Nano-Thymol Emulsion Inhibits Botrytis cinerea to Control Postharvest Gray Mold on Tomato Fruit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Nano-Thy
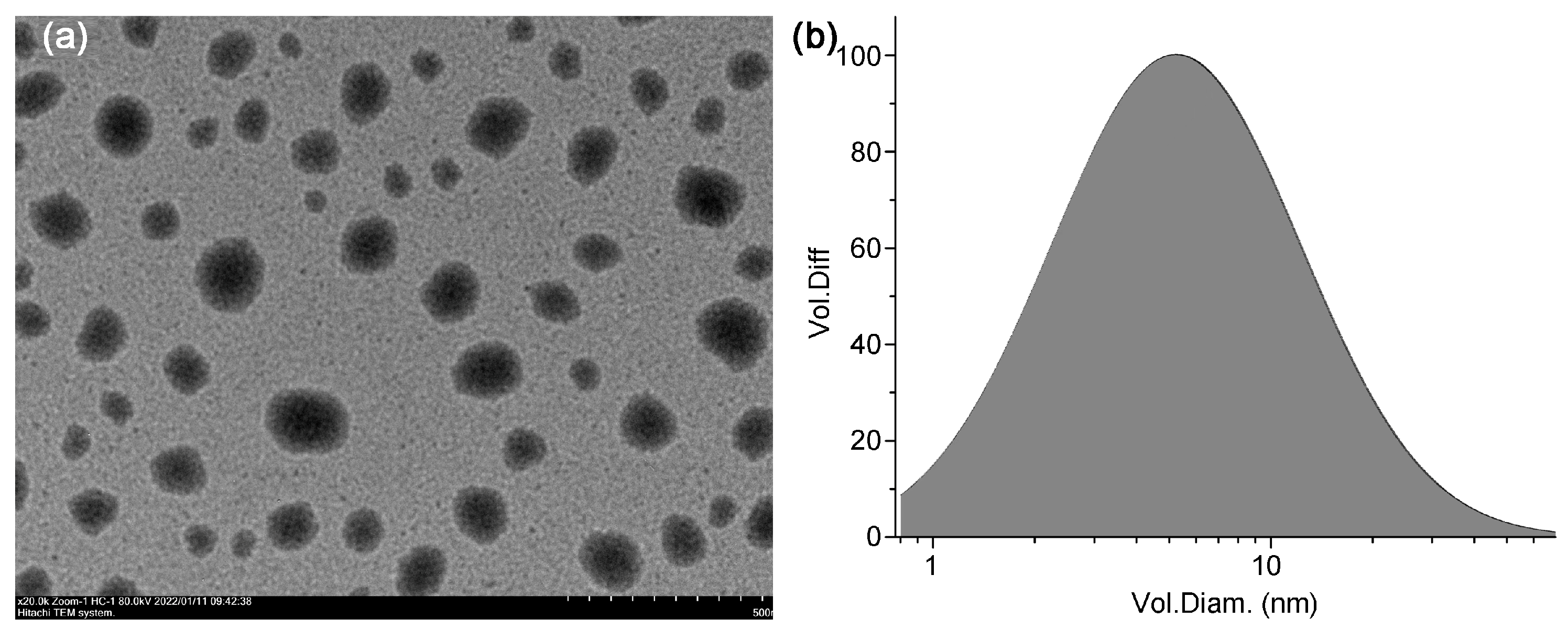
2.2. Characterization of Nano-Thy
2.2.1. Morphological Analysis
2.2.2. Determination of Structure
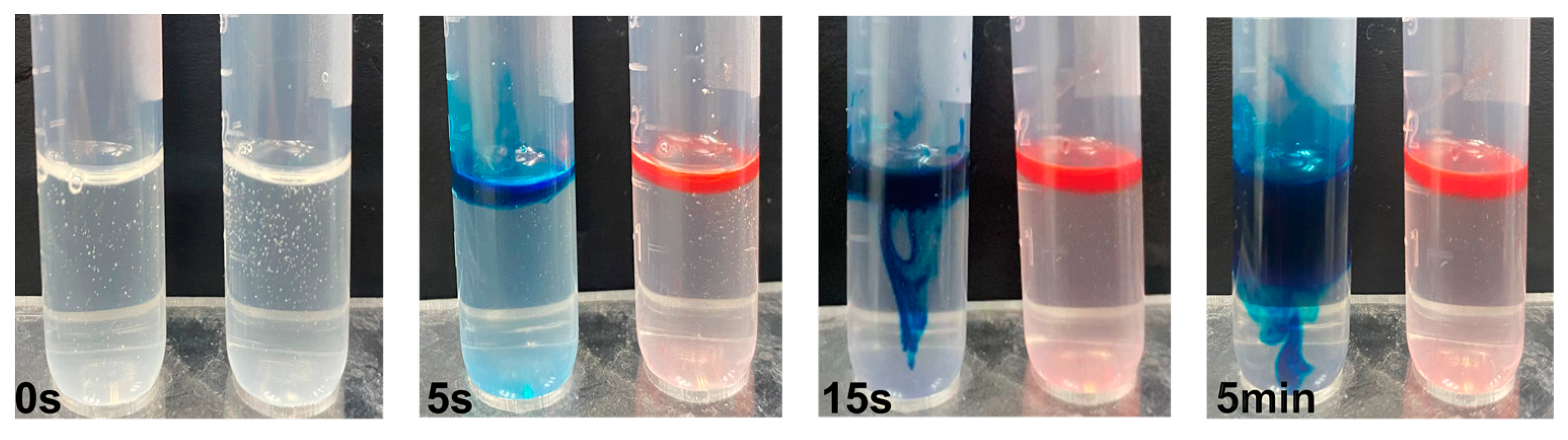
2.2.3. Determination of Stability
2.3. Determination of the Antifungal Activity of Nano-Thy
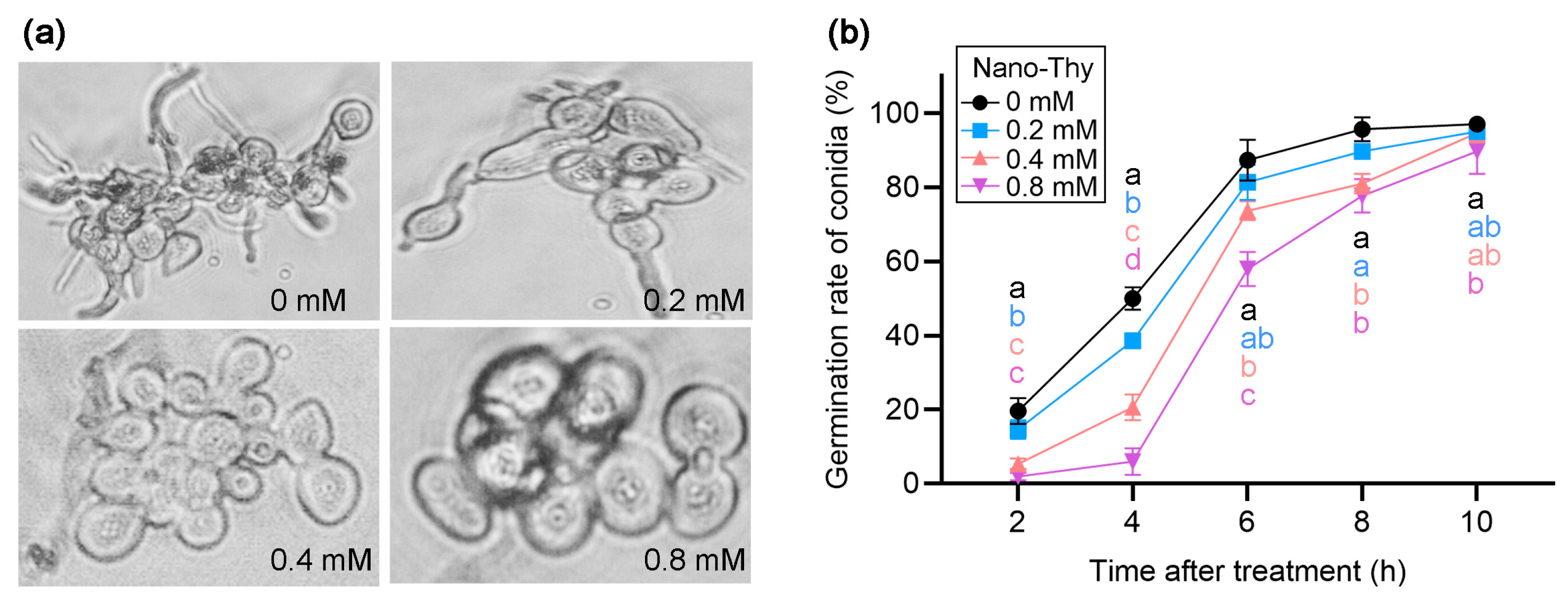
2.3.1. Test of B. cinerea Growth and Spore Germination
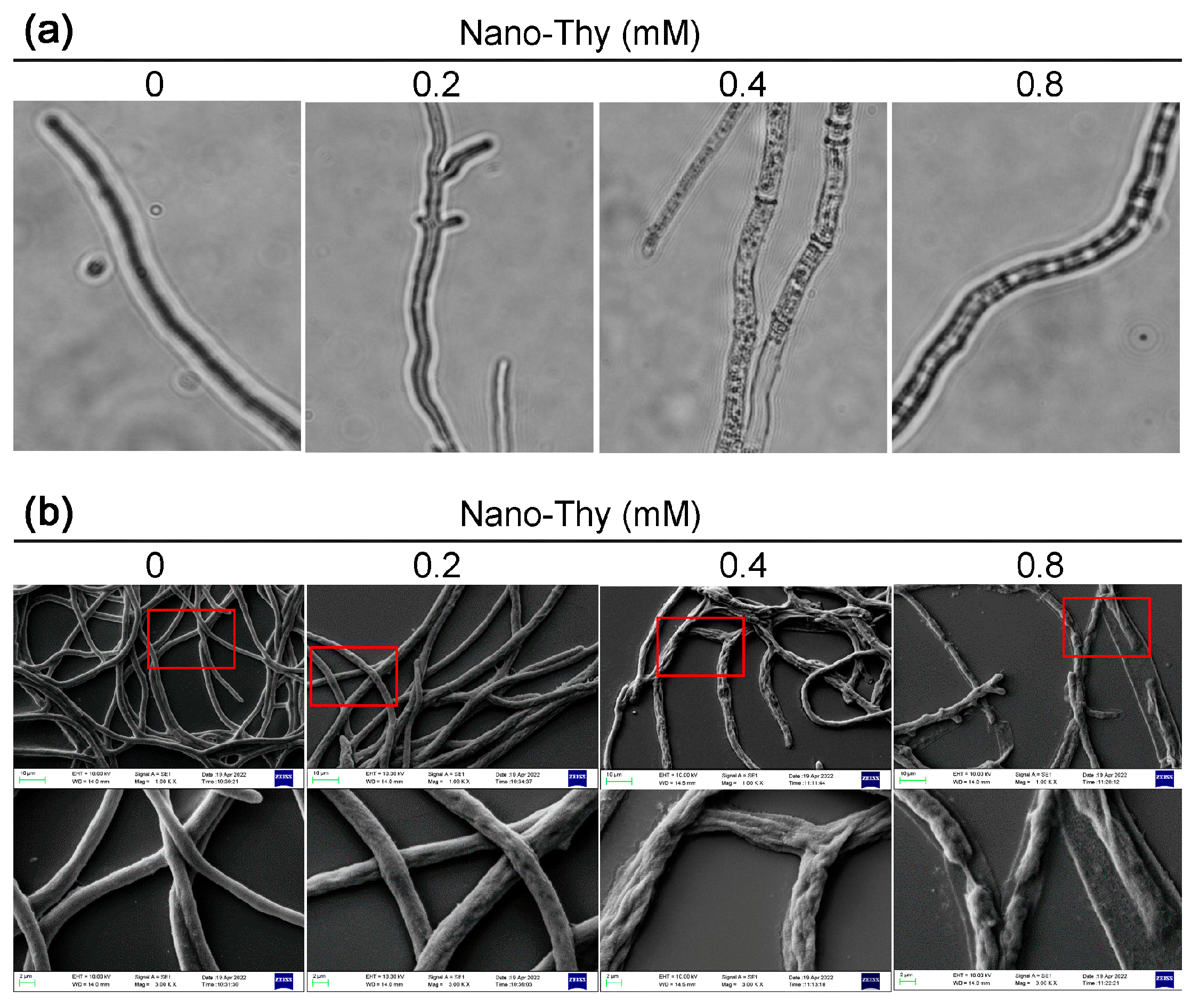
2.3.2. Morphological Observation of the Mycelia of B. cinerea
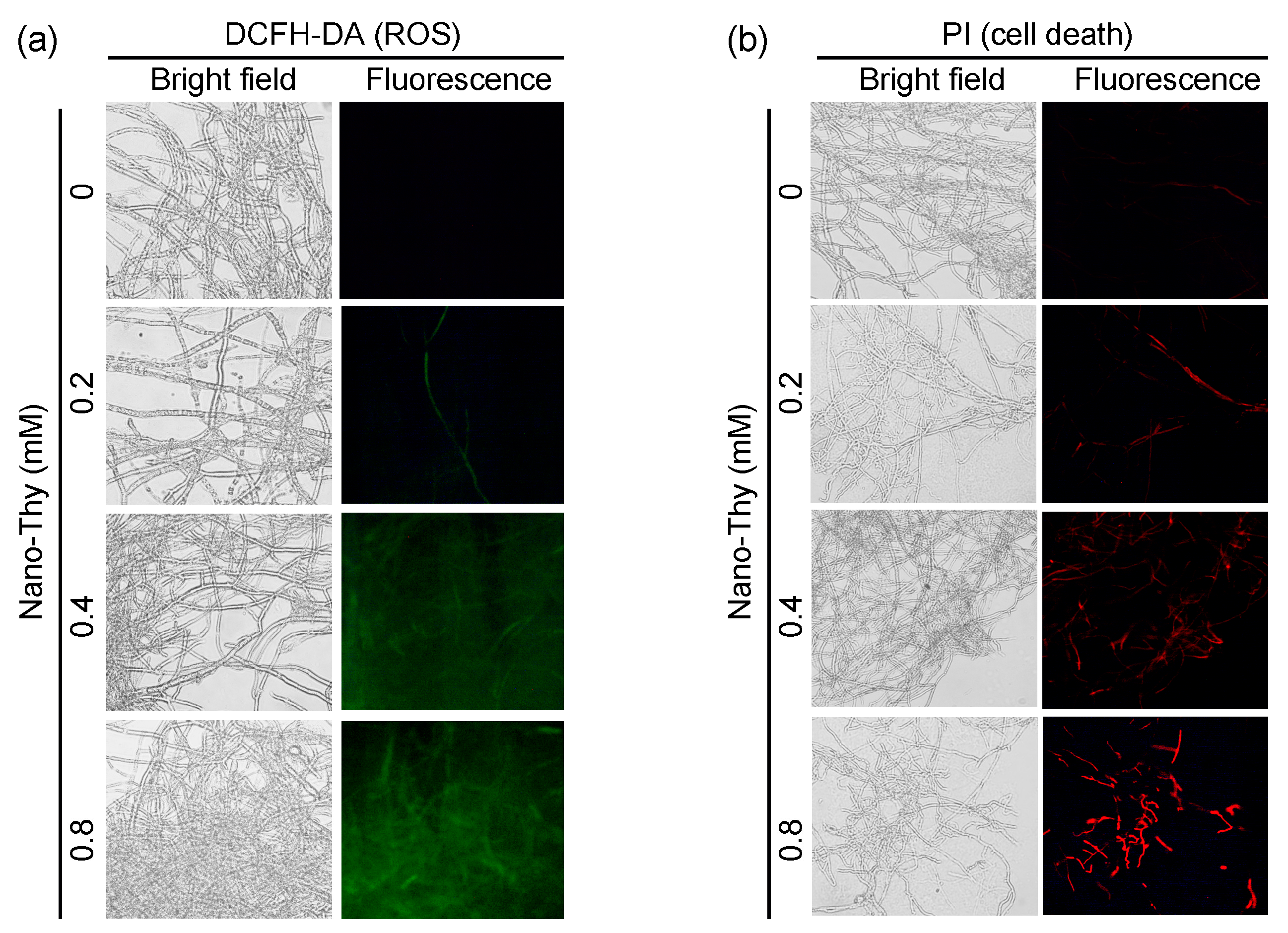
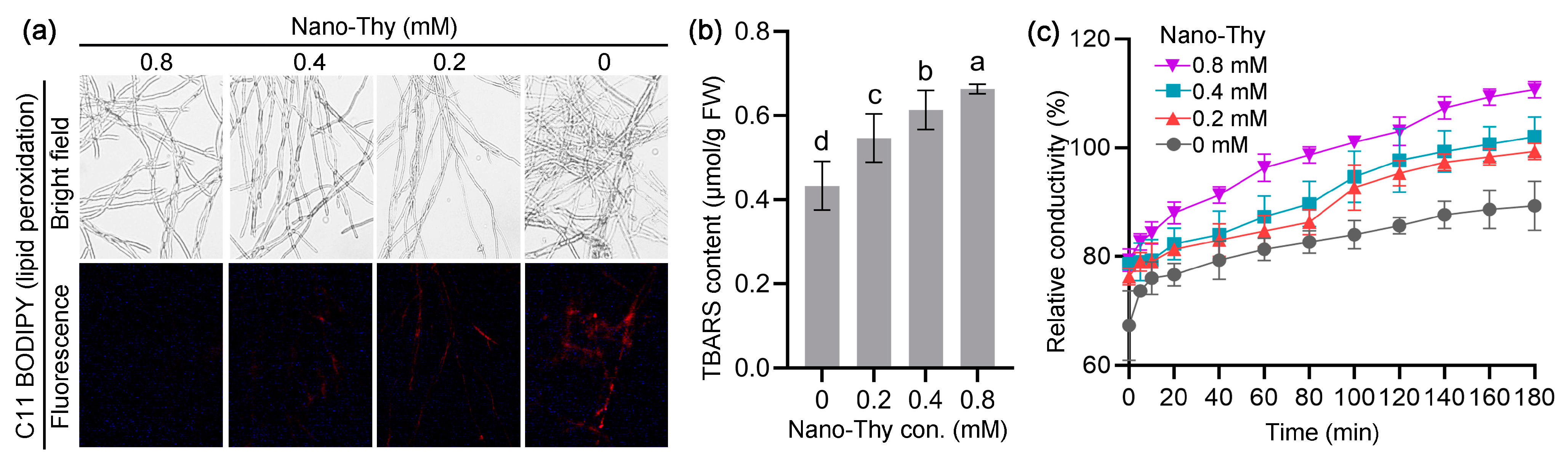
2.3.3. Fluorescent Detection of Reactive Oxygen Species (ROS), Cell Death, and Lipid Peroxidation in Mycelia
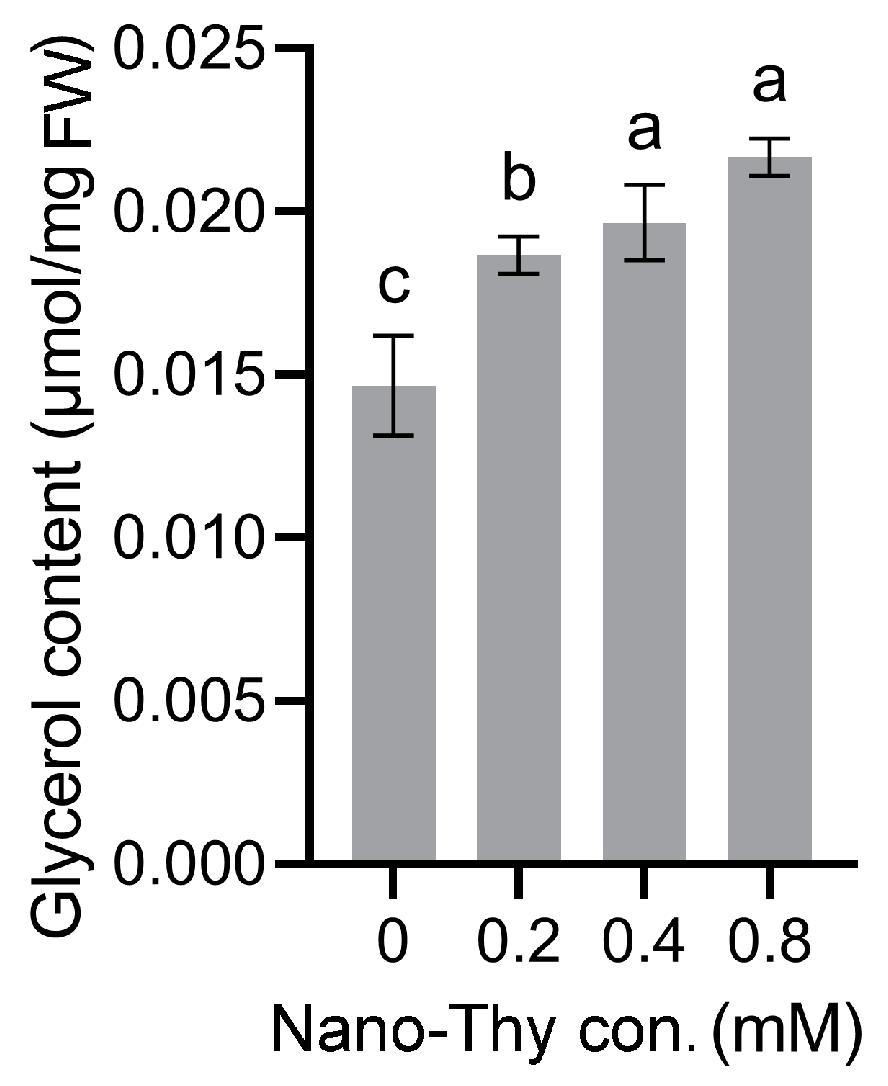
2.3.4. Determination of Glycerol Contents in Mycelia
2.3.5. Determination of Relative Conductivity of Mycelia
2.3.6. Measurement of TBARS Content in Mycelia
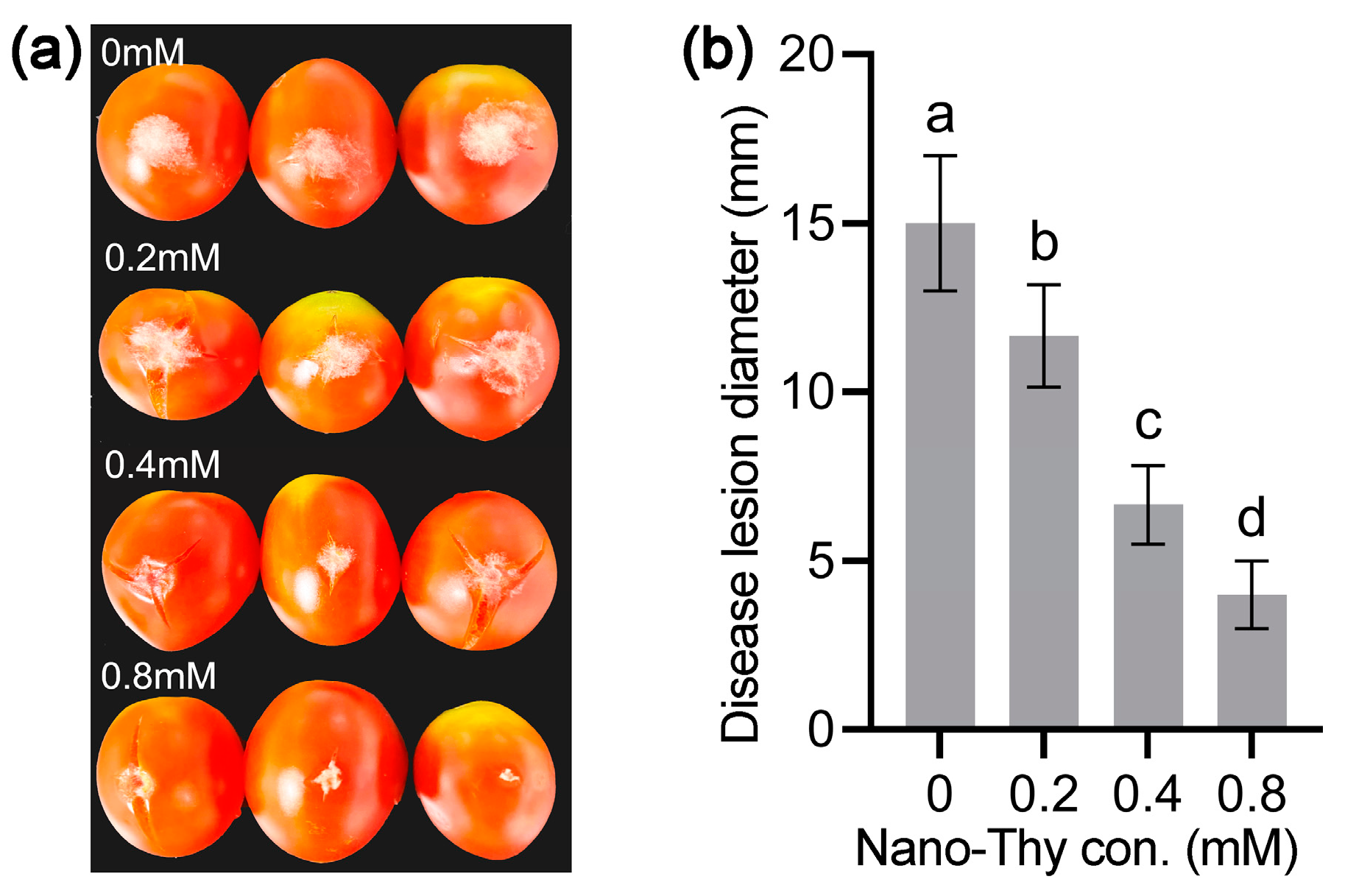
2.4. Evaluation of Gray Mold in Cherry Tomato upon Nano-Thy Treatment
2.5. Statistical Analysis
3. Results
3.1. Nano-Thy Was a Stable Nano Emulsion
3.2. Nano-Thy Inhibited the Growth and Spore Germination of B. cinerea
3.3. Nano-Thy Induced Morphological Alteration of B. cinerea
3.4. Nano-Thy Induced Oxidative Injury in B. cinerea
3.5. Nano-Thy Induced the Accumulation of Glycerol in the Mycelia of B. cinerea
3.6. Nano-Thy Inhibited the Infection of B. cinerea on Fresh Cherry Tomato Fruit
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mein, J.R.; Lian, F.; Wang, X.D. Biological activity of lycopene metabolites: Implications for cancer prevention. Nutr. Rev. 2008, 66, 667–683. [Google Scholar] [CrossRef]
