Abstract
Nitrogen (N) deposition is known to significantly affect plant growth. Mycorrhizas play an important role in plant productivity, and plants of different mycorrhizal types respond differently to global change, which will inevitably affect plant response to N deposition. However, little is known about the differences of different mycorrhizas in biomass allocation of host plants in response to N addition. Here, a meta-analysis of data from N addition experiments was carried out to analyze the response of biomass in arbuscular mycorrhiza (AM) and ectomycorrhiza (ECM) plants to N addition. The results showed that biomass of leaf, stem, fine root (FR), and litter between AM and ECM plants responded differently to N addition (p < 0.05). Among them, biomass of leaf and stem in ECM plants (leaf: 46.89%; stem: 45.59%) was more sensitive (positively) to N addition than AM plants (leaf: 27.84%; stem: 10.30%) (p < 0.05). N addition suppressed biomass of FR in AM plants (−11.22%) but promoted that in ECM plants (13.77%). The effects on biomass also varied with different functional groups between AM and ECM plants. However, the N responses were influenced by other resources. When other treatments were added, biomass was less varied in AM plants compared to ECM plants. In addition, the N response of WB (whole biomass) and root biomass were positively correlated with annual temperature in ECM plants, but that in AM plants did not. The effects on shoot biomass in AM and ECM plants to N addition both decreased with annual temperature. The N response of root biomass increased with annual precipitation. It can be seen that different mycorrhizal types regulate the response of different plant organ biomass to N addition, which is significant for predicting ecosystem responses and feedback to environmental change.
1. Introduction
Nitrogen (N) is usually considered an important plant nutrient, which directly affects ecosystem productivity [1,2]. The growth of plants and the production of terrestrial ecosystems are highly dependent on N [1,3,4]. Numerous studies have shown that the deposition rate of N in the atmosphere tends to increase rapidly with global climate changes [5,6], which alleviates N limitation in the ecosystem, thereby affecting biogeochemical cycles such as ecosystem carbon (C) sequestration [1,7]. N addition affects the quantity and allocation of photosynthetic products in plants and promotes the accumulation of plant biomass by improving the soil nutrient status [1,8,9,10]. Meanwhile, plants change their patterns of biomass allocation to adapt to changes in the environment [10]. Drought and elevated CO2 generally enhance the biomass allocation of roots over shoots [11]. The responses of biomass exhibit great variations with different plant organs to N addition. [1,12]. Li’s study pointed out that increased precipitation and N deposition elevated the biomass allocation to shoots for increased light competition [13]. Mao’s study showed that belowground biomass (BGB) was less promoted than aboveground biomass (AGB) to N addition; specifically, leaf and stem biomass increased significantly, but not coarse root (CR) and fine root (FR) biomass [12]. At the same time, some studies have suggested that N addition also indirectly affects plant growth and the whole ecological processes on a global scale by affecting other factors, including soil pH [14], microbial community [15], the absorption of other nutrients by plants [2], soil enzyme activity [16], etc. However, the effects of different mycorrhizal types on the responses of biomass of plant organs under N addition are still unclear, and more research in this field is needed.
Mycorrhizas, as mutualistic symbionts formed by mycorrhizal fungi and plant roots, are widely distributed in various ecological environments [17]. More than 92% of plant species are associated with mycorrhiza [18]. They play crucial roles for maintain biogeochemical cycles and ecosystem functions [19] and can maintain plant diversity [20,21], change soil structure [22], regulate water uptake [23], promote plant growth [21,24,25], etc. Arbuscular mycorrhiza (AM) and ectomycorrhiza (ECM) are the two most widespread and most studied mycorrhizal types in nature [26]. Arbuscular mycorrhizal fungi form symbiotic associations with 85% of all terrestrial plants [17], while ectomycorrhizas are most symbiotic with woody plants in boreal and temperate forests [27]. According to research, different types of mycorrhizas have different ecological functions [28,29]. The study by Vargas found differences in CO2 fluxes between AM and ECM types in ecosystems [30]. Yan’s study found that the soil carbon storage of ECM type was 1.5 times that of AM type [31]. While another study has shown that the N cycling and transform process was significantly different between AM and ECM forests, and soil concentrations of soluble organic N and nitrate N in AM forests were higher than that in ECM forests [32]. At the same time, the effects of different mycorrhizal types on different plant organs were also different. The net primary productivity (NPP) of leaves of AM-dominated forest was significantly higher than in ECM-dominated forest, while the NPP of trunk and branch of ECM-dominated forest was higher than that of AM-dominated forest [24]. In addition, a number of studies demonstrated that mycorrhiza also affects plant responses to environmental change [30,31,33,34]. In the ecosystem for AM-dominated plants, CO2 flux was mainly controlled by annual precipitation, while in the ecosystem for ECM-dominated plants, CO2 flux was more affected by interannual variations in precipitation [30]. Terrer’s study pointed out that ECM plants showed a strong biomass increase in response to elevated CO2 regardless of N availability, while the biomass of AM plants was not affected by CO2 fertilization when N availability was limited. Therefore, different mycorrhizas have different ecological functions in the ecosystem and affect the response of the ecosystem to environmental change.
Nitrogen deposition is expected to continue to increase in the 21st century and affect ecosystem productivity and biomass accumulation [4,5]. Although N deposition differs from the N addition experiment in many aspects, N addition experiments are still important ways to study the effects of N deposition on plant growth [1,4,35,36,37]. Furthermore, biomass allocation has been used as a basis for understanding and predicting terrestrial carbon storage [38], but the responses of biomass of organs with different mycorrhizal types to N deposition have rarely been reported. Considering the widespread distribution and multiple ecological functions of different mycorrhizas [39], mycorrhizal type is an indispensable factor for further study on the response and change of terrestrial plant growth to N addition. Therefore, in this study, mycorrhizal types were classified to explore the response of different mycorrhizal plants and to obtain the effects of different mycorrhizal types on plant biomass allocation under N addition. Moreover, it could provide a reference for the growth and development of plants affected by different mycorrhizas under N deposition in the future. In this study, we conducted a meta-analysis to assess the effects of nitrogen addition on plant biomass and proposed hypotheses: (1) responses of plant biomass to N addition varied according to different mycorrhizal types; (2) responses of plant biomass to N addition in relation to environmental changes were different in different mycorrhizal types.
