Abstract
Physiological, agricultural and toxicological impact of an excess of Zn in the soil is an important issue, as Zn is a heavy metal and impairs many processes of plants and animals. The novelty of this work is that it is a comprehensive approach to facilitate visualization not only of the condition of cabbage plants under Zn stress, but also prediction of the toxicity associated with consumption of such cabbages. We treated plants of two cabbage cultivars, differing in their earliness, with 50 and 200 mg Zn kg−1 soil (Zn50 and Zn200, respectively) above the natural Zn levels of 118.13 mg kg−1 soil (Zn0). Leaf cell membrane integrity, condition of the photosynthetic apparatus (reflected by relative chlorophyll content (SPAD) and Fv/Fm parameter of chlorophyll a fluorescence), head biomass, and Zn bioaccumulation in the heads were analyzed. Toxicological risk was also assessed by Daily Intake of Metal (DIM) and Health Risk Index (HRI) indicators. The data revealed that plants of the late cultivar were more sensitive to soil Zn than those of the early one. Detrimental effects of Zn (especially at the higher dose, Zn200) were manifested in the seedlings just after three weeks of treatment, and then reflected in the yield. We assume that, due to their fast and prolonged response to Zn, the seedlings of the late cultivar can be used as biomarkers of Zn toxicity. Although Zn plants did not indicate toxicological risk, based on DIM and HRI, Zn concentration in the soil should be taken into account prior to cabbage planting, because plants which do not reveal symptoms of injury can accumulate Zn above the permissible level.
1. Introduction
Zinc (Zn) is a microelement necessary for the proper growth and development of plants [1,2]. It is a structural component of ribosomes and cytoplasmic membranes and participates in many biological processes, including nitrogen metabolism, photosynthesis, auxin biosynthesis, nucleic acid metabolism, and protein biosynthesis and folding. Zn is a cofactor of enzymes belonging to all classes, e.g., carbonic anhydrase, carboxypeptidase, alcohol dehydrogenase and Cu/Zn-SOD [1,3].
The primary source of Zn in soil is bedrock. Zn can also be incorporated into the soil from the atmosphere along with volcanic ash, or as a result of forest fires [1]. The main anthropogenic sources of Zn are mining and metallurgy, which contribute to the contamination of soil, air and water [4,5]. Increased Zn content in soils and its accumulation in plants are also observed along motorways [5,6]. In the case of agricultural soils, contamination with Zn may occur as a result of the use of phosphorus fertilizers and sewage sludge, but also by distant wind emissions from road traffic [1,5,6,7].
In most crops, the necessary concentration of Zn in the leaves, for proper development, is approximately 15–30 mg Zn kg−1 DM. The symptoms of Zn toxicity in some plants can be visible on the leaves at just 100 mg Zn kg−1 [3]. These are less common than those caused by Zn deficiency [1,3]. Moreover, the threshold of toxicity can vary widely, even within the same species [1,8].
The most common symptoms of Zn toxicity include growth inhibition leading to a decrease in biomass, inhibition of root growth and development, and stem cracking, leaf deformation, changes in the leaf structure and disturbances in the gas exchange [9,10,11]. Zn, by interacting with other metals, such as Fe, Mg, Ca, or Mn, can cause their secondary deficiency, resulting in limitation of photosynthetic activity [1,12], among others, due to chlorophyll content dropping and the photosynthetic electron transport chain malfunctioning [10]. There are also frequent changes in the redox homeostasis, namely enhancement of lipid peroxidation and protein oxidation, which, together with diminished activity of antioxidants, leads to accelerated plant senescence [13]. Zn/P antagonism is widely known [1,14].
The aim of this experiment was to evaluate the influence of zinc-spiked soil on the condition of cabbage Brassica oleracea var. capitata (white form) and accumulation of zinc in its agricultural yield (heads). The choice of the species for this study was based on many premises. The Brassicaceae family comprises Zn-hyperaccumulating plants [1,15]. Among agricultural Brassica species used so far for Zn phytoremediation and phytoextraction, rapeseed (or canola) and Indian mustard (B. juncea) have been used [16,17]. The phytoremediation potential of cabbage has already been postulated [2,18], which encouraged us to examine it as a potential Cd accumulator and bioindicator [19]. The study on the impact of Zn on cabbage is the next step in our research, and we used the same cultivars as previously. We assumed that the cultivars could be useful in bioindication and phytoremediation of Zn-contaminated soils. Biofortification of cabbage with Zn, together with the risk for consumers, was also considered, based on the research by Liu et al. [20].
To emulate soil conditions occurring in the field, the plants were cultivated in agricultural soil collected from the field, in large vegetation pots (10 L). This is quite rare in heavy metal stress research. So far, hydroponics with a Zn-contaminated medium [21], waste water or sewage sludge irrigation [22,23] and bottom sediments [24] have already been used. Field trials in soils with naturally differentiated Zn content were performed in [18,25,26,27]. Pot experiments with different soils were conducted by Pongrac et al. [14], but in terms of elemental composition of red cabbage and Zn/P interactions. White et al. [8] and Chaudhry et al. [17] used a potting medium composed of peat and sand, but the soil volume was 1 L only. Hence, our approach allowed for assessing the plant response in soil conditions similar to natural ones, in the context of biomonitoring, phytoremediation, and biofortification of Zn. At the same time, it provided a basis for research on the mechanisms of Zn uptake and detoxification by plants. Concerning biofortification of plants with Zn, it is usually performed by foliar spraying with Zn salts, not by soil fertilization ([8], and the references therein). However, it was worth considering this aspect of the study in terms of ecotoxicology.