- Lin, F.; Huang, Z.; Chen, Y.; Zhou, L.; Chen, M.; Sun, J.; Lu, Z.; Lu, Y. Effect of combined Bacillomycin D and chitosan on growth of Rhizopus stolonifer and Botrytis cinerea and cherry tomato preservation. J. Sci. Food Agric. 2021, 101, 229–239. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, S.; Zhang, W.; Ng, T.; Ye, X. Buckwheat Antifungal Protein with Biocontrol Potential to Inhibit Fungal (Botrytis cinerea) Infection of Cherry Tomato. J. Agric. Food Chem. 2019, 67, 6748–6756. [Google Scholar] [CrossRef]
- Seow, Y.X.; Yeo, C.R.; Chung, H.L.; Yuk, H.G. Plant essential oils as active antimicrobial agents. Crit. Rev. Food Sci. Nutr. 2014, 54, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Singh, R.; Sharma, K.; Vishwakarma, R.K. Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, D.D.C.; Ribeiro, W.R.; Goncalves, D.C.; Menini, L.; Costa, H. Recent advances and future perspective of essential oils in control Colletotrichum spp.: A sustainable alternative in postharvest treatment of fruits. Food Res. Int. 2021, 150, 110758. [Google Scholar] [CrossRef]
- Tao, N.; Jia, L.; Zhou, H. Anti-fungal activity of Citrus reticulata Blanco essential oil against Penicillium italicum and Penicillium digitatum. Food Chem. 2014, 153, 265–271. [Google Scholar] [CrossRef]
- Govindachari, T.R.; Suresh, G.; Gopalakrishnan, G.; Masilamani, S.; Banumathi, B. Antifungal activity of some tetranortriterpenoids. Fitoterapia 2000, 71, 317–320. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Valdramidis, V.P.; O’ Donnell, C.P.