Effect of Mycorrhizal Inoculation on Melon Plants under Deficit Irrigation Regimes
Abstract
1. Introduction
2. Materials and Methods
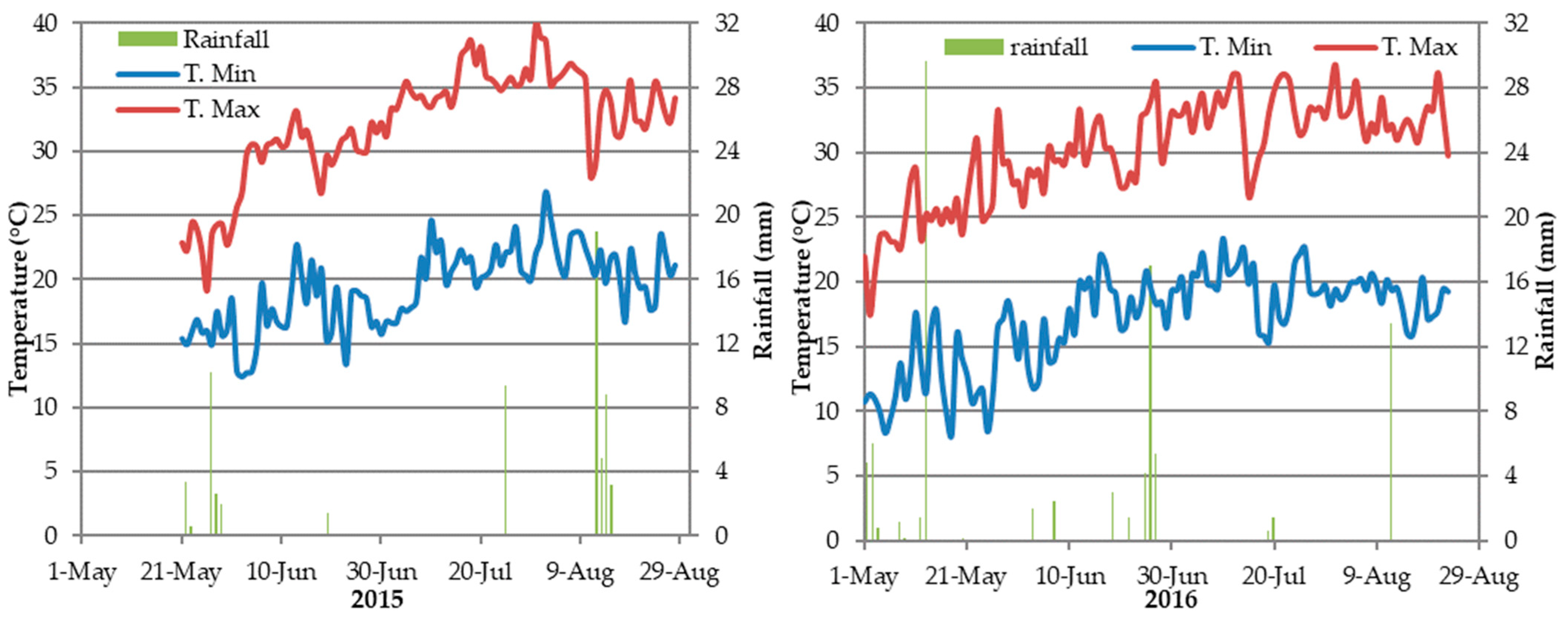
2.1. Experimental Site and Climate Data
2.2. Crop Management, Experimental Design, and Irrigation Treatments
2.3. Agronomical, Morpho-Physiological, Yield, and Quality Parameters
2.4. Statistics and Principal Component Analyses
3. Results
3.1. Amount of Applied Irrigation
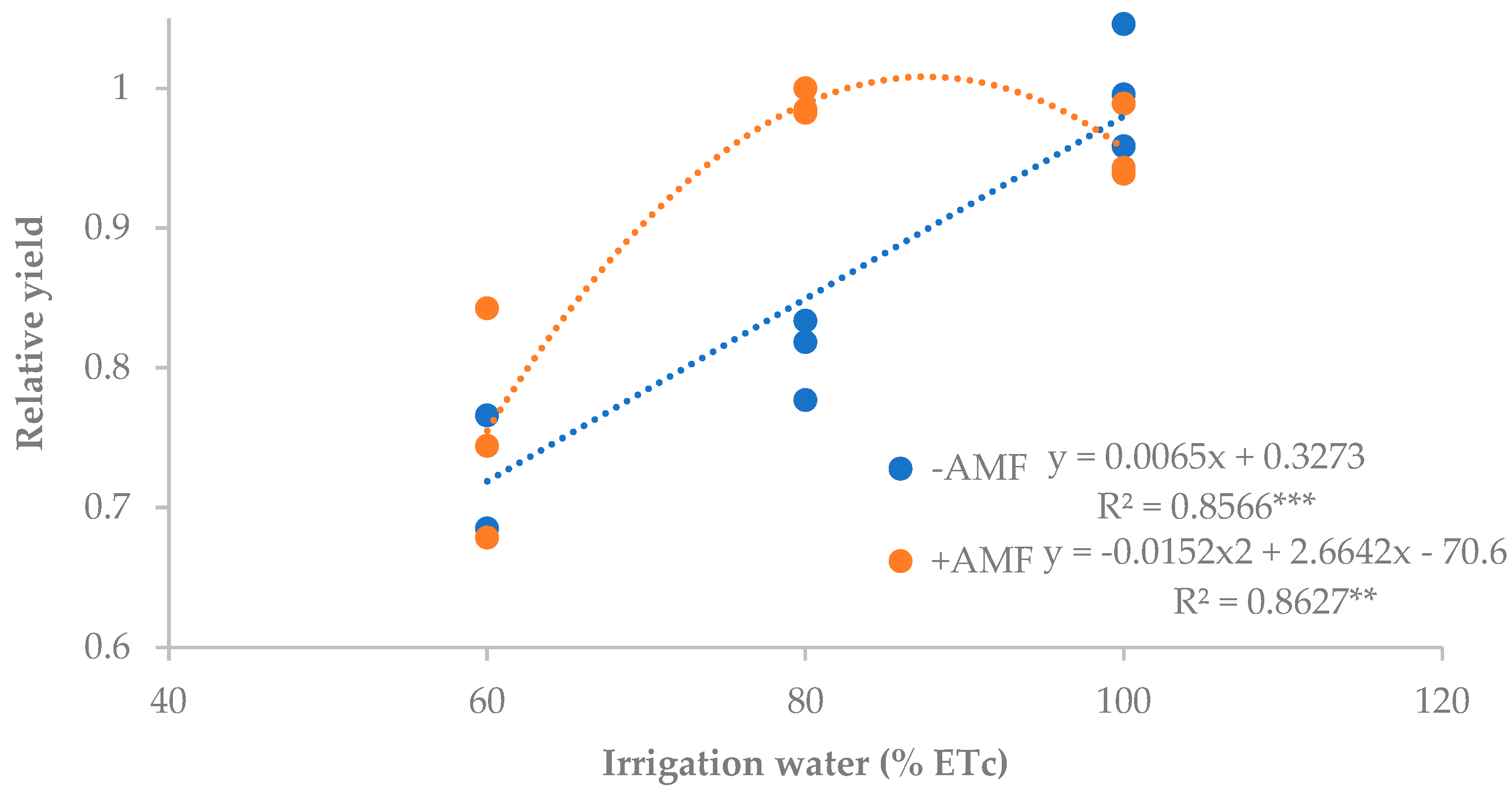
3.2. Plant Growth and Yield Components
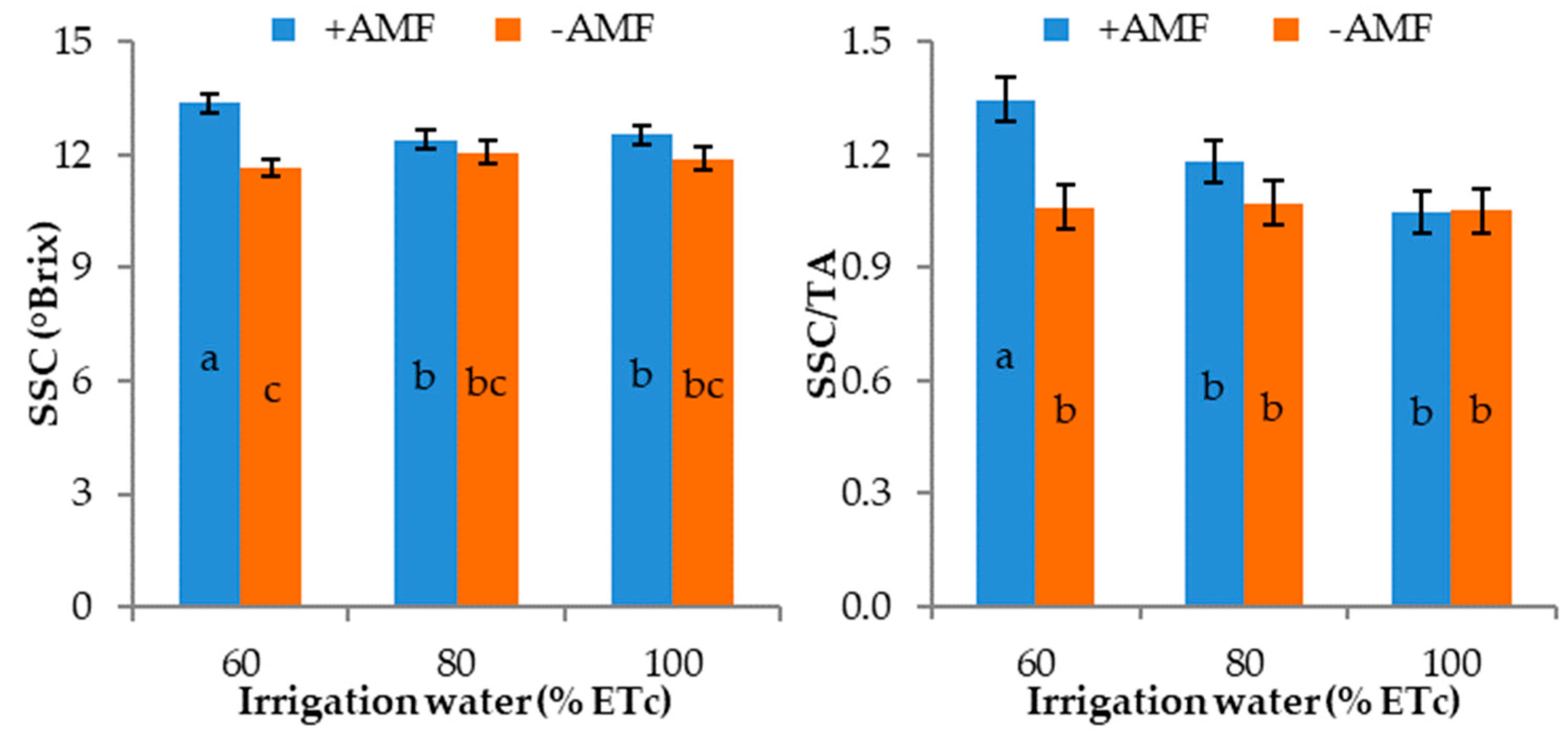
3.3. Fruit Characteristics
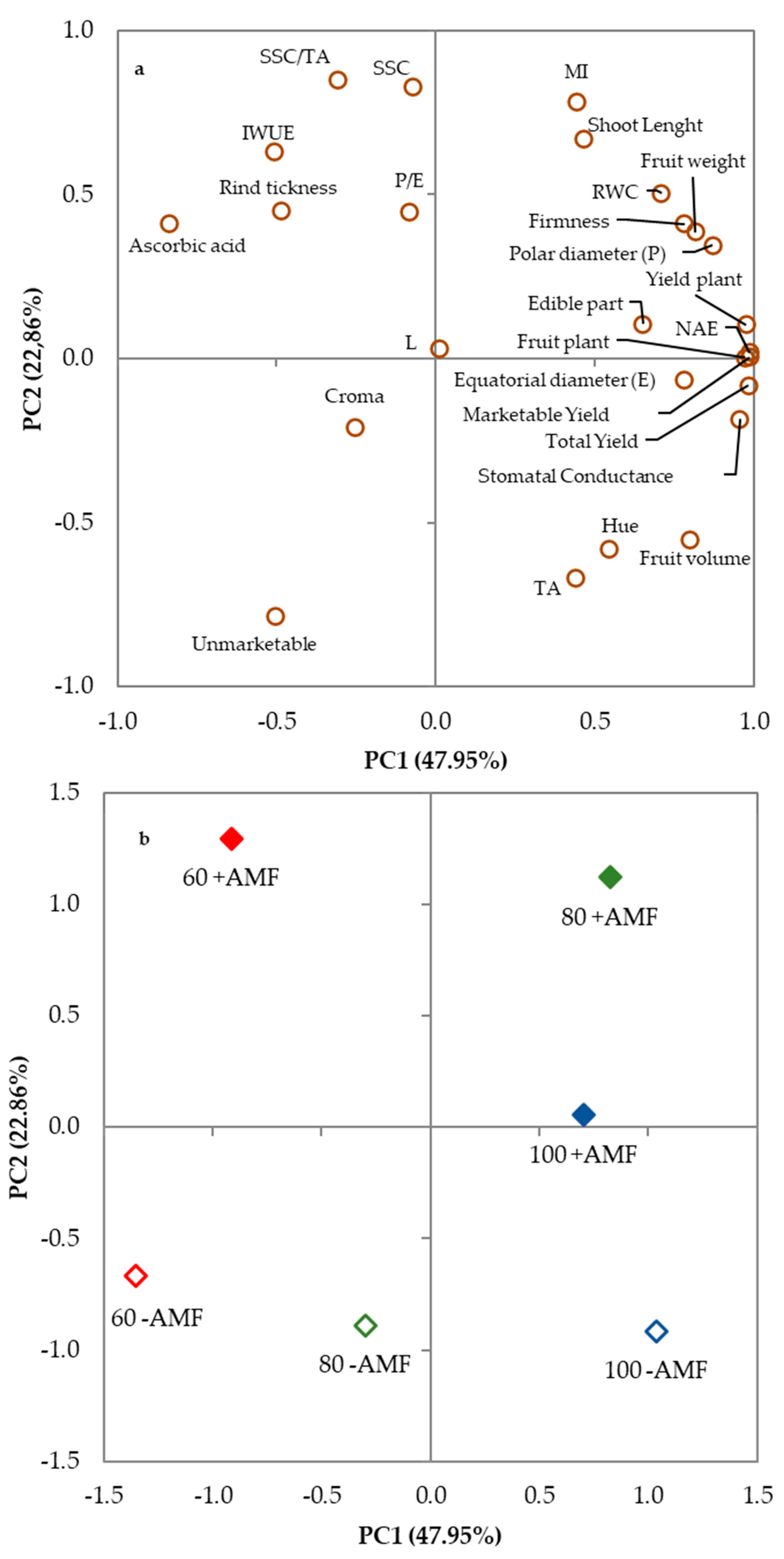
3.4. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jury, W.A.; Vaux, H., Jr. The role of science in solving the world’s emerging water problems. Proc. Natl. Acad. Sci. USA 2005, 102, 15715–15720. [Google Scholar] [CrossRef]
- Bogale, A.; Nagle, M.; Latif, S.; Aguila, M.; Müller, J. Regulated deficit irrigation and partial root-zone drying irrigation impact bioactive compounds and antioxidant activity in two select tomato cultivars. Sci. Hortic. 2016, 213, 115–124. [Google Scholar] [CrossRef]
- Moncada, A.; Vetrano, F.; Miceli, A. Alleviation of Salt Stress by Plant Growth-Promoting Bacteria in Hydroponic Leaf Lettuce. Agronomy 2020, 10, 1523. [Google Scholar] [CrossRef]
- Fereres, E.; Soriano, M.A. Deficit irrigation for reducing agricultural water use. J. Exp. Bot. 2007, 58, 147–159. [Google Scholar] [CrossRef]
- Rodriguez-Ramos, J.C.; Turini, T.; Wang, D.; Hale, L. Impacts of deficit irrigation and organic amendments on soil microbial populations and yield of processing tomatoes. Appl. Soil Ecol. 2022, 180, 104625. [Google Scholar] [CrossRef]
- Kuscu, H.; Turhan, A. Yield, Net Return and Fruit Quality Response of Melon to Deficit Irrigation. Gesunde Pflanz 2022, 74, 647–659. [Google Scholar] [CrossRef]
- Khapte, P.S.; Kumar, P.; Burman, U.; Kumar, P. Deficit irrigation in tomato: Agronomical and physio-biochemical implications. Sci. Hortic. 2019, 248, 256–264. [Google Scholar] [CrossRef]