Hence, before setting the experiment, and based on our previous research [19], we formulated a few hypotheses. First, we assumed that plants of the early cultivar would suffer more severe stress than those of the late cultivar, while the late cultivar plants could accumulate more zinc, due to their longer growing season. We also predicted that the physiological response to Zn would be stronger at a higher dose (Zn200); however, we took into account the potential Zn-excluder strategy [25]. Next, we stipulated a link between the early response of the seedlings to Zn and final head biomass. We also focused on widely used toxicological indicators to assess the potential health risk for consumers of cabbage grown in Zn-spiked soil [28,29,30].
2. Materials and Methods
2.1. Plant and Soil Material
The experiment was performed in a greenhouse of the University of Agriculture in Kraków. The seeds of two cabbage cultivars (Brassica oleracea ssp. capitata f. alba L.) were used: early cultivar ‘Ditmarska Najwcześniejsza’ and late cultivar ‘Kamienna Głowa’. The cultivation protocol was based on the agricultural protocols for fast-growing early cultivar and slow-growing late cultivar of longer vegetation period (see e.g., [18,31]). Before planting, the seeds were treated with seed dressing T 75/DS/WS (70% Thiuram), and immediately sown into 100 mL pots filled with commercial garden soil. The seedlings grew at 5–15/15–20 °C until the stage of 6–8 leaves and then were transplanted into large pots of 10 L capacity, filled with approximately 10 kg of local soil taken from an experimental field (humus horizon) of the University of Agriculture in Kraków, Poland.
The study involved Eutric Cambisol (Loamic) with clay silt texture [32], with alkaline reaction (pHKCl = 7.07, Table 1). Granulometric composition of the soil was 19% sand, 5% coarse silt, 41% fine silt, 24% coarse silt clay, 6% clay fine silt, and 5% colloidal clay. The detailed elemental analysis of the soil is presented in Table 1. The natural amount of Zn was 118.13 mg kg−1 DM (total; Table 1) and 49.5 mg kg−1 DM (1 M HCl-extractable, data not shown). After the transplantation, the plants were cultivated in a greenhouse, at 20–25 °C/17–20 °C (day/night), relative humidity 30–50%, the photoperiod 14/10 h (day/night), and natural light intensity (photosynthetic photon flux density, PPFD) 400–500 μmol (quanta) m−2 s−1. Additional automatic lighting during cloudy days was provided to obtain this value. The plants were fertilized with N (NO3-N + NH4-N) at 105 mg L−1 of soil (early cultivar) and 120 mg L−1 of soil (late cultivar), P (Ca(HPO4)2)—50 and 60 mg L−1, K (KCl, 60% potassium salt)—160 and 180 mg L−1, respectively. The doses were established according to Babik et al. [31]. To avoid wilting, all plants were irrigated daily with tap water. The early cultivar plants grew for thirteen weeks (91 days) and those of the late cultivar for twenty-one weeks (151 days), according to agricultural protocols [19,31].

Table 1.
Physico-chemical composition of soil used in the experiment.
2.2. Zn Treatment
Ten days before planting, Zn was added to the soil as ZnSO4·7H2O (Sigma-Aldrich, St. Louis, MO, USA), dissolved in 100 mL of deionized water, in the amount of 50 and 200 mg Zn kg−1 of soil, and mixed thoroughly with the soil. The concentrations of Zn used in the experiment, and the contamination procedure, were based on previous experiments [19]. The sulfate form was used as it is the most popular form and has been widely used in numerous experiments [10,16,17,21]. Control pots (Zn0) contained the same soil but treated with the same volume of deionized water without Zn. Each treatment (Zn0, Zn50 and Zn200) was prepared in four replications for each cultivar. Total amount of Zn in Zn200 treatment (318.5 mg kg−1 of soil) exceeded the lowest permissible level 300 mg kg−1 established by the Regulation of the Minister of the Environment of Poland [33].
2.3. Plant Analyses
2.3.1. Biometric Analysis
Leaf area of 3-week seedlings was measured photometrically using ImageJ software for scanned images. Fresh and dry mass of the same leaves (FM and DM, respectively) were assessed with a laboratory scale (AS 220.R2, Radwag, Radom, Poland). FM was obtained by immediate measurement after abscission, while DM was taken after drying the leaf samples at 80 °C for 24 h (until constant weight), and subsequent air-drying for the next 24 h. Specific Leaf Area was calculated as the measured leaf area to DM [34]. Biomass of the heads was determined at harvest using the scale C32.15.C2.K (Radwag Poland).
2.3.2. Membrane Disintegration
Membrane disintegration was measured in leaves at the third and eighth week of planting, with a conductometer with automatic temperature compensation (CC-315, Elmetron, Zabrze, Poland) and expressed as the electrolyte leakage index. Non-senescent, fully developed leaves (third to fifth from the top) were sampled. The plant material was washed with deionized water, closed in tubes with 15 mL of deionized water and gently shaken for 24 h. Conductivity was measured, then the samples were boiled at 100 °C for 15 min, shaken for 24 h and the assay was repeated [35]. The detrimental effect of Zn on leaf cell membrane status was described by Yang et al. 2012 in Brassica chinensis [36].
2.3.3. Relative Chl Content (SPAD) and Fv/Fm of Chl a Fluorescence
The parameters were obtained for three- and eight-week plants. Relative Chl was measured photometrically in non-senescent, fully developed leaves (third to fifth from the top) with a portable Chl meter SPAD-502 Plus (Konica Minolta, Tokyo, Japan). Based on the differential transmittance of red and infrared light by the sample, the method provides numerical values (SPAD readings) expressed as “Chl index” or “greenness index” that are proportional to Chl content (Konica Minolta, 2009). The method has already been successfully used for cabbage leaves [19].
