Water-Soluble Carbon Nanoparticles Improve Seed Germination and Post-Germination Growth of Lettuce under Salinity Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Sensitivity of Different Lettuce Varieties
2.2. Pretreatments of Salt-Sensitive Lettuce Seeds with Carbon Nanoparticles (CNPs)
2.3. Seed Priming with CNPs
2.4. Thermoinhibition Tests of CNP-Treated Seeds
2.5. Effects of CNPs on Post-Germination Growth
2.6. Determination of Chlorophyll Contents
2.7. Statistical Analysis
3. Results
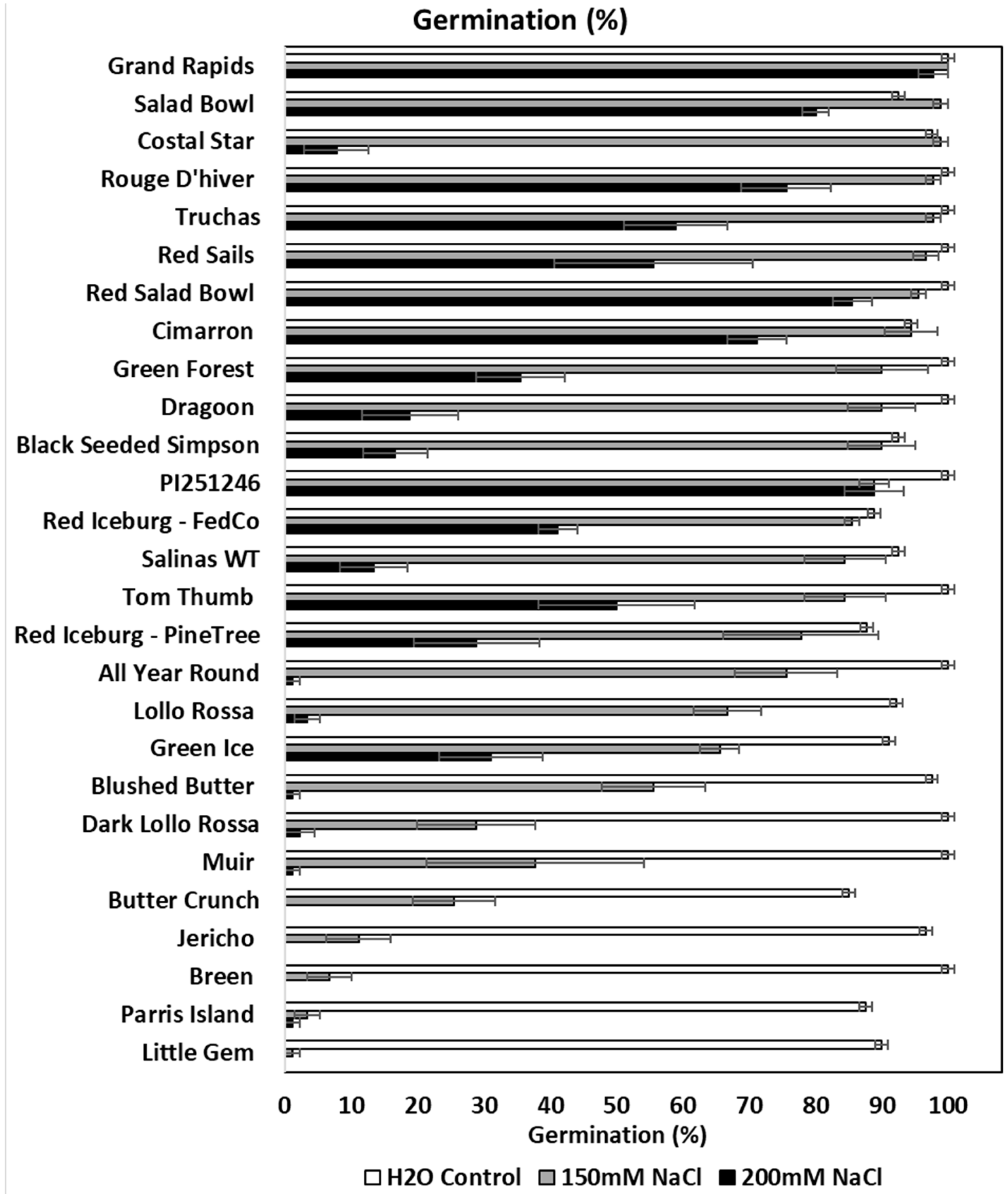
3.1. Distinct Responses of Lettuce Varieties to Salt Stress at Germination
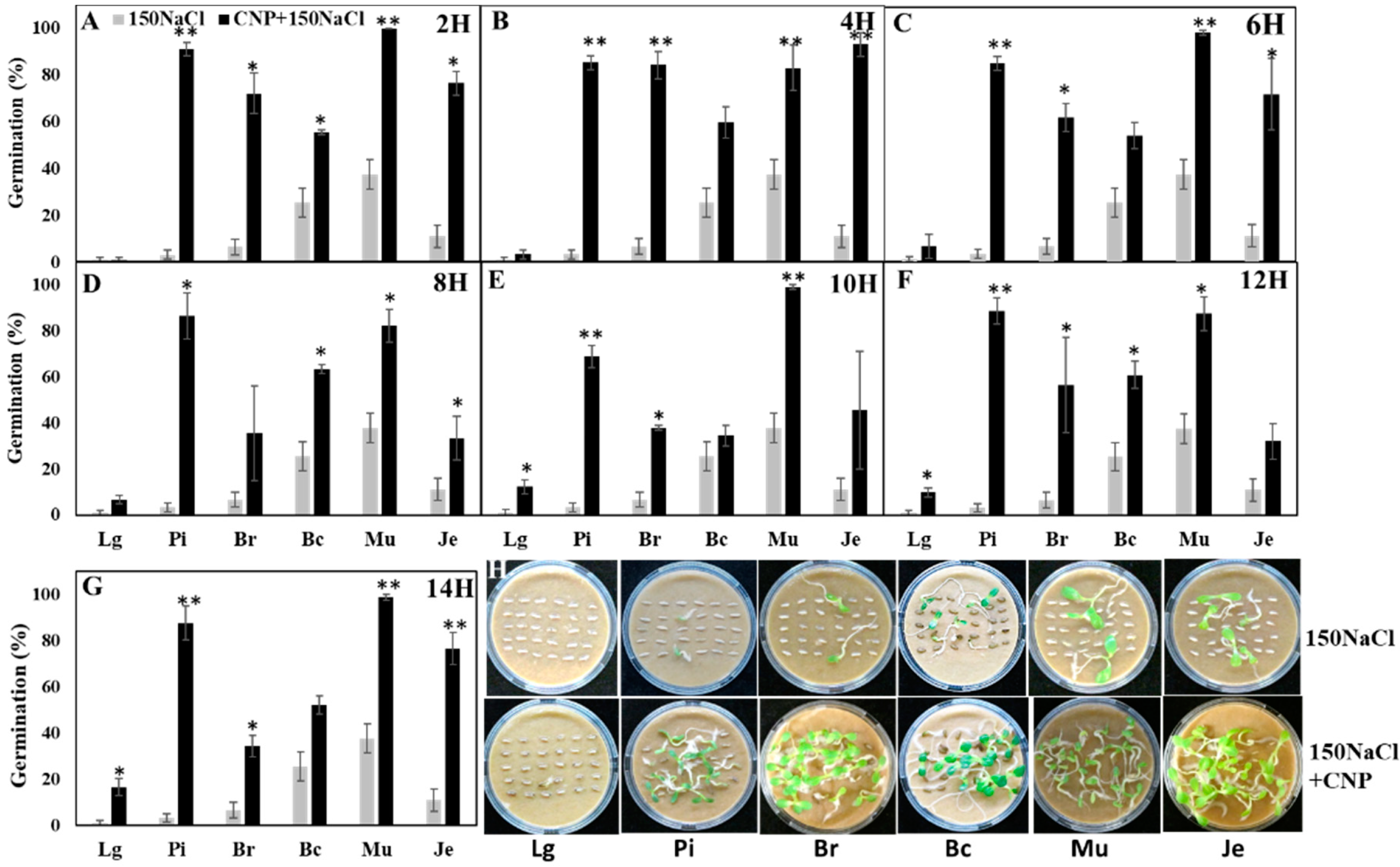
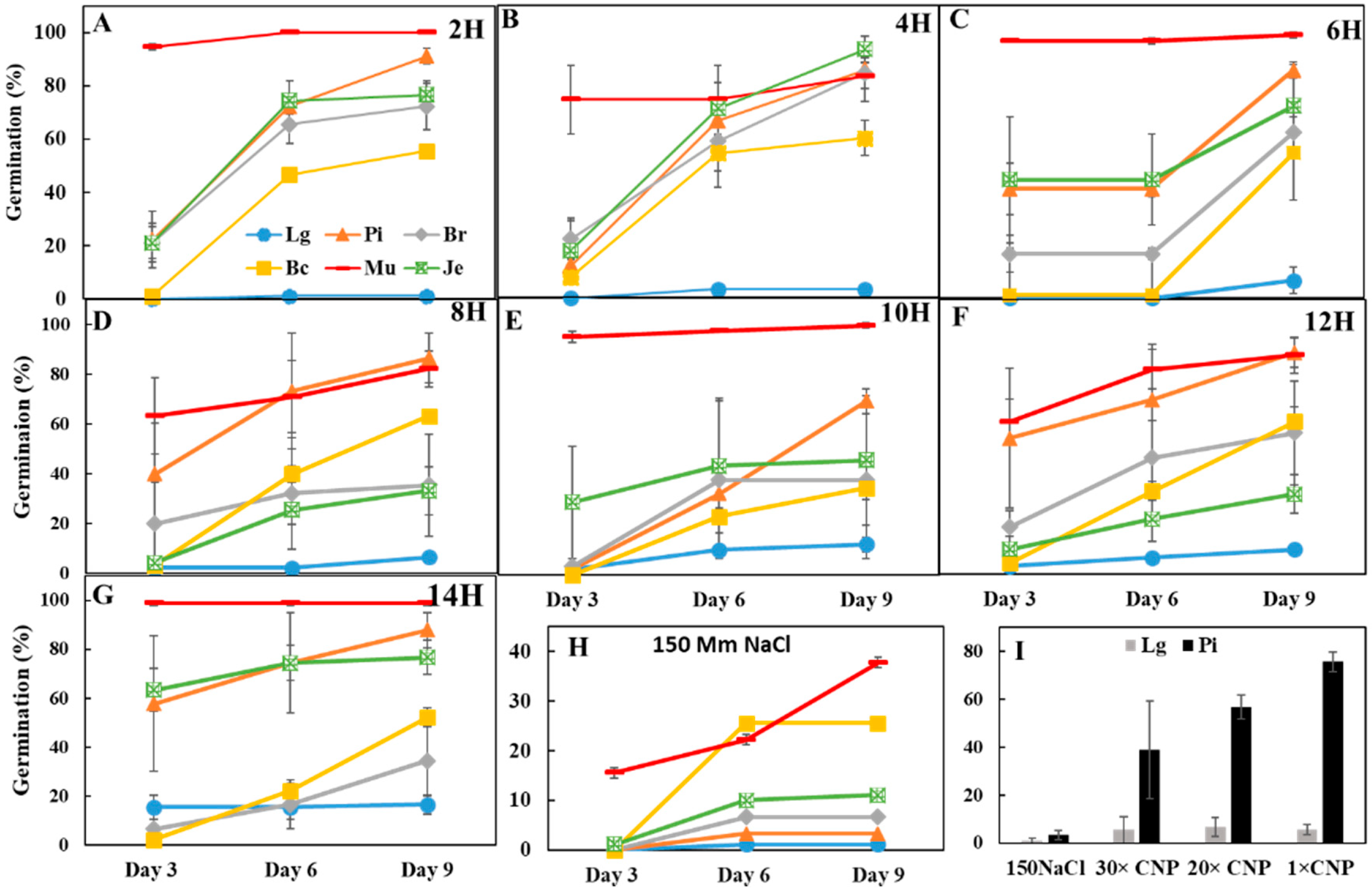
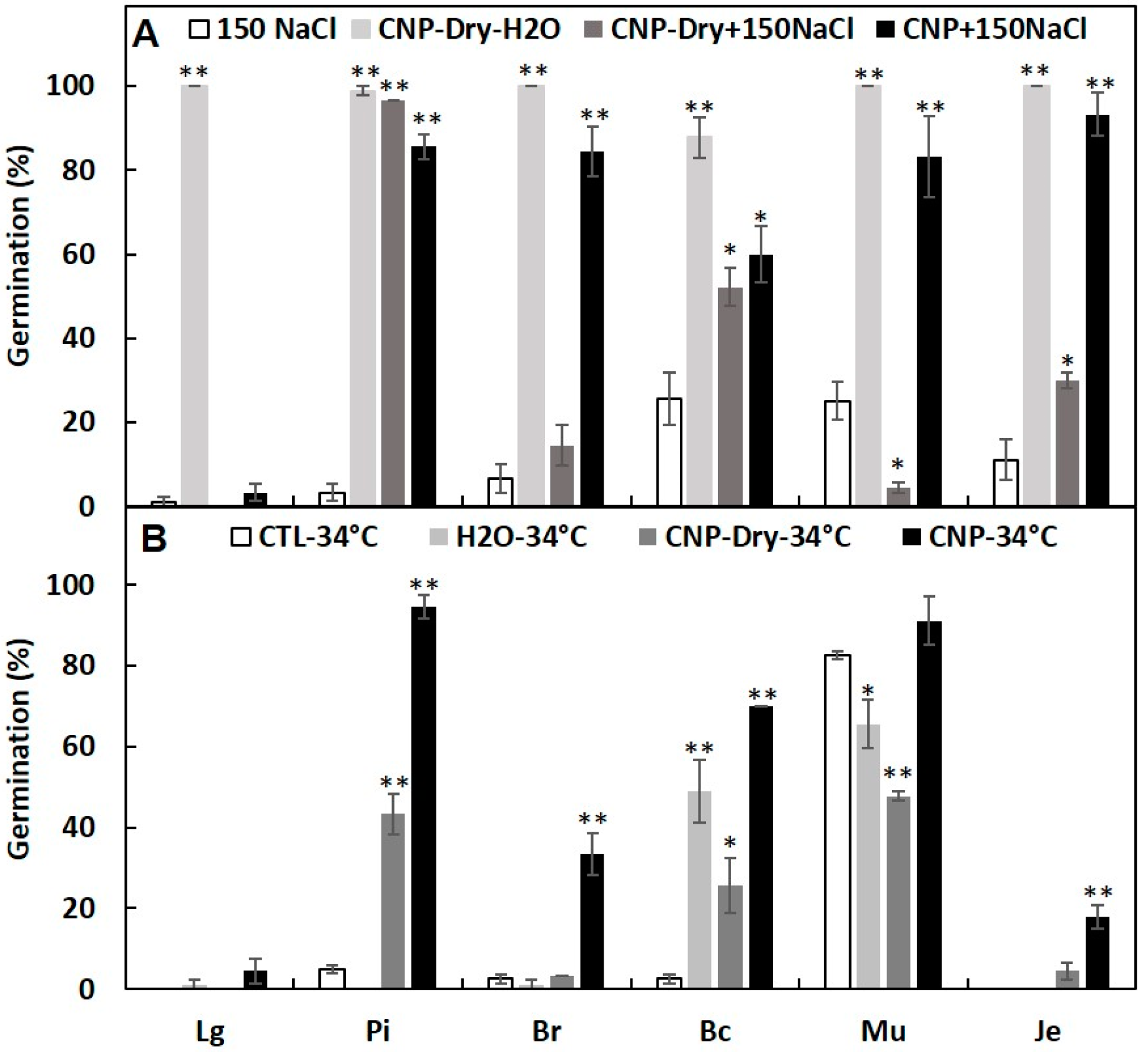
3.2. Effects of CNPs on Seed Germination of Salt-Sensitive Lettuce
