Effects of Trellis Systems on the Vegetative Growth and Fruit Quality of Muscat-Flavored Table Grapes
Abstract
1. Introduction
2. Materials and Methods
2.1. Growing Conditions
2.2. Plant Material and Experimental Design
2.3. Investigation of Vegetative Growth and Fruit Load
2.4. Determination of Fruit Physicochemical Indices
2.5. Measurement of the Polyphenolic Compounds Contents in the Fruits
2.6. Detection of Monoterpenes and Qualitative and Quantitative Analysis
2.7. Analysis of the Aromatic Profile
2.8. Data Processing and Statistical Analysis
3. Results
3.1. Effects of Trellis Systems on Fruit Load and Vine Growth Characteristics
3.2. Effects of Different Trellis Systems on Fruit Physicochemical Indicators and Polyphenolic Compounds
3.2.1. Effects of Trellis Systems on Fruit Physicochemical Indicators
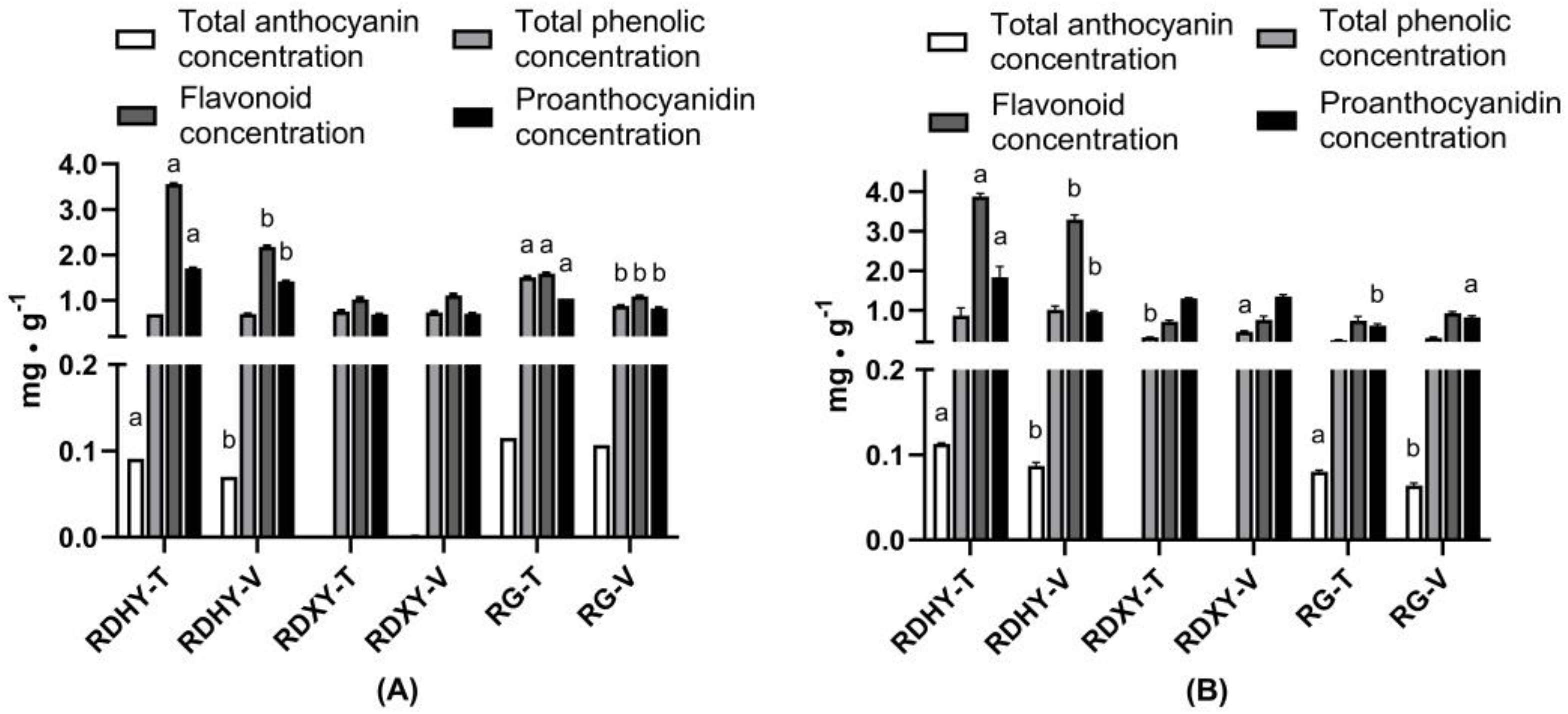
3.2.2. Effects of Trellis Systems on the Content of Polyphenolic Compounds in the Fruit
3.3. Effects of Trellis Systems on the Composition and Content of Monoterpenes in 3 Table Grape Cultivars
3.3.1. Effects of Trellis Systems on the Composition and Content of Monoterpenes
3.3.2. Multivariate Statistical Analysis of Monoterpenes
3.3.3. Evaluation of the Aromatic Profile
4. Discussion
4.1. Effects of Trellis Systems on the Viticultural Characteristics of Three Table Grape Cultivars
4.2. Effects of Trellis Systems on Basic Indicators of Fruit Quality
4.3. Effects of Trellis Systems on Monoterpenes in Grapes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gregan, S.M.; Jordan, B. Methoxypyrazine accumulation and o-methyltransferase gene expression in sauvignon blanc grapes: The role of leaf removal, light exposure, and berry development. J. Sci. Food Agric. 2016, 64, 2200–2208. [Google Scholar] [CrossRef] [PubMed]
- Kalua, C.M.; Boss, P.K. Evolution of volatile compounds during the development of cabernet sauvignon grapes (Vitis vinifera L.). J. Agric. Food Chem. 2009, 57, 3818–3830. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Garcia, L.; Hellin, P.; Flores, P.; Fenoll, J. Prediction of Muscat aroma in table grape by analysis of rose oxide. Food Chem. 2014, 154, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-Q.; Cheng, G.; Duan, L.-L.; Jiang, R.; Pan, Q.-H.; Duan, C.-Q.; Wang, J. Effect of training systems on fatty acids and their derived volatiles in Cabernet Sauvignon grapes and wines of the north foot of Mt. Tianshan. Food Chem. 2015, 181, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Fuentes, S.; Siebert, T.; Krstic, M.; Herderich, M.; Barlow, E.-W.-R.; Howell, K. Terpene evolution during the development of Vitis vinifera L. cv. Shiraz grapes. Food Chem. 2016, 204, 463–474. [Google Scholar] [CrossRef]
- Mateo, J.J.; Jiménez, M. Monoterpenes in grape juice and wines. J. Chromatogr. A 2000, 881, 557–567. [Google Scholar] [CrossRef]
- Wilson, B.; Strauss, C.R.; Williams, P.J. The distribution of free and glycosidically bound monoterpenes among skin, juice, and pulp fractions of some white grape varieties. Am. J. Enol. Vitic. 1986, 37, 107–111. [Google Scholar] [CrossRef]
