Morphophysiology, Productivity and Quality of Soybean (Glycine max (L.) Merr.) cv. Merlin in Response to Row Spacing and Seeding Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Soil Conditions
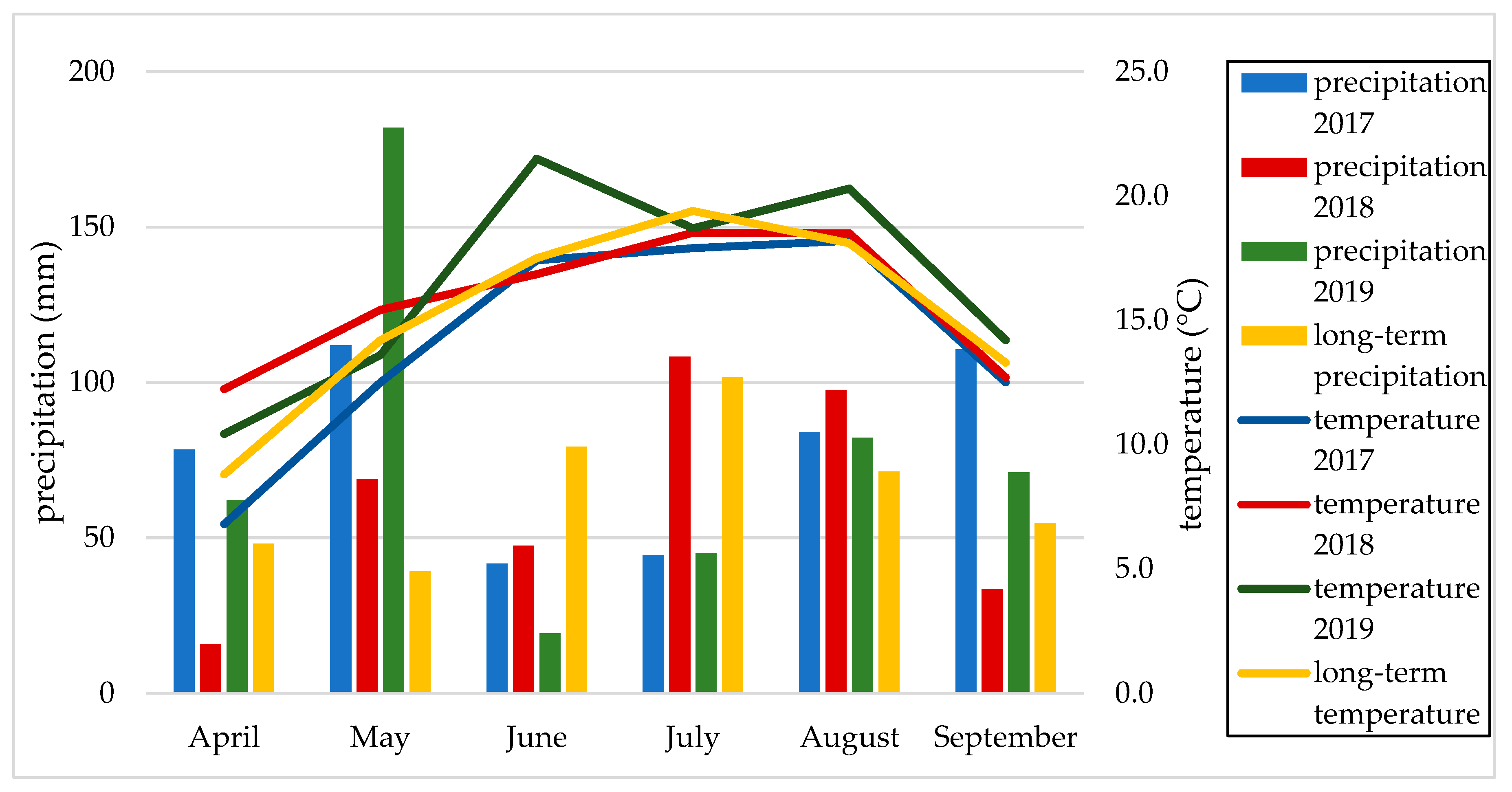
2.3. Weather Conditions
2.4. Physiological Measurements
2.4.1. Measuring Relative Chlorophyll Content