3.3. Pretreatment with CNP But Not H2O Improved Lettuce Seed Germination
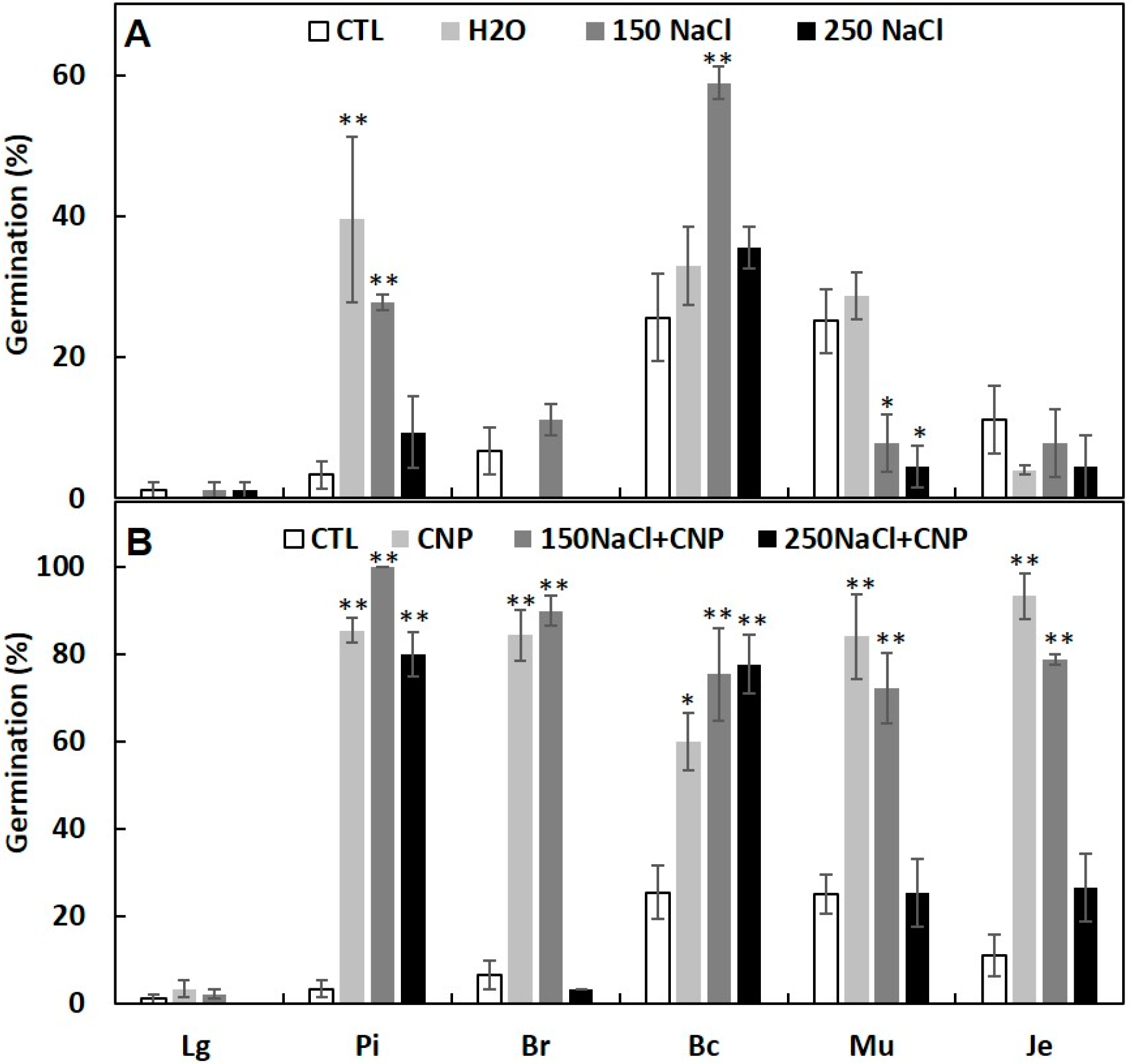
3.4. Seed Priming with CNPs Can Enhance Seed Germination under Salinity and High Temperature Stresses
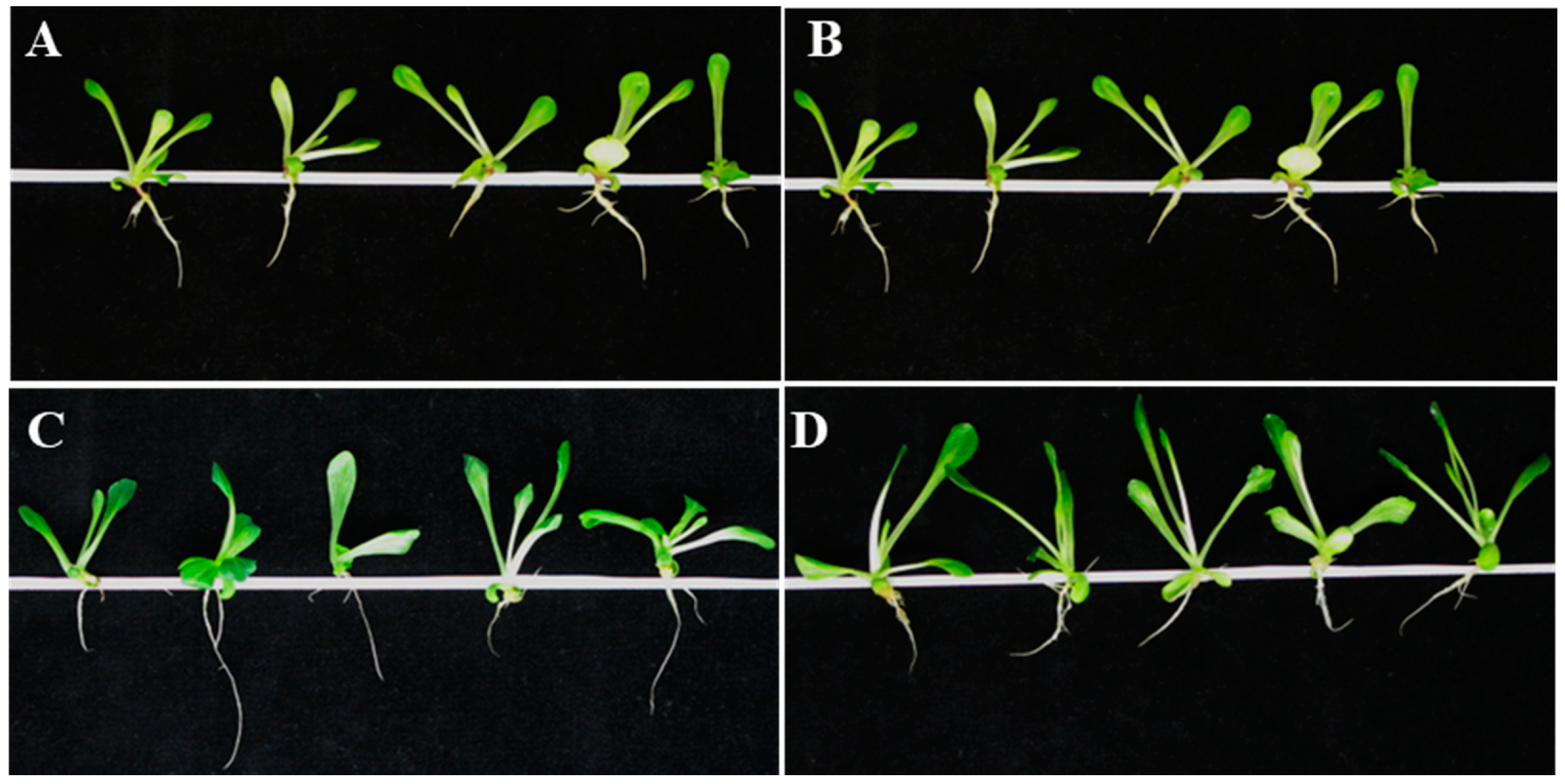
3.5. CNPs Treatment Positively Affected Post-Germination Seedling Growth under Salinity Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Veatch-Blohm, M.E.; Sawch, D.; Elia, N.; Pinciotti, D. Salinity Tolerance of Three Commonly Planted Narcissus Cultivars. HortScience 2014, 49, 1158–1164. [Google Scholar] [CrossRef]
- Kaushal, S.S.; Duan, S.W.; Doody, T.R.; Haq, S.; Smith, R.M.; Johnson, T.A.N.; Newcomb, K.D.; Gorman, J.; Bowman, N.; Mayer, P.M.; et al. Human-accelerated weathering increases salinization, major ions, and alkalinization in fresh water across land use. Appl. Geochem. 2017, 83, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Melillo, J.; Richmond, T.T.C.; Yohe, G. Climate Change Impacts in the United States: The Third National Climate Assessment, 2014. Global Change Information System. Available online: https://data.globalchange.gov/ (accessed on 21 August 2019).
