Individual and Combined Effects of High-Temperature Stress at Booting and Flowering Stages on Rice Grain Yield
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Measurement of Grain Yield Parameters
2.3. Calculation of Heat Degree Days
2.4. Statistical Analysis
3. Results
3.1. Analysis of Variance for Yield and Yield Components
3.2. Effects of Extreme High-Temperature Stress on Grain Yield
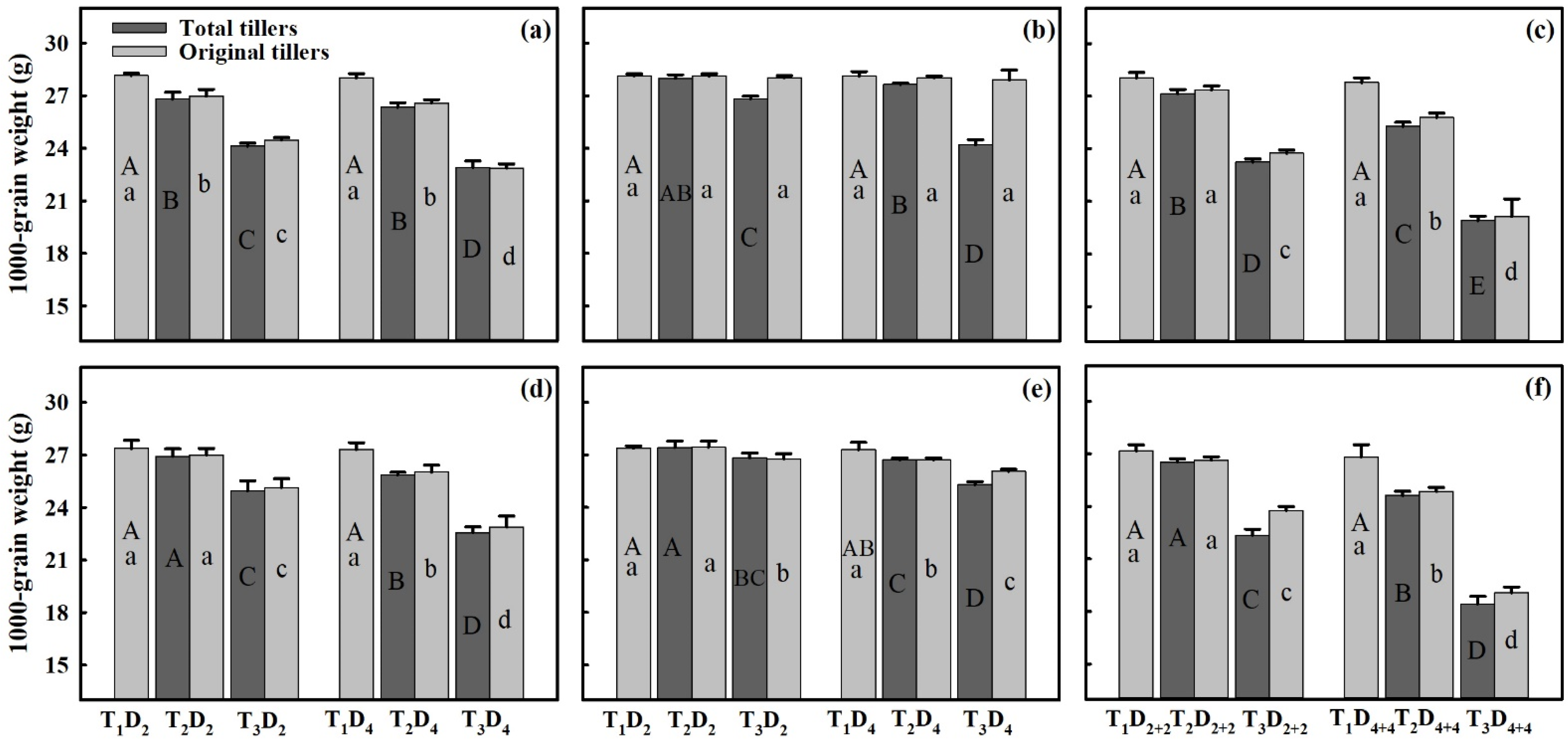
3.3. Effect of Extreme High-Temperature Stress on Yield Components
3.4. Multivariate Analysis between Grain Yield and Yield Components under Heat Stress
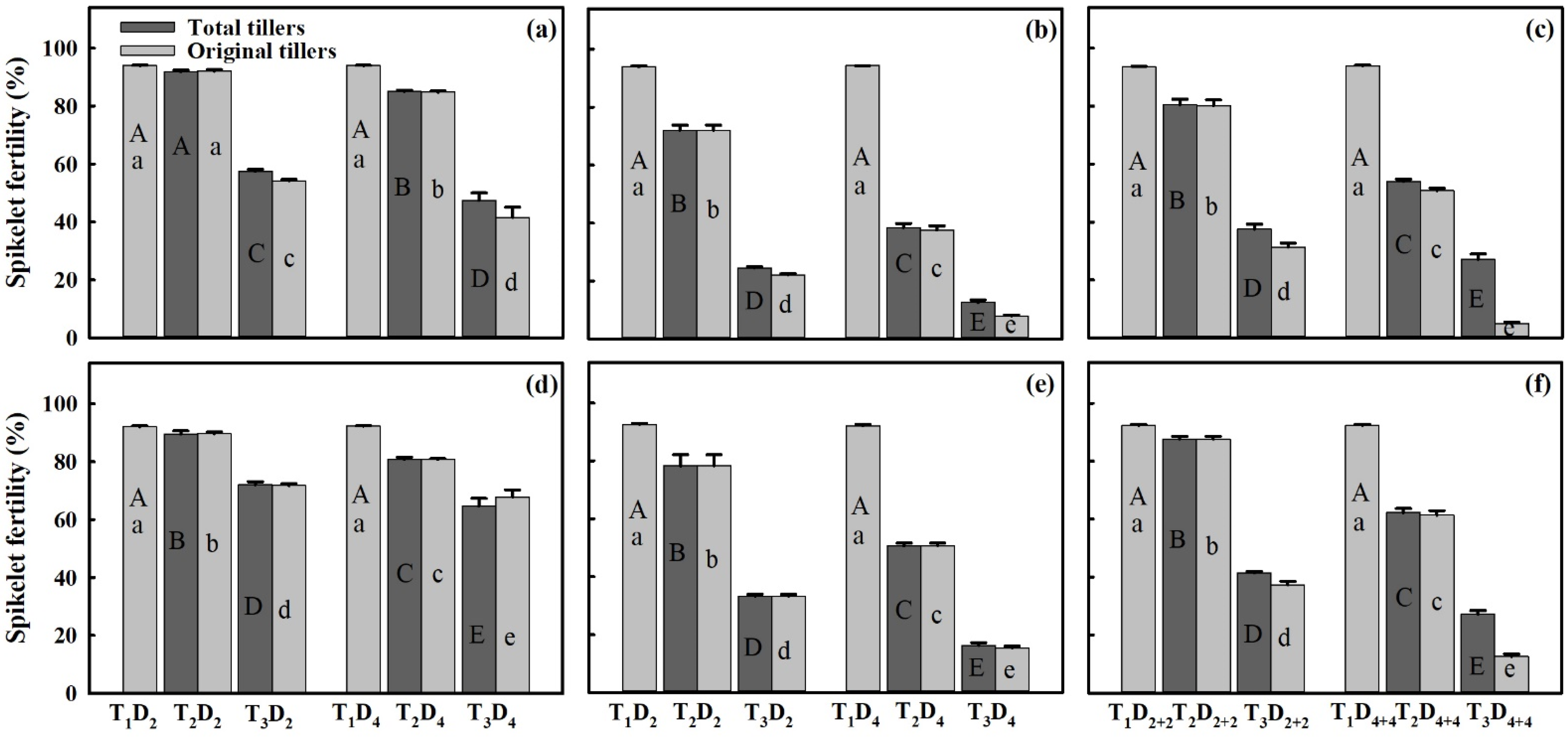
3.5. Spikelet Fertility (%) in Response to HDD at Various Panicle Positions
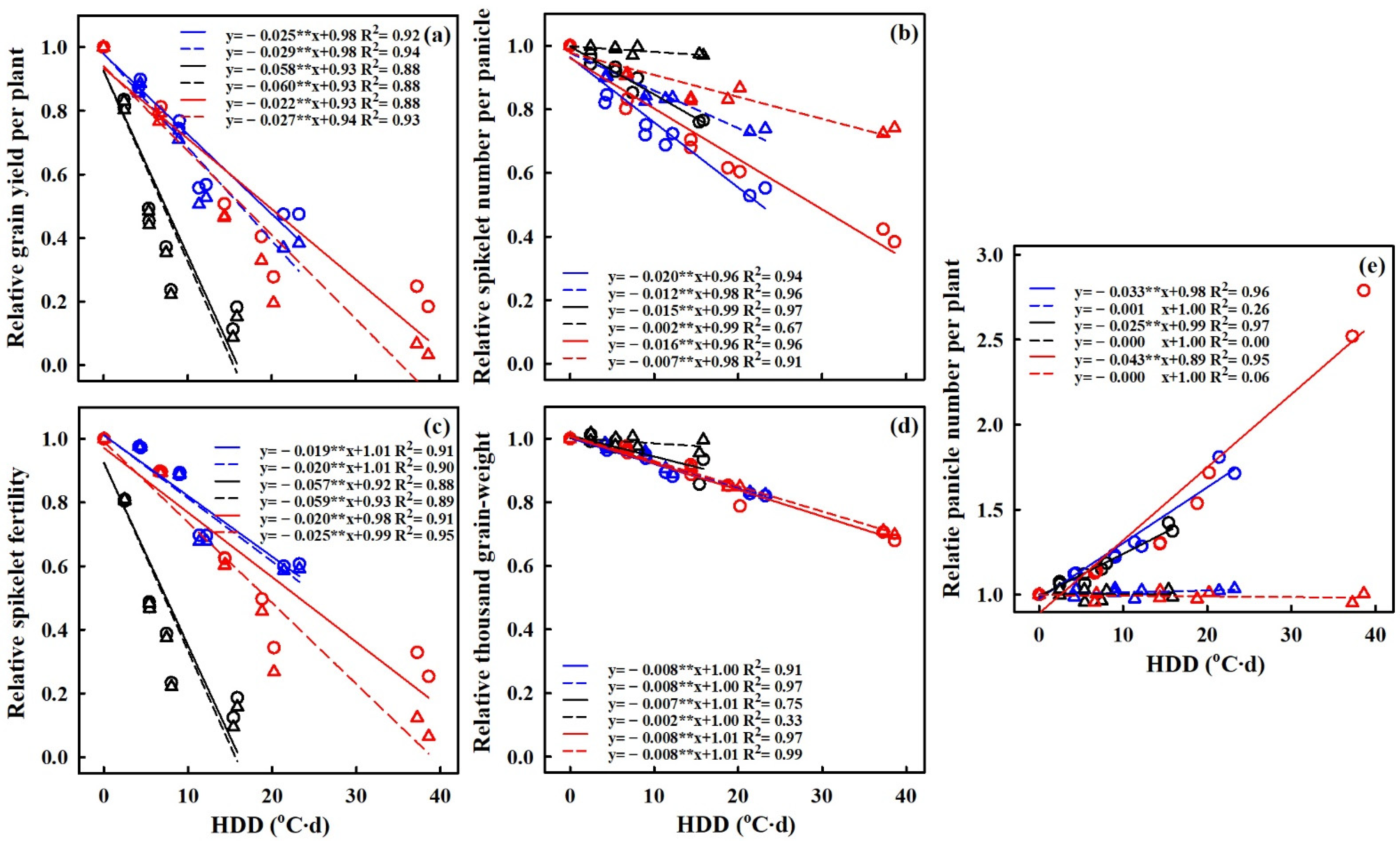
3.6. Quantification of Heat Stress Effects on Grain Yield and Yield Components
4. Discussion
4.1. Responses of Rice Yield and Yield Components to High Temperature
4.2. Impact of High Temperature on Grain Yield-Forming Traits
4.3. Comparison of Seed Setting Rate at Various Positions and Relationship of Spikelet Fertility (%) with Vapor Pressure Deficit
4.4. The Relationship of HDD with the Effect of Extreme High-Temperature Stress on Grain Yield and Yield Components
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- IPCC. Special Report in 2018 on the Impacts of Global Warming of 1.5 °C. The Physical Science Basis, Summary for Policymakers; IPCC: Geneva, Switzerland, 2018; Volume 15. [Google Scholar]
- Lobell, D.B.; Sibley, A.; Ortiz-Monasterio, J.I. Extreme heat effects on wheat senescence in India. Nat. Clim. Chang. 2012, 2, 186. [Google Scholar] [CrossRef]
- Krishnan, P.; Ramakrishnan, B.; Reddy, K.R.; Reddy, V. High-Temperature Effects on Rice Growth, Yield, and Grain Quality. Adv. Agron. 2011, 111, 87–206. [Google Scholar]
- Buckley, L.B.; Huey, R.B. Temperature extremes: Geographic patterns, recent changes, and implications for organismal vulnerabilities. Glob. Chang. Biol. 2016, 22, 3829–3842. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Hasegawa, T.; Liu, B.; Tang, L.; Liu, L.; Cao, W.; Zhu, Y. Current rice models underestimate yield losses from short-term heat stresses. Glob. Chang. Biol. 2021, 27, 402–416. [Google Scholar] [CrossRef] [PubMed]
- Fageria, N. Yield physiology of rice. J. Plant Nutr. 2007, 30, 843–879. [Google Scholar] [CrossRef]
- Fu, J.; Huang, Z.; Wang, Z.; Yang, J.; Zhang, J. Pre-anthesis non-structural carbohydrate reserve in the stem enhances the sink strength of inferior spikelets during grain filling of rice. Field Crops Res. 2011, 123, 170–182. [Google Scholar] [CrossRef]
- Prabhakar, S.V. Climate change impacts in Japan and Southeast Asia: Implications for crop adaptation. In Crop Adaptation to Climate Change; Wiley: Hoboken, NJ, USA, 2011; pp. 131–142. [Google Scholar] [CrossRef]
