The Roles of Arbuscular Mycorrhizal Fungi in Influencing Plant Nutrients, Photosynthesis, and Metabolites of Cereal Crops—A Review
Abstract
:1. Introduction
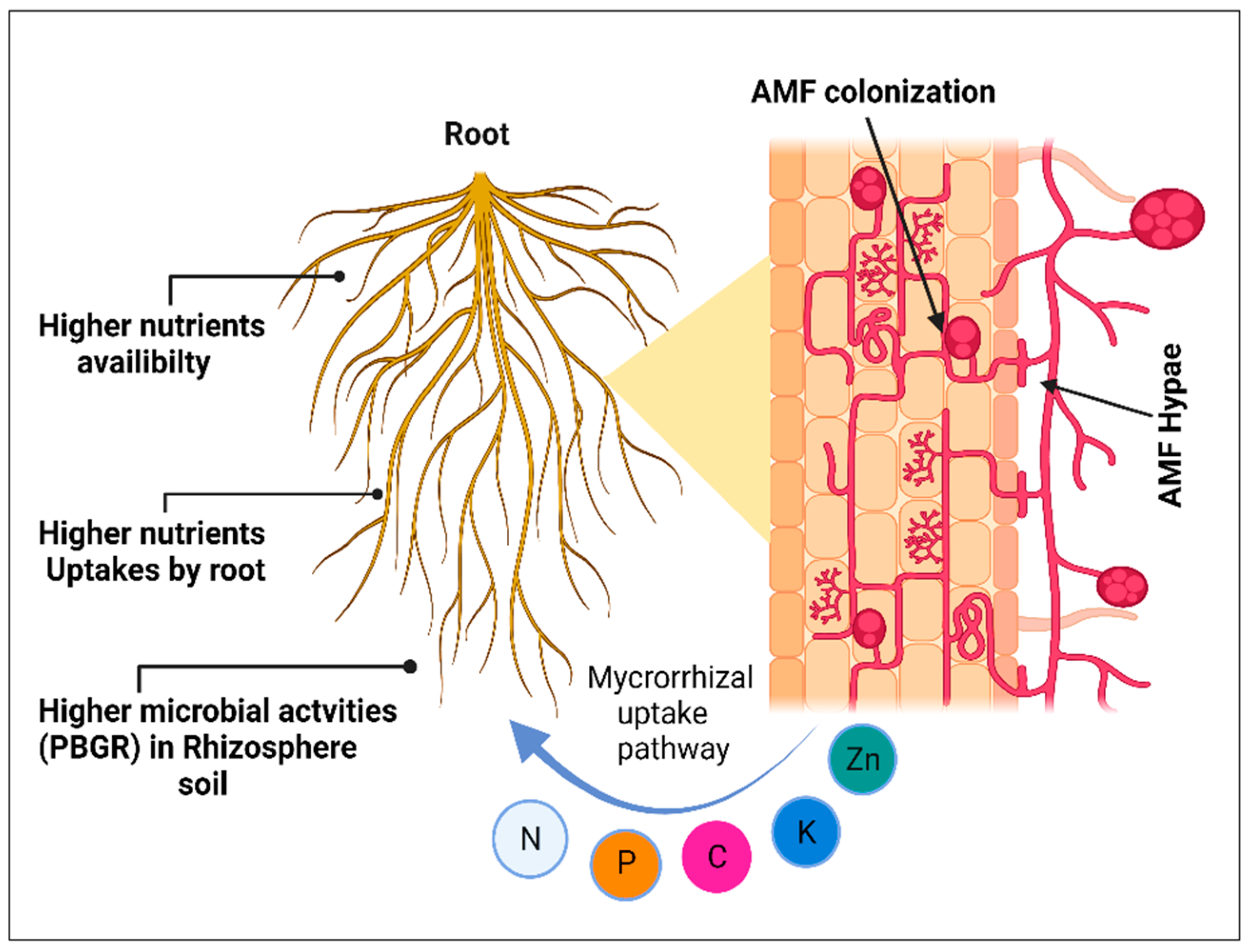
1.1. The Effects of AM on Nutrient Uptake
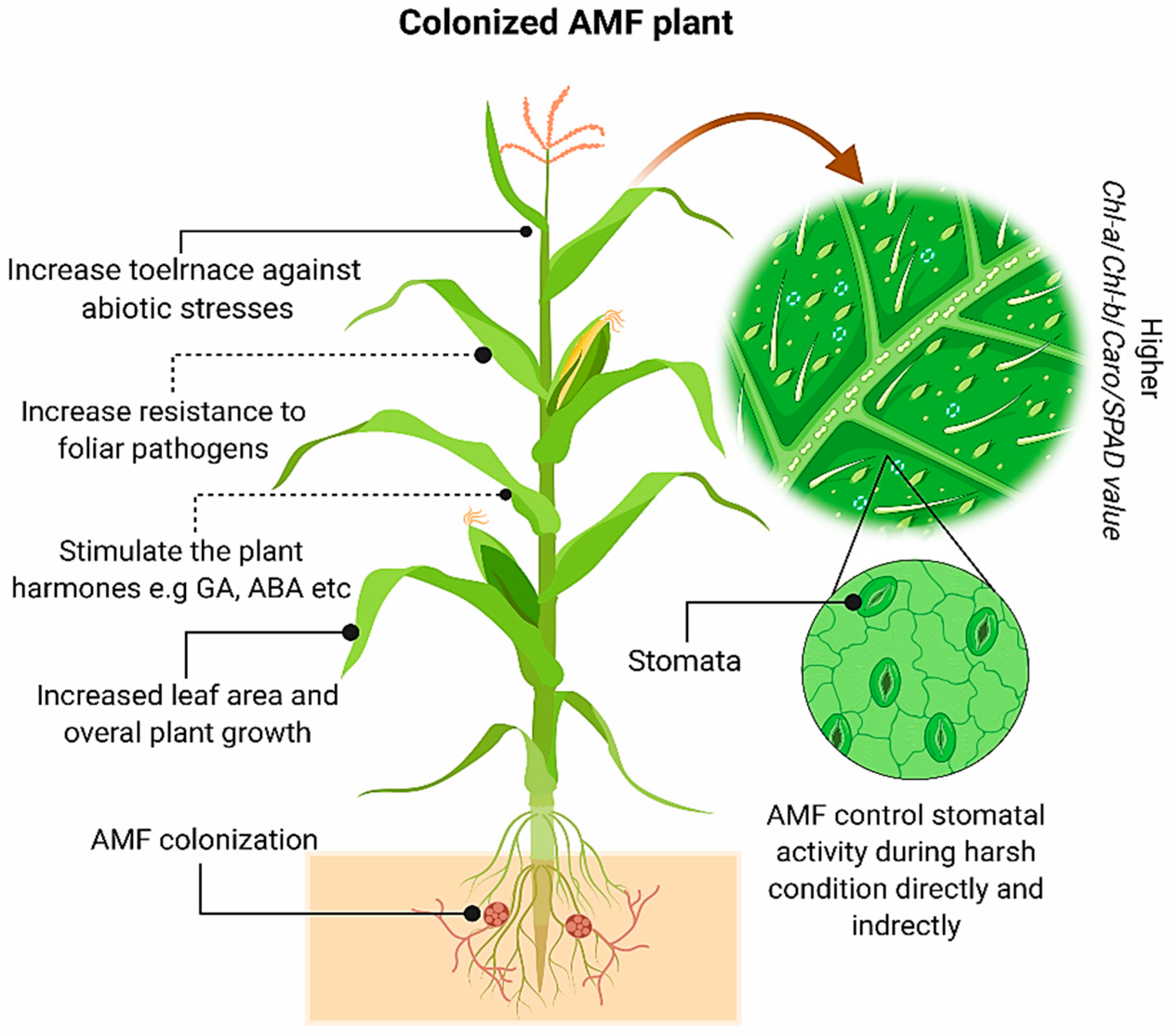
1.2. The Effects of AM Fungi on the Chlorophylls, Carotenoids, and Photosynthesis Rate
1.3. The Effects of AM Fungi on the Primary and Secondary Metabolites
| Plant | AMF Spore | Plant Stage | Function | Changes after AM Colonization | Environmental Conditions | References |
|---|---|---|---|---|---|---|
| Wheat | R. irregularis | Vegetative stage | Increased stress tolerance | Enhanced macro and micro nutrient concentration | Low- or high-temperature stress | [30] |
| Wheat | F. mosseae | Vegetative stage | Increased chlorophyll pigments | Increased the concentrations of P, N, K, and Mg | Saline soil condition | [31] |
| Wheat | F. geosporum | Seeding and vegetative stages | Maintenance of PSI and PSII | Upregulation of water and nutrient uptake | Salt, drought, and heavy metal conditions | [32] |
| Wheat | G. claroideum | Tillering stage | Increased photosynthesis | Enhanced total dry weight and leaf chlorophyll concentration | Drought stress condition | [33] |
| Wheat | R. intraradices, F. mosseae, F. geosporum | Tillering stage | Increased the activity of PSI and PSII | Increased relative water content (RWC) | Drought stress condition | [34] |
| Maize | F. mosseae | Pre-flowering stage | Increased stress tolerance | Increased N and P concentration | Under water deficit conditions | [35] |
| Maize | G. mosseae, G. clarum | Seedling stage | Improved plant growth | Increased P uptake by AM pathway | Under P-deficient conditions | [36] |
| Maize | G. etunicatum | Tillering stage | Increased plant biomass | Increased leaf water potential | Under P-deficient conditions | [36] |
| Maize | G. intraradices, G. intraradices | Vegetative stage | Increased water uptake and leaf water potential | Increased drought stress tolerance | Under sandy loam soil | [37] |
| Maize | R. irregularis | Seedling, tillering, and fruiting stages | Increased root length | Increased Cu tolerance | Under heavy metal condition | [38] |
| Rice | G. mosseae | Heading stage | Increased root and shoot growth | Increased N accumulation and protein content | Under greenhouse conditions | [39] |
| Rice | C. etunicatum | Heading and flowering stage | Improving nutrition status and plant growth | Increased net photosynthetic rate, stomatal conductance, and transpiration rate | Under salt stress conditions | [40] |
| Sorghum | R. irregularis | Fruiting stage | Improved their transpiration efficiency and drought tolerance | Increased uptake 15N | Under drought conditions | [41] |
| Sorghum | Glomus species | Flowering and fruiting stages | Increased root and shoot growth | Increased total dry matter yield | Under striga hermonthica conditions | [42] |
| Barley | G. mosseae | Flowering stage | Increased resistance against heavy metal conditions | Decreased Cd and Co uptake | Under heavy metal conditions | [43] |
| Barley | R. irregularis | Fruiting stage | Improved yield production | Improved Zn concentrations in grain | Under overexpression of HvZIP13 gene | [44] |
| Oat | Mix-AMF | Tillering and flowering stages | Increased nutrients and plant growth | Improved the P content in plants | Under fumigated soil | [45] |
| Oat | Mix-AMF with organic fertilizer | Early and late flowering stages | Increased photosynthesis and plant growth | Improved total N and P concentrations and dry weight of plant | Under organic farming conditions | [46] |
| Buckwheat | Mix-AMF | Fruiting stage | Increased growth and productivity | Improved the nutritional and functional quality | Under greenhouse conditions | [47] |
| Buckwheat | A. laevis | Fruiting stage | Increased plant growth | Increased plant height, leaf area, number of branches | Under organic conditions | [48] |
| Buckwheat | Glomus species | Harvesting stage | Plant biomass | Increased inorganic phosphorus uptake | Under organic garden conditions | [49] |
| Quinoa | G. mosseae | Fruiting stage | Plant growth | Decreased uptake of 137Cs | Under loamy sand conditions | [50] |
| Quinoa | G. mosseae | Fruiting stage | Shoot and root growth | Improved nutrient uptake | Under different nitrogen levels | [51] |
| Millet | G. mosseae | Harvesting stage | Plant height and dry weight of root | Improved P and N uptake | Under experimental pot conditions | [52] |
| Millet | R. fasciculatus | Fruiting stage and harvesting stage | Plant growth and grain yield | Increased the lengths and weights of shoots and roots | Under Benomyl conditions | [53] |
2. AM Fungi and Mineral Nutrition
| Plant | AM Fungi Spore | Plant Stage | Nutrient Uptake | Environmental Conditions | References |
|---|---|---|---|---|---|
| Wheat | Glomus species | Tillering stage, heading stage | Nitrogen | Under ozone stress conditions | [79] |
| Wheat | R. tenuis | Vegetative stage, fruiting stage | Phosphate | In a semi-arid field environment | [80] |
| Wheat | R. fasciculatus, F. mosseae | Fruiting stage | Zinc | Under drought stress conditions | [55] |
| Wheat | R. Intraradices | Tillering stage | Zinc | Under P application conditions | [81] |
| Maize | G. mosseae, G. etunicatum | Tillering stage | Nitrogen | Under zinc-deficient soil conditions | [82] |
| Maize | G. mosseae | Vegetative stage | Nitrogen | Under field conditions | [61] |
| Maize | R. irregularis | Fruiting stage | Phosphorus | Compartmented pots with radioactive P tracer conditions | [10] |
| Maize | G. clarum | Fruiting stage | Phosphorus | Under P deficient conditions | [36] |
| Rice | R. intraradices | Tillering and maturity stages | Nitrogen, phosphorus, and carbon | Under experimental greenhouse conditions | [39] |
| Rice | Glomus species | Early tillering stage | Nitrogen and phosphorus | Under wetland conditions | [83] |
| Rice | G. mosseae | Fruiting stage | Arsenic | Under As soil conditions | [84] |
| Rice | G. geosporum, G. mosseae | Fruiting stage | Phosphorus | Under As soil conditions | [84] |