- Geerts, S.; Raes, D. Deficit irrigation as an on-farm strategy to maximize crop water productivity in dry areas. Agric. Water Manag. 2009, 96, 1275–1284. [Google Scholar] [CrossRef]
- Zeng, C.Z.; Bie, Z.L.; Yuan, B.Z. Determination of optimum irrigation water amount for drip-irrigated muskmelon (Cucumis melo L.) in plastic greenhouse. Agric. Water Manag. 2009, 96, 595–602. [Google Scholar] [CrossRef]
- Wang, J.; Huang, G.; Li, J.; Zheng, J.; Huang, Q.; Liu, H. Effect of soil moisture-based furrow irrigation scheduling on melon (Cucumis melo L.) yield and quality in an arid region of Northwest China. Agric. Water Manag. 2017, 179, 167–176. [Google Scholar] [CrossRef]
- Bang, H.; Leskovar, D.I.; Bender, D.A.; Crosby, K. Deficit irrigation impact on lycopene, soluble solids, firmness and yield of diploid and triploid watermelon in three distinct environments. J. Hortic. Sci. Biotechnol. 2004, 79, 885–890. [Google Scholar] [CrossRef]
- Yildirim, O.; Halloran, N.; Çavuşoğlu, Ş.; Şengül, N. Effects of different irrigation programs on the growth, yield, and fruit quality of drip-irrigated melon. Turkish J. Agric. For. 2009, 33, 243–255. [Google Scholar] [CrossRef]
- Nangare, D.D.; Singh, Y.; Kumar, P.S.; Minhas, P.S. Growth, fruit yield and quality of tomato (Lycopersicon esculentum Mill.) as affected by deficit irrigation regulated on phenological basis. Agric. Water Manag. 2016, 171, 73–79. [Google Scholar] [CrossRef]
- Vetrano, F.; Fascella, S.; Moncada, A.; Incalcaterra, G. Testing various Sicilian melon landraces grown without irrigation. Italus Hortus 2010, 17, 97–98. [Google Scholar]
- Oweis, T.; Hachum, A. Water harvesting and supplemental irrigation for improved water productivity of dry farming systems in West Asia and North Africa. Agric. Water Manag. 2006, 80, 57–73. [Google Scholar] [CrossRef]
- Ali, M.H.; Hoque, M.R.; Hassan, A.A.; Khair, A. Effects of deficit irrigation on yield, water productivity, and economic returns of wheat. Agric. Water Manag. 2007, 92, 151–161. [Google Scholar] [CrossRef]
- Fabeiro, C.; Martín de Santa Olalla, F.; De Juan, J.A. Production of muskmelon (Cucumis melo L.) under controlled deficit irrigation in a semi-arid climate. Agric. Water Manag. 2002, 54, 93–105. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition—Current knowledge and future directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef]