2. Materials and Methods
2.1. Data Collection
Data for this study were drawn from the open-access database of measured plant biomass in N addition experiments (Plant NE) which were collected from publications in Web of Science until 2019 [4]. We determined the mycorrhizal types of plants in the database. All plants with typical arbuscular mycorrhizal structures were of AM type, and those with typical ectomycorrhizal structures were of ECM type. The mycorrhizal types of most plant species were determined according to Averill, Wang, and Stefan’s study [40,41,42], and others were ascertained from other published literature (Table S1). Some experiments were not included in the meta-analysis if they met any of the following exclusion criteria: (1) species did not form associations with AM and ECM; (2) biomass data for the control groups and treatment groups were not provided; (3) papers did not report standard deviation and sample size in each group. In addition, all plots containing N-fixing species were not included in the main analysis because they might be particularly responsive to N addition. We analyzed the role of N-fixing species in a separate meta-analysis (whole biomass (WB), aboveground biomass (AGB), and belowground biomass (BGB)) (Figure S1). For biomass analyses, different methods of biomass estimation were accepted because we did not consider this to be a significant source of error in this analysis [43]. For the meta-analysis, we only considered AM and ECM as the only difference. On the basis of these criteria, articles reported responses of plant biomass with different mycorrhizal types to N addition. Results from other proxy variables were not included in our analysis. For example, plant height or size was not included in biomass analysis.
In total, 298 plant species, including 217 AM plant species and 53 ECM plant species, were developed. The analyses were divided according to Xu’s study [4]. Finally, our dataset included 387 observations for WB, 287 for AGB, 84 for BGB, 39 for litter, 261 for shoot, 70 for leaf, 81 for stem, 249 for root, 20 for coarse root (CR), and 80 for fine root (FR). Moreover, other treatments with N addition and climatic factors such as mean annual temperature (MAT) and mean annual precipitation (MAP)) were also collected and recorded. Based on these, a database (Table S1) containing species, mycorrhizal type, growth form, mean biomass in the control groups (Mc) and treatment groups (Mt), standard deviations of means in the control groups (SDc) and treatment groups (SDt), the sample size in the control groups (Nc) and treatment groups (Nt), other treatments and climatic factors such as MAT and MAP was established about N addition experiments.
In addition, we extracted some data into two datasets: dataset 1 included only N addition without other additions (Table S2) and dataset 2 contained studies on N addition together with other treatments (Table S3). In dataset 2, the data which the other treatments are elements needed for plant growth or have a positive effect (e.g., CO2 addition) on plants.
2.2. Data Analysis
The meta-analysis method was, according to Han’s study, to analyze the response of plant biomass to N addition [39]. The responses of AM and ECM plant biomass to N addition were assessed by the response ratio (RR):
where Mt and Mc are the plant biomass in the treatment and the control groups, the variance (vi) of each response ratio (RR) was calculated as:
where SDt and SDc are the standard deviations of means in the treatment and control groups, respectively, and Nt and Nc are the sample size in the treatment and control groups.
We calculated the weighted response ratio and 95% confidence interval (CI) using the rma.mv function in the ‘metafor’ package by R 3.6.3 [39]. If the 95% CI values did not overlap zero, the effect of N addition was considered significant (p < 0.05). To test whether the responses differed between AM and ECM plants, we used R 3.6.3 to perform between-group heterogeneity tests (QB test). When QB values were significant (p < 0.05), the responses between groups were different [39]. Figures about N addition effects on plant biomass were drawn by Graphpad Prism 8 (https://www.graphpad.com (accessed on 26 July 2022)). Next, all the data of woody and herbaceous plants were extracted and performed analysis between functional groups. Then, we observed observations of N addition with other treatments (e.g., CO2 enrichment plus N addition) and only N addition separately and analyzed N addition effects on plant biomass under other treatments. The same method was used to draw plots of the response of N addition on plant biomass variables with or without other treatments.
Then, we applied linear regression to analyze the relationships of the effect of the plant biomass to N addition with MAT and MAP. For some observations without temperature or precipitation data, linear regression analysis was not carried out. If the MAT and MAP from the database by Xu’s study showed a range [4], we calculated its means as MAT and MAP to apply linear regression. For example, if MAT = 15.00–20.00 °C, we calculated that the means of MAT was 17.5 °C and if MAP = 250–600 mm, we calculated that the means of MAP was 425 mm to apply regression. The statistical analyses were conducted by Graphpad Prism 8 (https://www.graphpad.com (accessed on 26 July 2022)). When the correlation was significant (p < 0.05), it indicated that the response ratio to N addition changed with MAT or MAP.
3. Results
3.1. Effects of N Addition on Biomass in Different Organs of AM and ECM Plants
Analysis and comparison of results showed that litter biomass differed significantly from N addition between AM and ECM plants (p < 0.001) (Figure 1). For litter, N addition had an insignificant (negative) effect on the biomass of ECM plants (−7.98%) but significantly increased the biomass of AM plants (51.68%). Moreover, N addition significantly increased whole biomass (WB), aboveground biomass (AGB), and belowground biomass (BGB) of AM and ECM plants with no significant differences between the two groups. Next, we further tested the variations of N addition effects on six indicators of AGB (shoot, leaf, and stem) and BGB (root, coarse root (CR), and fine root (FR)) (Figure 2a,b).
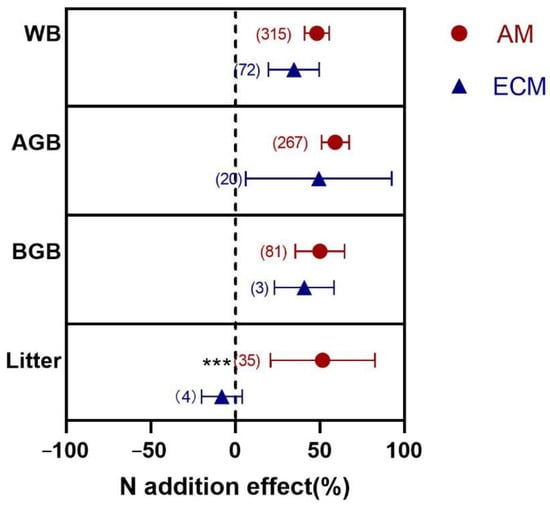
Figure 1.
Effects of N addition on WB, AGB, BGB, and litter biomass of AM and ECM plants. AM means arbuscular mycorrhizal type with typical AM structures. ECM means ectomycorrhiza type with typical ECM structures. The symbols represent the average of the 95% confidence intervals (CIs). The effect is considered to be significant when the 95% CI does not overlap with zero. The chi-square test was used to test the heterogeneity of all index effects between AM and ECM plants (*** p < 0.001). WB, whole plant biomass; AGB, aboveground biomass; BGB, belowground biomass. The numbers in parentheses represent the sample size of observations.
