Abstract
Salinity has a negative impact on the agricultural production of crops. It adversely affects the physiochemical properties of the soil and ecological balance of the area. Plant growth-promoting bacteria play a key role in the biological control of phyto-pathogens and abiotic stress including salinity. Four varieties of wheat crop (V1: Akbar 2019, V2: Dilkash 2021, V3: Faisalabad 2008, and V4: Subhani 2020) were compared for their salinity stress tolerance and response towards Bacillus subtilis NA2. A completely randomized design (4 wheat varieties × 3 salt stress levels × 3 replicate × 2 control and bacterial treatments = 72 pots) was adopted using distilled water as a control. Stress negatively affected the plant growth. However, plants primed with Bacillus subtilis NA2 showed improved growth (plant lengths 29.45% and increased biomass 33.23%). Overall, bacterial strain enhanced the levels of carotenoids (45.53%), anthocyanin (32.51%), ascorbic acid (41.53%), total soluble proteins (59.21%), chlorophyll contents (49.65%), and peroxidase activity (31.76%). Levels of malondialdehyde (27.42%) and hydrogen peroxide (20.37%), catalase (16.48%), and ascorbate peroxidase (19.24%) decreased. With commensurable benefits, it can be inferred from the above study that the Bacillus subtilis NA2 strain is beneficial for the better yield of wheat under salinity stress by improving the plant defense mechanism and may be adopted in future by farmers.
1. Introduction
Salinity has a negative impact on 9 to 34% of the world’s irrigated land [1,2]. Saline soils are a major issue in many nations. Human-induced soil salinization affects about 32 million ha of droughted lands and 60 million ha of watered land [3]. It is well known that salinity is among the most severe stresses of environment that hinder crop productivity [4]. Due to abiotic stress, ionic toxicity, and a decreased capacity to absorb necessary minerals, saline soils restrict plant growth [5]. In extreme situations, the soil solution’s hyperosmotic pressure may cause root cells to shed water instead of absorption. Through secondary mechanisms such as the encouragement of ionic, abiotic and oxidative stressors, salt stress can kill plants. The two salt ions that are most common in saline soil are sodium and chlorine, both of which are harmful to plants. An excessive buildup of salt ions makes plants poisonous and prevents them from absorbing other nutritional ions, which causes nutrient insufficiency [6]. Osmotic stress lowers the energetic state of the soil water and raises the osmotic pressure in plant cells [7]. Consequently, the height, density and structure of the roots of plants have been altered, which reduces the soil water and nutrient uptake and leads to physiological drought that slows or even kills the plant [8]. In response to abiotic stress like salinity plants goes into cellular oxidative stress and reactive oxygen species are produced [9]. These ROS can cause base modifications in nucleic acids, induce inter-strand and intra-strand crosslinks, crosslinks with proteins, and ultimately trigger double strand breaks [10]. They also cause lipid peroxidation, which increases membrane fluidity and permeability [11], and damage of functional and structural proteins [12].
Plant growth-promoting rhizobacteria (PGPR), among other helpful soil microbes, may help in the growth of plant under stress conditions. PGPR helps in nitrogen absorption, phytohormones production, minerals solubilization, siderophores antibiotics, and enzymes production [13]. Bacillus subtilis increases plant resilience to stress and generates a variety of plant hormones for growth promotion. Plant growth-promoting bacteria (PGPB) can also activate peroxidase and antimicrobial compounds to take part in plant defense mechanisms and increase plant tolerance against pathogens and pests. PGPB release osmotic regulators, extracellular polysaccharides (EPS) [14], and different volatile organic compounds (VOCs) [15], which change the structure and shape of roots and cause plants to develop IST-induced systemic tolerance [16,17].
Keeping in view the importance of wheat as a staple food and salinity, a major environmental issue, this project was planned to explore the potential of Bacillus subtilis to support and stimulate the growth of wheat plants in a saline soil. The development of wheat roots and leaves, oxidative damage, photosynthesis, ion and nutrient uptake and thus all other physical and non-physical aspects was correlated among uninoculated and inoculated wheat plants subjected to three disparate salt concentrations, that is, 0, 150 and 300 mM in soil.
2. Materials and Methods
2.1. Minimum Inhibitory Concentration
The Bacillus subtilis strain NA2 was examined for salt stress tolerance growing in salt containing media to estimate minimum inhibitory concentrations (MIC). To achieve this, various salt concentrations (0 to 10% sodium chloride) were applied. The lowest inhibitory concentration was determined to be the maximum concentration of NaCl that permitted no growth to be seen on media containing salt and nutrient broth.
2.2. In Vitro PGP Analysis
Using a peptone water broth medium, it was investigated whether bacterial strain NA2 could produce ammonia. The medium for peptone water broth was prepared, autoclaved and placed into culture tubes. Each culture tube was inoculated with the isolated strain NA2, then cultured in a shaking incubator at 37 °C and 120 rpm. About 1 mL of the cultivated media was transferred to a centrifuge tube after 48 and 72 h. Before mixing the supernatant and Nessler’s reagent, the culture was centrifuged at 4000 rpm for 5 min. Reagent’s color altered from white to brown or yellow. The absorbance at 450 nm was examined with a spectrophotometer. The produced stock solution was combined with the same Nessler’s reagent, and OD was measured at 450 nm using the same spectrophotometer in order to estimate the absorbance for each known concentration [18].
IAA was produced by utilizing LB medium with and without tryptophan as a substrate. The auxins (specifically indole-3-acetic acid) were generated by the isolates under normal and stressful conditions. Tryptophan was added to LB broth in flasks at a ratio of 0.3 g/L. Bacterial inoculation was incubated for the period of 72 h at 120 rpm and 37 °C in a shaking incubator. Growth was observed after 72 h. Cultured media were spun at 4000 rpm for 5 min, and the supernatants were separated. Salkowski’s reagent and two drops of orthophosphoric acid also were added to the supernatant. The appearance of a pink-red tint was a sign that IAA was being produced, and a spectrophotometer was used to detect the optical density at 530 nm [19]. A graph was drawn to determine the concentration of IAA synthesized by selected bacterial strains.
Estimates of nitrogen fixation were made to determine whether selected bacterial strain might convert atmospheric nitrogen gas into organic form that plants could use. Malate medium was prepared to test the ability of bacteria of nitrogen fixation. After being autoclaved, NF medium was solidified by adding about 15 g/L of agar before being placed into culture tubes and Petri plates. Culture tubes were incubated at 37 °C and 120 rpm for 7 days in an incubator shaker, and plates were incubated at 37 °C for 7 days as well. Growth was indicated by a change in color. Color shifted from green to blue. The growth of bacterial cells in nitrogen-free media was estimated using nitrogen-free broth (NFM) medium [20]. Using a spectrophotometer, the growth of a suspension of isolated bacteria was evaluated after 7 days at 600 nm.
2.3. Pot Experiment
Seeds of 4 wheat varieties (Triticum aestivum) cultivars (V1: Akbar 2019; V2: Dilkash 2020; V3: Faisalabad 2008; V4: Subhani 2021) were gathered at the Wheat Sub-section of the Ayub Agriculture Research Institute (AARI) in Faisalabad, Pakistan. The total 72 pots (Bacterial treatments (2), salt stress (3), replicates (3) cultivars (4) = 72), were employed in an entirely randomized greenhouse experiment (Table 1). Soil properties were studied as described previously [21] as shown in Table 2. For pot experiment, two treatments were used: (T0) sterile distilled water as the control, and (T1) Bacillus subtilis strain NA2. For every pot, 15 wheat seeds were planted in 350 g of soil. Each seed treatment under normal and salt stress had three replicate pots. With the application of Bacillus subtilis NA2, the untreated control was applied as a evaluation for the physiology and biochemical attributes of wheat under salinity stress. Pods were arranged randomly on a bench in a greenhouse with a temperature range of 31 °C during day and 24 °C at night. The humidity level was between 50 and 60%. For the duration of the experiment, pots were watered once per day, which lasted for 21 days. The seedlings were trimmed to seven per pod after germination. Once during the experiment, plants received nutrition from a half-strength Hoagland nutritional solution [22].

Table 1.
