The Agropyron mongolicum bHLH Gene AmbHLH148 Positively Involved in Transgenic Nicotiana benthamiana Adaptive Response to Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Growth Condition
2.2. Plant Drought Treatment
2.3. Homology Analysis, Expression Analysis and Cloning of the AmbHLH148 Gene
2.4. Subcellular Localization of AmbHLH148
2.5. AmbHLH148 Vector Construction and Genetic Transformation
2.6. qRT-PCR Analysis
2.7. Determination of Physiological Indexes
2.8. Statistical Analysis
3. Results
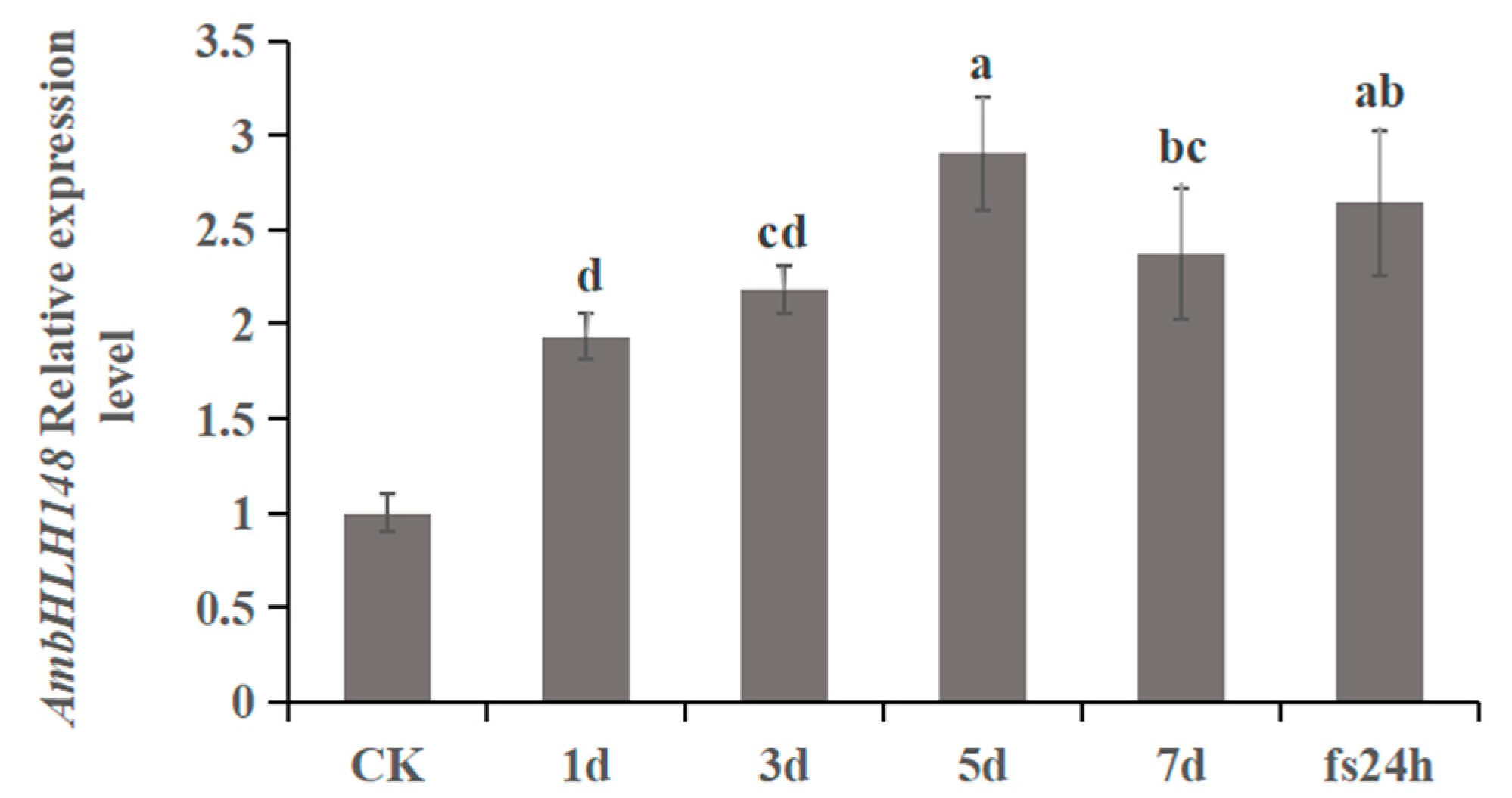
3.1. Analysis of AmbHLH148 Gene Expression in A. mongolicum under 25% PEG-6000 Stress
3.2. Homology Analysis of the AmbHLH148 Gene and Full-Field Amplification of ORF
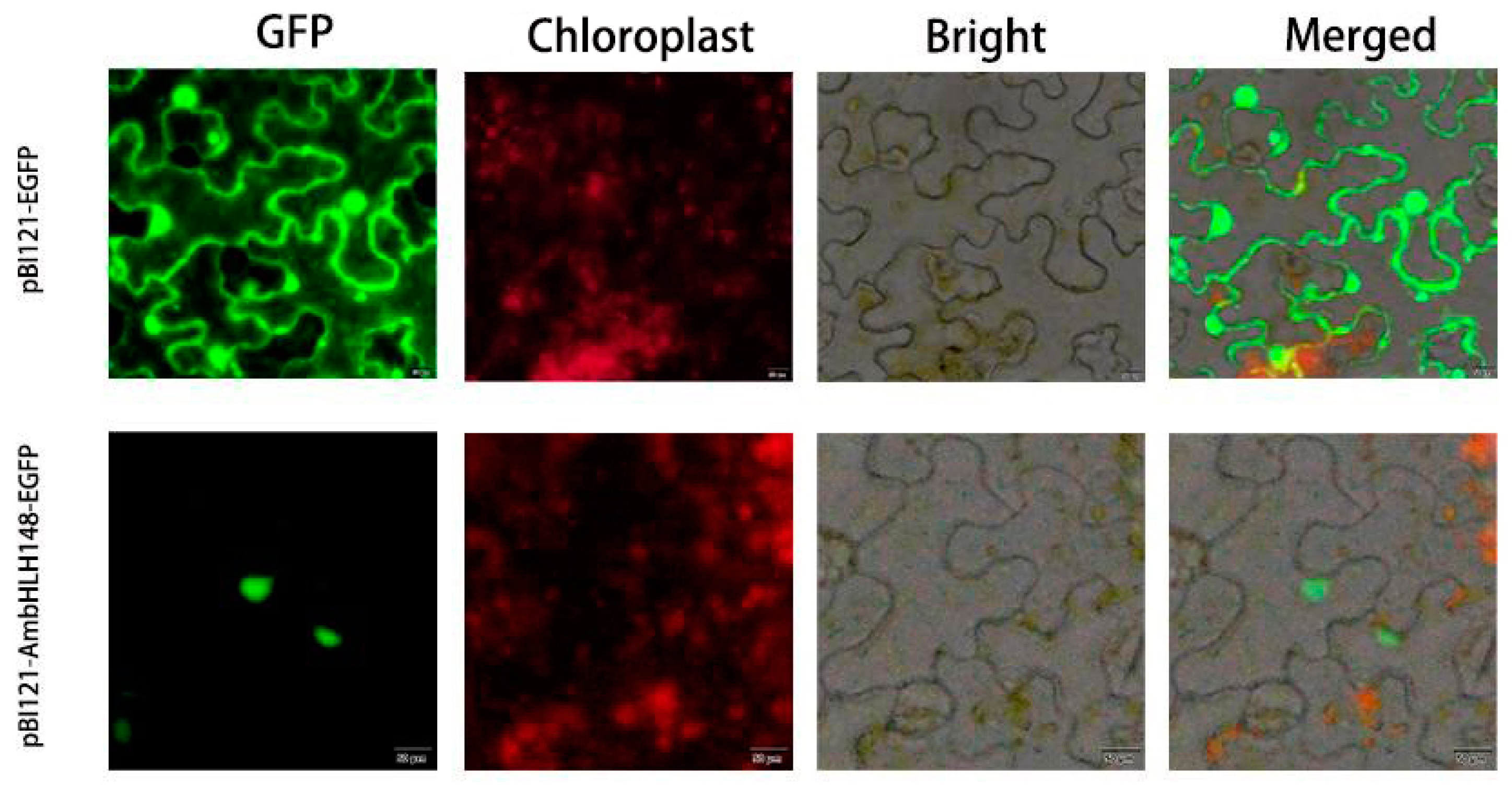
3.3. Subcellular Localization of AmbHLH148 Gene
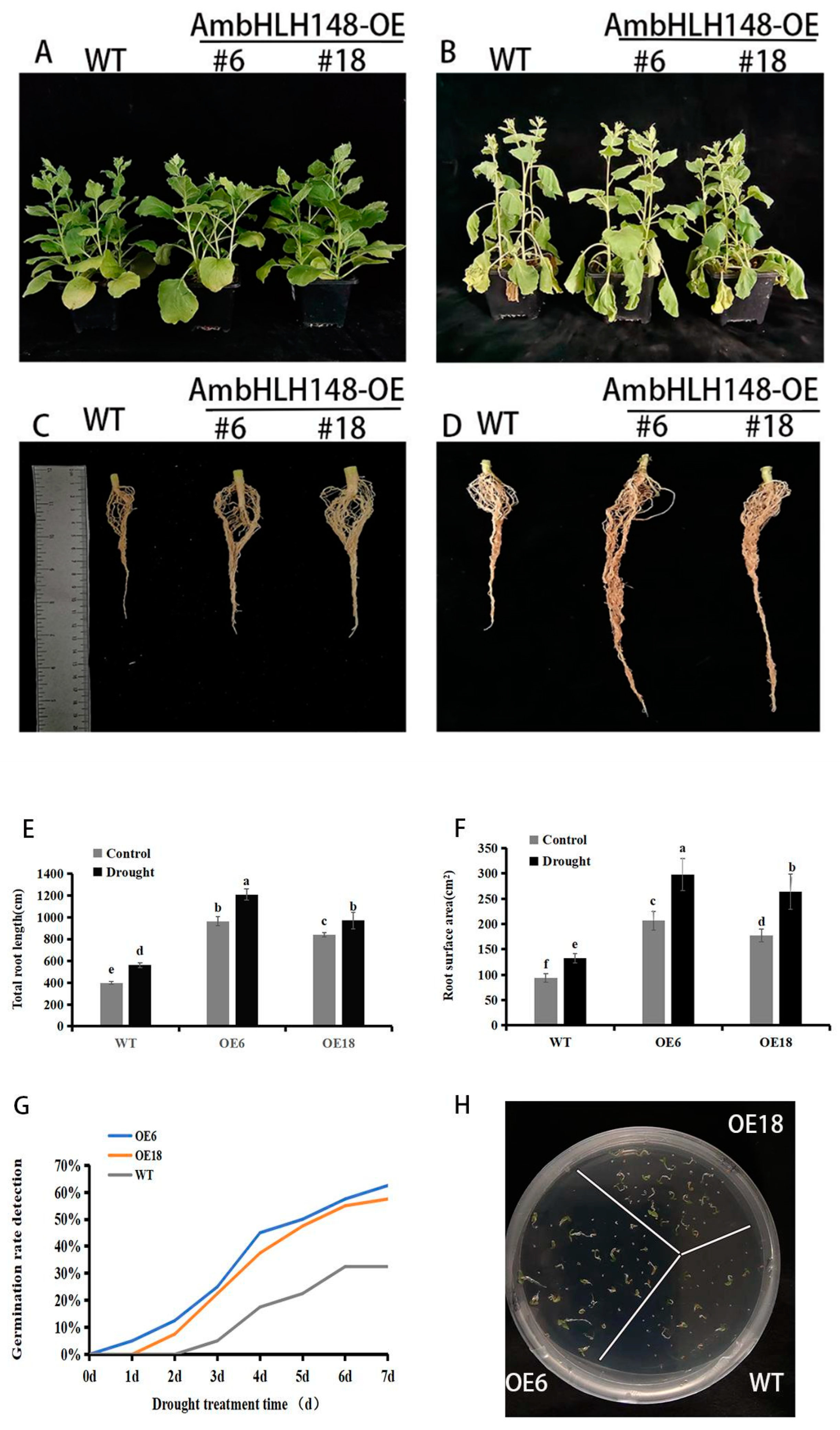
3.4. Production and Selection of AmbHLH148 Transgenic Plants
3.5. Observation of Tobacco Phenotypes under Drought Stress
3.6. AmbHLH148 Affects the Expression of Stress Response Genes
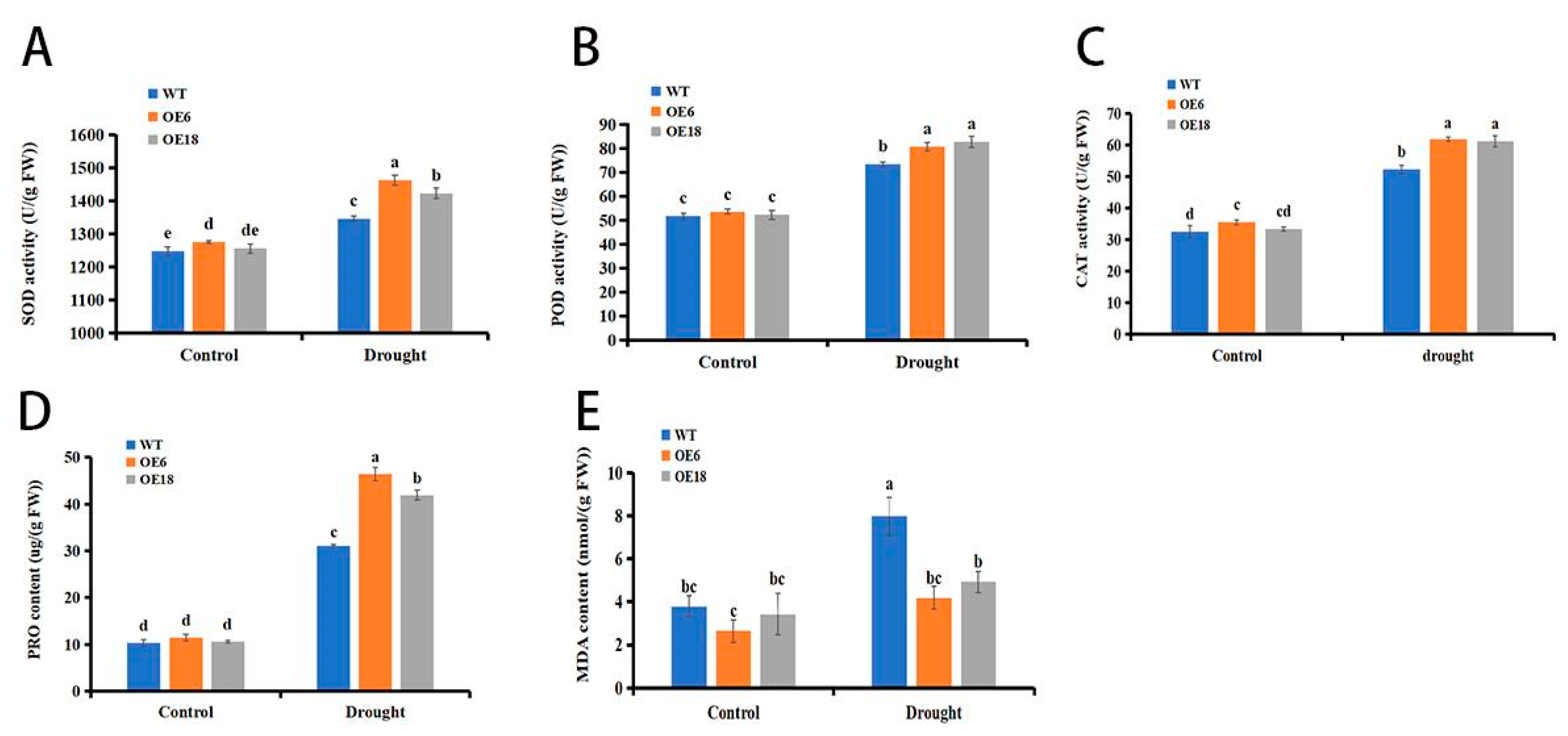
3.7. Overexpression of AmbHLH148 Enhanced Antioxidant Capacity of Tobacco
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, B.F.; Ma, Z.H.; Yan, N.N. Agricultural Drought Mitigating Indices Derived from the Changes in Drought Characteristics. Remote Sens. Environ. 2020, 244, 111813. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Wei, X.; Wang, M.P.; Zhu, X.F.; Zhao, Y.; Wei, F.J.; Xia, Z.L. Overexpression of NtabDOG1L promotes plant growth and enhances drought tolerance in Nicotiana tabacum. Plant Sci. 2019, 287, 110186. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, C.; Elliott, J.; Deryng, D.; Ruane, A.C.; Müller, C.; Arneth, A.; Boote, K.J.; Folberth, C.; Glotter, M.; Khabarov, N.; et al. Assessing agricultural risks of climate change in the 21st century in a global gridded crop model intercomparison. Proc. Natl. Acad. Sci. USA 2013, 111, 3268–3273. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.S.; Song, C.P.; Wang, B.S.; Zhou, J.M.; Kangasjarvi, J.; Zhu, J.K.; Gong, Z.Z. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 2018, 60, 805–826. [Google Scholar] [CrossRef]
