Vine Physiology, Yield Parameters and Berry Composition of Sangiovese Grape under Two Different Canopy Shapes and Irrigation Regimes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Physiological Measurements, Vegetative Evaluation and Yield Data
2.3. Must Analyses and Anthocyanin, Flavonols and Stilbenes Separation via HPLC
2.4. RNA Extraction and Gene Expression Analysis
2.5. Statistical Analysis
3. Results
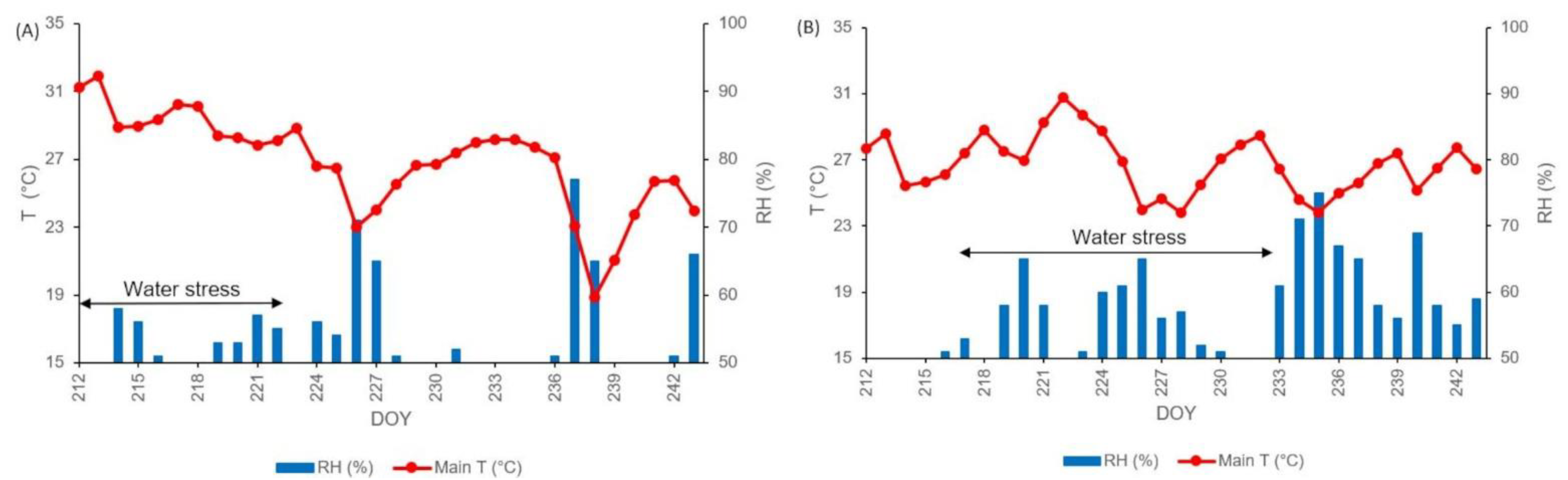
3.1. Weather Conditions Recorded during the Years of Study and during the Water Stress Treatment
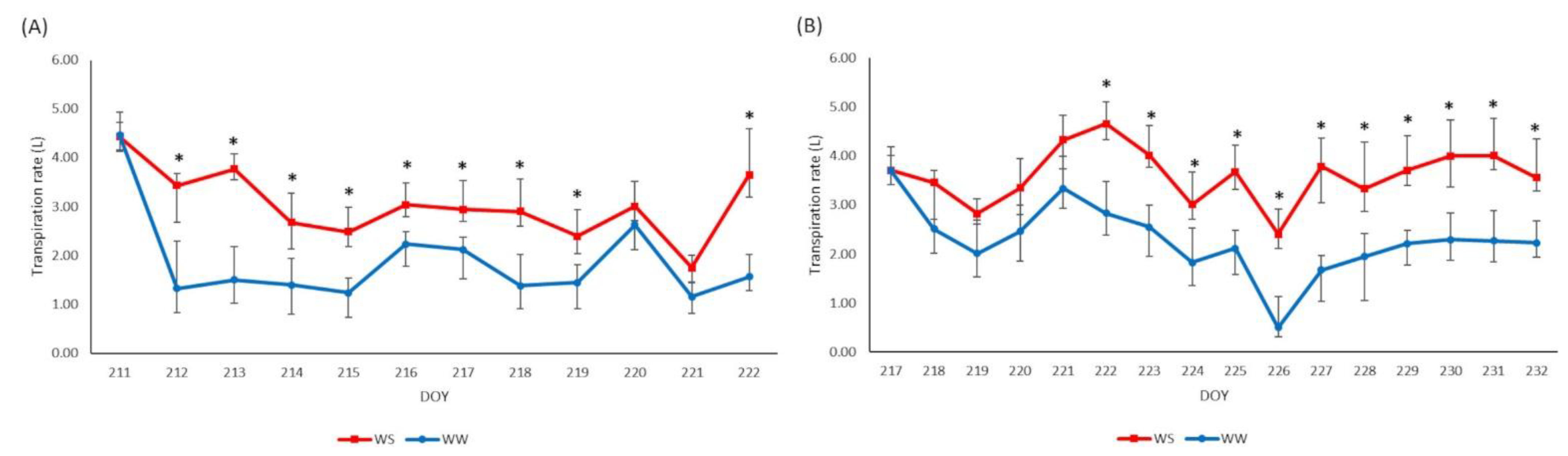
3.2. Physiological Analyses and Yield Data at Harvest
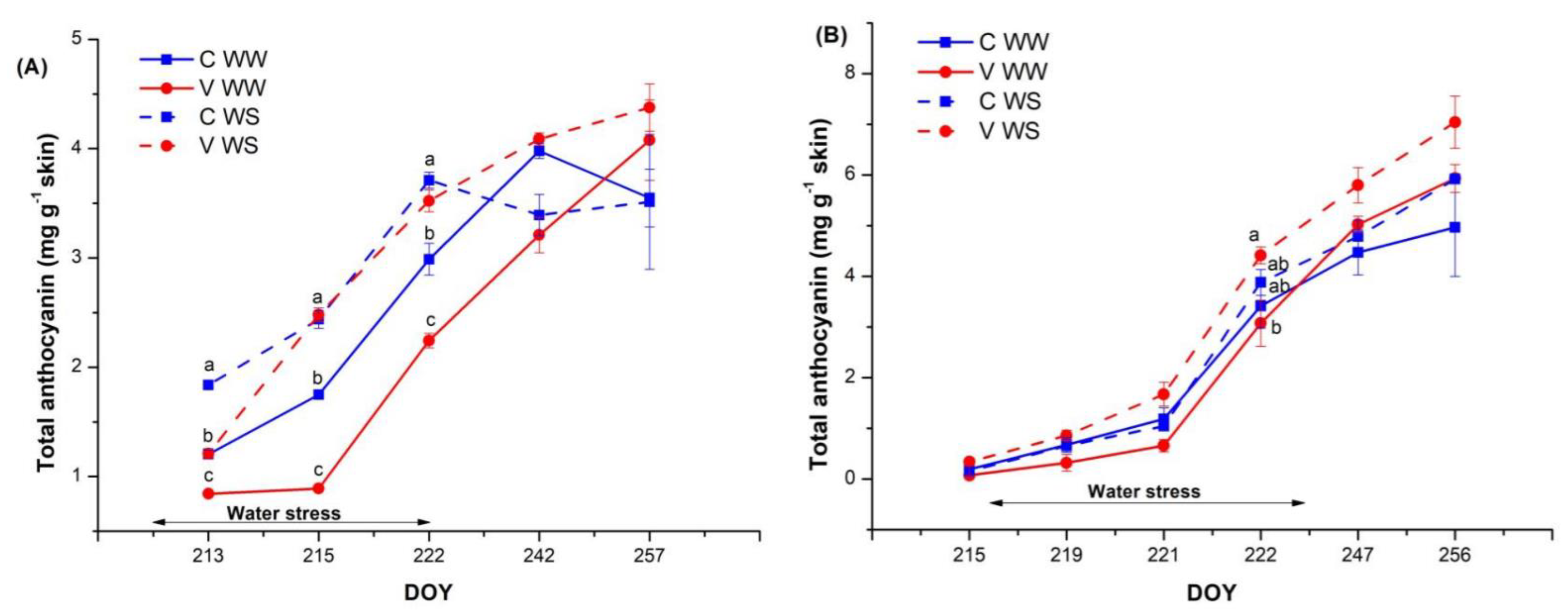
3.3. Berry Composition and Anthocyanin Accumulation during Ripening and Phenol Profiles at Harvest
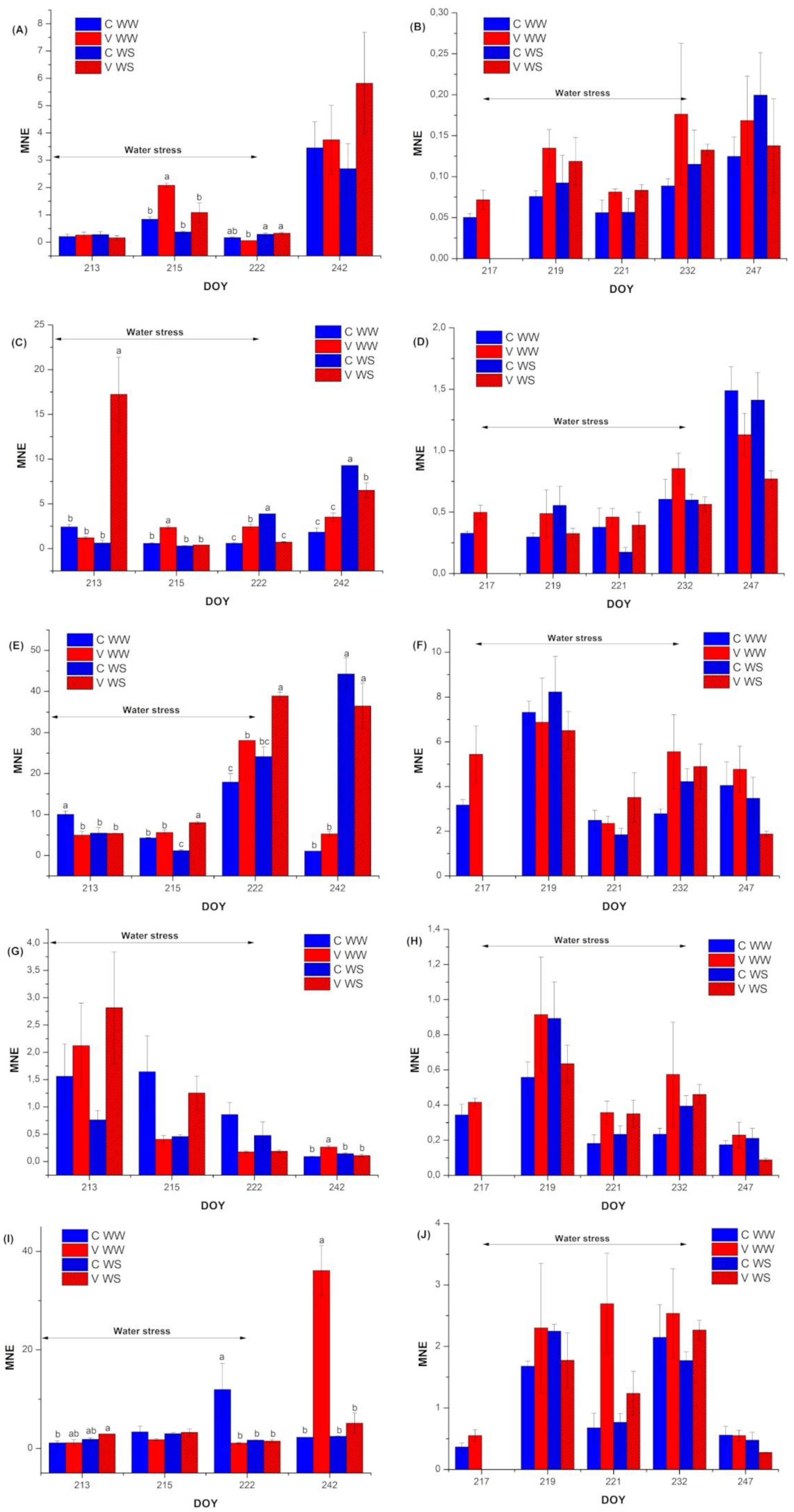
3.4. Gene Expression Analyses
4. Discussion
4.1. Effects of the Shape of the Canopy and of the Water Stress at Veraison on Gas Exchanges, Berry Ripening and Yield at Harvest
4.2. Effects of the Canopy Shape and of the Water Stress at Veraison on Anthocyanin Accumulation during Ripening and on Polyphenol Compounds at Harvest
4.3. Effects of the Canopy Shape and of the Water Stress at Veraison on the Expression of the Genes Involved in Anthocyanin Biosynthesis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Santos, J.A. An overview of climate change impacts on European viticulture. Food Sec. 2012, 1, 94–110. [Google Scholar] [CrossRef]
- Hannah, L.; Roehrdanz, P.R.; Ikegami, M.; Shepard, A.V.; Shaw, M.R.; Tabor, G.; Zhi, L.; Marquet, P.A.; Hijmans, R.J. Climate change, wine, and conservation. Proc. Natl. Acad. Sci. USA 2013, 110, 6907–6912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houghton, J.T.; Ding, Y.D.; Griggs, D.J.; Noguer, M.; van der Linden, P.J.; Dai, X.; Maskell, K.; Johnson, C.A. Climate Change 2001: The Scientific Basis: Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- IPCC. Climate Change 2013: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Support for Policymakers; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Jones, G.V.; White, M.A.; Cooper, O.R.; Storchmann, K. Climate change and global wine quality. Clim. Change 2005, 73, 319–343. [Google Scholar] [CrossRef]
- Duchêne, E. How can grapevine genetics contribute to the adaptation to climate change? Oeno One 2016, 50, 113–124. [Google Scholar] [CrossRef] [Green Version]
- OIV. Distribution of the World’s Grapevine Varieties. In International Organization of Vine and Wine (International Organisation of Vine and Wine, OIV); OIV: Paris, France, 2017. [Google Scholar]
- Mattivi, F.; Guzzon, R.; Vrhovsek, U.; Stefanini, M.; Velasco, R. Metabolite profiling of grape: Flavonols and anthocyanins. J. Agric. Food Chem. 2006, 54, 7692–7702. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.T.; Correia, C.; Moriondo, M.; Leolini, L.; Dibari, C.; Costafreda-Aumedes, S.; et al. A review of the potential climate change impacts and adaptation options for European viticulture. Appl. Sci. 2020, 10, 3092. [Google Scholar] [CrossRef]
- Allegro, G.; Pastore, C.; Valentini, G.; Filippetti, I. The evolution of phenolic compounds in Vitis vinifera L. red berries during ripening: Analysis and role on wine sensory—A review. Agronomy 2021, 11, 999. [Google Scholar] [CrossRef]