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of Natural Antimicrobials for Food Preservation. J. Agric. Food Chem. 2009, 57, 5987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shcherbakova, L.; Mikityuk, O.; Arslanova, L.; Stakheev, A.; Erokhin, D.; Zavriev, S.; Dzhavakhiya, V. Studying the Ability of Thymol to Improve Fungicidal Effects of Tebuconazole and Difenoconazole Against Some Plant Pathogenic Fungi in Seed or Foliar Treatments. Front. Microbiol. 2021, 12, 629429. [Google Scholar] [CrossRef]
- Gao, T.; Zhou, H.; Zhou, W.; Hu, L.; Chen, J.; Shi, Z. The Fungicidal Activity of Thymol against Fusarium graminearum via Inducing Lipid Peroxidation and Disrupting Ergosterol Biosynthesis. Molecules 2016, 21, 770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Chang, J.W.; Saenger, M.; Deering, A. Thymol nanoemulsions formed via spontaneous emulsification: Physical and antimicrobial properties. Food Chem. 2017, 232, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Robledo, N.; Vera, P.; Lopez, L.; Yazdani-Pedram, M.; Tapia, C.; Abugoch, L. Thymol nanoemulsions incorporated in quinoa protein/chitosan edible films; antifungal effect in cherry tomatoes. Food Chem. 2018, 246, 211–219. [Google Scholar] [CrossRef]
- Zunino, M.P.; Zygadlo, J.A. Effect of monoterpenes on lipid oxidation in maize. Planta 2004, 219, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Perumal, A.B.; Li, X.; Su, Z.; He, Y. Preparation and characterization of a novel green tea essential oil nanoemulsion and its antifungal mechanism of action against Magnaporthae oryzae. Ultrason. Sonochem. 2021, 76, 105649. [Google Scholar] [CrossRef]
- Suresh Kumar, R.S.; Shiny, P.J.; Anjali, C.H.; Jerobin, J.; Goshen, K.M.; Magdassi, S.; Mukherjee, A.; Chandrasekaran, N. Distinctive effects of nano-sized permethrin in the environment. Environ. Sci. Pollut. Res. Int. 2013, 20, 2593–2602. [Google Scholar] [CrossRef] [PubMed]