| Barley | G. mosseae | Seedling, flowering, and fruiting stages | Zinc | Under Cd conditions | [85] |
| Barley | G. intraradices | Fruiting stage | Zinc | Under drought and seat stress conditions | [86] |
| Sorghum | A. scrobiculata | Harvesting stage | Nitrogen | Under greenhouse conditions | [87] |
| Sorghum | Glomus species | Harvesting stage | Phosphorus | Under greenhouse conditions | [87] |
| Sorghum | R. irregularis | Fruiting stage | Phosphorus | Under low-phosphorus soil conditions | [69] |
| Sorghum | G. etunicatum and G. intraradices | Fruiting stage | Zinc, magnesium, and iron | Under micronutrient-deficient conditions | [88] |
| Oat | G. mosseae | Fruiting stage | Nitrogen and phosphorus | Under nutrient deficient condition | [89] |
| Oat | R. irregularis | Fruiting stage | Mineral nutrition content | Under conditions of different levels of P | [13] |
| Buckwheat | Glomus species | Harvesting stage | Phosphorus | Under low to medium soil pH conditions | [49] |
| Buckwheat | Glomus species | Harvesting stage | Nitrogen | Under low to medium soil pH conditions | [49] |
| Quinoa | G. mosseae | Fruiting stage | Cesium | Under loamy sand conditions | [50] |
| Quinoa | G. mosseae | Fruiting stage | Nitrogen | Under conditions of different nitrogen levels | [51] |
| Millet | G. mosseae | Fruiting stage | Nitrogen | Under experimental pot conditions | [52] |
| Millet | G. mosseae | Fruiting stage | Phosphorus | Under experimental pot conditions | [52] |
| Millet | G. mosseae | Fruiting stage | N, P, and K | Under cow dung (CD) or poultry manure (PM) conditions | [90] |
3. AM Fungi and Photosynthetic Activity of Cereal Crops
4. AM Fungi with Primary and Secondary Metabolites
| Plant | AM Fungi Spore | Primary Metabolites | Secondary Metabolites | Environmental Conditions | References |
|---|---|---|---|---|---|
| Wheat | Glomus species | Proline and glycinebetaine | _ | Under salt stress conditions | [105] |
| Wheat | Mix-AMF | γ-amino butyric acid | _ | Under rainfed field conditions | [72] |
| Wheat | F. mosseae | Amino acid (3-phospho-hydroxypyruvate) and carbohydrates (mannosylfructose-phosphate) | Flavonoids, terpenoids, and staurosporine (alkaloids) | Under water stress conditions | [120] |
| Wheat | F. Mosseae, C. claroideum | _ | Production of phenolic compounds | Under drought stress conditions | [121] |
| Maize | R. intraradices, F. mosseae, F. geosporum | Carbohydrates and photosynthates | _ | Under high-temperature stress conditions | [122] |
| Maize | G. albida, C. etunicatum, A. longula | Total proteins | Phenols and tannins | Under experimental greenhouse conditions | [123] |
| Maize | Glomus species | Carbohydrate, leaf soluble sugar, and proline content | _ | Under different temperature stress conditions | [30] |
| Maize | Mix-AMF | Protein levels | _ | Under field conditions | [124] |
| Rice | G. etunicatum, G. geosporum, G. mosseae | Cyanidin-3-glucoside and peonidin-3-glucoside | _ | Under salt stress conditions | [125] |
| Rice | Glomus species | Fatty acids, amino acids, and carotenoids | Terpenoids | Under Cd soil conditions | [126] |
| Barley | G. intraradices | Glucose and glyceraldehyde 3-phosphate | _ | Under feeding experimental conditions | [127] |
| Barley | G. mosseae, G. intraradices, G. rosea | _ | Cyclohexenone and its derivatives | Pot greenhouse experimental conditions | [128] |
| Barley | G. intraradices | _ | Hydroxycinnamic acid amides, yclohexenone derivatives, and blumenin | Under defined nutritional medium conditions | [129] |
| Sorghum | G. mosseae, G. intraradices | Octadecane, pentaethylene glycol, acetic acid, and pentaethylene glycol | _ | Under volatile organic compound (VOC) conditions | [130] |
| Sorghum | Mix-AMF | _ | Anthocyanin, polyphenols, and flavonoids | Under drought stress conditions | [131] |
| Sorghum | Glomus species | Glucose, fatty acids, and methionine | Ferulic acid and phenolic compounds, 3,4-dihydroxycinnamic acid | Under marginal soil conditions | [132] |
| Oat | R. irregularis | Ascorbic acid and glutathione content | _ | Under sulfur dioxide (SO2) exposure conditions | [111] |
| Oat | G. intraradices | _ | Terpenoid glycoside and phenolic compounds | Under defined nutritional medium condition | [133] |
| Oat | G. intraradices | _ | Sesquiterpenoid cyclohexenone derivatives | Under defined nutritional medium conditions | [134] |
| Oat | G. intraradices | Free amino acids and proteins | _ | Under N application conditions | [135] |
| Buckwheat | Glomus species | _ | Flavonoid content | Under UV-B radiation conditions | [136] |
| Buckwheat | Mix-AMF | Carbohydrates | _ | Under greenhouse conditions | [47] |
| Buckwheat | Mix-AMF | Fatty acids | _ | Under temperate agricultural soil conditions | [119] |
| Quinoa | G. mosseae | Soluble sugar | _ | Under conditions of different nitrogen levels | [51] |
| Quinoa | Mix-AMF | Natural lipids | _ | Under temperate agricultural soil conditions | [119] |
| Quinoa | Mix-AMF | _ | Polyphenol compounds | Under salt stress conditions | [137] |
| Millet | R. intraradices | _ | Benzenoid, phenol, and flavonoid content | Under sandy loam soil conditions | [121] |
| Millet | F. mosseae | Fatty acids | _ | Under sandy clay loam conditions | [121] |
| Millet | R. intraradices | Proline and soluble sugar content | Phenol and flavonoid content | Under drought stress conditions | [120] |
5. The Roles of AM Fungi in Grain Yield and the Quality of Cereal Crops
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, Y.; Yang, X.; Zhang, X.; Yaseen, T.; Shi, L.; Zhang, T. Arbuscular mycorrhizal fungi promote plant growth of Leymus chinensis (Trin.) Tzvelev by increasing the metabolomics activity under nitrogen addition. Grassl. Sci. 2021, 67, 128–138. [Google Scholar] [CrossRef]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Ravnskov, S.; Jiang, D.; Wollenweber, B. Changes in carbon and nitrogen allocation, growth and grain yield induced by arbuscular mycorrhizal fungi in wheat (Triticum aestivum L.) subjected to a period of water deficit. Plant Growth Regul. 2015, 75, 751–760. [Google Scholar] [CrossRef]