Chlorophyll fluorescence parameters were obtained with an FMS-2 fluorometer (Hansatech, King’s Lynn, UK) for the same leaves as used for the SPAD analysis. First, the leaves were adapted in darkness for 20 min, and then a saturating light pulse (10,000 μmol (photon) m−2 s−1 for 0.9 s) was applied to obtain Fm. Next, the leaves were irradiated for 270 s with actinic light (1500 μmol (photon) m−2 s−1). The source of a modulation beam (duration pulses 1.8 μs, 2.3 kHz) was an amber LED (peak wavelength 594 nm, PPFD ca. 0.05 μmol (photon) m−2 s−1). Actinic and pulse irradiations were provided by a halogen lamp (20 W). The signal detector was a PIN photodiode with a long-pass filter (>700 nm). The sampling rate was 10–20 kHz (depending on the instrument mode). Fv/Fm, widely known as maximal quantum yield of PSII photochemistry (or force of the light reactions, [27]), was used as a simple tool for estimation of the condition of the photosynthetic apparatus.
2.4. Zn Determination in the Soil and Plant Material
The air-dried and lyophilized samples were digested in a microwave-assisted Anton Paar digestion system. The digestion was performed at 240 °C and 60 bar for 0.5 g samples mixed with digesting solutions: 5 mL HNO3 (65%) + 2 mL H2O2 (30%) (plant material) or 7 mL HNO3 (65%) (soil material). The acidic solutions after digestion were transferred into 25 mL PMP volumetric flasks and filled up with deionized water. For Zn determination, a GBC SensAA atomic absorption spectrometer, equipped with a deuterium lamp background correction, single element hollow cathode lamps (HCL) and air-acetylene flame, was used. In the event of particularly low Zn content, a GBC SavantAA atomic absorption spectrometer with graphite furnace atomization was applied. The sample volume injected was 10 µL and the matrix modifier (1% solution of NH4H2PO4) volume was 5 µL. Zn HCl was used as the radiation source at the wavelength of 213.9 nm. For quantitative analysis, the calibration curve was prepared with the use of AAS/ICP grade standard stock solutions IAEA-V-10 Hay Powder (International Atomic Energy Agency, Vienna, Austria), ERM-CD281 Rye Grass (Institute for Reference Materials and Measurements, Geel, Belgium), and CRM023-050 Trace Metals–Sandy Loam 7 (RT Corporation).
2.5. Bioaccumulation Factor
Bioaccumulation factor was calculated as follows:
where: Cp is the metal concentration in the plant sample (mg/kg) and Cs is the metal concentration in the soil sample (mg/kg). If the BCF < 1 the plant can be an excluder, if the BCF > 1 the plant can be an accumulator, and at the BCF = 1 there is no uptake [37]. In our experiment, we used the Cp for heads, namely the edible parts of the plants. A similar approach was taken by Rehman et al. [29] for the comparison of heavy metal transfer from soil to vegetables.
BCF = Cp/Cs
2.6. Toxicological Risk Assessment
The daily intake of metals (DIM) was calculated as follows [29,30]:
where: C is the concentration of Zn (mg kg−1 DM), Cf is the conversion factor for conversion of FM to DM (0.085), IR is the cabbage ingestion rate of 0.345 kg d−1 for adults and 0.232 kg d−1 for children (1–6 years), and BW is the body weight (73 kg for adults and 32.7 kg for children) [28,29,38].
DIM = (C × Cf × IR)/BW,
The Health Risk Index (HRI) resulting from consumption of cabbage from the experiment was determined as follows:
where DIM is the daily intake of metal and RfD is the reference dose (0.3 mg kg −1 BW d −1) according to [38].
HRI = DIM/RfD,
2.7. Statistical Analysis
The Kolmogorov-Smirnov test was used to assess the distribution normality, then ANOVA and Tukey’s test (n = 4) were performed. In the comparison of data from the 3rd and 8th week of growth, ANOVA, with repeated measurements, was applied, followed by the Tukey’s test. The differences were considered significant if p was at least ≤ 0.05. All the analyses were carried out using Microsoft Office Excel 2007 and the Statistica 12.0 (SPSS, Chicago, IL, USA).
3. Results
3.1. Plant Condition after Three Weeks of Growth in Zn-Spiked Soil
The biometric parameters are presented in Table 2. The early cultivar showed no effect of Zn on fresh or dry mass of leaves, while both parameters dropped in the late cultivar in a dose-dependent manner. Leaf dry mass in the late cv. decreased more than the fresh mass (for Zn50 fresh mass was 71% and dry mass 63% of Zn0, and for Zn200 it was 61% and 51% of Zn0, respectively).

Table 2.
Biometric parameters of leaves of cabbages after 3 weeks of growth in unspiked soil and the soil spiked with zinc. The natural content of zinc in the Zn0 (control) object was 118.13 [mg kg−1 DM].
The leaf area of non-spiked plants (Zn0) was 146.4 cm2 in the early cultivar, and 89.7 cm2 in the late one. In the early cultivar the area was not diminished by Zn, while in the case of the late cv. the drop was irrespective of Zn dose (leaf area of Zn50 and Zn200 was between 60 and 70% of Zn0; Table 2). Specific Leaf Area (SLA) increased only in the plants of the late cv. exposed to Zn200, and it amounted to 133% of Zn0.
3.2. Plant Physiology between the Third and Eight Week
3.2.1. Membrane Disintegration
After three weeks of plant cultivation in Zn-spiked soil, membrane disintegration, assayed as the percentage of total electrolyte leakage from Zn0 leaf tissue, was 13.8 for the early cultivar and 15.6 for the late one (Figure 1A and Figure 1B, respectively). There was no impact of Zn on leaf membrane status of the early cultivar, as the values for Zn0, Zn50 and Zn200 were almost equal (Figure 1A). After eight weeks all means were slightly, but significantly, lower than before (Figure 1A).