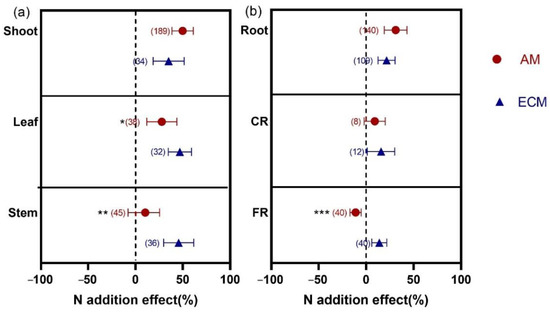
Figure 2.
Effects of N addition on biomass of shoot, leaf, and stem (a) and biomass of root, CR, and FR (b) of AM and ECM plants. AM means arbuscular mycorrhizal type with typical AM structures. ECM means ectomycorrhiza type with typical ECM structures. The symbols represent the average of the 95% confidence intervals (CIs). The effect is considered to be significant when the 95% CI does not overlap with zero. The chi-square test was used to test the heterogeneity of all index effects between AM and ECM plants (*** p < 0.001, ** p < 0.01, and * p < 0.05). CR, coarse root; FR, fine root. The numbers in parentheses represent the sample size of observations.
The N addition effects varied biomass between AM and ECM plants in aboveground organs (Figure 2a). In terms of leaf, the response of biomass to N addition was larger in ECM plants (46.89%) than in AM plants (27.84%) (p = 0.026). For stem, N addition stimulated biomass significantly in ECM plants (45.59%), with no effect in AM plants (10.30%) (p = 0.005). However, there was no significant difference in shoot biomass between AM and ECM shoots (p = 0.161).
For belowground organs, the results showed that only the response of FR biomass varied significantly to N addition between AM and ECM plants (p < 0.001). N addition significantly decreased FR biomass of AM plants (−11.22%); in contrast, the effect of FR biomass in ECM plants (13.77%) was increased. In addition, N addition significantly increased root biomass in AM and ECM by 31.02% and 21.09%, respectively. For CR, N addition increased the biomass in ECM plants (15.65%) but not in AM plants (9%), with no significant differences between them (p = 0.791).
3.2. Effects of N Addition on Biomass of AM and ECM Plants with Functional Groups
N addition had positive effects on WB, AGB, and BGB in AM and ECM woody plants (Figure 3a). Among them, the increase in BGB in AM plants (124.94%) was significantly higher than that in ECM plants (40.76%) (p = 0.016). There were no changes in litter biomass of AM and ECM woody plants. Further analysis of organs (Figure 3b), AM woody plants showed a significantly greater response (68.32%) than ECM woody plants (35.05%) in shoot (p = 0.006). For leaf, the response of biomass to N addition was larger in ECM woody plants (46.89%) than AM woody plants (26.91%) (p < 0.001). There were no significant differences in stem and root responses between AM and ECM plants. However, the effects of N addition on FR biomass in ECM woody plants were slightly promoted (13.77%), but N addition suppressed FR biomass in AM woody plants (−10.82%).
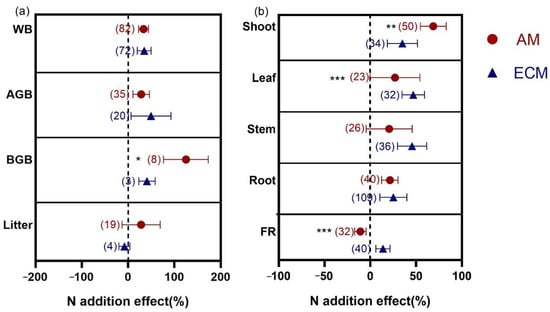
Figure 3.
Effects of N addition on WB, AGB, BGB and litter biomass (a) and biomass of shoot, leaf, stem, root and FR (b) in AM and ECM woody plants. AM means arbuscular mycorrhizal type with typical AM structures. ECM means ectomycorrhiza type with typical ECM structures. The symbols represent the average of the 95% confidence intervals (CIs). The effect is considered to be significant when the 95% CI does not overlap with zero. The chi-square test was used to test the heterogeneity of all index effects between AM and ECM plants (*** p < 0.001, ** p < 0.01, and * p < 0.05). WB, whole plant biomass; AGB, aboveground biomass; BGB, belowground biomass; FR, fine root. The numbers in parentheses represent the sample size of observations.
The responses of biomass to N addition were also different between AM woody and herbaceous plants (Figure 4). The responses of WB and AGB in herbaceous plants increased more than in woody plants to N addition, while BGB in woody plants (124.94%) promoted more than that in herbaceous plants (38.26%). For organs (Figure 4b), N addition stimulated more shoot biomass in woody plants (68.72%) than in herbaceous plants (41.97%). The responses of biomass in other organs were not significant differences between woody and herbaceous plants.
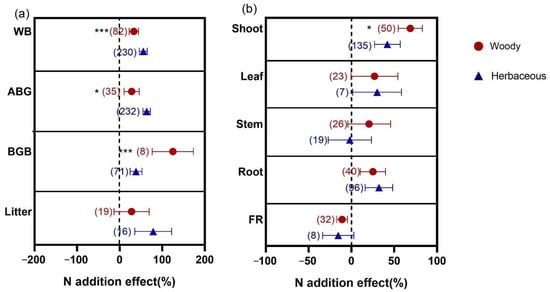
Figure 4.
Effects of N addition on WB, AGB, BGB and litter biomass (a) and biomass of shoot, leaf, stem, root and FR (b) in Woody and Herbaceous in AM. AM means arbuscular mycorrhizal type with typical AM structures. The symbols represent the average of the 95% confidence intervals (CIs). The effect is considered to be significant when the 95% CI does not overlap with zero. The chi-square test was used to test the heterogeneity of all index effects between AM and ECM plants (*** p < 0.001 and * p < 0.05). WB, whole plant biomass; AGB, aboveground biomass; BGB, belowground biomass. FR, fine root. The number in parentheses represent the sample size of observations.