- Donsi, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef]
- Heydari, M.; Amirjani, A.; Bagheri, M.; Sharifian, I.; Sabahi, Q. Eco-friendly pesticide based on peppermint oil nanoemulsion: Preparation, physicochemical properties, and its aphicidal activity against cotton aphid. Environ. Sci. Pollut. Res. Int. 2020, 27, 6667–6679. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- Mansouri, S.; Pajohi-Alamoti, M.; Aghajani, N.; Bazargani-Gilani, B.; Nourian, A. Stability and antibacterial activity of Thymus daenensis L. essential oil nanoemulsion in mayonnaise. J. Sci. Food Agric. 2021, 101, 3880–3888. [Google Scholar] [CrossRef]
- Hashemi Gahruie, H.; Ziaee, E.; Eskandari, M.H.; Hosseini, S.M. Characterization of basil seed gum-based edible films incorporated with Zataria multiflora essential oil nanoemulsion. Carbohydr. Polym. 2017, 166, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Sugumar, S.; Clarke, S.K.; Nirmala, M.J.; Tyagi, B.K.; Mukherjee, A.; Chandrasekaran, N. Nanoemulsion of eucalyptus oil and its larvicidal activity against Culex quinquefasciatus. Bull Entomol Res. 2014, 104, 393–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawakami, K.; Yoshikawa, T.; Moroto, Y.; Kanaoka, E.; Takahashi, K.; Nishihara, Y.; Masuda, K. Microemulsion formulation for enhanced absorption of poorly soluble drugs. I. Prescription design. J. Control Release 2002, 81, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Watnasirichaikul, S.; Davies, N.M.; Rades, T.; Tucker, I.G. Preparation of Biodegradable Insulin Nanocapsules from Biocompatible Microemulsions. Pharm. Res. 2000, 17, 684–689. [Google Scholar] [CrossRef]