2.4.2. Measuring Chlorophyll Fluorescence
2.4.3. Measuring Leaf Area Index
2.5. Biometric Measurements
2.6. Analytical Methods
2.7. Statistical Analyses
3. Results and Discussion
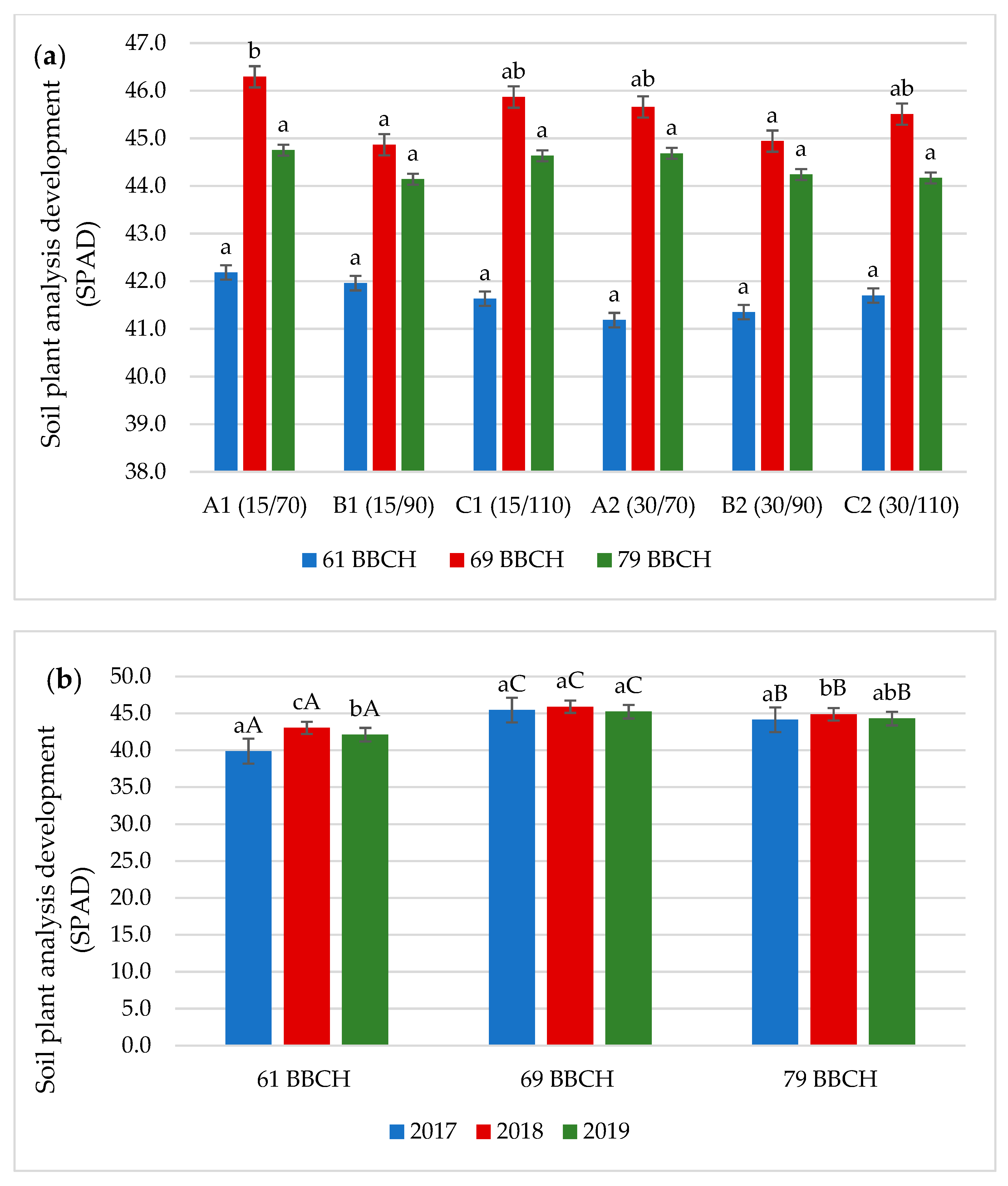
3.1. Chlorophyll Content
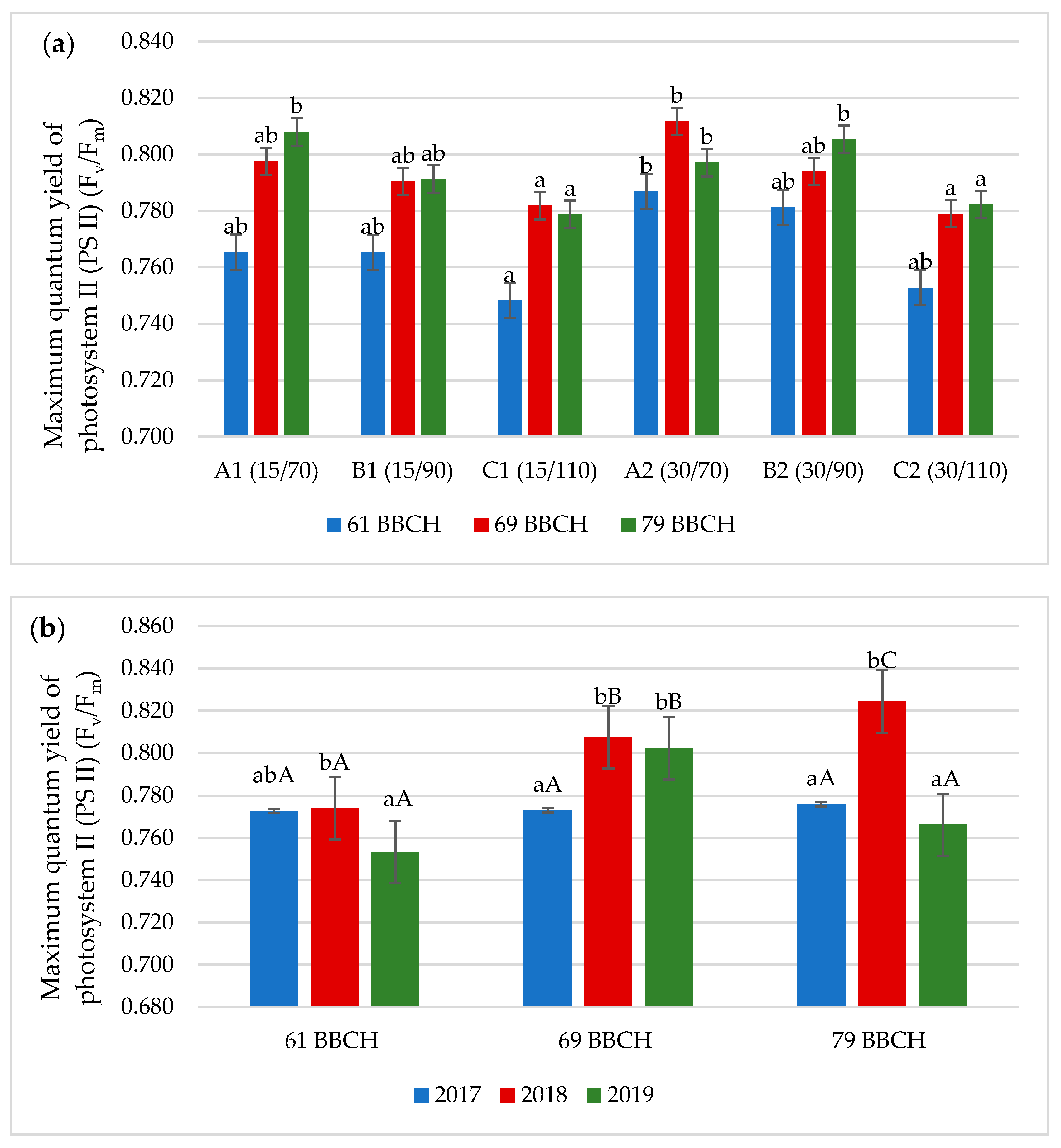
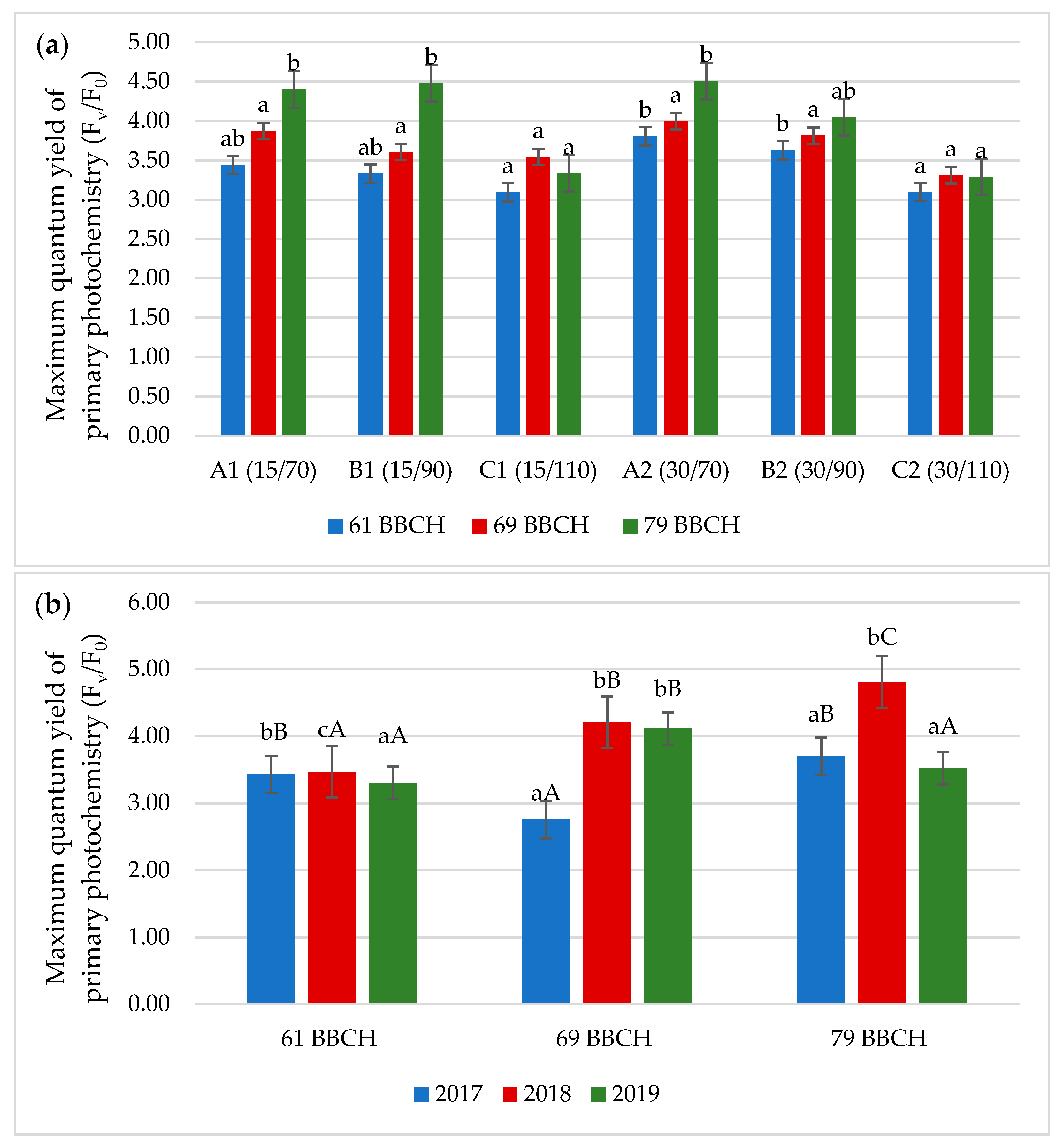