- Poni, S.; Intrieri, C.; Silvestroni, O. Interactions of leaf age, fruiting, and exogenous cytokinins in Sangiovese grapevines under non-irrigated conditions. I. Gas exchange. Am. J. Enol. Vitic. 1994, 45, 71–78. [Google Scholar]
- Spayd, S.E.; Tarara, J.M.; Mee, D.L.; Ferguson, J.C. Separation of sunlight and temperature effects on the composition of Vitis vinifera cv. Merlot berries. Am. J. Enol. Vitic. 2002, 53, 171–182. [Google Scholar]
- Tarara, J.M.; Lee, J.; Spayd, S.E.; Scagel, C.F. Berry temperature and solar radiation alter acylation, proportion, and concentration of anthocyanin in Merlot grapes. Am. J. Enol. Vitic. 2008, 59, 235–247. [Google Scholar]
- De Orduña, R. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Movahed, N.; Pastore, C.; Cellini, A.; Allegro, G.; Valentini, G.; Zenoni, S.; Cavallini, E.; D’Incà, E.; Tornielli, G.B.; Filippetti, I. The grapevine VviPrx31 peroxidase as a candidate gene involved in anthocyanin degradation in ripening berries under high temperature. J. Plant. Res. 2016, 129, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Pastore, C.; Dal Santo, S.; Zenoni, S.; Movahed, N.; Allegro, G.; Valentini, G.; Tornielli, G.B. Whole plant temperature manipulation affects flavonoid metabolism and the transcriptome of grapevine berries. Front. Plant. Sci. 2017, 8, 929. [Google Scholar] [CrossRef] [PubMed]
- Palliotti, A.; Tombesi, S.; Silvestroni, O.; Lanari, V.; Gatti, M.; Poni, S. Changes in vineyard establishment and canopy management urged by earlier climate-related grape ripening: A review. Sci. Hortic. 2014, 178, 43–54. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Coito, J.L.; Gonçalves, E.F.; Chaves, M.M.; Amâncio, S. Differential physiological response of the grapevine varieties Touriga Nacional and Trincadeira to combined heat, drought and light stresses. Plant. Biol. 2016, 18, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Lovisolo, C.; Perrone, I.; Carra, A.; Ferrandino, A.; Flexas, J.; Medrano, H.; Schubert, A. Drought induced changes in development and function of grapevine (Vitis spp.) organs and in their hydraulic and non-hydraulic interactions at the whole plant level: A physiological and olecular update. Funct. Plant Biol. 2010, 37, 98–111. [Google Scholar] [CrossRef]
- Costa, J.M.; Ortuño, M.F.; Lopes, C.M.; Chaves, M.M. Grapevine varieties exhibiting differences in stomatal response to water deficit. Funct. Plant Biol. 2012, 39, 179–189. [Google Scholar] [CrossRef]
- Hoffmann, M.; Lux, R.; Schultz, H.R. Constructing a framework for risk analyses of climate change effects on the water budget of differently sloped vineyards with a numeric simulation using the Monte Carlo method coupled to a water balance model. Front. Plant Sci. 2014, 5, 645. [Google Scholar] [CrossRef] [Green Version]
- Schultz, H.R. Differences in hydraulic architecture account for near-isohydric and anisohydric behavior of two field-grown Vitis vinifera L. cultivars during drought. Plant Cell. Environ. 2003, 26, 1393–1405. [Google Scholar] [CrossRef]
- Tombesi, S.; Poni, S.; Palliotti, A. Water stress in Vitis vinifera: Variability in intraspecific physiological behaviours and their potential exploiting in the mitigation of climate change effects. Italus Hortus 2016, 23, 45–53. [Google Scholar]
