Seedling Growth and Phosphorus Uptake in Response to Different Phosphorus Sources
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Plant Material and Growth Conditions
2.2. Sampling and Measurements
2.3. P Analysis
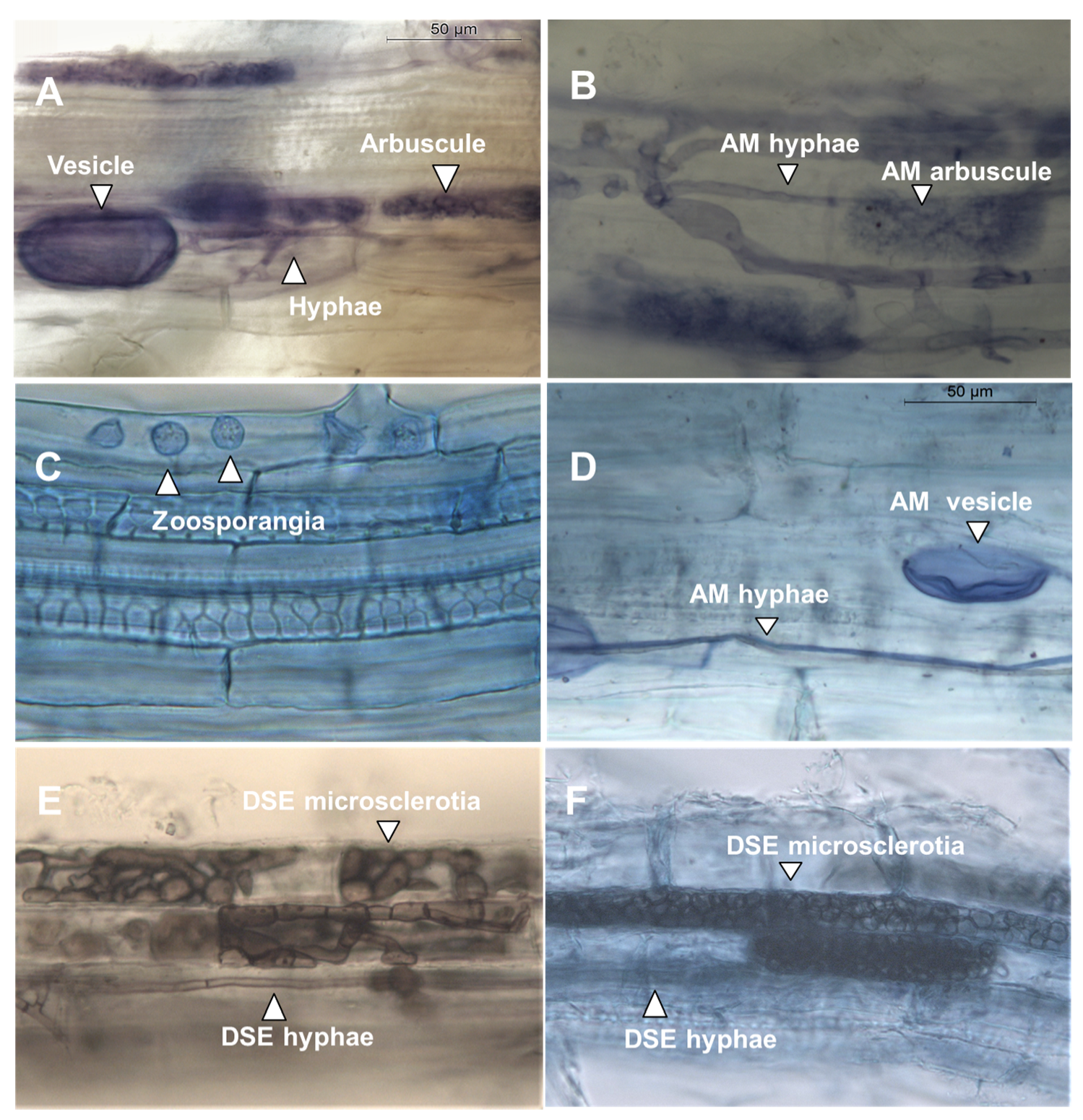
2.4. Mycorrhiza Analyses
2.5. Statistics
3. Results
3.1. Growth-Related Traits and P Content of Shoots
3.2. Soil pH and Mycorrhiza
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lambers, H.; Shane, M.W.; Cramber, M.D.; Pearse, S.J.; Veneklaas, E.J. Root structure and functioning for efficient acquisition of phosphorus: Matching morphological and physiological traits. Ann. Bot. 2006, 98, 693–713. [Google Scholar] [CrossRef] [PubMed]
- Hable, M. Mycorrhizal fungi and plant nutrition. In Plant Nutrient Management in Hawaii’s Soils, Approaches for Tropical and Subtropical Agriculture; Silva, J.A., Uchida, R., Eds.; College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa: Honolulu, HI, USA, 2000; pp. 127–132. [Google Scholar]
- Seleiman, M.F.; Santanen, A.; Mäkelä, P.S.A. Recycling sludge on cropland as fertilizer—Advantages and risks. Resour. Conserv. Recycl. 2020, 155, 104647. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Syers, J.K.; Johnston, A.E.; Curtin, D. Efficiency of soil and fertilizer phosphorus use. In FAO Fertilizer and Plant Nutrition Bulletin 18; Food and Agriculture Organization of the United Nations: Rome, Italy, 2008. [Google Scholar]
- Grant, C.A.; Flaten, D.N.; Tomasiewicz, D.J.; Sheppard, S.C. The importance of early season phosphorus nutrition. Can. J. Plant Sci. 2001, 81, 211–224. [Google Scholar] [CrossRef]
- Grant, C.A.; Bailey, L.D. Fertility management in canola production. Can. J. Plant Sci. 1993, 73, 651–670. [Google Scholar] [CrossRef]
- Veneklaas, E.J.; Lambers, H.; Bragg, J.; Finnegan, P.M.; Lovelock, C.E.; Plaxton, W.C.; Price, C.A.; Scheible, W.R.; Shane, M.W.; White, P.J.; et al. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol. 2012, 195, 306–320. [Google Scholar] [CrossRef]
- Lynch, J.P.; Brown, K.M. Topsoil foraging—An architectural adaptation of plants to low phosphorus availability. Plant Soil 2001, 237, 225–237. [Google Scholar] [CrossRef]
- Chen, Y.P.; Rekha, P.D.; Arun, A.B.; Shen, F.T.; Lai, W.-A.; Young, C.C. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil Ecol. 2006, 34, 33–41. [Google Scholar] [CrossRef]
- Grant, C.; Bittman, S.; Montreal, M.; Plenchette, C.; Morel, C. Soil and fertilizer phosphorus: Effects on plant P supply and mycorrhizal development. Can. J. Plant Sci. 2005, 85, 3–14. [Google Scholar] [CrossRef]