Randomized experiment design for wheat plants inoculated with Bacillus subtilis strain NA2 under control and stressed conditions.

Table 2.
Physical and chemical properties of the soil that were used in the study to promote plant growth under salinity stress.
2.4. Plant Physiological Parameters
The fresh and dry weights of wheat roots and shoot were calculated after 21 days of the plant’s growth. After harvesting, plant roots were cleaned with distilled water. Manual ruler measurements were made on the size of the leaves, the lengths of the roots and shoots, fresh weights and dry weights of roots and shoots, which were afterwards oven-dried, were recorded.
2.5. Plant Photosynthetic Pigments
About 10 mL of methanol (80%) were used to homogenize the 0.5 g of fresh leaf sample. The samples were centrifuged at 12,000× g for 10 min before being kept at 4 °C overnight [23]. The extract’s absorbance was examined through the use of an ultraviolet visible spectrophotometer at 663, 645, 480, 530, and 657 nm. The photosynthetic pigments contents of chlorophyll a, b, total chlorophyll, carotenoids, and anthocyanin were calculated by using the following formulas:
Chlorophyll a (mg/g F.Wt) = [12.7(OD 663) − 2.69 (OD 645)] × V/1000 × W
Chlorophyll b (mg/g F.Wt) = [22.9(OD 663) − 4.68 (OD 645)] × V/1000 × W
Carotenoid (mg/g F.Wt) = [(OD 480) − 0.114(OD 663) − 0.638 (OD 645)] × 1000/2500
Anthocyanin (units/g F.Wt) = [(OD 530) − 0.25 (OD 657)]/0.25 (F.Wt: Fresh weight, V: volume and W: Weight)
2.6. Biochemical Attributes
Fresh leaf sample (0.5 g) from each replicate of all experimental treatments was homogenized in about 10 mL of methanol (80%) for the estimation of plant biochemical attributes. The leaf extracted samples of wheat were centrifuged at 12,000 rpm for 10 min before being kept at 4 °C overnight. Total flavonoid contents and total soluble sugars were analyzed followed by this preparation. Total flavonoid contents were measured using the technique of [24]. Then, 0.3 mL of NaNO2 (1%) and 0.3 mL of AlCl3 were combined with about 1 mL of sample (1%); 2 mL of NaOH (4% concentration) was added after 10 min. Using a UV spectrophotometer, the reaction mixture’s absorbance was determined at 510 nm. The Julkunen-Tiitto [25] method was used to determine the total phenolic contents of the samples. The phenolic reagent from Folin–Ciocalteu was added. After a quick shaking, 5 mL of 20% caustic soda was added to the mixture, followed by the addition of distilled water to make the final volume 10 mL. At 750 nm the absorbance of each treated sample was measured. A sample of roughly 0.1 mL for total soluble sugars (TSS) was mixed with 1 mL of Anthrone’s reagent [26]. The mixture boiled for fifteen minutes and then cooled to room temperature. At 625 nm, the absorbance of each treated sample was assessed.
For lipid peroxidation, the accumulation of hydrogen peroxide (H₂O₂) level was analyzed using Velikova’s [23] method. In a cool mortar and pestle, 0.5 g of the fresh leaf sample was crushed with 5 mL of 0.1% trichloroacetic acid. After successful filtering of extract, 1 mL of 1 M potassium iodide and 0.5 mL of phosphate buffer were combined with 1 mL of the supernatant. The sample mixes were vortexed, and a spectrophotometer was used to determine their absorbance at 390 nm. Tannic acid was used to generate a standard curve that was used to calculate H₂O₂. A fresh leaf sample (0.5 g) from the plant was used to measure ascorbic acid (AsA), which was then homogenised in 10 mL of TCA (6%). Then, 4 mL of the extract and 2 mL of dinitrophenyl hydrazine were combined with 1 drop of thio-urea. After boiling for fifteen minutes, the mixture reached room temperature. The mixture was mixed with five millilitres of 80% H2SO4. The optical density of all treated samples was assessed at 530 nm in accordance with [27] and in comparison, to a standard curve premeditated with ascorbic acid concentrations ranging from 10 to 100 mg/L. Malondialdehyde (MDA) levels were calculated using the method described in [28]. In a cold mortar and pestle, a fresh wheat leaf sample (0.5 g) was pulverized with 5 mL of 1 percent (w/v) TCA. For 10 min, the mixture was centrifuged at 15,000 rpm. 1 mL of 0.5 percent thio-barbituric acid (TBA) was added to 0.5 mL of supernatant. The liquid was heated to a boil and then quickly cooled. All treated samples’ optical density was assessed using a spectrophotometer at 532 and 600 nm.
MDA = ∆ (OD532 − OD600)/1.56 × 105
2.7. Antioxidant Enzyme Activity
A 0.5 g fresh wheat leaf sample was grinded in 10 mL potassium phosphate buffer for enzyme extraction (pH 7.8). The extract supernatant was separated by centrifugation for 15 min at 15,000 rpm before being frozen at 20 °C in an ultra-low freezer. Using the approach of Bradford, the amount of total soluble protein (TSP) was estimated as described by [29]. The absorbance at 595 nm was assessed after combining 50 µL of extracted supernatant with 1 mL of Bradford reagent. By comparing the protein content to the BSA standard curve, protein content was calculated. The activity of catalase enzyme was ascertained using the method suggested by [30]. A plant extract of about 0.1 mL, 1 mL of 5.9 mM H₂O₂ with 1.9 mL of 50 mM phosphate buffer (7.0 pH) were used. The optical density at 240 nm was assessed for total two minutes at 20 s gaps. A change of 0.01 A240 Units/min was equated to one unit of catalase activity. The Peroxidase activity (POD) enzyme’s activity was assessed using a technique suggested by [31]. To determine the peroxidase enzyme’s activity, A reaction mixture of 750 µL phosphate buffer solutions (7.0 pH), 100 µL H₂O₂ (5.9 mM), 100 µL guaiacol (0.5 percent), and 50 µL extracted wheat enzyme was prepared. The optical density at 470 nm was then assessed using a spectrophotometer for 2 min at 20-s gaps. Ascorbate peroxidase activity was assessed using a reaction mixture of 100 µL ascorbate (0.5 mM) + 700 µL phosphate buffers (7.0 pH) + 100 µL enzyme extract + 100 µL H₂O₂ (5.9 mM). A spectrophotometer was then used to measure the optical density at 290 nm for 2 min at 20 s gaps. Then, the activity of catalase, peroxidase, and ascorbate peroxidase were examined and reported in units/mg of total soluble protein.