- Palliotti, A.; Tombesi, S.; Frioni, T.; Silvestroni, O.; Lanari, V.; D’Onofrio, C.; Matarese, F.; Bellincontro, A.; Poni, S. Physiological parameters and protective energy dissipation mechanism expressed in the leaves of two Vitis vinifera L. genotypes under multiple summer stresses. J. Plant Physiol. 2015, 185, 84–92. [Google Scholar] [CrossRef]
- Palliotti, A.; Silvestroni, O.; Petoumenou, D. Photosynthetic and photoinhibition behavior of two field-grown grapevine cultivars under multiple summer stresses. Am. J. Enol. Vitic. 2009, 60, 189–198. [Google Scholar]
- Medrano, H.; Tomás, M.; Martorell, S.; Escalona, J.M.; Pou, A.; Fuentes, S.; Flexas, J.; Bota, J. Improving water use efficiency of vineyards in semi-arid regions. A review. Agron. Sustain. Dev. 2015, 35, 499–517. [Google Scholar] [CrossRef] [Green Version]
- Valentini, G.; Allegro, G.; Pastore, C.; Colucci, E.; Magnanini, E.; Filippetti, I. Climate change and vine training systems: The influence different spatial distribution of shoots may have on sugar accumulation in Sangiovese grapevines. BIO Web Conf. 2019, 13, 04006. [Google Scholar] [CrossRef]
- Naulleau, A.; Gary, C.; Prévot, L.; Hossard, L. Evaluating Strategies for Adaptation to Climate Change in Grapevine Production—A Systematic Review. Front. Plant Sci. 2021, 11, 607859. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.E.; Ayars, J.E. Grapevine water use and the crop coefficient are linear functions of the shaded area measured beneath the canopy. Agric. For. Meteorol. 2005, 132, 201–211. [Google Scholar] [CrossRef]
- Gladstone, E.A.; Dokoozlian, N.K. Influence of leaf area density and trellis/training system on the light microclimate within grapevine canopies. Vitis 2003, 43, 123–131. [Google Scholar]
- Smart, R.E. Photosynthesis by grapevine canopies. J. App. Ecol. 1974, 11, 997–1006. [Google Scholar] [CrossRef]
- Poni, S.; Lakso, A.; Intrieri, C.; Rebucci, B.; Filippetti, I. Laser scanning estimation of relative light interception by canopy components in different grapevine training systems. Vitis 1996, 35, 177–182. [Google Scholar]
- Escalona, J.M.; Flexas, J.; Bota, J.; Medrano, H. Distribution of leaf photosynthesis and transpiration within grapevine canopies under different drought conditions. Vitis 2003, 42, 57–64. [Google Scholar]
- Intrieri, C.; Poni, S.; Rebucci, B.; Magnanini, E. Effects of canopy manipulations on whole-vine photosynthesis: Results from pot and field experiments. Vitis 1997, 36, 167–173. [Google Scholar]
- Intrigliolo, D.S.; Lakso, A.N. Effects of light interception and canopy orientation on grapevine water status and canopy gas exchange. Acta Hortic. 2011, 889, 99–104. [Google Scholar] [CrossRef]
- Medrano, H.; Pou, A.; Tomás, M.; Martorell, S.; Gulias, J.; Flexas, J.; Escalona, J.M. Average daily light interception determines leaf water use efficiency among different canopy locations in grapevine. Agric. Water Manag. 2012, 114, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Buckley, T.N.; Martorell, S.; Diaz-Espejo, A.; Tomàs, M.; Medrano, H. Is stomatal conductance optimized over both time and space in plant crowns? A field test in grapevine (Vitis vinifera). Plant Cell. Environ. 2014, 37, 2707–2721. [Google Scholar] [CrossRef] [Green Version]