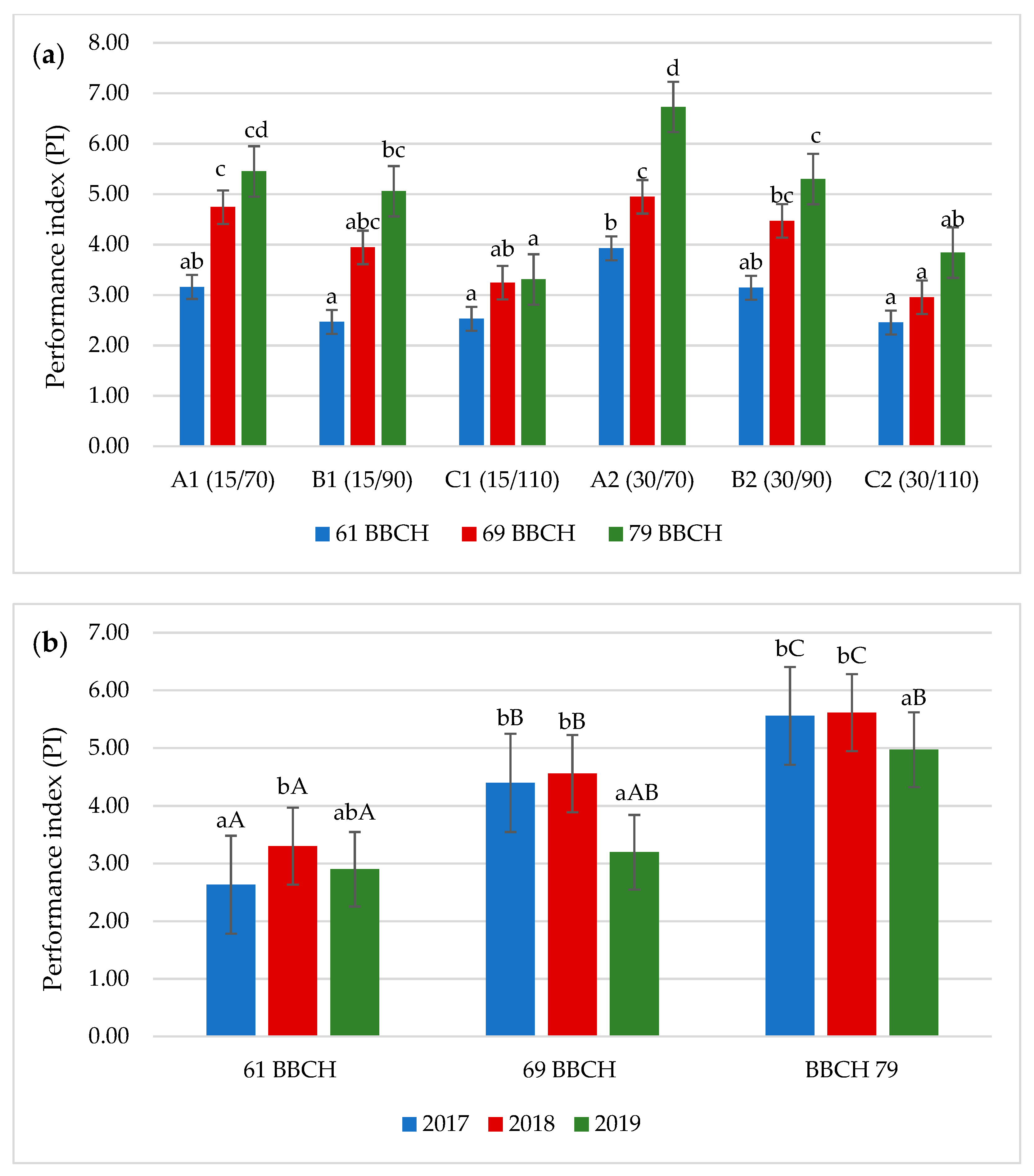
3.2. Chlorophyll Fluorescence
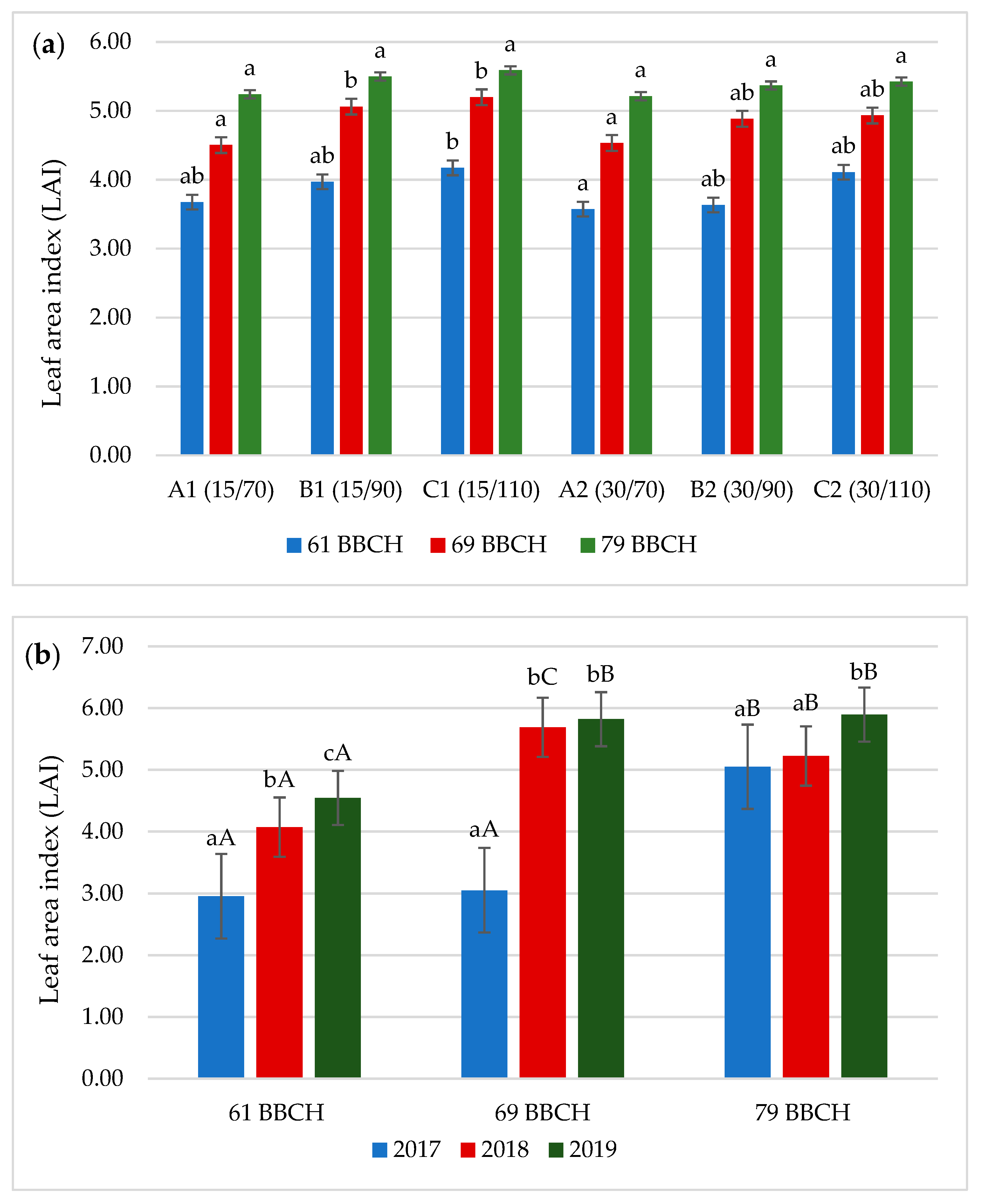
3.3. Leaf Area Index (LAI)
3.4. Soybean Seed Yield and Yield Components
3.5. Plant Morphology and Nodulation
3.6. Protein and Fat Content and Their Efficiency
3.7. Canonical Variate Analysis (CVA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panasiewicz, K.; Faligowska, A.; Szymańska, G.; Szukała, J.; Ratajczak, K.; Sulewska, H. The effect of various tillage systems on productivityof narrow-leaved lupin-winter wheat-winter triticale-winter barley rotation. Agronomy 2020, 10, 304. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 2 February 2021).
