Analysis of Physiological Status in Response to Water Deficit of Spelt (Triticum aestivum ssp. spelta) Cultivars in Reference to Common Wheat (Triticum aestivum ssp. vulgare)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Gas Exchange Parameters
2.3. Chlorophyll Fluorescence
2.4. Chlorophyll Content Index
2.5. Relative Water Content (RWC)
2.6. Lipid Peroxidation Index (MDA)
2.7. Free Proline Analysis
2.8. Anthocyanin Analyses
2.9. Estimation of Drought Resistance Index (DRI)
2.10. Statistical Analysis
3. Results
3.1. Physiological State of Plants
3.1.1. Gas Exchange Parameters
3.1.2. Chlorophyll Fluorescence and Chlorophyll Content Index
3.2. Relative Water Content (RWC)
3.3. Lipid Peroxidation Index (MDA)
3.4. Free Proline Accumulation
3.5. Anthocyanins Accumulation
3.6. Physiological Plant Activity (PPA) and Drought Resistance Index (DRI)
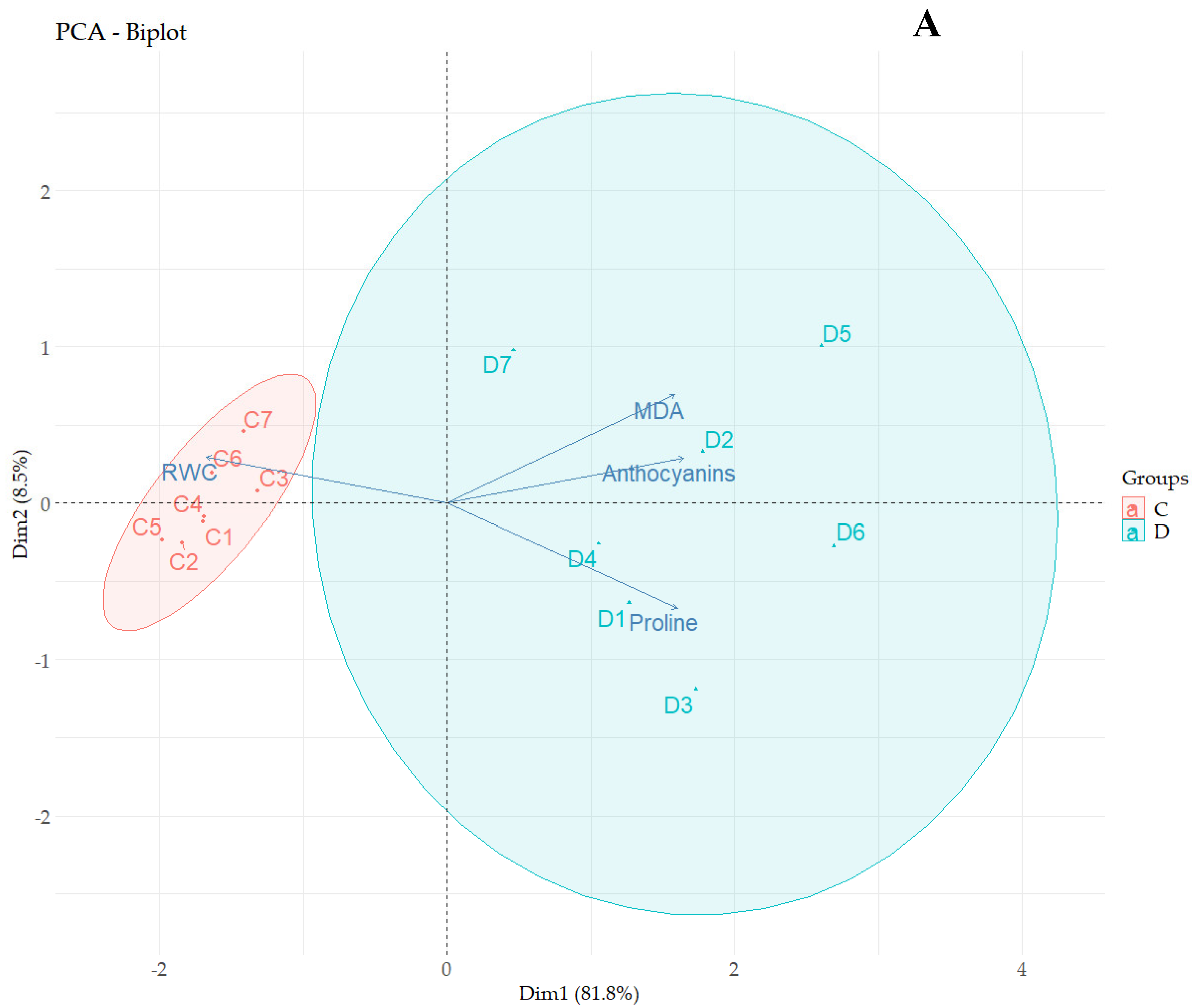
3.7. Principal Component Analysis
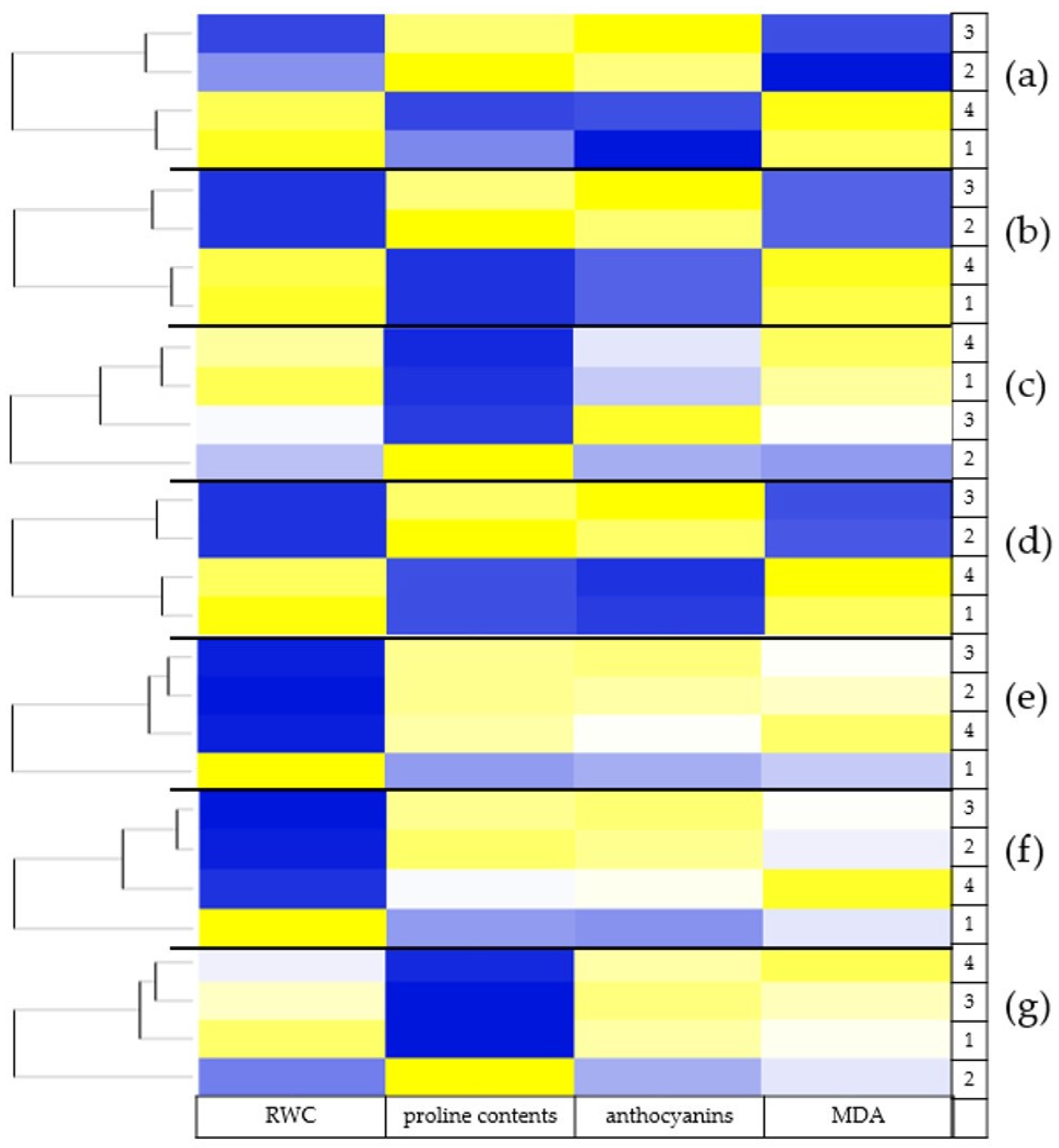
3.8. Correlation Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kowalczewski, P.Ł.; Radzikowska, D.; Ivanišová, E.; Szwengiel, A.; Kačániová, M.; Sawinska, Z. Influence of Abiotic Stress Factors on the Antioxidant Properties and Polyphenols Profile Composition of Green Barley (Hordeum vulgare L.). Int. J. Mol. Sci. 2020, 21, 397. [Google Scholar] [CrossRef]
- Nguyen, L.T.T.; Osanai, Y.; Anderson, I.C.; Bange, M.P.; Tissue, D.T.; Singh, B.K. Flooding and prolonged drought have differential legacy impacts on soil nitrogen cycling, microbial communities and plant productivity. Plant Soil 2018, 431, 371–387. [Google Scholar] [CrossRef]
- Jajor, E.; Zamojska, J.; Dworzańska, D.; Horoszkiewicz-Janka, J.; Danielewicz, J.; Węgorek, P.; Korbas, M.; Ciecierski, W.; Bocianowski, J.; Wilk, R. Yield, volume, quality and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat and maize cultivations. Open Chem. 2020, 19, 1089–1095. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Konvalina, P.; Suchý, K.; Stehno, Z.; Capouchová, I.; Moudrý, J. Drought Tolerance of Different Wheat Species (Triticum L.). In Vulnerability of Agriculture, Water and Fisheries to Climate Change; Springer: Dordrecht, The Netherlands, 2014; pp. 207–216. [Google Scholar]