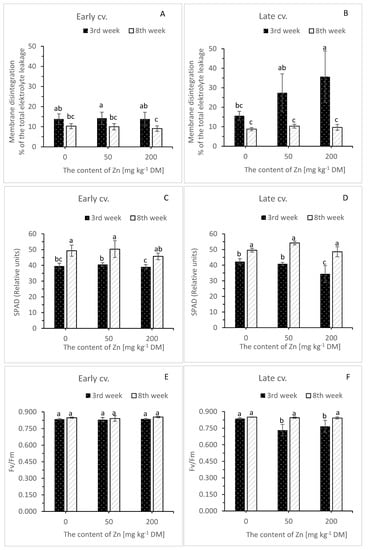
Figure 1.
The influence of zinc on leaf membrane disintegration (A,B), SPAD values (C,D) and Fv/Fm parameter (E,F) of chlorophyll fluorescence (CF) of white cabbage at the 3rd and 8th weeks of growth. The natural content of zinc in the Zn0 (control) object was 118.13 [mg kg−1 DM]. SPAD—Soil–Plant Analyses Development (greenness index), Fv/Fm—photochemical efficiency of PSII at the dark-adapted state. Note that n = 4, means ± SD are given. Means labelled with different letters are significantly differentiated within a date for a cultivar (p < 0.05; Tukey’s test).
3.2.2. SPAD and Fv/Fm Values
For SPAD values, three weeks after Zn addition to the soil, there was no difference between treatments in the early cultivar (Figure 1C). In the late cultivar, we witnessed a decrease for Zn200 plants at this stage of the experiment (Figure 1D). Then, during the eight weeks, there was a concerted increment (Figure 1C,D).
Three-week exposure to Zn-spiked soil resulted in a decrease in Fv/Fm ratio of chlorophyll a fluorescence (photochemical efficiency of PSII at the dark-adapted state) (Figure 1F) in the late cultivar, and there was no effect of Zn in the early cultivar (Figure 1E). During the eight weeks all values were similar (Figure 1E,F).
3.3. Final Head Biomass, and Zn Bioaccumulation
Biomass of cabbage heads after 13 and 21 weeks of cultivation (for early and late cv., respectively), and corresponding bioavailable Zn content and bioaccumulation factor values, are given in Table 3.

Table 3.
Biomass of heads and zinc accumulation by cabbages grown in unspiked soil and the soil spiked with zinc. The natural content of zinc in the Zn0 (control) object was 118.13 [mg kg−1 DM]. The early cultivar was grown for 13 weeks, the late one for 21 weeks.
Heads of the early cv. treated with Zn200 accumulated more biomass than the respective Zn0 and Zn50 plants. It was 119% of the Zn0 value. The response of the late cv. was reverse, namely head biomass of Zn200 plants diminished (87% of the respective Zn0). Constitutive biomass (Zn0) was bigger for plants of the late cultivar than for the early cultivar.
Bioavailable Zn related to head dry mass greatly increased in both cultivars with Zn200 (Table 3). The absolute bioavailable Zn was, in general, higher in the late cv. This meant that the Zn0 heads of the late cv. accumulated more Zn than those of the early cv. (48.8 vs. 39.1 mg kg−1 DM, respectively), and the amount of Zn in the Zn50 and Zn200 heads was also higher in the late cv. than in the early one. The response was different when comparing Zn amounts in relation to the respective controls, which meant that Zn-treated plants of the early cv. accumulated more Zn than Zn-treated plants of the late cv. In the case of Zn200, it was 389% for the early cultivar and 350% for the late one (Table 3).
The bioaccumulation factor, calculated for the heads (head amount of Zn vs. soil amount of Zn), revealed a similar pattern to that of the bioavailable Zn. It was manifested as higher absolute values for the late cultivar, and the relative ones higher for the early cultivar (Table 3).
3.4. Toxicological Risk Assessment of Cabbage Consumption
Estimated Daily Intake of Metal for Zn, for adults and children, was higher in the late cultivar, and greatly increased with the dose of the metal (Table 4). Health Risk Index (HRI) for adults did not exceed 1 in any case (Table 4).

Table 4.
Toxicological risk assessment (DIM and HRI) resulting from consumption of cabbages grown in unspiked soil and the soil spiked with zinc. The natural content of zinc in the Zn0 (control) object was 118.13 [mg kg−1 DM]. The early cultivar was grown for 13 weeks, the late one for 21 weeks.
4. Discussion
4.1. Seedlings’ Response to Zn
At this stage, the cabbage seedlings initiated their growth in the contaminated medium, and it was interesting how fast they revealed the symptoms of Zn intoxication. The data from the first stage of vegetation (third week) clearly indicated that the fast-growing early cultivar was resistant to increased doses of Zn, while the plants of the slower-growing late one suffered from Zn stress, as their leaf cell membranes were injured, fresh and dry mass, as well as the area of the leaves, diminished, and the leaf blades were thinner.
Zn can affect plant water management [39]. For this reason, we considered both mass-related parameters, because leaves contain approximately 90% of water, and lower water content means diminished fresh mass. In the late cv. leaf dry mass dropped more than the fresh mass. This suggested that not only water content in the leaves was altered, but the biomass production and/or assimilate allocation was impaired with the increasing Zn doses, resulting in leaf thinning, indicated as higher SLA [34,40].
