Seed Characteristics Affect Low-Temperature Stress Tolerance Performance of Rapeseed (Brassica napus L.) during Seed Germination and Seedling Emergence Stages
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment 1: Germination and Seedling Test of Rapeseed Germplasm Resources under Normal- and Low-Temperature Conditions
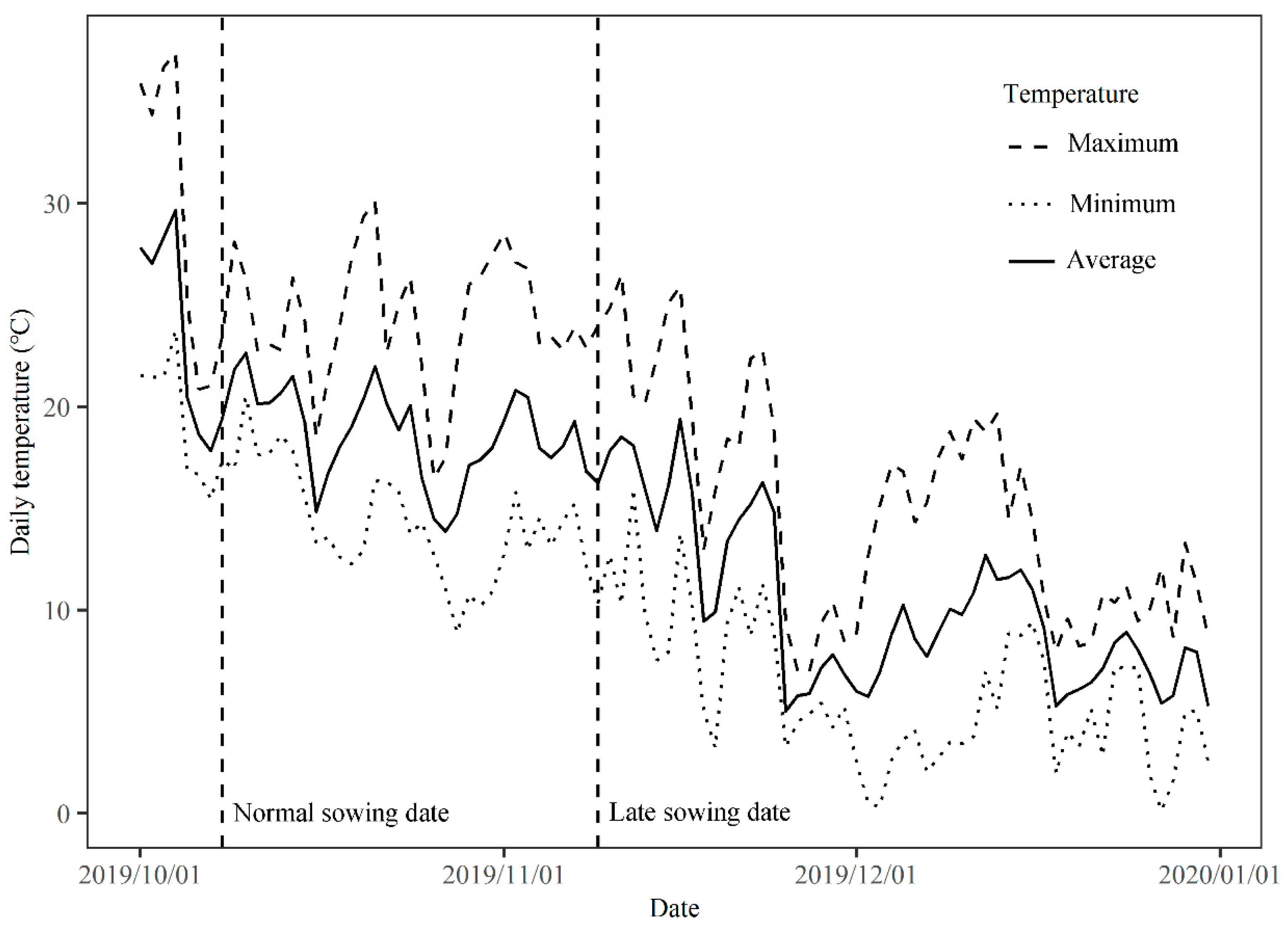
2.2. Experiment 2: Pot Experiment to Evaluate the Performance of Low-Temperature-Resistant and Low-Temperature-Sensitive Genotypes under Normal and Late Sowing Conditions
2.3. Data Analysis
3. Results
3.1. The Effect of Low Temperature on the Functional Traits of Rapeseed during Germination and Seedling Emergence Stages
3.2. Distance Correlation and Weight of Low-Temperature Stress Index Related to Seed Germination- and Seedling Emergence-Related Traits
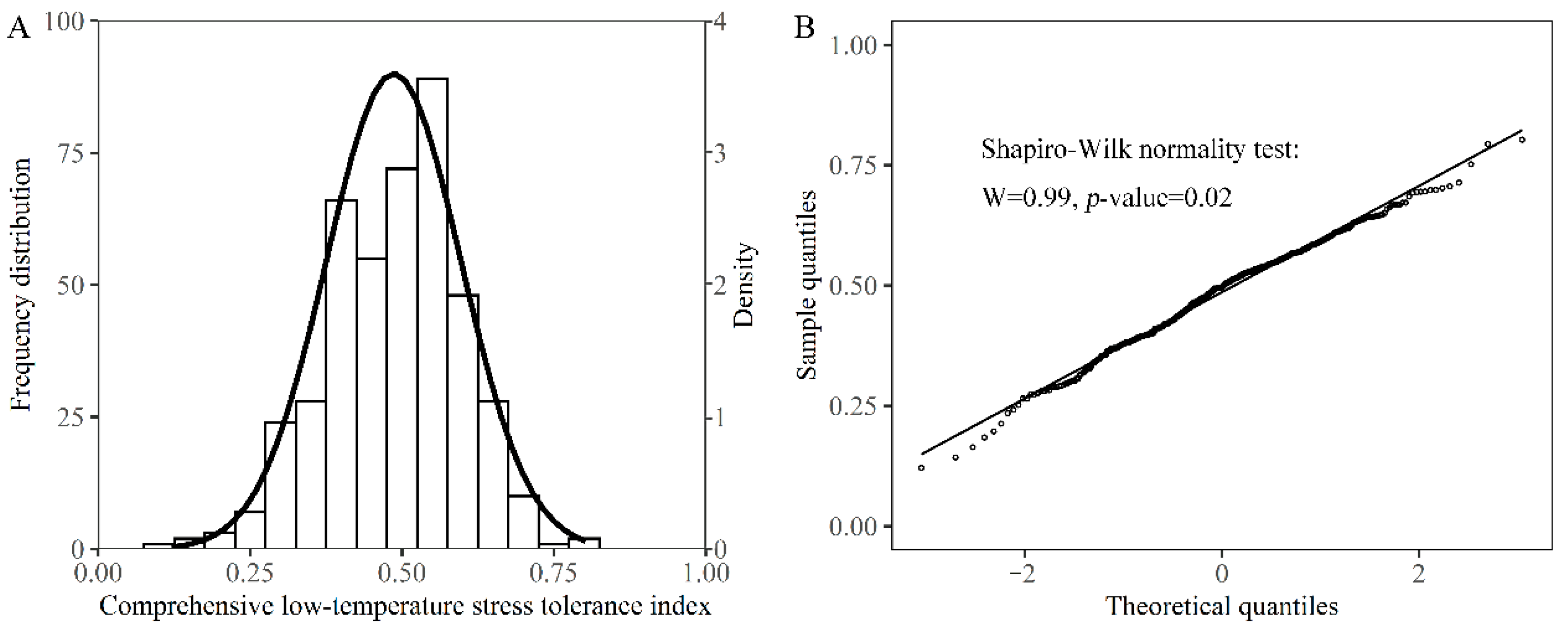
3.3. A Comprehensive Evaluation of Low-Temperature Stress Tolerance of Different Rapeseed Genotypes during Seed Germination and Seedling Emergence Stages
3.4. Effects of Rapeseed Characteristics on Low-Temperature Stress Tolerance during the Seed Germination and Seedling Emergence Stages
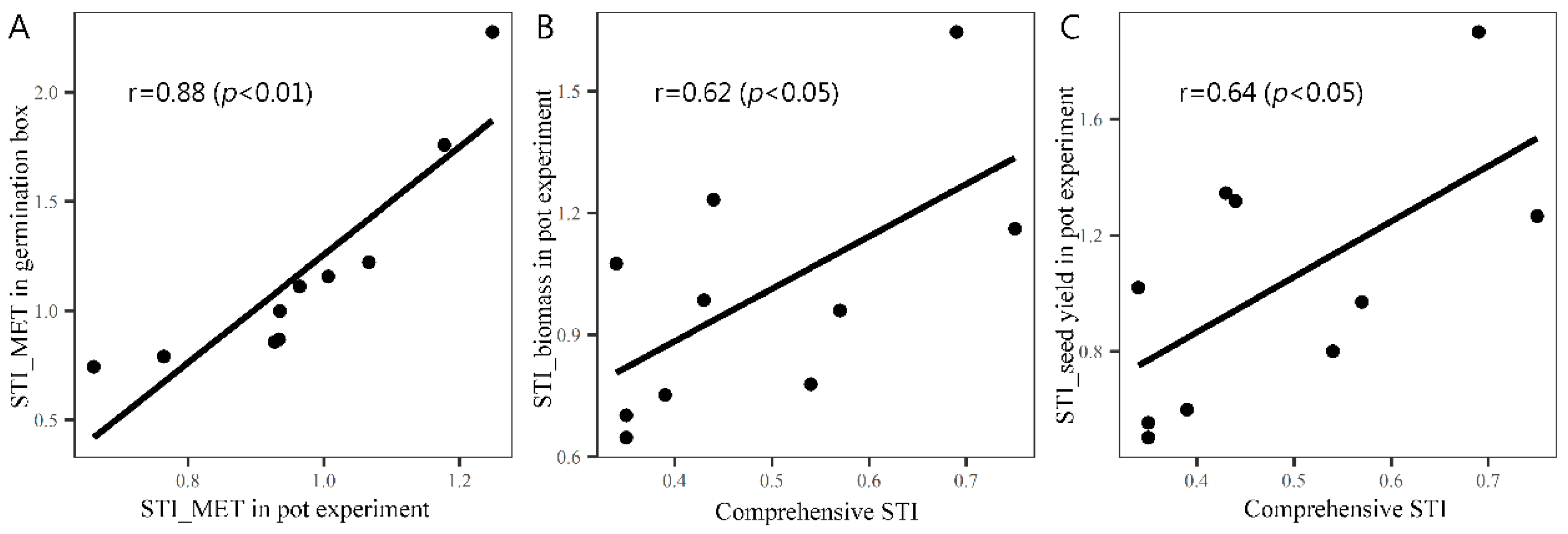
3.5. The Performance of Genotypes with Different Comprehensive Low-Temperature Stress Indices under the Conditions of Early and Late Sowing in Pots
4. Discussion
4.1. Evaluation of Low-Temperature Tolerance of Seed Germination- and Seedling Emergence-Related Indicators of Rapeseed
4.2. The Seed Characteristics Affected the Low-Temperature Tolerance of Rapeseed Seed Germination and Seedling Indicators
4.3. Genotypes with a High Comprehensive Low-Temperature Stress Index Have a Strong Ability to Adapt to Late Sowing Low-Temperature Conditions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Feng, Z.C.; Xiao, T.H.; Ma, X.M.; Zhou, G.S.; Huang, F.H.; Li, J.N.; Wang, H.Z. Development, potential and adaptation of Chinese rapeseed industry. Chin. J. Oil Crop Sci. 2019, 41, 485–489. [Google Scholar]
