Chemical Composition of Cynara Cardunculus L. var. altilis Heads: The Impact of Harvesting Time
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Plant Material
2.3. Chemical Composition Analysis
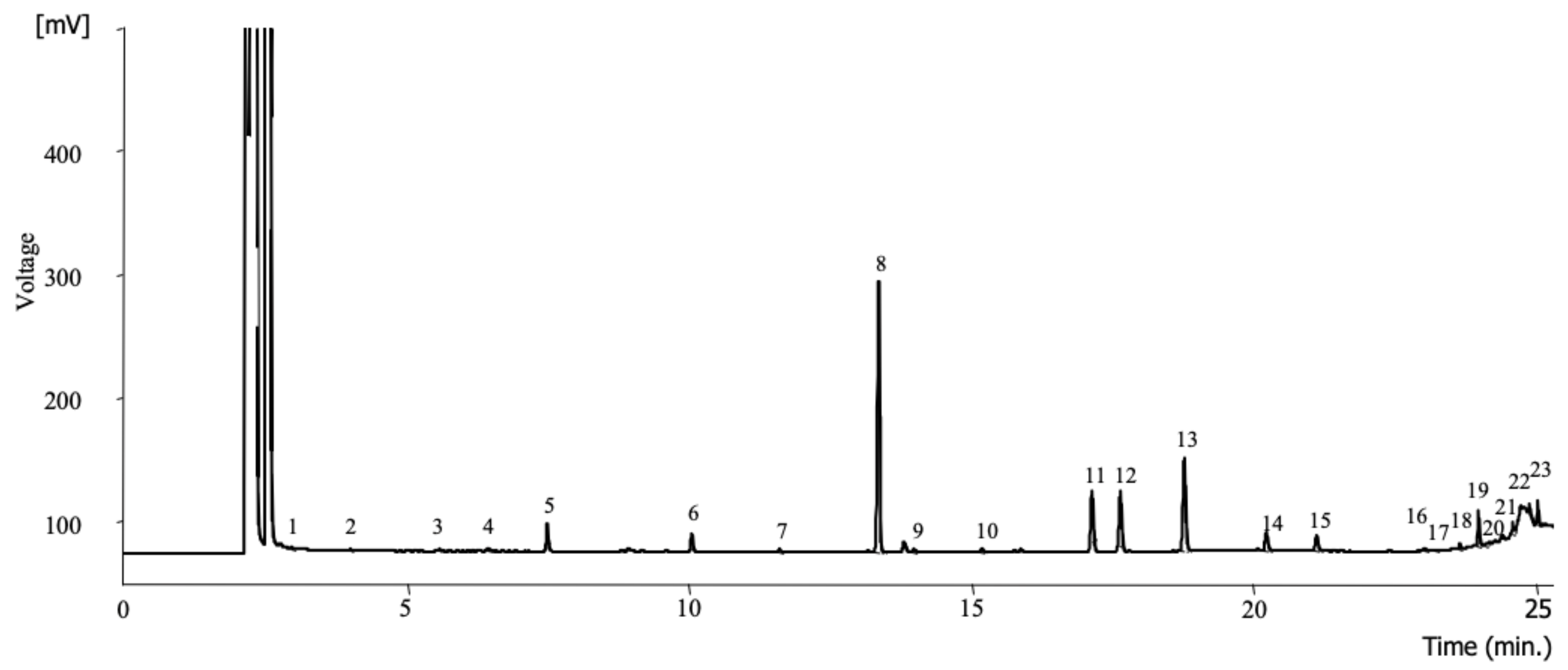
2.3.1. Fatty Acids
2.3.2. Tocopherols
2.3.3. Organic Acids
2.3.4. Free Sugars
2.4. Statistical Analysis
3. Results
3.1. Lipid Fraction and Fatty Acids Composition
3.2. Organic Acids and Free Sugars
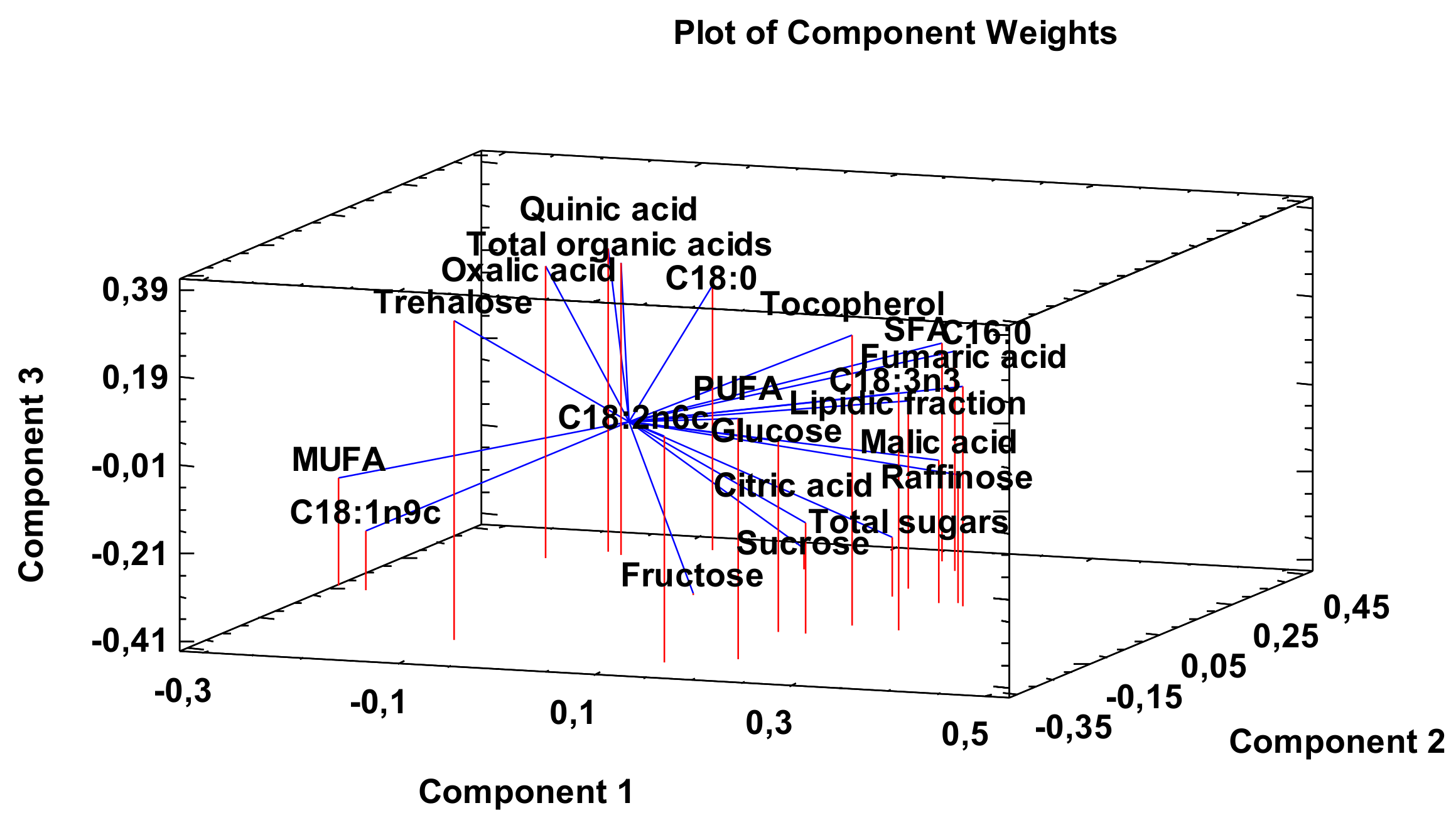
3.3. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gominho, J.; Curt, M.D.; Lourenço, A.; Fernández, J.; Pereira, H. Cynara cardunculus L. as a biomass and multi-purpose crop: A review of 30 years of research. Biomass Bioenergy 2018, 109, 257–275. [Google Scholar] [CrossRef]
- Zayed, A.; Serag, A.; Farag, M.A. Cynara cardunculus L.: Outgoing and potential trends of phytochemical, industrial, nutritive and medicinal merits. J. Funct. Foods 2020, 69, 103937. [Google Scholar] [CrossRef]
- De Falco, B.; Incerti, G.; Amato, M.; Lanzotti, V. Artichoke: Botanical, agronomical, phytochemical, and pharmacological overview. Phytochem. Rev. 2015, 14, 993–1018. [Google Scholar] [CrossRef]
- Gostin, A.I.; Waisundara, V.Y. Edible flowers as functional food: A review on artichoke (Cynara cardunculus L.). Trends Food Sci. Technol. 2019, 86, 381–391. [Google Scholar] [CrossRef]
- Brás, T.; Paulino, A.F.C.; Neves, L.A.; Crespo, J.G.; Duarte, M.F. Ultrasound assisted extraction of cynaropicrin from Cynara cardunculus leaves: Optimization using the response surface methodology and the effect of pulse mode. Ind. Crops Prod. 2020, 150, 112395. [Google Scholar] [CrossRef]
- Amira, A.B.; Besbes, S.; Attia, H.; Blecker, C. Milk-clotting properties of plant rennets and their enzymatic rheological, and sensory role in cheese making: A review. Int. J. Food Prop. 2017, 28, 576–593. [Google Scholar]
- Gomes, S.; Belo, A.T.; Alvarenga, N.; Dias, J.; Lage, P.; Pinheiro, C.; Pinto-Cruz, C.; Brás, T.; Duarte, M.F.; Martins, A.P.L. Characterization of Cynara cardunculus L. flower from Alentejo as a coagulant agent for cheesemaking. Int. Dairy J. 2019, 91, 178–184. [Google Scholar] [CrossRef]
- Jiménez-Moreno, N.; Cimminelli, M.J.; Volpe, F.; Ansó, R.; Esparza, I.; Mármol, I.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. Phenolic composition of Artichoke waste and its antioxidant capacity on differentiated Caco-2 cells. Nutrients 2019, 11, 1723. [Google Scholar] [CrossRef]
- Pari, L.; Alfano, V.; Mattei, P.; Santangelo, E. Pappi of cardoon (Cynara cardunculus L.): The use of wetting during the harvesting aimed at recovering for the biorefinery. Ind. Crops Prod. 2017, 108, 722–728. [Google Scholar] [CrossRef]
- Francaviglia, R.; Bruno, A.; Falcucci, M.; Farina, R.; Renzi, G.; Russo, D.E.; Sepe, L.; Neri, U. Yields and quality of Cynara cardunculus L. wild and cultivated cardoon genotypes. A case study from a marginal land in Central Italy. Eur. J. Agron. 2016, 72, 10–19. [Google Scholar] [CrossRef]