- Buscaill, P.; Rivas, S. Transcriptional control of plant defence responses. Curr. Opin. Plant Biol. 2014, 20, 35–46. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene Networks Involved in Abiotic Stress Responses. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Zhu, C.J.K. From laboratory to field. Using Information from Arabidopsis to Engineer Salt, Cold, and Drought Tolerance in Crops. Plant Physiol. 2004, 135, 615–621. [Google Scholar] [CrossRef]
- Joshi, R.; Wani, S.H.; Singh, B.; Bohra, A.; Dar, Z.A.; Lone, A.A.; Pareek, A.; Pareek, S.L.S. Transcription Factors and Plants Response to Drought Stress: Current Understanding and Future Directions. Front. Plant Sci. 2016, 7, 1029. [Google Scholar] [CrossRef]
- Guo, J.R.; Sun, B.X.; He, H.R.; Zhang, Y.F.; Tian, H.Y.; Wang, B.S. Current Understanding of bHLH Transcription Factors in Plant Abiotic Stress Tolerance. Int. J. Mol. Sci. 2021, 22, 4921. [Google Scholar] [CrossRef]
- Bailey, P.C.; Martin, C.; Toledo-Ortiz, G.; Quail, P.H. Update on the Basic Helix-Loop-Helix Transcription Factor Gene Family in Arabidopsis thaliana. Plant Cell 2003, 15, 2497–2501. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Chen, J.; Liang, C.L.; Liu, F. Genome-Wide Identification and Characterization of the bHLH Transcription Factor Family in Pepper (Capsicum annuum L.). Front. Genet. 2020, 11, 570156. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Duan, X.; Jiang, H.; Sun, Y.; Tang, Y.; Zheng, Y.; Guo, J.; Liang, W.; Yin, J. Genome-Wide Analysis of Basic/Helix-Loop-Helix Transcription Factor Family in Rice and Arabidopsis. Plant Physiol. 2006, 141, 1167–1184. [Google Scholar] [CrossRef]
- Wang, R.Q.; Zhao, P.; Kong, N.N.; Lu, R.Z.; Yue, P.; Huang, C.X. Genome-Wide Identification and Characterization of the Potato bHLH Transcription Factor Family. Genes 2018, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Wang, T.; Han, J.; Ren, Z.H. Genome-Wide Identification and Characterization of Cucumber bHLH Family Genes and the Functional Characterization of CsbHLH041 in NaCl and ABA Tolerance in Arabidopsis and Cucumber. BMC Plant Biol. 2020, 20, 272. [Google Scholar] [CrossRef]
- Gao, C.; Sun, J.L.; Wang, C.Q.; Dong, Y.M.; Xiao, S.H.; Wang, X.J.; Jiao, Z.G.; Chen, Z.H. Genome-Wide Analysis of Basic/Helix-Loop-Helix Gene Family in Peanut and Assessment of Its Roles in Pod Development. PLoS ONE 2017, 12, e0181843. [Google Scholar] [CrossRef]