- Bonjean, A.P.; Dequidt, C.; Sang, T. Rapeseed in China. OCL 2016, 23, D605. [Google Scholar] [CrossRef][Green Version]
- Xian, M.Z.; Yang, P.; Hu, L.Y.; Xu, Z.H. Comprehensive evaluation of low temperature tolerance in rapeseed during germination and emergence periods. Crops 2015, 5, 116–122. [Google Scholar]
- Gorzin, M.; Ghaderi-Far, F.; Sadeghipour, H.R.; Zeinali, E. Induced thermo-dormancy in rapeseed (Brassica napus L.) cultivars by sub- and supra-optimal temperatures. J. Plant Growth Regul. 2020, 40, 2164–2177. [Google Scholar] [CrossRef]
- Marcos Filho, J. Seed vigor testing: An overview of the past, present and future perspective. Sci. Agric. 2015, 72, 363–374. [Google Scholar] [CrossRef]
- Nonogaki, H.; Bassel, G.W.; Bewley, J.D. Germination-Still a mystery. Plant Sci. 2010, 179, 574–581. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Xue, H.; Pritchard, H.W.; Wang, X. Changes in the mitochondrial protein profile due to ROS eruption during ageing of elm (Ulmus pumila L.) seeds. Plant Physiol. Biochem. 2017, 114, 72–87. [Google Scholar] [CrossRef]
- Xu, H.H.; Li, N.; Liu, S.J.; Wang, W.Q.; Wang, W.P.; Zhang, H.; Cheng, H.Y.; Song, S.Q. Research progress in seed germination and its control. Acta Agron. Sin. 2014, 40, 1141–1156. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Wang, Z.X.; Yang, W.S.; Yang, C.C.; Wang, J.; Yang, S.L.; Sun, Q.Q. Influences of temperature and PEG treatment on enzymatic characteristics during wheat seed germination. Shandong Agric. Sci. 2013, 45, 42–45. [Google Scholar]
- Xian, M.; Luo, T.; Khan, M.N.; Hu, L.; Xu, Z. Identifying differentially expressed genes associated with tolerance against low temperature stress in Brassica napus through transcriptome analysis. Int. J. Agric. Biol. 2017, 19, 273–281. [Google Scholar] [CrossRef]
- Nykiforuk, C.L.; Johnson-Flanagan, A.M. Storage reserve mobilization during low temperature germination and early seedling growth in Brassica napus. Plant Physiol. Biochem. 1999, 37, 939–947. [Google Scholar] [CrossRef]