- Gourdji, S.M.; Sibley, A.M.; Lobell, D.B. Global crop exposure to critical high temperatures in the reproductive period: Historical trends and future projections. Environ. Res. Lett. 2013, 8, 024041. [Google Scholar] [CrossRef]
- Shi, P.; Zhu, Y.; Tang, L.; Chen, J.; Sun, T.; Cao, W.; Tian, Y. Differential effects of temperature and duration of heat stress during anthesis and grain filling stages in rice. Environ. Exp. Bot. 2016, 132, 28–41. [Google Scholar] [CrossRef]
- Zhao, C.; Piao, S.; Wang, X.; Huang, Y.; Ciais, P.; Elliott, J.; Huang, M.; Janssens, I.A.; Li, T.; Lian, X. Plausible rice yield losses under future climate warming. Nat. Plants 2016, 3, 16202. [Google Scholar] [CrossRef] [Green Version]
- Ceccarelli, S.; Grando, S.; Maatougui, M.; Michael, M.; Slash, M.; Haghparast, R.; Rahmanian, M.; Taheri, A.; Al-Yassin, A.; Benbelkacem, A. Plant breeding and climate changes. J. Agric. Sci. 2010, 148, 627–637. [Google Scholar] [CrossRef]
- Wahid, A. Physiological implications of metabolite biosynthesis for net assimilation and heat-stress tolerance of sugarcane (Saccharum officinarum) sprouts. J. Plant Res. 2007, 120, 219–228. [Google Scholar] [CrossRef]
- Wassmann, R.; Jagadish, S.; Heuer, S.; Ismail, A.; Redona, E.; Serraj, R.; Singh, R.; Howell, G.; Pathak, H.; Sumfleth, K. Climate change affecting rice production: The physiological and agronomic basis for possible adaptation strategies. Adv. Agron. 2009, 101, 59–122. [Google Scholar]
- Sánchez, B.; Rasmussen, A.; Porter, J.R. Temperatures and the growth and development of maize and rice: A review. Glob. Chang. Biol. 2014, 20, 408–417. [Google Scholar] [CrossRef]
- Espe, M.B.; Hill, J.E.; Hijmans, R.J.; McKenzie, K.; Mutters, R.; Espino, L.A.; Leinfelder-Miles, M.; van Kessel, C.; Linquist, B.A. Point stresses during reproductive stage rather than warming seasonal temperature determine yield in temperate rice. Glob. Chang. Biol. 2017, 23, 4386–4395. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S. Fundamentals of Rice Crop Science; The International Rice Research Institute: Manilla, Philippines, 1981. [Google Scholar]
- Counce, P.A.; Keisling, T.C.; Mitchell, A.J. A uniform, objective, and adaptive system for expressing rice development. Crop Sci. 2000, 40, 436–443. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, A.; Wang, W.; Ali, I.; Zhen, F.; Osman, R.; Liu, B.; Liu, L.; Zhu, Y.; Cao, W.; Tang, L. Individual and Combined Effects of Booting and Flowering High-Temperature Stress on Rice Biomass Accumulation. Plants 2021, 10, 1021. [Google Scholar] [CrossRef]
- Peterson, M.; Lin, S.; Jones, D.; Rutger, J. Cool night temperatures cause sterility in rice. Calif. Agric. 1974, 28, 12–14. [Google Scholar]