- Ratajczak, K.; Sulewska, H.; Błaszczyk, L.; Basińska-Barczak, A.; Mikołajczak, K.; Salamon, S.; Szymańska, G.; Dryjański, L. Growth and Photosynthetic Activity of Selected Spelt Varieties (Triticum aestivum ssp. spelta L.) Cultivated under Drought Conditions with Different Endophytic Core Microbiomes. Int. J. Mol. Sci. 2020, 2, 7987. [Google Scholar] [CrossRef] [PubMed]
- Kosakivska, I.V.; Vasyuk, V.A.; Voytenko, L.V. Drought stress effects on growth characteristics of two relative wheats Triticum aestivum L. and Triticum spelta L. Fiziol. Rastenij Genet. 2018, 50, 241–252. [Google Scholar] [CrossRef]
- Le Gouis, J.; Oury, F.X.; Charmet, G. How changes in climate and agricultural practices influenced wheat production in Western Europe. J. Cereal Sci. 2020, 93, 102960. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.; Wang, L.; Farooq, M.; Saleem, F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 69, 2026–2032. [Google Scholar]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and drought: Can we make metabolic connections from available data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef]
- Sharma, P.; Bhushan, A.; Rama, J.; Dubey, S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Laxa, C.M.; Sonnewald, U. Signalling events in plants: Stress factors in combination change the picture. Environ. Exp. Bot. 2015, 114, 4–14. [Google Scholar]
- Rybka, K.; Nita, Z. Modern phenotypes of cereals for growing in areas endangered with drought. Biul. Inst. Hod. Aklim. Roślin 2014, 273, 55–72. [Google Scholar]
- El Gataa, Z.; El Hanafi, S.; El Messoadi, K.; Samir, K.; Kehel, Z.; Tadesse, W. Genome wide association and prediction studies of agronomic and quality traits in spring beard wheat (Triticum aestivum L.) under rain-fed environment with terminal moisture stress. J. Cereal Sci. 2021, 101, 103278. [Google Scholar] [CrossRef]