3. Results
3.1. In Vitro Plant Growth Promoting (PGP) Characteristics of Bacillus subtilis NA2
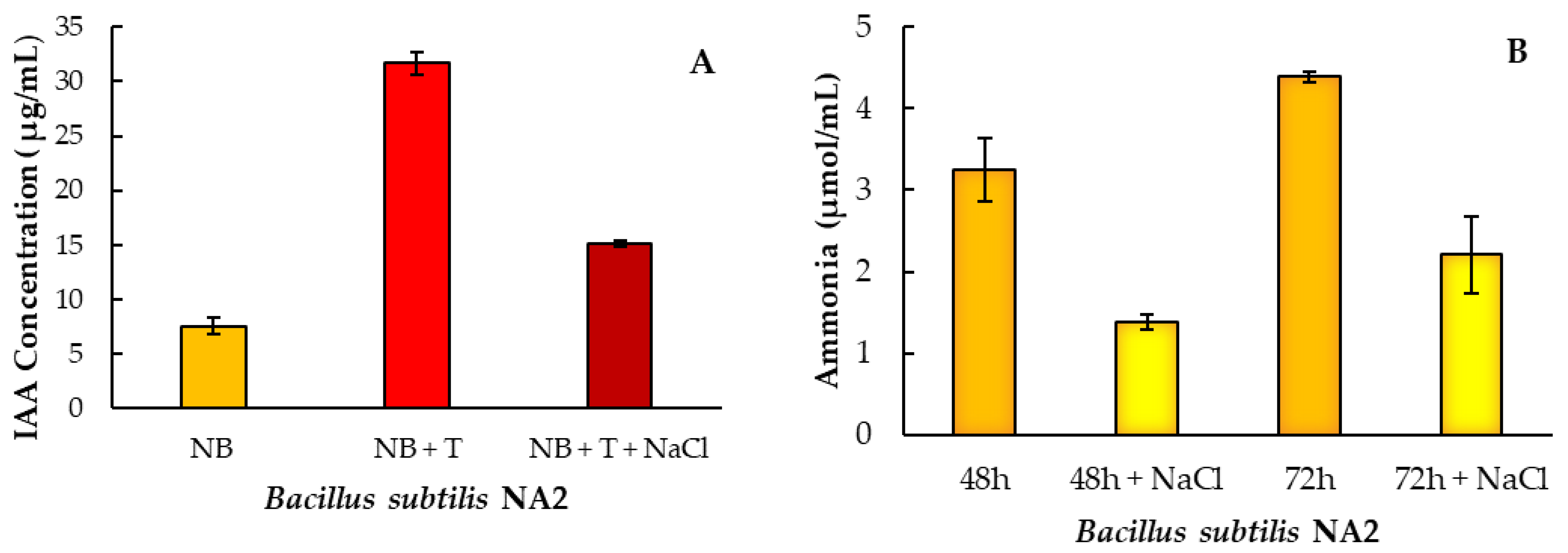
Ammonia production by the Bacillus subtilis NA2 was examined under control and salinity stress by the mixing of supernatant of bacterial culture with Nessler’s reagent. Strain NA2 was able to produce a sufficient amount of ammonia when cultured in peptone water broth. The maximum level of ammonia production by Bacillus subtilis NA2 was observed within 48 h (51.48 μmol/mL) but ammonia production reduced to 20.24 μmol/mL when checked at 72 h. (Table 3, Figure 1B). IAA synthesis followed the same trend of ammonia production under control and salt stress in bacterial culture. Bacillus subtilis NA2 synthesize IAA only in broth culture supplemented with L-Tryptophan (1 g/L) as a potential substrate. Bacillus subtilis NA2 confirmed IAA production 7.87 ± 1.36 and 31.68 ± 1.79 (μg/mL) with and without L-Tryptophan, respectively (Figure 1A, Table 3). Positive nitrogenase activity of Bacillus subtilis NA2 was examined in nitrogen free malate agar media. At 600 nm, nitrogen free liquid broth media demonstrated the greatest amount of atmospheric nitrogen fixation in terms of positive bacterial cell growth. (Table 3).

Table 3.
Characterization of in vitro PGP traits of Bacillus subtilis NA2 for atmospheric nitrogen fixation, IAA synthesis, and ammonia production.
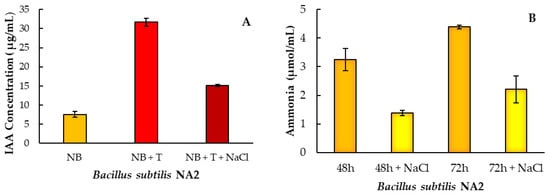
Figure 1.
IAA (A) and ammonia (B) production after 48 and 72 h with inoculation of Bacillus subtilis NA2 under normal and stressed condition (with and without NaCl). Here, NB = nutrient broth, T = tryptophan. Bar shows means of three replicates ± standard error.
3.2. In Vitro Salt Tolerance Testing of Bacillus subtilis NA2
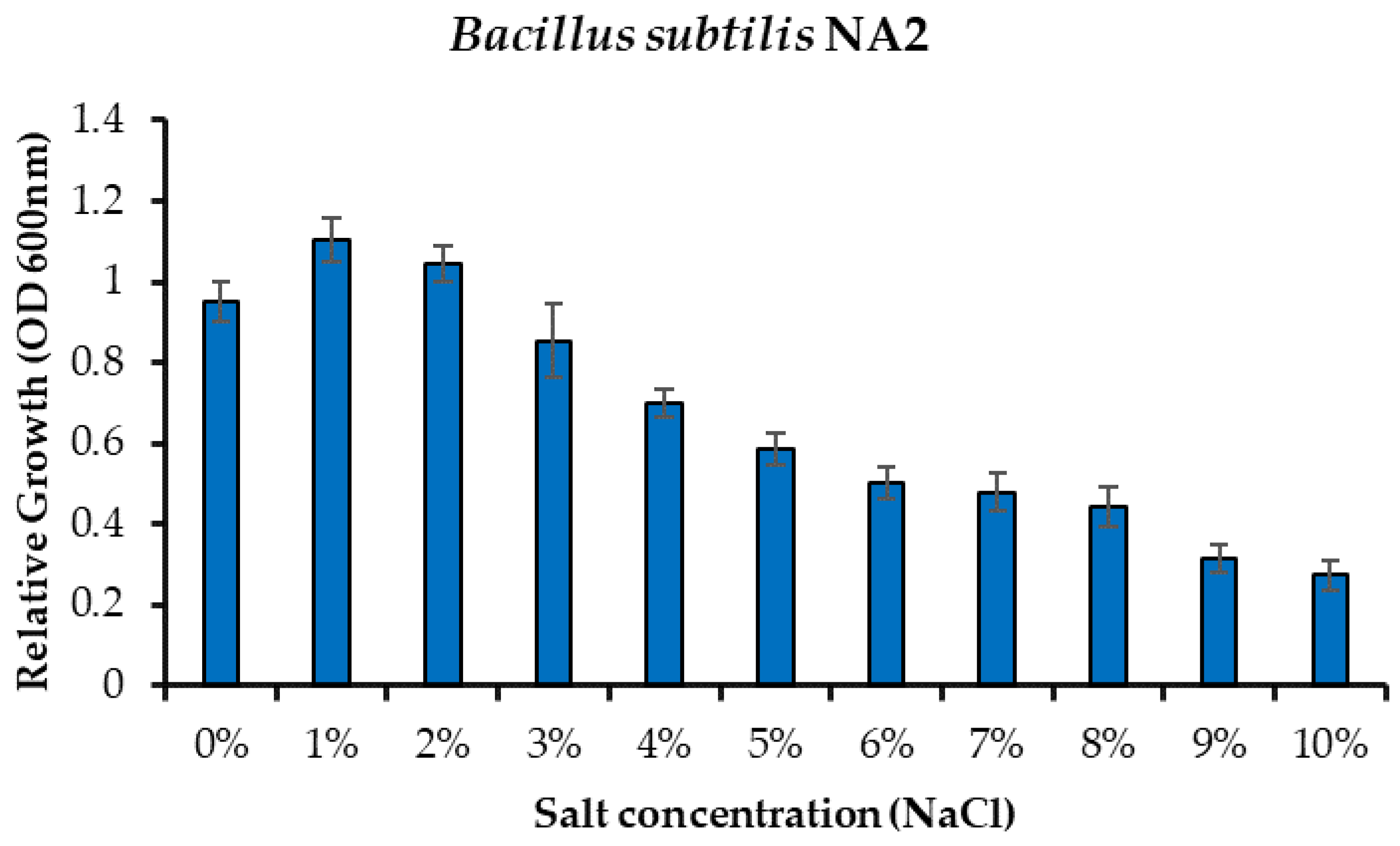
Minimum inhibitory concentration of bacteria was done to test the tolerating capacity of bacteria in different salt concentrations. Liquid media were prepared with a nutrient broth medium to check the salt tolerance of bacteria. Minimum inhibitory was observed at different salt concentrations that were from 0% to 10% salt of NaCl. Bacteria (Bacillus subtilis NA2) showed tolerance up to 8% against salt. Bacterial growth was observed at 0 to 8%, and then it started declined as clearly shown in Figure 2.
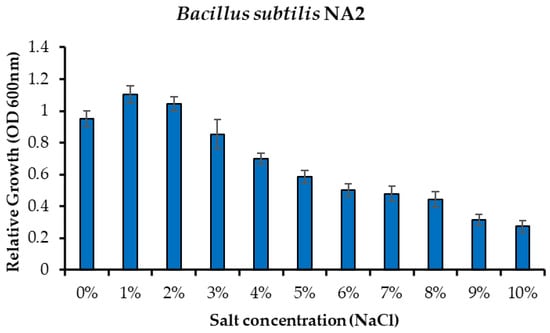
Figure 2.