- Ismail, A.M.; Horie, T. Genomics, Physiology, and Molecular Breeding Approaches for Improving Salt Tolerance. Annu. Rev. Plant Biol. 2017, 68, 405–434. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Shweta; Singh, S.; Singh, S.; Pandey, R.; Singh, V.P.; Sharma, N.C.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation and phytotoxicity. Plant Physiol. Bioch. 2017, 110, 2–12. [Google Scholar] [CrossRef]
- Zaytseva, O.; Neumann, G. Carbon nanomaterials: Production, impact on plant development, agricultural and environmental applications. Chem. Biol. Technol. 2016, 3, 17. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Adrees, M.; Ibrahim, M.; Zia-ur-Rehmand, M.; Farid, M.; Abbas, F. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J. Hazard Mater. 2017, 322, 2–16. [Google Scholar] [CrossRef]
- Maity, A. Influence of Metal Nanoparticles (NPs) on Germination and Yield of Oat (Avena sativa) and Berseem (Trifolium alexandrinum). Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 595–607. [Google Scholar] [CrossRef]
- Mahakham, W.; Theerakulpisut, P.; Maensiri, S.; Phumying, S.; Sarmah, A.K. Environmentally benign synthesis of phytochemicals-capped gold nanoparticles as nanopriming agent for promoting maize seed germination. Sci. Total Environ. 2016, 573, 1089–1102. [Google Scholar] [CrossRef]
- Zafar, H.; Ali, A.; Zia, M. CuO Nanoparticles Inhibited Root Growth from Brassica nigra Seedlings but Induced Root from Stem and Leaf Explants. Appl. Biochem. Biotechnol. 2017, 181, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.C.; Zhang, H.B.; Tu, C.; Hu, X.F.; Li, L.Z.; Luo, Y.M.; Christie, P. Phytotoxicity of ZnO nanoparticles and the released Zn(II) ion to corn (Zea mays L.) and cucumber (Cucumis sativus L.) during germination. Environ. Sci. Pollut. Res. 2015, 22, 11109–11117. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.K.; Hossain, Z. Impact of nano-CuO stress on rice (Oryza sativa L.) seedlings. Chemosphere 2013, 93, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, M.R.; Giorgetti, L.; Geri, C.; Cremonini, R. The effects of nano-TiO2 on seed germination, development and mitosis of root tip cells of Vicia narbonensis L. and Zea mays L. J. Nanoparticle Res. 2011, 13, 2443–2449. [Google Scholar] [CrossRef]
- Moon, Y.S.; Park, E.S.; Kim, T.O.; Lee, H.S.; Lee, S.E. SELDI-TOF MS-based discovery of a biomarker in Cucumis sativus seeds exposed to CuO nanoparticles. Environ. Toxicol. Pharmacol. 2014, 38, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.M.G.; Chung, I.M. Impact of copper oxide nanoparticles exposure on Arabidopsis thaliana growth, root system development, root lignificaion, and molecular level changes. Environ. Sci. Pollut. Res. 2014, 21, 12709–12722. [Google Scholar] [CrossRef]
- Lee, C.W.; Mahendra, S.; Zodrow, K.; Li, D.; Tsai, Y.C.; Braam, J.; Alvarez, P.J.J. Developmental Phytotoxicity of Metal Oxide Nanoparticles to Arabidopsis Thaliana. Environ. Toxicol. Chem. 2010, 29, 669–675. [Google Scholar] [CrossRef]
- Garcia-Gomez, C.; Babin, M.; Obrador, A.; Alvarez, J.; Fernandez, M. Integrating ecotoxicity and chemical approaches to compare the effects of ZnO nanoparticles, ZnO bulk, and ZnCl2 on plants and microorganisms in a natural soil. Environ. Sci. Pollut. Res. 2015, 22, 16803–16813. [Google Scholar] [CrossRef]
- Ma, Y.H.; He, X.; Zhang, P.; Zhang, Z.Y.; Guo, Z.; Tai, R.Z.; Xu, Z.J.; Zhang, L.J.; Ding, Y.Y.; Zhao, Y.L.; et al. Phytotoxicity and biotransformation of La2O3 nanoparticles in a terrestrial plant cucumber (Cucumis sativus). Nanotoxicology 2011, 5, 743–753. [Google Scholar] [CrossRef]
- Wu, S.G.; Huang, L.; Head, J.; Chen, D.R.; Kong, I.C.; Tang, Y.J. Phytotoxicity of metal oxide nanoparticles is related to both dissolved metals ions and adsorption of particles on seed surfaces. J. Pet. Environ. Biotechnol. 2012, 3, 4–8. [Google Scholar]