- Castro Marin, A.; Chinnici, F. Physico-Chemical Features of Sangiovese Wine as Affected by a Post-Fermentative Treatment with Chitosan. Appl. Sci. 2020, 10, 6877. [Google Scholar] [CrossRef]
- Pastore, C.; Zenoni, S.; Fasoli, M.; Pezzotti, M.; Tornielli, G.B.; Filippetti, I. Selective defoliation affects plant growth, fruit transcriptional ripening program and flavonoid metabolism in grapevine. BMC Plant Biol. 2013, 13, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belhadj, A.; Telef, N.; Saigne, C.; Cluzet, S.; Barrieu, F.; Hamdi, S.; Mérillon, J.M. Effect of methyl jasmonate in combination with carbohydrates on gene expression of PR proteins, stilbene and anthocyanin accumulation in grapevine cell cultures. Plant Physiol. Bioch. 2008, 46, 493–499. [Google Scholar] [CrossRef]
- Goto-Yamamoto, N.; Wan, G.H.; Masaki, K.; Kobayashi, S. Structure and transcription of three chalcone synthase genes of grapevine (Vitis vinifera). Plant Sci. 2022, 162, 867–872. [Google Scholar] [CrossRef]
- Jeong, S.T.; Goto-Yamamoto, N.; Kobayashi, S.; Esaka, M. Expression of VvmybA1 gene and anthocyanin accumulation in various grape organs. Plant Sci. 2004, 167, 247–252. [Google Scholar] [CrossRef]
- Simon, P. Q-Gene: Processing quantitative real-time RT–PCR data. Bioinformatics 2003, 19, 1439–1440. [Google Scholar] [CrossRef] [Green Version]
- Tombesi, S.; Nardini, A.; Frioni, T.; Soccolini, M.; Zadra, C.; Farinelli, D.; Poni, S.; Palliotti, A. Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Sci. Rep. 2015, 5, 12449. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Bota, J.; Escalona, J.M.; Sampol, B.; Medrano, H. Effects of drought on photosynthesis in grapevines under field conditions: An evaluation of stomatal and mesophyll limitations. Funct. Plant Biol. 2002, 29, 461–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medrano, H.; Escalona, J.M.; Bota, J.; Gulias, J.; Flexas, J. Regulation of photosynthesis of C3 plants in response to progressive drought: Stomatal conductance as a reference parameter. Ann. Bot. 2002, 89, 895–905. [Google Scholar] [CrossRef]
- Riou, C.; Pieri, P.; Le Clech, B. Water use of grapevines well supplied with water. Simplified expression of transpiration. Vitis 1994, 33, 109–115. [Google Scholar]
- Trambouze, W.; Voltz, M. Measurement and modelling of the transpiration of a Mediterranean vineyard. Agric. For. Meteorol. 2001, 107, 153–166. [Google Scholar] [CrossRef]
- Heilman, J.L.; McInnes, K.J.; Gesch, R.W.; Lascano, R.J.; Savage, M.J. Effects of trellising on the energy balance of a vineyard. Agric. For. Meteorol. 1996, 81, 79–93. [Google Scholar] [CrossRef]
- Flexas, J.; Ribas-Carbó, M.; Diaz-Espejo, A.; Galmés, J.; Medrano, H. Mesophyll conductance to CO2: Current knowledge and future prospects. Plant Cell. Environ. 2008, 31, 602–621. [Google Scholar] [CrossRef]
- Hale, C.R.; Buttrose, M.S. Effect of temperature on ontogeny of berries of Vitis vinifera L. cv. cabernet Sauvignon. J. Am. Soc. Hortic. Sci. 1974, 99, 390–394. [Google Scholar] [CrossRef]
- Kliewer, W.M.; Torres, R.E. Effect of controlled day and night temperatures on grape coloration. Am. J. Enol. Vitic. 1972, 23, 71–77. [Google Scholar]
- Dokoozlian, N.K.; Kliewer, W.M. The light environment within grapevine canopies. II. Influence of leaf area density on fruit zone light environment and some canopy assessment parameters. Am. J. Enol. Vitic. 1993, 46, 219–226. [Google Scholar]