- Dinakar, C.; Djilianov, D.; Bartels, D. Photosynthesis in desiccation tolerant plants: Energy metabolism and antioxidative stress defense. Plant Sci. 2012, 182, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Dodd, I.C.; Ryan, A.C. Whole-plant physiological responses to water-deficit stress. eLS 2016, 1–9. [Google Scholar]
- Fábregas, N.; Fernie, A.R. The metabolic response to drought. J. Exp. Bot. 2019, 70, 1077–1085. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: Multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Shah, A.; Smith, D.L. Flavonoids in agriculture: Chemistry and roles in, biotic and abiotic stress responses, and microbial associations. Agron. J. 2020, 10, 1209. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Wang, C.; Li, Y.; Guo, T. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol. Biochem. 2014, 80, 60–66. [Google Scholar] [CrossRef]
- Bandurska, H. Drought Stress Responses: Coping Strategy and Resistance. Plants 2022, 11, 922. [Google Scholar] [CrossRef] [PubMed]
- Razavi, F.; Pollet, B.; Steppe, K.; van Labeke, M.C. Chlorophyll fluorescence as a tool for evaluation of drought stress in strawberry. Photosynthetica 2008, 46, 631–633. [Google Scholar] [CrossRef]
- Mathobo, R.; Marais, D.; Steyn, J.M. The effect of drought stress on yield, leaf gaseous exchange and chlorophyll fluorescence of dry beans (Phaseolus vulgaris L.). Agric. Water Manag. 2017, 180, 118–125. [Google Scholar] [CrossRef]
- Callejo, M.J.; Vargas-Kostiuk, M.-E.; Rodríguez-Quijano, M. Selection, training and validation process of a sensory panel for bread analysis: Influence of cultivar on the quality of breads made from common wheat and spelt wheat. J. Cereal Sci. 2015, 61, 55–62. [Google Scholar] [CrossRef]
- Frakolaki, G.; Giannou, V.; Topakas, E.; Tzia, C. Chemical characterization and breadmaking potential of spelt versus wheat flour. J. Cereal Sci. 2018, 79, 50–56. [Google Scholar] [CrossRef]
- Ruibal-Mendieta, N.L.; Delacroix, D.L.; Mignolet, E.; Pycke, J.-M.; Marques, C.; Rozenberg, R.; Petitjean, G.; Habib-Jiwan, J.L.; Meurens, M.; Quetin-Leclercq, J.; et al. Spelt (Triticum aestivum ssp. spelta) as a Source of Breadmaking Flours and Bran Naturally Enriched in Oleic Acid and Minerals but Not Phytic Acid. J. Agric. Food Chem. 2005, 53, 2751–2759. [Google Scholar] [CrossRef]