- Lahiani, M.H.; Dervishi, E.; Chen, J.; Nima, Z.; Gaume, A.; Biris, A.S.; Khodakovskaya, M.V. Impact of carbon nanotube exposure to seeds of valuable crops. ACS Appl. Mater. Interfaces 2013, 5, 7965–7973. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.; Lahiani, M.H.; Hicks, V.K.; Hudson, M.K.; Green, M.J.; Khodakovskaya, M. Effects of carbon-based nanomaterials on seed germination, biomass accumulation and salt stress response of bioenergy crops. PLoS ONE 2018, 13, e0202274. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Kaur, S.; Dharamvir, K.; Nayyar, H.; Verma, G. Multi-walled carbon nanotubes applied through seed-priming influence early germination, root hair, growth and yield of bread wheat (Triticum aestivum L.). J. Sci. Food Agric. 2018, 98, 3148–3160. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Mohamed, M.S.; Gao, W.; Maekawa, T.; Yoshida, Y.; Ajayan, P.M.; Kumar, D.S. Effect of Carbon Nanomaterials on the Germination and Growth of Rice Plants. J. Nanosci. Nanotechnol. 2012, 12, 2212–2220. [Google Scholar] [CrossRef]
- Husen, A.; Siddiqi, K.S. Carbon and fullerene nanomaterials in plant system. J. Nanobiotechnol. 2014, 12, 16. [Google Scholar] [CrossRef]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; AlMutairi, K.A.; Siddiqui, Z.H. Role of nanomaterials in plants under challenging environments. Plant Physiol. Biochem. 2017, 110, 194–209. [Google Scholar] [CrossRef]
- Taylor, A.G.; Allen, P.S.; Bennett, M.A.; Bradford, K.J.; Burris, J.S.; Misra, M.K. Seed enhancements. Seed Sci. Res. 1998, 8, 245–256. [Google Scholar] [CrossRef]
- Argyris, J.; Dahal, P.; Hayashi, E.; Still, D.W.; Bradford, K.J. Genetic variation for lettuce seed thermoinhibition is associated with temperature-sensitive expression of abscisic acid, gibberellin, and ethylene biosynthesis, metabolism, and response genes. Plant Physiol. 2008, 148, 926–947. [Google Scholar] [CrossRef]
- Xu, C.P.; Mou, B.Q. Evaluation of Lettuce Genotypes for Salinity Tolerance. Hortscience 2015, 50, 1441–1446. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of Accurate Extinction Coefficients and Simultaneous-Equations for Assaying Chlorophyll-a and Chlorophyll-b Extracted with 4 Different Solvents-Verification of the Concentration of Chlorophyll Standards by Atomic-Absorption Spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 8263. [Google Scholar] [CrossRef] [PubMed]
- Feizi, H.; Rezvani Moghaddam, P.; Shahtahmassebi, N.; Fotovat, A. Impact of bulk and nanosized titanium dioxide (TiO2) on wheat seed germination and seedling growth. Biol. Trace Element Res. 2012, 146, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Sivritepe, N.; Sivritepe, H.O.; Eris, A. The effects of NaCl priming on salt tolerance in melon seedlings grown under saline conditions. Sci. Hortic. 2003, 97, 229–237. [Google Scholar] [CrossRef]
- Yan, K.; Xu, H.L.; Cao, W.; Chen, X.B. Salt priming improved salt tolerance in sweet sorghum by enhancing osmotic resistance and reducing root Na+ uptake. Acta Physiol. Plant. 2015, 37, 203. [Google Scholar] [CrossRef]
- Martinez-Ballesta, M.C.; Zapata, L.; Chalbi, N.; Carvajal, M. Multiwalled carbon nanotubes enter broccoli cells enhancing growth and water uptake of plants exposed to salinity. J. Nanobiotechnol. 2016, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.Q.; Dahal, P.; Kunusoth, K.; McCallum, C.M.; Bradford, K.J. Expression of 9-cis-EPOXYCAROTENOID DIOXYGENASE4 Is Essential for Thermoinhibition of Lettuce Seed Germination but Not for Seed Development or Stress Tolerance. Plant Cell 2013, 25, 884–900. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; de Silva, K.; Nedosekin, D.A.; Dervishi, E.; Biris, A.S.; Shashkov, E.V.; Galanzha, E.I.; Zharov, V.P. Complex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 1028–1033. [Google Scholar] [CrossRef]
- Zhao, L.J.; Peng, B.; Hernandez-Viezcas, J.A.; Rico, C.; Sun, Y.P.; Peralta-Videa, J.R.; Tang, X.L.; Niu, G.H.; Jin, L.X.; Varela-Ramirez, A.; et al. Stress Response and Tolerance of Zea mays to CeO2 Nanoparticles: Cross Talk among H2O2, Heat Shock Protein, and Lipid Peroxidation. ACS Nano 2012, 6, 9615–9622. [Google Scholar] [CrossRef]
- Lin, D.H.; Xing, B.S. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef]
- Canas, J.E.; Long, M.Q.; Nations, S.; Vadan, R.; Dai, L.; Luo, M.X.; Ambikapathi, R.; Lee, E.H.; Olszyk, D. Effects of functionalized and nonfunctionalized single-walled carbon nanotubes on root elongation of select crop species. Environ. Toxicol. Chem. 2008, 27, 1922–1931. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Hatami, M. Spray treatment with silver nanoparticles plus thidiazuron increases anti-oxidant enzyme activities and reduces petal and leaf abscission in four cultivars of geranium (Pelargonium zonale) during storage in the dark. J. Hortic. Sci. Biotech. 2014, 89, 712–718. [Google Scholar] [CrossRef]