- Kavas, M.; Baloglu, M.C.; Atabay, E.S.; Ziplar, U.T.; Dasgan, H.Y.; Ünver, T. Genome-Wide Characterization and Expression Analysis of Common Bean bHLH Transcription Factors in Response to Excess Salt Concentration. Mol. Genet. Genom. 2016, 291, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Groszmann, M.; Bylstra, Y.; Lampugnani, E.R.; Smyth, D.R. Regulation of tissue-specific expression of SPATULA, a bHLH gene involved in carpel development, seedling germination, and lateral organ growth in Arabidopsis. J. Exp. Bot. 2010, 61, 1495–1508. [Google Scholar] [CrossRef]
- Mertens, J.; Pollier, J.; Vanden Bossche, R.; Lopez-Vidriero, I.; Franco-Zorrilla, J.M.; Goossens, A. The bHLH transcription factors TSAR1 and TSAR2 regulate triterpene saponin biosynthesis in Medicago truncatula. Plant Physiol. 2016, 170, 194–210. [Google Scholar] [CrossRef]
- Yu, J.Q.; Gu, K.D.; Sun, C.H.; Zhang, Q.Y.; Wang, J.H.; Ma, F.F.; You, C.X.; Hu, D.G.; Hao, Y.J. The apple bHLH transcription factor MdbHLH3 functions in determining the fruit carbohydrates and malate. Plant Biotechnol. J. 2020, 19, 285–299. [Google Scholar] [CrossRef]
- Pires, N.; Dolan, L. Origin and diversification of basic-helix-loop-helix proteins in plants. Mol. Biol. Evol. 2010, 27, 862–874. [Google Scholar] [CrossRef]
- Qi, T.C.; Song, S.S.; Ren, Q.C.; Wu, D.W.; Huang, H.; Chen, Y.; Fan, M.; Peng, W.; Ren, C.M.; Xie, D.X. The Jasmonate-ZIM-Domain Proteins Interact with the WD-Repeat/bHLH/MYB Complexes to Regulate Jasmonate-Mediated Anthocyanin Accumulation and Trichome Initiation in Arabidopsis thaliana. Plant Cell 2011, 23, 1795–1814. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, Z.; Zeng, Q.; Wang, S.; Luo, Y.; Huang, Y.; Xin, Y.; He, N. Abnormal expression of bHLH3 disrupts a flavonoid homeostasis network, causing differences in pigment composition among mulberry fruits. Hortic. Res. 2020, 7, 83. [Google Scholar] [CrossRef]
- Shen, H.; Moon, J.; Huq, E. PIF1 is regulated by light-mediated degradation through the ubiquitin-26S proteasome pathway to optimize photomorphogenesis of seedlings in Arabidopsis. Plant J. 2005, 44, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Paik, I.; Chen, F.; Ngoc Pham, V.; Zhu, L.; Kim, J.I.; Huq, E. A phyB-PIF1-SPA1 kinase regulatory complex promotes photomor-phogenesis in Arabidopsis. Nat. Commun. 2019, 10, 4216. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Q.; Liu, Y.; Zhao, X.; Imaizumi, T.; Somers, D.E.; Tobin, E.M.; Lin, C. Arabidopsis CRY2 and ZTL mediate blue-light regulation of the transcription factor CIB1 by distinct mechanisms. Proc. Natl. Acad. Sci. USA 2013, 110, 17582–17587. [Google Scholar] [CrossRef]