3.3. Effects of N Addition on Biomass of AM and ECM Plants under Other Treatments
Terrestrial plants are under the influence of other treatments in addition to N availability. We separately detected differences in the response of plant biomass between AM and ECM plants under only N addition and N addition with other treatments (Figure 5). When there was only N addition, the response of WB in ECM plants was significantly improved (49.39%), while no effect was observed in AM plants (12.32%) (p < 0.001). To be further analyzed, a greater increase in AGB in ECM plants (123.27%) than in AM plants (59.82%), and N addition stimulated the FR biomass in ECM plants (15.8%) but suppressed that in AM plants (−11.22%) (p < 0.001). However, there were no differences between AM and ECM plants in shoot, leaf, stem, and root biomass when only N addition.
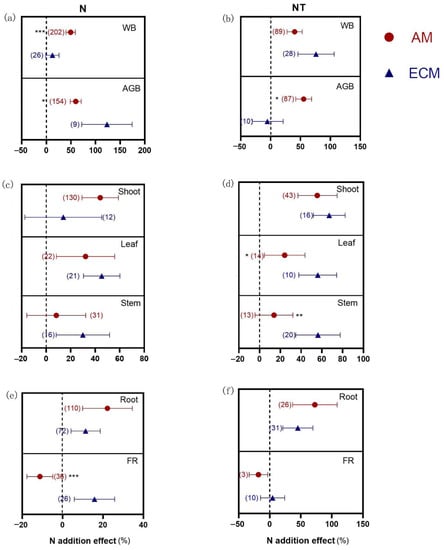
Figure 5.
N addition effects on biomass of AM and ECM plants in response to N addition under added N alone (a,c,e) and added N together with other treatments (b,d,f). AM means arbuscular mycorrhizal type with typical AM structures. ECM means ectomycorrhiza type with typical ECM structures. N means only N addition. NT means N addition with other treatments. The symbols represent the average of the 95% confidence intervals (CIs). The effect is considered to be significant when the 95% CI does not overlap with zero. The chi-square test was used to test the heterogeneity of all index effects between AM and ECM plants (*** p < 0.001, ** p < 0.01, and * p < 0.05). WB, whole plant biomass; AGB, aboveground biomass; FR, fine root. The numbers in parentheses represent the sample size of observations.
When N with other treatments was added, AGB in AM plants was significantly increased by 55.39%, but that in ECM plants was not. Furthermore, the leaf biomass of ECM plants showed a significantly greater response (56.07%) than that of AM plants (24.20%) (p = 0.016). Under other treatments, N addition also improved stem biomass in ECM plants (56.29%), while stem biomass in AM plants was not affected (11.88%) (p = 0.002). The WB and biomass of root and FR were not differently varied between AM and ECM plants to N addition with other treatments.
3.4. Climatic Factors Influencing the Responses of AM and ECM Plants to N Addition
The response ratio in AGB of AM and ECM plants did not exhibit any significant trend with MAT (p > 0.5) (Figure 6b). However, the response ratio in WB of ECM plants significantly increased with MAT (Figure 6a). Furthermore, the response ratio of shoot biomass decreased significantly with MAT, with a higher slope in AM plants (r2 = 0.5978) than in ECM plants (r2 = 0.1997). When root was considered, only the response of biomass in ECM plants increased significantly as MAT increased (Figure 6f). There were no regressions between the response ratios of biomass of leaf, stem, and FR and MAT.
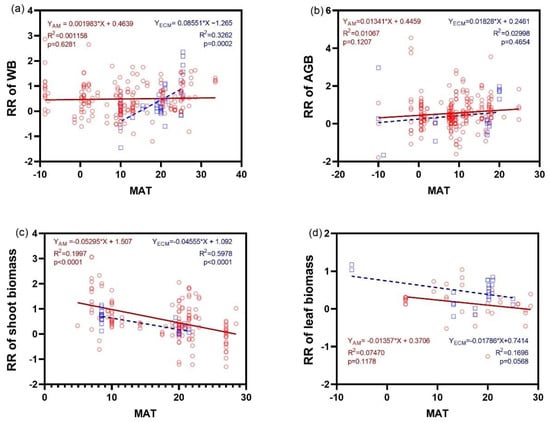
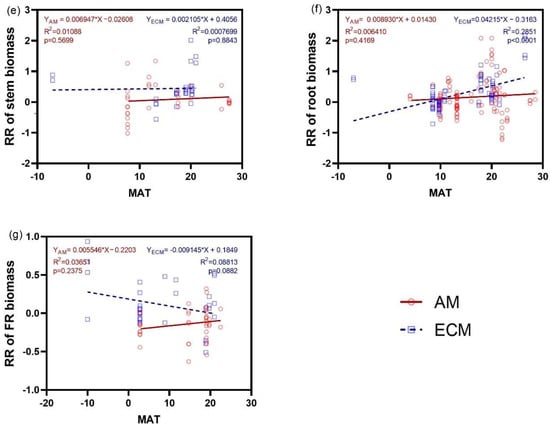
Figure 6.
Relationship between the response ratio (RR) of WB (a), AGB (b), the biomass of shoot (c), leaf (d), stem (e), root (f), and FR (g) and changes of MAT (Mean annual temperature) under N addition between AM and ECM plants. Each point in the figure represents a different observation. AM, arbuscular mycorrhiza; ECM, ectomycorrhiza; WB, whole plant biomass; AGB, aboveground biomass; FR, fine root.
It can be concluded from the response ratio of AM and ECM plants to N addition with MAP changes that only the response ratio (positive) of root biomass in ECM plants was related to the MAP (r2 = 0.2396) (Figure 7c). In addition, no matter AM or ECM plants, there were no regressions between the response ratios of WB and AGB and FR biomass to N addition and MAP (Figure 5a,b,d).
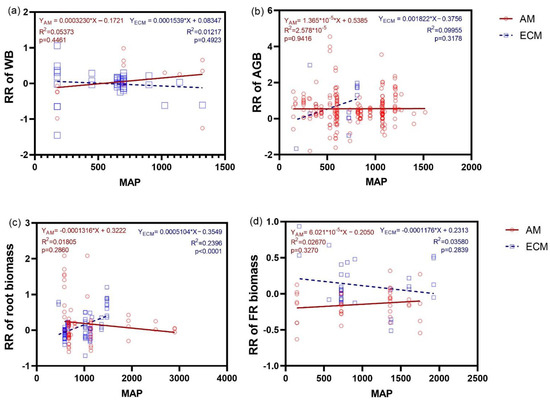
Figure 7.