- Omidi, H.; Tahmasebi, Z.; Badi HA, N.; Torabi, H.; Miransari, M. Fatty acid composition of canola (Brassica napus L.), as affected by agronomical, genotypic and environmental parameters. Comptes Rendus Biol. 2010, 333, 248–254. [Google Scholar] [CrossRef]
- Pritchard, F.; Eagles, H.; Norton, R.; Salisbury, P.; Nicolas, M. Environmental effects on seed composition of Victorian canola. Aust. J. Exp. Agric. 2000, 40, 679–685. [Google Scholar] [CrossRef]
- Wang, T.Y.; Yu, K.J.; Wan, W.; Ye, B.T.; Khattak, A.N.; Yang, R.Q.; Tian, E.T. Variation and correlation analyisis of erucic acid and glucoside content in germplasm groups of Brassica napus. Seed 2020, 39, 59–62. [Google Scholar]
- Wei, D.Y.; Cui, Y.X.; Mei, J.Q.; Tang, Q.L.; Li, J.N.; Qian, W. Genome-wide association study on seed oil content in rapeseed and construction of integration system for oil content loci. Acta Agron. Sin. 2018, 44, 1311–1319. [Google Scholar] [CrossRef]
- Hou, W.H.; Wang, J.L.; Dan, B.; Hu, D. Correlation of oilbody, protein body, oil content and protein content of Brassica rapa L. in Tibet. Chin. J. Oil Crop Sci. 2017, 39, 634–639. [Google Scholar]
- Gajardo, H.A.; Wittkop, B.; Soto-Cerda, B.; Higgins, E.E.; Parkin, I.A.; Snowdon, R.J.; Federico, M.L.; Iniguez-Luy, F.L. Association mapping of seed quality traits in Brassica napus L. using GWAS and candidate QTL approaches. Mol. Breed. 2015, 35, 143. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, T.; Liu, J.; Xian, M.; Yuan, J.; Hu, L.; Xu, Z. Evaluation of the low-temperature tolerance of rapeseed genotypes at the germination and seedling emergence stages. Crop Sci. 2019, 59, 1709–1717. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, W.; Jiang, M.; Yang, L.; Zhou, X. QTL mapping for low temperature germination in rapeseed. Sci. Rep. 2021, 11, 23382. [Google Scholar] [CrossRef]
- Elias, S.; Copeland, L. Evaluation of seed vigor tests for canola. Seed Technol. 1997, 19, 78–87. [Google Scholar]