- Satake, T.; Yoshida, S. High temperature-induced sterility in indica rices at flowering. Jpn. J. Crop Sci. 1978, 47, 6–17. [Google Scholar] [CrossRef] [Green Version]
- Jagadish, S.; Craufurd, P.; Wheeler, T. High temperature stress and spikelet fertility in rice (Oryza sativa L.). J. Exp. Bot. 2007, 58, 1627–1635. [Google Scholar] [CrossRef] [Green Version]
- Endo, M.; Tsuchiya, T.; Hamada, K.; Kawamura, S.; Yano, K.; Ohshima, M.; Higashitani, A.; Watanabe, M.; Kawagishi-Kobayashi, M. High temperatures cause male sterility in rice plants with transcriptional alterations during pollen development. Plant Cell Physiol. 2009, 50, 1911–1922. [Google Scholar] [CrossRef]
- Zahra, N.; Wahid, A.; Hafeez, M.B.; Ullah, A.; Siddique, K.H.; Farooq, M. Grain development in wheat under combined heat and drought stress: Plant responses and management. Environ. Exp. Bot. 2021, 188, 104517. [Google Scholar] [CrossRef]
- Wu, C.; Cui, K.; Fahad, S. Heat stress decreases rice grain weight: Evidence and physiological mechanisms of heat effects prior to flowering. Int. J. Mol. Sci. 2022, 23, 10922. [Google Scholar] [CrossRef] [PubMed]
- Larkindale, J.; Hall, J.D.; Knight, M.R.; Vierling, E. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 2005, 138, 882–897. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.-Y.; Chai, K.-H.; Ko, S.-S.; Kuang, L.-Y.; Lur, H.-S.; Charng, Y.-Y. A positive feedback loop between HEAT SHOCK PROTEIN101 and HEAT STRESS-ASSOCIATED 32-KD PROTEIN modulates long-term acquired thermotolerance illustrating diverse heat stress responses in rice varieties. Plant Physiol. 2014, 164, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, R.P.; Misra, H.S.; Saini, A. Heat-stress priming and alternative splicing-linked memory. J. Exp. Bot. 2018, 69, 2431–2434. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.A.; Li, Z.-G.; Hoque, T.S.; Burritt, D.J.; Fujita, M.; Munné-Bosch, S. Heat or cold priming-induced cross-tolerance to abiotic stresses in plants: Key regulators and possible mechanisms. Protoplasma 2018, 255, 399–412. [Google Scholar] [CrossRef]
- Wang, X.; Cai, J.; Jiang, D.; Liu, F.; Dai, T.; Cao, W. Pre-anthesis high-temperature acclimation alleviates damage to the flag leaf caused by post-anthesis heat stress in wheat. J. Plant Physiol. 2011, 168, 585–593. [Google Scholar] [CrossRef]
- Shi, W.; Lawas, L.; Raju, B.; Jagadish, S. Acquired thermo-tolerance and trans-generational heat stress response at flowering in rice. J. Agron. Crop Sci. 2016, 202, 309–319. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, C.; Huang, Z.; Abid, M.; Jiang, S.; Dai, T.; Zhang, W.; Ma, S.; Jiang, D.; Han, X. Heat priming during early reproductive stages enhances thermo-tolerance to post-anthesis heat stress via improving photosynthesis and plant productivity in winter wheat (Triticum aestivum L.). Front. Plant. Sci. 2018, 9, 805. [Google Scholar] [CrossRef]