Relationship between the ratio response (RR) of WB (a), AGB (b), the biomass of root (c) and FR (d), and changes of MAP (Mean annual precipitation) under N addition between AM and ECM plants. Each point in the figure represents a different observation. AM, arbuscular mycorrhiza; ECM, ectomycorrhiza; WB, whole plant biomass; AGB, aboveground biomass; FR, fine root.
4. Discussion
Increased atmospheric nitrogen deposition is an important component of global change, which has an important impact on plant growth and has been widely studied [4,5,6,12]. Mycorrhizas are widespread in various ecological environments, which should not be ignored. Our results showed that the responses of organ (leaf, stem, and fine root (FR)) biomass to N addition were different between AM and ECM plants, and the biomass was more increased in ECM plants than in AM plants. The effects on biomass also varied between woody and herbaceous plants. In addition, responses of plant biomass to N addition in relation to other treated and climatic changes were different between AM and ECM plants. The significance of this study is to reveal the biomass allocation to N addition under different mycorrhizal types. The findings in this study provide new research ideas and perspectives for terrestrial plant biomass changes under N deposition and are valuable for predicting ecosystem responses and feedback to environmental change.
It is illustrated from previous studies that mycorrhizas play an important role in plant growth and development [21,24,25,44]. In addition, the role of mycorrhizas varies with the type of mycorrhizas [29], and the response of plants to global changes also varies with different mycorrhizal types [30,31,34]. Our study showed that plants with different mycorrhizal types responded differently to N addition (Figure 2a,b). After N addition, the aboveground and belowground biomass of ECM plants all increased significantly, but that of AM plants did not. It can be seen that ECM plants are more sensitive to N addition than AM plants. One reason may be related to mycorrhizal ecological functions. Ectomycorrhiza plants allocate more C to support mycorrhizas than AM plants, and ECM has a great ability to mobilize and capture N [27]. Furthermore, ECM fungi produce lignocellulolytic enzymes to degrade soil organic matter, and transfer N from degraded soil organic matter to host plants with N needed to sustain their growth, but AM fungi do not [27,29]. Secondly, ECM plants are mainly found in N-deficient environments, while AM plants exist in areas that P is deficient, but N is abundant [17]. Therefore, ECM plants uptake more N after N addition than AM plants. Moreover, the external environment may also affect the response of different mycorrhizal plants to N addition. However, on a macro level (whole biomass (WB), aboveground biomass (AGB), and belowground biomass (BGB)), the responses of biomass showed no significant difference in N addition between AM and ECM plants (Figure 1). N addition had a significant positive effect on plant biomass, whether it was WB, AGB, or BGB (Figure 1), which is consistent with the findings of Mao’s study [12]. This indicated that the differences of mycorrhizal types (AM and ECM) did not or less lead to differences in biomass allocation of AM and ECM plants to N addition from a macro perspective.
Nitrogen addition increased AGB and BGB in AM and ECM plants; litter input also increased accordingly [45]. However, in this study, the biomass of ECM plants was almost unaffected by N addition (Figure 1). It may be related to litter decomposition. When N was limited, some ECM fungi can directly obtain organic N from soil organic matter, becoming a strong competitor as saprophytic microorganisms [28,46]. It reduces saprophytic fungi activity and the rate of litter decomposition [47]. N addition improved microbial nutrient utilization efficiency [48], accelerating the decomposition of ECM litter. The differences in growth forms between AM and ECM plants may also result in the different responses of litter biomass in AM and ECM plants to N addition. AM fungi are symbiotic with plants of various growth forms, including woody plants [49], while ECM is mostly symbiotic with woody plants. In our study, N addition did not affect the litter biomass on woody plants regardless of mycorrhizal types (AM and ECM) (Figure 3a). However, N addition increased litter biomass in AM herbaceous plants (Figure 4a). We boldly speculated that the litter biomass was increased in AM plants mainly due to the litter biomass of herbaceous plants in AM plants rather than woody plants. The specific demonstration needs to be further studied.
We noted that plant functional types it was one of the factors that influenced the response of plants to N addition in AM and ECM plants. AM fungi are symbiotic with herbaceous and woody plants [49], while ECM is rarely symbiotic with herbaceous plants and more symbiotic with woody plants. Although the response of BGB in AM woody plants was greater than that in ECM woody plants (Figure 3a), there was no difference in N addition in AM and ECM plants for BGB (Figure 1). This was due to the relatively smaller increase in N addition in AM herbaceous plants (Figure 4a), which maybe because herbaceous species are smaller in stature than woody plants [43] and don’t need to provide more biomass for belowground organs (especially CR) to support the plants. The specific reasons need to be further explored. In addition, our finding of greater WB and AGB responses in AM herbaceous plants than in AM woody plants was consistent with Xia’s study [43]. Mao’s study also confirmed that the increase in biomass in grasslands due to N deposition was significantly greater than in forests [12].
Where biological processes in many ecosystems were once limited by N, plant growth is more limited by other resources as N is added [50]. Many other resources can act by moderating or exacerbating N deposition effects on host C fixation and belowground C flux (CO2, warm) or by altering soil resource availability (warm, H2O) [51]. Our study found that other resources also influence the response of different mycorrhizal types to N addition (Figure 5) which was consistent with Terrer’s study [27]. In this study, for WB, AM plants had no significant difference in response to N addition with or without other treatments, while ECM plants did, and the WB increases of ECM plants were not significant when only N was added (Figure 5a). These results indicated that other treatments had a greater impact on the response of ECM plants to N addition, while AM plants had a more stable response to other treatments. It may be due to the different mycorrhizal plants responding differently to nutrient uptake. In general, AM plants show better performance due to enhanced uptake of mineral nutrients [18]. The symbiotic associations between AM fungi and plants can promote nutrient absorption by host plants [17]. Mycorrhizal fungal hyphae can obtain mineral nutrients by exploring soil volume and providing these nutrients to plants in return for carbon [18], so plants need relatively little from the outside. To further analyzed the changes of the AGB under the same conditions, we found that AGB in AM and ECM plants were significantly different from N addition with or without other treatments (Figure 5b). It was further verified that compared with AM plants, ECM plants were more sensitive in response to other treatments under N addition. For belowground organs, FR biomass of AM and ECM plants was significantly different when only N was added (Figure 5e), which was in line with Jia’s study [52]. It may be because different mycorrhiza adopted different adaptation strategies to the change of soil N availability. Jia’s study showed that after N addition, AM plants did not change FR morphological traits through the trade-off between nutrient absorption efficiency and root construction cost and that nutrient acquisition depended more on the complementarity between AM fungi and FR architecture [52]. However, the FR of ECM plants relies on the FR to improve nutrient absorption efficiency, spatial expansion, and in situ nutrient absorption capacity, and rapid nutrient absorption is the main strategy [52]. Thus, it is implied that mycorrhizas play an important role in regulating plant biomass response to N deposition and adopt different mycorrhizal strategies for other treatments.