- Lu, J.; Shao, G.; Cui, J.; Wang, X.; Keabetswe, L. Yield, fruit quality and water use efficiency of tomato for processing under regulated deficit irrigation: A meta-analysis. Agric. Water Manag. 2019, 222, 301–312. [Google Scholar] [CrossRef]
- Manzoni, S.; Schimel, J.P.; Porporato, A. Responses of soil microbial communities to water stress: Results from a meta-analysis. Ecology 2012, 93, 930–938. [Google Scholar] [CrossRef]
- Bastida, F.; Torres, I.F.; Romero-Trigueros, C.; Baldrian, P.; Větrovský, T.; Bayona, J.M.; Alarcón, J.J.; Hernández, T.; García, C.; Nicolás, E. Combined effects of reduced irrigation and water quality on the soil microbial community of a citrus orchard under semi-arid conditions. Soil Biol. Biochem. 2017, 104, 226–237. [Google Scholar] [CrossRef]
- Schimel, J.P. Life in dry soils: Effects of drought on soil microbial communities and processes. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 409–432. [Google Scholar] [CrossRef]
- Hale, L.; Curtis, D.; Leon, N.; McGiffen, M., Jr.; Wang, D. Organic amendments, deficit irrigation, and microbial communities impact extracellular polysaccharide content in agricultural soils. Soil Biol. Biochem. 2021, 162, 108428. [Google Scholar] [CrossRef]
- Zhang, X.; Myrold, D.D.; Shi, L.; Kuzyakov, Y.; Dai, H.; Hoang, D.T.T.; Dippold, M.A.; Meng, X.; Song, X.; Li, Z. Resistance of microbial community and its functional sensitivity in the rhizosphere hotspots to drought. Soil Biol. Biochem. 2021, 161, 108360. [Google Scholar] [CrossRef]
- Hijri, M. Analysis of a large dataset of mycorrhiza inoculation field trials on potato shows highly significant increases in yield. Mycorrhiza 2016, 26, 209–214. [Google Scholar] [CrossRef]
- Reid, A.; Greene, S.E. How Microbes Can Help Feed the World: Report on an American Academy of Microbiology Colloquium Washington, DC//December 2012. Am. Soc. Microbiol. 2012, 1–36. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559436/ (accessed on 29 November 2022).