- Lawrence, M.J. Surfactant Systems: Microemulsions and Vesicles as Vehicles for Drug Delivery. Eur. J. Drug. Metab. Pharmacokinet. 1994, 19, 257–269. [Google Scholar] [CrossRef]
- Thomulka, K.W.; Abbas, C.G.; Young, D.A.; Lange, J.H. Evaluating Median Effective Concentrations of Chemicals with Bioluminescent Bacteria. Bull. Environ. Contam. Toxicol. 1996, 56, 446–452. [Google Scholar] [CrossRef]
- Fan, J.; Cai, H.; Tan, W.S. Role of the plasma membrane ROS-generating NADPH oxidase in CD34+ progenitor cells preservation by hypoxia. J. Biotechnol. 2007, 130, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Zeng, W.; Dai, H.; Yan, M.; Cai, X.; Luo, H.; Ke, M.; Liu, Z. Decitabine-Induced Changes in Human Myelodysplastic Syndrome Cell Line SKM-1 Are Mediated by FOXO3A Activation. J. Immunol. Res. 2017, 2017, 4302320. [Google Scholar] [CrossRef]
- Angelova, P.R.; Choi, M.L.; Berezhnov, A.V.; Horrocks, M.H.; Hughes, C.D.; De, S.; Rodrigues, M.; Yapom, R.; Little, D.; Dolt, K.S.; et al. Alpha synuclein aggregation drives ferroptosis: An interplay of iron, calcium and lipid peroxidation. Cell Death Differ. 2020, 27, 2781–2796. [Google Scholar] [CrossRef]
- Ye, X.; Ling, T.; Xue, Y.; Xu, C.; Zhou, W.; Hu, L.; Chen, J.; Shi, Z. Thymol Mitigates Cadmium Stress by Regulating Glutathione Levels and Reactive Oxygen Species Homeostasis in Tobacco Seedlings. Molecules 2016, 21, 1339. [Google Scholar] [CrossRef]
- Ji, J.Y.; Yang, J.; Zhang, B.W.; Wang, S.R.; Zhang, G.C.; Lin, L.N. Sodium pheophorbide a controls cherry tomato gray mold (Botrytis cinerea) by destroying fungal cell structure and enhancing disease resistance-related enzyme activities in fruit. Pestic. Biochem. Physiol. 2020, 166, 104581. [Google Scholar] [CrossRef]