- Lourenço, A.; Neiva, D.M.; Gominho, J.; Curt, M.D.; Fernández, J.; Marques, A.V.; Pereira, H. Biomass production of four Cynara cardunculus clones and lignin composition analysis. Biomass Bioenergy 2015, 76, 86–95. [Google Scholar] [CrossRef]
- Conceição, C.; Martins, P.; Alvarenga, N.; Dias, J.; Lamy, E.; Garrido, L.; Gomes, S.; Freiras, S.; Belo, A.; Brás, T.; et al. Cynara cardunculus: Use in cheesemaking and pharmaceutical applications. In Technological Approaches for Novel Applications in Dairy Processing; Intech Open: London, UK, 2016; Volume 1, pp. 73–107. [Google Scholar]
- D’Antuono, I.; Carola, A.; Sena, L.M.; Linsalata, V.; Cardinali, A.; Logrieco, A.F.; Colucci, M.G.; Apone, F. Artichoke polyphenols produce skin anti-age effects by improving endothelial cell integrity and functionality. Molecules 2018, 23, 2729. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.B.; Affes, H.; Athmouni, K.; Ksouda, K.; Dhouibi, R.; Sahnoun, Z.; Hammami, S.; Zeghal, K.M. Chemicals compositions, antioxidant and anti-inflammatory activity of Cynara scolymus leaves extracts, and analysis of major bioactive polyphenols by HPLC. Evid. Based Complement. Altern. Med. 2017, 2017, 4951937. [Google Scholar]
- Marques, P.; Marto, J.; Gonçalves, L.M.; Pacheco, R.; Fitas, M.; Pinto, P.; Serralheiro, M.L.M.; Ribeiro, H. Cynara scolymus L.: A promising Mediterranean extract for topical anti-aging prevention. Ind. Crops Prod. 2017, 109, 699–706. [Google Scholar] [CrossRef]
- Almeida, C.M.; Simões, I. Cardoon-based rennets for cheese production. Appl. Microbiol. Biotechnol. 2018, 102, 4675–4686. [Google Scholar] [CrossRef] [PubMed]
- Faria-Silva, C.; Ascenso, A.; Costa, A.M.; Marto, J.; Carvalheiro, M.; Ribeiro, H.M.; Simões, S. Feeding the skin: A new trend in food and cosmetics convergence. Trends Food Sci. Technol. 2020, 95, 21–32. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Barreira, J.C.M.; Alves, M.J.; Barracosa, P.; Ferreira, I.C.F.R. Phenolic profile and bioactivity of cardoon (Cynara cardunculus L.) inflorescence parts: Selecting the best genotype for food applications. Food Chem. 2018, 268, 196–202. [Google Scholar] [CrossRef]
- Mandim, F.; Petropoulos, S.A.; Giannoulis, K.D.; Dias, M.I.; Fernandes, Â.; Pinela, J.; Kostic, M.; Soković, M.; Barros, L.; Santos-Buelga, C.; et al. Seasonal variation of bioactive properties and phenolic composition of Cynara cardunculus var. altilis. Food Res. Int. 2020, 134, 109281. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Lo Monaco, A.; Mauromicale, G. Choice of time of harvest influences the polyphenol profile of globe artichoke. J. Funct. Foods 2013, 5, 1822–1828. [Google Scholar] [CrossRef]