Effect of salinity stress on comparative growth of Bacillus subtilis NA2 under different concentrations of sodium chloride.
3.3. Plant Physiological Parameters
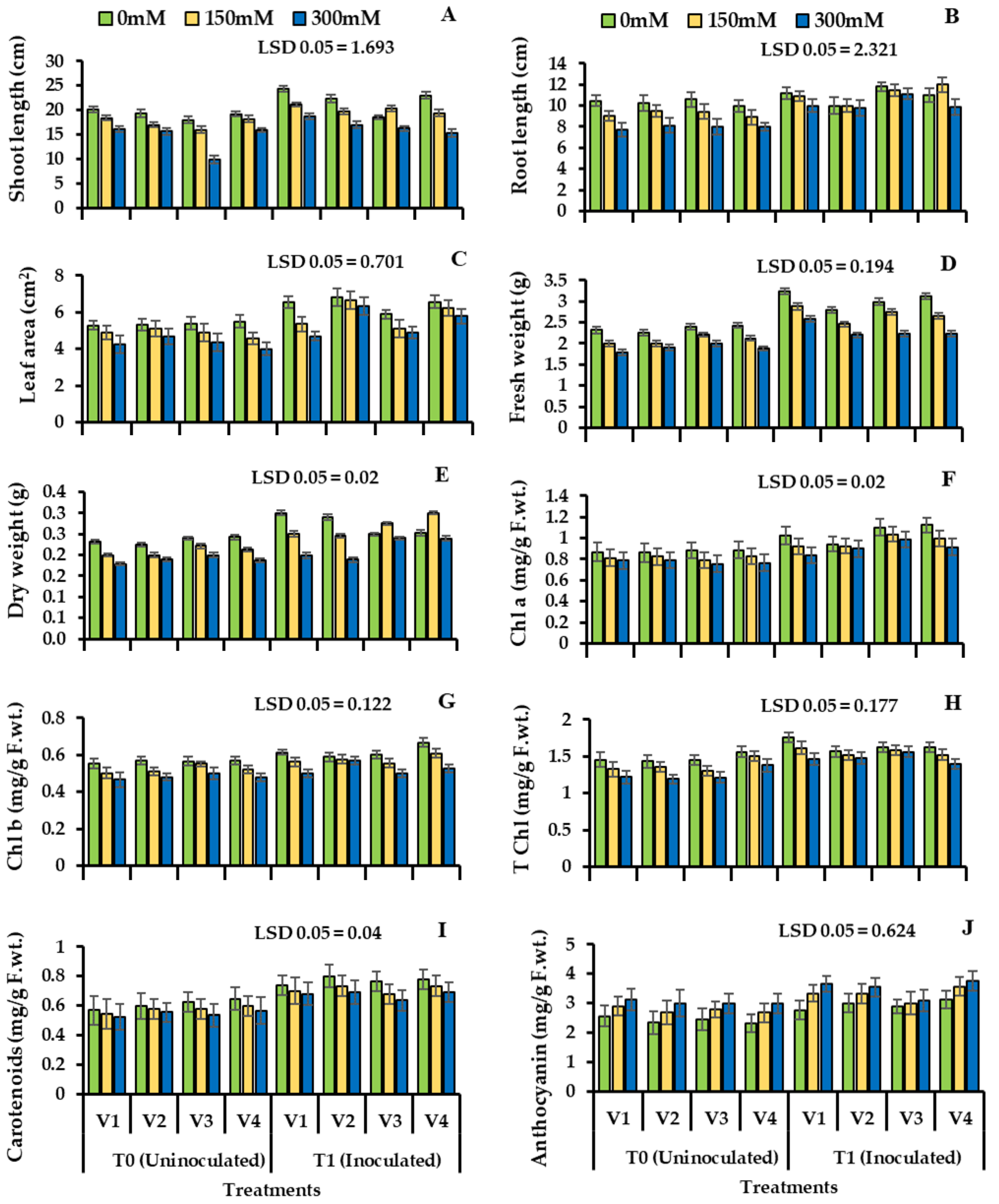
Plant growth decreased significantly under the salinity stress. Plant root length decreased in the uninoculated plants (V1: 13.11%, V2: 20.23%, V3: 10.22%, and V4:19.43%) and shoot length (V1: 19.36%, V2: 17.67%, V3: 11.02%, V4: 17.82%) also decreased in the uninoculated plants (Figure 3, Table 4). However, bacterial inoculation improved the root and shoot length of four cultivars of wheat under salinity stress. Bacillus subtilis NA2 inoculation showed improvement in shoot length in S2 and S3 in V1 (21.43%, 16.44%), V2 (23.65%, 15.47%), V3 (19.42%, 12.26%), and V4 (33.53%, 21.76%) in inoculated plants when compared with uninoculated. Plant root length increased significantly in all four cultivars of wheat (V1: 19.73%, 12.11%; V2: 25.51%, 20.78%; V3: 17.47%, 11.25%; and V4: 29.25%, 21.64%) under S2 and S3 as compared to control plants shown in Table 4, Figure 3A,B, respectively.
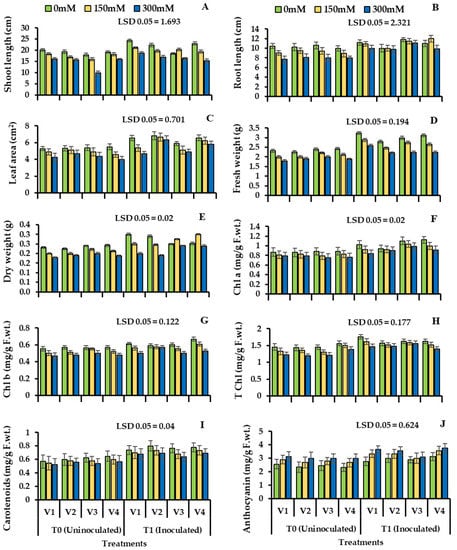
Figure 3.
Shoot length (A), root length (B), leaf area (C), plant fresh weight (D), plant dry weight (E), chlorophyll a (F), chlorophyll b (G), total chlorophyll (H), carotenoids (I), and anthocyanin content (J) of four cultivars of wheat (Triticum aestivum L.) inoculated with Bacillus subtilis strain NA2. Treated with bacteria under salinity stress condition (Mean ± S.E). Here, T0 = uninoculated control, T1 = inoculated with Bacillus subtilis NA2. All LSD values are at 0.05.

Table 4.
Mean squares from a three-way evaluation of variance of data for diverse physiological, biological, and antioxidant parameters of wheat (Triticum aestivum L.) plants inoculated with Bacillus subtilis strain NA2 under control and stress condition.
Plant seeds inoculated with bacterial strain improved the fresh weights of all four varieties of wheat under salinity stress S2 and S3 (V1: 22.32%, 13.54%), (V2: 20.62%, 15.37%), (V3: 16.26%, 8.41%), and (V4: 24.71%, 14.52%). Fresh and dry weights of wheat varieties increased after inoculation. Stressed plants when treated with bacteria showed improved dry weight (V1: 32.16%, 24.51%), (V2: 29.83%, 19.63%), (V3: 22.27%, 14.46%), and (V4: 25.36%, 16.72%) shown in Table 4, Figure 3D,E, respectively.
3.4. Plant Photosynthetic Pigments
Under uninoculated plants with salinity stress, photosynthetic pigment decreased, and there was a visible decrease in chlorophyll a, b, total chlorophyll, and carotenoids content as compared to control plants. The Bacillus subtilis strain significantly improved photosynthetic pigments, that is, chlorophyll a, b, and total chlorophyll and carotenoid content as well, shown in Table 4, Figure 3. Wheat plant treated with bacteria showed an increase in chlorophyll a (V1: 18.33%, 10.64%), (V2: 22.37%, 16.26%), (V4: 28.72%, 20.47%), and there was little change in the third variety (V3: 12.65%, 7.36%). Inoculation with Bacillus subtilis NA2 showed improvement in chlorophyll b (V1: 24.83%, 16.64%), (V2: 27.87%, 21.57%), (V3: 17.65%, 9.24%) and (V4: 29.55%, 22.41%) and maximum improvement in total chlorophyll was observed in (V4: 29.06%, 22.43%) as compared to plants without inoculation. Plants bio-primed with bacteria showed an increase in carotenoid content (V1: 40.76%, 28.74%) with respect to control. V2 showed high content of anthocyanin in S2 and S3 (32.92% and 20.44%). V1 showed high content of anthocyanin under (S2: 35.61%; S3: 28.74%) when compared to inoculated plants (Table 4, Figure 3I,J).