- Saboor, A.; Ali, M.A.; Hussain, S.; El Enshasy, H.A.; Hussain, S.; Ahmed, N.; Datta, R. Zinc nutrition and arbuscular mycorrhizal symbiosis effects on maize (Zea mays L.) growth and productivity. Saudi J. Biol. Sci. 2021, 28, 6339–6351. [Google Scholar] [CrossRef]
- Bernaola, L.; Cange, G.; Way, M.O.; Gore, J.; Hardke, J.; Stout, M. Natural colonization of rice by arbuscular mycorrhizal fungi in different production areas. Rice Sci. 2018, 25, 169–174. [Google Scholar] [CrossRef]
- Douds, D.D.; Lee, J.; Rogers, L.; Lohman, M.E.; Pinzon, N.; Ganser, S. Utilization of inoculum of AMfungi producedon-farm for the production of Capsicum annuum: A summary of seven years of field trials on a conventional vegetable farm. Biol. Agric. Hortic. 2012, 28, 129–145. [Google Scholar] [CrossRef]
- Gaur, A.; Adholeya, A.; Mukerji, K.G. On-farm production of VAM inoculum and vegetable crops in marginal soil amended with organic matter. Trop. Agric. 2000, 77, 21–26. [Google Scholar]
- Wertheim, F.; Douds, D.; Handley, D.; Hutton, M. Evaluating the potential of arbuscular mycorrhizal fungi to boost yields in field-grown leeks. J. NACAA 2014, 7, 1. [Google Scholar]
- Khan, Y.; Sohail, A.; Yaseen, T.; Rehman, K.U.; Noor, M.; Akbar, K. Arbuscular mycorrhizal fungi improved the growth and yield productivity of lens esculenta under the influence of poultry litter. Pak. J. Phytopathol. 2021, 33, 145–151. [Google Scholar] [CrossRef]
- Battini, F.; Grønlund, M.; Agnolucci, M.; Giovannetti, M.; Jakobsen, I. Facilitation of phosphorus uptake in maize plants by mycorrhizosphere bacteria. Sci. Rep. 2017, 7, 4686. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mineral nutrition, toxic element accumulation and water relations of arbuscular mycorrhizal plants. In Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2008; pp. 145–148. [Google Scholar]
- Mei, L.; Yang, X.; Cao, H.; Zhang, T.; Guo, J. Arbuscular mycorrhizal fungi alter plant and soil C: N: P stoichiometries under warming and nitrogen input in a semiarid meadow of China. Int. J. Environ. Res. Public Health 2019, 16, 397. [Google Scholar] [CrossRef]
- Mustafa, G.; Randoux, B.; Tisserant, B.; Fontaine, J.; Magnin-Robert, M.; Sahraoui, A.L.H.; Reignault, P. Phosphorus supply, arbuscular mycorrhizal fungal species, and plant genotype impact on the protective efficacy of mycorrhizal inoculation against wheat powdery mildew. Mycorrhiza 2016, 26, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, C.; Huang, Y. Interactive impacts of earthworms (Eisenia fetida) and arbuscular mycorrhizal fungi (Funneliformis mosseae) on the bioavailability of calcium phosphates. Plant Soil 2015, 396, 45–57. [Google Scholar] [CrossRef]
- Miransari, M. Soil microbes and the availability of soil nutrients. Acta Physiol. Plant. 2013, 35, 3075–3084. [Google Scholar] [CrossRef]
- Frew, A.; Powell, J.R.; Glauser, G.; Bennett, A.E.; Johnson, S.N. Mycorrhizal fungi enhance nutrient uptake but disarm defences in plant roots, promoting plant-parasitic nematode populations. Soil Biol. Biochem. 2018, 126, 123–132. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Biol. Annu. Rev. Plant 2011, 62, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.; Iqbal, H. Maize in Pakistan–an overview. Agric. Nat. Resour. 2010, 44, 757–763. [Google Scholar]
- Smith, S.E.; Gianinazzi-Pearson, V. Physiological interactions between symbionts in vesicular-arbuscular mycorrhizal plants. Annu. Rev. Plant physiol. Plant Mol. Biol. 1988, 39, 221–244. [Google Scholar] [CrossRef]
- Pepe, A.; Giovannetti, M.; Sbrana, C. Lifespan and functionality of mycorrhizal fungal mycelium are uncoupled from host plant lifespan. Sci. Rep. 2018, 8, 10235. [Google Scholar] [CrossRef]
- Ryan, M.H.; Angus, J.F. Arbuscular mycorrhizae in wheat and field pea crops on a low P soil: Increased Zn-uptake but no increase in P-uptake or yield. Plant Soil 2003, 250, 225–239. [Google Scholar] [CrossRef]
- Latz, M.A.; Jensen, B.; Collinge, D.B.; Jørgensen, H.J. Endophytic fungi as biocontrol agents: Elucidating mechanisms in disease suppression. Plant Ecol. Divers. 2018, 11, 555–567. [Google Scholar] [CrossRef]
- Card, S.; Johnson, L.; Teasdale, S.; Caradus, J. Deciphering endophyte behaviour: The link between endophyte biology and efficacious biological control agents. FEMS Microbiol. Ecol. 2016, 92, fiw114. [Google Scholar] [CrossRef]
- Kaddes, A.; Fauconnier, M.L.; Sassi, K.; Nasraoui, B.; Jijakli, M.H. Endophytic fungal volatile compounds as solution for sustainable agriculture. Molecules 2019, 24, 1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, S.; Goswami, A. Effect of Excess Fertilizers and Nutrients: A Review on Impact on Plants and Human Population. In Proceedings of the International Conference on Sustainable Computing in Science, Technology and Management (SUSCOM), Jaipur, India, 26–28 February 2019. [Google Scholar]
- Rejeb, I.; Pastor, V.; Mauch-Mani, B. Plant responses to simultaneous biotic and abiotic stress: Molecular mechanisms. Plants 2014, 3, 458–475. [Google Scholar] [CrossRef]
- Ganugi, P.; Masoni, A.; Pietramellara, G.; Benedettelli, S. A Review of Studies from the Last Twenty Years on Plant–Arbuscular Mycorrhizal Fungi Associations and Their Uses for Wheat Crops. Agronomy 2019, 9, 840. [Google Scholar] [CrossRef]
- Khan, A.; Sharif, M.; Ali, A.; Shah, S.N.M.; Mian, I.A.; Wahid, F.; Ali, N. Potential of AM fungi in phytoremediation of heavy metals and effect on yield of wheat crop. Am. J. Plant Sci. 2014, 5, 1578–1586. [Google Scholar] [CrossRef]
- Al-Mutairi, A.A.; Cavagnaro, T.R.; Khor, S.F.; Neumann, K.; Burton, R.A.; Watts-Williams, S.J. The effect of zinc fertilization and arbuscular mycorrhizal fungi on grain quality and yield of contrasting barley cultivars. Funct. Plant Biol. 2020, 47, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Song, F.; Liu, F. Arbuscular mycorrhizal fungi and tolerance of temperature stress in plants. In Arbuscular Mycorrhizas and Stress Tolerance of Plants; Springer: Singapore, 2017; pp. 163–194. [Google Scholar]
- Abdel-Fattah, G.M.; Asrar, A.A. Arbuscular mycorrhizal fungal application to improve growth and tolerance of wheat (Triticum aestivum L.) plants grown in saline soil. Acta Physiol. Plant. 2012, 34, 267–277. [Google Scholar] [CrossRef]