- Keller, M.; Tarara, J.M.; Mills, L.J. Spring temperatures alter reproductive development in grapevines. Aust. J. Grape Wine Res. 2010, 16, 445–454. [Google Scholar] [CrossRef]
- Zhang, G.-J.; Wang, X.-Y.; Sun, L.; Yan, A.-L.; Wang, H.-L.; Ren, J.-C.; Xu, H.-Y. Grapevine vigor control theory and coping strategy for grape growing under mainland monsoon type climate. Sino-Overseas Grapevine Wine 2016, 3, 30–33. [Google Scholar]
- Li, H.; Wang, H. Chinese Wine; Northwest Agriculture and Forestry University Press: Yangling, China, 2010; pp. 26–29. [Google Scholar]
- Wolf, T.K.; Dry, P.R.; Iland, P.G.; Botting, D.; Dick, J.; Kennedy, U.; Ristic, R. Response of Shiraz grapevines to five different training systems in the Barossa Valley, Australia. Aust. J. Grape Wine Res. 2003, 9, 82–95. [Google Scholar] [CrossRef]
- Song, J.-Q.; Shellie, K.C.; Wang, H.; Qian, M.-C. Influence of deficit irrigation and kaolin particle film on grape composition and volatile compounds in Merlot grape (Vitis vinifera L.). Food Chem. 2012, 134, 841–850. [Google Scholar] [CrossRef]
- Naylor, A.P. The Effects of Row Orientation, Trellis, Shoot and Bunch Position on the Variability of Sauvignon Blanc Vitis vinifera L. Juice Composition. Master Thesis, Lincoln University, Lincoln, New Zealand, 2001. [Google Scholar]
- Reynolds, A.G.; Heuvel, J.E.V. Influence of grapevine training systems on vine growth and fruit composition: A review. Am. J. Enol. Viticult 2009, 60, 251–268. [Google Scholar] [CrossRef]
- Zhou, Y.-M.; Han, J.-Y.; Zhang, J.; Cheng, G.; Xie, T.-L. Effects of different trellis systems on grapevine growth and fruit quality of Vitis vinifera L. cv. Guipu No.6. South China Fruits 2018, 47, 101–103. [Google Scholar]
- Geng, K.-Q.; Li, D.-M.; Zhang, J.; Zhang, Y.-X.; Zhan, Z.-N.; Wang, Z.-P. Evolution of volatile aroma compounds and amino acids in Cabernet Gernischt grape berries (Vitis vinifera L.): Comparison of different training systems for mechanical soil burial. Foods 2022, 11, 1568. [Google Scholar] [CrossRef]
- Bernardo, N.P.; Oliveira, A.; Mota, R.V.; Camara, F.M.M.; Peregrinp, I.; Regina, M.L.; Purgatto, E. Volatile composition of sparkling wines of cv. Chardonnay cultivated under different training systems in Serra da Mantiqueira (Brazil). Foods 2022, 11, 1529. [Google Scholar] [CrossRef]
- Swanepoel, J.J.; Hunter, J.J.; Archer, E. The Effect of trellis systems on the performance of Vitis vinifera L. cvs. Sultanina and Chenel in the lower Orange River region. S. Afr. J. Enol. Vitic 2017, 11, 59–66. [Google Scholar]
- Mota, R.V.D.; Amorim, D.D.; Favero, A.C.; Purgatto, E.; Regina, M.D.A. Effect of trellising system on grape and wine composition of Syrah vines grown in the cerrado region of Minas Gerais. Food Sci. Technol. 2011, 31, 967–972. [Google Scholar] [CrossRef]
- Nan, L.; Liu, L.; Zhao, X.; Qiu, S.; Wang, H.; Li, H. Effect of alternative new pruning system and harvesting times on aroma compounds of young wines from Ecolly (Vitis vinifera) in a new grape growing region of the Weibei Plateau in China. Sci. Hortic. 2013, 162, 181–187. [Google Scholar] [CrossRef]
- He, P.-C.; Cheng, G.-L. Studies of different training systems on wine grapes. J. Fruit Sci. 1994, 11, 14–18. [Google Scholar]