- Miceli, A.; Romano, C.; Moncada, A.; Piazza, G.; Torta, L.; D’Anna, F.; Vetrano, F. Yield and quality of mini-watermelon as affected bygrafting and mycorrhizal inoculum. J. Agric. Sci. Technol. 2016, 18, 505–516. [Google Scholar]
- Redecker, D.; Kodner, R.; Graham, L.E. Glomalean fungi from the Ordovician. Science 2000, 289, 1920–1921. [Google Scholar] [CrossRef]
- Bona, E.; Cantamessa, S.; Massa, N.; Manassero, P.; Marsano, F.; Copetta, A.; Lingua, G.; D’Agostino, G.; Gamalero, E.; Berta, G. Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads improve yield, quality and nutritional value of tomato: A field study. Mycorrhiza 2017, 27, 1–11. [Google Scholar] [CrossRef]
- Bowles, T.M.; Barrios-Masias, F.H.; Carlisle, E.A.; Cavagnaro, T.R.; Jackson, L.E. Effects of arbuscular mycorrhizae on tomato yield, nutrient uptake, water relations, and soil carbon dynamics under deficit irrigation in field conditions. Sci. Total Environ. 2016, 566, 1223–1234. [Google Scholar] [CrossRef]
- Poulton, J.L.; Bryla, D.; Koide, R.T.; Stephenson, A.G. Mycorrhizal infection and high soil phosphorus improve vegetative growth and the female and male functions in tomato. New Phytol. 2002, 154, 255–264. [Google Scholar] [CrossRef]
- Gianinazzi, S.G. Domestication of beneficial soil microorganisms: An innovative technology for agriculture. In Proceedings of the International Congress on Mycorrhizae, Marrakesh, Morocco, 15–17 October 2014; p. 26. [Google Scholar]
- Nzanza, B.; Marais, D.; Soundy, P. Yield and nutrient content of tomato (Solanum lycopersicum L.) as influenced by Trichoderma harzianum and Glomus mosseae inoculation. Sci. Hortic. 2012, 144, 55–59. [Google Scholar] [CrossRef]
- Yang, P.; He, S. The effects of arbuscular mycorrhizal fungi and deficit irrigation on the yield and sugar content of watermelons (Citrullus lanatus). Hortic. Sci. 2022, 49, 225–233. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Sonmez, O.; Aydemir, S.; Tuna, A.L.; Cullu, M.A. The influence of arbuscular mycorrhizal colonisation on key growth parameters and fruit yield of pepper plants grown at high salinity. Sci. Hortic. 2009, 121, 1–6. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef]
- Higo, M.; Azuma, M.; Kamiyoshihara, Y.; Kanda, A.; Tatewaki, Y.; Isobe, K. Impact of phosphorus fertilization on tomato growth and arbuscular mycorrhizal fungal communities. Microorganisms 2020, 8, 178. [Google Scholar] [CrossRef]
- Doorenbos, J.; Pruitt, W.O. Crop Water Requirements; Irrig. Drain. Pap. No. 24; FAO: Rome, Italy, 1974. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Requirements; Irrig. Drain. Pap. No. 56; FAO: Rome, Italy, 1998. [Google Scholar]
- Smith, M. CROPWAT, a Computer Program for Irrigation Planning and Management; FAO Irrigation and Drainage Publications No 46; FAO: Rome, Italy, 1992. [Google Scholar]
- Cabello, M.J.; Castellanos, M.T.; Romojaro, F.; Martínez-Madrid, C.; Ribas, F. Yield and quality of melon grown under different irrigation and nitrogen rates. Agric. Water Manag. 2009, 96, 866–874. [Google Scholar] [CrossRef]
- Miceli, A.; Vetrano, F.; Moncada, A. Effects of Foliar Application of Gibberellic Acid on the Salt Tolerance of Tomato and Sweet Pepper Transplants. Horticulturae 2020, 6, 93. [Google Scholar] [CrossRef]
- Moncada, A.; Vetrano, F.; Esposito, A.; Miceli, A. Fertigation Management and Growth-Promoting Treatments Affect Tomato Transplant Production and Plant Growth after Transplant. Agronomy 2020, 10, 1504. [Google Scholar] [CrossRef]
- Vetrano, F.; Miceli, C.; Angileri, V.; Frangipane, B.; Moncada, A.; Miceli, A. Effect of Bacterial Inoculum and Fertigation Management on Nursery and Field Production of Lettuce Plants. Agronomy 2020, 10, 1477. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–IN18. [Google Scholar] [CrossRef]
- Rajapakse, S.; Miller, J.C. Methods for Studying Vesicular-arbuscular Mycorrhizal Root Colonization and Related Root Physical Properties. Methods Microbiol. 1992, 24, 301–316. [Google Scholar] [CrossRef]
- Liguori, G.; Gaglio, R.; Settanni, L.; Inglese, P.; D’Anna, F.; Miceli, A. Effect of Opuntia ficus-indica Mucilage Edible Coating in Combination with Ascorbic Acid, on Strawberry Fruit Quality during Cold Storage. J. Food Qual. 2021, 2021, 9976052. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239. [Google Scholar] [CrossRef]
- Kirnak, H.; Higgs, D.; Kaya, C.; Tas, I. Effects of Irrigation and Nitrogen Rates on Growth, Yield, and Quality of Muskmelon in Semiarid Regions. J. Plant Nutr. 2005, 28, 621–638. [Google Scholar] [CrossRef]
- Hong, T.; Cai, Z.; Li, R.; Liu, J.; Li, J.; Wang, Z.; Zhang, Z. Effects of water and nitrogen coupling on watermelon growth, photosynthesis and yield under CO2 enrichment. Agric. Water Manag. 2022, 259, 107229. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Colla, G.; Rea, E. Yield, Mineral Composition, Water Relations, and Water Use Efficiency of Grafted Mini-watermelon Plants Under Deficit Irrigation. HortScience 2008, 43, 730–736. [Google Scholar] [CrossRef]