- Tripathi, S.; Sarkar, S. Influence of water soluble carbon dots on the growth of wheat plant. Appl. Nanosci. 2015, 5, 609–616. [Google Scholar] [CrossRef]
| Leafy | Romaine | Iceberg | Butterhead | Seed Company |
|---|---|---|---|---|
| Muir, Rouge D’hiver, Green Forest | Truchas, Dragoon Breen, Costal Star | Johnny’s Seeds | ||
| Red Salad Bowl Black Seeded Simpson Salad Bowl, Red Sails, Lollo Rossa, Dark Lolla Rossa | Blushed Butter, Parris Island | Red Iceberg | Tom Thumb Butter Crunch | Fedco Seeds |
| Green Ice | Cimarron Jericho | Red Iceberg | Little Gem *, All Year Round | Pinetree Garden Seeds |
| Grand Rapids | Eden Brothers | |||
| Grand Rapids | Stokes Seeds | |||
| PI251246 ** | Salinas | Self-produced |
| Variety | Treatment | Leaf Length (cm) | Root Length (cm) | No. of Leaf | No. of Lateral Roots | Fresh Weight (mg) | Dry Weight (mg) |
|---|---|---|---|---|---|---|---|
| Lg | control | 2.68 ± 0.47 | 2.27 ± 0.62 | 4.42 ± 0.67 | 4.08 ± 4.27 | 125.33 ± 10.01 | 7.53 ± 0.52 |
| CNP | 3.22 ± 0.36 * | 2.21 ± 0.46 | 5.17 ± 0.58 ** | 7.17 ± 2.62 | 151.9 ± 10.97 | 8.6 ± 0.49 | |
| Pi | control | 2.91 ± 0.45 | 2.71 ± 0.97 | 4.33 ± 0.89 | 2.17 ± 1.03 | 91.83 ± 5.29 | 4.98 ± 0.34 |
| CNP | 4.18 ± 0.43 ** | 2.13 ± 0.51 * | 4.83 ± 0.39 * | 5.58 ± 1.08 ** | 166.68 ± 9.71 ** | 8.83 ± 0.4 ** | |
| Br | control | 3.45 ± 0.67 | 3.06 ± 0.78 | 4.25 ± 0.87 | 2.83 ± 1.4 | 149.04 ± 7.71 | 6.78 ± 0.35 |
| CNP | 2.96 ± 0.38 | 1.96±0.55 ** | 4.85 ± 0.79 | 4.42 ± 1.93 * | 111.58 ± 7.98 ** | 6.35 ± 0.35 * | |
| Bc | control | 3.26 ± 0.49 | 2.3 ± 0.43 | 4.58 ± 0.51 | 2.92 ± 2.15 | 123.84 ± 7.19 | 7.26 ± 0.46 |
| CNP | 2.98 ± 0.58 | 2.31 ± 0.53 | 4.75 ± 0.97 | 2.92 ± 2.91 | 131.22 ± 10.5 | 7.13 ± 0.51 | |
| Mu | control | 4.35 ± 1.15 | 2.77 ± 0.77 | 6.33 ± 1.15 | 5.33 ± 1.77 | 362.28 ± 82.74 | 13.32 ± 1.66 |
| CNP | 4.38 ± 0.62 | 2.65 ± 0.37 | 4.42 ± 0.67 ** | 5.67 ± 1.49 | 182.18 ± 71.9 * | 6.88 ± 0.62 ** | |
| Je | control | 3.55 ± 0.68 | 2.09 ± 0.64 | 3.83 ± 0.58 | 3.33 ± 1.3 | 57.12 ± 2.61 | 2.62 ± 0.21 |
| CNP | 3.63 ± 0.24 | 2.18 ± 0.57 | 3.25 ± 0.45 ** | 2.58 ± 1.31 | 70.65 ± 27.8 | 2.2 ± 0.13 * |
| Sample | Chl a −CNP (ug/g FW) | Chl a +CNP (ug/g FW) | Chl b −CNP (ug/g FW) | Chl b +CNP (ug/g FW) | Total Chl −CNP (ug/g FW) | Total Chl +CNP (ug/g FW) |
|---|---|---|---|---|---|---|
| Little Gem | 20.50 ± 5.18 | 25.60 ± 9.97 | 188.00 ± 62.19 | 399.19 ± 163.7 * | 231.54 ± 64.63 | 424.79 ± 172.87 * |
| Parris Island | 29.58 ± 6.79 | 30.07 ± 12.46 | 548.52± 104.29 | 776.15 ± 123.07 ** | 593.41 ± 107.90 | 806.22 ± 131.44 ** |
| Breen | 16.37 ± 4.02 | 23.64 ± 8.38 | 163.80 ± 63.02 | 295.85 ± 96.60 * | 200.48 ± 66.78 | 319.46 ± 103.98 * |
| Buttercrunch | 24.77 ± 3.73 | 23.66 ± 13.52 | 674.52 ± 139.01 | 889.80 ± 118.60 ** | 700.89 ± 139.54 | 913.46 ± 106.67 ** |
| Muir | 18.25 ± 6.97 | 26.71 ± 5.99 * | 248.96 ± 106.79 | 801.35 ± 212.21 ** | 299.18 ± 113.33 | 828.06 ± 215.44 ** |
| Jericho | 37.53 ± 11.39 | 39.33 ± 9.66 | 554.44 ± 184.83 | 939.01 ± 125.84 ** | 624.78 ± 194.19 | 978.35 ± 132.15 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baz, H.; Creech, M.; Chen, J.; Gong, H.; Bradford, K.; Huo, H. Water-Soluble Carbon Nanoparticles Improve Seed Germination and Post-Germination Growth of Lettuce under Salinity Stress. Agronomy 2020, 10, 1192. https://doi.org/10.3390/agronomy10081192
Baz H, Creech M, Chen J, Gong H, Bradford K, Huo H. Water-Soluble Carbon Nanoparticles Improve Seed Germination and Post-Germination Growth of Lettuce under Salinity Stress. Agronomy. 2020; 10(8):1192. https://doi.org/10.3390/agronomy10081192
Chicago/Turabian StyleBaz, Hanna, Matthew Creech, Jianjun Chen, Haijun Gong, Kent Bradford, and Heqiang Huo. 2020. "Water-Soluble Carbon Nanoparticles Improve Seed Germination and Post-Germination Growth of Lettuce under Salinity Stress" Agronomy 10, no. 8: 1192. https://doi.org/10.3390/agronomy10081192
APA StyleBaz, H., Creech, M., Chen, J., Gong, H., Bradford, K., & Huo, H. (2020). Water-Soluble Carbon Nanoparticles Improve Seed Germination and Post-Germination Growth of Lettuce under Salinity Stress. Agronomy, 10(8), 1192. https://doi.org/10.3390/agronomy10081192