- Liu, H.; Yu, X.; Li, K.; Klejnot, J.; Yang, H.; Lisiero, D.; Lin, C. Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science 2008, 322, 1535–1539. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, X.; Zhu, D.; Cui, S.; Li, X.; Cao, Y.; Ma, L.G. A single amino acid substitution in IIIf subfamily of basic helix-loop-helix transcription factor AtMYC1 leads to trichome and root hair patterning defects by abolishing its interaction with partner proteins in Arabidopsis. J. Biol. Chem. 2012, 287, 14109–14121. [Google Scholar] [CrossRef]
- Qi, Y.; Zhou, L.; Han, L.L.; Zou, H.Z.; Miao, K.; Wang, Y. PsbHLH1, a Novel Transcription Factor Involved in Regulating Anthocyanin Biosynthesis in Tree Peony (Paeonia Suffruticosa). Plant Physiol. Biochem. 2020, 154, 396–408. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, B.; Deyholos, M.K. Functional characterization of the Arabidopsis bHLH92 transcription factor in abiotic stress. Mol. Genet. Genom. 2009, 282, 503–516. [Google Scholar] [CrossRef]
- Waseem, M.; Rong, X.; Li, Z. Dissecting the role of a basic helix-loop-helix transcription factor, SlbHLH22, under salt and drought stresses in transgenic Solanum lycopersicum L. Front. Plant Sci. 2019, 10, 734. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, N.; Jiao, Y.; Li, R.; Xiao, D.; Wang, Z. The grapevine basic helix-loop-helix (bHLH) transcription factor positively modulates CBF-pathway and confers tolerance to cold-stress in Arabidopsis. Mol. Biol. Rep. 2014, 41, 5329–5342. [Google Scholar] [CrossRef]
- Yao, P.; Sun, Z.; Li, C.; Zhao, X.; Li, M.; Deng, R.; Huang, Y.; Zhao, H.; Chen, H.; Wu, Q. Overexpression of Fagopyrum tataricum FtbHLH2 enhances tolerance to cold stress in transgenic Arabidopsis. Plant Physiol. Biochem. 2018, 125, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Qiu, J.Y.; Hui, Q.L.; Xu, Y.Y.; He, Y.Z.; Peng, L.Z.; Fu, X.Z. Systematic analysis of the basic/helix-loop-helix (bHLH) transcription factor family in pummelo (Citrus grandis) and identification of the key members involved in the response to iron deficiency. BMC Genom. 2020, 21, 233. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Li, Y.; Liang, G. FIT and bHLH Ib transcription factors modulate iron and copper crosstalk in Arabidopsis. Plant Cell Environ. 2021, 44, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Itai, R.N.; Nozoye, T.; Kobayashi, T.; Nishizawa, N.K.; Nakanishi, H. The bHLH protein OsIRO3 is critical for plant survival and iron (Fe) homeostasis in rice (Oryza sativa L.) under Fe-deficient conditions. Soil Sci. Plant Nutr. 2020, 66, 579–592. [Google Scholar] [CrossRef]
- Nakata, M.; Mitsuda, N.; Herde, M.; Koo, A.J.K.; Moreno, J.E.; Suzuki, K.; Howe, G.A.; Ohme-Takagi, M. A bHLH-type transcription factor, ABAINDUCIBLE BHLH-TYPE TRANSCRIPTION FACTOR/JA-ASSOCIATED MYC2-LIKE1, acts as a repressor to negatively regulate jasmonate signaling in Arabidopsis. Plant Cell 2013, 25, 1641–1656. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Urao, T.; Ito, T.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB)function as transcriptional activators in abscisic acid signaling. Plant Cell 2003, 15, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Babitha, K.C.; Vemanna, R.S.; Nataraja, K.N.; Udayakumar, M. Overexpression of EcbHLH57 Transcription Factor from Eleusine coracana L. in Tobacco Confers Tolerance to Salt, Oxidative and Drought Stress. PLoS ONE 2015, 10, e0137098. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, C.; Han, X.; Tang, S.; Liu, S.; Xia, X.; Yin, W. A novel bHLH Transcription Factor PebHLH35 from Populus Euphratica Confers Drought Tolerance through Regulating Stomatal Development, Photosynthesis and Growth in Arabidopsis. Biochem. Biophys. Res. Commun. 2014, 450, 453–458. [Google Scholar] [CrossRef]
- Liu, W.W.; Tai, H.H.; Li, S.S.; Gao, W.; Zhao, M.; Xie, C.X.; Li, W.X. bHLH122 is important for drought and osmotic stress resistance in Arabidopsis and in the repression of ABA catabolism. New Phytol. 2013, 201, 1192–1204. [Google Scholar] [CrossRef]
- Yang, T.R.; Yao, S.F.; Hao, L.; Zhao, W.J.; Lu, W.J.; Xiao, K. Wheat bHLH-type Transcription Factor Gene TabHLH1 is Crucial in Mediating Osmotic Stresses Tolerance through Modulating Largely the ABA-Associated Pathway. Plant Cell Rep. 2016, 35, 2309–2323. [Google Scholar] [CrossRef]