- Ibrahim, A.H.; Abdel-Fattah, G.M.; Eman, F.M.; Abd El_Aziz, M.H.; Shohr, A.E. Arbuscular mycorrhizal fungi and spermine alleviate the adverse effects of salinity stress on electrolyte leakage and productivity of wheat plants. New Phytol. 2011, 51, 261–276. [Google Scholar]
- Beltrano, J.; Ronco, M. Improved tolerance of wheat plants (Triticum aestivum L.) to drought stress and rewatering by the arbuscular mycorrhizal fungus Glomus claroideum: Effect on growth and cell membrane stability. Braz. J. Plant Physiol. 2008, 20, 29–37. [Google Scholar] [CrossRef]
- Mathur, S.; Tomar, R.S.; Jajoo, A. Arbuscular mycorrhizal fungi (AMF) protects photosynthetic apparatus of wheat under drought stress. Photosynth. Res. 2018, 139, 227–238. [Google Scholar] [CrossRef]
- Ghorchiani, M.; Etesami, H.; Alikhani, H.A. Improvement of growth and yield of maize under water stress by co-inoculating an arbuscular mycorrhizal fungus and a plant growth promoting rhizobacterium together with phosphate fertilizers. Agric. Ecosyst. Environ. 2018, 258, 59–70. [Google Scholar] [CrossRef]
- Almagrabi, O.A.; Abdelmoneim, T.S. Using of arbuscular mycorrhizal fungi to reduce the deficiency effect of phosphorous fertilization on maize plants (Zea mays L.). Life Sci. J. 2012, 9, 1648–1654. [Google Scholar]
- Amerian, M.R.; Stewart, W.S.; Griffiths, H. Effect of two species of arbuscular mycorrhizal fungi on growth, assimilation and leaf water relations in maize (Zea mays). Asp. Appl. Biol. 2001, 63, 71–76. [Google Scholar]
- Merlos, M.A.; Zitka, O.; Vojtech, A.; Azcón-Aguilar, C.; Ferrol, N. The arbuscular mycorrhizal fungus Rhizophagus irregularis differentially regulates the copper response of two maize cultivars differing in copper tolerance. Plant Sci. 2016, 253, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Ma, F.; Yang, J.; Su, M. Effects of arbuscular mycorrhizal fungi inoculation on carbon and nitrogen distribution and grain yield and nutritional quality in rice (Oryza sativa L.). J. Sci. Food Agric. 2017, 97, 2919–2925. [Google Scholar] [CrossRef]
- Porcel, R.; Redondo-Gómez, S.; Mateos-Naranjo, E.; Aroca, R.; Garcia, R.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis ameliorates the optimum quantum yield of photosystem II and reduces non-photochemical quenching in rice plants subjected to salt stress. J. Plant physiol. 2015, 185, 75–83. [Google Scholar] [CrossRef]
- Symanczik, S.; Lehmann, M.F.; Wiemken, A.; Boller, T.; Courty, P.-E. Effects of two contrasted arbuscular mycorrhizal fungal isolates on nutrient uptake by Sorghum bicolor under drought. Mycorrhiza 2018, 28, 779–785. [Google Scholar] [CrossRef]
- Gworgwor, N.A.; Weber, H.C. Arbuscular mycorrhizal fungi-parasite-host interaction for the control of Striga hermonthica (Del.) Benth. in sorghum [Sorghum bicolor (L.) Moench]. Mycorrhiza 2003, 13, 277–281. [Google Scholar] [CrossRef]
- Watts-Williams, M.; Ardakani, M.R.; Rejali, F.; Zaefarian, F.; Teimouri, S.; Noormohammadi, G.; Miransari, M. Uptake of heavy metals by mycorrhizal barley (Hordeum vulgare L.). J. Plant Nutr. 2015, 38, 904–919. [Google Scholar]
- Watts-Williams, S.J.; Cavagnaro, T.R. Arbuscular mycorrhizal fungi increase grain zinc concentration and modify the expression of root ZIP transporter genes in a modern barley (Hordeum vulgare) cultivar. Plant Sci. 2018, 274, 163–170. [Google Scholar] [CrossRef]
- Plenchette, C.; Fortin, J.A.; Furlan, V. Growth responses of several plant species to mycorrhizae in a soil of moderate P-fertility. Plant Soil 1983, 70, 199–209. [Google Scholar] [CrossRef]
- Jannoura, R.; Joergensen, R.G.; Bruns, C. Organic fertilizer effects on growth, crop yield, and soil microbial biomass indices in sole and intercropped peas and oats under organic farming conditions. Eur. J. Agron. 2014, 52, 259–270. [Google Scholar] [CrossRef]
- Zhao, J.; Zhong, L.; Zou, L.; Zhang, C.; Peng, L.; Xiao, W.; Zhao, G. Efficient promotion of the sprout growth and rutin production of tartary buckwheat by associated fungal endophytes. Cereal Res. Commun. 2014, 42, 401–412. [Google Scholar] [CrossRef]
- Tummaramatti, S.; Hegde, L.; Vijaykumar, N. Pot experiment: Studies on effect of bio-fertilizers on Buckwheat (Fagopyrum esculentum Moench). Ecol. Environ. Conserv. 2016, 22, S55–S58. [Google Scholar]
- Boglaienko, D.; Soti, P.; Shetty, K.G.; Jayachandran, K. Buckwheat as a cover crop in Florida: Mycorrhizal Status and soil analysis. Agroecol. Sustain. Food Syst. 2014, 38, 1033–1046. [Google Scholar] [CrossRef]
- Vinichuk, M.; Mårtensson, A.; Ericsson, T.; Rosén, K. Effect of arbuscular mycorrhizal (AM) fungi on 137Cs uptake by plants grown on different soils. J. Environ. Radioact. 2013, 115, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Pang, C.; Zhang, Y.; Hua, Y.; He, X.; Yang, Y. Growth and physiological characteristics of quinoa inoculated with arbuscular mycorrhizal fungi under different nitrogen levels. Acta Bot. Boreali-Occidentalia Sin. 2017, 37, 1323–1330. [Google Scholar]
- Ramakrishnan, K.; Bhuvaneswari, G. Effect of inoculation of am fungi and beneficial microorganisms on growth and nutrient uptake of Eleusine coracana (L.) Gaertn. (Finger millet). Int. Lett. Nat. Sci. 2014, 13, 59–69. [Google Scholar] [CrossRef]
- Channabasava, A.; Lakshman, H.C.; Jorquera, M.A. Effect of fungicides on association of arbuscular mycorrhiza fungus Rhizophagus fasciculatus and growth of Proso millet (Panicum miliaceum L.). J. Soil Sci. Plant Nutr. 2015, 15, 35–45. [Google Scholar] [CrossRef]
- Mardukhi, B.; Rejali, F.; Daei, G.; Ardakani, M.R.; Malakouti, M.J.; Miransari, M. Arbuscular mycorrhizas enhance nutrient uptake in different wheat genotypes at high salinity levels under field and greenhouse conditions. C. R. Biol. 2011, 334, 564–571. [Google Scholar] [CrossRef]
- Pellegrino, E.; Opik, M.; Bonari, E.; Ercoli, L. Responses of wheat to arbuscular mycorrhizal fungi: A meta-analysis of field studies from 1975 to 2013. Soil Biol. Biochem. 2015, 84, 210–217. [Google Scholar] [CrossRef]
- Schweiger, P.F.; Jakobsen, I. Direct measurement of arbuscular mycorrhizal phosphorus uptake into field-grown winter wheat. J. Agron. 1999, 91, 998–1002. [Google Scholar] [CrossRef]
