Genetic Diversity and Population Structure of Brachiaria (syn. Urochloa) Ecotypes from Uganda
Abstract
1. Introduction
2. Materials and Methods
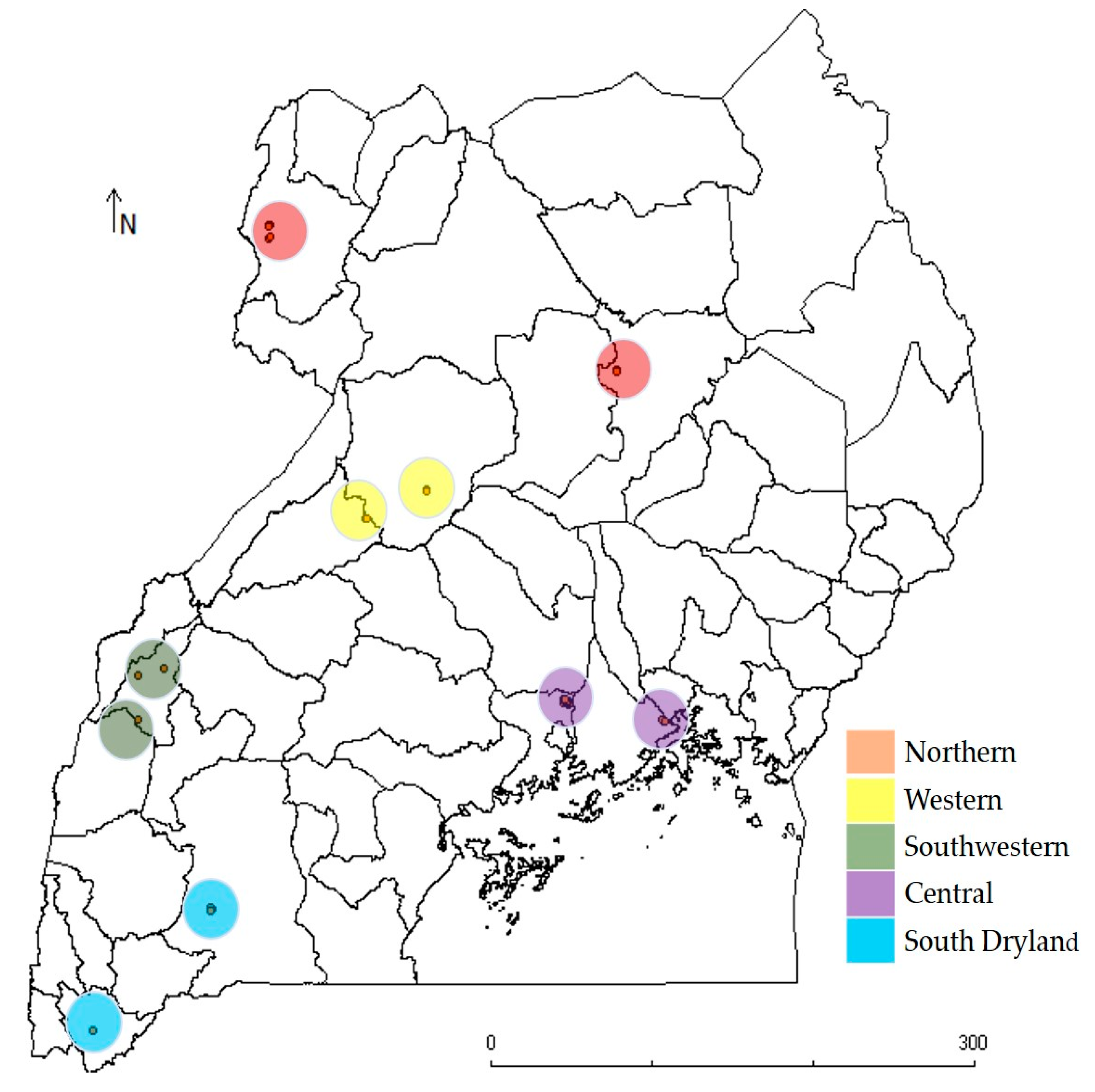
2.1. Source of Plant Materials
2.2. DNA Extraction
2.3. PCR Amplification and Capillary Electrophoresis
2.4. Allelic Scoring
2.5. Population Genetic Analyses
3. Results
3.1. Microsatellite Diversity and Analysis of Molecular Variance
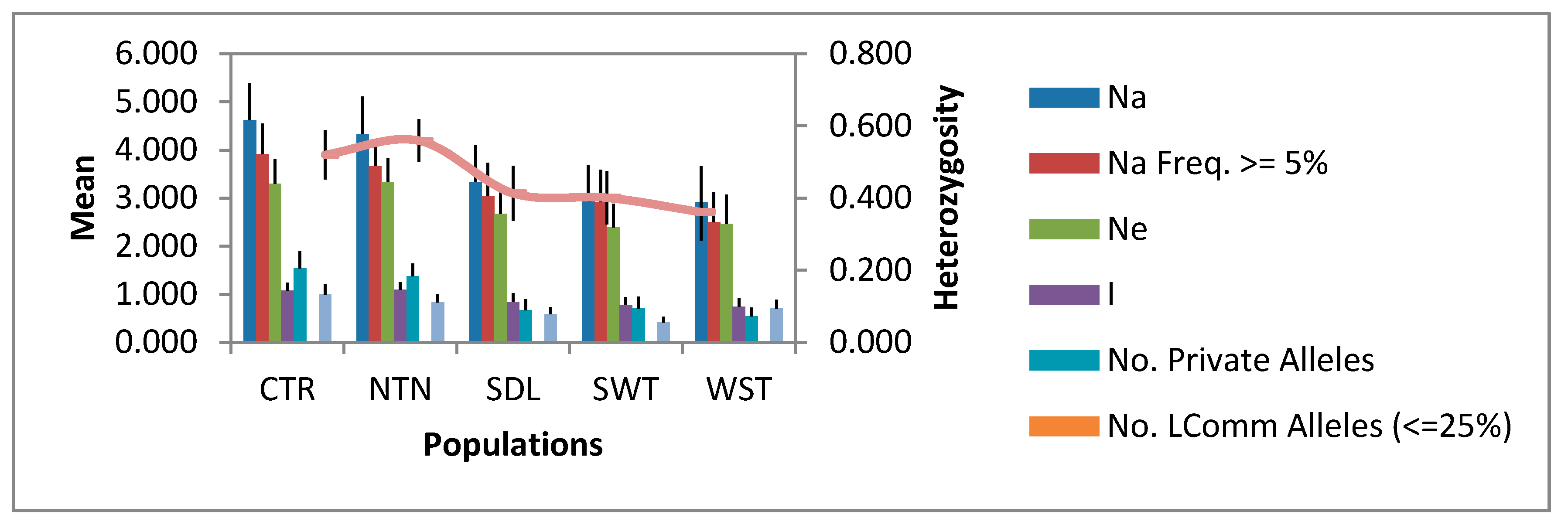
3.2. Allelic Diversity in the Regional Populations
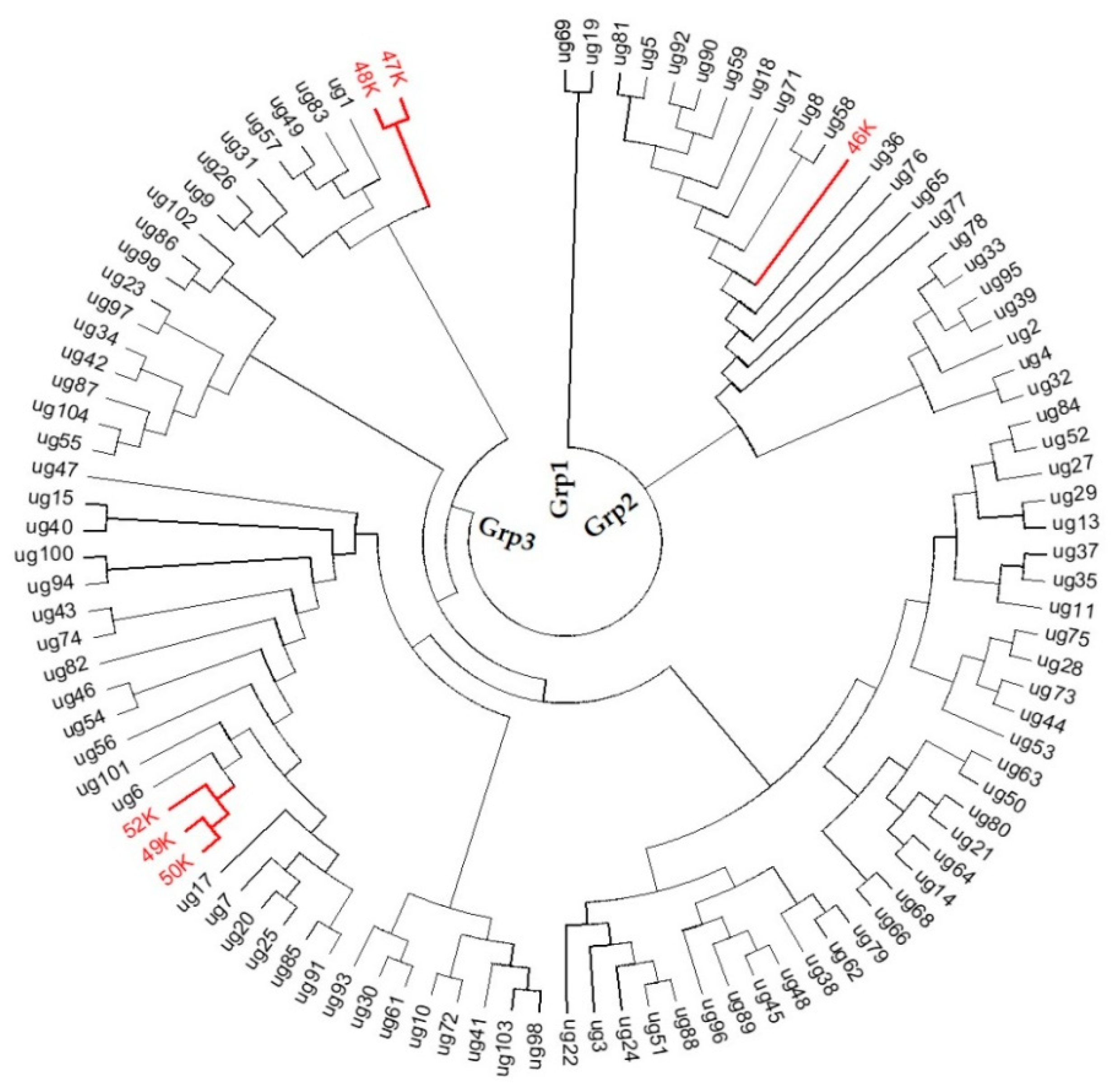
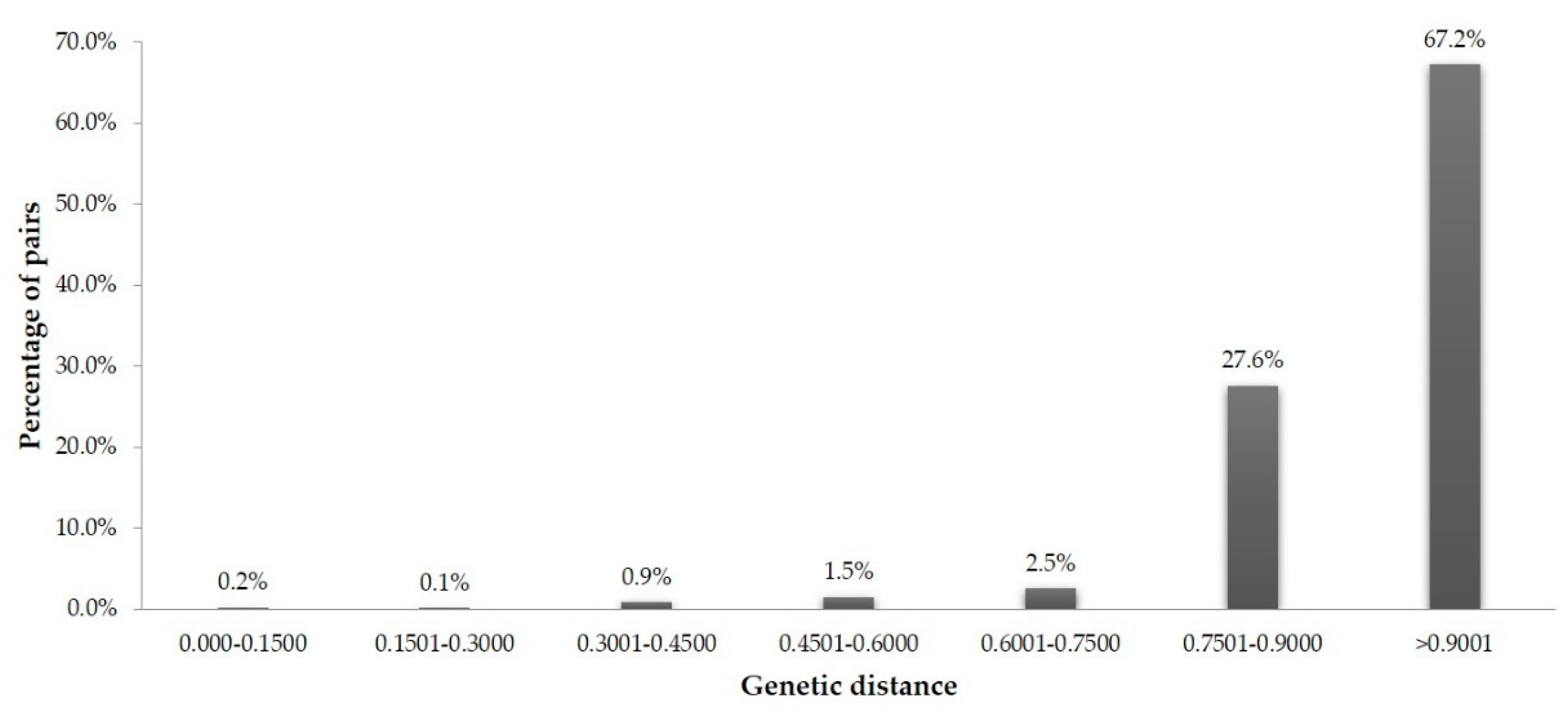
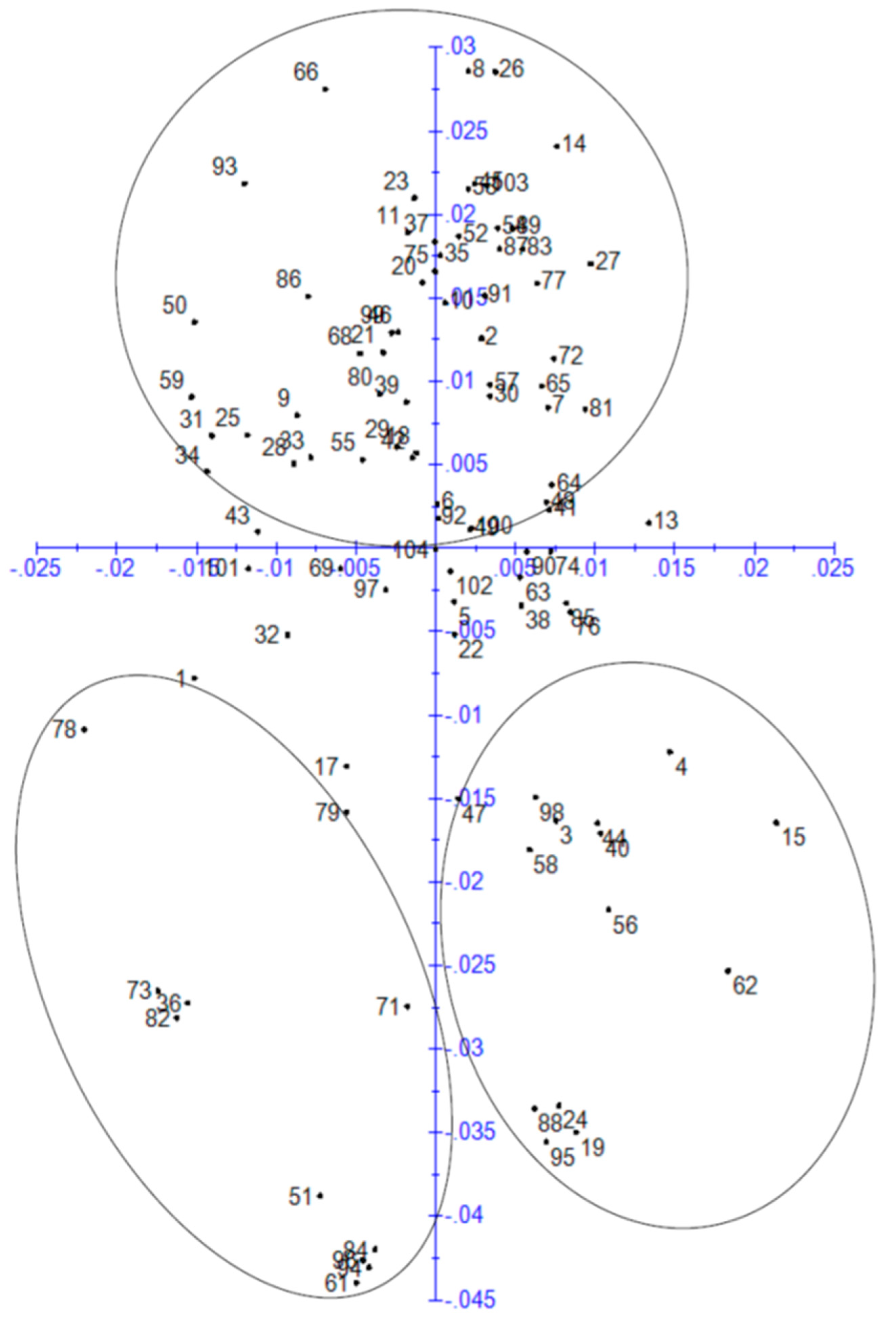
3.3. Similarity-Based Analysis
3.4. Principal Component Analysis
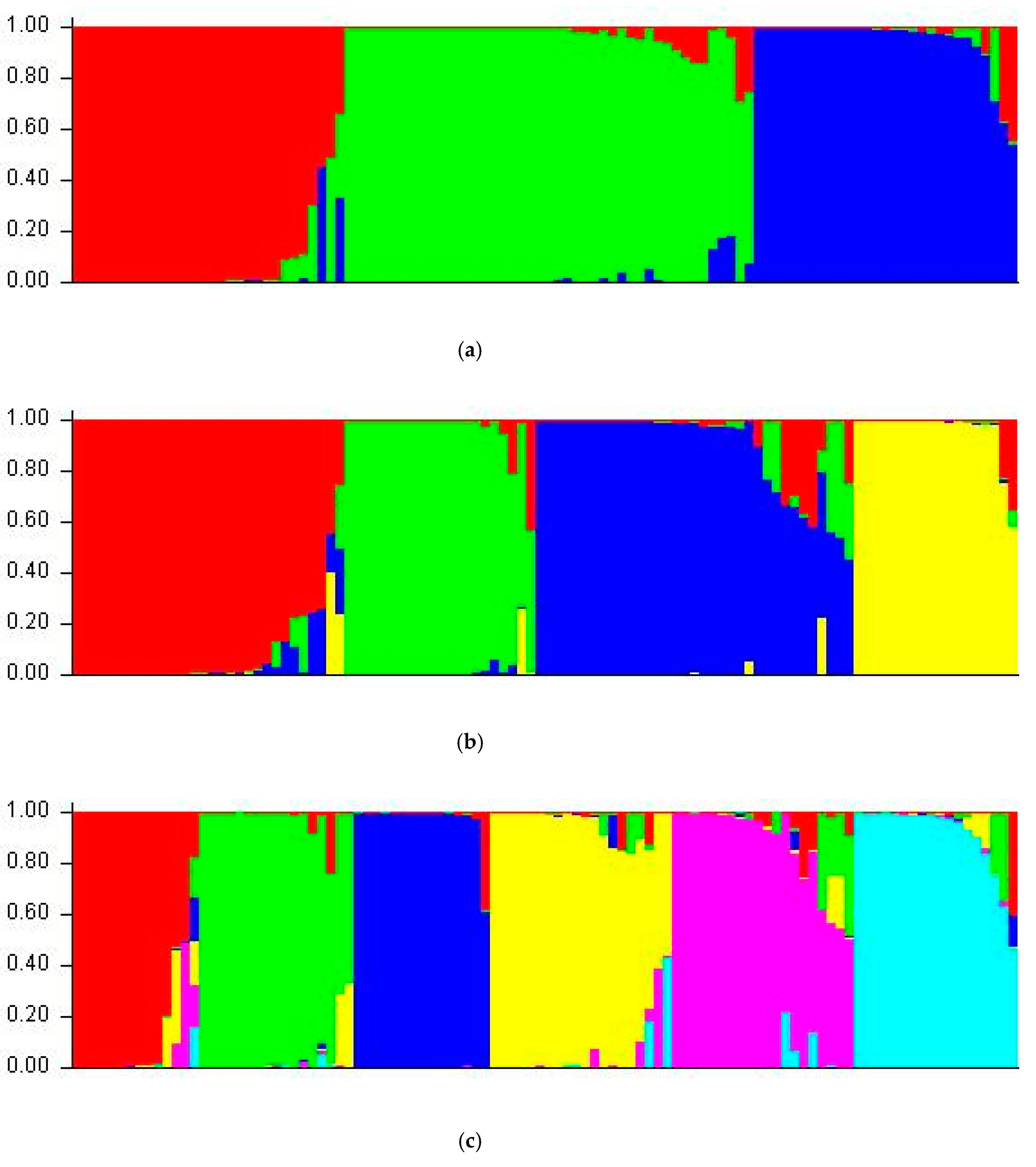
3.5. Structure Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jungmann, L.; Vigna, B.B.Z.; Paiva, J.; Sousa, A.C.B.; do Valle, C.B.; Laborda, P.R.; Zucchi, M.I.; de Souza, A.P. Development of microsatellite markers for Brachiaria humidicola (Rendle) Schweik. Conserv. Genet. Res. 2009, 1, 475. [Google Scholar] [CrossRef]