- Bolan, N.S. A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants. Plant Soil 1991, 134, 189–207. [Google Scholar] [CrossRef]
- Gavito, M.E.; Miller, M.H. Early phosphorus nutrition, mycorrhizae development, dry matter partitioning and yield of maize. Plant Soil 1998, 199, 177–186. [Google Scholar] [CrossRef]
- Zhu, J.; Lynch, J.P. The contribution of lateral rooting to phosphorus acquisition efficiency in maize (Zea mays) seedlings. Funct. Plant Biol. 2004, 31, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Amann, C.; Amberger, A. Phosphorus efficiency of buckwheat (Fagopyrum esculentum). J. Plant Nutr. Soil Sci. 1989, 152, 181–189. [Google Scholar] [CrossRef]
- Talboys, P.J.; Heppell, J.; Roose, T.; Healey, J.R.; Jones, D.L.; Withers, P.J. Struvite: A slow-release fertiliser for sustainable phosphorus management? Plant Soil 2016, 401, 109–123. [Google Scholar] [CrossRef]
- Watt, M.; Evans, J.R. Proteoid roots. Physiology and development. Plant Phys. 1999, 121, 317–323. [Google Scholar] [CrossRef]
- Müller, J.; Gödde, V.; Niehaus, K.; Zörb, C. Metabolic adaptations of white lupin roots and shoots under phosphorus deficiency. Front. Plant Sci. 2015, 6, 1014. [Google Scholar] [CrossRef]
- Tommerup, I.C. Development of infection by a vesicular-arbuscular mycorrhizal fungus in Brassica napus L. and Trifolium subterraneum L. New Phytol. 1984, 98, 487–495. [Google Scholar] [CrossRef]
- Miller, M.H. Arbuscular mycorrhizae and the phosphorus nutrition of maize: A review of Guelph studies. Can. J. Plant Sci. 2000, 80, 47–52. [Google Scholar] [CrossRef]
- Evans, J.; Neeson, R.; Burnett, V.; Luckett, D.J.; Fettell, N.A. Phosphorus-use efficiency, growth and yield of spelt wheat (Triticum aestivum ssp. spelta) compared with standard wheat (T. aestivum ssp. vulgare) in south-eastern Australia. J. Org. Syst. 2014, 9, 63–78. [Google Scholar]
- Sarapatka, B.; Dudová, L.; Kršková, M.E. Effect of pH and phosphate supply on acid phosphatase activity in cereal roots. Biologia 2003, 59, 127–131. [Google Scholar]
- Tammeorg, P.; Simojoki, A.; Mäkelä, P.; Stoddard, F.L.; Alakukku, L.; Helenius, J. Short-term effects of biochar on soil properties and wheat yield formation with meat bone meal and inorganic fertiliser on a boreal loamy sand. Agric. Ecosyst. Environ. 2014, 191, 108–116. [Google Scholar] [CrossRef]
- HSY Water Treatment Laboratory. Average Water Quality at Pitkäkoski and Vanhankaupunki Water Treatment Plants. Available online: https://vanha.hsy.fi/en/experts/water-services/drinking-water-quality/Documents/Water%20quality%202020_1.pdf (accessed on 25 May 2020).