- Dai, M.; Hamel, C.; Bainard, L.D.; Arnaud, M.S.; Grant, C.A.; Lupwayi, N.Z.; Lemke, R. Negative and positive contributions of arbuscular mycorrhizal fungal taxa to wheat production and nutrient uptake efficiency in organic and conventional systems in the Canadian prairie. Soil Biol. Biochem. 2014, 74, 156–166. [Google Scholar] [CrossRef]
- Graham, J.H.; Abbott, L.K. Wheat responses to aggressive and non-aggressive arbuscular mycorrhizal fungi. Plant Soil 2000, 220, 207–218. [Google Scholar] [CrossRef]
- Subramanian, K.S.; Tenshia, V.; Jayalakshmi, K.; Ramach, V. Role of arbuscular mycorrhizal fungus (Glomus intraradices) (fungus aided) in zinc nutrition of maize. J. Agric. Biotech. Sustain. Dev. 2009, 1, 029–038. [Google Scholar]
- Azaizeh, H.A.; Marschner, H.; Römheld, V.; Wittenmayer, L. Effects of a vesicular-arbuscular mycorrhizal fungus and other soil microorganisms on growth, mineral nutrient acquisition and root exudation of soil-grown maize plants. Mycorrhiza 1995, 5, 321–327. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, A.; Wang, F.; Han, X.; Wang, D.; Li, S. Arbuscular mycorrhizal fungi and rhizobium facilitate nitrogen uptake and transfer in soybean/maize intercropping system. Front. Plant Sci. 2015, 6, 339. [Google Scholar] [CrossRef]
- Chareesri, A.; De Deyn, G.B.; Sergeeva, L.; Polthanee, A.; Kuyper, T.W. Increased arbuscular mycorrhizal fungal colonization reduces yield loss of rice (Oryza sativa L.) under drought. Mycorrhiza 2020, 30, 315–328. [Google Scholar] [CrossRef]
- He, M.; Dijkstra, F.A. Drought effect on plant nitrogen and phosphorus: A meta-analysis. New Phytol. 2014, 204, 924–931. [Google Scholar] [CrossRef]
- Li, Y.; Ran, W.; Zhang, R.; Sun, S.; Xu, G. Facilitated legume nodulation, phosphate uptake and nitrogen transfer by arbuscular inoculation in an upland rice and mung bean intercropping system. Plant Soil 2009, 315, 285–296. [Google Scholar] [CrossRef]
- Li, H.; Ye, Z.H.; Chan, W.F.; Chen, X.W.; Wu, F.Y.; Wu, S.C.; Wong, M.H. Can arbuscular mycorrhizal fungi improve grain yield, As uptake and tolerance of rice grown under aerobic conditions? Environ. Pollut. 2011, 159, 2537–2545. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhao, R.; Zhao, W.; Fu, R.; Guo, J.; Bi, N.; Zhang, J. Effects of arbuscular mycorrhizal fungi on maize (Zea mays L.) and sorghum (Sorghum bicolor L. Moench) grown in rare earth elements of mine tailings. Appl. Soil Ecol. 2013, 72, 85–92. [Google Scholar] [CrossRef]
- Caris, C.; Hördt, W.; Hawkins, H.J.; Römheld, V.; George, E. Studies of iron transport by arbuscular mycorrhizal hyphae from soil to peanut and sorghum plants. Mycorrhiza 1998, 8, 35–39. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, J.; Lu, S.; Li, Y.; Wang, F. Arbuscular mycorrhizal fungi improve the performance of sweet sorghum grown in a mo-contaminated soil. J. Fungi 2020, 6, 44. [Google Scholar] [CrossRef]
- Watts-Williams, S.J.; Gill, A.R.; Jewell, N.; Brien, C.J.; Berger, B.; Tran, B.T.; Cavagnaro, T.R. Enhancement of sorghum grain yield and nutrition: A role for arbuscular mycorrhizal fungi regardless of soil phosphorus availability. Plants People Planet 2022, 4, 143–156. [Google Scholar] [CrossRef]
- Cameron, J.C.; Lehman, R.M.; Sexton, P.; Osborne, S.L.; Taheri, W.I. Fungicidal seed coatings exert minor effects on arbuscular mycorrhizal fungi and plant nutrient content. Agron. J. 2017, 109, 1005–1012. [Google Scholar] [CrossRef]
- Will, M.E.; Sylvia, D.M. Interaction of rhizosphere bacteria, fertilizer, and vesicular-arbuscular mycorrhizal fungi with sea oats. Appl. Environ. Microbiol. 1990, 56, 2073–2079. [Google Scholar] [CrossRef]
- Saia, S.; Ruisi, P.; Amato, G.; Di, M.G.; Frenda, A.S.; Giambalvo, D. Effects of soil inoculation with arbuscular mycorrhizal fungi on plant growth and nutrient uptake of some Mediterranean species grown under rainfed field conditions. Nitrogen Workshop 2012. In Proceedings of the 17th International Nitrogen Workshop, Wexford, Ireland, 26–29 June 2012; pp. 90–91. [Google Scholar]
- Watts-Williams, S.J.; Nguyen, T.D.; Kabiri, S.; Losic, D.; McLaughlin, M.J. Potential of zinc-loaded graphene oxide and arbuscular mycorrhizal fungi to improve the growth and zinc nutrition of Hordeum vulgare and Medicago truncatula. Appl. Soil Ecol. 2020, 150, 103464. [Google Scholar] [CrossRef]
- Maji, D.; Misra, P.; Singh, S.; Kalra, A. Humic acid rich vermicompost promotes plant growth by improving microbial community structure of soil as well as root nodulation and mycorrhizal colonization in the roots of Pisum sativum. Appl. Soil Ecol. 2017, 110, 97–108. [Google Scholar] [CrossRef]
- Mohammad, M.J.; Malkawi, H.I.; Shibli, R. Effects of arbuscular mycorrhizal fungi and phosphorus fertilization on growth and nutrient uptake of barley grown on soils with different levels of salts. J. Plant Nutr. 2003, 26, 125–137. [Google Scholar] [CrossRef]
- Bagayoko, M.; George, E.; Römheld, V.; Buerkert, A. Effects of mycorrhizae and phosphorus on growth and nutrient uptake of millet, cowpea and sorghum on a West African soil. J. Agric. Sci. 2000, 135, 399–407. [Google Scholar] [CrossRef]
- Yadav, B.K.; Tarafdar, J.C. Ability of Emericella rugulosa to mobilize unavailable P compounds during Pearl millet [Pennisetum glaucum (L.) R. Br.] crop under arid condition. Ind. J. Microbiol. 2007, 47, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Pandey, S. Symbiosis of arbuscular mycorrhizal fungi and Pennisetum glaucum l. improves plant growth and glomalin-related soil protein in barren soil. Int. J. Sci. Invent. Today 2017, 6, 783–792. [Google Scholar]
- Cui, X.C.; Hu, J.L.; Lin, X.G.; Wang, F.Y.; Chen, R.R.; Wang, J.H.; Zhu, J.G. Arbuscular mycorrhizal fungi alleviate ozone stress on nitrogen nutrition of field wheat. J. Agric. Sci. Technol. 2013, 15, 1043–1052. [Google Scholar]
- Smith, S.E.; Manjarrez, M.; Stonor, R.; McNeill, A.; Smith, F.A. Indigenous arbuscular mycorrhizal (AM) fungi contribute to wheat phosphate uptake in a semi-arid field environment, shown by tracking with radioactive phosphorus. Appl. Soil Ecol. 2015, 96, 68–74. [Google Scholar] [CrossRef]