- Scavo, A.; Pandino, G.; Restuccia, C.; Parafati, L.; Cirvilleri, G.; Mauromicale, G. Antimicrobial activity of cultivated cardoon (Cynara cardunculus L. var. altilis DC.) leaf extracts against bacterial species of agricultural and food interest. Ind. Crops Prod. 2019, 129, 206–211. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant. Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Mandim, F.; Dias, M.I.; Pinela, J.; Barracosa, P.; Ivanov, M.; Ferreira, I.C.F.R. Chemical composition and in vitro biological activities of cardoon (Cynara cardunculus L. var. altilis DC.) seeds as influenced by viability. Food Chem. 2020, 323, 126838. [Google Scholar] [CrossRef] [PubMed]
- Ben Amira, A.; Blecker, C.; Richel, A.; Arias, A.A.; Fickers, P.; Francis, F.; Besbes, S.; Attia, H. Influence of the ripening stage and the lyophilization of wild cardoon flowers on their chemical composition, enzymatic activities of extracts and technological properties of cheese curds. Food Chem. 2018, 245, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Claus, T.; Maruyama, S.A.; Palombini, S.V.; Montanher, P.F.; Bonafé, E.G.; de Oliveira Santos Junior, O.; Matsushita, M.; Visentainer, J.V. Chemical characterization and use of artichoke parts for protection from oxidative stress in canola oil. LWT Food Sci. Technol. 2015, 61, 346–351. [Google Scholar] [CrossRef]
- Archontoulis, S.V.; Struik, P.C.; Vos, J.; Danalatos, N.G. Phenological growth stages of Cynara cardunculus: Codification and description according to the BBCH scale. Ann. Appl. B 2013, 156, 253–270. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Pereira, C.; Tzortzakis, N.; Barros, L.; Ferreira, I.C.F.R. Nutritional value and bioactive compounds characterization of plant parts from Cynara cardunculus L. (Asteraceae) cultivated in central Greece. Front. Plant. Sci. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, E.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Bioactivity and chemical characterization in hydrophilic and lipophilic compounds of Chenopodium ambrosioides L. J. Funct. Foods 2013, 5, 1732–1740. [Google Scholar] [CrossRef]
- Mandim, F.; Barros, L.; Calhelha, R.C.; Abreu, R.M.V.; Pinela, J.; Alves, M.J.; Heleno, S.; Santos, P.F.; Ferreira, I.C.F.R. Calluna vulgaris (L.) Hull: Chemical characterization, evaluation of its bioactive properties and effect on the vaginal microbiota. Food Funct. 2019, 10, 78–89. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Morales, P.; Sánchez-Mata, M.C.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Nutritional parameters of infusions and decoctions obtained from Fragaria vesca L. roots and vegetative parts. LWT Food Sci. Technol. 2015, 62, 32–38. [Google Scholar] [CrossRef]
- Pereira, E.; Barros, L.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Bioactivity and phytochemical characterization of Arenaria Montana L. Food Funct. 2014, 5, 1848–1855. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Petropoulos, S.A.; Pannico, A.; Kyriacou, M.C.; Giordano, M.; Colla, G.; Troise, A.D.; Vitaglione, P.; De Pascale, S.; Rouphael, Y. The bioactive profile of lettuce produced in a closed soilless system as configured by combinatorial effects of genotype and macrocation supply composition. Food Chem. 2020, 309, 125713. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Fernandes, Â.; Calhelha, R.C.; Danalatos, N.; Barros, L.; Ferreira, I.C.F.R. How extraction method affects yield, fatty acids composition and bioactive properties of cardoon seed oil? Ind. Crops Prod. 2018, 124, 459–465. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Pereira, C.; Ntatsi, G.; Danalatos, N.; Barros, L.; Ferreira, I.C.F.R. Nutritional value and chemical composition of Greek artichoke genotypes. Food Chem. 2018, 267, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Dosi, R.; Daniele, A.; Guida, V.; Ferrara, L.; Severino, V.; Di Maro, A. Nutritional and metabolic profiling of the globe artichoke (Cynara scolymus L. “Capuanella” heads) in province of caserta, Italy. Aust. J. Crop. Sci. 2013, 7, 1927–1934. [Google Scholar]
- Petropoulos, S.; Fernandes, Â.; Pereira, C.; Tzortzakis, N.; Vaz, J.; Soković, M.; Barros, L.; Ferreira, I.C.F.R. Bioactivities, chemical composition and nutritional value of Cynara cardunculus L. seeds. Food Chem. 2019, 289, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, A.M.R.C.; Dias, A.M.A.; Seabra, I.J.; Portugal, A.A.T.G.; De Sousa, H.C.; Braga, M.E.M. Biodiesel obtained from supercritical carbon dioxide oil of Cynara cardunculus L. J. Supercrit. Fluids 2012, 68, 52–63. [Google Scholar] [CrossRef]
- Raccuia, S.A.; Piscioneri, I.; Sharma, N.; Melilli, M.G. Genetic variability in Cynara cardunculus L. domestic and wild types for grain oil production and fatty acids composition. Biomass Bioenergy 2011, 35, 3167–3173. [Google Scholar] [CrossRef]
- Ramos, P.A.B.; Guerra, A.R.; Guerreiro, O.; Freire, C.S.R.; Silva, A.M.S.; Duarte, M.F.; Silvestre, A.J.D. Lipophilic extracts of Cynara cardunculus L. var. altilis (DC): A source of valuable bioactive terpenic compounds. J. Agric. Food Chem. 2013, 61, 8420–8429. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Perreira, C.; Barros, L.; Ferreira, I.C.F.R. Leaf parts from Greek artichoke genotypes as a good source of bioactive compounds and antioxidants. Food Funct. 2017, 8, 2022–2029. [Google Scholar] [CrossRef]
- Liu, L.; Hu, Q.; Wu, H.; Xue, Y.; Cai, L.; Fang, M.; Liu, Z.; Yao, P.; Wu, Y.; Gong, Z. Protective role of n6/n3 PUFA supplementation with varying DHA/EPA ratios against atherosclerosis in mice. J. Nutr. Biochem. 2016, 32, 171–180. [Google Scholar] [CrossRef]
- Ierna, A.; Mauromicale, G. Cynara cardunculus L. genotypes as a crop for energy purposes in a Mediterranean environment. Biomass Bioenergy 2010, 34, 754–760. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G.; Williamson, G. Profile of polyphenols and phenolic acids in bracts and receptacles of globe artichoke (Cynara cardunculus var. scolymus) germplasm. J. Food Compos. Anal. 2011, 24, 148–153. [Google Scholar] [CrossRef]