- Bencze, S.; Bamberger, Z.; Janda, T.; Balla, K.; Varga, B.; Bedő, Z.; Veisz, O. Physiological response of wheat varieties to elevated atmospheric CO 2 and low water supply levels. Photosynthetica 2014, 52, 71–82. [Google Scholar] [CrossRef]
- Sun, G.; Liu, X.; Yu, X.; Peng, Y.; Leng, X.; Huang, Y.; Yang, Q. Effects of moistube patterns and fertilization levels on growth and physiological characteristics of blueberry. Chin. J. Ecol. 2019, 38, 604. [Google Scholar]
- Dias, M.C.; Brüggemann, W. Differential inhibition of photosynthesis under drought stress in Flaveria species with different degrees of development of the C4 syndrome. Photosynthetica 2007, 45, 75–84. [Google Scholar] [CrossRef]
- Niu, Y.H.; Cao, H.X.; Shi, X.H.; Wang, X.M. Effects of water deficit on growth-development and physiological characteristics of greenhouse tomato. J. Irrig. Drain. 2013, 32, 67–70. [Google Scholar]
- Li, H.; Liu, H.; Pang, J.; Li, S.; Cui, Y.; Sun, J. Effects of water and nitrogen interaction on growth and nutrient accumulation of potted tomatoes. Trans. Chin. Soc. Agric. Mach. 2019, 50, 272–279. [Google Scholar]
- Wang, Y.; Liu, F.; Jensen, L.S.; de Neergaard, A.; Jensen, C.R. Alternate partial root-zone irrigation improves fertilizer-N use efficiency in tomatoes. Irrig. Sci. 2013, 31, 589–598. [Google Scholar] [CrossRef]
- Ribas, F.; Cabello, M.J.; Moreno, M.M.; Moreno, A.; López-Bellido, L. Influencia del riego y de la aplicación de potasio en la producción del melón (Cucumis melo L.). I: Rendimiento. Invest. Agric. Prod. Prot. Veg. 2001, 16, 283–297. [Google Scholar]
- Sçimsçek, M.; Kacçura, M.; Tonkaz, T. The effects of different irrigation regimes on watermelon [Citrillus lanatus (Thunb.)] yield and yield components under semi-arid climatic conditions. Aust. J. Agric. Res 2004, 55, 1149–1157. [Google Scholar] [CrossRef]
- Zotarelli, L.; Dukes, M.D.; Scholberg, J.M.; Hanselman, T.; Le Femminella, K.; Munoz-Carpena, R. Nitrogen and water use efficiency of zucchini squash for a plastic mulch bed system on a sandy soil. Sci. Hortic. 2008, 116, 8–16. [Google Scholar] [CrossRef]
- Kirnak, H.; Tas, I.; Kaya, C.; Higgs, D. Effects of deficit irrigation on growth, yield and fruit quality of eggplant under semi-arid conditions. Aust. J. Agric. Res. 2002, 53, 1367–1373. [Google Scholar] [CrossRef]
- Dalla Costa, L.; Gianquinto, G. Water stress and watertable depth influence yield, water use efficiency, and nitrogen recovery in bell pepper: Lysimeter studies. Aust. J. Agric. Res. 2002, 53, 201–210. [Google Scholar] [CrossRef]
- Bloch, D.; Hoffmann, C.M.; Märländer, B. Impact of water supply on photosynthesis, water use and carbon isotope discrimination of sugar beet genotypes. Eur. J. Agron. 2006, 24, 218–225. [Google Scholar] [CrossRef]
- Kaya, C.; Higgs, D.; Kirnak, H.; Tas, I. Mycorrhizal colonisation improves fruit yield and water use efficiency in watermelon (Citrullus lanatus Thunb.) grown under well-watered and water-stressed conditions. Plant Soil 2003, 253, 287–292. [Google Scholar] [CrossRef]
- Dogan, E.; Kirnak, H.; Berekatoglu, K.; Bilgel, L.; Surucu, A. Water stress imposed on muskmelon (Cucumis melo L.) with subsurface and surface drip irrigation systems under semi-arid climatic conditions. Irrig. Sci. 2008, 26, 131–138. [Google Scholar] [CrossRef]
- Long, R.L.; Walsh, K.B.; Midmore, D.J.; Rogers, G. Irrigation scheduling to increase muskmelon fruit biomass and soluble solids concentration. HortScience 2006, 41, 367–369. [Google Scholar] [CrossRef]
- Al-Mefleh, N.K.; Samarah, N.; Zaitoun, S.; Al-Ghzawi, A. Effect of irrigation levels on fruit characteristics, total fruit yield and water use efficiency of melon under drip irrigation system. J. Food Agric. Environ. 2012, 10, 540–545. [Google Scholar]
- Liu, X.; Peng, Y.; Yang, Q.; Wang, X.; Cui, N. Determining optimal deficit irrigation and fertilization to increase mango yield, quality, and WUE in a dry hot environment based on TOPSIS. Agric. Water Manag. 2021, 245, 106650. [Google Scholar] [CrossRef]
- Ribas, F.; Cabello, M.J.; Moreno, M.M.; Moreno, A.; López-Bellido, L. Effect of irrigation and potassium application in melon (Cucumis melo L.) production. II: Quality. Span. J. Agric. Res. 2003, 1, 79. [Google Scholar] [CrossRef]
- Leite, V.M.; Rosolem, C.A.; Rodrigues, J.D. Gibberellin and cytokinin effects on soybean growth. Sci. Agric. 2005, 60, 537–541. [Google Scholar] [CrossRef]
- Barzegar, T.; Heidaryan, N.; Lotfi, H.; Ghahremani, Z. Yield, fruit quality and physiological responses of melon cv. Khatooni under deficit irrigation. Adv. Hortic. Sci. 2018, 32, 451–458. [Google Scholar]
- Sharma, S.P.; Leskovar, D.I.; Crosby, K.M.; Volder, A.; Ibrahim, A.M.H. Root growth, yield, and fruit quality responses of reticulatus and inodorus melons (Cucumis melo L.) to deficit subsurface drip irrigation. Agric. Water Manag. 2014, 136, 75–85. [Google Scholar] [CrossRef]