- Huang, H.; Yan, L.; Lyu, Y.; Ding, X.Y.; Cai, J.S.; Cheng, Y.; Zhang, X.K.; Zou, X.L. Screening and evaluation of low temperature tolerance of rapeseed (Brassica napus L.) at germination stage. Chin. J. Oil Crop Sci. 2019, 41, 723–734. [Google Scholar]
- Zhao, Y.; Liu, X.; Zheng, L.; Chen, L.; Qin, Y. Effect of heavy metal stress on seed germination of Albizia julibrissin. Agric. Biotechnol. 2021, 10, 48–51. [Google Scholar]
- Zhang, R.; Hussain, S.; Wang, Y.; Liu, Y.; Li, Q.; Chen, Y.; Wei, H.; Gao, P.; Dai, Q. Comprehensive evaluation of salt tolerance in rice (Oryza sativa L.) germplasm at the germination stage. Agronomy 2021, 11, 1569. [Google Scholar] [CrossRef]
- Luo, T.; Zhang, Y.; Zhang, C.; Nelson, M.N.; Yuan, J.; Guo, L.; Xu, Z. Genome-wide association mapping unravels the genetic control of seed vigor under low-temperature conditions in rapeseed (Brassica napus L.). Plants 2021, 10, 426. [Google Scholar] [CrossRef]
- Khoshgoftarmanesh, A.H.; Sadrarhami, A.; Sharifi, H.R.; Afiuni, D.; Schulin, R. Selecting zinc-efficient wheat genotypes with high grain yield using a stress tolerance index. Agron. J. 2009, 101, 1409. [Google Scholar] [CrossRef]
- Krishnan, A.R.; Kasim, M.M.; Hamid, R.; Ghazali, M.F. A modified CRITIC method to estimate the objective weights of decision criteria. Symmetry 2021, 13, 973. [Google Scholar] [CrossRef]
- Diepenbrock, W. Yield analysis of winter oilseed rape (Brassica napus L): A review. Field Crop Res. 2000, 67, 35–49. [Google Scholar] [CrossRef]
- Yi, F.; Wa Ng, Z.; Baskin, C.; Baskin, J.M.; Ye, R.; Sun, H.; Zhang, Y.; Ye, X.; Liu, G.; Yang, X. Seed germination responses to seasonal temperature and drought stress are species-specific but not related to seed size in a desert steppe: Implications for effect of climate change on community structure. Ecol. Evol. 2019, 9, 2149–2159. [Google Scholar] [CrossRef]
- Yang, P.; Xian, M.Z.; Hu, L.Y.; Xu, Z.H. Effects of low-temeprature on seed germination and seedling development of rapeseed. J. Huazhong Agric. Univ. 2015, 34, 7–13. [Google Scholar]