- Li, Y.-D.; Zhang, G.-D.; Ma, J.-P. A new training system for wine grapes in soil-bury areas: Slant trunk with horizontal cordons. Sino-Overseas Grapevine Wine 2006, 6, 25–27. [Google Scholar]
- Coombe, B.G. Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Yang, F.-C.; Wu, J.; Cheng, J.-H.; Xu, K.; Chen, J.-W. Studies on extraction and physical-chemical properties of anthocyanin from red globe grape peel. J. Fruit Sci. 2007, 24, 287–292. [Google Scholar]
- Arnous, A.; Makris, D.P.; Kefalas, P. Correlation of pigment and flavanol content with antioxidant properties in selected aged regional wines from Greece. J. Food Compos. Anal. 2002, 15, 655–665. [Google Scholar] [CrossRef]
- Dai, H.; Qin, C.; Ding, L. Effects of salicylic acid on the contents of total flavonoids and resveratrol and related enzyme activities in ‘Cabernet Sauvignon’. J. China Agric. Univ. 2016, 21, 37–42. [Google Scholar]
- Ramchandani, A.G.; Chettiyar, R.S.; Pakhale, S. Evaluation of antioxidant and anti-initiating activities of crude polyphenolic extracts from seedless and seeded Indian grapes. Food Chem. 2010, 119, 298–305. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Wang, H.-L.; Zhang, G.-J.; Yan, A.-L.; Ren, J.-C.; Liu, Z.-H.; Xu, H.-Y.; Sun, L. Effects of Fruit Bagging Treatment with Different Types of Bags on the Contents of Phenolics and Monoterpenes in Muscat-Flavored Table Grapes. Horticulturae 2022, 8, 411. [Google Scholar] [CrossRef]
- Wu, Y.-W.; Zhu, B.-Q.; Tu, C.; Duan, C.-Q.; Pan, Q.-H. Generation of volatile compounds in litchi wine during winemaking and short-term bottle storage. J. Agric. Food Chem. 2011, 59, 4923–4931. [Google Scholar] [CrossRef]
- Sun, L.; Zhu, B.-Q.; Zhang, X.-Y.; Zhang, G.-J.; Yan, A.-L.; Wang, H.-L.; Wang, X.-Y.; Xu, H.-Y. The accumulation profiles of terpene metabolites in three Muscat table grape cultivars through HS-SPME-GCMS. Sci. Data 2020, 7, 5. [Google Scholar] [CrossRef]
- Bowen, A.J.; Reynolds, A.G. Odor potency of aroma compounds in Riesling and Vidal blanc table wines and ice wines by gas chromatography-olfactometry-mass spectrometry. J. Agric. Food Chem. 2012, 60, 2874–2883. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. Aroma composition and new chemical markers of Spanish citrus honeys. Food Chem. 2007, 103, 601–606. [Google Scholar] [CrossRef]
- Fenoll, J.; Manso, A.; Hellin, P.; Ruiz, L.; Flores, P. Changes in the aromatic composition of the Vitis vinifera grape Muscat Hamburg during ripening. Food Chem. 2009, 114, 420–428. [Google Scholar] [CrossRef]
- Han, Z.-X.; Rana, M.M.; Liu, G.-F.; Gao, M.-J.; Li, D.-X.; Wu, F.-G.; Li, X.-B.; Wan, X.-C.; Wei, S. Data on green tea flavor determinantes as affected by cultivars and manufacturing processes. Data Brief 2017, 10, 492–498. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.; Yang, W.; Sun, B.; Zhou, Y.; Zheng, Y.; Huang, M.; Yang, W. Characterization of the potent odorants in Zanthoxylum armatum DC Prodr. pericarp oil by application of gas chromatography-mass spectrometry-olfactometry and odor activity value. Food Chem. 2020, 319, e126564. [Google Scholar] [CrossRef]