3.5. Biochemical Attributes of Plant
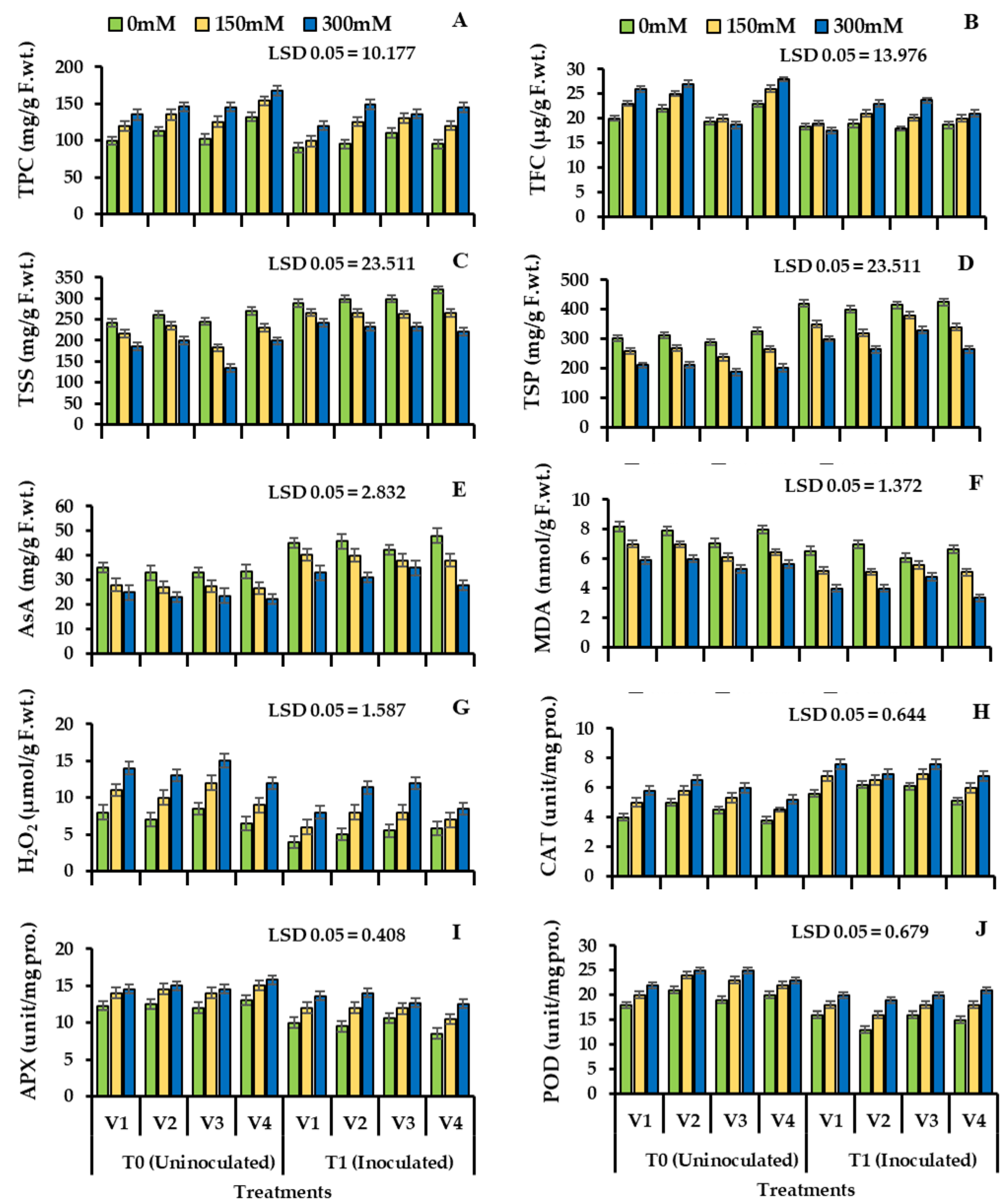
Phenolic content was significantly reduced in all varieties of wheat when inoculated with Bacillus subtilis and S3 (V1: 33.34%; V2: 40.52%; V3: 20.73%; V4: 42.37%). Total flavonoids were increased significantly due to the salinity stress in wheat cultivars in uninoculated plants (V1: 45.50%; V2: 48.36%; V3: 36.73%; V3: 43.58%). Plant seeds bio-primed with bacteria showed (Table 4, Figure 4) reduced value for flavonoids (V1: 18.35%; V2: 21.72%; V3: 28.57; V4: 19.59%).
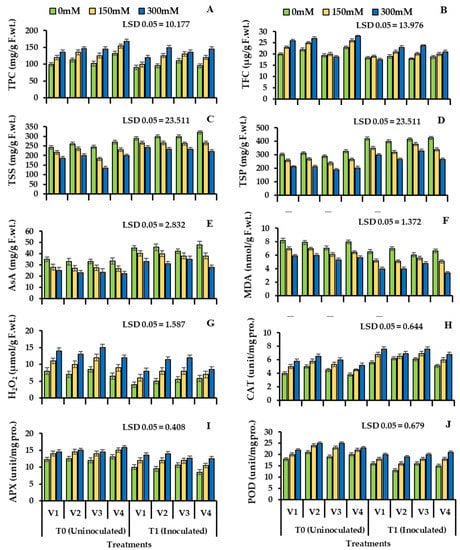
Figure 4.
Total phenolic contents (A), total flavonoid contents (B), total soluble sugars (C), total soluble proteins (D), ascorbic acid (E), malondialdehyde (F), hydrogen peroxide contents (G), catalase (H), peroxidase (I), ascorbate peroxidase (J), enzyme activity of four cultivars of wheat (Triticum aestivum L.) inoculated with Bacillus subtilis (NA2). Treated with salinity stress condition (Mean ± S.E). Here, T0 = uninoculated control, T1 = inoculated with Bacillus subtilis. All LSD values are at 0.05.
Plant biochemical parameters showed decreased value for salinity stress and a reduction in plant total soluble sugar under S3 (V1: 24.62%; V2: 22.37%; V3: 44.52%; V4: 26.62%), total soluble protein (V1: 19.72; V2: 20.62; V3: 10.73%; V3: 22.61%) and ascorbic acid content (V1: 33.72%; V2: 29.79%; V3: 20.42%; V4: 35.63%) was observed in uninoculated plants. The inoculation with Bacillus subtilis NA2 increased the total soluble sugar content under S3 (V1: 29.74%; V2: 32.52%; V3: 16.77%; V4: 28.49%), total soluble protein (V1: 40.69%; V2: 36.36%; V3: 14.73%; V4: 38.58%) and ascorbic acid (V1: 41.47%; V2: 38.55%; V3: 22.93%; V4: 36.28%) (Table 4, Figure 4C–E) respectively.
In uninoculated plants, all four cultivars of wheat showed a significant increase in malondialdehyde (MDA) (V1: 45.36%; V2: 42.57%; V3: 53.63%; V4: 40.0%) and hydrogen peroxide (H2O2) (V1: 55.82%; V2: 46.16%; V3: 36.73%; V3: 50.19%) under S3. Inoculation with Bacillus subtilis NA2 lowered the malondialdehyde (Table 4, Figure 4F) content (V1: 19.47%; V2: 15.82%; V3: 9.45%; V3: 21.75%), H2O2 (Table 4, Figure 4G) content was also lower (V1: 22.92%; V2: 25.16%; V3: 12.50%; V3: 28.58%) when compared to uninoculated control plants under S3. All wheat cultivars showed a major difference, and bacterial treatment was found to be varying significantly in wheat cultivars under salinity stress (Table 4, Figure 4).