- Bahrami, F.; Arzani, A.; Karimi, V. Evaluation of yield-based drought tolerance indices for screening safflower genotypes. Agron. J. 2014, 106, 1219. [Google Scholar] [CrossRef]
- Farshadfar, E.; Mohammadi, R.; Farshadfar, M.; Dabiri, S. Relationships and repeatability of drought tolerance indices in wheat-rye disomic addition lines. Aust. J. Crop Sci. 2013, 7, 130–138. [Google Scholar]
- Dehghani, G.H.; Shhi, F.M.; Alizadeh, B. A study of drought tolerance indices in canola (Brassica napus L.) genotypes. J. Sci. Technol. Agric. Nat. Res. 2009, 13, 77–90. [Google Scholar]
- Kharrazi, M.A.S.; Rad, M.R.N. Evaluation of sorghum genotypes under drought stress conditions using some stress tolerance indices. Afr. J. Biotechnol. 2011, 10, 13086–13089. [Google Scholar]
- He, Y.; Guo, H.; Jin, M.; Ren, P. A linguistic entropy weight method and its application in linguistic multi-attribute group decision making. Nonlinear Dyn. 2016, 84, 399–404. [Google Scholar] [CrossRef]
- Li, H.-X.; Li, L.-X.; Wang, J.-Y.; Mo, Z.-W.; Li, Y.-D. Fuzzy decision making based on variable weights. Math. Comput. Model. 2004, 39, 163–179. [Google Scholar] [CrossRef]
- Luo, T.; Xian, M.; Zhang, C.; Zhang, C.; Hu, L.; Xu, Z. Associating transcriptional regulation for rapid germination of rapeseed (Brassica napus L.) under low temperature stress through weighted gene co-expression network analysis. Sci. Rep. 2019, 9, 55. [Google Scholar] [CrossRef]
- Gomes, M.; Garcia, Q. Reactive oxygen species and seed germination. Biologia 2013, 68, 351–357. [Google Scholar] [CrossRef]
- Nykiforuk, C.L.; Johnson-Flanagan, A.M. Germination and early seedling development under low temperature in canola. Crop Sci. 1994, 34, 1047–1054. [Google Scholar] [CrossRef]
- Wu, X.; Liu, H.; Wang, W.; Chen, S.; Hu, X.; Li, C. Proteomic analysis of seed viability in maize. Acta Physiol. Plant 2011, 33, 181–191. [Google Scholar] [CrossRef]
- Yan, S.J.; Liu, J.; Si, L.T.; Ma, Z.G.; Yang, J.M. Study on the relationship oil content in cucumber seed and chilling tolerance. Acta Agric. Boreali Simica 2007, 22, 33–35. [Google Scholar]