- Olguín, M.A.V.; De la Fuente, M.C.; Mendoza, A.B.; Maldonado, A.J.; Rangel, A.S.; Cusimamani, E.F. Commercial and nutraceutical quality of grafted melon cultivated under hydric stress. Hortic. Sci. 2020, 47, 139–149. [Google Scholar] [CrossRef]
- Cui, N.; Du, T.; Kang, S.; Li, F.; Zhang, J.; Wang, M.; Li, Z. Regulated deficit irrigation improved fruit quality and water use efficiency of pear-jujube trees. Agric. Water Manag. 2008, 95, 489–497. [Google Scholar] [CrossRef]
- Barzegar, T.; Lotfi, H.; Rabiei, V.; Ghahremani, Z.; Nikbakht, J. Effect of water-deficit stress on fruit yield, antioxidant activity, and some physiological traits of four Iranian melon genotypes. Iran. J. Hortic. Sci. 2017, 48, 13–25. [Google Scholar] [CrossRef]
- Li, Y.-J.; Yuan, B.-Z.; Bie, Z.-L.; Kang, Y. Effect of drip irrigation criteria on yield and quality of muskmelon grown in greenhouse conditions. Agric. Water Manag. 2012, 109, 30–35. [Google Scholar] [CrossRef]
- Talukdar, N.C.; Germida, J.J. Occurrence and isolation of vesicular–arbuscular mycorrhizae in cropped field soils of Saskatchewan, Canada. Can. J. Microbiol. 1993, 39, 567–575. [Google Scholar] [CrossRef]
- Błaszkowski, J. Comparative studies of the occurrence of arbuscular fungi and mycorrhizae (Glomales) in cultivated and uncultivated soils of Poland. Acta Mycol. 2014, 28, 93–140. [Google Scholar] [CrossRef]
- Douds, D.D.; Janke, R.R.; Peters, S.E. VAM fungus spore populations and colonization of roots of maize and soybean under conventional and low-input sustainable agriculture. Agric. Ecosyst. Environ. 1993, 43, 325–335. [Google Scholar] [CrossRef]
- Gosling, P.; Hodge, A.; Goodlass, G.; Bending, G.D. Arbuscular mycorrhizal fungi and organic farming. Agric. Ecosyst. Environ. 2006, 113, 17–35. [Google Scholar] [CrossRef]
- Lekberg, Y.; Koide, R.T. Is plant performance limited by abundance of arbuscular mycorrhizal fungi? A meta-analysis of studies published between 1988 and 2003. New Phytol. 2005, 168, 189–204. [Google Scholar] [CrossRef]
- Westphal, A.; Snyder, N.L.; Xing, L.; Camberato, J.J. Effects of Inoculations with Mycorrhizal Fungi of Soilless Potting Mixes During Transplant Production on Watermelon Growth and Early Fruit Yield. HortScience 2008, 43, 354–360. [Google Scholar] [CrossRef]
- Theodorou, C. Soil moisture and the mycorrhizal association of Pinus radiata D. don. Soil Biol. Biochem. 1978, 10, 33–37. [Google Scholar] [CrossRef]
- Boucher, N.L.; Malajczuk, N. Effects of high soil moisture on formation of ectomycorrhizas and growth of karri (Eucalyptus diversicolor) seedlings inoculated with Descolea maculata, Pisolithus tinctorius and Laccaria laccata. New Phytol. 1990, 114, 87–91. [Google Scholar] [CrossRef]
- Barnes, C.J.; van der Gast, C.J.; McNamara, N.P.; Rowe, R.; Bending, G.D. Extreme rainfall affects assembly of the root-associated fungal community. New Phytol. 2018, 220, 1172–1184. [Google Scholar] [CrossRef]
- Huang, Z.; Zou, Z.; He, C.; He, Z.; Zhang, Z.; Li, J. Physiological and photosynthetic responses of melon (Cucumis melo L.) seedlings to three Glomus species under water deficit. Plant Soil 2011, 339, 391–399. [Google Scholar] [CrossRef]
- Jacobson, K.M. Moisture and substrate stability determine VA-mycorrhizal fungal community distribution and structure in an arid grassland. J. Arid Environ. 1997, 35, 59–75. [Google Scholar] [CrossRef]
- Estaun, M.V. Effect of sodium chloride and mannitol on germination and hyphal growth of the vesicular-arbuscular mycorrhizal fungus Glomus mosseae. Agric. Ecosyst. Environ. 1990, 29, 123–129. [Google Scholar] [CrossRef]
- Bolgiano, N.C.; Safir, G.R.; Warncke, D.D. Mycorrhizal infection and growth of onion in the field in relation to phosphorus and water availability. J. Am. Soc. Hortic. Sci. 1983, 108, 819–825. [Google Scholar] [CrossRef]
- Sensoy, S.; Demir, S.; Tufenkci, S.; Erdinç, C.; Demirer, E.; Unsal, H.; Halifeoglu, G.; Ekincialp, A. Response of four zucchini (Cucurbita pepo L.) hybrids to different arbuscular mycorrhizal fungi. J. Anim. Plant Sci. 2011, 21, 31. [Google Scholar]
- Abdel Latef, A.A. Growth and some physiological activities of pepper (Capsicum annuum L.) in response to cadmium stress and mycorrhizal symbiosis. J. Agric. Sci. Technol. 2013, 15, 1437–1448. [Google Scholar]
- Zhao, R.; Guo, W.; Bi, N.; Guo, J.; Wang, L.; Zhao, J.; Zhang, J. Arbuscular mycorrhizal fungi affect the growth, nutrient uptake and water status of maize (Zea mays L.) grown in two types of coal mine spoils under drought stress. Appl. Soil Ecol. 2015, 88, 41–49. [Google Scholar] [CrossRef]
- Subramanian, K.S.; Santhanakrishnan, P.; Balasubramanian, P. Responses of field grown tomato plants to arbuscular mycorrhizal fungal colonization under varying intensities of drought stress. Sci. Hortic. 2006, 107, 245–253. [Google Scholar] [CrossRef]