- Noguerol-Pato, R.; Gonzalez-Barreiro, C.; Cancho-Grande, B.; Martinez, M.C.; Santiago, J.L.; Simal-Gandara, J. Floral, spicy and herbaceous active odorants in Gran Negro grapes from shoulders and tips into the cluster, and comparison with Brancellao and Mouraton varieties. Food Chem. 2012, 135, 2771–2782. [Google Scholar] [CrossRef]
- Pino, J.A.; Mesa, J. Contribution of volatile compounds to mango (Mangifera indica L.) aroma. Flavour Fragr. J. 2006, 21, 207–213. [Google Scholar] [CrossRef]
- Tamura, H.; Boonbumrung, S.; Yoshizawa, T.; Varanyanond, D.W. The Volatile Constituents in the Peel and Pulp of a Green Thai Mango, KhieoSawoei Cultivar (Mangifera indica L.). Food Sci. Technol. Res. 2001, 7, 72–77. [Google Scholar] [CrossRef]
- Wu, Y.; Duan, S.; Zhao, L.; Gao, Z.; Luo, M.; Song, S.; Xu, W.; Zhang, C.; Ma, C.; Wang, S. Aroma characterization based on aromatic series analysis in table grapes. Sci. Rep. 2016, 6, 31116. [Google Scholar] [CrossRef]
- Cai, J. Study on Aroma Quality Improvement for Cabernet Sauvignon Wines based on Pre-Fermentation Technology from North Slope of Tianshan Mountains. Ph.D. Thesis, China Agriculture University, Beijing, China, 2014. [Google Scholar]
- Cai, X.; Mai, R.-Z.; Zou, J.-J.; Zhang, H.-Y.; Zeng, X.-L.; Zheng, R.-R.; Wang, C.-Y. Analysis of aroma-active compounds in three sweet osmanthus (Osmanthus fragrans) cultivars by GC-olfactometry and GC-MS. J. Zhejiang Univ.-Sci. B 2014, 15, 638–648. [Google Scholar] [CrossRef]
- Percival, D.C.; Fisher, K.H.; Sullivan, J.-A. Use of fruit zone leaf removal with Vitis vinifera L. cv. Riesling grapevines. I. Effect on canopy structure, microclimate, bud survival, shoot density, and vine vigor. Am. J. Enol. Vitic. 1994, 45, 123–132. [Google Scholar] [CrossRef]
- Huang, H.-H. Application of T-trellis system and rain-shelter cultivation on ‘Kyoho’. Mod. Hortic. 2006, 5, 16–17. [Google Scholar]
- Chao, W.-J.; Zhang, W.; Yao, L.-Q. Effects of different pruning methods on flower initiation of red globe grape. Sino-Overseas Grapevine Wine 2009, 1, 31–33. [Google Scholar]
- Jayasena, V.; Cameron, I. Brix/acid ratio as a predictor of consumer acceptability of Crimson Seedless table grapes. J. Food Qual. 2008, 31, 736–750. [Google Scholar] [CrossRef]
- Nelson, K.E.; Schutz, H.G.; Ahmedullah, M.; Mcpherson, J. Flavour preferences of supermarket customers for ‘Thompson Seedless’ grapes. Am. J. Enol. Vitic. 1973, 24, 31–40. [Google Scholar] [CrossRef]
- Bai, S.-J.; Li, C.; Cai, J.-S.; Zhao, R.-H.; Chen, G. Principal factor analysis and Euclidean cluster diagram of new grape cultivars’ quality traits in Turpan area. Acta Agric.-Occident. Sin. 2016, 25, 1006–1016. [Google Scholar]
- Zhang, L.; Zhang, X.-Y.; Kang, Y.-L.; Ma, G.-F.; Yuan, H.-Y. The effect of soil fertility on wine grape quality. Acta Agric. Jiangxiensis 2008, 2, 226–229. [Google Scholar]
- Makris, D.P.; Kallithraka, S.; Kefalas, P. Flavonols in grapes, grape products and wines: Burden, profile and influential parameters. J. Food Compos. Anal. 2006, 19, 396–404. [Google Scholar] [CrossRef]
- Xia, L.-L.; Xu, C.-M.; Huang, K.-L.; Lu, J.; Zhang, Y.-L. Evaluation of phenolic compounds, antioxidant and antiproliferative activities of 31 grape cultivars with different genotypes. J. Food Biochem. 2019, 43, e12626. [Google Scholar] [CrossRef]