- Renvoize, S.A.; Clay, W.D.; Kabuye, C.H.S. Morphology, taxonomy and natural distribution of Brachiaria (Trin.) Griesb. In Brachiaria: Biology, Agronomy and Improvement; Miles, J.W., Maass, B.L., do Valle, C.B., Eds.; International Center for Tropical Agriculture: Cali, Colombia, 1996; Volume 1, pp. 1–15. [Google Scholar]
- Keller-Grein, G.; Maass, B.L.; Hanson, J. Natural variation in Brachiaria and existing germplasm collections. In Brachiaria: Biology, Agronomy and Improvement; Miles, J.W., Maass, B.L., do Valle, C.B., Eds.; International Center for Tropical Agriculture: Cali, Colombia, 1996; Volume 1, pp. 16–42. [Google Scholar]
- Barnard, C. Herbage Plant Species, Australia Herbage Plant Registration Authority; CSIRO: Canberra, Australia, 1969; Volume 154. [Google Scholar]
- Oram, R.N. Register of Australian herbage plant cultivars. In Australian Herbage Plant Registration Authority, Division of Plant Industry; CSIRO: East Melbourne, Australia, 1990; Volume 304. [Google Scholar]
- Parsons, J.J. Spread of African pasture grasses to the American tropics. J. Range Manag. 1972, 25, 12–17. [Google Scholar] [CrossRef]