- Singh, G. The Soybean: Botany, Production and Uses; CABI: Wallingford, CT, USA, 2010; ISBN 978-1-84593-644-0. [Google Scholar]
- Qin, J.; Zhang, J.; Liu, D.; Yin, C.; Wang, F.; Chen, P.; Chen, H.; Ma, J.; Zhang, B.; Xu, J. iTRAQ-based analysis of developmental dynamics in the soybean leaf proteome reveals pathways associated with leaf photosynthetic rate. Mol. Genet. Genom. 2016, 291, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- de Portes, T.A.; de Araújo, B.R.B. Comparison of the allocation of phytomass in soybean and bean and its potential role in biological nitrogen fixation. Acta Sci. Agron. 2012, 34, 285–292. [Google Scholar] [CrossRef]
- Zou, H.; Zhang, N.-N.; Pan, Q.; Zhang, J.-H.; Chen, J.; Wei, G.-H. Hydrogen sulfide promotes nodulation and nitrogen fixation in soybean—Rhizobia symbiotic system. Mol. Plant Microbe Interact. 2019, 32, 972–985. [Google Scholar] [CrossRef]
- Soares, I.O.; de Rezende, P.M.; Bruzi, A.T.; Zuffo, A.M.; Zambiazzi, E.V.; Fronza, V.; Teixeira, C.M. Interaction between soybean cultivars and seed density. Am. J. Plant Sci. 2015, 6, 1425–1434. [Google Scholar] [CrossRef][Green Version]
- Souza, R.T.G.; Teixeira, I.R.; Jesus, F.F.; Reis, E.F. Spray droplet spectrum and spray deposition in different soybean sowing systems. Aust. J. Crop Sci. 2017, 11, 1195–1202. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hossain, M.M. Plant density effects on growth, yield and yield components of two soybean varieties under equidistant planting arrangement. Asian J. Plant Sci. 2011, 10, 278–286. [Google Scholar] [CrossRef]
- Ribeiro, A.B.M.; Bruzi, A.T.; Zuffo, A.M.; Zambiazzi, E.V.; Soares, I.O.; Vilela, N.J.D.; de Pereira, J.L.A.; Moreira, S.G. Productive performance of soybean cultivars grown in different plant densities. Cienc. Rural 2017, 47, 1–8. [Google Scholar] [CrossRef]
- Sobko, O.; Hartung, J.; Zikeli, S.; Claupein, W.; Gruber, S. Effect of sowing density on grain yield, protein and oil content and plant morphology of soybean (Glycine max L. Merrill). Plant Soil Environ. 2019, 65, 594–601. [Google Scholar] [CrossRef]
- Cox, W.J.; Cherney, J.H. Growth and yield responses of soybean to row spacing and seeding rates. Agron. J. 2011, 103, 123–128. [Google Scholar] [CrossRef]
- Kozak, M.; Malarz, W.; Serafin-Andrzejewska, M.; Kotecki, A. The effects of sowing rate and Asahi SL biostimulator on soybean growth and yield. In Biostimulators in Modern Agriculture. Field Crops; Dąbrowski, Z.T., Ed.; Editorial House Wieś Jutra: Warsaw, Poland, 2008; pp. 77–84. [Google Scholar]
- Kozak, M.; Malarz, W.; Serafin-Andrzejewska, M.; Kotecki, A. The effects of different sowing rate and Asahi SL treatments on soybean seed sowing value. In Biostimulators in Modern Agriculture. Field Crops; Dąbrowski, Z.T., Ed.; Editorial House Wieś Jutra: Warsaw, Poland, 2008; pp. 85–91. [Google Scholar]
- Dima, D.C. Results of soybean time of sowing and distance between rows in Romania in the experimental years 2015 and 2016. Sci. Pap. Ser. A Agron. 2017, 60, 229–235. [Google Scholar]
- Devlin, D.L.; Fjell, D.L.; Shroyer, J.P.; Gorden, W.B.; Marsh, B.H.; Maddux, L.D.; Martin, V.; Duncan, S.R. Row spacing and rates of soybean in low and high yielding environments. J. Prod. Agric. 1995, 8, 215–222. [Google Scholar] [CrossRef]
- De Bruin, J.L.; Pedersen, P. Effect of row spacing and seeding rate on soybean yield. Agron. J. 2008, 100, 704–710. [Google Scholar] [CrossRef]
- Prusiński, J.; Nowicki, R. Effect of planting density and row spacing on the yielding of soybean (Glycine max L. Merrill). Plant Soil Environ. 2020, 66, 616–623. [Google Scholar] [CrossRef]
- Randelović, P.; Ðordević, V.; Milić, S.; Balešević-Tubić, S.; Petrović, K.; Miladinović, J.; Ðukić, V. Prediction of soybean plant density using a machine learning model and vegetation indices extracted from RGB images taken with a UAV. Agronomy 2020, 10, 1108. [Google Scholar] [CrossRef]
- Cheng, B.; Raza, A.; Wang, L.; Xu, M.; Lu, J.; Gao, Y.; Qin, S.; Zhang, Y.; Ahmad, I.; Zhou, T.; et al. Effects of multiple planting densities on lignin metabolism and lodging resistance of the strip intercropped soybean stem. Agronomy 2020, 10, 1177. [Google Scholar] [CrossRef]
- Liu, X.; Rahman, T.; Yang, F.; Yang, S.C.; Yong, T.; Jiang, L.; Zhang, C.; Yang, W. PAR interception and utilization in different maize and soybean intercropping patterns. PLoS ONE 2017, 12, 0169218. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gong, W.; Yang, W. Shade inhibits leaf size by controlling cell proliferation and enlargement in soybean. Sci. Rep. 2017, 7, 9259. [Google Scholar] [CrossRef] [PubMed]