- Liu, X.; Guo, S.; Xiao, Q.; Du, Y.; Zhai, H. Effect of canopy micro-environment on anthocyanins of ‘Moldova’ grape. Food Sci. 2018, 39, 98–106. [Google Scholar]
- Palliotti, A. A new closing Y-shaped training system for grapevines. Aust. J. Grape Wine Res. 2012, 18, 57–63. [Google Scholar] [CrossRef]
- Shens, T.; Shan, S.-M.; Sun, Y.; Li, Y.-L.; Zhang, J.-X. The effect of Chang trellis system on photosynthetic efficiency and fruit quality of Cabernet Sauvignon. North. Hortic. 2015, 1, 27–30. [Google Scholar]
- Song, J.; Forney, C.F. Flavour volatile production and regulation in fruit. Can. J. Plant Sci. 2008, 88, 537–550. [Google Scholar] [CrossRef]
- Nykänen, L. Formation and occurrence of flavor compounds in wine and distilled alcoholic beverages. Am. J. Enol. Viticult. 1986, 37, 184–196. [Google Scholar] [CrossRef]
- Friedel, M.; Frotscher, J.; Nitsch, M.; Hofmann, M.; Bogs, J.; Stoll, M.; Dietrich, H. Light promotes expression of monoterpene and flavonol metabolic genes andenhances flavour of winegrape berries (Vitis vinifera L. cv. Riesling). Aust. J. Grape Wine Res. 2016, 22, 409–421. [Google Scholar] [CrossRef]
- Luo, J.; Brotchie, J.; Pang, M.; Marriott, P.J.; Howell, K.; Zhang, P. Freeterpene evolution during the berry maturation of five Vitis vinifera L. cultivars. Food Chem. 2019, 299, 125101. [Google Scholar] [CrossRef]




| Percentage of Budbreak/% | Percentage of Bearing Shoots/% | Flower Clusters per Bearing Shoot | ||
|---|---|---|---|---|
| Year 2019 | ||||
| Treatment | ||||
| T | 59.7 | 72.4 | 1.5 | |
| V | 54.5 | 68.2 | 1.34 | |
| Cultivar | ||||
| RDHY | 51.3 b | 84.4 a | 1.53 a | |
| RDXY | 65.9 a | 88.2 a | 1.64 a | |
| RG | 54.0 b | 38.3 b | 1.09 b | |
| Treatment × Cultivar | ||||
| RDHY | ||||
| T | 46.8 b | 91.4 a | 1.68 a | |
| V | 55.8 b | 77.4 a | 1.38 a | |
| RDXY | ||||
| T | 73.4 a | 84.7 a | 1.63 a | |
| V | 58.5 b | 91.8 a | 1.65 a | |
| RG | ||||
| T | 59.0 b | 41.0 b | 1.18 b | |
| V | 49.1 b | 35.6 b | 1.00 b | |
| Significance | ||||
| Treatment | 0.217 ns | 0.357 ns | 0.120 ns | |
| Cultivar | 0.026 * | <0.001 *** | <0.001 *** | |
| Treatment × Cultivar | 0.072 ns | 0.179 ns | 0.409ns | |
| Year 2020 | ||||
| Treatment | ||||
| T | 66.4 | 59.2 | 1.5 | |
| V | 71 | 67.1 | 1.57 | |
| Cultivar | ||||
| RDHY | 64.1 b | 71.0 b | 1.72 a | |
| RDXY | 76.2 a | 85.2 a | 1.74 a | |
| RG | 65.9 b | 33.2 c | 1.15 b | |
| Treatment × Cultivar | ||||
| RDHY | ||||
| T | 62.7 | 67.9 a | 1.67 a | |
| V | 65.5 | 74.2 a | 1.76 a | |
| RDXY | ||||
| T | 73 | 82.0 a | 1.59 a | |
| V | 79.4 | 88.5 a | 1.90 a | |
| RG | ||||
| T | 63.6 | 27.9 b | 1.23 b | |
| V | 68.2 | 38.5 b | 1.06 b | |
| Significance | ||||
| Treatment | 0.161 ns | 0.098 ns | 0.374ns | |
| Cultivar | 0.016 * | <0.001 *** | <0.001 *** | |
| Treatment × Cultivar | 0.897 ns | 0.904 ns | 0.097ns | |
| Year 2021 | ||||
| Treatment | ||||
| T | 79 | 67.5 a | 1.55 | |
| V | 82.5 | 57.6 b | 1.5 | |
| Cultivar | ||||
| RDHY | 81.9 | 75.6 a | 1.63 b | |
| RDXY | 80 | 78.0 a | 1.81 a | |
| RG | 80.3 | 34.0 b | 1.15 c | |
| Treatment × Cultivar | ||||