- Argel, P.J.; Keller-Grein, G. Regional experience with Brachiaria: Tropical America—Humid lowlands. In Brachiaria: Biology, Agronomy and Improvement; Miles, J.W., Maass, B.L., do Valle, C.B., Eds.; International Center for Tropical Agriculture: Cali, Colombia, 1996; Volume 1, pp. 205–224. [Google Scholar]
- Fisher, M.J.; Kerridge, P.C. The agronomy and physiology of Brachiaria species. In Brachiaria: Biology, Agronomy and Improvement; Miles, J.W., Maass, B.L., do Valle, C.B., Eds.; International Center for Tropical Agriculture: Cali, Colombia, 1996; Volume 1, pp. 43–52. [Google Scholar]
- Jank, L.; Barrios, S.C.; do Valle, C.B.; Simeão, R.M.; Alves, G.F. The value of improved pastures to Brazilian beef production. Crop Pasture Sci. 2014, 65, 1132–1137. [Google Scholar] [CrossRef]
- Arango, J.; Moreta, D.; Nunez, J.; Hartmann, K.; Dominguez, M.; Ishitani, M.; Miles, J.; Subbarao, G.; Peters, M.; Rao, I. Developing methods to evaluate phenotypic variability in biological nitrification inhibition (BNI) capacity of Brachiaria grasses. Trop. Grassl. 2014, 2, 6–8. [Google Scholar] [CrossRef]