- Evans, J.; Neeson, R.; Burnett, V.; Luckett, D.J.; Fettell, N.A. Phosphorus-use efficiency, growth and yield of spelt wheat (Triticum aestivum ssp. spelta) compared with standard wheat (T. aestivum ssp. vulgare) in south-eastern Australia. J. Org. Syst. 2014, 9, 63–78. [Google Scholar]
- Troccoli, A.; Codianni, P. Appropriate seeding rate for einkorn, emmer, and spelt grown under rainfed condition in southern Italy. Eur. J. Agron. 2005, 22, 293–300. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Wang, H.; Arakawa, O.; Motomura, Y. Influence of maturity and bagging on the relationship between anthocyanin accumulation and phenylalanine ammonia-lyase (PAL) activity in ‘Jonathan’ apples. Postharvest Biol. Technol. 2000, 19, 123–128. [Google Scholar] [CrossRef]
- Balboa, K.; Ballesteros, G.I.; Molina-Montenegro, M.A. Integration of Physiological and Molecular Traits Would Help to Improve the Insights of Drought Resistance in Highbush Blueberry Cultivars. Plants 2020, 9, 1457. [Google Scholar] [CrossRef] [PubMed]
- Radzikowska, D.; Grzanka, M.; Kowalczewski, P.Ł.; Głowicka-Wołoszyn, R.; Blecharczyk, A.; Nowicki, M.; Sawinska, Z. Influence of SDHI Seed Treatment on the Physiological Conditions of Spring Barley Seedlings under Drought Stress. Agronomy 2020, 10, 731. [Google Scholar] [CrossRef]
- Michael, G. Biplots in Practice; Fundacion BBVA: Bilbao, Spain, 2010; p. 2011113. [Google Scholar]
- Ward, J.H. Hierarchical grouping to optimize anobjective function. J. Am. Stat. Assoc. 1963, 69, 236–244. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought Stress in Plants: An Overview. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–33. [Google Scholar]
- Kollist, H.; Zandalinas, S.I.; Sengupta, S.; Nuhkat, M.; Kangasjärvi, J.; Mittler, R. Rapid Responses to Abiotic Stress: Priming the Landscape for the Signal Transduction Network. Trends Plant Sci. 2019, 24, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Bota, J.; Galmés, J.; Medrano, H.; Ribas-Carbó, M. Keeping a positive carbon balance under adverse conditions: Responses of photosynthesis and respiration to water stress. Physiol. Plant. 2006, 127, 343–352. [Google Scholar] [CrossRef]