| RDHY | ||||
| T | 77 | 85.4 a | 1.69 a,b | |
| V | 86.8 | 65.8 b | 1.56 b | |
| RDXY | ||||
| T | 84.2 | 73.8 a,b | 1.78 a | |
| V | 75.7 | 82.3 a | 1.83 a | |
| RG | ||||
| T | 75.7 | 43.3 c | 1.19 c | |
| V | 84.9 | 24.7 d | 1.12 c | |
| Significance | ||||
| Treatment | 0.797 ns | 0.034 * | 0.346ns | |
| Cultivar | 0.182 ns | <0.001 *** | <0.001 *** | |
| Treatment × Cultivar | 0.017 * | 0.027 * | 0.393ns | |
| Cultivar | Treatment | Vine Thickness (mm) | Vine Length (mm) | Shoot Thickness (mm) | Maximum Shoot Thickness (mm) | Minimum Shoot Thickness (mm) | Difference between Max and Min (mm) |
|---|---|---|---|---|---|---|---|
| Treatment | |||||||
| T | 59.5 | 596.2 | 11.2 b | ||||
| V | 58.8 | 464.6 | 11.9 a | ||||
| Cultivar | |||||||
| RDHY | 61.0 | 492.7 | 11.3 | ||||
| RDXY | 58.5 | 568.7 | 11.5 | ||||
| RG | 57.9 | 529.8 | 11.7 | ||||
| Treatment × Cultivar | |||||||
| RDHY | |||||||
| T | 61.1 | 574.7 | 10.4 b | 14.8 | 4.3 | 10.5 | |
| V | 61.0 | 410.7 | 12.2 a | 17.8 | 4.6 | 13.2 | |
| RDXY | |||||||
| T | 56.7 | 662.7 | 11.6 a | 16.8 | 5.3 | 11.5 | |
| V | 60.2 | 474.7 | 11.4 a | 16.2 | 5.0 | 11.2 | |
| RG | |||||||
| T | 60.7 | 551.3 | 11.5 a | 16.3 | 7.7 | 8.6 | |
| V | 55.1 | 508.3 | 12.0 a | 19.2 | 7.2 | 12.1 | |
| Significance | |||||||
| Treatment | 0.865 ns | 0.089 ns | <0.001 *** | ||||
| Cultivar | 0.819 ns | 0.692 ns | 0.246 ns | ||||
| Treatment × Cultivar | 0.682 ns | 0.680 ns | <0.001 *** | ||||
| Cluster Weight (g) | Berry Weight (g) | Berry Width (cm) | Berry Length (cm) | Fruit Shape Index | Total Soluble Solids (°Brix) | TA (g·L−1) | TSS/TA | ||
|---|---|---|---|---|---|---|---|---|---|
| 2019 | |||||||||
| Treatment | |||||||||
| T | 632.4 | 17.1 | 4.93 b | 34.9 a | |||||
| V | 574.7 | 16.9 | 5.22 a | 32.9 b | |||||
| Cultivar | |||||||||
| RDHY | 478.9 b | 17.7 a | 4.56 c | 39.4 a | |||||
| RDXY | 542.9 b | 16.5 b | 5.23 b | 31.5 b | |||||
| RG | 788.8 a | 16.7 b | 5.44 a | 30.8 b | |||||
| Treatment × Cultivar | |||||||||
| RDHY | T | 503 b | 6.1 | 1.96 | 2.35 | 1.2 | 17.4 a | 4.00 c | 43.1 a |
| V | 455 b | 5.8 | 1.97 | 2.32 | 1.18 | 18.0 a | 5.11 b | 35.7 b | |
| RDXY | T | 637 a | 7.5 | 2.29 | 2.48 | 1.08 | 16.2 b | 5.17 b | 30.6 c |
| V | 516 b | 7.1 | 2.2 | 2.34 | 1.06 | 16.8 b | 5.28 b | 32.4 c | |
| RG | T | 758 a | 9.4 | 2.44 | 2.63 | 1.08 | 17.7 a | 5.63 a | 31.1 c |
| V | 820 a | 9.7 | 2.36 | 2.48 | 1.05 | 15.7 c | 5.25 b | 30.4 c | |
| Significance | |||||||||
| Treatment | 0.249 ns | 0.418 ns | <0.001 *** | 0.035 * | |||||
| Cultivar | <0.001 *** | 0.006 ** | <0.001 *** | <0.001 *** | |||||
| Treatment × Cultivar | 0.144 ns | <0.001 *** | <0.001 *** | 0.003 ** | |||||
| 2020 | |||||||||
| Treatment | |||||||||
| T | 576.4 | 18.9 a | 4.91 b | 39.3 a | |||||
| V | 526.6 | 17.8 b | 5.04 a | 35.9 b | |||||
| Cultivar | |||||||||
| RDHY | 322.8 c | 20.5 a | 4.88 c | 42.9 a | |||||
| RDXY | 542.9 b | 17.5 b | 5.07 a | 34.8 b | |||||
| RG | 788.8 a | 17.2 b | 4.98 b | 35.1 b | |||||
| Treatment × Cultivar | |||||||||
| RDHY | T | 368 b | 6.4 | 2.05 | 2.5 | 1.22 | 21.0 a | 4.70 c | 46.2 a |
| V | 344 b | 6.1 | 2.04 | 2.44 | 1.2 | 20.0 a | 5.05 a | 39.6 b | |