- Moreta, D.; Arango, J.; Soleto, M.; Vergara, D.; Rincón, A.; Ishitani, M.; Castro, A.; Miles, J.; Peters, M.; Tohme, J.; et al. Biological nitrification inhibition (BNI) in Brachiaria pastures: A novel strategy to improve eco-efficiency of crop- livestock systems and to mitigate climate change. Trop. Grassl. 2014, 2, 88–91. [Google Scholar] [CrossRef]
- Ghimire, S.; Njarui, D.; Mutimura, M.; Cardoso, J.; Johnson, L.; Gichangi, E.; Teasdale, S.; Odokonyero, K.; Caradus, J.; Rao, I.; et al. Climate-smart Brachiaria for improving livestock production in East Africa: Emerging opportunities. In Sustainable Use of Grassland Resources for Forage Production, Biodiversity and Environmental Protection, Proceedings of 23rd International Grassland Congress, New Delhi, India, 20–24 November 2015; Vijay, D., Srivastava, M., Gupta, C., Malaviya, D., Roy, M., Mahanta, S., Singh, J., Maity, A., Ghos, P., Eds.; Range Management of Society of India: Jhansi, India, 2015; pp. 361–370. [Google Scholar]
- Subbarao, G.V.; Nakahara, K.; Hurtado, M.P.; Ono, H.; Moreta, D.E.; Salcedo, A.F.; Yoshihashi, A.T.; Ishikawa, T.; Ishitani, M.; Ohnishi-Kameyama, M.; et al. Evidence for Biological nitrification inhibition in Brachiaria pastures. Proc. Nat. Acad. Sci. USA 2009, 106, 17302–17307. [Google Scholar] [CrossRef]
- Djikeng, A.; Rao, I.; Njarui, D.; Mutimura, M.; Caradus, J.; Ghimire, S.R.; Linda, J.; Ahonsi, M.; Kelemu, S. Climate-Smart Brachiaria grasses for improving livestock production in East Africa. Trop. Grassl. 2014, 2, 38–39. [Google Scholar] [CrossRef]
- Bogdan, A.V. Tropical Pastures and Fodder Plants; Longman: London, UK, 1977; Volume 475. [Google Scholar]
- Sotomayor-Ríos, A.; Vélez-Fortuño, J.; Woodbury, R.; Schertz, K.F.; Sierra-Bracero, A. Description and cytology of a form of Signal grass (Brachiaria brizantha Stapf.) and its agronomic behavior compared to Guinea grass (Panicum maximum Jack.). J. Agric. Univ. P R. 1960, 44, 208–220. Available online: https://revistas.upr.edu/index.php/jaupr/article/view/13809 (accessed on 29 September 2019).
- Vendramini, J.; Seller, B.; Sullenberger, L.E.; Silveira, M. Mulato II (Brachiaria sp.). Available online: https://edis.ifas.ufl.edu/ag310 (accessed on 29 September 2019).