- Su, Y.; Liang, W.; Liu, Z.; Wang, Y.; Zhao, Y.; Ijaz, B.; Hua, J. Overexpression of GhDof1 improved salt and cold tolerance and seed oil content in Gossypium hirsutum. J. Plant Physiol. 2017, 218, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Theimer, R.R. Degradation of oil bodies isolated from cotyledons during germination of rapeseed seedlings. J. Plant Physiol. 1997, 151, 471–478. [Google Scholar] [CrossRef]
- Gu, J.; Chao, H.; Gan, L.; Guo, L.; Zhang, K.; Li, Y.; Wang, H.; Raboanatahiry, N.; Li, M. Proteomic dissection of seed germination and seedling establishment in Brassica napus. Front. Plant Sci. 2016, 7, 1482. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, P.J.; Holford, P.; Mcglasson, W.B.; Newman, S. Temperature and seed weight affect the germination of peach rootstock seeds and the growth of rootstock seedlings. Sci. Hortic. 2003, 98, 247–256. [Google Scholar] [CrossRef]
- Willenborg, C.J.; Wildeman, J.C.; Miller, A.K.; Rossnagel, B.G.; Shirtliffe, S.J. Oat germination characteristics differ among genotypes, seed sizes, and osmotic potentials. Crop Sci. 2005, 45, 2023–2029. [Google Scholar] [CrossRef]
- Kidson, R.; Westoby, M. Seed mass and seedling dimensions in relation to seedling establishment. Oecologia 2000, 125, 11–17. [Google Scholar] [CrossRef]
- Ambika, S.; Manonmani, V.; Somasundaram, G. Review on effect of seed size on seedling vigour and seed yield. Res. J. Seed Sci. 2014, 7, 31–38. [Google Scholar] [CrossRef]
- He, Y.; Yang, Z.; Tang, M.; Yang, Q.Y.; Zhang, Y.; Liu, S. Enhancing canola breeding by editing a glucosinolate transporter gene lacking natural variation. Plant Physiol. 2022, 188, 1848–1851. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, G.L. Study on changes of fatty acids composition from radish seed germination. Cereals Oils 2020, 33, 73–75. [Google Scholar]
- Zhao, X.A.; Wu, M.F. Anti-nutritional effect and mechanism of glucosinolates. Chin. Lives Breed. 2011, 10, 34–37. [Google Scholar]