| RDXY | T | 509 a | 8.2 | 2.35 | 2.46 | 1.05 | 18.3 b | 5.09 a | 36.0 b |
| V | 457 b | 6.7 | 2.18 | 2.35 | 1.08 | 16.6 c | 5.05 a | 33.7 b | |
| RG | T | 905 a | 12.2 | 2.67 | 2.94 | 1.1 | 17.5 b,c | 4.93 b | 35.7 b |
| V | 906 a | 13.1 | 2.75 | 3.03 | 1.1 | 16.9 b,c | 5.03 ab | 34.5 b | |
| Significance | |||||||||
| Treatment | 0.296 ns | 0.025 * | 0.001 ** | 0.026 * | |||||
| Cultivar | <0.001 *** | <0.001 *** | 0.001 ** | <0.001 *** | |||||
| Treatment × Cultivar | 0.118 ns | 0.671 ns | <0.001 *** | 0.254ns | |||||
| 2021 | |||||||||
| Treatment | |||||||||
| T | 734.9 | 17.2 | 5.16 | 33.9 | |||||
| V | 722.2 | 16.9 | 5.15 | 32.4 | |||||
| Cultivar | |||||||||
| RDHY | 395.8 c | 18.3 a | 5.15 b | 36.5 a | |||||
| RDXY | 712.5 b | 17.0 b | 5.56 a | 29.8 c | |||||
| RG | 1077.4 a | 15.9 b | 4.74 c | 33.3 b | |||||
| 333Treatment × Cultivar | |||||||||
| RDHY | T | 422 c | 6 | 2.05 | 2.32 | 1.13 | 18.4 a | 5.33 b | 36.9 a |
| V | 369 c | 6.2 | 2.05 | 2.4 | 1.17 | 18.2 a | 4.98 c | 36.1 a | |
| RDXY | T | 752 b | 8.5 | 2.33 | 2.6 | 1.12 | 17.2 a,b | 5.31 b | 32.2 a |
| V | 673 b | 8.5 | 2.4 | 2.6 | 1.08 | 16.7 a,b | 5.80 a | 28.3 b | |
| RG | T | 1031 a | 11.9 | 2.5 | 2.9 | 1.16 | 16.0 b | 4.83 c | 34.0 a |
| V | 1124 a | 10.8 | 2.4 | 2.6 | 1.08 | 15.8 b | 4.65 d | 33.8 a | |
| Significance | |||||||||
| Treatment | 0.782 ns | 0.539 ns | 0.730 ns | 0.208 ns | |||||
| Cultivar | <0.001 *** | 0.003 ** | <0.001 *** | 0.002 ** | |||||
| Treatment × Cultivar | 0.295 ns | 0.976 ns | <0.001 *** | 0.157 ns |
| Code | Compound | Olfactory Threshold (µg∙L−1) a | Cultivar | Treatment b | Aroma Description | Aromatic Category c | Category No. | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|
| T | V | |||||||||
| M1 | β-Myrcene | 36 | RDHY | 11.9 | 5.73 | Fruity flavor | Other fruity flavor | 10 | [37] | |
| RDXY | 13.31 | 16.46 | ||||||||
| RG | \ | \ | ||||||||
| M2 | Limonene | 10 | RDHY | 2.95 | 0.52 | Floral, grassy and citrus flavor | Other floral, grassy and citrus flavor | 2, 4, 9 | [40] | |
| RDXY | 3.67 | 5.4 | ||||||||
| RG | \ | \ | ||||||||
| M3 | Phellandrene | 40 | RDHY | 0.73 | 0.65 | Sweet and rose flavor | Muscat and sweet flavor | 1, 8 | [39] | |
| RDXY | 1.69 | 1.96 | ||||||||
| RG | \ | \ | ||||||||
| M4 | β-trans-Ocimene | 34 | RDHY | 4.00 | 2.11 | Herbal | Grassy and sweet flavor | 2, 8 | [41] | |
| RDXY | 4.74 | 2.43 | ||||||||
| RG | \ | \ | ||||||||
| M5 | γ-Terpinen | 1000 | RDHY | 0.05 | 0.03 | |||||
| RDXY | 0.06 | 0.17 | ||||||||
| RG | \ | \ | ||||||||
| M6 | β-cis-Ocimene | 34 | RDHY | 5.78 | 2.76 | Herbal, floral | Grassy and other floral | 2, 9 | [41] | |
| RDXY | 7.02 | 6.23 | ||||||||
| RG | \ | \ | ||||||||
| M7 | Terpinolen | 200 | RDHY | 17.67 | 15.77 | Pine oil flavor | Grassy flavor | 2 | [39] | |
| RDXY | 18.87 | 19.12 | ||||||||
| RG | \ | \ | ||||||||
| M8 | cis-Rose oxide | 0.5 | RDHY | 106.51 | 103.59 | Rose flavor | Muscat flavor | 1 | [33] | |
| RDXY | 115.16 | 124.71 | ||||||||
| RG | \ | \ | ||||||||
| M9 | trans-Rose oxide | 0.5 | RDHY | 76.39 | 76.38 | Rose flavor | Muscat flavor | 1 | [33] | |
| RDXY | 74.40 | 76.96 | ||||||||
| RG | \ | \ | ||||||||