- Gruszka-Vendruscolo, E.C.; Schuster, I.; Pileggi, M.; Scapim, C.A.; Molinari, H.B.C.; Marur, C.J.; Vieira, L.G.E. Stress-induced synthesis of proline confers tolerance to water deficit in transgenic wheat. J. Plant Physiol. 2007, 164, 1367–1376. [Google Scholar] [CrossRef]
- Chen, W.P.; Li, P.H. Membrane stabilization by abscisic acid under cold aids proline in alleviating chilling injury in maize (Zea mays L.) cultured cells. Plant. Cell Environ. 2002, 25, 955–962. [Google Scholar] [CrossRef]
- Nayyar, H. Accumulation of osmolytes and osmotic adjustment in water-stressed wheat (Triticum aestivum) and maize (Zea mays) as affected by calcium and its antagonists. Environ. Exp. Bot. 2003, 50, 253–264. [Google Scholar] [CrossRef]
- Zhu, X.; Gong, H.; Chen, G.; Wang, S.; Zhang, C. Different solute levels in two spring wheat cultivars induced by progressive field water stress at different developmental stages. J. Arid Environ. 2005, 62, 1–14. [Google Scholar] [CrossRef]
- Chandler, J.W.; Bartels, D. Drought avoidance and drought adaptation. In Encyclopedia Water Science, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2003; Volume 55, pp. 163–165. [Google Scholar]
- Dhanda, S.S.; Sethi, G.S.; Behl, R.K. Indices of Drought Tolerance in Wheat Genotypes at Early Stages of Plant Growth. J. Agron. Crop Sci. 2004, 190, 6–12. [Google Scholar] [CrossRef]
- Raghavendra, A.S.; Reddy, K.B. Action of Proline on Stomata Differs from That of Abscisic Acid, G-Substances, or Methyl Jasmonate. Plant Physiol. 1987, 83, 732–734. [Google Scholar] [CrossRef]
- Shao, H.B.; Liang, Z.S.; Shao, M.A.; Wang, B.C. Changes of anti-oxidative enzymes and membrane peroxidation for soil water deficits among 10 wheat genotypes at seedling stage. Colloids Surf. B 2005, 42, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Pietragalla, J.; Mullan, D. Physiological Breeding II: A Field Guide to Wheat Phenotyping; Pask, A., Pietragalla, J., Mullan, D., Reynolds, M., Eds.; The International Maize and Wheat Improvement Center, CIMMYT: Mexico City, Mexico, 2012; pp. 25–27. [Google Scholar]