- Seo, J.S.; Joo, J.; Kim, M.J.; Kim, Y.K.; Nahm, B.H.; Song, S.I.; Cheong, J.J.; Lee, J.S.; Kim, J.K.; Choi, Y.D. OsbHLH148, a basic helix-loop-helix protein, interacts with OsJAZ proteins in a jasmonate signaling pathway leading to drought tolerance in rice. Plant J. 2011, 65, 907–921. [Google Scholar] [CrossRef]
- Wang, F.B.; Zhu, H.; Chen, D.H.; Li, Z.J.; Peng, R.H.; Yao, Q.H. A grape bHLH transcription factor gene, VvbHLH1, increases the accumulation of flavonoids and enhances salt and drought tolerance in transgenic Arabidopsis thaliana. Plant Cell Tissue Organ. Cult. 2016, 125, 387–398. [Google Scholar] [CrossRef]
- Ao, T.G.B.Y.; Lang, M.L.; Li, Y.Q.; Wang, L.C.; Yang, X.J. Cloning and Expression Analysis of Cysteine Protease Gene (MwCP) in Agropyron mongolicum Keng. Genet. Mol. Res. 2015, 15, gmr.15017424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.T.; Fan, B.B.; Yu, Z.; Nie, L.Z.; Zhao, Y.; Yu, X.X.; Sun, F.C.; Lei, X.F.; Ma, Y.H. Functional Analysis of Three miRNAs in Agropyron mongolicum Keng under Drought Stress. Agronomy 2019, 9, 661. [Google Scholar] [CrossRef]
- Ma, Y.H.; Yu, X.X.; Zhuo, Y.; Sun, F.C.; Li, X.D.; Li, X.L. RNA-Seq of Agropyron mongolicum Keng in Response to Drought Stress. Grassl. Sci. 2018, 64, 3–5. [Google Scholar] [CrossRef]
- Du, J.C.; Li, X.Q.; Li, T.T.; Yu, D.Y.; Han, B. Genome-Wide Transcriptome Profiling Provides Overwintering Mechanism of Agropyron mongolicum. BMC Plant Biol. 2017, 17, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yun, J.F.; Shi, F.M.; Wang, J.J.; Yang, Q.C.; Chao, Y.H. Molecular cloning and characterization of agroup 3LEA gene from Agropyron mongolicum Keng. Afr. J. Biotechnol. 2010, 9, 6040–6048. [Google Scholar]
- Ao, T.G.B.Y.; Gao, L.J.; Wang, L.C.; Li, Y.Q.; Zhao, Y.; Lang, M.L.; Yang, X.J. Cloning and Expression Analysis of AP2/EREBP Transcription Factor Gene (MwAP2/EREBP) in Agropyron mongolicum Keng. Nanosci. Nanotechnol. Lett. 2017, 9, 2031–2038. Available online: https://www.nstl.gov.cn/paper_detail.html?id=912f15371934bf7ddd34ba65c16344a3 (accessed on 23 November 2023). [CrossRef]
- Fan, B.B.; Sun, F.C.; Yu, Z.; Zhang, X.F.; Yu, X.X.; Wu, J.; Yan, X.X.; Zhao, Y.; Nie, L.Z.; Fang, Y.Y.; et al. Integrated analysis of small RNAs, transcriptome and degradome sequencing reveal the drought stress network in Agropyron mongolicum Keng. Front. Plant Sci. 2022, 13, 976684. [Google Scholar] [CrossRef]