Biomass production depends on many factors, mainly the uptake and utilization of macro- and micro-nutrients. High doses of Zn can lower the uptake of P, Fe and Cu, but increase that of Mn, leading to inhibition and alteration of many physiological processes [14], which can be manifested as reduced leaf area and leaf thickening. Stuiver et al. [41] indicated that Zn positively affected S uptake but limited absorption of N in Chinese cabbage. However, in rice, Zn positively affected root-to-shoot N translocation and its distribution in the leaves [42]. The hypothesis about different impacts of Zn on N and S management in both cultivars at the seedling stage could be worthy of verifying in further experiments.
Focusing on the differential responses of cabbage seedlings to Zn, some possible explanations follow: (i) different growth pattern of the cultivars, (ii) different root growth and architecture, and (iii) different molecular mechanisms of Zn uptake and translocation. As far as growth patterns are concerned, the early cultivar plants grow fast because they can be harvested early, so it can be assumed they intensively utilized Zn for growth and development, while the slowly-growing late cultivar exhibited Zn toxicity symptoms. Root properties, namely the changes in the root architecture and ultrastructure [1,3], can be either the cause or the result of Zn impact on the aerial parts, as already established in Datura [9]. The uptake of Zn from the soil and Zn translocation within plants can also differ due to different transport mechanisms. Although both phloem and xylem, and symplastic as well and apoplastic pathways are possible [1,43], some Zn-specific root transporters may operate in different manners [3,13]. Zn surplus can be deposited into cortical parenchyma, epidermis, bundle sheath, leaf vacuoles and/or apoplast, complexed with histidine and organic acids, like malate or oxalate, and nicotianamine [1,3,17].
4.2. Plant Physiology between the Third and Eighth Week
At this stage of the experiment, it was of interest to estimate the progress of Zn toxicity to developing plants. We continued the analysis of membrane disintegration, together with measurements related to the photosynthetic apparatus, due to its impact on plant biomass and head formation.
Three weeks after planting, leaf membrane disintegration in the late cultivar plants subjected to additional Zn was distinctive. It corresponded with, as already discussed, diminished fresh and dry mass and leaf area, confirming Zn susceptibility. However, plants tended to cope with stress, as the measurements performed five weeks later (eight weeks after planting) revealed no injury. Membrane disintegration at 10–15% is typical of plants grown in conditions close to optimal and indicating no injury (e.g., [35]). Such values were recorded for both cultivars at the eighth week of the experiment.
Our SPAD results indicated diminished chlorophyll amounts in the leaves and confirmed susceptibility of the late cultivar seedlings to Zn. The negative impact of high Zn doses on chlorophyll formation has been established in many experiments, and it is known that the reason is Mg, Fe and Mn deficiency, because Zn negatively affects the uptake of these nutrients necessary for chlorophyll molecule formation [12]. SPAD, in other words, the “greenness index”, has been used in numerous studies on plant response to different stress factors, also in cabbages [19]. Contrary to the membrane integrity assay, it is a non-destructive method. For this reason, it was particularly valuable to us at the early stage of the vegetation.
SPAD also helps to interpret chlorophyll fluorescence parameters allowing assess to the condition of the photosynthetic apparatus [19]. A decline of Fv/Fm in the third week of vegetation with increasing concentration of Zn indicated that the leaves of the late cultivar seedlings suffered from heavy metal stress [19]. However, after eight weeks the plants recovered from the stress, as their Fv/Fm values were close to that of Zn0. In general, Fv/Fm was quite high, which complied with our previous work on cabbages [19]. It should be kept in mind that Fv/Fm is both constitutively and environmentally determined [35].
4.3. Final Head Biomass and Zn Bioaccumulation and Distribution
At this stage of the experiment, the most important issue was to analyze the impact of Zn on head biomass reflecting the yield, and Zn accumulation in terms of its potential toxicity to consumers.
The percentage values of head biomass can be referred to as the so-called Tolerance Index [16,17]. The data clearly indicated that the biomass accumulation in the Zn200 variant was stimulated in the early cultivar but diminished in the late cultivar. This corresponded to the effects obtained at the seedling stage and prompted us to conclude that the investigated cultivars greatly differed in their susceptibility to Zn. Therefore, the hypothesis put forward at the beginning of this study, that the stress initiated at the seedling stage would be reflected in the size of the heads, was confirmed. In general, the heads were rather small but this was typical of the greenhouse conditions [19]. The larger plant biomass of late over that of the early cv. at Zn0 is common, and was already established in similar research [19]. Leaves were tightly packed in heads, and it would not have been possible to remove them without damage. That is why neither biometry of the leaves, nor their photosynthetic parameters, were studied.
Regarding Zn bioaccumulation, Tsonev and Cebola Lidon [11] established that Zn concentration in plants grown in unpolluted soils ranges from 0.02 to 0.04 mg g−1 DM. In our experiment, the amount of Zn in the heads of the early cultivar from the Zn0 object fell within this range. In the late cultivar Zn0 plants it was 48.8 mg kg−1, i.e., approximately 0.050 mg g−1 DM, so it tended to exceed the aforementioned value. However, when compared with the values obtained by Czech et al. [26], it was still lower than the range for cabbages sold in industrial and agricultural regions of Poland (54.3–78.9 mg kg−1). It was also within the range obtained for cabbages from waste water-irrigated plants from Pakistan [28] and India [29].
The bioavailable Zn in the heads of the Zn200 variant exceeded 100 mg g−1 DM, i.e., the permissible concentration of Zn, according to the directive for feed [44,45]. The differentiation within the cultivars (more Zn in plants of the late cultivar) was probably due to the longer vegetation period of the late cultivar. To prove this, the plants should have been harvested at the same time, which could be done in another experiment.