- Shi, C.; Jin, Z.; Zheng, J.; Tang, R. Quantitative analysis on the effects of high temperature at meiosis stage on seed-setting rate of rice florets. Acta Agron. Sin. 2008, 34, 627. [Google Scholar] [CrossRef]
- Wu, C.; Cui, K.; Tang, S.; Li, G.; Wang, S.; Fahad, S.; Nie, L.; Huang, J.; Peng, S.; Ding, Y. Intensified pollination and fertilization ameliorate heat injury in rice (Oryza sativa L.) during the flowering stage. Field Crops Res. 2020, 252, 107795. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Li, L.; Zhou, Q.; Xiao, Y.; Wei, X.; Zhou, M. Short-term complete submergence of rice at the tillering stage increases yield. PLoS ONE 2015, 10, e0127982. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Liu, F.-L.; Jiang, D. Priming: A promising strategy for crop production in response to future climate. J. Integr. Agric. 2017, 16, 2709–2716. [Google Scholar]
- Wu, C.; Cui, K.; Wang, W.; Li, Q.; Fahad, S.; Hu, Q.; Huang, J.; Nie, L.; Peng, S. Heat-induced phytohormone changes are associated with disrupted early reproductive development and reduced yield in rice. Sci. Rep. 2016, 6, 34978. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Cai, J.; Liu, F.; Jin, M.; Yu, H.; Jiang, D.; Wollenweber, B.; Dai, T.; Cao, W. Pre-anthesis high temperature acclimation alleviates the negative effects of post-anthesis heat stress on stem stored carbohydrates remobilization and grain starch accumulation in wheat. J. Cereal Sci. 2012, 55, 331–336. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Xiao, L.; Xia, Y.; Song, H.; Liu, B.; Tang, L.; Cao, W.; Zhu, Y.; Liu, L. Effects of jointing and booting low temperature stresses on grain yield and yield components in wheat. Agric. For. Meteorol. 2017, 243, 33–42. [Google Scholar] [CrossRef]
- Chimenti, C.; Hall, A. Grain number responses to temperature during floret differentiation in sunflower. Field Crops Res. 2001, 72, 177–184. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Zhou, J.; Hu, S.; Chen, H.; Xiang, J.; Zhang, Y.; Zeng, Y.; Shi, Q.; Zhu, D. Research progress on heat stress of rice at flowering stage. Rice Sci. 2019, 26, 1–10. [Google Scholar] [CrossRef]
- Yun-Ying, C.; Hua, D.; Li-Nian, Y.; Zhi-Qing, W.; Shao-Chuan, Z.; Jian-Chang, Y. Effect of heat stress during meiosis on grain yield of rice cultivars differing in heat tolerance and its physiological mechanism. Acta Agron. Sin. 2008, 34, 2134–2142. [Google Scholar]
- Zhen, F.; Zhou, J.; Mahmood, A.; Wang, W.; Chang, X.; Liu, B.; Liu, L.; Cao, W.; Zhu, Y.; Tang, L. Quantifying the effects of short-term heat stress at booting stage on nonstructural carbohydrates remobilization in rice. Crop J. 2020, 8, 194–212. [Google Scholar] [CrossRef]