- Ma, X.; Luo, W.; Li, J.; Wu, F. Arbuscular mycorrhizal fungi increase both concentrations and bioavilability of Zn in wheat (Triticum aestivum L) grain on Zn-spiked soils. Appl. Soil Ecol. 2019, 135, 91–97. [Google Scholar] [CrossRef]
- Watts-Williams, S.J.; Tyerman, S.D.; Cavagnaro, T.R. The dual benefit of arbuscular mycorrhizal fungi under soil zinc deficiency and toxicity: Linking plant physiology and gene expression. Plant Soil 2017, 420, 375–388. [Google Scholar] [CrossRef]
- Solaiman, M.Z.; Hirata, H. Responses of directly seeded wetland rice to arbuscular mycorrhizal fungi inoculation. J. Plant Nut. 1997, 20, 1479–1487. [Google Scholar] [CrossRef]
- Chan, W.F.; Li, H.; Wu, F.Y.; Wu, S.C.; Wong, M.H. Arsenic uptake in upland rice inoculated with a combination or single arbuscular mycorrhizal fungi. J. Hazard. Mater. 2013, 262, 1116–1122. [Google Scholar] [CrossRef]
- Garg, N.; Kaur, H. Impact of cadmium-zinc interactions on metal uptake, translocation and yield in pigeonpea genotypes colonized by arbuscular mycorrhizal fungi. J. Plant Nutr. 2013, 36, 67–90. [Google Scholar] [CrossRef]
- Bhantana, P.; Malla, R.; Vista, S.P.; Rana, M.S.; Mohamed, G.; Joshi, B.D.; Hu, C.X. Use of Arbuscular Mycorrhizal Fungi (AMF) and Zinc Fertilizers in An Adaptation of Plant from Drought and Heat Stress. Biomed. J. Sci. Tech. Res. 2021, 38, 30357–30373. [Google Scholar] [CrossRef]
- Nakmee, P.S.; Techapinyawat, S.; Ngamprasit, S. Comparative potentials of native arbuscular mycorrhizal fungi to improve nutrient uptake and biomass of Sorghum bicolor Linn. Agric. Nat. Resour. 2016, 50, 173–178. [Google Scholar] [CrossRef]
- Aliasgharzad, N.; Shirmohamadi, E.; Oustan, S. Siderophore production by mycorrhizal sorghum roots under micronutrient deficient condition. Soil Environ. 2009, 28, 119–123. [Google Scholar]
- Zhang, S.; Lehmann, A.; Zheng, W.; You, Z.; Rillig, M.C. Arbuscular mycorrhizal fungi increase grain yields: A meta-analysis. New Phytol. 2019, 222, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, R.; Sheriff, H.H.; Buba, A. Effect of biofertilizer and organic manure on growth and nutrients content of pearl millet. ARPN J. Agric. Biol. Sci. 2014, 9, 351–355. [Google Scholar]
- Peterson, R.L.; Massicotte, H.B.; Melville, L.H. Mycorrhizas: Anatomy and cell biology. Mycologist 2004, 19, 133. [Google Scholar]
- Xu, H.; Lu, Y.; Tong, S. Effects of arbuscular mycorrhizal fungi on photosynthesis and chlorophyll fluorescence of maize seedlings under salt stress. Emir. J. Food Agric. 2018, 30, 199–204. [Google Scholar]
- De-Andrade, S.A.L.; Domingues, A.P.; Mazzafera, P. Photosynthesis is induced in rice plants that associate with arbuscular mycorrhizal fungi and are grown under arsenate and arsenite stress. Chemosphere 2015, 134, 141–149. [Google Scholar] [CrossRef]
- Gernns, H.; Alten, H.; Poehling, H.M. Arbuscular mycorrhiza increased the activity of a biotrophic leaf pathogen–is a compensation possible? Mycorrhiza 2001, 11, 237–243. [Google Scholar] [CrossRef]
- Achatz, B.; von Rüden, S.; Andrade, D.; Neumann, E.; Pons-Kühnemann, J.; Kogel, K.H.; Waller, F. Root colonization by Piriformospora indica enhances grain yield in barley under diverse nutrient regimes by accelerating plant development. Plant Soil 2010, 333, 59–70. [Google Scholar] [CrossRef]
- Shamshiri, M.H.; Fattahi, M. Effects of arbuscular mycorrhizal fungi on photosystem II activity of three pistachio rootstocks under salt stress as probed by the OJIP-test. Russ. J. Plant Physiol. 2016, 63, 101–110. [Google Scholar] [CrossRef]
- Meddich, A.; Ouhaddou, R.; Anli, M.; Boutasknit, A. Role of Phosphorus and Arbuscular Mycorrhizal Fungi in the Growth Performances and Tolerance of Barley to Water Stress. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 45–67. [Google Scholar]
- Ruiz-Lozano, J.M.; Aroca, R. Host response to osmotic stresses: Stomatal behaviour and water use efficiency of arbuscular mycorrhizal plants. In Arbuscular mycorrhizas: Physiology and Function; Springer: Dordrecht, The Netherlands, 2010; pp. 239–256. [Google Scholar]
- Kamali, S.; Mehraban, A. Effects of Nitroxin and arbuscular mycorrhizal fungi on the agro-physiological traits and grain yield of sorghum (Sorghum bicolor L.) under drought stress conditions. PLoS ONE 2020, 15, e0243824. [Google Scholar] [CrossRef]
- García-Parra, M.Á.; Plazas-Leguizamón, N.Z. Análisis del ciclo de vida de las publicaciones sobre la producción de quinua (Chenopodium quinoa Willd), a través de curvas en S. Rev. Investig. Desarro. Innovación 2019, 9, 379–391. [Google Scholar] [CrossRef]
- Borde, M.; Dudhane, M.; Jite, P. Growth photosynthetic activity and antioxidant responses of mycorrhizal and non-mycorrhizal bajra (Pennisetum glaucum) crop under salinity stress condition. Crop Prot. 2011, 30, 265–271. [Google Scholar] [CrossRef]
- Bowles, T.M.; Barrios-Masias, F.H.; Carlisle, E.A.; Cavagnaro, T.R.; Jackson, L.E. Efects of arbuscular mycorrhizae on tomato yield, nutrient uptake, water relations, and soil carbon dynamics under defcit irrigation in feld conditions. Sci. Total Environ. 2016, 12, 566–567. [Google Scholar]
- Talaat, N.B.; Shawky, B.T. Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ. Exp. Bot. 2014, 98, 20–31. [Google Scholar] [CrossRef]
- Li, T.; Hu, J.L.; Hao, Z.P.; Li, H.; Wang, Y.S.; Chen, B.D. First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 2013, 197, 617–630. [Google Scholar] [CrossRef]
- Rabie, G.H. Contribution of arbuscular mycorrhizal fungus to red kidney and wheat plants tolerance grown in heavy in metal polluted soil. Afr. J. Biotechnol. 2005, 4, 332–345. [Google Scholar]
- Shahabivand, S.; Maivan, H.Z.; Goltapeh, E.M.; Sharifi, M.; Aliloo, A.A. The effects of root endophyte and arbuscular mycorrhizal fungi on growth and cadmium accumulation in wheat under cadmium toxicity. Plant Physiol. Biochem. 2012, 60, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R.; Joudmand, A.; Aliasgharzad, N.; Tolrá, R.; Poschenrieder, C. Arbuscular mycorrhizal fungi alleviate low-temperature stress and increase freezing resistance as a substitute for acclimation treatment in barley. Crop Past. Sci. 2019, 70, 218–233. [Google Scholar] [CrossRef]