3.6. Antioxidant Enzyme Activities of Plant
The response of antioxidants to salinity stress in two stress levels, i.e., S2 and S3, was increased in uninoculated plants that increased the levels of catalase (29.61%, 40.79%), ascorbate peroxidase (16.27%, 35.15%), and peroxidase (37.36%, 55.62%). Diversity of trend was observed in all four wheat varieties. However, when treated with Bacillus subtilis NA2, a decrease in catalase activity (19.35%, 11.62%) and ascorbate peroxidase activity (20.18%, 12.46%) was observed. Greater reduction was observed in peroxidase activity (25.82%, 18.31%) under S2 and S3 (Table 4, Figure 4H,J) respectively).
4. Discussion
This study explored how inoculating salt-tolerant bacteria could influence the growth, photosynthetic pigments, other biochemical constituents, and antioxidant enzyme activity of wheat grown in various salinity levels [32]. Results showed that halophytes could resist salty conditions due to adaptive evolution, while glycophytes cannot and begin to die. Additionally, this adaptation enabled microorganisms associated with the halophytes to thrive in salty environments. Increases in soil salinity have significantly reduced plant growth and nutrient uptake. Studies both in vitro and in vivo showed that plants inoculated with the Bacillus subtilis NA2 strain of PGPRs experienced more significant growth parameters (root length, shoot length, total biomass, and photosynthetic pigments) as well as increased biochemical values (total flavonoid content, total soluble sugars, total phenolic content, total soluble proteins, and vitamin C) (Table 4, Figure 3). Furthermore, PGPRs were found to reduce the damaging effects of salinity stress, such as the accumulation of malondialdehyde and hydrogen peroxide, as well as to modulate antioxidant enzyme activity (Table 4, Figure 4). Salt stress has been known to induce nutrient deficiencies in plants, resulting in stunted growth and development. However, it has also been observed that halotolerant bacteria can increase the uptake of nutrients by plants and improve their growth, as seen in wheat, rice, meadows, cucumber, tomato, and maize plants [27,33,34,35,36,37,38].
Inoculation of the PGPR microbe NA2 induced salt tolerance and growth promotion in four wheat cultivars, namely AKBAR 2019, DILKASH 2020, FAISALABAD 2008, and SUBHANI 2021. Additionally, bacteria having potential to ACC deaminase such as Bacillus cereus strain Y5, Bacillus sp. Y14 and Bacillus subtilis strain Y16 effectively enhanced wheat growth when grown in saline soils [39]. Similarly, Klebsiella sp. SBP-8 was observed to affect plant growth under salinity and temperature stresses positively. The present study found that wheat plants exposed to the PGPR Bacillus subtilis NA2 and salt showed significantly increased growth, which is in line with other studies on the positive effects of PGPRs on plant development under salinity stress [27,40,41]. This suggests that these beneficial microorganisms can mitigate some of the negative impacts of salt on crop production. In this research, wheat plants pre-treated with the Bacillus subtilis strain revealed remarkable tolerance to salt stress with a notable increase in root and leaf biomass, more efficient nutrient uptake, and higher plant height. The probable mechanism of PGPB’s ability to reduce salinity stress for wheat may be associated with auxin synthesis, atmospheric nitrogen fixation, and ammonia production for the host plant [42].
The effects of the Bacillus subtilis NA2 strain on plant growth were tested to evaluate its potential as a plant growth promoter. Tests such as nitrogen fixation, ammonia, and indole-3-acetic acid (IAA) production were conducted, with the results indicating that it may be beneficial in stimulating plant growth in vitro [21,43]. Moreover, the results were in line with previous studies-a zone diameter of blue halo was observed on a nitrogen-free malate medium, which demonstrated the atmospheric nitrogen fixing on a qualitative level [44]. Research has revealed that certain bacterial strains, such as Bacillus, Pseudomonas, and Rhizobium, can establish close associations with plants that can significantly promote their growth. This is due to the production of various factors, such as phosphate solubilization, nitrogen fixation, ammonia, and indole acetic acid, which protect plants from abiotic stresses and diseases [29]. Many species of bacteria rely on tryptophan as a source for the synthesis of indole acetic acid (IAA). IAA is essential in controlling plant growth and development, including tissue differentiation, cell division, apical dominance, elongation and responsiveness to light, gravity, and pathogens. Our research results concur with earlier studies suggesting that IAA production depends on an appropriate substrate [30]. Decreased synthesis of IAA in adverse environmental conditions may be due to reduced microbial metabolism [45]. A minimum inhibitory concentration test was conducted to determine the tolerance of different bacteria to saline conditions. Results showed that most bacterial species grew under 5% NaCl concentration, while less growth was observed at 8% and none at 10% NaCl [46,47]. Bacilli are an essential component of soil microbial composition. Due to its robustness and widespread distribution, Bacillus has been proven to possess advantageous features that allow it to thrive in various environments [48]. Its ability to survive in these settings makes it an effective plant growth-promoting rhizobacterium (PGPR). Moreover, studies have shown that under more selective environmental conditions, microbiome variability and taxonomic diversity are significantly reduced [49].
Across a variety of cereal crops, researchers have observed a general decline in plant growth and drastically reduced yield when subject to stress conditions such as salinity [31]. A study showed that salinity significantly (p ≤ 0.001) decreased the fresh and dry weight of the shoots and roots of four wheat cultivars. Additionally, elevated lipid peroxidation levels and alteration in the wheat seedlings’ nutritional composition could be contributing factors to the lowered overall growth and development seen in stressed plants [50]. Salinity has been seen to affect plant growth and decrease yield [51]. Reductions evidence this in photosynthetic pigments, height, root length, shoot length, and fresh and dry biomass [52,53]. Salinity can lead to tissue necrosis, impair cell integrity, cause turgor loss and slowed growth, smaller leaves, and earlier senescence. These effects are consistent with the findings of various studies [37].
Salinity affects several photosynthetic parameters, including osmotic and leaf water potentials, transpiration rate, temperature, relative leaf water content, and carbon assimilation. It also reduces chlorophyll and carotenoid content in plants. Consequently, photosynthesis is impaired by salinity [37]. The findings of this study are comparable to those reported earlier, which indicated a considerable reduction in the total chlorophyll content, as well as chlorophyll a and b, across all wheat varieties tested. The enhanced solubilization and bioavailability of organic elements such as magnesium and the activation of enzymatic pathways responsible for chlorophyll biosynthesis likely account for the higher photosynthetic pigments observed in abiotically stressed maize plants [21,54,55,56].
Under salt stress conditions, the increased levels of ROS caused increased lipid peroxidation and damage to plant ultra-structures. In the present study, wheat plants subjected to salt stress had a high concentration of H₂O₂ and MDA. Nevertheless, the exogenous application of PGPR bacterial strains reduced the amount of H₂O₂ and MDA noticeably [21,57]. Under abiotic stress, such as salinity, H₂O₂ is formed through increased oxidative stress [58]. In both salt-stressed and non-stressed wheat, bacterial treatments significantly increased the ascorbic acid content. This rise in concentration was observed in various portions of cereal and other crops like wheat [59], tomato [60], and canola [61], which are gradually becoming more resilient to salt stress. The increase of ascorbic acid levels has been shown to significantly influence the fresh and dry weights of all four wheat cultivars grown under salt-stress conditions. This suggests that auxin secretion from these strains could be responsible for increased growth and tolerance towards salt. The growth-regulator IAA, a plant hormone, has been known to stimulate the development of lateral roots during different stages. Moreover, it has also been seen to enter plant cells and promote their growth [17].