| M10 | Allo-ocimene | \ | RDHY | \ | \ | |||||
| RDXY | \ | \ | ||||||||
| RG | \ | \ | ||||||||
| M11 | (E,Z)-Allo-ocimene | \ | RDHY | \ | \ | |||||
| RDXY | \ | \ | ||||||||
| RG | \ | \ | ||||||||
| M12 | cis-Furan linalool oxide | 6 | RDHY | 18.42 | 8.29 | Floral | Floral | 9 | [41] | |
| RDXY | 61.56 | 48.68 | ||||||||
| RG | \ | \ | ||||||||
| M13 | trans-Furan linalool oxide | 6 | RDHY | 9.18 | 5.67 | Citrus, green | Citrus and grassy flavor | 2, 4 | [41] | |
| RDXY | 9.02 | 8.77 | ||||||||
| RG | \ | \ | ||||||||
| M14 | Nerol oxide | 3000 | RDHY | 0.02 | 0.01 | |||||
| RDXY | 0.02 | 0.02 | ||||||||
| RG | \ | \ | ||||||||
| M15 | Linalool | 6 | RDHY | 821.81 | 384.69 | Floral, lavender, citrus and bayberry flavor | Other floral, citrus and fruity flavor | 4, 9, 10 | [33] | |
| RDXY | 656.72 | 555.13 | ||||||||
| RG | 89.24 | 89.54 | ||||||||
| M16 | hortrineol | 110 | RDHY | 4.07 | 3.22 | Floral and fruity flavor | Other floral and fruity flavor | 9, 10 | [39] | |
| RDXY | 3.10 | 2.81 | ||||||||
| RG | \ | \ | ||||||||
| M17 | 4-Terpineol | 130 | RDHY | 0.08 | 0.07 | |||||
| RDXY | 0.07 | 0.07 | ||||||||
| RG | \ | \ | ||||||||
| M18 | Neral | 1000 | RDHY | 0 | 0 | |||||
| RDXY | 0 | 0 | ||||||||
| RG | \ | \ | ||||||||
| M19 | α-Terpineol | 330 | RDHY | 0.28 | 0.2 | |||||
| RDXY | 0.3 | 0.28 | ||||||||
| RG | \ | \ | ||||||||
| M20 | Geranial | 32 | RDHY | 0.76 | 0.77 | |||||
| RDXY | 0.78 | 0.79 | ||||||||
| RG | \ | \ | ||||||||
| M21 | β-Citronellol | 40 | RDHY | 0.54 | 0.56 | |||||
| RDXY | 0.7 | 0.78 | ||||||||
| RG | \ | \ | ||||||||
| M22 | Nerol | 300 | RDHY | 0.13 | 0.13 | |||||
| RDXY | 0.15 | 0.15 | ||||||||
| RG | \ | \ | ||||||||
| M23 | Geraniol | 40 | RDHY | 3.05 | 3.20 | Rose, geranium, peach and lemon flavor | Muscat, lemon and other fruity flavor | 1, 3, 10 | [40] | |
| RDXY | 3.16 | 3.05 | ||||||||
| RG | \ | \ | ||||||||
| M24 | Geranic acid | 40 | RDHY | 46.64 | 47.14 | Green flavor | Grassy flavor | 2 | [36] | |
| RDXY | 52.19 | 51.79 | ||||||||
| RG | 45.89 | 45.69 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.-Y.; Zhang, G.-J.; Wang, H.-L.; Ren, J.-C.; Yan, A.-L.; Liu, Z.-H.; Xu, H.-Y.; Sun, L. Effects of Trellis Systems on the Vegetative Growth and Fruit Quality of Muscat-Flavored Table Grapes. Agronomy 2023, 13, 1090. https://doi.org/10.3390/agronomy13041090
Wang X-Y, Zhang G-J, Wang H-L, Ren J-C, Yan A-L, Liu Z-H, Xu H-Y, Sun L. Effects of Trellis Systems on the Vegetative Growth and Fruit Quality of Muscat-Flavored Table Grapes. Agronomy. 2023; 13(4):1090. https://doi.org/10.3390/agronomy13041090
Chicago/Turabian StyleWang, Xiao-Yue, Guo-Jun Zhang, Hui-Ling Wang, Jian-Cheng Ren, Ai-Ling Yan, Zhen-Hua Liu, Hai-Ying Xu, and Lei Sun. 2023. "Effects of Trellis Systems on the Vegetative Growth and Fruit Quality of Muscat-Flavored Table Grapes" Agronomy 13, no. 4: 1090. https://doi.org/10.3390/agronomy13041090
APA StyleWang, X.-Y., Zhang, G.-J., Wang, H.-L., Ren, J.-C., Yan, A.-L., Liu, Z.-H., Xu, H.-Y., & Sun, L. (2023). Effects of Trellis Systems on the Vegetative Growth and Fruit Quality of Muscat-Flavored Table Grapes. Agronomy, 13(4), 1090. https://doi.org/10.3390/agronomy13041090