- Kukić, J.; Popovic, V.; Petrović, S.; Mucaji, P.; Ćirić, A.; Stojković, D.; Soković, M. Antioxidant and antimicrobial activity of Cynara cardunculus extracts. Food Chem. 2008, 107, 861–868. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Dias, M.I.; Vasilakoglou, I.B.; Petrotos, K.; Barros, L.; Ferreira, I.C.F.R. Nutritional value, chemical composition and cytotoxic properties of common purslane (Portulaca oleracea L.) in relation to harvesting stage and plant part. Antioxidants 2019, 8, 293. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.P.; Pereira, E.; Pires, T.C.S.P.; Alves, M.J.; Pereira, O.R.; Barros, L.; Ferreira, I.C.F.R. Rubus ulmifolius Schott fruits: A detailed study of its nutritional, chemical and bioactive properties. Food Res. Int. 2019, 119, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Dias, S.; Gominho, J.; Baptista, I.; Pereira, H. Pattern recognition of cardoon oil from different large-scale field trials. Ind. Crops Prod. 2018, 118, 236–245. [Google Scholar] [CrossRef]
- Raccuia, S.A.; Melilli, M.G. Biomass and grain oil yields in Cynara cardunculus L. genotypes grown in a Mediterranean environment. Field Crops Res. 2007, 101, 187–197. [Google Scholar] [CrossRef]
- Maccarone, E.; Fallico, B.; Fanella, F.; Mauromicale, G.; Raccuia, S.A. Possible alternative utilization of Cynara spp. II. Chemical characterization of their grain oil. Ind. Crops Prod. 1999, 10, 229–237. [Google Scholar] [CrossRef]
- Chihoub, W.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Harzallah-Skhiri, F.; Ferreira, I.C.F.R. Valorisation of the green waste parts from turnip, radish and wild cardoon: Nutritional value, phenolic profile and bioactivity evaluation. Food Res. Int. 2019, 126, 108651. [Google Scholar] [CrossRef]
- Petropoulos, S.A.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Edible halophytes of the Mediterranean basin: Potential candidates for novel food products. Trends Food Sci. Technol. 2018, 74, 69–84. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Dias, M.I.; Pereira, C.; Calhelha, R.C.; Chrysargyris, A.; Tzortzakis, N.; Ivanov, M.; Sokovic, M.D.; Barros, L.; et al. Chemical composition and plant growth of Centaurea raphanina subsp. mixta plants cultivated under saline conditions. Molecules 2020, 25, 2204. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Barros, L.; Ferreira, I.C.F.R. Analytical tools used to distinguish chemical profiles of plants widely consumed as infusions and dietary supplements: Artichoke, milk thistle, and borututu. Food Anal. Methods 2014, 7, 1604–1611. [Google Scholar] [CrossRef]
- Šušaníková, I.; Balleková, J.; Štefek, M.; Hošek, J.; Mučaji, P. Artichoke leaf extract, as AKR1B1 inhibitor, decreases sorbitol level in the rat eye lenses under high glucose conditions ex vivo. Phyther. Res. 2018, 32, 2389–2395. [Google Scholar] [CrossRef]
- Miláčková, I.; Kapustová, K.; Mučaji, P.; Hošek, J. Artichoke Leaf Extract Inhibits AKR1B1 and Reduces NF-κB Activity in Human Leukemic Cells. Phyther. Res. 2017, 31, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hernández, O.; Ruiz-Aceituno, L.; Sanz, M.L.; Martínez-Castro, I. Determination of free inositols and other low molecular weight carbohydrates in vegetables. J. Agric. Food Chem. 2011, 59, 2451–2455. [Google Scholar] [CrossRef] [PubMed]