- Ndikumana, J.; de Leeuw, P.N. Regional experience with Brachiaria: Sub-Saharan Africa. In Brachiaria: Biology, Agronomy and Improvement; Miles, J.W., Maass, B.L., do Valle, C.B., Eds.; International Center for Tropical Agriculture: Cali, Colombia, 1996; Volume 1, pp. 247–257. [Google Scholar]
- Miles, J.W.; Maass, B.L.; do Valle, C.B. Brachiaria: Biology, Agronomy and Improvement; International Center for Tropical Agriculture: Cali, Colombia, 1996; Volume 288. [Google Scholar]
- Jungmann, L.; Sousa, A.C.B.; Paiva, J.; Francisco, P.M.; Vigna, B.B.Z.; do Valle, C.B.; Zucchi, M.; de Souza, A.P. Isolation and characterization of microsatellite markers for Brachiaria brizantha (Hochst. ex A. Rich.) Stap. Conserv. Genet. 2009, 10, 1873–1876. [Google Scholar] [CrossRef]
- Silva, P.; Martins, A.; Gouvea, E.; Marco, P.; Ferreira, M. Development and validation of microsatellite markers for Brachiaria ruziziensis obtained by partial genome assembly of Illumina single end reads. BMC Genomics 2013, 14, 17. [Google Scholar] [CrossRef]
- Maass, B.L.; Midega, C.A.O.; Mutimura, M.; Rahetlah, V.B.; Salgado, P.; Kabirizi, J.M.; Khan, Z.R.; Ghimire, S.R.; Rao, I. Home coming of Brachiaria: Improved hybrids prove useful for African Animal Agricultural. East Afr. Agric. For. J. 2015, 81, 71–78. [Google Scholar] [CrossRef]
- Njarui, D.M.G.; Gichangi, E.M.; Ghimire, S.R.; Muinga, R.W. Climate smart Brachiaria grasses for improving livestock production in East Africa—Kenya experience. In Proceedings of the End of the Project Workshop, Naivasha, Kenya, 14–15 September 2016; Kenya Agricultural and Livestock Research Organization: Nairobi, Kenya, 2016; Volume 271. [Google Scholar]
- USAID. Uganda Biodiversity and Tropical Forest Assessment. Final Report; 2006. Available online: https://www.vub.be/klimostoolkit/sites/default/files/documents/uganda_biodiversity_assessment_usaid.pdf (accessed on 22 July 2020).
- FAO. Crop Calendar—An Information Tool for Seed Security. 2010. Available online: http://www.fao.org/agriculture/seed/cropcalendar/welcome.do (accessed on 22 July 2020).
- da Costa, T.R.; Filho, P.S.V.; Gonçalves-Vidigal, M.C.; Galvan, M.Z.; Lacanallo, G.F.; da Silva, L.I.; Kvitschal, M.V. Genetic diversity and population structure of sweet cassava using simple sequence repeat (SSR) molecular markers. Afr. J. Biotechnol. 2013, 12, 1040–1048. [Google Scholar]
- Garcia, M.; Vigna, B.B.Z.; Sousa, A.C.B.; Jungmann, L.; Cidade, F.W.; Toledo-Silva, G.; Francisco., P.M.; Chiari, L.; Carvalho, M.A.; Karia, C.T.; et al. Molecular genetics variability, population structure and mating system in tropical forages. Trop. Grassl. Forrajes 2013, 1, 25–30. [Google Scholar] [CrossRef]
- Kawube, G.; Kabirizi, J.; Namazzi, C. Genetic characterization of Alupe Napier grass accessions based on simple sequence repeat (SSR). Am. J. Exp. Agric. 2015, 7, 205–213. [Google Scholar]
- Ondabu, N.; Maina, S.; Kimani, W.; Njarui, D.; Djikeng, A.; Ghimire, S. Molecular characterization of Kenyan Brachiaria grass ecotypes with microsatellites (SSR) markers. Agronomy 2017, 7, 8. [Google Scholar] [CrossRef]
- Kuwi, S.O.; Kyalo, M.; Mutai, C.; Mwilawa, A.; Hanson, J.; Djikeng, A.; Ghimire, S.R. Genetic diversity and population structure of Urochloa grass accessions from Tanzania using simple sequence repeat (SSR) markers. Braz. J. Bot. 2018, 41, 699–709. [Google Scholar] [CrossRef]
- Tegegn, A.; Kyalo, M.; Mutai, C.; Hanson, J.; Asefa, G.; Djikeng, A.; Ghimire, S.R. Genetic diversity and population structure of Brachiaria brizantha (A. Rich) Stapf accessions from Ethiopia. Afr. J. Range For. Sci. 2019, 36, 129–133. [Google Scholar] [CrossRef]
- Prasantha, V.P.; Chandra, S. ALS-Binary: A Program for Converting Allele Size in Microsatellite Markers to 0–1 (Binary Data). Biometrics; ICRISAT: Hyderabad, India, 1997; Available online: http://www.icrisat.org/bt-software-d-als.htm (accessed on 12 May 2015).
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure from multi-locus genotype data. Genetics 2000, 155, 945–959. [Google Scholar]
- Earl, D.A.; Von Holdt, B.M. Structure harvester: A website and program for visualizing structure output and implementing Evanno method. Conserv. Genet. Res. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Evanno, G.S.; Goudet, R. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Roger, J.S. Measures of genetic similarity and genetic distance. In Studies in Genetic VII; University of Texas: Austin, TX, USA, 1972; Publication 7213; pp. 145–153. [Google Scholar]
- Liu, K.; Muse, S.V. PowerMarker: An integrated analysis environment for genetic marker analysis. Bioinformatics 2005, 21, 2128–2129. [Google Scholar] [CrossRef] [PubMed]
- Addinsoft. XLSTAT Statistical and Data Analysis Solution; Addinsoft: New York, NY, USA, 2020; Available online: https://www.xlstat.com (accessed on 3 June 2020).
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin (version 3.0): An integrated software package for population genetics data analysis. Evol. Bioinform. 2005, 1, 47–50. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. Available online: https://bioinformatics.oxfordjournals.org/content/28/19/2537 (accessed on 28 May 2018). [CrossRef]
- Meirmans, P.G. Using the AMOVA framework to estimate a standardized genetics differentiation measure. Evolution 2007, 60, 2399–2402. [Google Scholar] [CrossRef]
- Perrier, X.; Jacquemoud-Collet, J.P. DARwin Software: Dissimilarity Analysis and Representation for Windows (Version 6.0.15). 2006. Available online: http://darwin.cirad.fr/darwin (accessed on 17 April 2020).