- Zhang, X.F.; Ma, Y.H.; Fan, B.B.; Zhao, Y.; Wu, X.J.; Lei, X.F. RNA-Seq-based analysis of bHLH transcription factor families in A. mongolicum. Mol. Plant Breed. 2023, 21, 1154–1163. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Japelaghi, R.H.; Haddad, R.; Valizadeh, M.; Uliaie, E.D.; Javaran, M.J. High-Efficiency Agrobacterium-Mediated Transformation of Tobacco (Nicotiana tabacum). J. Plant Mol. Breed. 2018, 6, 38–50. [Google Scholar]
- Dheda, K.; Huggett, J.F.; Bustin, S.A.; Johnson, M.A.; Rook, G.; Zumla, A. Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques 2004, 37, 112–119. [Google Scholar] [CrossRef]
- Xu, X.Y.; Yao, X.Z.; Lu, L.T. Overexpression of the Transcription Factor NtNAC2 Confers Drought Tolerance in Tobacco. Plant Mol. Biol. Rep. 2018, 36, 941026. [Google Scholar] [CrossRef]
- Song, Z.P.; Li, Y.L.; Jia, Y.H.; Lian, W.L. An endoplasmic reticulum-localized NtHSP70-8 confers drought tolerance in tobacco by regulating water loss and antioxidant capacity. Environ. Exp. Bot. 2021, 188, 104519. [Google Scholar] [CrossRef]
- Molinari, H.B.C.; Marur, C.J.; Daros, E.; Campos, M.K.F.; Carvalho, J.F.R.P.; Filho, J.C.B.; Pereira, L.F.P.; Vieira, L.G.E. Evaluation of the stress-inducible production of proline in transgenic sugarcane (Saccharum spp.): Osmotic adjustment, chlorophyll fluorescence and oxidative stress. Physiol. Plant. 2007, 130, 218–229. [Google Scholar] [CrossRef]
- Cui, X.; Wang, Y.X.; Liu, Z.W.; Wang, W.L.; Li, H.; Zhuang, J. Transcriptome-wide identification and expression profile analysis of the bHLH family genes in Camellia sinensis. Funct. Integr. Genom. 2018, 18, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.R.; Huang, Z.; Xiang, X.Y.; Xu, W.X.; Wang, J.T.; Chen, J.; Song, L.; Xiao, Y.; Li, X.; Ma, J.; et al. MfbHLH38, a Myrothamnus flabellifolia bHLH transcription factor, confers tolerance to drought and salinity stresses in Arabidopsis. BMC Plant Biol. 2020, 20, 542–556. [Google Scholar] [CrossRef]
- Zhao, Q.; Fan, Z.; Qiu, L.; Qiu, L.N.; Che, Q.Q.; Wang, T.; Li, Y.Y.; Wang, Y.Z. MdbHLH130, an Apple bHLH Transcription Factor, Confers Water Stress Resistance by Regulating Stomatal Closure and ROS Homeostasis in Transgenic Tobacco. Front. Plant Sci. 2020, 11, 543696. [Google Scholar] [CrossRef] [PubMed]
- Hir, R.L.; Castelain, M.; Chakraborti, D.; Moritz, T.; Dinant, S.; Bellini, C. AtbHLH68 transcription factor contributes to the regulation of ABA homeostasis and drought stress tolerance in Arabidopsis thaliana. Physiol. Plant. 2017, 160, 312–327. [Google Scholar] [CrossRef]