- Baum, C.; El-Tohamy, W.; Gruda, N. Increasing the productivity and product quality of vegetable crops using arbuscular mycorrhizal fungi: A review. Sci. Hortic. 2015, 187, 131–141. [Google Scholar] [CrossRef]
- Huang, L.L.; Yang, C.; Zhao, Y.; Xu, X.; Xu, Q.; Li, G.Z.; Hao, L. Antioxidant defenses of mycorrhizal fungus infection against SO2-induced oxidative stress in Avena nuda seedlings. Bull. Environ. Contam. Toxicol. 2008, 81, 440–444. [Google Scholar] [CrossRef]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef]
- Alarcon, A.; Davies, F.T.; Autenrieth, R.L.; Zuberer, D.A. Arbuscular mycorrhiza and petroleum-degrading microorganisms enhance phytoremediation of petroleum-contaminated soil. Int. J. Phytoremediation 2008, 10, 251–263. [Google Scholar] [CrossRef]
- Xun, F.; Xie, B.; Liu, S.; Guo, C. Effect of plant growth-promoting bacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) inoculation on oats in saline-alkali soil contaminated by petroleum to enhance phytoremediation. Environ. Sci. Pollut. Res. 2015, 22, 598–608. [Google Scholar] [CrossRef]
- Kumar, P. Soil applied glycine betaine with Arbuscular mycorrhizal fungi reduces chromium uptake and ameliorates chromium toxicity by suppressing the oxidative stress in three genetically different Sorghum (Sorghum bicolor L.) cultivars. BMC Plant Biol. 2021, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Evelin, H.; Giri, B.; Kapoor, R. Ultrastructural evidence for AMF mediated salt stress mitigation in Trigonella foenum-graecum. Mycorrhiza 2013, 23, 71–86. [Google Scholar] [CrossRef]
- Sarwat, M.; Hashem, A.; Ahanger, M.A.; Abd-Allah, E.F.; Alqarawi, A.A.; Alyemeni, M.N.; Gucel, S. Mitigation of NaCl stress by arbuscular mycorrhizal fungi through the modulation of osmolytes, antioxidants and secondary metabolites in mustard (Brassica juncea L.) plants. Front. Plant Sci. 2016, 7, 869. [Google Scholar] [CrossRef]
- Prity, S.A.; Sajib, S.A.; Das, U.; Rahman, M.M.; Haider, S.A.; Kabir, A.H. Arbuscular mycorrhizal fungi mitigate Fe deficiency symptoms in sorghum through phytosiderophore-mediated Fe mobilization and restoration of redox status. Protoplasma 2020, 257, 1373–1385. [Google Scholar] [CrossRef]
- Vestberg, M.; Palojärvi, A.; Pitkänen, T.; Kaipainen, S.; Puolakka, E.; Keskitalo, M. Neutral lipid fatty acid analysis is a sensitive marker for quantitative estimation of arbuscular mycorrhizal fungi in agricultural soil with crops of different mycotrophy. Agric. Food Sci. 2012, 21, 12–27. [Google Scholar] [CrossRef]
- Tyagi, J.; Varma, A.; Pudake, R.N. Evaluation of comparative effects of arbuscular mycorrhiza (Rhizophagus intraradices) and endophyte (Piriformospora indica) association with finger millet (Eleusine coracana) under drought stress. Eur. J. Soil Biol. 2017, 81, 1–10. [Google Scholar] [CrossRef]
- Mythili, M.; Ramalakshmi, A. Unraveling the distribution of AMF communities and their metabolites associated with soils of minor millets. Rhizosphere 2022, 21, 100473. [Google Scholar] [CrossRef]
- Bernardo, L.; Carletti, P.; Badeck, F.W.; Rizza, F.; Morcia, C.; Ghizzoni, R.; Rouphael, Y.; Colla, G.; Terzi, V.; Lucini, L. Metabolomic responses triggered by arbuscular mycorrhiza enhance tolerance to water stress in wheat cultivars. Plant Physiol. Biochem. 2019, 137, 203–212. [Google Scholar] [CrossRef]
- Tereucán, G.; Ruiz, A.; Nahuelcura, J.; Oyarzún, P.; Santander, C.; Winterhalter, P.; Cornejo, P. Shifts in biochemical and physiological responses by the inoculation of arbuscular mycorrhizal fungi in Triticum aestivum growing under drought conditions. J. Sci. Food Agric. 2022, 102, 1927–1938. [Google Scholar] [CrossRef]
- Mathur, S.; Sharma, M.P.; Jajoo, A. Improved photosynthetic efficacy of maize (Zea mays) plants with arbuscular mycorrhizal fungi (AMF) under high temperature stress. J. Photochem. Photobiol. B Biol. 2018, 180, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.A.; Maia, L.C.; Silva, F.S. Arbuscular mycorrhizal fungi as biotechnology alternative to increase concentrate of secondary metabolites in Zea mays L. Braz. J. Bot. 2019, 42, 189–193. [Google Scholar] [CrossRef]
- Bona, E.; Scarafoni, A.; Marsano, F.; Boatti, L.; Copetta, A.; Massa, N.; Berta, G. Arbuscular mycorrhizal symbiosis affects the grain proteome of Zea mays: A field study. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Tisarum, R.; Theerawitaya, C.; Samphumphuang, T.; Polispitak, K.; Thongpoem, P.; Singh, H.P.; Cha-Um, S. Alleviation of salt stress in upland rice (Oryza sativa L. ssp. indica cv. Leum Pua) using arbuscular mycorrhizal fungi inoculation. Front. Plant Sci. 2020, 11, 348. [Google Scholar] [CrossRef]
- Vilela, L.A.F.; Barbosa, M.V. Contribution of arbuscular mycorrhizal fungi in promoting cadmium tolerance in plants. In Cadmium Tolerance Plants; Academic Press: Cambridge, MA, USA, 2019; pp. 553–586. [Google Scholar]
- Maier, W.; Schneider, B.; Strack, D. Biosynthesis of sesquiterpenoid cyclohexenone derivatives in mycorrhizal barley roots proceeds via the glyceraldehyde 3-phosphate/pyruvate pathway. Tetrahedron Lett. 1998, 39, 521–524. [Google Scholar] [CrossRef]
- Vierheilig, H.; Gagnon, H.; Strack, D.; Maier, W. Accumulation of cyclohexenone derivatives in barley, wheat and maize roots in response to inoculation with different arbuscular mycorrhizal fungi. Mycorrhiza 2000, 9, 291–293. [Google Scholar] [CrossRef]
- Peipp, H.; Maier, W.; Schmidt, J.; Wray, V.; Strack, D. Arbuscular mycorrhizal fungus-induced changes in the accumulation of secondary compounds in barley roots. Phytochem. 1997, 44, 581–587. [Google Scholar] [CrossRef]
- Sun, X.G.; Tang, M. Effect of arbuscular mycorrhizal fungi inoculation on root traits and root volatile organic compound emissions of Sorghum bicolor. S. Afr. J. Bot. 2013, 88, 373–379. [Google Scholar] [CrossRef]
- Zhang, Q.; Gong, M.; Yuan, J.; Hou, Y.; Zhang, H.; Wang, Y.; Hou, X. Dark septate endophyte improves drought tolerance in Sorghum. Int. J. Agric. Biol. 2017, 19, 53–60. [Google Scholar] [CrossRef]