- Keyvan, S. The effects of drought stress on yield, relative water content, proline, soluble carbohydrates and chlorophyll of bread wheat cultivars. J. Anim. Plant Sci. 2010, 8, 1051–1060. [Google Scholar]
- Dib, T.A.; Monneveux, P.; Acevedo, E.; Nachit, M.M. Evaluation of proline analysis and chlorophyll fluorescence quenching measurements as drought tolerance indicators in durum wheat (Triticum turgidum L. var. durum). Euphytica 1994, 79, 65–73. [Google Scholar] [CrossRef]
- Krause, G.H.; Weis, E. Chlorophyll fluorescence as a tool in plant physiology. Photosynth. Res. 1984, 5, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Cetner, M.D.; Dąbrowski, P.; Samborska, I.A.; Łukasik, I.; Swoczyna, T.; Pietkiewicz, S.; Bąba, W.; Kalaji, H.M. Chlorophyll fluorescence measurements in environmental studies. Kosmos 2016, 65, 197–205. [Google Scholar]
- Havaux, M.; Lannoye, R. Drought resistance of hard wheat cultivars measured by a rapid chlorophyll fluorescence test. J. Agric. Sci. 1985, 104, 501–504. [Google Scholar] [CrossRef]
- Gilmore, A.; Björkman, O. Temperature-sensitive coupling and uncoupling of ATPase-mediated, nonradiative energy dissipation: Similarities between chloroplasts and leaves. Planta 1995, 197, 646–654. [Google Scholar] [CrossRef]
- Zlatev, Z. Drought-Induced Changes in Chlorophyll Fluorescence of Young Wheat Plants. Biotechnol. Biotechnol. Equip. 2009, 23, 438–441. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Łoboda, T. Fluorescencja Chlorofilu w Badaniach Stanu Fizjologicznego Roślin; Wydawnictwo SGGW: Warszawa, Poland, 2010; pp. 1–116. [Google Scholar]
- Lu, C.; Zhang, J. Effects of water stress on photosynthesis, chlorophyll fluorescence and photoinhibition in wheat plants. Funct. Plant Biol. 1998, 25, 883–892. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta Gen. Subj. 1989, 1, 87–92. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Grieco, M.; Tikkanen, M.; Paakkarinen, V.; Kangasja¨rvi, S.; Aro, E.M. Steady-state phosphorylation of light-harvesting complex II proteins preserves photosystem I under fluctuating white light. Plant Physiol. 2012, 160, 1896–1910. [Google Scholar] [CrossRef] [PubMed]