- Das, S.; Krishnan, P.; Nayak, M.; Ramakrishnan, B. High temperature stress effects on pollens of rice (Oryza sativa L.) genotypes. Environ. Exp. Bot. 2014, 101, 36–46. [Google Scholar] [CrossRef]
- Weerakoon, W.; Maruyama, A.; Ohba, K. Impact of humidity on temperature-induced grain sterility in rice (Oryza sativa L.). J. Agron. Crop Sci. 2008, 194, 135–140. [Google Scholar] [CrossRef]
- Wu, C.; Tang, S.; Li, G.; Wang, S.; Fahad, S.; Ding, Y. Roles of phytohormone changes in the grain yield of rice plants exposed to heat: A review. PeerJ 2019, 7, e7792. [Google Scholar] [CrossRef] [PubMed]
- Howarth, C.J. Abiotic Stresses: Plant Resistance through Breeding Molecular Approaches; Ashraf, M., Harris, P., Eds.; Food Products Press®, an Imprint of The Howarth Press, Inc.: Binghamton, NY, USA, 2005; Volume 10, p. 1920. [Google Scholar]
- Prasad, P.V.; Pisipati, S.; Mutava, R.; Tuinstra, M. Sensitivity of grain sorghum to high temperature stress during reproductive development. Crop Sci. 2008, 48, 1911–1917. [Google Scholar] [CrossRef] [Green Version]
- Morita, S.; Nakano, H. Nonstructural carbohydrate content in the stem at full heading contributes to high performance of ripening in heat-tolerant rice cultivar Nikomaru. Crop Sci. 2011, 51, 818–828. [Google Scholar] [CrossRef]
- Oh-e, I.; Saitoh, K.; Kuroda, T. Effects of high temperature on growth, yield and dry-matter production of rice grown in the paddy field. Plant Prod. Sci. 2007, 10, 412–422. [Google Scholar] [CrossRef]
- Wu, Q.-X.; Yang, L.; Shao, H.; Ran, C.-F.; Yang, Z.-B.; Yu, J.; Li, L.-Q.; Li, X.-J. Effects of low temperature stress at anther connective formation phase on physiological characteristics an yield of wheat. J. Triticeae Crops 2013, 3, 752–757. [Google Scholar]
- Talukder, A.; McDonald, G.K.; Gill, G.S. Effect of short-term heat stress prior to flowering and early grain set on the grain yield of wheat. Field Crops Res. 2014, 160, 54–63. [Google Scholar] [CrossRef]
- Zheng, D.; Yang, X.; Mínguez, M.I.; Mu, C.; He, Q.; Wu, X. Effect of freezing temperature and duration on winter survival and grain yield of winter wheat. Agric. For. Meteorol. 2018, 260, 1–8. [Google Scholar] [CrossRef]
- Prasad, P.; Boote, K.; Allen, L., Jr.; Sheehy, J.; Thomas, J. Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. Field Crops Res. 2006, 95, 398–411. [Google Scholar] [CrossRef]
- Lawas, L.M.; Bheemanahalli, R.; Solis, C.A.; Krishna Jagadish, S. Sheathed panicle phenotype (cv. Sathi) maintains normal spikelet fertility and grain filling under prolonged heat stress in rice. Crop Sci. 2018, 58, 1693–1705. [Google Scholar] [CrossRef]
- Sun, T.; Hasegawa, T.; Tang, L.; Wang, W.; Zhou, J.; Liu, L.; Liu, B.; Cao, W.; Zhu, Y. Stage-dependent temperature sensitivity function predicts seed-setting rates under short-term extreme heat stress in rice. Agric. For. Meteorol. 2018, 256, 196–206. [Google Scholar] [CrossRef]