Zn uptake and bioavailability greatly depend on soil factors. Soil pH in the experiment was above 7.0 and the soil was rich in Ca and P. It is known that a pH above 7.0, and high amounts of Ca and P, reduce Zn bioavailability [11]. Hence, the soil used in our experiment was rather advantageous to plants in terms of potential Zn toxicity, because Zn could have been partially bound in the soil. However, the results presented in Table 3 indicated that the higher the Zn content in the soil, the higher its bioavailability. It should be remembered that Zn uptake depends on the activity of ion channels and Zn transporters [13]. Plants of the same family as cabbage, with well-known response to Zn, rely on multiple copies of heavy metal transporter genes belonging to HMA4 and MTP1 [3,13].
Regarding the bioaccumulation factor (BCF), in our experiment it was approximately 0.3 for Zn0 plants of the early cultivar, which meant that Zn was accumulated mostly in the roots. The value was close to that obtained by Niedźwiecka and Zamorska-Wojdyła [45] with rapeseed plants grown around a power station that was a source of heavy metals. Similar results were also obtained by Sękara et al. [18] in white cabbage cv. ‘Krautman F1’. The value obtained by Rehman et al. [29] in Pakistan was lower (0.08), but one has to remember that BCF may depend on many environmental factors that differ in distant geographic zones. Little is known about the mechanisms of Zn accumulation and storage in the aerial parts of plants [46].
4.4. Toxicological Risk of Cabbage Consumption
Contamination of vegetables with Zn leads to potential health risks for humans in the event of their consumption [20,29,30]. Despite the fact that the cabbage plants used in our experiment were planted in a greenhouse, Daily Intake of Metal index for Zn was calculated to estimate the potential health risks associated with their consumption. As already mentioned, the Zn amount in the Zn200 cabbage heads exceeded the permissible concentration of this metal according to the directive for feed [44,45]. However, the Health Risk Index (HRI) for human adults indicated no risk.
It has to be emphasized that cabbage is not the only source of Zn in the human diet. For example, nuts and sprouts, which are popularly consumed, contain relatively large amounts of Zn [30]. Moreover, intake of Zn-containing off-the counter medications increased during the COVID-19 pandemic [47]. For this reason, undesirable, and sometimes dangerous, side-effects of Zn interaction with some medications can occur [48]. Hence, despite the low risk of the increasing amount of Zn in cabbage, it should be of concern to growers and consumers.
5. Conclusions
Our work indicated that plants of the early cultivar of cabbage showed no signs of Zn toxicity. On the other hand, the late cultivar plants experienced Zn stress, which they overcame gradually, finally producing smaller heads at a higher dose of Zn. Zn accumulation in the heads from the spiked soil did not result in toxicological risks to consumers. Although the results of our paper cannot be directly extrapolated to the growers’ fields, they bring some useful information. Firstly, due to their fast and prolonged response to Zn, the seedlings of the late cabbage can be used as biomarkers of Zn toxicity. Secondly, molecular mechanisms of Zn uptake from the soil and its redistribution within the plant are worth investigating in different cabbage genotypes. Finally, considering the risk of plant contamination with Zn, it was found to be low.
Author Contributions
Conceptualization, methodology, software, validation, formal analysis investigation resources, data curation, visualization, supervision, project administration, funding acquisition R.B.-K. and J.A; writing—original draft preparation, R.B.-K.; writing—review and editing, R.B.-K. and J.A. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the research programs of the University of Agriculture in Krakow funded by the Ministry of Science and Higher Education of the Republic of Poland and the European Regional Development Fund under the Innovative Economy Operational Programme 2007–2013, project “The exploitation of white cabbage for phytoremediation and biofumigation of soils (AGROBIOKAP)”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available by contacting the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- Barrameda-Medina, Y.; Montesinos-Pereira, D.; Romero, L.; Ruiz, J.M.; Blasco, B. Comparative study of the toxic effect of Zn in Lactuca sativa and Brassica oleracea plants: I. Growth, distribution, and accumulation of Zn, and metabolism of carboxylates. Environ. Exp. Bot. 2014, 107, 98–104. [Google Scholar] [CrossRef]
- Gupta, N.; Ram, H.; Kumar, B. Mechanism of Zinc absorption in plants: Uptake, transport, translocation and accumulation. Rev. Environ. Sci. Biotechnol. 2016, 15, 89–109. [Google Scholar] [CrossRef]
- Kicińska, A.; Smreczak, B.; Jadczyszyn, J. Soil Bioavailability of Cadmium, Lead, and Zinc in the Areas of Zn–Pb Ore Mining and Processing (Bukowno, Olkusz). J. Ecol. Eng. 2019, 20, 84–92. [Google Scholar] [CrossRef]