- Laryea, M.; Jiang, Y.; Xu, G.; Lombeck, I. Fatty acid composition of blood lipids in Chinese children consuming high erucic acid rapeseed oil. Ann. Nutr. Metab. 1992, 36, 273–278. [Google Scholar] [CrossRef]
- Li, Y.J.; Chang, R.Z.; Zhao, Y.T.; Sun, J.Y. Laboratory screening on tolerance to cold in soybean and application in field. Soyb. Sci. 1992, 11, 49–57. [Google Scholar]
- Chang, B.W.; Zhong, P.; Liu, J.; Tang, Z.H.; Gao, Y.B.; Yu, H.J.; Guo, W. Effect of low-temperature stress and gibberellin on seed germination and seedling physiological responses in peanut. Acta Agron. Sin. 2019, 45, 118–130. [Google Scholar] [CrossRef]
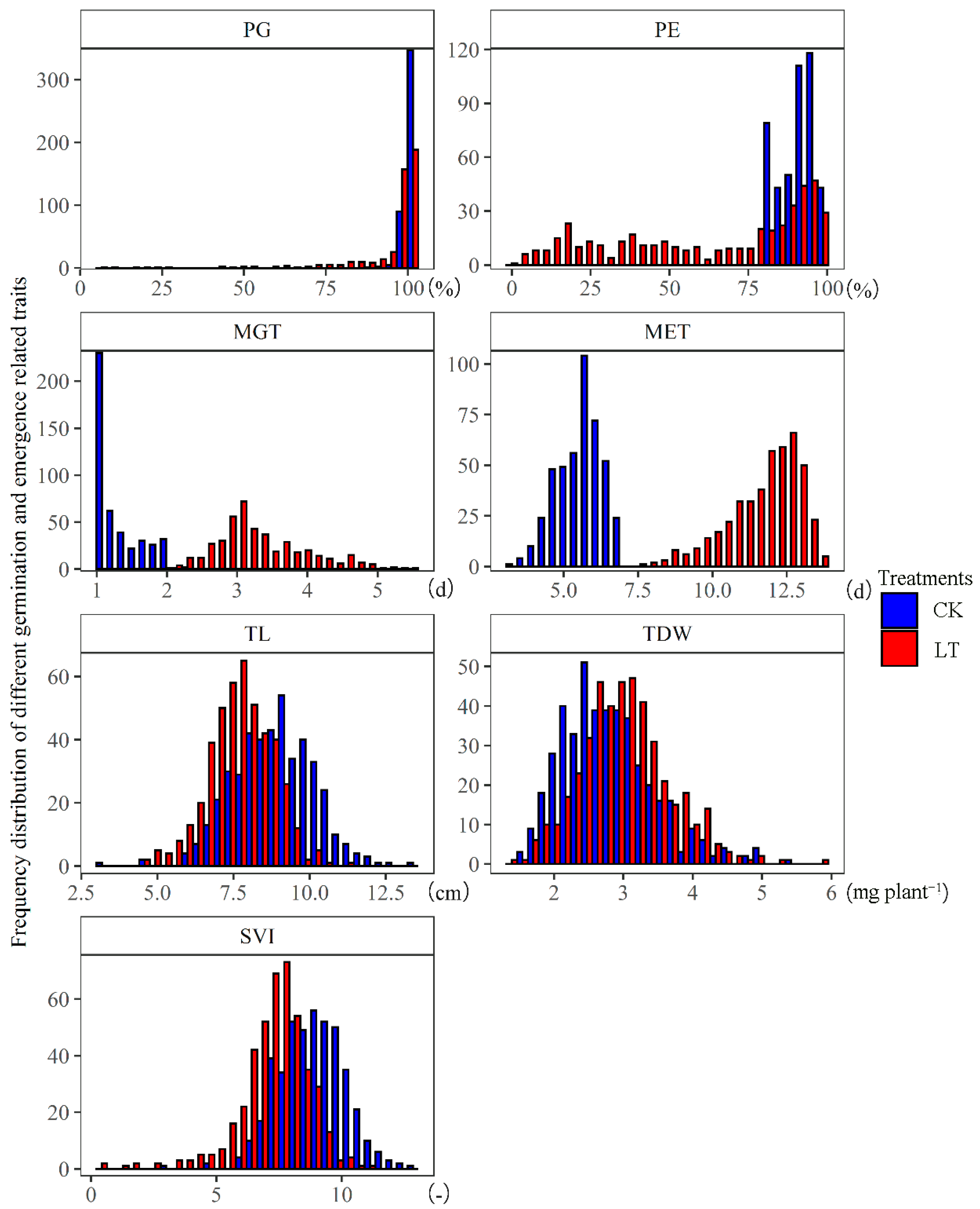



| Statistic Variable | PG | PE | MGT | MET | TL | TDW | SVI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CK | LT | CK | LT | CK | LT | CK | LT | CK | LT | CK | LT | CK | LT | |
| Mean | 99.7 | 94.7 | 90.4 | 62.3 | 1.29 | 3.34 | 5.59 | 11.75 | 8.84 | 7.70 | 2.79 | 3.03 | 8.82 | 7.29 |
| Min | 89.0 | 5.0 | 80.0 | 1.0 | 1.00 | 2.01 | 3.43 | 7.69 | 3.16 | 4.52 | 1.60 | 1.44 | 3.16 | 0.43 |
| Max | 100.0 | 100.0 | 99.3 | 99.6 | 2.30 | 5.43 | 6.93 | 13.90 | 13.38 | 11.11 | 5.42 | 5.93 | 12.85 | 11.11 |
| CV | 1% | 14% | 6% | 49% | 25% | 21% | 13% | 10% | 15% | 14% | 24% | 22% | 15% | 19% |
| Skewness | −7.84 | −4.03 | −0.52 | −0.44 | 1.08 | 1.43 | −0.46 | −0.87 | −0.13 | −0.14 | 0.82 | 0.53 | −0.15 | −1.23 |
| Kurtosis | 73.81 | 18.42 | −0.93 | −1.31 | −0.11 | 5.55 | −0.38 | 0.31 | 0.62 | 0.28 | 0.82 | 0.79 | 0.54 | 0.77 |
| Wilcoxon test | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | |||||||
| Statistic Index | STI_PG | STI_PE | STI_MGT | STI_MET | STI_TL | STI_TDW | STI_SVI |
|---|---|---|---|---|---|---|---|
| Standard deviation | 0.14 | 0.30 | 0.19 | 0.15 | 0.17 | 0.16 | 0.16 |
| Information content | 0.62 | 1.36 | 0.87 | 0.74 | 0.92 | 0.67 | 0.75 |
| Weight | 10.4% | 22.9% | 14.7% | 12.5% | 15.6% | 11.3% | 12.6% |
| Variety | STI_PG | STI_PE | STI_MGT | STI_MET | STI_TL | STI_TDW | STI_SVI | Comprehensive STI |