- Nicoletto, C.; Santagata, S.; Tosini, F.; Sambo, P. Qualitative and healthy traits of different Italian typical artichoke genotypes. CyTA J. Food 2013, 11, 108–113. [Google Scholar] [CrossRef]
- Lombardo, S.; Restuccia, C.; Muratore, G.; Barbagallo, R.N.; Licciardello, F.; Pandino, G.; Scifò, O.; Mazzaglia, A.; Ragonese, F.; Mauromicale, G. Effect of nitrogen fertilization on the overall quality of minimally processed globe artichoke heads. J. Sci. Food Agric. 2017, 97, 650–658. [Google Scholar] [CrossRef]
- Di Salvo, R.; Fadda, C.; Sanguinetti, A.M.; Naes, T.; Del Caro, A. Effect of harvest time and geographical area on sensory and instrumental texture profile of a PDO artichoke. Int. J. Food Sci. Technol. 2014, 49, 1231–1237. [Google Scholar] [CrossRef]
- Leroy, G.; Grongnet, J.F.; Mabeau, S.; le Corre, D.; Baty-Julien, C. Changes in inulin and soluble sugar concentration in artichokes (Cynara scolymus L.) during storage. J. Sci. Food Agric. 2010, 90, 1203–1209. [Google Scholar] [CrossRef]
- Barracosa, P.; Barracosa, M.; Pires, E. Cardoon as a Sustainable Crop for Biomass and Bioactive Compounds Production. Chem. Biodivers. 2019, 16, e1900498. [Google Scholar] [CrossRef]
- Vergauwen, R.; Van Laere, A.; Van Den Ende, W. Properties of fructan:fructan 1-fructosyltransferases from chicory and globe thistle, two asteracean plants storing greatly different types of inulin. Plant. Physiol. 2003, 133, 391–401. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Introducing inulin-type fructans. Br. J. Nutr. 2005, 93, S13–S25. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Fernandes, Â.; Dias, M.I.; Pereira, C.; Calhelha, R.; Gioia, F.D.; Tzortzakis, N.; Ivanov, M.; Sokovic, M.; Barros, L.; et al. Wild and cultivated Centaurea raphanina subsp. mixta: A valuable source of bioactive compounds. Antioxidants 2020, 9, 314. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, D.; Leitch, M.; Fatehi, P. Lignin-carbohydrate complexes: Properties, applications, analyses, and methods of extraction: A review. Biotechnol. Biofuels 2018, 11, 1–28. [Google Scholar]



| Sample | Total Lipidic Fraction (g/100 g dw) | α-Tocopherol (µg/100 g dw) |
|---|---|---|
| Car A | 17.5 ± 0.1 a | 264 ± 1 b |
| Car B | 5.1 ± 0.1 b | 619 ± 4 a |
| Car C | 3.5 ± 0.2 d | 25 ± 2 f |
| Car D | 4.31 ± 0.04 c | 107 ± 1 e |
| Car E | 1.9 ± 0.2 e | 162 ± 1 c |
| Car F | 1.6 ± 0.1 f | 117 ± 5 d |
| Car A | Car B | Car C | Car D | Car E | Car F | |
|---|---|---|---|---|---|---|
| Fatty Acids (Relative Percentage, %) | ||||||
| C6:0 | 0.49 ± 0.01 c | 0.082 ± 0.006 f | 0.123 ± 0.001 d | 0.094 ± 0.002 e | 3.71 ± 0.01 a | 0.84 ± 0.02 b |
| C8:0 | 0.250 ± 0.002 c | 0.19 ± 0.01 d | 0.057 ± 0.003 e | 0.059 ± 0.006 e | 1.314 ± 0.004 a | 0.29 ± 0.01 b |
| C10:0 | 0.205 ± 0.008 cd | 0.198 ± 0.001 d | 0.254 ±0.006 b | 0.21 ± 0.02 c | 0.473 ± 0.006 a | 0.186 ± 0.002 e |