- Julia, C.; Dingkuhn, M. Predicting temperature induced sterility of rice spikelets requires simulation of crop-generated microclimate. Eur. J. Agron. 2013, 49, 50–60. [Google Scholar] [CrossRef]
- Jagadish, S.; Craufurd, P.; Wheeler, T. Phenotyping parents of mapping populations of rice for heat tolerance during anthesis. Crop Sci. 2008, 48, 1140–1146. [Google Scholar] [CrossRef]
- Julia, C.; Dingkuhn, M. Variation in time of day of anthesis in rice in different climatic environments. Eur. J. Agron. 2012, 43, 166–174. [Google Scholar] [CrossRef]
- Matsui, T.; Omasa, K.; Horie, T. Mechanism of anther dehiscence in rice (Oryza sativa L.). Ann. Bot. 1999, 84, 501–506. [Google Scholar] [CrossRef] [Green Version]
- Jung, W.-S.; Lee, K.-J.; Lee, B.-W. Responses of spikelet fertility to air, spikelet, and panicle temperatures and vapor pressure deficit in rice. J. Crop Sci. Biotechnol. 2015, 18, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Matsui, T.; Kobayasi, K.; Yoshimoto, M.; Hasegawa, T. Stability of rice pollination in the field under hot and dry conditions in the Riverina region of New South Wales, Australia. Plant Prod. Sci. 2007, 10, 57–63. [Google Scholar] [CrossRef]
- Sathishraj, R.; Bheemanahalli, R.; Ramachandran, M.; Dingkuhn, M.; Muthurajan, R.; Krishna, J.S. Capturing heat stress induced variability in spikelet sterility using panicle, leaf and air temperature under field conditions. Field Crops Res. 2016, 190, 10–17. [Google Scholar] [CrossRef]
- Shi, P.; Tang, L.; Lin, C.; Liu, L.; Wang, H.; Cao, W.; Zhu, Y. Modeling the effects of post-anthesis heat stress on rice phenology. Field Crops Res. 2015, 177, 26–36. [Google Scholar] [CrossRef]
- González-Schain, N.; Dreni, L.; Lawas, L.M.; Galbiati, M.; Colombo, L.; Heuer, S.; Jagadish, K.S.; Kater, M.M. Genome-wide transcriptome analysis during anthesis reveals new insights into the molecular basis of heat stress responses in tolerant and sensitive rice varieties. Plant Cell Physiol. 2016, 57, 57–68. [Google Scholar] [CrossRef] [PubMed]
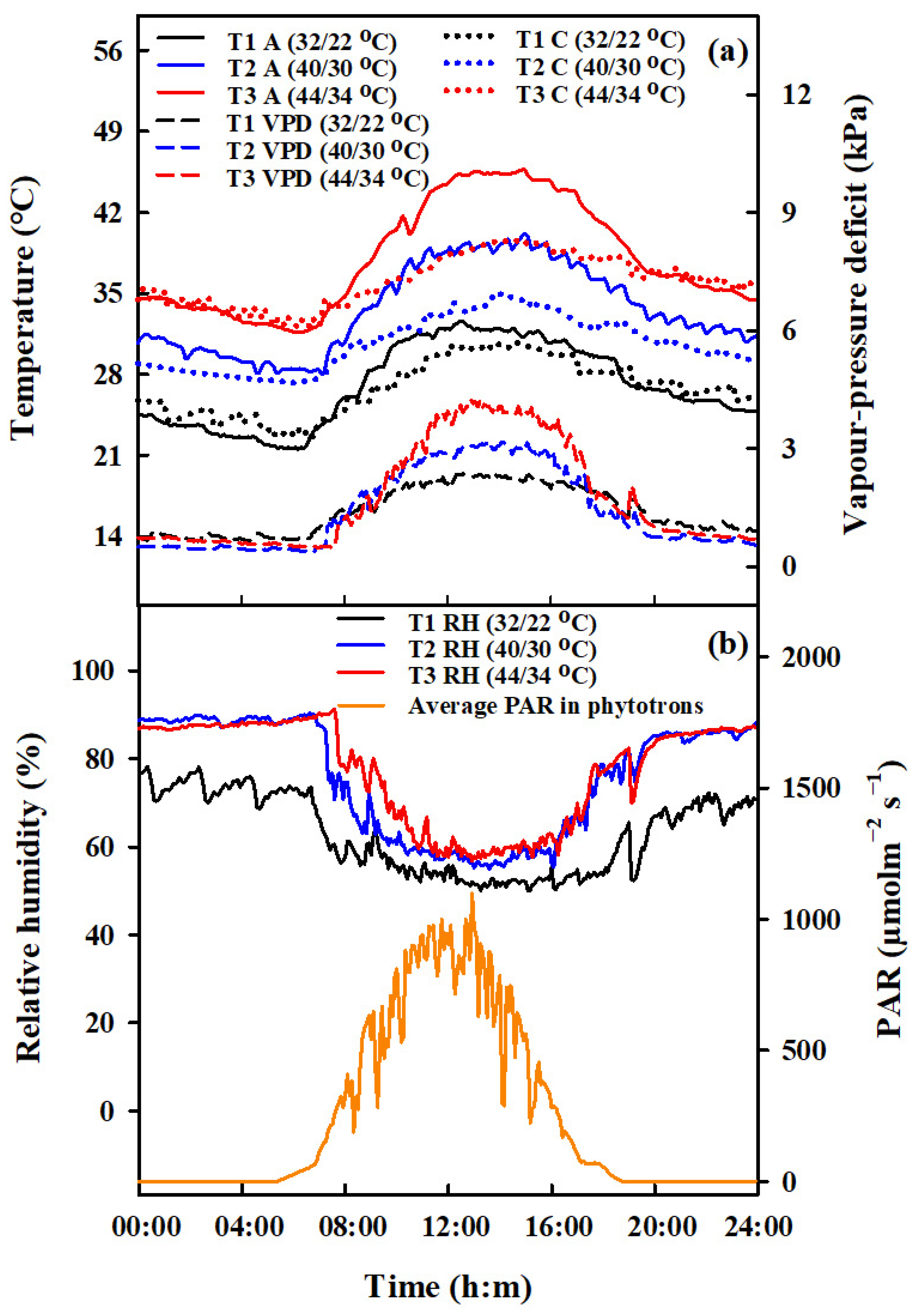
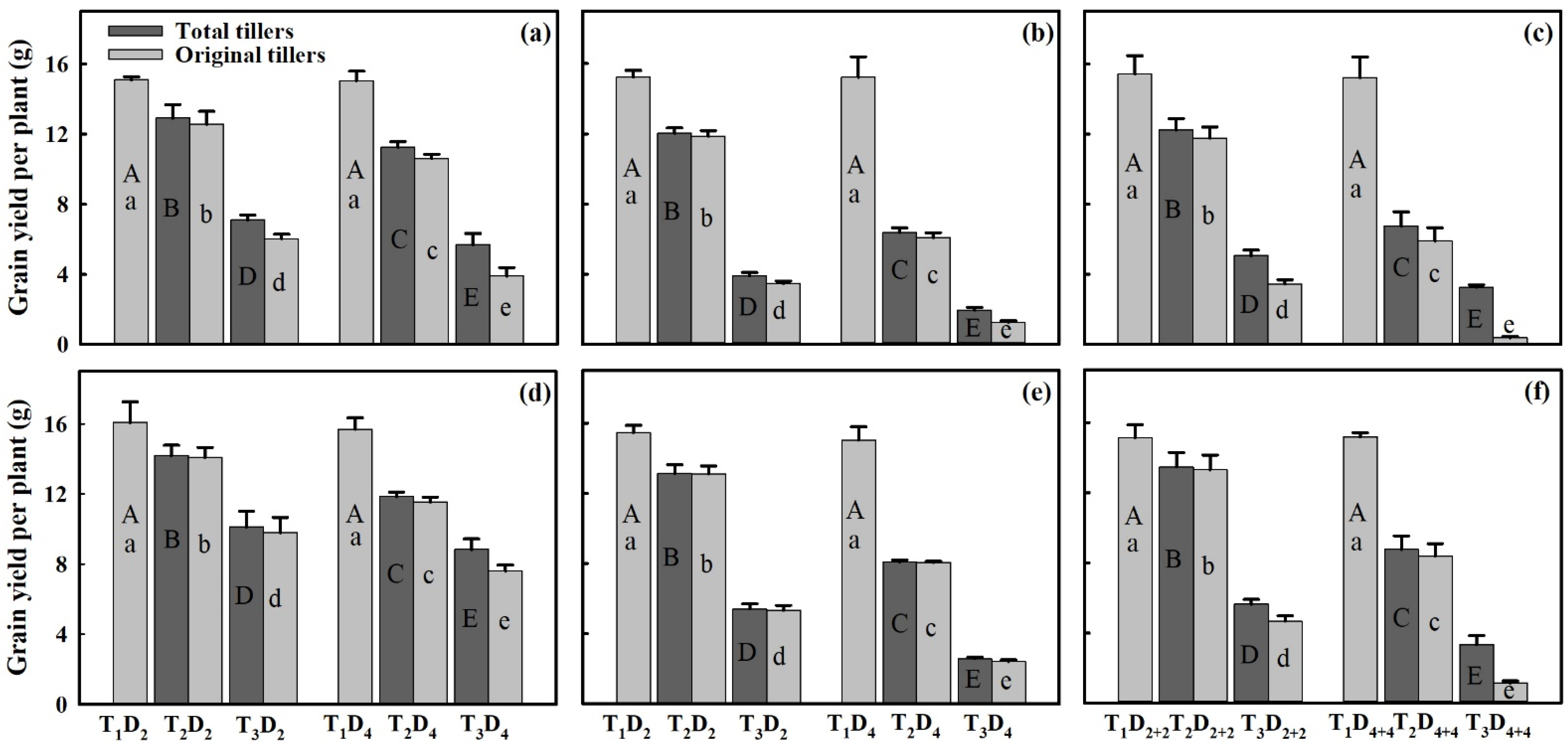
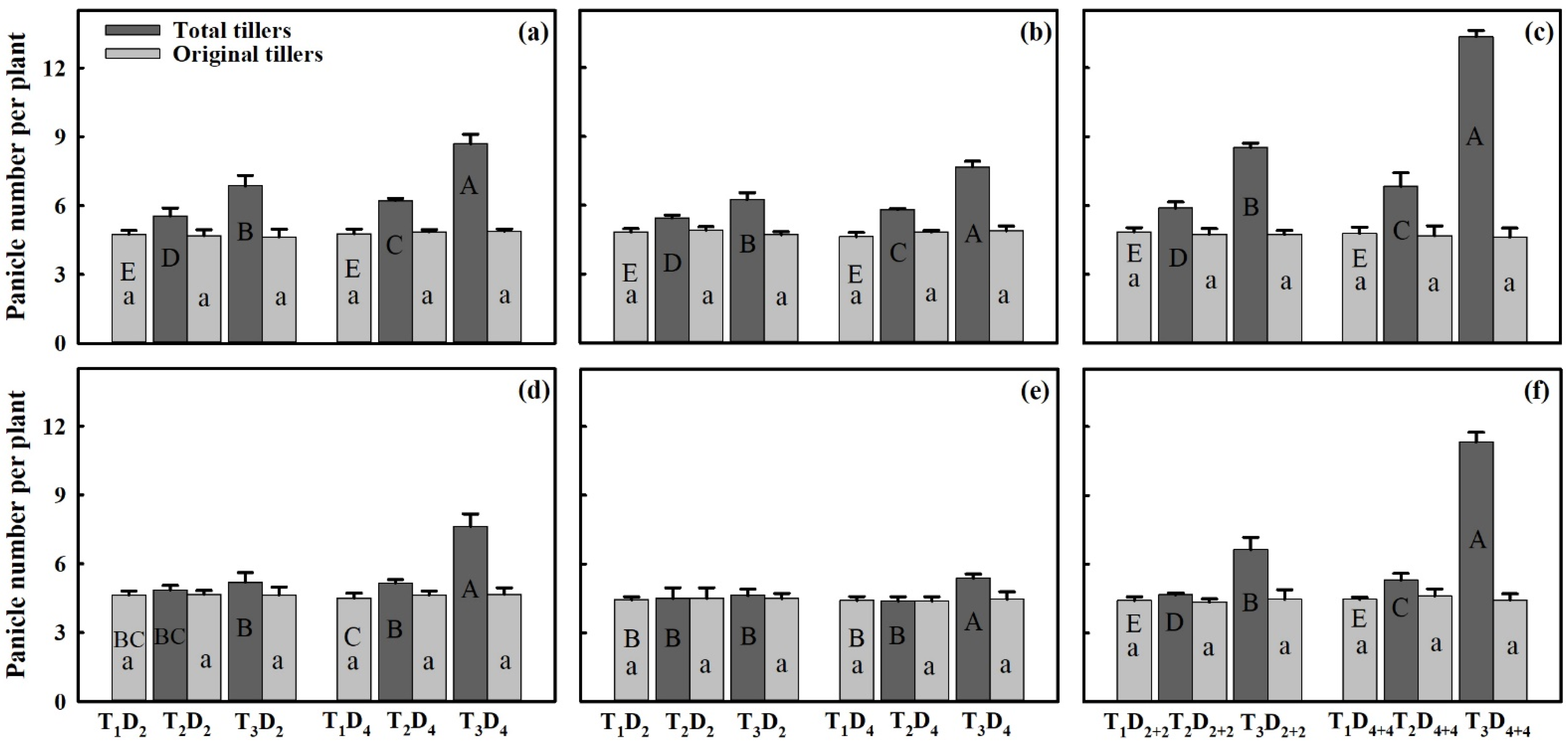
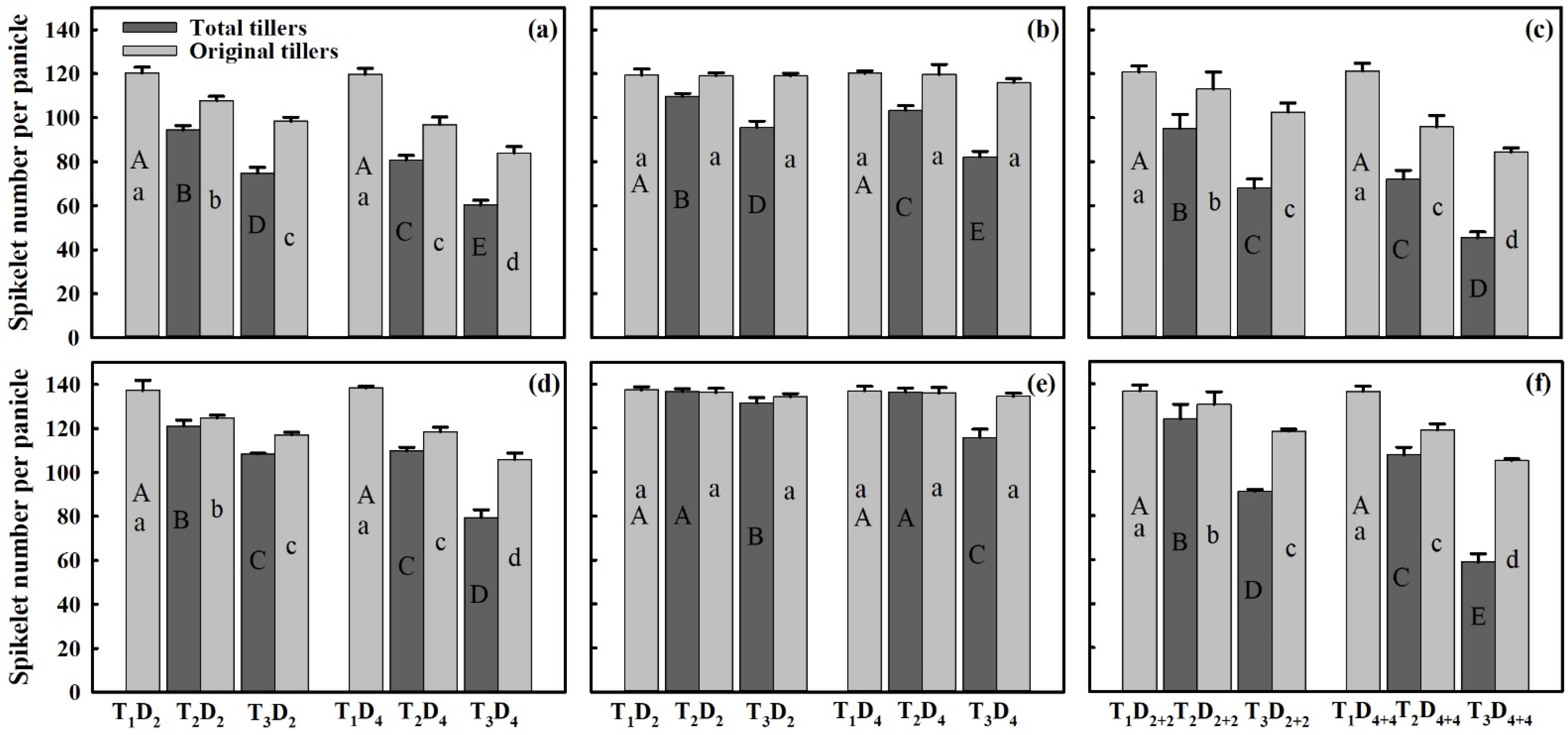

| Cultivar | Temperature Levels | Stage | Durations |
|---|---|---|---|
| Huaidao-5 and Wuyunjing-24 | T1 (32/22 °C) T2 (40/30 °C) T3 (44/34 °C) | Booting (BT) | D2 (2 days) and D4 (4 days) |
| Flowering (FL) | D2 (2 days) and D4 (4 days) | ||
| Combined (BT + FL) | D2+2 (4 days = 2 days BT + 2 days FL) and D4+4 (8 days = 4 days BT + 4 days FL) |
| Stage | Cultivar | Yield and Yield Components | T | D | T × D |
|---|---|---|---|---|---|
| Booting | Huaidao-5 | YPP | <0.001 | 0.001 | 0.034 |
| PNPP | <0.001 | <0.001 | 0.001 | ||
| SNPP | <0.001 | <0.001 | <0.001 | ||