Our results suggested that the response of WB in ECM plants to N addition increased with the increase in mean annual temperature (MAT) (Figure 6a). This may be due to the increased response of root biomass in ECM plants to N addition with increasing temperature (Figure 6f). It suggested limitations of low temperature rather than the high temperature on plant growth and its N response across the globe, which was consistent with Xia’s study [43]. Furthermore, with the increase in MAT, the response of shoot biomass to N addition decreased whether AM or ECM plants. It indicated ECM plant changed its allocation strategy with the increase in MAT, in which the increased biomass was more allocated from aboveground organ to belowground organ. Under low temperatures, nutrient availability in the soil was low. Because low temperature usually mitigates the mineralization and decomposition of organic matter, resulting in less nitrogen and phosphorus in the soil [53]. N addition increases N availability in soil, allowing plants to allocate more biomass to aboveground organs [12]. With the increase in temperature, nutrient availability increased, and the whole biomass also accordingly improved. The huge increase in biomass requires a larger root system to support the plants, leading to increases in root biomass [54]. Furthermore, the responses of the N addition effect between AM and ECM plants to N addition were different with MAT changes. It may be related to the different responses of different mycorrhizal plants to temperature, and different mycorrhizal types have different effects on nutrient uptake and utilization of host plants [33]. For example, our results indicated that the organ biomass of ECM plants responded more strongly to N addition than to AM plants. Previous studies have shown that AM mainly improves P nutrition in plants [17], while ECM is beneficial to N absorption [55].
The linear increases in the responses of root biomass in ECM plants to N addition along the precipitation gradient (Figure 7c). With low annual precipitation, the positive responses of plant growth to N addition were likely to be suppressed by water limitation [43]. Plants often show greater stimulation of biomass production in response to increased soil N availability when water availability is not a limiting factor [43]. In addition, the responses of the N addition effect between AM and ECM plants were different with MAP changes. It may be that different mycorrhizal plants have different responses to precipitation [34] and N addition. Different mycorrhizas have different abilities to improve the water status of plants, and AM can increase water uptake by expanding absorption area and also increase stomatal conductance by improving phosphorus nutrient status [56], while ECM not only expands absorption area but also increases aquaporin gene expression [57,58,59].
5. Conclusions
This study showed that mycorrhizal types modulated the response of plant organ biomass to N addition, and different plant mycorrhizal types induced different responses to N addition. The biomass of ECM plants was more sensitive to N addition than AM plants in leaf and stem. The effects on biomass also varied with different functional groups between AM and ECM plants. We further explored the relationship between environmental changes and the responses of different mycorrhizal plants to nitrogen; it demonstrated that the response of N addition effects to other treatments was also different with different mycorrhizal types, and biomass was less varied in AM plants compared to ECM plants. Furthermore, the response ratio to N addition in different mycorrhizal plants to climate change was also different. These findings partly explain past findings of why some plants respond differently to N addition, providing new research ideas and perspectives for terrestrial plant biomass changes under N deposition. This study illustrates again the importance of mycorrhizal types in ecosystems and provides theoretical guidance and a scientific basis for projecting vegetation and ecosystem responses and feedback to environmental change.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12102357/s1, Table S1: Plant biomass with N addition experiences-related variable and mycorrhizal type. Table S2: Dataset 1: N addition without other additions. Table S3: Dataset 2: N addition with other treatments. Table S4: Introduction—Table S1. Table S5: Abbreviation. Figure S1: N addition effects on biomass of N-fixing species in response to N addition.
Author Contributions
M.L. and Z.S.: conceptualization, validation, writing—review and editing, and visualization. M.L.: methodology and writing—original draft preparation. Z.S.: software, investigation, data curation, supervision, project administration, and funding acquisition. M.L., S.Y., M.Z. (Menghan Zhang), S.W. and M.Z. (Mengge Zhang): resources. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NSFC (32171620, 31670499).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
References
- Chen, J.; Dong, C.; Yao, X.; Wang, W. Effects of nitrogen addition on plant biomass and tissue elemental content in different degradation stages of temperate steppe in northern China. J. Plant Ecol. 2018, 11, 730–739. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Sun, W.; Shen, Y.; Ren, T.; Huang, J.; Wang, C. Effects of different forms and levels of N additions on soi potential net N mineralization rate in meadow steppe. Chin. J. Plant Ecol. 2019, 43, 174–184. [Google Scholar]
- Wang, J.; Wang, S.; Li, R.; Yan, J.; Sha, L.; Han, S. C:N:P stoichiometric characteristics of four forest types’ dominant tree species in China. Chin. J. Plant Ecol. 2011, 35, 587–595. [Google Scholar]
- Xu, X.; Lu, R.; Yan, L.; Xia, J. PlantNE: A global database of plant biomass from nitrogen-addition experiments. Ecol. Appl. EA 2019, 100, e02840-1. [Google Scholar]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef]