Salinity can cause osmotic and ionic stress, leading to reactive oxygen species (ROS) forming, resulting in extensive oxidative damage to cellular organelles and membranes. Antioxidants can reduce this damage by directly reacting with free radicals or indirectly increasing the activity or expression of intracellular antioxidant enzymes such as catalase, peroxidase, and ascorbate peroxidase. Application of the Bacillus subtilis NA2 bacteria led to a decrease in the activity of these enzymes in four different wheat cultivars. Inoculation with B. subtilis was found to reduce the production of reactive oxygen species (ROS) and the activities of certain enzymes, which is consistent with previous research that has shown increased antioxidant enzyme activities in wheat cultivars under salt stress. These results highlight the importance of genetic variation among wheat cultivars in their ability to respond to stress and treatment conditions [30,62,63,64,65]. Our findings in wheat varieties have indicated that the application of Bacillus subtilis NA2 could positively affect plant growth promotion, even under unfavorable saline conditions. Moreover, there is an interaction between the specific strain used and the kind of crop that results in different antioxidant responses. This suggests that this strain can be used in the management of salt and other abiotic stresses in cereal and other crops. Overall, this strain has been a successful treatment for dealing with these issues.
5. Conclusions
It was concluded that an isolated bacterial strain of Bacillus subtilis NA2 is capable of tolerating stress though efficient nitrogen fixation, ammonia production and IAA synthesis. Our results confirmed that salinity stress inhibited the plant biomass and nutrition in wheat cultivars in a dose-dependent manner. The application of bacterial species confers more advantages and fruitful growth of plant seedlings as reflected from the shoot biomass (33%), photosynthetic pigments (40%), lower lipid peroxidation while maintaining lower levels of lipid peroxidation (48%), and antioxidants (28%). Additionally, it was suggested that using bacteria in conjunction with agricultural crops might be a natural technique for the production of sustainable food.
Author Contributions
Conceptualization, S.J. and B.Z.; methodology, M.A. and T.M.; software, M.A. and S.G.; validation, S.G., A.A. and N.A.; formal analysis, S.G.; investigation, S.G. and M.A.; resources, S.J.; data curation, S.G.; writing—original draft preparation, S.G.; writing—review and editing, S.J. and M.A.; visualization, S.G.; supervision, S.J.; project administration, S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
Authors are thankful to University Malaysia Sabah (UMS), Sabah, Malaysia, for their technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Farhangi-Abriz, S.; Ghassemi-Golezani, K. How can salicylic acid and jasmonic acid mitigate salt toxicity in soybean plants? Ecotoxicol. Environ. Saf. 2018, 147, 1010–1016. [Google Scholar] [CrossRef]
- Ghassemi, F.; Jakeman, A.J.; Nix, H.A. Salinisation of Land and Water Resources: Human Causes, Extent, Management and Case Studies; CAB International: Wallingford, UK, 1995. [Google Scholar]
- Zhang, H.-X.; Hodson, J.N.; Williams, J.P.; Blumwald, E. Engineering salt-tolerant Brassica plants: Characterization of yield and seed oil quality in transgenic plants with increased vacuolar sodium accumulation. Proc. Natl. Acad. Sci. USA 2001, 98, 12832–12836. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.; Grattan, S.R. How salinity damages citrus: Osmotic effects and specific ion toxicities. HortTechnology 2005, 15, 95–99. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Pang, C.-H.; Wang, B.-S. Oxidative stress and salt tolerance in plants. In Progress in Botany; Springer: Berlin/Heidelberg, Germany, 2008; pp. 231–245. [Google Scholar]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Smirnoff, N. Plant resistance to environmental stress. Curr. Opin. Biotechnol. 1998, 9, 214–219. [Google Scholar] [CrossRef]
- Jena, N. DNA damage by reactive species: Mechanisms, mutation and repair. J. Biosci. 2012, 37, 503–517. [Google Scholar] [CrossRef]
- Jabborova, D.P.; Enakiev, Y.I.; Davranov, K.D.; Begmatov, S.A. Effect of co-inoculation with Bradyrhizobium japonicum and Pseudomonas putida on root morph-architecture traits, nodulation and growth of soybean in response to phosphorus supply under hydroponic conditions. Bulg. J. Agric. Sci. 2018, 24, 1004–1011. [Google Scholar]
- Crosby, N.J.A. Determination of ammonia by the Nessler method in waters containing hydrazine. Analyst 1968, 93, 406–408. [Google Scholar] [CrossRef]
- Dobbelaere, S.; Croonenborghs, A.; Thys, A.; Vande Broek, A.; Vanderleyden, J. Phytostimulatory effect of Azospirillum brasilense wild type and mutant strains altered in IAA production on wheat. Plant Soil 1999, 212, 153–162. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Tariq, M.; Noman, M.; Ahmed, T.; Hameed, A.; Manzoor, N.; Zafar, M. Antagonistic features displayed by plant growth promoting rhizobacteria (PGPR): A review. J. Plant Sci. Phytopathol. 2017, 1, 38–43. [Google Scholar]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 4, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Antoun, H.; Prévost, D. Ecology of plant growth promoting rhizobacteria. In PGPR: Biocontrol Biofertilization; Springer: Dordrecht, Switzerland, 2005; pp. 1–38. [Google Scholar]
- Bilal, R.; Rasul, G.; Qureshi, J.A.; Malik, K.A. Characterization of Azospirillum and related diazotrophs associated with roots of plants growing in saline soils. World J. Microbiol. Biotechnol. 1990, 6, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Irigoyen, J.; Einerich, D.; Sánchez-Díaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativd) plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Azeem, M.; Haider, M.Z.; Javed, S.; Saleem, M.H.; Alatawi, A. Drought Stress Amelioration in Maize (Zea mays L.) by Inoculation of Bacillus spp. Strains under Sterile Soil Conditions. Agriculture 2022, 12, 50. [Google Scholar] [CrossRef]
- Läuchli, A. Electron probe analysis. In Microautoradiography and Electron Probe Analysis; Springer: Berlin/Heidelberg, Germany, 1972; pp. 191–236. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Mukherjee, S.; Choudhuri, M. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R. Phenolic constituents in the leaves of northern willows: Methods for the analysis of certain phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, W.J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Akram, M.S.; Shahid, M.; Tariq, M.; Azeem, M.; Javed, M.T.; Saleem, S.; Riaz, S. Deciphering Staphylococcus sciuri SAT-17 mediated anti-oxidative defense mechanisms and growth modulations in salt stressed maize (Zea mays L.). Front. Microbiol. 2016, 7, 867. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Karthika, S.; Midhun, S.J.; Jisha, M. A potential antifungal and growth-promoting bacterium Bacillus sp. KTMA4 from tomato rhizosphere. Microb. Pathog. 2020, 142, 104049. [Google Scholar] [CrossRef]
- Abbas, S.; Javed, M.T.; Shahid, M.; Hussain, I.; Haider, M.Z.; Chaudhary, H.J.; Tanwir, K.; Maqsood, A. Acinetobacter sp. SG-5 inoculation alleviates cadmium toxicity in differentially Cd tolerant maize cultivars as deciphered by improved physio-biochemical attributes, antioxidants and nutrient physiology. Plant Physiol. Biochem. 2020, 155, 815–827. [Google Scholar] [CrossRef]
- Edgerton, M.D. Increasing crop productivity to meet global needs for feed, food, and fuel. Plant Physiol. 2009, 149, 7–13. [Google Scholar] [CrossRef]
- Kerbab, S.; Silini, A.; Chenari Bouket, A.; Cherif-Silini, H.; Eshelli, M.; El Houda Rabhi, N.; Belbahri, L. Mitigation of NaCl stress in wheat by rhizosphere engineering using salt habitat adapted PGPR halotolerant bacteria. Appl. Sci. 2021, 11, 1034. [Google Scholar] [CrossRef]