- Keller, M.; Hrazdina, G. Interaction of nitrogen availability during bloom and light intensity during veraison. ii. effects on anthocyanin and phenolic development during grape ripening. Am. J. Enol. Vitic. 1998, 49, 341–349. [Google Scholar]
- Bravdo, B.; Hepner, Y. Water management and effect on fruit quality in grapevines. In Proceedings of the Australian Wine Industry Technical Conference, Adelaide, Australia, 14–17 July 1986; Volume 14, pp. 150–158. [Google Scholar]
- Matthews, M.A.; Anderson, M.M. Fruit ripening in Vitis vinifera L.: Responses to seasonal water deficits. Am. J. Enol. Vitic. 1988, 39, 313–320. [Google Scholar]
- Reynolds, G.A.; Heuvel, V.E. Influence of grapevine training systems on vine growth and fruit composition: A review. Am. J. Enol. Vitic. 2009, 60, 251–268. [Google Scholar]
- Castellarin, S.D.; Pfeiffer, A.; Sivilotti, P.; Degan, M.; Peterlunger, E.; Di Gaspero, G. Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant Cell Environ. 2007, 30, 1381–1399. [Google Scholar] [CrossRef] [Green Version]
- Williams, L.E.; Matthews, M.A. Grapevine. In Irrigation of Agricultural Crops; Agronomy monograph 30; Stewart, B.A., Nielsen, D.R., Eds.; ASA-CSSA-SSSA: Madison, WI, USA, 1990; pp. 1019–1055. [Google Scholar]
- Antolin, M.C.; Ayari, M.; Sanchez-Diaz, M. Effects of partial rootzone drying on yield, ripening and berry ABA in potted Tempranillo grapevines with split roots. Aust. J. Grape Wine Res. 2006, 12, 13–20. [Google Scholar] [CrossRef]
- Schultz, H.R. Water relations and photosynthetic responses of two grapevine cultivars of different geographical origin during water stress. Strategies to optimize wine grape quality. Acta Hort. 1995, 427, 251–266. [Google Scholar]
- Poni, S.; Intrieri, C. Grapevine photosynthesis: Effects linked to light radiation and leaf age. Adv. Hort. Sci. 2001, 15, 5–15. [Google Scholar]
- Howell, G.S. Sustainable grape productivity and the growth-yield relationship: A Review. Am. J. Enol. Vitic. 2001, 52, 165–174. [Google Scholar]
- Guidoni, S.; Oggero, G.; Cravero, S.; Rabino, M.; Cravero, M.C.; Balsari, P. Manual and mechanical leaf removal in the bunch zone (Vitis Vinifera, L., cv Barbera): Effects on berry composition, health, yield and wine quality, in a warm temperate area. J. Int. Sci. Vigne Vin. 2008, 42, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Castellarin, S.D.; Di Gaspero, G. Transcriptional control of anthocyanin biosynthetic genes in extreme phenotypes for berry pigmentation of naturally occurring grapevines. BMC Plant Biol. 2007, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Koundouras, S.; Hatzidimitriou, E.; Karamolegkou, M.; Dimopoulou, E.; Kallithraka, S.; Tsialtas, J.T.; Zioziou, E.; Nikolaou, N.; Kotseridis, Y. Irrigation and rootstock effects on the phenolic concentration and aroma potential of Vitis vinifera L. cv. Cabernet Sauvignon grapes. J. Agric. Food Chem. 2009, 57, 7805–7813. [Google Scholar] [CrossRef] [PubMed]