- Xin, Z.; OuYang, Q.; Wan, C.; Che, J.; Li, L.; Chen, J.; Tao, N. Isolation of antofine from Cynanchum atratum BUNGE (Asclepiadaceae) and its antifungal activity against Penicillium digitatum. Postharvest. Biol. Technol. 2019, 157, 110961. [Google Scholar] [CrossRef]
- Kong, W.; Huang, C.; Chen, Q.; Zou, Y.; Zhang, J. Nitric oxide alleviates heat stress-induced oxidative damage in Pleurotus eryngii var. tuoliensis. Fungal Genet. Biol. 2012, 49, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Duran, R.; Cary, J.W.; Calvo, A.M. Role of the osmotic stress regulatory pathway in morphogenesis and secondary metabolism in filamentous fungi. Toxins 2010, 2, 367–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raveau, R.; Fontaine, J.; Lounes-Hadj Sahraoui, A. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamilvanan, S. Formulation of multifunctional oil-in-water nanosized emulsions for active and passive targeting of drugs to otherwise inaccessible internal organs of the human body. Int. J. Pharm. 2009, 381, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.; Singh, V.K.; Das, S.; Prasad, J.; Kedia, A.; Upadhyay, N.; Dubey, N.K.; Dwivedy, A.K. Essential Oil Nanoemulsion as Eco-Friendly and Safe Preservative: Bioefficacy Against Microbial Food Deterioration and Toxin Secretion, Mode of Action, and Future Opportunities. Front. Microbiol. 2021, 12, 751062. [Google Scholar] [CrossRef]
- Gill, T.A.; Li, J.; Saenger, M.; Scofield, S.R. Thymol-based submicron emulsions exhibit antifungal activity against Fusarium graminearum and inhibit Fusarium head blight in wheat. J. Appl. Microbiol. 2016, 121, 1103–1116. [Google Scholar] [CrossRef] [Green Version]