| C11:0 | 0.72 ± 0.02 a | 0.335 ± 0.001 c | 0.237 ± 0.001 d | 0.16 ± 0.02 e | 0.579 ± 0.005 b | 0.17 ± 0.01 e |
| C12:0 | 1.8 ± 0.1 b | 2.57 ± 0.04 a | 0.406 ± 0.002 e | 0.82 ± 0.07 d | 1.53 ± 0.07 c | 0.326 ± 0.004 f |
| C13:0 | n.d. | 0.028 ± 0.001 d | 0.0375 ± 0.0007 b | 0.084 ± 0.004 a | 0.030 ± 0.001 c | 0.027 ± 0.003 d |
| C14:0 | 1.90 ± 0.02 b | 0.58 ± 0.02 f | 1.27 ± 0.02 d | 1.450 ± 0.002 c | 2.69 ± 0.01 a | 1.0 ± 0.1 e |
| C14:1 | n.d. | 0.53 ± 0.01 b | n.d. | n.d. | 0.54 ± 0.01 a | 0.23 ± 0.01 c |
| C15:0 | 0.48 ± 0.01 a | n.d. | 0.193 ± 0.001 d | 0.176 ± 0.009 e | 0.427 ± 0.006 b | 0.28 ± 0.02 c |
| C15:1 | n.d. | n.d. | n.d. | n.d. | 1.36 ± 0.02* | 0.122 ± 0.003 * |
| C16:0 | 43.8 ± 0.1 a | 30.4 ± 0.8 b | 22.577 ± 0.003 d | 14.62 ± 0.03 f | 25.60 ± 0.08 c | 17.8 ± 0.2 e |
| C16:1 | 0.43 ± 0.01 e | 0.317 ± 0.003 f | 0.827 ± 0.002 d | 12.62 ± 0.03 b | 12.76 ± 0.03 a | 6.69 ± 0.04 c |
| C17:0 | 0.779 ± 0.001 a | 0.666 ± 0.001 b | 0.313 ± 0.003 e | 0.239 ± 0.001 f | 0.462 ± 0.001 d | 0.579 ± 0.004 c |
| C18:0 | 6.0 ± 0.1 a | 2.96 ± 0.05 e | 3.236 ± 0.008 d | 2.687 ± 0.004 f | 5.68 ± 0.01 b | 4.599 ± 0.001 c |
| C18:1n9 | 7.7 ± 0.1 e | 4.48 ± 0.04 f | 46.6 ± 0.1 a | 32.8 ± 0.1 c | 32.47 ± 0.08 d | 33.7 ± 0.8 b |
| C18:2n6c | 20.1 ± 0.1 d | 30.6 ± 0.4 a | 6.23 ± 0.03 e | 25.82 ± 0.08 c | 0.748 ± 0.002 f | 27.2 ± 0.4 b |
| C18:3n6 | n.d. | 0.176 ± 0.006 a | 0.049 ± 0.004 d | n.d. | 0.067 ± 0.001 c | 0.145 ± 0.005 b |
| C18:3n3 | 5.55 ± 0.05 b | 7.5 ± 0.1 a | 1.02 ± 0.01 e | 2.705 ± 0.008 c | 0.3675 ± 0.0007 f | 1.38 ± 0.06 d |
| C20:0 | 2.18 ± 0.02 b | 3.225 ± 0.005 a | 0.655 ± 0.007 e | 0.377 ± 0.001 f | 0.882 ± 0.009 c | 0.672 ± 0.005 d |
| C20:1 | n.d. | 0.159 ± 0.002 c | 0.196 ± 0.004 b | 0.114 ± 0.006 e | 4.52 ± 0.01 a | 0.138 ± 0.001 d |
| C20:2 | n.d. | 0.223 ± 0.001 b | 0.107 ± 0.001 d | 0.182 ± 0.005 c | 0.0845 ± 0.0007 e | 0.31 ± 0.02 a |
| C21:0 | 0.276 ± 0.002 b | 0.324 ± 0.004 a | 0.092 ± 0.004 e | 0.070 ± 0.001 f | 0.2695 ± 0.0007 c | 0.169 ± 0.005 d |
| C20:3n6 | 0.23 ± 0.02 b | 8.9 ± 0.3 a | 0.103 ± 0.009 c | 0.101 ± 0.006 c | n.d. | n.d. |
| C20:3n3 | 1.14 ± 0.04 b | 0.142 ± 0.001 d | 0.12 ± 0.01 d | 1.38 ± 0.08 a | n.d. | 0.22 ± 0.02 c |
| C22:0 | n.d. | 0.81 ± 0.01 d | 2.6365 ± 0.0007 a | 1.56 ± 0.08 c | 1.645 ± 0.004 b | n.d. |
| C22:1 | 2.249 ± 0.008 b | 0.12 ± 0.01 f | 4.9505 ± 0.0007 a | 1.22 ± 0.02 c | 0.44 ± 0.01 e | 0.82 ± 0.06 d |
| C20:5n3 | 0.38 ± 0.04 c | n.d. | 0.036 ± 0.001 e | 0.32 ± 0.03 d | 0.6285 ± 0.002 a | 0.51 ± 0.01 b |
| C22:2 | 0.30 ± 0.01* | 0.184 ± 0.001 * | n.d. | n.d. | n.d. | n.d. |
| C23:0 | 1.61 ± 0.09 a | 1.47 ± 0.09 b | 0.26 ± 0.02 e | 0.2615 ± 0.0007 e | 0.308 ± 0.001 d | 0.52 ± 0.02 c |
| C24:0 | 1.4 ± 0.1 c | 2.88 ± 0.05 b | 7.411 ± 0.001 a | n.d. | 0.413 ± 0.001 e | 1.1 ± 0.1 d |
| SFA | 61.9 ± 0.4 a | 46.7 ± 0.7 b | 39.75 ± 0.06 d | 22.9 ± 0.2 e | 46.0 ± 0.1 c | 28.5 ± 0.5 f |
| MUFA | 10.4 ± 0.1 e | 5.61 ± 0.04 f | 52.6 ± 0.1 a | 46.73 ± 0.07 c | 52.1 ± 0.1 b | 41.7 ± 0.9 d |