|---|---|---|---|---|---|---|---|---|
| 11-9-703 | 0.06 | 0.05 | 0.00 | 0.30 | 0.35 | 0.11 | 0.02 | 0.12 |
| 97096 | 0.00 | 0.04 | 0.20 | 0.09 | 0.44 | 0.24 | 0.00 | 0.14 |
| Zhongshuang No.4 | 0.47 | 0.08 | 0.17 | 0.07 | 0.16 | 0.18 | 0.14 | 0.16 |
| 90750 | 0.91 | 0.02 | 0.20 | 0.07 | 0.06 | 0.13 | 0.19 | 0.18 |
| wx1025 | 0.16 | 0.16 | 0.09 | 0.20 | 0.57 | 0.14 | 0.11 | 0.20 |
| Huahang 901 | 0.99 | 0.92 | 0.50 | 0.21 | 0.76 | 0.66 | 0.84 | 0.71 |
| Caoyou No.2 | 1.00 | 0.86 | 0.86 | 0.19 | 0.77 | 0.46 | 0.85 | 0.71 |
| Qianyou No.4 | 1.00 | 0.98 | 0.86 | 1.00 | 0.42 | 0.37 | 0.54 | 0.75 |
| SWU63 | 1.00 | 0.97 | 0.85 | 0.62 | 0.84 | 0.35 | 0.92 | 0.79 |
| Fengding 240 | 1.00 | 0.95 | 0.67 | 0.75 | 0.81 | 0.56 | 0.89 | 0.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, T.; Sheng, Z.; Zhang, C.; Li, Q.; Liu, X.; Qu, Z.; Xu, Z. Seed Characteristics Affect Low-Temperature Stress Tolerance Performance of Rapeseed (Brassica napus L.) during Seed Germination and Seedling Emergence Stages. Agronomy 2022, 12, 1969. https://doi.org/10.3390/agronomy12081969
Luo T, Sheng Z, Zhang C, Li Q, Liu X, Qu Z, Xu Z. Seed Characteristics Affect Low-Temperature Stress Tolerance Performance of Rapeseed (Brassica napus L.) during Seed Germination and Seedling Emergence Stages. Agronomy. 2022; 12(8):1969. https://doi.org/10.3390/agronomy12081969
Chicago/Turabian StyleLuo, Tao, Ziwei Sheng, Chunni Zhang, Qin Li, Xiaoyan Liu, Zhaojie Qu, and Zhenghua Xu. 2022. "Seed Characteristics Affect Low-Temperature Stress Tolerance Performance of Rapeseed (Brassica napus L.) during Seed Germination and Seedling Emergence Stages" Agronomy 12, no. 8: 1969. https://doi.org/10.3390/agronomy12081969
APA StyleLuo, T., Sheng, Z., Zhang, C., Li, Q., Liu, X., Qu, Z., & Xu, Z. (2022). Seed Characteristics Affect Low-Temperature Stress Tolerance Performance of Rapeseed (Brassica napus L.) during Seed Germination and Seedling Emergence Stages. Agronomy, 12(8), 1969. https://doi.org/10.3390/agronomy12081969