- Bitterlich, M.; Franken, P.; Graefe, J. Arbuscular Mycorrhiza Improves Substrate Hydraulic Conductivity in the Plant Available Moisture Range Under Root Growth Exclusion. Front. Plant Sci. 2018, 9, 301. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, G.; Erice, G.; Aroca, R.; Zamarreño, Á.M.; García-Mina, J.M.; Ruiz-Lozano, J.M. Radial water transport in arbuscular mycorrhizal maize plants under drought stress conditions is affected by indole-acetic acid (IAA) application. J. Plant Physiol. 2020, 246–247, 153115. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, K.; Bi, Q.; Zhu, J.; Zhang, Q.; Jin, C.; Lu, L.; Lin, X. Effects of organic amendment on soil aggregation and microbial community composition during drying-rewetting alternation. Sci. Total Environ. 2017, 574, 735–743. [Google Scholar] [CrossRef]
- Liu, C.; Ravnskov, S.; Liu, F.; Rubæk, G.H.; Andersen, M.N. Arbuscular mycorrhizal fungi alleviate abiotic stresses in potato plants caused by low phosphorus and deficit irrigation/partial root-zone drying. J. Agric. Sci. 2018, 156, 46–58. [Google Scholar] [CrossRef]
- Augé, R.M. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Azcon, R.; Gomez, M. Effects of arbuscular-mycorrhizal glomus species on drought tolerance: Physiological and nutritional plant responses. Appl. Environ. Microbiol. 1995, 61, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Koide, R.T. Nutrient supply, nutrient demand and plant response to mycorrhizal infection. New Phytol. 1991, 117, 365–386. [Google Scholar] [CrossRef]
- Ames, R.N.; Reid, C.P.P.; Porter, L.K.; Cambardella, C. Hyphal uptake and transport of nitrogen from two 15n-labelled sources by glomus mosseae, a vesicular-arbuscular mycorrhizal fungus. New Phytol. 1983, 95, 381–396. [Google Scholar] [CrossRef]
- Zuccarini, P. Mycorrhizal infection ameliorates chlorophyll content and nutrient uptake of lettuce exposed to saline irrigation. Plant Soil Environ. 2008, 53, 283–289. [Google Scholar] [CrossRef]
- Tawaraya, K.; Naito, M.; Wagatsuma, T. Solubilization of Insoluble Inorganic Phosphate by Hyphal Exudates of Arbuscular Mycorrhizal Fungi. J. Plant Nutr. 2006, 29, 657–665. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Roldán, A.; Albacete, A.; Pascual, J.A. The interaction with arbuscular mycorrhizal fungi or Trichoderma harzianum alters the shoot hormonal profile in melon plants. Phytochemistry 2011, 72, 223–229. [Google Scholar] [CrossRef]
- Kays, S.J. Preharvest factors affecting appearance. Postharvest Biol. Technol. 1999, 15, 233–247. [Google Scholar] [CrossRef]
- Tshibangu Kazadi, A.; Lwalaba wa Lwalaba, J.; Kirika Ansey, B.; Mavungu Muzulukwau, J.; Manda Katabe, G.; Iband Karul, M.; Baert, G.; Haesaert, G.; Mukobo Mundende, R.-P. Effect of Phosphorus and Arbuscular Mycorrhizal Fungi (AMF) Inoculation on Growth and Productivity of Maize (Zea mays L.) in a Tropical Ferralsol. Gesunde Pflanz. 2022, 74, 159–165. [Google Scholar] [CrossRef]
- Mathur, S.; Tomar, R.S.; Jajoo, A. Arbuscular Mycorrhizal fungi (AMF) protects photosynthetic apparatus of wheat under drought stress. Photosynth. Res. 2019, 139, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Copetta, A.; Todeschini, V.; Massa, N.; Bona, E.; Berta, G.; Lingua, G. Inoculation with arbuscular mycorrhizal fungi improves melon (Cucumis melo) fruit quality under field conditions and plant performance in both field and greenhouse. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2021, 155, 1063–1074. [Google Scholar] [CrossRef]
- Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Egamberdieva, D. Bioremediation of adverse impact of cadmium toxicity on Cassia italica Mill by arbuscular mycorrhizal fungi. Saudi J. Biol. Sci. 2016, 23, 39–47. [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.A.; Su, Y.; Lei, Y.; Mustafa, N.S.A.; Ahmad, P.; Zhang, L. Improved Drought Tolerance by AMF Inoculation in Maize (Zea mays) Involves Physiological and Biochemical Implications. Plants 2019, 8, 579. [Google Scholar] [CrossRef]
- Mo, Y.; Wang, Y.; Yang, R.; Zheng, J.; Liu, C.; Li, H.; Ma, J.; Zhang, Y.; Wei, C.; Zhang, X. Regulation of Plant Growth, Photosynthesis, Antioxidation and Osmosis by an Arbuscular Mycorrhizal Fungus in Watermelon Seedlings under Well-Watered and Drought Conditions. Front. Plant Sci. 2016, 7, 644. [Google Scholar] [CrossRef]
- Alarcón, A.L.; Gómez-Bellot, M.J.; Bernabe, A.J.; Calvo, G.; Fernández Martín, F. Changes in root architecture and productivity of melon (Cucumis melo L. cv. Hispano Nunhems) promoted by Glomus iranicum var. tenuihypharum. J. Hortic. Sci. Biotechnol. 2020, 95, 364–373. [Google Scholar] [CrossRef]
- Mohammadi, S.A.; Prasanna, B.M. Analysis of Genetic Diversity in Crop Plants—Salient Statistical Tools and Considerations. Crop Sci. 2003, 43, 1235–1248. [Google Scholar] [CrossRef]








| pH | 7.20 |
| ECe * (µS cm−1) | 480 |
| Organic matter (%) | 1.81 |
| Organic C (%) | 0.82 |
| N Kjeldahl (%) | 0.22 |
| P (ppm) | 98 |
| K (ppm) | 370 |
| Mg (ppm) | 460 |
| Ca (ppm) | 1890 |
| Na (ppm) | 190 |
| Cation exchange capacity (meq 100 g−1) | 13.3 |
| Treatments | MI (%) | Main Shoot Length (cm) | RWC (%) | Stomatal Conductance (mmol m−2 s−1) | ||