| PUFA | 27.7 ± 0.3 d | 47.7 ± 0.8 a | 7.66 ± 0.05 e | 30.4 ± 0.1 b | 1.895 ± 0.006 f | 29.8 ± 0.4 c |
| PUFA/SFA | 0.45 ± 0.01 d | 1.02 ± 0.03 c | 0.193 ± 0.001 e | 1.33 ± 0.01 a | 0.0412 ± 0.0002 f | 1.043 ± 0.002 b |
| n-6/n-3 | 2.76 ± 0.04 d | 1.88 ± 0.02 e | 5.02 ± 0.04 c | 5.8 ± 0.1 b | 0.902 ± 0.002 f | 13.1 ± 0.3 a |
| Organic Acids (g/100 g dw) | ||||||
|---|---|---|---|---|---|---|
| Car A | Car B | Car C | Car D | Car E | Car F | |
| Oxalic acid | 0.324 ± 0.002 f | 0.98 ± 0.01 b | 0.3994 ± 0.0001 e | 0.650 ± 0.001 c | 12.1± 0.1 a | 0.44 ± 0.01 d |
| Quinic acid | 0.87 ± 0.04 b | 0.46 ± 0.01 c | 0.17 ± 0.01 e | 0.017 ± 0.001 f | 3.3 ± 0.1 a | 0.45 ± 0.02 d |
| Malic acid | 1.45 ± 0.06 c | 2.31 ± 0.02 a | 1.495 ± 0.001 b | 0.0149 ± 0.0005 e | 0.36 ± 0.02 d | n.d. |
| Citric acid | 0.70 ± 0.04 b | 0.86 ± 0.05 a | 0.66 ± 0.02 c | 0.84 ± 0.01 a | tr | n.d. |
| Fumaric acid | 0.046 ± 0.001 b | 0.0542 ± 0.0003 a | 0.0110 ± 0.0003 c | tr | 0.0045 ± 0.0001 d | n.d. |
| Total | 3.39 ± 0.06 c | 4.67 ± 0.06 b | 2.74 ± 0.03 d | 1.52 ± 0.01 e | 15.7 ± 0.2 a | 0.89 ± 0.01 f |
| Free Sugars (g/100 g dw) | ||||||
|---|---|---|---|---|---|---|
| Car A | Car B | Car C | Car D | Car E | Car F | |
| Fructose | 0.13 ± 0.03 d | 0.51 ± 0.04 b | 1.64 ± 0.06 a | 0.013 ± 0.004 e | 0.14 ± 0.02 d | 0.184 ± 0.001 c |
| Glucose | n.d. | 2.02 ± 008 a | 0.68 ± 0.03 b | 0.06 ± 0.01 e | 0.27 ± 0.01 d | 0.26 ± 0.02 c |
| Sucrose | 2.39 ± 0.06 b | n.d. | 3.0 ± 0.1 a | n.d. | 0.28 ± 0.02 c | 0.11 ± 0.01 d |
| Trehalose | 0.23 ± 0.04 d | 0.98 ± 0.02 a | 0.26 ± 0.02 d | 0.96 ± 0.09 a | 0.34 ± 0.02 b | 0.797 ± 0.005 c |
| Raffinose | 2.24 ± 0.07 b | 2.62 ± 0.06 a | 1.8 ± 0.1 c | n.d. | n.d. | n.d. |
| Total | 5.0 ± 0.2 c | 6.12 ± 0.08 b | 7.4 ± 0.1 a | 1.03 ± 0.09 e | 1.03 ± 0.02 d | 1.35 ± 0.03 d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandim, F.; Petropoulos, S.A.; Fernandes, Â.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Barros, L. Chemical Composition of Cynara Cardunculus L. var. altilis Heads: The Impact of Harvesting Time. Agronomy 2020, 10, 1088. https://doi.org/10.3390/agronomy10081088
Mandim F, Petropoulos SA, Fernandes Â, Santos-Buelga C, Ferreira ICFR, Barros L. Chemical Composition of Cynara Cardunculus L. var. altilis Heads: The Impact of Harvesting Time. Agronomy. 2020; 10(8):1088. https://doi.org/10.3390/agronomy10081088
Chicago/Turabian StyleMandim, Filipa, Spyridon A. Petropoulos, Ângela Fernandes, Celestino Santos-Buelga, Isabel C. F. R. Ferreira, and Lillian Barros. 2020. "Chemical Composition of Cynara Cardunculus L. var. altilis Heads: The Impact of Harvesting Time" Agronomy 10, no. 8: 1088. https://doi.org/10.3390/agronomy10081088
APA StyleMandim, F., Petropoulos, S. A., Fernandes, Â., Santos-Buelga, C., Ferreira, I. C. F. R., & Barros, L. (2020). Chemical Composition of Cynara Cardunculus L. var. altilis Heads: The Impact of Harvesting Time. Agronomy, 10(8), 1088. https://doi.org/10.3390/agronomy10081088