- Li, C.J.; Yan, C.X.; Sun, Q.X.; Wang, J.; Yuan, C.L.; Mou, Y.F.; Shan, S.H. The bHLH transcription factor AhbHLH112 improves the drought tolerance of peanut. BMC Plant Biol. 2021, 21, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, Y.; Liu, D.; Wang, X.; Zhang, L. Transcription factor TabHLH49 positively regulates dehydrin WZY2 gene expression and enhances drought stress tolerance in wheat. BMC Plant Biol. 2020, 20, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Manavalan, L.P.; Guttikonda, S.K.; Tran, L.P.; Nguyen, H. Physiological and molecular approaches to improve drought resistance in soybean. Plant Cell Physiol. 2009, 50, 1260–1276. [Google Scholar] [CrossRef] [PubMed]
- Sahid, S.; Roy, C.; Shee, D.; Shee, R.; Datta, R.; Paul, S. ZFP37, C3H, NAC94, and bHLH148 transcription factors regulate cultivar-specific drought response by modulating r40C1 gene expression in rice. Environ. Exp. Bot. 2023, 214, 105480. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Ma, Y.; Fan, B.; Sun, F.; Zhai, Y.; Zhao, Y.; Nie, L.; Fang, Y.; Yu, Z.; Qi, B. The Agropyron mongolicum bHLH Gene AmbHLH148 Positively Involved in Transgenic Nicotiana benthamiana Adaptive Response to Drought Stress. Agronomy 2023, 13, 2918. https://doi.org/10.3390/agronomy13122918
Zhang X, Ma Y, Fan B, Sun F, Zhai Y, Zhao Y, Nie L, Fang Y, Yu Z, Qi B. The Agropyron mongolicum bHLH Gene AmbHLH148 Positively Involved in Transgenic Nicotiana benthamiana Adaptive Response to Drought Stress. Agronomy. 2023; 13(12):2918. https://doi.org/10.3390/agronomy13122918
Chicago/Turabian StyleZhang, Xuefeng, Yanhong Ma, Bobo Fan, Fengcheng Sun, Yongqing Zhai, Yan Zhao, Lizhen Nie, Yongyu Fang, Zhuo Yu, and Bingjie Qi. 2023. "The Agropyron mongolicum bHLH Gene AmbHLH148 Positively Involved in Transgenic Nicotiana benthamiana Adaptive Response to Drought Stress" Agronomy 13, no. 12: 2918. https://doi.org/10.3390/agronomy13122918
APA StyleZhang, X., Ma, Y., Fan, B., Sun, F., Zhai, Y., Zhao, Y., Nie, L., Fang, Y., Yu, Z., & Qi, B. (2023). The Agropyron mongolicum bHLH Gene AmbHLH148 Positively Involved in Transgenic Nicotiana benthamiana Adaptive Response to Drought Stress. Agronomy, 13(12), 2918. https://doi.org/10.3390/agronomy13122918