- Kumari, S.; Kumaraswamy, R.V.; Choudhary, R.C.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Thymol nanoemulsion exhibits potential antibacterial activity against bacterial pustule disease and growth promotory effect on soybean. Sci. Rep. 2018, 8, 6650. [Google Scholar] [CrossRef] [Green Version]
- Elshamy, S.; Khadizatul, K.; Uemura, K.; Nakajima, M.; Neves, M.A. Chitosan-based film incorporated with essential oil nanoemulsion foreseeing enhanced antimicrobial effect. J. Food Sci. Technol. 2021, 58, 3314–3327. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Sharifzadeh, A.; Shokri, H.; Khosravi, A.R.; Abbaszadeh, A. Antifungal efficacy of thymol, carvacrol, eugenol and menthol as alternative agents to control the growth of food-relevant fungi. J. Mycol. Med. 2014, 24, e51–e56. [Google Scholar] [CrossRef]
- Romanazzi, G.; Smilanick, J.L.; Feliziani, E.; Droby, S. Integrated management of postharvest gray mold on fruit crops. Postharvest. Biol. Technol. 2016, 113, 69–76. [Google Scholar] [CrossRef]
- Kaewklin, P.; Siripatrawan, U.; Suwanagul, A.; Lee, Y.S. Active packaging from chitosan-titanium dioxide nanocomposite film for prolonging storage life of tomato fruit. Int. J. Biol. Macromol. 2018, 112, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.; Shin, S.; Kim, J.; Kim, J.; Lee, D.; Lee, H.; Lee, E.J.; Hyun, J. Protective coating of strawberries with cellulose nanofibers. Carbohydr. Polym. 2021, 258, 117688. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Gulati, N.; Makhija, M.; Purohit, D.; Dureja, H. Nanoemulsion: A Novel Drug Delivery Approach for Enhancement of Bioavailability. Recent Pat. Nanotechnol. 2020, 14, 276–293. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Belenky, P.; Camacho, D.; Collins, J.J. Fungicidal drugs induce a common oxidative-damage cellular death pathway. Cell Rep. 2013, 3, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Ji, D.; Chen, T.; Ma, D.; Liu, J.; Xu, Y.; Tian, S. Inhibitory effects of methyl thujate on mycelial growth of Botrytis cinerea and possible mechanisms. Postharvest. Biol. Technol. 2018, 142, 46–54. [Google Scholar] [CrossRef]