- Munger, P.; Bleuholder, H.; Hack, H.; Hess, M.; Stauss, R.; van den Boom, T.; Weber, E. Phenological growth stages of soybean plant (Glycine max L. Merr.): Codification and description according to the BBCH scale. J Agron. Crop Sci. 1997, 179, 209–217. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. International soil classification system for naming soils and creating legends for soil maps. In Word Reference Base for Soil Resources 2014, Update 2015; Word Soil Resources Reports No. 106; FAO: Rome, Italy, 2015; pp. 172–173. [Google Scholar]
- Skowera, B. Changes of hydrothermal conditions in the Polish area (1971–2010). Fragm. Agron. 2014, 31, 74–87. (In Polish) [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Campbell, N.A.; Atchley, W.R. The geometry of canonical variate analysis. Syst. Zool. 1981, 30, 268–280. [Google Scholar] [CrossRef]
- Lepš, J.; Šmilauer, T. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination (Version 5.0); Microcomputer Power: Ithaca, NY, USA, 2012; 496p. [Google Scholar]
- Fan, Y.; Chen, J.; Wang, Z.; Tan, T.; Li, S.; Li, J.; Wang, B.; Zhang, J.; Cheng, Y.; Wu, X.; et al. Soybean (Glycine max L. Merr.) seedlings response to shading: Leaf structure, photosynthesis and proteomic analysis. BMC Plant Biol. 2019, 19, 34. [Google Scholar] [CrossRef]
- Gong, W.; Qi, P.; Du, J.; Sun, X.; Wu, X.; Song, C.; Liu, W.; Wu, Y.; Yu, X.; Yong, T.; et al. Transcriptome analysis of shade-induced inhibition on leaf size in relay intercropped soybean. PLoS ONE. 2014, 9, e98465. [Google Scholar] [CrossRef]
- Evans, J.R.; Poorter, H. Photosynthetic acclimation of plants to growth irradiance: The relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ. 2001, 24, 755–767. [Google Scholar] [CrossRef]
- Fritschi, F.B.; Ray, J.D. Soybean leaf nitrogen, chlorophyll content, and chlorophyll a/b ratio. Photosynthetica 2007, 45, 92–98. [Google Scholar] [CrossRef]
- Hussain, S.; Iqbal, N.; Brestic, M.; Raza, M.A.; Pang, T.; Langham, D.R.; Safdar, M.E.; Ahmeda, S.; Wena, B.; Gao, Y.; et al. Changes in morphology, chlorophyll fluorescence performance and Rubisco activity of soybean in response to foliar application of ionic titanium under normal light and shade environment. Sci. Total Environ. 2019, 658, 626–637. [Google Scholar] [CrossRef]
- Yao, X.; Li, C.; Li, S.; Zhu, Q.; Zhang, H.; Wang, H.; Yu, C.; Martin, S.K.S.; Xie, F. Effect of shade on leaf photosynthetic capacity, light-intercepting, electron transfer and energy distribution of soybeans. Plant Growth Regul. 2017, 83, 1–8. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 13, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
- Gratani, L.; Catoni, R.; Pirone, G.; Frattaroli, A.R.; Varone, L. Physiological and morphological leaf trait variations in two Apennine plant species in response to different altitudes. Photosynthetica 2012, 50, 15–23. [Google Scholar] [CrossRef]
- Khalid, M.H.B.; Raza, M.A.; Yu, H.Q.; Sun, F.A.; Zhang, Y.Y.; Lu, F.Z.; Si, L.; Iqbal, N.; Khan, I.; Fu, F.L.; et al. Effect of shade treatments on morphology, photosynthetic and chlorophyll fluorescence characteristics of soybeans (Glycine max L. Merr.). Appl. Ecol. Environ. Res. 2019, 17, 2551–2569. [Google Scholar] [CrossRef]
- De Luca, M.J.; Nogueira, M.A.; Hungria, M. Feasibility of lowering soybean planting density without compromising nitrogen fixation and yield. Agron. Soil Environ. Qulit. 2014, 106, 2118–2124. [Google Scholar] [CrossRef]
- De Luca, M.J.; Hungria, M. Plant densities and modulation of symbiotic nitrogen fixation in soybean. Sci. Agric. 2014, 71, 181–187. [Google Scholar] [CrossRef]
- Piper, E.L.; Boote, K.J. Temperature and cultivar effects on soybean seed oil and protein concentrations. J. Am. Oil Chem. Soc. 1999, 76, 1233–1241. [Google Scholar] [CrossRef]
- Carciochi, W.D.; Schwalbert, R.; Andrade, F.H.; Corassa, G.M.; Carter, P.; Gaspar, A.P.; Schmidt, J.; Ciampitti, I.A. Soybean seed yield response to plant density by yield environment in North America. J. Agron. 2019, 111, 1923–1932. [Google Scholar] [CrossRef]
- Souza, R.; Teixeira, I.; Reis, E.; Silva, A. Soybean morphophysiology and yield response to seeding systems and plant populations. Chil. J. Agric. Res. 2016, 76, 1. [Google Scholar] [CrossRef]