- Sayed, O.H. Chlorophyll Fluorescence as a Tool in Cereal Crop Research. Photosynthetica 2003, 41, 321–330. [Google Scholar] [CrossRef]
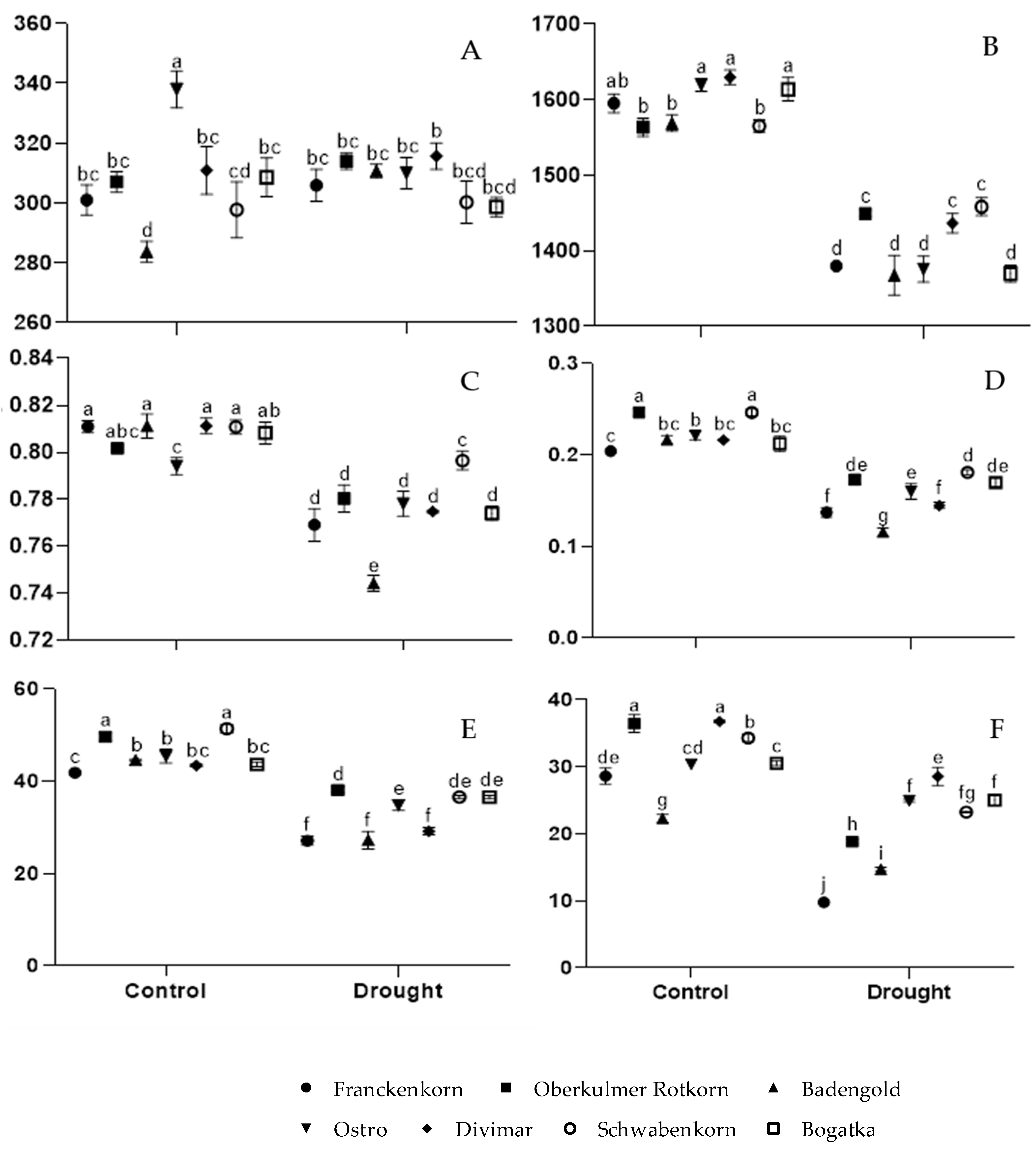


| Cultivars | A (µmol·m−2·s−1) | E (mmol·m−2·s−1) | Gs (mol·m−2·s−1) | Ci (vpm) | ||||
|---|---|---|---|---|---|---|---|---|
| Control | Drought | Control | Drought | Control | Drought | Control | Drought | |
| Franckenkorn | 6.89 d | 5.02 e | 1.18 e | 1.18 e | 0.07 e | 0.04 f | 182.6 f | 160.0 g |
| Oberkulmer Rotkorn | 4.29 f | 0.34 j | 0.79 fg | 0.06 l | 0.04 f | 0.00 i | 373.9 a | 162.1 g |
| Badengold | 9.67 a | 2.95 g | 1.33 c | 1.23 d | 0.11 a | 0.02 g | 201.1 e | 169.2 g |
| Ostro | 7.63 c | 2.69 gh | 1.49 b | 0.51 h | 0.08 d | 0.02 g | 291.7 c | 143.9 g |
| Divimar | 4.24 f | 1.67 i | 0.84 f | 0.33 j | 0.04 f | 0.01 h | 298.7 c | 136.3 gh |
| Schwabenkorn | 7.62 c | 0.16 j | 1.61 a | 0.14 k | 0.09 c | 0.00 i | 321.3 b | 193.1 f |
| Bogatka | 8.26 b | 2.50 h | 1.50 b | 0.39 i | 0.08 d | 0.01 h | 270.1 d | 137.3 gh |
| LSD α0.05 | 0.533 | 0.065 | 0.005 | 29.05 | ||||
| Cultivars | RWC (%) | MDA (μmol·g−1 d.m.) | ||
|---|---|---|---|---|
| Control | Drought | Control | Drought | |
| Franckenkorn | 89.2 b | 72.1 d | 0.26 c | 0.31 c |
| Oberkulmer Rotkorn | 88.3 b | 71.3 d | 0.25 c | 0.40 b |
| Badengold | 87.5 b | 71.0 d | 0.29 c | 0.39 b |
| Ostro | 88.4 b | 64.1 e | 0.27 c | 0.33 b |
| Divimar | 91.5 ab | 64.1 e | 0.25 c | 0.50 a |
| Schwabenkorn | 91.2 ab | 54.1 f | 0.29 c | 0.44 a |
| Bogatka | 93.1 a | 85.9 c | 0.31 c | 0.42 ab |
| LSD α0.05 | 2.99 | 0.09 | ||
| Cultivars | Proline (mg·g−1 d.m.) | Anthocyanins (µg∙g−1 d.m.) | ||
|---|---|---|---|---|
| Control | Drought | Control | Drought | |
| Franckenkorn | 0.6 e | 11.8 b | 159.5 e | 514.1 a |
| Oberkulmer Rotkorn | 0.4 e | 9.8 c | 119.4 f | 547.6 a |
| Badengold | 1.0 e | 19.7 a | 199.3 e | 302.1 d |