- Kapila, R.K.; Yadav, R.S.; Plaha, P.; Rai, K.N.; Yadav, O.P.; Hash, C.T.; Howarth, C.J. Genetic diversity among pearl millet maintainers using microsatellite markers. Plant Breed. 2008, 127, 33–37. [Google Scholar] [CrossRef]
- Singh, R.K.; Sharma, R.K.; Singh, A.K.; Singh, V.P.; Tiwari, S.P.; Mohapatra, T. Sustainability of mapped sequence tagged microsatellite site markers for establishing distinctness, uniformity and stability in aromatic rice. Euphytica 2004, 135, 135–143. [Google Scholar] [CrossRef]
- Pessoa-Filho, M.; Azevedo, A.L.S.; Sobrinho, F.S.; Gouvea, E.G.; Martins, A.M.; Ferreira, M.E. Genetic diversity and structure of Ruzigrass germplasm collected in Africa and Brazil. Crop Sci. 2015, 55, 2736–2745. [Google Scholar] [CrossRef]
- Sousa, A.C.B.; Carvalho, M.A.; Campos, T.; Sforca, D.A.; Zucchi, M.I.; Jank, L.; Souza, A.P. Molecular diversity, genetic structure and mating system of Calopogonium mucunoides Desv. Genet. Res. Crop. Evol. 2012, 59, 1449–1464. [Google Scholar] [CrossRef]
- Vigna, B.B.Z.; Jungmann, L.; Francisco, P.M.; Zucchi, M.I.; do Valle, C.B.; de Souza, A.P. Genetic diversity and population structure of Brachiaria brizantha germplasm. Trop. Plant Biol. 2011, 4, 157–169. [Google Scholar] [CrossRef]
- Trivino, N.J.; Perez, J.G.; Recio, M.E.; Ebina, M.; Yamanaka, N.; Tsuruta, S.; Ishitani, M.; Worthington, M. Genetic diversity and population structure of Brachiaria species and breeding populations. Crop Sci. 2017, 57, 2633–2644. [Google Scholar] [CrossRef]
- White, F. The vegetation of Africa. In A Descriptive Memoir to Accompany the UNESCO/AETFAT/UNSO Vegetation Map of Africa; UNESCO: Paris, France, 1983. [Google Scholar]
- DeWoody, J.; Nason, J.D.; Hipkins, V.D. Mitigating scoring errors in microsatellite data from wild population. Mol. Ecol. Notes 2006, 6, 951–957. [Google Scholar] [CrossRef]
- Palop-Esteban, M.; Segarra-Moragues, J.; Gonzalez-Candelas, F. Polyploid origin, genetic diversity and population structure in the tetraploid sea lavender Limomnium narbonense Miller (Plumbaginaceae) from eastern Spain. Genetica 2011, 139, 1309–1322. [Google Scholar] [CrossRef] [PubMed]
- Szpiech, Z.A.; Jakobsson, M.; Rosenberg, N.A. ADZE: A rarefaction approach for counting alleles private to combinations of populations. Bioinformatics 2008, 24, 2498–2504. [Google Scholar] [CrossRef]
- Salema, K.F.M.; Sallam, A. Analysis of population structure and genetic diversity of Egyptian and exotic rice (Oryza sativa L.) genotypes. C. R. Biol. 2016, 339, 1–9. [Google Scholar] [CrossRef]
- Ozias-Akins, P.; van Dijk, P.J. Mendelian genetics of apomixes in plants. Annu. Rev. Genet. 2007, 41, 509–537. [Google Scholar] [CrossRef]
- Santo, F.C.; Guyot, R.; do Valle, C.B.; Chiari, L.; Techio, V.H.; Heslop-Harrison, P.; Vanzela, A.L. Chromosome distribution and evolution of abundant retrotransposons in plants: Gypsy elements in diploid and polyploidy Brachiaria forage grasses. Chromosome Res. 2015, 23, 571–582. [Google Scholar] [CrossRef]
- Pagliarini, M.S.; do Valle, C.B.; Santos, E.M.; Mendes, D.V.; Bernardo, Z.H.; Mendes-Bonato, A.B.; Silva, N.; Calisto, V. Microsporogenesis in Brachiaria brizantha (Poaceae) as a selection tool for breeding. Genet. Mol. Res. 2012, 11, 1309–1318. [Google Scholar] [CrossRef]
- Comai, L. The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef]
- Teixiera, H.; Rodriguez-Echeverria, S.; Nabais, C. Genetic diversity and differentiation of Juniperus thurifera in Spain and Morocco as determined by SSR. PLoS ONE 2014, 9, e88996. [Google Scholar] [CrossRef] [PubMed]
| Marker | Forward Primer Sequence (5′ to 3′) | Reverse Primer Sequence (5′ to 3′) | Annealing Temp. (°C) | Expected Product Size | Repeat Motif | Summary Statistics | |
|---|---|---|---|---|---|---|---|
| No. of Allele | PIC | ||||||
| Br0012 | ACTCAAACAATCTCCAACACG | CCCACAAATGGTGAATGTAAC | 59 | 144–196 | (CA)6 | 14 | 0.91 |
| Br0028 | CATGGACAAGGAGAAGATTGA | TGGGAGTTAAACATTAGTGTTTT | 58 | 111–197 | (TA)8 | 38 | 0.96 |