- Chakraborty, U.; Chakraborty, B.N.; Chakraborty, A.P.; Dey, P.L. Water stress amelioration and plant growth promotion in wheat plants by osmotic stress tolerant bacteria. World J. Microbiol. Biotechnol. 2013, 29, 789–803. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmad, I.; Hilger, T.H.; Nadeem, S.M.; Akhtar, M.F.; Jamil, M.; Hussain, A.; Zahir, Z.A. Preliminary study on phosphate solubilizing Bacillus subtilis strain Q3 and Paenibacillus sp. strain Q6 for improving cotton growth under alkaline conditions. PeerJ 2018, 6, e5122. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Asghar, H.N.; Arshad, M. Rhizobacteria capable of producing ACC-deaminase may mitigate salt stress in wheat. Soil Sci. Soc. Am. J. 2010, 74, 533–542. [Google Scholar] [CrossRef]
- Sharma, S.; Kulkarni, J.; Jha, B. Halotolerant rhizobacteria promote growth and enhance salinity tolerance in peanut. Front. Microbiol. 2016, 7, 1600. [Google Scholar] [CrossRef]
- Greenway, H.; Munns, R. Mechanisms of salt tolerance in nonhalophytes. Annu. Rev. Plant Physiol. 1980, 31, 149–190. [Google Scholar] [CrossRef]
- Han, H.; Lee, K. Plant growth promoting rhizobacteria effect on antioxidant status, photosynthesis, mineral uptake and growth of lettuce under soil salinity. Res. J. Agric Biol. Sci. 2005, 1, 210–215. [Google Scholar]
- Khan, M.Y.; Zahir, Z.A.; Asghar, H.N.; Waraich, E.A. Preliminary investigations on selection of synergistic halotolerant plant growth promoting rhizobacteria for inducing salinity tolerance in wheat. Pak. J. Bot 2017, 49, 1541–1551. [Google Scholar]
- Barra, P.J.; Inostroza, N.G.; Acuña, J.J.; Mora, M.L.; Crowley, D.E.; Jorquera, M.A. Formulation of bacterial consortia from avocado (Persea americana Mill.) and their effect on growth, biomass and superoxide dismutase activity of wheat seedlings under salt stress. Appl. Soil Ecol. 2016, 102, 80–91. [Google Scholar] [CrossRef]
- Abd_Allah, E.F.; Alqarawi, A.A.; Hashem, A.; Radhakrishnan, R.; Al-Huqail, A.A.; Al-Otibi, F.O.N.; Malik, J.A.; Alharbi, R.I.; Egamberdieva, D. Endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by regulating the plant defense mechanisms. J. Plant Interact. 2018, 13, 37–44. [Google Scholar] [CrossRef]
- Hidri, R.; Metoui-Ben Mahmoud, O.; Debez, A.; Abdelly, C.; Barea, J.-M.; Azcon, R. Modulation of C:N:P stoichiometry is involved in the effectiveness of a PGPR and AM fungus in increasing salt stress tolerance of Sulla carnosa Tunisian provenances. Appl. Soil Ecol. 2019, 143, 161–172. [Google Scholar] [CrossRef]
- Shahzad, Q.; Mahmood, S.; Javed, S.; Mushtaq, T.J.M. Chromium Stress Tolerance of a C4 (Zea mays L.) and C3 (Vigna radiata L.) Plants Primed with UV and Gamma-Treated Bacillus subtilis. Microorganisms 2021, 9, 2313. [Google Scholar] [CrossRef]
- Bashir, S.; Javed, S.; Al-Anazi, K.M.; Farah, M.A.; Ali, S. Bioremediation of Cadmium Toxicity in Wheat (Triticum aestivum L.) Plants Primed with L-Proline, Bacillus subtilis and Aspergillus niger. Int. J. Environ. Res. Public Health 2022, 19, 12683. [Google Scholar] [CrossRef]
- Idris, E.E.; Iglesias, D.J.; Talon, M.; Borriss, R. Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol. Plant Microbe Interact. 2007, 20, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Bukhari, S.A.; Ashraf, M.Y.; Mahmood, S.; Iftikhar, T. Effect of salinity on growth, biochemical parameters and fatty acid composition in safflower (Carthamus tinctorius L.). Pak. J. Bot 2014, 46, 1153–1158. [Google Scholar]
- Javed, S.; Yasin Ashraf, M.; Meraj, M.; Anwer Bukhari, S.; Zovia, I.J.C.P.B. Salinity and drought induced antioxidant responses in different cultivars of safflower (Carthamus tinctorius L.). Curr. Pharm. Biotechnol. 2013, 14, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Akbar, A.; Luo, Q.; Khan, A.H.; Manghwar, H.; Shaban, M.; Yang, X. Microbiome diversity in cotton rhizosphere under normal and drought conditions. Microb. Ecol. 2019, 77, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Chanway, C. Plant growth promotion by Bacillus and relatives. In Applications and Systematics of Bacillus and Relatives; 2002; pp. 219–235. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9780470696743.ch15 (accessed on 9 December 2022).
- Yao, L.; Wu, Z.; Zheng, Y.; Kaleem, I.; Li, C. Growth promotion and protection against salt stress by Pseudomonas putida Rs-198 on cotton. Eur. J. Soil Biol. 2010, 46, 49–54. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Berg, G.; Lindström, K.; Räsänen, L.A. Alleviation of salt stress of symbiotic Galega officinalis L. (goat’s rue) by co-inoculation of Rhizobium with root-colonizing Pseudomonas. Plant Soil 2013, 369, 453–465. [Google Scholar] [CrossRef]
- Kruasuwan, W.; Thamchaipenet, A. 1-aminocyclopropane-1-carboxylate (ACC) deaminase-producing endophytic diazotrophic Enterobacter sp. EN-21 modulates salt–stress response in sugarcane. J. Plant Growth Regul. 2018, 37, 849–858. [Google Scholar] [CrossRef]
- Ghorbanli, M.; Gafarabad, M.; Amirkian, T.; Allahverdi, M.B. Investigation of proline, total protein, chlorophyll, ascorbate and dehydroascorbate changes under drought stress in Akria and Mobil tomato cultivars. Iran. J. Plant Physiol. 2013, 3, 651–658. [Google Scholar]
- Rahdari, P.; Hosseini, S.M.; Tavakoli, S. The studying effect of drought stress on germination, proline, sugar, lipid, protein and chlorophyll content in purslane (Portulaca oleracea L.) leaves. J. Med. Plants Res. 2012, 6, 1539–1547. [Google Scholar]
- Moradi, F.; Ismail, A.M. Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann. Bot. 2007, 99, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Khanna, K.; Jamwal, V.L.; Kohli, S.K.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R.; Abd_Allah, E.F.; Hashem, A.; Ahmad, P. Plant growth promoting rhizobacteria induced Cd tolerance in Lycopersicon esculentum through altered antioxidative defense expression. Chemosphere 2019, 217, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Exogenous jasmonic acid modulates the physiology, antioxidant defense and glyoxalase systems in imparting drought stress tolerance in different Brassica species. Plant Biotechnol. Rep. 2014, 8, 279–293. [Google Scholar] [CrossRef]
- Amirjani, M.R.; Mahdiyeh, M. Antioxidative and biochemical responses of wheat to drought stress. J. Agric. Biol. Sci. 2013, 8, 291–301. [Google Scholar]
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef]
- Shafiq, S.; Akram, N.A.; Ashraf, M.; Arshad, A. Synergistic effects of drought and ascorbic acid on growth, mineral nutrients and oxidative defense system in canola (Brassica napus L.) plants. Acta Physiol. Plant. 2014, 36, 1539–1553. [Google Scholar] [CrossRef]
- Gueta-Dahan, Y.; Yaniv, Z.; Zilinskas, B.A.; Ben-Hayyim, G. Salt and oxidative stress: Similar and specific responses and their relation to salt tolerance in citrus. Planta 1997, 203, 460–469. [Google Scholar] [CrossRef]
- Zahir, Z.A.; Ghani, U.; Naveed, M.; Nadeem, S.M.; Asghar, H.N. Comparative effectiveness of Pseudomonas and Serratia sp. containing ACC-deaminase for improving growth and yield of wheat (Triticum aestivum L.) under salt-stressed conditions. Arch. Microbiol. 2009, 191, 415–424. [Google Scholar] [CrossRef]
- Tiwari, G.; Duraivadivel, P.; Sharma, S.; Hariprasad, P. 1-Aminocyclopropane-1-carboxylic acid deaminase producing beneficial rhizobacteria ameliorate the biomass characters of Panicum maximum Jacq. by mitigating drought and salt stress. Sci. Rep. 2018, 8, 17513. [Google Scholar] [CrossRef]
- Malik, S.; Ashraf, M. Exogenous application of ascorbic acid stimulates growth and photosynthesis of wheat (Triticum aestivum L.) under drought. Soil Environ. 2012, 31, 72–77. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).