| Ostro | 0.4 e | 6.7 c | 141.9 ef | 426.8 b |
| Divimar | 0.7 e | 9.7 c | 112.2 f | 513.2 a |
| Schwabenkorn | 0.4 e | 13.8 b | 142.9 ef | 426.4 b |
| Bogatka | 0.7 e | 5.5 d | 191.4 e | 378.2 c |
| LSD α0.05 | 3.97 | 79.88 | ||
| Cultivars | PPAC (%) | PPAwd (%) | DRI (PPAwd/PPAc) |
|---|---|---|---|
| Franckenkorn | 73 | 58.9 | 0.811 |
| Oberkulmer Rotkorn | 73 | 43.6 | 0.595 |
| Badengold | 73 | 46.1 | 0.636 |
| Ostro | 72 | 48.3 | 0.666 |
| Divimar | 74 | 48.9 | 0.664 |
| Schwabenkorn | 74 | 38.5 | 0.518 |
| Bogatka | 74 | 52.6 | 0.713 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radzikowska, D.; Sulewska, H.; Bandurska, H.; Ratajczak, K.; Szymańska, G.; Kowalczewski, P.Ł.; Głowicka-Wołoszyn, R. Analysis of Physiological Status in Response to Water Deficit of Spelt (Triticum aestivum ssp. spelta) Cultivars in Reference to Common Wheat (Triticum aestivum ssp. vulgare). Agronomy 2022, 12, 1822. https://doi.org/10.3390/agronomy12081822
Radzikowska D, Sulewska H, Bandurska H, Ratajczak K, Szymańska G, Kowalczewski PŁ, Głowicka-Wołoszyn R. Analysis of Physiological Status in Response to Water Deficit of Spelt (Triticum aestivum ssp. spelta) Cultivars in Reference to Common Wheat (Triticum aestivum ssp. vulgare). Agronomy. 2022; 12(8):1822. https://doi.org/10.3390/agronomy12081822
Chicago/Turabian StyleRadzikowska, Dominika, Hanna Sulewska, Hanna Bandurska, Karolina Ratajczak, Grażyna Szymańska, Przemysław Łukasz Kowalczewski, and Romana Głowicka-Wołoszyn. 2022. "Analysis of Physiological Status in Response to Water Deficit of Spelt (Triticum aestivum ssp. spelta) Cultivars in Reference to Common Wheat (Triticum aestivum ssp. vulgare)" Agronomy 12, no. 8: 1822. https://doi.org/10.3390/agronomy12081822
APA StyleRadzikowska, D., Sulewska, H., Bandurska, H., Ratajczak, K., Szymańska, G., Kowalczewski, P. Ł., & Głowicka-Wołoszyn, R. (2022). Analysis of Physiological Status in Response to Water Deficit of Spelt (Triticum aestivum ssp. spelta) Cultivars in Reference to Common Wheat (Triticum aestivum ssp. vulgare). Agronomy, 12(8), 1822. https://doi.org/10.3390/agronomy12081822