- Dhawi, F.; Datta, R.; Ramakrishna, W. Mycorrhiza and heavy metal resistant bacteria enhance growth, nutrient uptake and alter metabolic profile of sorghum grown in marginal soil. Chemosphere 2016, 157, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Maier, W.; Peipp, H.; Schmidt, J.; Wray, V.; Strack, D. Levels of a terpenoid glycoside (blumenin) and cell wall-bound phenolics in some cereal mycorrhizas. Plant Physiol. 1995, 109, 465–470. [Google Scholar] [CrossRef]
- Maier, W.; Hammer, K.; Dammann, U.; Schulz, B.; Strack, D. Accumulation of sesquiterpenoid cyclohexenone derivatives induced by an arbuscular mycorrhizal fungus in members of the Poaceae. Planta 1997, 202, 36–42. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Pfeffer, P.E.; Jin, H.; Abubaker, J.; Douds, D.D.; Allen, J.W.; Shachar-Hill, Y. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 2005, 435, 819–823. [Google Scholar] [CrossRef]
- Regvar, M.; Bukovnik, U.; Likar, M.; Kreft, I. UV-B radiation affects flavonoids and fungal colonisation in Fagopyrum esculentum and F. tataricum. Open Life Sci. 2012, 7, 275–283. [Google Scholar] [CrossRef]
- Karimi, G.; Pourakbar, L.; Moghaddam, S.S.; Popović-Djordjević, J. Integrated effects of bacteria and fungi biofertilizers on morphological traits, antioxidants indices, and polyphenol compounds of quinoa (Chenopodium quinoa Willd.) under salinity condition. Res. Sq. 2020; preprint. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Bonini, P.; Cardarelli, M. Coating seeds with endophytic fungi enhances growth, nutrient uptake, yield and grain quality of winter wheat. Int. J. Plant Prod. 2015, 9, 171–190. [Google Scholar]
- Sukumaran, S.; Li, X.; Li, X.; Zhu, C.; Bai, G.; Perumal, R.; Tuinstra, M.R.; Vara Prasad, P.V.; Mitchell, S.E.; Tesso, T.T.; et al. QTL mapping for grain yield, flowering time, and stay-green traits in sorghum with genotyping-by-sequencing markers. Crop Sci. 2006, 56, 1429–1442. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhu, Y.G.; Marschner, P.; Smith, F.A.; Smith, S.E. Wheat responses to arbuscular mycorrhizal fungi in a highly calcareous soil differ from those of clover, and change with plant development and P supply. Plant Soil 2005, 277, 221–232. [Google Scholar] [CrossRef]
- Celebi, S.Z.; Demir, S.; Celebi, R.; Durak, E.D.; Yilmaz, I.H. The effect of Arbuscular Mycorrhizal Fungi (AMF) applications on the silage maize (Zea mays L.) yield in different irrigation regimes. Eur. J. Soil Biol. 2010, 46, 302–305. [Google Scholar] [CrossRef]
- Gao, C.; El-Sawah, A.M.; Ali, D.F.I.; Alhaj Hamoud, Y.; Shaghaleh, H.; Sheteiwy, M.S. The integration of bio and organic fertilizers improve plant growth, grain yield, quality and metabolism of hybrid maize (Zea mays L.). Agronomy 2020, 10, 319. [Google Scholar] [CrossRef]
- Parvin, S.; Van Geel, M.; Yeasmin, T.; Verbruggen, E.; Honnay, O. Effects of single and multiple species inocula of arbuscular mycorrhizal fungi on the salinity tolerance of a Bangladeshi rice (Oryza sativa L.) cultivar. Mycorrhiza 2020, 30, 431–444. [Google Scholar] [CrossRef]
- Jerbi, M.; Labidi, S.; Laruelle, F.; Tisserant, B.; Jeddi, F.B.; Sahraoui, A.L.H. Mycorrhizal biofertilization improves grain yield and quality of hulless Barley (Hordeum vulgare ssp. nudum L.) under water stress conditions. J. Cereal Sci. 2022, 104, 103436. [Google Scholar] [CrossRef]
- Cobb, A.B.; Wilson, G.W.; Goad, C.L.; Bean, S.R.; Kaufman, R.C.; Herald, T.J.; Wilson, J.D. The role of arbuscular mycorrhizal fungi in grain production and nutrition of sorghum genotypes: Enhancing sustainability through plant-microbial partnership. Agric. Ecosyst. Environ. 2016, 233, 432–440. [Google Scholar] [CrossRef]
- Kahiluoto, H.; Ketoja, E.; Vestberg, M. Contribution of arbuscular mycorrhiza to soil quality in contrasting cropping systems. Agric. Ecosyst. Environ. 2009, 134, 36–45. [Google Scholar] [CrossRef]
- Castillo, C.G.; Puccio, F.; Morales, D.; Borie, F.; Sieverding, E. Early arbuscular mycorrhiza colonization of wheat, barley and oats in Andosols of southern Chile. J. Soil Sci. Plant Nutr. 2012, 12, 511–524. [Google Scholar] [CrossRef]
- Toubali, S.; Ait-El-Mokhtar, M.; Boutasknit, A.; Anli, M.; Ait-Rahou, Y.; Benaffari, W.; Meddich, A. Root Reinforcement Improved Performance, Productivity, and Grain Bioactive Quality of Field-Droughted Quinoa (Chenopodium quinoa). Front. Plant Sci. 2022, 13, 860484. [Google Scholar] [CrossRef]
- Mathimaran, N.; Jegan, S.; Thimmegowda, M.N.; Prabavathy, V.R.; Yuvaraj, P.; Kathiravan, R.; Mäder, P. Intercropping transplanted pigeon pea with finger millet: Arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria boost yield while reducing fertilizer input. Front. Sustain. Food Syst. 2020, 4, 88. [Google Scholar] [CrossRef]
- Shen, W.; Feng, Z.; Song, H.; Jin, D.; Fu, Y.; Cheng, F. Effects of solid waste-based soil conditioner and arbuscular mycorrhizal fungi on crop productivity and heavy metal distribution in foxtail millet (Setaria italica). J. Environ. Manag. 2022, 313, 114974. [Google Scholar] [CrossRef]
- Schütz, L.; Saharan, K.; Mäder, P.; Boller, T.; Mathimaran, N. Rate of hyphal spread of arbuscular mycorrhizal fungi from pigeon pea to finger millet and their contribution to plant growth and nutrient uptake in experimental microcosms. Appl. Soil Ecol. 2022, 169, 104156. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, Y.; Shah, S.; Hui, T. The Roles of Arbuscular Mycorrhizal Fungi in Influencing Plant Nutrients, Photosynthesis, and Metabolites of Cereal Crops—A Review. Agronomy 2022, 12, 2191. https://doi.org/10.3390/agronomy12092191
Khan Y, Shah S, Hui T. The Roles of Arbuscular Mycorrhizal Fungi in Influencing Plant Nutrients, Photosynthesis, and Metabolites of Cereal Crops—A Review. Agronomy. 2022; 12(9):2191. https://doi.org/10.3390/agronomy12092191
Chicago/Turabian StyleKhan, Yaseen, Sulaiman Shah, and Tian Hui. 2022. "The Roles of Arbuscular Mycorrhizal Fungi in Influencing Plant Nutrients, Photosynthesis, and Metabolites of Cereal Crops—A Review" Agronomy 12, no. 9: 2191. https://doi.org/10.3390/agronomy12092191
APA StyleKhan, Y., Shah, S., & Hui, T. (2022). The Roles of Arbuscular Mycorrhizal Fungi in Influencing Plant Nutrients, Photosynthesis, and Metabolites of Cereal Crops—A Review. Agronomy, 12(9), 2191. https://doi.org/10.3390/agronomy12092191