- Gambetta, G.A.; Matthews, M.A.; Shaghasi, T.H.; McElrone, J.A.; Castellarin, S.D. Sugar and abscisic acid signaling orthologs are activated at the onset of ripening in grape. Planta 2010, 232, 219–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ollé, D.; Guiraud, J.L.; Souquet, J.M.; Terrier, N.; Ageorges, A.; Cheynier, V.; Verries, C. Effect of pre- and post-veraison water deficit on proanthocyanidin and anthocyanin accumulation during Shiraz berry development. Aust. J. Grape Wine Res. 2011, 17, 90–100. [Google Scholar] [CrossRef]
- Guo, S.H.; Yang, B.H.; Wang, X.W.; Li, J.N.; Li, S.; Yang, X.; Ren, R.H.; Fang, Y.L.; Xu, T.F.; Zhang, Z.H.; et al. ABA signaling plays a key role in regulated deficit irrigation-driven anthocyanins accumulation in ‘Cabernet Sauvignon’ grape berries. Environ. Exp. Bot. 2021, 181, 104290. [Google Scholar] [CrossRef]
- Haselgrove, L.; Botting, D.; Van Heeswijck, R.; Hoj, P.; Dry, P.R.; Ford, C.; Land, P.G.I. Canopy microclimate and berry composition: The effect of bunch exposure on the phenolic composition of Vitis vinifera L. cv. Shiraz grape berries. Aust. J. Grape Wine Res. 2000, 6, 141–149. [Google Scholar] [CrossRef]
- Lemut, M.S.; Sivilotti, P.; Franceschi, P.; Wehrens, R.; Vrhovsek, U. Use of metabolic profiling to study grape skin polyphenol behavior as a result of canopy microclimate manipulation in a ‘Pinot noir’ vineyard. J. Agric. Food Chem. 2013, 61, 8976–8986. [Google Scholar] [CrossRef]
- Deluc, L.G.; Decendit, A.; Papastamoulis, Y.; Mérillon, J.M.; Cushman, J.C.; Cramer, G.R. Water deficit increases stilbene metabolism in Cabernet Sauvignon berries. J. Agric. Food Chem. 2011, 59, 289–297. [Google Scholar] [CrossRef]
- Vezzulli, S.; Battilani, P.; Bavaresco, L. Stilbene-Synthase gene expression after Aspergillus carbonarius infection in grapes. Am. J. Enol. Vitic. 2007, 58, 132–134. [Google Scholar]
- Versari, A.; Parpinello, G.P.; Tornielli, G.B.; Ferrarini, R.; Giulivo, C. Stilbene compounds and stilbene synthase expression during ripening, wilting, and UV treatment in grape cv. Corvina. J. Agric. Food Chem. 2001, 49, 5531–5536. [Google Scholar] [CrossRef]
- Savoi, S.; Wong, D.C.J.; Degu, A.; Herrera, J.C.; Bucchetti, B.; Peterlunger, E.; Fait, A.; Mattivi, F.; Castellarin, S.D. Multi-omics and integrated network analyses reveal new insights into the systems relationships between metabolites, structural genes, and transcriptional regulators in developing grape berries (Vitis vinifera L.) exposed to water deficit. Front. Plant Sci. 2017, 8, 124. [Google Scholar] [CrossRef] [Green Version]
- Yu, R.; Cook, M.G.; Yacco, R.S.; Watrelot, A.A.; Gambetta, G.; Kennedy, J.A.; Kurtural, S.K. Effects of leaf removal and applied water on flavonoid accumulation in grapevine (Vitis vinifera L. cv. Merlot) berry in a hot climate. J. Agric. Food Chem. 2016, 64, 8118–8127. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, H.; Andary, C.; Kraeva, E.; Carbonneau, A.; Deloire, A. Influence of pre- and postveraison water deficit on synthesis and concentration of skin phenolic compounds during berry growth of Vitis vinifera cv. Shiraz. Am. J. Enol. Vitic. 2002, 53, 261–267. [Google Scholar]
- Deluc, L.G.; Quilici, D.R.; Decendit, A.; Grimplet, J.; Wheatley, M.D.; Schlauch, K.A.; Mérillon, J.M.; Cushman, J.C.; Cramer, G.R. Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Gen. 2009, 10, 212. [Google Scholar] [CrossRef] [Green Version]