- Rębilas, K.; Bacior, M.; Klimek-Kopyra, A.; Zając, T. A mode for the yield losses estimation in an early soybean (Glycine max L. Merr.) cultivar depending on the cutting height at harvest. Field Crops Res. 2020, 254, 107846. [Google Scholar] [CrossRef]
- Gong, W.Z.; Jiang, C.D.; Wu, Y.S.; Chen, H.H.; Liu, W.Y.; Yang, W.Y. Tolerance vs. avoidance: Two strategies of soybean (Glycine max) seedlings in response to shade in intercropping. Photosynthetica 2015, 53, 259–268. [Google Scholar] [CrossRef]
- Jarecki, W. Reaction of soybean (Glycine max (L.) Merr.) to seed inoculation with Bradyrhizobium japonicum bacteria. Plant Soil Environ. 2020, 66, 242–247. [Google Scholar] [CrossRef]
- Bellaloui, H.; Bruns, H.A.; Abbas, H.K.; Mengistu, A.; Fisher, D.K.; Reddy, N.K. Effects of row-type, row-spacing, seeding rate, soil-type, and cultivar differences on soybean seed nutrition under us Mississippi Delta conditions. PLoS ONE 2015, 10, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.S.; Balbinot, A.A., Jr.; Werner, F.; Zucareli, C.; Franchini, J.C.; Debiasi, H. Plant density and mineral nitrogen fertilization influencing yield, yield components and concentration of oil and protein in soybean grains. Soil Sci. Plant Nutr. 2016, 75, 362–370. [Google Scholar] [CrossRef]

| Treatment | 2017 | 2018 | 2019 |
|---|---|---|---|
| Sowing date | 2 May | 24 April | 24 April |
| Herbicide | 2 May Afalon dyspersyjny 450 SC (linuron) 1 dm3 ha−1 | 27 April Boxer 800 EC (prosulfocarb) 4 dm3 ha−1 | 26 April Boxer 800 EC (prosulfocarb) 4 dm3 ha−1 |
| Insecticide | - | - | 10 June Cyperkill Max 500 EC (cypermrthrin) 0.05 dm3 ha−1 |
| Fungicide | - | 25 May Topsin M 500 SC (methyl thiophanate) 1.5 dm3 ha−1 | - |
| Harvest date | 11.09. | 14.09. | 12.09. |
| Traits | Years | ||
|---|---|---|---|
| 2017 | 2018 | 2019 | |
| pH KCl | 6.92 | 6.03 | 6.35 |
| Humus content (%) | 1.16 | 1.16 | 1.38 |
| Content of available nutrients (mg kg−1) | |||
| P | 155.2 | 153.0 | 207.1 |
| K | 196.4 | 154.1 | 147.0 |
| Mg | 238.1 | 102.3 | 179.1 |
| Fe | 2885.3 | 1035.0 | 2079.3 |
| Zn | 13.54 | 10.90 | 14.50 |
| Mn | 370.40 | 116.00 | 341.90 |
| Cu | 11.43 | 3.79 | 8.21 |
| Year | Month | Mean | |||||
|---|---|---|---|---|---|---|---|
| April | May | June | July | August | September | ||
| 2017 | 3.79 (eh) | 2.88 (vh) | 0.80 (d) | 0.80 (d) | 1.50 (o) | 2.94 (vh) | 2.12 (h) |
| 2018 | 0.42 (vd) | 1.43 (o) | 0.94 (d) | 1.88 (rh) | 1.70 (rh) | 0.88 (d) | 1.21 (rd) |
| 2019 | 2.93 (vh) | 4.63 (eh) | 0.31 (ed) | 0.82 (d) | 1.47 (o) | 1.86 (rh) | 2.00 (rh) |
| long term | 1.75 (rh) | 1.81(rh) | 1.55 (o) | 1.45 (o) | 1.50 (o) | 1.62 (rh) | 1.61(rh) |
| Factor | Seed Yield [tha−1] | Number of Pods Per Plant [pcs.] | Number of Seeds Per Plant [pcs.] | Seed Weight Per Plant [g] | Thousand Seeds Weight [g] | |
|---|---|---|---|---|---|---|
| Row Spacing [cm] (S) | Sowing Density [pcs.m−2] (D) | |||||
| 15 | 70 | 4.84 a ± 0.54 | 27.6 b ± 9.5 | 57.0 c ± 10.6 | 8.23 c ± 1.27 | 146 a ± 18 |
| 90 | 4.95 a ± 0.48 | 20.4 ab ± 4.5 | 45.1 ab ± 7.4 | 7.61 c ± 0.97 | 173 b ± 39 | |
| 110 | 4.91 a ± 0.42 | 19.9 a ± 9.0 | 42.9 a ± 8.7 | 6.01 ab ± 1.05 | 142 a ± 18 | |
| 30 | 70 | 4.73 a ± 0.48 | 24.6 ab ± 8.6 | 50.8 bc ± 9.6 | 7.82 c ± 0.99 | 157 a ± 23 |
| 90 | 4.87 a ± 0.40 | 23.6 ab ± 5.8 | 45.4 ab ± 6.9 | 6.65 b ± 0.80 | 148 a ± 17 | |
| 110 | 4.85 a ± 0.35 | 18.5 a ± 5.4 | 41.0 a ± 9.0 | 5.75 a ± 1.17 | 141 a ± 12 | |
| 15 | 4.90 a ± 0.47 | 22.6 a ± 8.5 | 48.3 a ± 10.8 | 7.28 b ± 1.43 | 154 a ± 30 | |
| 30 | 4.82 a ± 0.41 | 22.2 a ± 7.1 | 45.7 a ± 9.3 | 6.74 a ± 1.29 | 148 a ± 19 | |
| 70 | 4.78 a ± 0.50 | 26.1 b ± 9.0 | 53.9 b ± 10.4 | 8.02 c ± 1.13 | 151 b ± 21 | |
| 90 | 4.91 a ± 0.43 | 22.0 ab ± 5.3 | 45.2 a ± 7.0 | 7.13 b ± 1.00 | 161 c ± 32 | |
| 110 | 4.88 a ± 0.38 | 19.2 a ± 7.3 | 42.0 a ± 8.7 | 5.88 a ± 1.10 | 141 a ± 15 | |
| Year (Y) | ||||||
| 2017 | 4.51 a ± 0.30 | 20.1 a ± 4.2 | 42.6 a ± 9.1 | 5.98 a ± 1.35 | 140 a ± 5 | |
| 2018 | 5.33 c ± 0.29 | 19.3 a ± 5.8 | 42.9 a ± 6.1 | 7.31 b ± 0.92 | 173 b ± 30 | |
| 2019 | 4.74 b ± 0.22 | 27.9 b ± 9.4 | 55.5 b ± 8.8 | 7.74 b ± 1.22 | 140 a ± 14 | |
| Mean | 4.86 ± 0.44 | 22.4 ± 7.8 | 47.0 ± 10.0 | 7.01 ± 1.38 | 151 ± 25 | |
| S | ns | ns | ns | ** | ns | |
| D | ns | ** | *** | *** | *** | |
| Y | *** | *** | *** | *** | *** | |
| S × D | ns | ns | ns | ns | *** | |
| S × Y | ns | ns | ns | ns | ns | |
| D × Y | ns | ns | ** | ns | ** | |
