronomy13020439
El-Beltagi HS, Ahmed AR, Mohamed HI, Al-Otaibi HH, Ramadan KMA, Elkatry HO. Utilization of Prickly Pear Peels Flour as a Natural Source of Minerals, Dietary Fiber and Antioxidants: Effect on Cakes Production. Agronomy. 2023; 13(2):439. https://doi.org/10.3390/agronomy13020439
Chicago/Turabian StyleEl-Beltagi, Hossam S., Abdelrahman R. Ahmed, Heba I. Mohamed, Hala Hazam Al-Otaibi, Khaled M. A. Ramadan, and Haiam O. Elkatry. 2023. "Utilization of Prickly Pear Peels Flour as a Natural Source of Minerals, Dietary Fiber and Antioxidants: Effect on Cakes Production" Agronomy 13, no. 2: 439. https://doi.org/10.3390/agronomy13020439
APA StyleEl-Beltagi, H. S., Ahmed, A. R., Mohamed, H. I., Al-Otaibi, H. H., Ramadan, K. M. A., & Elkatry, H. O. (2023). Utilization of Prickly Pear Peels Flour as a Natural Source of Minerals, Dietary Fiber and Antioxidants: Effect on Cakes Production. Agronomy, 13(2), 439. https://doi.org/10.3390/agronomy13020439