- Savoi, S.; Wong, D.C.J.; Arapitsas, P.; Miculan, M.; Bucchetti, B.; Peterlunger, E.; Fait, A.; Mattivi, F.; Castellarin, S.D. Transcriptome and metabolite profiling reveals that prolonged drought modulates the phenylpropanoid and terpenoid pathway in white grapes (Vitis vinifera L.). BMC Plant Biol. 2016, 16, 67. [Google Scholar] [CrossRef] [Green Version]
- Gambetta, G.A.; Herrera, J.C.; Dayer, S.; Feng, Q.; Hochberg, U.; Castellarin, S.D. The physiology of drought stress in grapevine: Towards an integrative definition of drought tolerance. J. Exp. Bot. 2020, 71, 4658–4676. [Google Scholar] [CrossRef] [PubMed]
- Dayer, S.; Herrera, J.C.; Dai, Z.; Burlett, R.; Lamarque, L.J.; Delzon, S.; Bortolami, G.; Cochard, H.; Gambetta, G.A. The sequence and thresholds of leaf hydraulic traits underlying grapevine varietal differences in drought tolerance. J. Exp. Bot. 2020, 71, 4333–4344. [Google Scholar] [CrossRef] [Green Version]
- Gagné, S.; Cluzet, S.; Mérillon, J.M.; Gény, L. ABA initiates anthocyanin production in grape cell cultures. Plant Growth Regul. 2011, 30, 1–10. [Google Scholar] [CrossRef]
- Villalobos-González, L.; Peña-Neira, A.; Ibáñez, F.; Pastenes, C. Long-term effects of abscisic acid (ABA) on the grape berry phenylpropanoid pathway: Gene expression and metabolite content. Plant Physiol. Biochem. 2016, 105, 213–223. [Google Scholar] [CrossRef]

| 2018 | 2019 | |||||||
|---|---|---|---|---|---|---|---|---|
| T Mean (°C) | T Min (°C) | T Max (°C) | Rainfall (mm) | T Mean (°C) | T Min (°C) | T Max (°C) | Rainfall (mm) | |
| April | 16.7 | 12.2 | 21.4 | 18.6 | 14.3 | 8.8 | 19.7 | 53.6 |
| May | 19.3 | 15.3 | 24.2 | 59.4 | 15.3 | 10.9 | 19.9 | 185.5 |
| June | 23.2 | 18.3 | 28.7 | 97 | 25.2 | 18.4 | 31.4 | 24.4 |
| July | 26.0 | 20. 8 | 31.6 | 49.2 | 25.8 | 19.1 | 32.1 | 30.2 |
| August | 26.1 | 20.8 | 31.7 | 28.2 | 25.6 | 19.3 | 32.3 | 30.5 |
| September | 22.1 | 16.8 | 26.9 | 32.2 | 20.4 | 15.0 | 26.1 | 48.9 |
| October | 16.7 | 12. 8 | 21.1 | 61.8 | 16.6 | 12.1 | 21.1 | 45 |
| 2018 | 2019 | |||||||
|---|---|---|---|---|---|---|---|---|
| DOY | 213 | 215 | 222 | 242 | 219 | 225 | 231 | 247 |
| C WW | −8.5 | −7.5 | −6.4 | −7.2 | −5.7 | −5.7 | −7.6 | −6.1 |
| V WW | −8.3 | −7.6 | −7.1 | −7.7 | −6.5 | −6.1 | −6.7 | −6.5 |
| C WS | −11.5 | −13.0 | −13.5 | −7.4 | −6.8 | −6.6 | −8.8 | −6.4 |
| V WS | −11.3 | −12.5 | −16.0 | −6.5 | −7.8 | −7.7 | −9.7 | −6.1 |
| Training system effect | ns | ns | ns | ns | ns | ns | ns | ns |
| Water stress effect | * | * | * | ns | ns | ns | * | ns |
| Significance of training system × water stress interaction | ns | ns | ns | ns | ns | ns | ns | ns |
| 2018 | 2019 | ||||
|---|---|---|---|---|---|
| DOY | 215 | 222 | 219 | 225 | 231 |
| C | 17.0 a | 13.0 | 17.6 | 11.5 | 14.6 |
| V | 11.9 b | 12.9 | 16.5 | 11.6 | 14.0 |
| C WS | 6.4 c | 6.4 | 14.4 | 11.4 | 12.6 |
| V WS | 8.0 bc | 7.5 | 15.8 | 10.6 | 13.1 |
| Training system effect | ns | ns | ns | ns | ns |
| Water stress effect | * | * | ns | ns | * |
| Significance of training system × water stress interaction | * | ns | ns | ns | ns |
| 2018 | 2019 | ||||
|---|---|---|---|---|---|
| DOY | 215 | 222 | 219 | 225 | 231 |
| C WW | 0.23 a | 0.22 | 0.20 | 0.17 | 0.16 |
| V WW | 0.17 b | 0.25 | 0.18 | 0.14 | 0.15 |
| C WS | 0.07 c | 0.07 | 0.16 | 0.12 | 0.12 |
| V WS | 0.07 c | 0.08 | 0.17 | 0.12 | 0.11 |
| Training system effect | ns | ns | ns | ns | ns |