- Kicińska, A. Assessment of the road traffic impact on accumulation of selected elements in soils developed on Krynica and Bystrica subunit (Magura Nappe, Polish Outer Carpathians). Carpathian J. Earth Environ. Sci. 2016, 11, 245–254. [Google Scholar]
- Malinowska, E.; Jankowski, K.; Wiśniewska-Kadżajan, B.; Sosnowski, J.; Kolczarek, R.; Jankowska, J.; Ciepiela, G.A. Content of Zinc and Copper in Selected Plants Growing Along a Motorway. Bull. Environ. Contam. Toxicol. 2015, 95, 638–643. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Antonkiewicz, J.; Pełka, R.; Bik-Małodzińska, M.; Żukowska, G.; Gleń-Karolczyk, K. The effect of cellulose production waste and municipal sewage sludge on biomass and heavy metal uptake by a plant mixture. Environ. Sci. Pollut. Res. 2018, 25, 31101–31112. [Google Scholar] [CrossRef]
- White, P.J.; Pongrac, P.; Sneddon, C.C.; Thompson, J.A.; Wright, G. Limits to the Biofortification of Leafy Brassicas with Zinc. Agriculture 2018, 8, 32. [Google Scholar] [CrossRef]
- Vaillant, N.; Monnet, F.; Hitmi, A.; Sallanon, H.; Coudret, A. Comparative study of responses in four Datura species to a zinc stress. Chemosphere 2005, 59, 1005–1013. [Google Scholar] [CrossRef]
- Shi, G.R.; Cai, Q.S. Photosynthetic and anatomic responses of peanut leaves to zinc stress. Biol. Plant. 2009, 53, 391–394. [Google Scholar] [CrossRef]
- Tsonev, T.; Lidon, F. Zinc in plants—An overview. Emir. J. Food Agric. 2012, 24, 322–333. [Google Scholar]
- Mourato, M.P.; Moreira, I.N.; Leitão, I.; Pinto, F.R.; Sales, J.R.; Martins, L.L. Effect of Heavy Metals in Plants of the Genus Brassica. Int. J. Mol. Sci. 2015, 16, 17975–17998. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Garg, N. Zinc toxicity in plants: A review. Planta 2021, 253, 129. [Google Scholar] [CrossRef] [PubMed]
- Pongrac, P.; McNicol, J.W.; Lilly, A.; Thompson, J.A.; Wright, G.; Hillier, S.; White, P.J. Mineral element composition of cabbage as affected by soil type and phosphorus and zinc fertilisation. Plant Soil 2019, 434, 151–165. [Google Scholar] [CrossRef]
- Sarret, G.; Willems, G.; Isaure, M.-P.; Marcus, M.A.; Fakra, S.C.; Frérot, H.; Pairis, S.; Geoffroy, N.; Manceau, A.; Saumitou-Laprade, P. Zinc distribution and speciation in Arabidopsis halleri × Arabidopsis lyrata progenies presenting various zinc accumulation capacities. New Phytol. 2009, 184, 581–595. [Google Scholar] [CrossRef]
- Belouchrani, A.S.; Mameri, N.; Abdi, N.; Grib, H.; Lounici, H.; Drouiche, N. Phytoremediation of soil contaminated with Zn using Canola (Brassica napus L.). Ecol. Eng. 2016, 95, 43–49. [Google Scholar] [CrossRef]
- Chaudhry, H.; Nisar, N.; Mehmood, S.; Iqbal, M.; Nazir, A.; Yasir, M. Indian Mustard Brassica juncea efficiency for the accumulation, tolerance and translocation of zinc from metal contaminated soil. Biocatal. Agric. Biotechnol. 2020, 23, 101489. [Google Scholar] [CrossRef]
- Sękara, A.; Poniedziałek, M.; Ciura, J.; Jędrszczyk, E. Zinc and Copper Accumulation and Distribution in the Tissues of Nine Crops: Implications for Phytoremediation. Pol. J. Environ. Stud. 2005, 14, 829–835. [Google Scholar]
- Bączek-Kwinta, R.; Juzoń, K.; Borek, M.; Antonkiewicz, J. Photosynthetic response of cabbage in cadmium–spiked soil. Photosynthetica 2019, 57, 731–739. [Google Scholar] [CrossRef]
- Liu, C.; Xiao, R.; Huang, F.; Yang, X.; Dai, W.; Xu, M. Physiological responses and health risks of edible amaranth under simultaneous stresses of lead from soils and atmosphere. Ecotox. Environ. Saf. 2021, 223, 112543. [Google Scholar] [CrossRef]
- Bashmakov, D.I.; Lukatkin, A.S.; Anjum, N.A.; Ahmad, I.; Pereira, E. Evaluation of zinc accumulation, allocation, and tolerance in Zeamays L. seedlings: Implication for zinc phytoextraction. Environ. Sci. Pollut. Res. 2015, 22, 15443–15448. [Google Scholar] [CrossRef] [PubMed]
- Viqar-un-Nisa; Ahmed, R.; Mohammad, M. Levels of selected essential and toxic elements in the food stuffs of Islamabad as analyzed by DPASV. Toxicol. Environ. Chem. 2005, 87, 67–75. [Google Scholar] [CrossRef]
- Mishra, A.; Tripathi, B.D. Heavy metal contamination of soil, and bioaccumulation in vegetables irrigated with treated waste water in the tropical city of Varanasi, India. Toxicol. Environ. Chem. 2008, 90, 861–871. [Google Scholar] [CrossRef]
- Kazberuk, W.; Szulc, W.; Rutkowska, B. Use Bottom Sediment to Agriculture–Effect on Plant and Heavy Metal Content in Soil. Agronomy 2021, 11, 1077. [Google Scholar] [CrossRef]
- Gisbert, C.; Clemente, R.; Navarro-Aviñó, J.; Baixauli, C.; Ginér; Serrano, R.; Walker, D.J.; Bernal, M.P. Tolerance and accumulation of heavy metals by Brassicaceae species grown in contaminated soils from Mediterranean regions of Spain. Environ. Exp. Bot. 2006, 56, 19–27. [Google Scholar] [CrossRef]
- Czech, A.; Pawlik, M.; Rusinek, E. Contents of Heavy Metals, Nitrates, and Nitritesin Cabbage. Pol. J. Environ. Stud. 2012, 21, 321–329. [Google Scholar]