- Liu, X.; Duan, L.; Mo, J.; Du, E.; Shen, J.; Lu, X.; Zhang, Y.; Zhou, X.; He, C.; Zhang, F. Nitrogen deposition and its ecological impact in China: An overview. Environ. Pollut. 2011, 159, 2251–2264. [Google Scholar] [CrossRef]
- Thomas, D.C.; Zak, D.R.; Filley, T.R. Chronic N deposition does not apparently alter the biochemical composition of forest floor and soil organic matter. Soil Boil. Biochem. 2012, 54, 7–13. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Wang, Z.; Wu, X.; Huang, R.; Zhu, J. Photosynthetic characteristics, biomass allocation, C, N and P distribution of Schima superba seedlings in response to simulated nitrogen deposition. Acta Ecol. Sin. 2013, 33, 1569–1577. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, J.; Wang, G.; Yang, L.; Yang, Y. Effects of drought and nitrogen addition on photosynthetic characteristics and resource allocation of Abies fabri seedlings in eastern Tibetan Plateau. New For. 2011, 43, 505–518. [Google Scholar] [CrossRef]
- Fang, Y.; Zou, X.; Lie, Z.; Xue, L. Variation in Organ Biomass with Changing Climate and Forest Characteristics across Chinese Forests. Forests 2018, 9, 521. [Google Scholar] [CrossRef]
- Anwar, E.; Zhengbing, Y.; Di, T.; Wenxuan, H.; Zhiyao, T.; Jingyun, F. Drought effect on plant biomass allocation: A meta-analysis. Ecol. Evol. 2017, 7, 11002–11010. [Google Scholar]
- Mao, J.; Xing, Y.; Yan, G.; Wang, Q. A meta-analysis of the response of terrestrial plant biomass allocation to simulated N deposition. Acta Ecol. Sin. 2018, 38, 3183–3194. [Google Scholar]
- Li, C.; Zheng, Z.; Peng, Y.; Nie, X.; Yang, L.; Xiao, Y.; Zhou, G. Precipitation and nitrogen addition enhance biomass allocation to aboveground in an alpine steppe. Ecol. Evol. 2019, 9, 12193–12201. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Rui, J.; Li, J.; Dai, Y.; Bai, Y.; Heděnec, P.; Wang, J.; Zhang, S.; Pei, K.; Liu, C.; et al. Rate-specific responses of prokaryotic diversity and structure to nitrogen deposition in the Leymus chinensis steppe. Soil Boil. Biochem. 2014, 79, 81–90. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; Zhong, H.; Chen, X.; Sui, X. Effects of simulated nitrogen deposition on the soil microbial community diversity of a Deyeuxia angustifolia wetland in the Sanjiang Plain, Northeastern China. Ann. Microbiol. 2022, 72, 11. [Google Scholar] [CrossRef]
- Paolo, Z.; Dolores, A.; Jordi, S.; Romà, O.; Josep, P. Changes in soil enzymatic activity in a P-limited Mediterranean shrubland subject to experimental nitrogen deposition. Appl. Soil Ecol. 2021, 168, 104159. [Google Scholar]
- Smith, S.E.; Read, F.D. Mycorrhizal Symbiosis, 3rd ed.; Elsevier Ltd.: London, UK, 2008; pp. 1–787. [Google Scholar]
- Yang, S.; Shi, Z.; Zhang, M.; Li, Y.; Gao, J.; Wang, X.; Liu, D. Stoichiometry of Carbon, Nitrogen and Phosphorus in Shrub Organs Linked Closely With Mycorrhizal Strategy in Northern China. Front. Plant Sci. 2021, 12, 687347. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.-A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef]
- Bever, J.D.; Dickie, I.A.; Facelli, E.; Facelli, J.M.; Klironomos, J.; Moora, M.; Rillig, M.C.; Stock, W.D.; Tibbett, M.; Zobel, M. Rooting theories of plant community ecology in microbial interactions. Trends Ecol. Evol. 2010, 25, 468–478. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Klironomos, J.N.; Margot, U.; Peter, M.; Ruth, S.-E.; Thomas, B.; Andres, W.; Sanders, I.R. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 1998, 396, 69–72. [Google Scholar] [CrossRef]
- Rillig Matthias, C.; Mummey Daniel, L. Mycorrhizas and soil structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Augé, R.M. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, D.; Wang, F.; Ding, X. Effect of mycorrhizal strategy on net primary productivity of trees in global forest ecosystem. Ecol. Environ. Sci. 2012, 21, 404–408. [Google Scholar]
- Zhang, Y.; Li, Y.; Chen, X.; Guo, S.; Lee, Y.-I. Effect of different mycobionts on symbiotic germination and seedling growth of Dendrobium officinale, an important medicinal orchid. Bot. Stud. 2020, 61, 2. [Google Scholar] [CrossRef] [PubMed]
- Kilpeläinen, J.; Aphalo, P.J.; Barbero-López, A.; Adamczyk, B.; Nipu, S.A.; Lehto, T. Are arbuscular-mycorrhizal Alnus incana seedlings more resistant to drought than ectomycorrhizal and nonmycorrhizal ones? Tree Physiol. 2020, 40, 782–795. [Google Scholar] [CrossRef]
- Terrer, C.; Vicca, S.; Hungate, B.A.; Phillips, R.P.; Prentice, I.C. Mycorrhizl association as a primary control of the CO2 fertilization effect. Science 2016, 353, 72–74. [Google Scholar] [CrossRef]
- Duan, J.; Zhang, Y.; Hao, L.; Wang, Q.; Yan, L.; He, R. Acting Mechanisms of AMF and EMF Regulating Litter Decomposition. World For. Res. 2022, 35, 21–27. [Google Scholar]
- Yan, G.; Lu, J.; Qiu, L.; Huang, M.; Xing, Y.; Wang, Q. Mycorrhizal characteristics of AM and ECM and their response to environmental changes. J. Qufu Norm Univ. 2022, 48, 106–112. [Google Scholar]
- Vargas, R.; Baldocchi, D.D.; Querejeta, J.I.; Curtis, P.S.; Hasselquist, N.J.; Janssens, I.A.; Allen, M.F.; Montagnani, L. Ecosystem CO2 fluxes of arbuscular and ectomycorrhizal dominated vegetation types are differentially influenced by precipitation and temperature. New Phytol. 2010, 185, 226–236. [Google Scholar] [CrossRef]
- Lu, S.; Shi, Z.; Zhang, M.; Yang, M.; Wang, X.; Xu, X. Discrepancies of Leaf Ash Concentration in Arbuscular and Etco-mycorrhizal Different Plants Associated Arbuscular and Etco-mycorrhizas and Their Response to Climate Change. Ecol. Environ. Sci. 2020, 29, 35–40. [Google Scholar]
- Chen, L.; Cai, Y.; Lei, H.; Qi, X.; Lin, J.; Liao, W.; Huang, Z. Comparison of soil nitrogen cycling between arbuscular mycorrhizal and ectomycorrhizal forests in a subtropical region. Chin. J. Ecol. 2022, 41, 218–226. [Google Scholar]