|---|---|---|---|---|---|---|
| 20 dat 3 | 30 dat | 40 dat | ||||
| Irrigation treatment (% ETc) | ||||||
| 60 | 18.8 1 | 26.4 b | 87.2 | 152.6 | 89.5 b | 163.5 |
| 80 | 30.3 | 29.1 a | 94.8 | 154.4 | 90.9 ab | 233.8 |
| 100 | 20.3 | 26.4 b | 90.2 | 153.0 | 92.8 a | 306.1 |
| Plant noculation | ||||||
| +AMF | 30.3 | 26.9 | 91.8 | 154.8 | 92.8 a | 241.0 |
| −AMF | 15.9 | 27.7 | 89.7 | 151.8 | 89.3 b | 227.9 |
| Significance2 | ||||||
| Irrigation treatment (I) | ** | * | ns | ns | *** | *** |
| Plant Inoculation (M) | *** | ns | ns | ns | *** | ns |
| I × M | ** | ns | ns | ns | ns | * |
| Treatments | Total Yield | Marketable Yield | Unmarketable Yield (%) | Fruits Plant−1 (pcs) | IWUE (kg m−3) | NAE (kg kg−1 N) | |
|---|---|---|---|---|---|---|---|
| (t ha−1) | (t ha−1) | (kg Plant−1) | |||||
| Irrigation treatment (% ETc) | |||||||
| 60 | 36.1 b 1 | 34.1 | 7.1 | 5.6 | 3.2 | 12.5 | 189.6 |
| 80 | 43.0 a | 40.9 | 8.2 | 4.9 | 3.6 | 11.5 | 228.3 |
| 100 | 46.4 a | 44.7 | 9.0 | 3.7 | 3.7 | 10.2 | 248.4 |
| Plant inoculation | |||||||
| +AMF | 42.5 | 41.0 | 8.4 | 3.5 | 3.6 | 11.7 | 228.6 |
| −AMF | 41.2 | 38.8 | 7.8 | 5.9 | 3.5 | 11.1 | 215.7 |
| Significance2 | |||||||
| Irrigation treatment (I) | *** | *** | ** | *** | ns | ** | *** |
| Plant Inoculation (M) | ns | ns | ns | * | ns | ns | * |
| I × M | ns | * | * | ** | ns | * | ** |
| Treatments | Fruit Weight (g) | Fruit Volume (mL) | Equatorial Diameter (E) (mm) | Polar Diameter (P) (mm) | P/E | Rind Thickness (mm) | Edible Part (%) | Pulp Firmness (N) | SSC (°Brix) | TA 3 (mg 100 g−1) | SSC/TA | Ascorbic Acid (mg 100 g−1 f.w.) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Irrigation treatment (% ETc) | ||||||||||||
| 60 | 2148.8 1 | 2186.0 | 153 b | 232 b | 1.51 | 8.2 | 58.9 | 17.6 b | 12.5 | 10.6 | 1.20 | 67.5 a |
| 80 | 2269.5 | 2394.0 | 160 a | 237 ab | 1.48 | 8.0 | 60.0 | 19.7 a | 12.2 | 10.9 | 1.13 | 33.0 ab |
| 100 | 2405.0 | 2479.4 | 159 a | 239 a | 1.50 | 7.9 | 60.2 | 19.6 a | 12.2 | 11.8 | 1.05 | 26.8 b |
| Plant inoculation | ||||||||||||
| +AMF | 2236.4 | 2312.8 | 158 | 238 a | 1.51 | 8.2 | 59.7 | 19.8 a | 12.8 | 10.9 | 1.19 | 44.7 |
| −AMF | 2286.8 | 2367.3 | 157 | 233 b | 1.48 | 7.9 | 59.7 | 18.1 b | 11.9 | 11.3 | 1.06 | 40.2 |
| Significance2 | ||||||||||||
| Irrigation treatment (I) | * | ** | * | * | ns | ns | ns | * | ns | ns | ns | * |
| Plant Inoculation (M) | ns | ns | ns | ** | ns | ns | ns | * | ** | ns | * | ns |
| I × M | * | * | ns | ns | ns | ns | ns | ns | * | ns | * | ns |
| Variable | PC1 | PC2 | PC3 | PC4 | PC5 |
|---|---|---|---|---|---|
| MI | 0.445 | 0.781 | −0.226 | 0.031 | −0.375 |
| Shoot Length | 0.467 | 0.668 | −0.575 | 0.063 | −0.035 |
| RWC | 0.709 | 0.504 | 0.317 | 0.370 | 0.074 |
| Stomatal Conductance | 0.957 | −0.185 | 0.054 | 0.168 | 0.139 |
| Total Yield | 0.984 | −0.082 | −0.129 | 0.086 | 0.032 |
| Marketable Yield | 0.988 | 0.005 | −0.114 | 0.079 | 0.068 |
| Unmarketable | −0.505 | −0.785 | −0.083 | 0.028 | −0.347 |
| Yield plant | 0.977 | 0.104 | −0.087 | 0.029 | 0.159 |
| Fruit plant | 0.972 | 0.003 | −0.193 | −0.123 | 0.060 |
| IWUE | −0.508 | 0.632 | −0.573 | −0.105 | 0.055 |
| NAE | 0.987 | 0.019 | −0.128 | 0.078 | 0.057 |
| Fruit weight | 0.816 | 0.385 | 0.406 | −0.092 | 0.113 |
| Fruit volume | 0.798 | −0.553 | −0.222 | −0.090 | −0.013 |
| Equatorial diameter (E) | 0.783 | −0.065 | 0.361 | −0.235 | −0.444 |
| Polar diameter (P) | 0.871 | 0.346 | 0.120 | 0.317 | −0.090 |
| P/E | −0.083 | 0.447 | −0.334 | 0.666 | 0.487 |
| Rind thickness | −0.484 | 0.451 | 0.615 | 0.359 | −0.236 |
| Edible part | 0.652 | 0.105 | −0.175 | −0.699 | 0.210 |
| Firmness | 0.782 | 0.411 | 0.364 | −0.069 | −0.288 |
| SSC | −0.072 | 0.829 | 0.521 | −0.153 | 0.110 |
| TA | 0.440 | −0.668 | 0.370 | 0.467 | 0.071 |
| SSC/TA | −0.308 | 0.848 | 0.105 | −0.403 | 0.112 |
| Ascorbic acid | −0.836 | 0.410 | −0.162 | −0.065 | 0.320 |
| L | 0.012 | 0.030 | 0.875 | −0.462 | 0.138 |
| Croma | −0.252 | −0.210 | 0.798 | 0.151 | 0.483 |
| Hue | 0.544 | −0.581 | −0.151 | −0.388 | 0.440 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miceli, A.; Vetrano, F.; Torta, L.; Esposito, A.; Moncada, A. Effect of Mycorrhizal Inoculation on Melon Plants under Deficit Irrigation Regimes. Agronomy 2023, 13, 440. https://doi.org/10.3390/agronomy13020440
Miceli A, Vetrano F, Torta L, Esposito A, Moncada A. Effect of Mycorrhizal Inoculation on Melon Plants under Deficit Irrigation Regimes. Agronomy. 2023; 13(2):440. https://doi.org/10.3390/agronomy13020440
Chicago/Turabian StyleMiceli, Alessandro, Filippo Vetrano, Livio Torta, Alessandro Esposito, and Alessandra Moncada. 2023. "Effect of Mycorrhizal Inoculation on Melon Plants under Deficit Irrigation Regimes" Agronomy 13, no. 2: 440. https://doi.org/10.3390/agronomy13020440
APA StyleMiceli, A., Vetrano, F., Torta, L., Esposito, A., & Moncada, A. (2023). Effect of Mycorrhizal Inoculation on Melon Plants under Deficit Irrigation Regimes. Agronomy, 13(2), 440. https://doi.org/10.3390/agronomy13020440