- Żurek, G.; Rybka, K.; Pogrzeba, M.; Krzyżak, J.; Prokopiuk, K. Chlorophyll a Fluorescence in Evaluation of the Effect of Heavy Metal Soil Contamination on Perennial Grasses. PLoS ONE 2014, 9, e91475. [Google Scholar] [CrossRef] [PubMed]
- Jan, F.A.; Ishaq, M.; Khan, S.; Ihsanullah, I.; Ahmad, I.; Shakirullah, M. A comparative study of human health risks via consumption of food crops grown on waste water irrigated soil (Peshawar) and relatively clean water irrigated soil (lower Dir). J. Hazard. Mater. 2010, 179, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Rehman, U.Z.; Khan, S.; Shah, M.T.; Brusseau, M.L.; Khan, S.A.; Mainhagu, J. Transfer of Heavy Metals from Soils to Vegetables and Associated Human Health Risks at Selected Sites in Pakistan. Pedosphere 2018, 28, 666–679. [Google Scholar] [CrossRef]
- Bączek-Kwinta, R.; Baran, A.; Simlat, M.; Lang, J.; Bieniek, M.; Florek, B. Enrichment of Different Plant Seeds with Zinc and Assessment of Health Risk of Zn-Fortified Sprouts Consumption. Agronomy 2020, 10, 937. [Google Scholar] [CrossRef]
- Babik, I.; Adamicki, F.; Dobrzański, A.J.; Nawrocki, B.; Robak, J.; Szwejda, J. Ekologiczne Metody Uprawy Kapusty—Materiały dla Rolników; Krajowe Centrum Rolnictwa Ekologicznego–Regionalne Centrum Doradztwa Rozwoju Rolnictwa I Obszarów Wiejskich w Radomiu: Radom, Poland, 2004; ISBN 83–89060–32–9. (In Polish)
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; 234p, ISBN 979–8–9862451–1–9. [Google Scholar]
- Regulation 2016. Regulation of the Minister of Environment on How to Conduct Land Surface Pollution Assessment Dated 1 September 2016. Journal of Laws of Poland, Item 1395. Available online: https://dziennikustaw.gov.pl/DU/rok/2016/pozycja/1395 (accessed on 12 September 2022). (In Polish)
- Vile, D.; Garnier, É.; Shipley, B.; Laurent, G.; Navas, M.L.; Roumet, C.; Lavorel, S.; Diaz, S.; Hodgson, J.G.; Lloret, F.; et al. Specific Leaf Area and Dry Matter Content Estimate Thickness in Laminar Leaves. Ann. Bot. 2005, 96, 1129–1136. [Google Scholar] [CrossRef]
- Bączek–Kwinta, R.; Kościelniak, J. The mitigating role of environmental factors in seedling injury and chill–dependent depression of catalase activity in maize leaves. Biol. Plant. 2009, 53, 278–284. [Google Scholar] [CrossRef]
- Yang, H.F.; Zhang, J.; Li, J.L. Physiological response to zinc pollution of rape (Brassica chinensis L.) in paddy soil ecosystem. Adv. Mater. Res. 2012, 356–360, 39–43. [Google Scholar] [CrossRef]
- Baker, A.J.M. Accumulators and excluders–strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- USEPA 2019. (United States Environmental Protection Agency). Regional Screening Levels (RSLs)–Generic Tables. Risk Based Screening Table. Composite Table: Summary Tab 0615.USEPA. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 12 September 2022).
- Poschenrieder, C.; Barceló, J. Water relations in heavy metal stressed plants. In Heavy Metal Stress in Plants, 3rd ed.; Prasad, M.N.V., Ed.; Springer: Berlin, Germany, 1999; pp. 249–270. [Google Scholar]
- Wu, F.Z.; Bao, W.K.; Li, F.L.; Wu, N. Effects of water stress and nitrogen supply on leaf gas exchange and fluorescence parameters of Sophora davidii seedlings. Photosynthetica 2008, 46, 40–48. [Google Scholar] [CrossRef]
- Stuiver, C.E.E.; Posthumus, F.S.; Parmar, S.; Shahbaz, M.; Hawkesford, M.J.; Kok, L.J.D. Zinc exposure has differential effects on uptake and metabolism of sulfur and nitrogen in Chinese cabbage. J. Plant Nutr. Soil Sci. 2014, 177, 748–757. [Google Scholar] [CrossRef]
- Ji, C.; Li, J.; Jiang, C.; Zhang, L.; Shi, L.; Xu, F.; Cai, H. Zinc and nitrogen synergistic act on root–to–shoot translocation and preferential distribution in rice. J. Adv. Res. 2021, 35, 187–198. [Google Scholar] [CrossRef]
- Milner, M.J.; Kochian, L.V. Investigating heavy–metal hyperaccumulation using Thlaspi caerulescens as a model system. Ann. Bot. 2008, 102, 3–13. [Google Scholar] [CrossRef]
- Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on Undesirable Substances in Animal Feed–Council Statement. Official Journal L140, 30/05/2002P.0010–0022. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02002L0032-20131227&from=ES (accessed on 12 September 2022).
- Niedźwiecka, A.; Zamorska–Wojdyła, D. The bioaccumulation of heavy metals in Brassica napus L. in the area around Turów Power Station, Poland. In Proceedings of the 9th Conference on Interdisciplinary Problems in Environmental Protection and Engineering EKO–DOK201. E3S Web of Conferences, Boguszow-Gorce, Poland, 23–25 April 2017; Volume 17, p. 00065. [Google Scholar]
- Ricachenevsky, F.K.; Punshon, T.; Salt, D.E.; Fett, J.P.; Guerinot, M.L. Arabidopsis thaliana zinc accumulation in leaf trichomes is correlated with zinc concentration in leaves. Sci. Rep. 2021, 11, 5278. [Google Scholar] [CrossRef]
- Szarpak, Ł.; Pruc, M.; Gasecka, A.; Jaguszewski; Michalski, T.; Peacock, F.W.; Smereka, J.; Pytkowska, K.; Filipiak, K.J. Should we supplement zinc in COVID-19 patients? Evidence from meta–analysis. Pol. Arch. Intern. Med. 2021, 131, 802–807. [Google Scholar] [CrossRef]
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).