- Shi, Z.; Wang, F.; Miao, Y. Responses of net primary productivity to air temperature change in forests dominated by different mycorrhizal strategies. Chin. J. Plant Ecol. 2012, 36, 1165–1171. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, K.; Miao, Y.; Wang, F. Responses of Net Primary Productivity to Precipitation in Forests Dominated by Different Mycorrhizal Types. Bull. Soil Water Conser. 2014, 34, 14–19. [Google Scholar]
- Fu, G.; Shen, Z. Response of Alpine Plants to Nitrogen Addition on the Tibetan Plateau: A Meta-analysis. J. Plant Growth Regul. 2016, 35, 974–979. [Google Scholar] [CrossRef]
- Ren, H.; Xu, Z.; Huang, J.; Clark, C.; Chen, S.; Han, X. Nitrogen and water addition reduce leaf longevity of steppe species. Ann. Bot. 2011, 107, 145–155. [Google Scholar] [CrossRef]
- Li, J.; Lin, S.; Taube, F.; Pan, Q.; Dittert, K. Above and belowground net primary productivity of grassland influenced by supplemental water and nitrogen in Inner Mongolia. Plant Soil 2011, 340, 253–264. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, X.; He, Y.; Fu, Y.; Du, Z.; Lu, M.; Sun, X.; Li, C.; Lu, C.; Liu, R.; et al. Global systematic review with meta-analysis shows that warming effects on terrestrial plant biomass allocation are influenced by precipitation and mycorrhizal association. Nat. Commun. 2022, 13, 4914. [Google Scholar] [CrossRef]
- Han, Y.; Feng, J.; Han, M.; Zhu, B. Responses of arbuscular mycorrhizal fungi to nitrogen addition: A meta-analysis. Glob. Chang. Biol. 2020, 26, 7229–7241. [Google Scholar] [CrossRef]
- Averill, C.; Bhatnagar Jennifer, M.; Dietze Michael, C.; Pearse William, D.; Kivlin Stephanie, N. Global imprint of mycorrhizal fungi on whole-plant nutrient economics. Proc. Natl. Acad. Sci. USA 2019, 116, 23163–23168. [Google Scholar] [CrossRef]
- Wang, B.; Qiu, Y. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef]
- Stefan, H.; Lars, G.; Ingolf, K.; Michalski Stefan, G.; Rillig Matthias, C.; Martin, Z.; Mari, M. Mycorrhizas in the Central European flora: Relationships with plant life history traits and ecology. Ecology 2013, 94, 1389–1399. [Google Scholar]
- Xia, J.; Wan, S. Global response patterns of terrestrial plant species to nitrogen addition. New Phytol. 2008, 179, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Shi, Z.; Sun, Y.; Wang, X.; Yang, W.; Gao, J.; Wang, X. Stoichiometric Ratios of Carbon, Nitrogen and Phosphorus of Shrub Organs Vary with Mycorrhizal Type. Agriculture 2022, 12, 1061. [Google Scholar] [CrossRef]
- Liu, L.; Greaver, T.L. A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecol. Lett. 2010, 13, 819–828. [Google Scholar] [CrossRef]
- Firoz, S.; César, N.; Johan, B.; Magnus, E.; Mark, S.; Francois, R.; Björn, C.; Dimitrios, F.; Robert, C.; Gerald, L.; et al. Ectomycorrhizal fungi decompose soil organic matter using oxidative mechanisms adapted from saprotrophic ancestors. New Phytol. 2016, 209, 1705–1719. [Google Scholar]
- Bödeker, I.T.M.; Lindahl, B.D.; Olson, Å.; Clemmensen, K.E. Mycorrhizal and saprotrophic fungal guilds compete for the same organic substrates but affect decomposition differently. Funct. Ecol. 2016, 30, 1967–1978. [Google Scholar] [CrossRef]
- Manzoni, S.; Taylor, P.; Richter, A.; Porporato, A.; Agren, G.I. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 2012, 196, 79–91. [Google Scholar] [CrossRef]
- Hao, W.; Ai-Jiao, W.; Liu, B.-X.; Liu, R.-J.; Chen, Y.-L. Interactions between mycorrhizal fungal diversity and plant diversity: A review. Microbiol. China 2020, 47, 3918–3932. [Google Scholar]
- Vitousek, P.M.; Aber, J.D.; Howarth, R.W.; Likens, G.E.; Matson, P.A.; Schindler, D.W.; Schlesinger, W.H.; Tilman, D.G. Human Alteration of The Global Nitrogen Cycle—Sources And Consequences. Ecol. Appl. 1997, 7, 737–750. [Google Scholar] [CrossRef]
- Lilleskov, E.A.; Kuyper, T.W.; Bidartondo, M.I.; Hobbie, E.A. Atmospheric nitrogen deposition impacts on the structure and function of forest mycorrhizal communities: A review. Environ. Pollut. 2019, 246, 148–162. [Google Scholar] [CrossRef]
- Jia, L.; Chen, G.; Zhang, L.; Chen, T.; Jiang, Q.; Chen, Y.; Fan, A.; Wang, X. Plastic responses of fine root morphology and architecture traits to nitrogen addition in ecto-mycorrhizal and arbuscular mycorrhizal tree species in an evergreen broadleaved forest. Chin. J. Appl. Ecol. 2021, 32, 529–537. [Google Scholar]
- Liu, S.; Wang, H. N, P, and K characteristics of different age groups of temperate coniferous tree species in northwestern China. J. For. Res. 2018, 29, 471–478. [Google Scholar] [CrossRef]
- Li, X.J.; Liu, X.F.; Lin, C.F.; Chen, S.D.; Xiong, D.C.; Lin, W.S.; Xu, C.; Xie, J.S.; Yang, Y.S. Effects of experimental soil warming on plant biomass allocation during the early stages of succession in a subtropical forest in China. Acta Ecol. Sin. 2017, 37, 25–34. [Google Scholar]
- Read, D.J. Mycorrhizas in ecosystems. Experientia 1991, 47, 376–391. [Google Scholar] [CrossRef]
- Fitter, A.H. Water Relations of Red Clover Trifolium pratense L. as Affected by VA Mycorrhizal Infection and Phosphorus Supply Before and During Drought. J. Exp. Bot. 1988, 39, 595–603. [Google Scholar] [CrossRef]
- Querejeta, J.I.; Egerton-Warburton, L.M.; Allen Michael, F. Direct nocturnal water transfer from oaks to their mycorrhizal symbionts during severe soil drying. Oecologia 2003, 134, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Marjanović, Ž.; Uehlein, N.; Kaldenhoff, R.; Zwiazek, J.J.; Weiß, M.; Hampp, R.; Nehls, U. Aquaporins in poplar: What a difference a symbiont makes! Planta 2005, 222, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Marjanovi, Z.; Uwe, N.; Hampp, R. Mycorrhiza Formation Enhances Adaptive Response of Hybrid Poplar to Drought. Ann. N. Y. Acad. Sci. 2005, 1048, 496–499. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).