| Br0029 | TTTGTGCCAAAGTCCAAATAG | TATTCCAGCTTCTTCTGCCTA | 59 | 132–178 | (CT)14 | 14 | 0.84 |
| Br0031 | CCCCCATTTAACACCATAGTT | GCTCAAAATGCAATGTACGTG | 59 | 139–179 | (AC)7 | 21 | 0.93 |
| Br0067 | TTAGATTCCTCAGGACATTGG | TCCTATATGCCGTCGTACTCA | 59 | 130–171 | (AT)11 | 25 | 0.91 |
| Br0076 | CCTAGAATGCGGAAGTAGTGA | TTACGTGTTCCTCGACTCAAC | 59 | 120–262 | (CA)7 | 14 | 0.88 |
| Br0087 | TTCCCCCACTACTCATCTCA | AACAGCACACCGTAGCAACT | 59 | 229–261 | (AT)8 | 36 | 0.88 |
| Br0092 | TTGATCAGTGGGAGGTAGGA | TGAAACTTGTCCCTTTTTCG | 58 | 200–295 | (AT)6 | 14 | 0.76 |
| Br0100 | CCATCTGCAATTATTCAGGAAA | GTTCTTGGTGCTTGACCATT | 58 | 229–286 | (AT)11 | 23 | 0.95 |
| Br0115 | AATTCATGATCGGAGCACAT | TGAACAATGGCTTTGAATGA | 59 | 231–315 | (GA)8 | 28 | 0.93 |
| Br0117 | AGCTAAGGGGCTACTGTTGG | CGCGATCTCCAAAATGTAAT | 58 | 233–345 | (TA)5 | 27 | 0.83 |
| Br0118 | AGGAGGTCCAAATCACCAAT | CGTCAGCAAATTCGTACCAC | 59 | 237–321 | (TC)11 | 21 | 0.60 |
| Br0122 | CATTGCTCCTCTCGCACTAT | CTGCAGTTAGCAGGTTGGTT | 58 | 223–279 | (CT)11 | 18 | 0.88 |
| Br0130 | TCCTTTCATGAACCCCTGTA | CATCGCACGCTTATATGACA | 58 | 199–299 | (AG)14 | 26 | 0.95 |
| Br0149 | GCCAAGACCGCTGTTAGAGAA | CTAACATGGACACCGCTCTT | 58 | 231–299 | (AT)9 | 26 | 0.91 |
| Br0152 | ATGCTGCACTTACTGGTTCA | GGCTATCAATTCGAAGACCA | 58 | 233–301 | (AT)7 | 28 | 0.93 |
| Br0156 | GCCATGATGTTTCATTGGTT | TTTTGCACCTTTCATTGCTT | 58 | 231–286 | (AT)6 | 28 | 0.95 |
| Br0203 | CGCTTGAGAAGCTAGCAAGT | TAGCCTTTTGCATGGGTTAG | 58 | 208–310 | (GA)9 | 23 | 0.88 |
| Br0212 | ACTCATTTTCACACGCACAA | CGAAGAATTGCAGCAGAAGT | 59 | 248–330 | (AAT)10 | 21 | 0.89 |
| Br0213 | TGAAGCCCTTTCTAAATGATG | GAACTAGGAAGCCATGGACA | 58 | 212–337 | (AT)9 | 25 | 0.83 |
| Br0214 | TCTGGTGTCTCTTTGCTCCT | TCCATGGTACCTGAATGACA | 59 | 241–358 | (CA)5 | 24 | 0.96 |
| Br0235 | CACACTCACACACGGAGAGA | CATCCAGAGCCTGATGAAGT | 59 | 239–330 | (TC)9 | 33 | 0.87 |
| Br3002 | GCTGGAATCAGAATCGATGA | GAACTGCAGTGGCTGATCTT | 59 | 143–187 | (AAT)7 | 17 | 0.92 |
| Br3009 | AGACTCTGTGCGGGAAATTA | ACTTCGCTTGTCCTACTTGG | 58 | 116–199 | (AT)8 | 40 | 0.94 |
| Mean | 24.3 | 0.89 | |||||
| Source | DF | SS | MS | Est. var. | (%) |
|---|---|---|---|---|---|
| Among populations | 4 | 942826.981 | 235706.745 | 2905.353 | 2% |
| Within populations | 94 | 16796439.605 | 178685.528 | 178685.528 | 98% |
| Total | 98 | 17739266.586 | |||
| Genetic differentiation among ecotype populations (PhiPT) = 0.016; p = 0.142 | |||||
| Population | Number of Individual | Estimated Membership Coefficient | ||
|---|---|---|---|---|
| CI | CII | CIII | ||
| Central (CTR) | 25 | 0.384 (10) | 0.294 (7) | 0.321 (8) |
| Northern (NTN) | 23 | 0.354 (8) | 0.451 (10) | 0.194 (4) |
| South dryland (SDL) | 19 | 0.227 (4) | 0.413 (8) | 0.361 (7) |
| Southwestern (SWT) | 15 | 0.284 (4) | 0.437 (7) | 0.280 (4) |
| Western (WST) | 17 | 0.186 (3) | 0.483 (8) | 0.331 (6) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namazzi, C.; Sserumaga, J.P.; Mugerwa, S.; Kyalo, M.; Mutai, C.; Mwesigwa, R.; Djikeng, A.; Ghimire, S. Genetic Diversity and Population Structure of Brachiaria (syn. Urochloa) Ecotypes from Uganda. Agronomy 2020, 10, 1193. https://doi.org/10.3390/agronomy10081193
Namazzi C, Sserumaga JP, Mugerwa S, Kyalo M, Mutai C, Mwesigwa R, Djikeng A, Ghimire S. Genetic Diversity and Population Structure of Brachiaria (syn. Urochloa) Ecotypes from Uganda. Agronomy. 2020; 10(8):1193. https://doi.org/10.3390/agronomy10081193
Chicago/Turabian StyleNamazzi, Clementine, Julius Pyton Sserumaga, Swidiq Mugerwa, Martina Kyalo, Collins Mutai, Robert Mwesigwa, Appolinaire Djikeng, and Sita Ghimire. 2020. "Genetic Diversity and Population Structure of Brachiaria (syn. Urochloa) Ecotypes from Uganda" Agronomy 10, no. 8: 1193. https://doi.org/10.3390/agronomy10081193
APA StyleNamazzi, C., Sserumaga, J. P., Mugerwa, S., Kyalo, M., Mutai, C., Mwesigwa, R., Djikeng, A., & Ghimire, S. (2020). Genetic Diversity and Population Structure of Brachiaria (syn. Urochloa) Ecotypes from Uganda. Agronomy, 10(8), 1193. https://doi.org/10.3390/agronomy10081193