| Water stress effect | * | * | * | * | * |
| Significance of training system × water stress interaction | * | ns | ns | ns | ns |
| 2018 | 2019 | ||||
|---|---|---|---|---|---|
| DOY | 215 | 222 | 219 | 225 | 231 |
| C WW | 70 | 59 | 88 | 67 | 91 |
| V WW | 74 | 52 | 92 | 84 | 93 |
| C WS | 91 | 91 | 90 | 95 | 105 |
| V WS | 114 | 94 | 93 | 90 | 119 |
| Training system effect | ns | ns | ns | ns | ns |
| Water stress effect | * | * | ns | ns | * |
| Significance of training system × water stress interaction | ns | ns | ns | ns | ns |
| 2018 | 2019 | |||||||
|---|---|---|---|---|---|---|---|---|
| Bunches (n°) | Yield (kg) | Berry Weight (g) | Pruning Weight (kg) | Bunches (n°) | Yield (kg) | Berry Weight (g) | Pruning Weight (kg) | |
| C WW | 10 | 0.79 | 1.84 | 355 | 15 | 0.96 | 1.23 | 308 |
| V WW | 11 | 0.93 | 1.87 | 285 | 15 | 0.91 | 1.08 | 268 |
| C WS | 10 | 0.56 | 1.58 | 410 | 15 | 0.83 | 1.09 | 285 |
| V WS | 11 | 0.63 | 1.55 | 320 | 15 | 0.81 | 1.05 | 298 |
| Training system effect | ns | ns | ns | ns | ns | ns | ns | ns |
| Water stress effect | ns | ns | ns | ns | ns | ns | ns | ns |
| Significance of training system × water stress interaction | ns | ns | ns | ns | ns | ns | ns | ns |
| 2018 | 2019 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Soluble Solids (°Brix) | Total Acidity (g/L) | pH | Total Anthocyanins (mg/kg) | Soluble Solids (°Brix) | Total Acidity (g/L) | pH | Total Anthocyanins (mg/kg) | Total Flavonols (mg/kg) | Total Stilbenes (mg/kg) | |
| C WW | 22.7 | 4.89 | 3.7 | 708.8 | 20.0 | 6.47 | 3.5 | 728.5 | 240.1 | 4.60 |
| V WW | 20.8 | 5.39 | 3.6 | 764.2 | 19.5 | 6.60 | 3.4 | 885.9 | 287.4 | 5.30 |
| C WS | 23.0 | 5.58 | 3.7 | 688.5 | 20.6 | 6.08 | 3.5 | 959.0 | 313.6 | 5.70 |
| V WS | 22.4 | 5.54 | 3.6 | 701.9 | 21.1 | 7.04 | 3.4 | 1115.1 | 320.2 | 5.82 |
| Training system effect | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Water stress effect | ns | ns | ns | ns | ns | ns | ns | ns | * | * |
| Significance of training system × water stress interaction | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentini, G.; Pastore, C.; Allegro, G.; Mazzoleni, R.; Chinnici, F.; Filippetti, I. Vine Physiology, Yield Parameters and Berry Composition of Sangiovese Grape under Two Different Canopy Shapes and Irrigation Regimes. Agronomy 2022, 12, 1967. https://doi.org/10.3390/agronomy12081967
Valentini G, Pastore C, Allegro G, Mazzoleni R, Chinnici F, Filippetti I. Vine Physiology, Yield Parameters and Berry Composition of Sangiovese Grape under Two Different Canopy Shapes and Irrigation Regimes. Agronomy. 2022; 12(8):1967. https://doi.org/10.3390/agronomy12081967
Chicago/Turabian StyleValentini, Gabriele, Chiara Pastore, Gianluca Allegro, Riccardo Mazzoleni, Fabio Chinnici, and Ilaria Filippetti. 2022. "Vine Physiology, Yield Parameters and Berry Composition of Sangiovese Grape under Two Different Canopy Shapes and Irrigation Regimes" Agronomy 12, no. 8: 1967. https://doi.org/10.3390/agronomy12081967
APA StyleValentini, G., Pastore, C., Allegro, G., Mazzoleni, R., Chinnici, F., & Filippetti, I. (2022). Vine Physiology, Yield Parameters and Berry Composition of Sangiovese Grape under Two Different Canopy Shapes and Irrigation Regimes. Agronomy, 12(8), 1967. https://doi.org/10.3390/agronomy12081967