| SF | <0.001 | <0.001 | <0.001 | ||
| TGW | <0.001 | 0.001 | 0.016 | ||
| Wuyunjing-24 | YPP | <0.001 | 0.003 | ns | |
| PNPP | <0.001 | <0.001 | <0.001 | ||
| SNPP | <0.001 | <0.001 | <0.001 | ||
| SF | <0.001 | <0.001 | <0.001 | ||
| TGW | <0.001 | <0.001 | 0.001 | ||
| Flowering | Huaidao-5 | YPP | <0.001 | <0.001 | <0.001 |
| PNPP | <0.001 | <0.001 | <0.001 | ||
| SNPP | <0.001 | <0.001 | 0.001 | ||
| SF | <0.001 | <0.001 | <0.001 | ||
| TGW | <0.001 | <0.001 | <0.001 | ||
| Wuyunjing-24 | YPP | <0.001 | <0.001 | <0.001 | |
| PNPP | 0.003 | ns | 0.025 | ||
| SNPP | <0.001 | <0.001 | <0.001 | ||
| SF | <0.001 | <0.001 | <0.001 | ||
| TGW | <0.001 | <0.001 | 0.002 | ||
| Combined stages | Huaidao-5 | YPP | <0.001 | <0.001 | <0.001 |
| PNPP | <0.001 | <0.001 | <0.001 | ||
| SNPP | <0.001 | <0.001 | <0.001 | ||
| SF | <0.001 | <0.001 | <0.001 | ||
| TGW | <0.001 | <0.001 | <0.001 | ||
| Wuyunjing-24 | YPP | <0.001 | <0.001 | <0.001 | |
| PNPP | <0.001 | <0.001 | <0.001 | ||
| SNPP | <0.001 | <0.001 | <0.001 | ||
| SF | <0.001 | <0.001 | <0.001 | ||
| TGW | <0.001 | <0.001 | <0.001 |
| Stage | Cultivar | Yield Components | Original Tillers | Total Tillers | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T2 | T3 | ||||||||
| SRC | Partial R2 | SRC | Partial R2 | SRC | Partial R2 | SRC | Partial R2 | SRC | Partial R2 | |||
| Booting | Huaidao-5 | SNPP | 0.347 ** | 0.316 | 0.650 ** | 0.572 | 0.465 ** | 0.427 | 1.104 ** | 0.976 | 0.998 ** | 0.844 |
| TGW | 0.218 ** | 0.048 | 0.119 ** | 0.028 | 0.062 | 0.057 | 0.178 ** | 0.049 | 0.251 * | 0.191 | ||
| SF | 0.041 ** | 0.006 | 0.419 ** | 0.378 | 0.525 ** | 0.510 | 0.504 ** | 0.445 | 0.794 ** | 0.752 | ||
| PNPP | 0.679 ** | 0.631 | 0.476 ** | 0.021 | 0.278 ** | 0.001 | 0.931 ** | −0.473 | 1.095 ** | −0.798 | ||
| Wuyunjing-24 | SNPP | 0.404 ** | 0.213 | 0.341 ** | 0.261 | 0.511 ** | 0.369 | 0.585 ** | 0.517 | 1.963 ** | 1.305 | |
| TGW | 0.118 ** | 0.023 | 0.259 ** | 0.152 | 0.375 ** | 0.291 | 0.258 ** | 0.178 | 0.555 * | 0.387 | ||
| SF | 0.038 ** | 0.004 | 0.486 ** | 0.471 | 0.178 ** | 0.148 | 0.565 ** | 0.539 | 0.445 * | 0.363 | ||
| PNPP | 0.855 ** | 0.760 | 0.407 ** | 0.116 | 0.537 ** | 0.190 | 0.470 ** | −0.234 | 2.240 ** | −1.096 | ||
| Flowering | Huaidao-5 | SNPP | 0.348 ** | 0.269 | 0.045 ** | 0.004 | 0.053 * | 0.011 | 0.087 ** | 0.070 | 0.198 ** | 0.139 |
| TGW | 0.224 ** | 0.004 | 0.047 ** | 0.010 | 0.043 | 0.016 | 0.037 * | 0.021 | 0.119 ** | 0.113 | ||
| SF | 0.042 ** | 0.025 | 0.950 ** | 0.943 | 0.974 ** | 0.967 | 1.021 ** | 1.012 | 0.966 ** | 0.961 | ||
| PNPP | 0.791 ** | 0.702 | 0.134 ** | 0.043 | 0.118 ** | 0.003 | 0.174 ** | −0.104 | 0.261 ** | −0.213 | ||
| Wuyunjing-24 | SNPP | 0.211 ** | 0.178 | 0.111 * | 0.001 | 0.025 ** | 0.001 | 0.103 * | 0.002 | 0.172 ** | 0.080 | |