| S × D × Y | ns | ns | ns | ns | * | |
| Factor | Plant Height [cm] | 1st. Pod Height [cm] | Number of Nodules Per Plant [pcs.] | Dry Weight of Nodules Per Plant [g] | |
|---|---|---|---|---|---|
| Row Spacing [cm] (S) | Sowing Density [pcs.m−2] (D) | ||||
| 15 | 70 | 85.7 a–c ± 14.2 | 13.9 bc ± 1.9 | 22.1 ab ± 5.8 | 0.325 ab ± 0.062 |
| 90 | 87.9 bc ± 15.4 | 14.0 bc ± 1.3 | 22.1 ab ± 6.1 | 0.295 ab ± 0.074 | |
| 110 | 91.2 c ± 18.4 | 14.5 c ± 1.3 | 20.7 ab ± 4.2 | 0.280 ab ± 0.097 | |
| 30 | 70 | 80.2 a ± 11.8 | 12.2 a ± 1.3 | 25.0 b ± 4.2 | 0.330 b ± 0.105 |
| 90 | 83.7 ab ± 14.7 | 12.8 ab ± 1.1 | 23.0 a ± 6.4 | 0.250 a ± 0.036 | |
| 110 | 87.0 bc ± 15.1 | 13.3 a-c ± 1.4 | 18.9 a ± 4.2 | 0.249 a ± 0.091 | |
| 15 | 88.3 b ± 15.8 | 14.1 b ± 1.5 | 21.6 a ± 5.3 | 0.300 a ± 0.078 | |
| 30 | 83.6 a ± 13.8 | 12.8 a ± 1.3 | 22.3 a ±5.5 | 0.277 a ± 0.089 | |
| 70 | 82.9 a ± 13.1 | 13.1 a ± 1.7 | 23.5 b ± 5.1 | 0.328 b ± 0.084 | |
| 90 | 85.8 a ± 14.9 | 13.4 ab ± 1.4 | 22.5 ab ± 6.1 | 0.273 a ± 0.061 | |
| 110 | 89.1 b ± 16.6 | 13.9 b ± 1.4 | 19.8 a ± 4.2 | 0.265 a ± 0.093 | |
| Year (Y) | |||||
| 2017 | 67.0 a ± 1.1 | 12.9 a ± 1.1 | 18.5 a ± 5.0 | 0.237 a ± 0.071 | |
| 2018 | 98.6 c ± 9.3 | 13.0 a ± 1.6 | 24.7 b ± 5.6 | 0.359 b ± 0.078 | |
| 2019 | 92.2 b ± 4.3 | 14.5 b ± 1.3 | 22.7 b ± 3.4 | 0.269 a ± 0.046 | |
| Mean | 85.9 ± 14.9 | 13.5 ± 1.5 | 22.0 ± 5.3 | 0.288 ± 0.084 | |
| S | *** | *** | ns | ns | |
| D | *** | * | * | ** | |
| Y | *** | *** | *** | *** | |
| S × D | ns | ns | ns | ns | |
| S × Y | ** | ** | ns | * | |
| D × Y | ns | ns | ns | ns | |
| S × D × Y | ns | ns | ** | ns | |
| Factor | Protein Content [% Dry Matter] | Protein Yield [kgha−1] | Oil Content [% Dry Matter] | Oil Yield [kgha−1] | |
|---|---|---|---|---|---|
| Row Spacing [cm] (S) | Plant Density [pcs.m−2] (D) | ||||
| 15 | 70 | 35.4 a ± 2.5 | 1673 a ± 267 | 22.6 a ± 0.8 | 1066 a ± 152 |
| 90 | 36.8 b ± 3.4 | 1736 a ± 218 | 22.4 a ± 1.1 | 1063 a ± 153 | |
| 110 | 36.1 ab ± 2.9 | 1685 a ± 178 | 22.6 a ± 1.1 | 1057 a ± 117 | |
| 30 | 70 | 36.7 b ± 3.6 | 1810 a ± 173 | 22.5 a ± 1.3 | 1113 a ± 131 |
| 90 | 36.9 b ± 3.0 | 1725 a ± 215 | 22.5 a ± 0.7 | 1053 a ± 87 | |
| 110 | 36.7 b ± 2.5 | 1846 a ± 148 | 22.4 a ± 0.7 | 1134 a ± 138 | |
| 15 | 36.1 a ± 2.8 | 1698 a ± 212 | 22.5 a ± 1.0 | 1062 a ± 133 | |
| 30 | 36.8 b ± 2.9 | 1794 b ± 178 | 22.5 a ± 0.9 | 1100 a ± 119 | |
| 70 | 36.0 a ± 3.0 | 1742 a ± 226 | 22.5 a ± 1.1 | 1089 a ± 138 | |
| 90 | 36.8 b ± 3.1 | 1730 a ± 207 | 22.5 a ± 0.9 | 1058 a ± 118 | |
| 110 | 36.4 ab ± 2.6 | 1765 a ± 177 | 22.5 a ± 0.9 | 1095 a ± 129 | |
| Year (Y) | |||||
| 2017 | 34.6 a ± 1.0 | 1526 a ± 129 | 23.3 c ± 0.5 | 1030 a ± 77 | |
| 2018 | 40.2 b ± 1.2 | 1854 b ± 137 | 21.4 a ± 0.5 | 990 a ± 78 | |
| 2019 | 34.5 a ± 0.5 | 1858 b ± 106 | 22.7 b ± 0.3 | 1222 b ± 70 | |
| Mean | 36.4 ± 2.9 | 1746 ± 199 | 22.5 ± 0.9 | 1081 ± 126 | |
| S | ** | * | ns | ns | |
| D | * | ns | ns | ns | |
| Y | *** | *** | *** | *** | |
| S × D | ns | ns | ns | ns | |
| S × Y | * | ns | ns | ns | |
| D × Y | * | ns | ns | ns | |
| S × D × Y | * | ns | ns | ns | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jańczak-Pieniążek, M.; Buczek, J.; Bobrecka-Jamro, D.; Szpunar-Krok, E.; Tobiasz-Salach, R.; Jarecki, W. Morphophysiology, Productivity and Quality of Soybean (Glycine max (L.) Merr.) cv. Merlin in Response to Row Spacing and Seeding Systems. Agronomy 2021, 11, 403. https://doi.org/10.3390/agronomy11020403
Jańczak-Pieniążek M, Buczek J, Bobrecka-Jamro D, Szpunar-Krok E, Tobiasz-Salach R, Jarecki W. Morphophysiology, Productivity and Quality of Soybean (Glycine max (L.) Merr.) cv. Merlin in Response to Row Spacing and Seeding Systems. Agronomy. 2021; 11(2):403. https://doi.org/10.3390/agronomy11020403
Chicago/Turabian StyleJańczak-Pieniążek, Marta, Jan Buczek, Dorota Bobrecka-Jamro, Ewa Szpunar-Krok, Renata Tobiasz-Salach, and Wacław Jarecki. 2021. "Morphophysiology, Productivity and Quality of Soybean (Glycine max (L.) Merr.) cv. Merlin in Response to Row Spacing and Seeding Systems" Agronomy 11, no. 2: 403. https://doi.org/10.3390/agronomy11020403
APA StyleJańczak-Pieniążek, M., Buczek, J., Bobrecka-Jamro, D., Szpunar-Krok, E., Tobiasz-Salach, R., & Jarecki, W. (2021). Morphophysiology, Productivity and Quality of Soybean (Glycine max (L.) Merr.) cv. Merlin in Response to Row Spacing and Seeding Systems. Agronomy, 11(2), 403. https://doi.org/10.3390/agronomy11020403