- Heller, J.; Tudzynski, P. Reactive oxygen species in phytopathogenic fungi: Signaling, development, and disease. Annu Rev. Phytopathol. 2011, 49, 369–390. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, B.; Li, J.; Zhang, B.; Wang, H.; Li, M. Endoplasmic reticulum-derived reactive oxygen species (ROS) is involved in toxicity of cell wall stress to Candida albicans. Free Radic. Biol. Med. 2016, 99, 572–583. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Hao, Y.; Lu, H.; Li, P.; Chen, J.; Shi, Z.; Xie, Y.; Mo, H.; Hu, L. Nano-Thymol Emulsion Inhibits Botrytis cinerea to Control Postharvest Gray Mold on Tomato Fruit. Agronomy 2022, 12, 2973. https://doi.org/10.3390/agronomy12122973
Zhang J, Hao Y, Lu H, Li P, Chen J, Shi Z, Xie Y, Mo H, Hu L. Nano-Thymol Emulsion Inhibits Botrytis cinerea to Control Postharvest Gray Mold on Tomato Fruit. Agronomy. 2022; 12(12):2973. https://doi.org/10.3390/agronomy12122973
Chicago/Turabian StyleZhang, Jiao, Yini Hao, Haiyan Lu, Pan Li, Jian Chen, Zhiqi Shi, Yuhua Xie, Haizhen Mo, and Liangbin Hu. 2022. "Nano-Thymol Emulsion Inhibits Botrytis cinerea to Control Postharvest Gray Mold on Tomato Fruit" Agronomy 12, no. 12: 2973. https://doi.org/10.3390/agronomy12122973
APA StyleZhang, J., Hao, Y., Lu, H., Li, P., Chen, J., Shi, Z., Xie, Y., Mo, H., & Hu, L. (2022). Nano-Thymol Emulsion Inhibits Botrytis cinerea to Control Postharvest Gray Mold on Tomato Fruit. Agronomy, 12(12), 2973. https://doi.org/10.3390/agronomy12122973