| TGW | 0.311 ** | 0.110 | 0.158 * | 0.072 | 0.044 ** | 0.029 | 0.144 * | 0.067 | 0.050 | 0.040 | ||
| SF | 0.120 ** | 0.018 | 0.803 ** | 0.760 | 0.970 ** | 0.967 | 0.812 ** | 0.768 | 1.025 ** | 1.016 | ||
| PNPP | 0.777 ** | 0.693 | 0.447 ** | 0.165 | 0.079 ** | 0.003 | 0.439 ** | 0.161 | 0.211 ** | −0.138 | ||
| Combined stages | Huaidao-5 | SNPP | 0.342 ** | 0.311 | 0.239 ** | 0.221 | 0.069 | 0.057 | 0.411 ** | 0.402 | 0.883 * | 0.771 |
| TGW | 0.218 ** | 0.047 | 0.076 * | 0.060 | 0.273 * | 0.254 | 0.101 ** | 0.088 | 0.151 | 0.142 | ||
| SF | 0.033 ** | 0.003 | 0.706 ** | 0.694 | 0.674 ** | 0.669 | 0.680 ** | 0.665 | 0.905 ** | 0.862 | ||
| PNPP | 0.686 ** | 0.639 | 0.135 ** | 0.024 | 0.104 * | 0.017 | 0.224 ** | −0.155 | 0.869 ** | −0.778 | ||
| Wuyunjing-24 | SNPP | 0.345 ** | 0.285 | 0.235 ** | 0.212 | 0.089 | 0.070 | 0.444 ** | 0.424 | 1.042 ** | 0.859 | |
| TGW | 0.243 ** | 0.037 | 0.169 * | 0.156 | 0.021 | 0.019 | 0.200 ** | 0.185 | 0.181 * | 0.162 | ||
| SF | 0.055 * | 0.043 | 0.648 ** | 0.620 | 0.912 ** | 0.903 | 0.561 ** | 0.535 | 0.906 ** | 0.886 | ||
| PNPP | 0.654 ** | 0.635 | 0.205 ** | 0.007 | 0.099 ** | 0.004 | 0.214 ** | −0.145 | 1.102 ** | −0.916 | ||
| Treatment | VPD (Min/Max/Ave) | SFO (Booting) | SFT (Booting) | SFO (Flowering) | SFT (Flowering) | SFO (Combined) | SFT (Combined) |
|---|---|---|---|---|---|---|---|
| T1 | 0.64/2.36/1.42 | 93.04a | 92.97a | 93.25a | 93.25a | 92.97a | 92.97a |
| T2 | 0.37/3.20/1.43 | 86.84b | 86.87b | 59.64b | 59.85b | 70.02b | 71.10b |
| T3 | 0.47/4.22/1.76 | 58.87c | 60.45c | 19.73c | 21.69c | 21.57c | 33.30c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmood, A.; Ali, I.; Wang, W.; Ata-Ul-Karim, S.T.; Liu, B.; Liu, L.; Zhu, Y.; Cao, W.; Tang, L. Individual and Combined Effects of High-Temperature Stress at Booting and Flowering Stages on Rice Grain Yield. Agronomy 2022, 12, 3092. https://doi.org/10.3390/agronomy12123092
Mahmood A, Ali I, Wang W, Ata-Ul-Karim ST, Liu B, Liu L, Zhu Y, Cao W, Tang L. Individual and Combined Effects of High-Temperature Stress at Booting and Flowering Stages on Rice Grain Yield. Agronomy. 2022; 12(12):3092. https://doi.org/10.3390/agronomy12123092
Chicago/Turabian StyleMahmood, Aqib, Iftikhar Ali, Wei Wang, Syed Tahir Ata-Ul-Karim, Bing Liu, Leilei Liu, Yan Zhu, Weixing Cao, and Liang Tang. 2022. "Individual and Combined Effects of High-Temperature Stress at Booting and Flowering Stages on Rice Grain Yield" Agronomy 12, no. 12: 3092. https://doi.org/10.3390/agronomy12123092
APA StyleMahmood, A., Ali, I., Wang, W., Ata-Ul-Karim, S. T., Liu, B., Liu, L., Zhu, Y., Cao, W., & Tang, L. (2022). Individual and Combined Effects of High-Temperature Stress at Booting and Flowering Stages on Rice Grain Yield. Agronomy, 12(12), 3092. https://doi.org/10.3390/agronomy12123092