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants. BBCH Monograph, 2nd ed.; Federal Biological Research Centre for Agriculture and Forestry: Berlin, Germany, 2001. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Santanen, A.; Stoddard, F.L.; Mäkelä, P.S.A. Feedstock quality and growth of bioenergy crops fertilized with sewage sludge. Chemosphere 2012, 89, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.F.; Moore, T.S.; Christensen, J.R.M. Growth of vesicular-arbuscular mycorrhizal and non-mycorrhizal Bouteloua gracilis in a defined medium. Mycologia 1979, 71, 666–669. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Brundrett, M. Mycorrhizal Associations. The Web Resource. Available online: http://mycorrhizas.info/index.html (accessed on 29 May 2020).
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–163. [Google Scholar] [CrossRef]
- Grace, C.; Stribley, D.P. A safer procedure for routine staining of vesicular-arbuscular mycorrhizal fungi. Mycol. Res. 1991, 95, 1160–1162. [Google Scholar] [CrossRef]
- Inamullah, S.G.; Ayub, M.; Ali Khan, A.; Anwar, S.; Alam Khan, S. Response of common buckwheat to nitrogen and phosphorus fertilization. Sarhad J. Agric 2012, 28, 171–178. [Google Scholar]
- Neumann, G.G.; Massonneau, A.; Martinoia, E.; Römheld, V. Physiological adaptations to phosphorus deficiency during proteoid root development in white lupin. Planta 1999, 208, 373–382. [Google Scholar] [CrossRef]
- Weisskoppf, L.; Abou-Mansour, E.; Fromin, N.; Tomasi, N.; Santelia, D.; Edelkott, I.; Neumann, G.; Arango, M.; Tabacchi, R.; Martinoia, E. White lupin has developed a complex strategy to limit microbial degradation of secreted citrate required for phosphate acquisition. Plant Cell Environ. 2006, 29, 919–927. [Google Scholar] [CrossRef]
- Lambers, H.; Clements, J.C.; Nelson, M.N. How a phosphorus-acquisition strategy based on carboxylate exudation powers the success and agronomic potential of lupines (Lupinus, Fabaceae). Am. J. Bot. 2013, 100, 263–288. [Google Scholar] [CrossRef]
- Sulieman, S.; Ha, C.V.; Schulze, I.; Tran, I.S.P. Growth and nodulation of symbiotic Medicago truncatula at different levels of phosphorus availability. J. Exp. Bot. 2013, 64, 2701–2712. [Google Scholar] [CrossRef] [PubMed]
- Sulieman, S.; Tran, I.S.P. Phosphorus homeostasis in legume nodules as an adaptive strategy to phosphorus deficiency. Plant Sci. 2015, 239, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Schulze, I.; Temple, G.; Temple, S.I.; Beschow, H.; Vance, C.P. Nitrogen fixation by white lupin under phosphorus deficiency. Ann. Bot. 2006, 98, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-G.; He, Y.-Q.; Smith, S.E.; Smith, F.A. Buckwheat (Fagopyrum esculentum Moench) has high capacity to take up phosphorus (P) from a calcium (Ca)-bound source. Plant Soil 2001, 239, 1–8. [Google Scholar] [CrossRef]
- Nogalska, A.; Zalewska, M. The effect of meat and bone meal on phosphorus concentrations in soil and crop plants. Plant Soil Environ. 2013, 59, 575–580. [Google Scholar] [CrossRef]
- Warren, G.P.; Robinson, J.S.; Someus, E. Dissolution of phosphorus from animal bone char in 12 soils. Nutr. Cycl. Agroecosyst. 2009, 84, 167–178. [Google Scholar] [CrossRef]
- Toljander, J.F.; Santos-González, J.C.; Tehler, A.; Finlay, R.D. Community analysis of arbuscular mycorrhizal fungi and bacteria in the maize mycorrhizosphere in a long-term fertilization trial. FEMS Microb. Ecol. 2008, 65, 323–338. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Santanen, A.; Kleemola, J.; Stoddard, F.L.; Mäkelä, P.S.A. Improved sustainability of feedstock production with sludge and interacting mycorrhiza. Chemosphere 2013, 91, 1236–1242. [Google Scholar] [CrossRef]
- Demars, B.G.; Boerner, R.E.J. Vesicular arbuscular mycorrhizal development in the Brassicacea in relation to plant life span. Flora 1996, 191, 179–189. [Google Scholar] [CrossRef]
- Lay, C.-Y.; Hamel, C.; St-Arnaud, M. Taxonomy and pathogenity of Olpidium brassicae and its allied species. Fungal Biol. 2018, 122, 837–846. [Google Scholar] [CrossRef]
- McArthur, D.A.I.; Knowles, N.R. Resistance responses of potato to vesicular arbuscular mycorrhizal fungi under varying abiotic phosphorus levels. Plant Physiol. 1992, 100, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Ban, Y.; Tang, M.; Chen, H.; Xu, Z.; Zhang, H.; Yang, H. The response of dark septate endophytes (DES) to heavy metals in pure culture. PLoS ONE 2012, 7, e47968. [Google Scholar] [CrossRef] [PubMed]
- Yakti, W.; Kowacs, G.M.; Vagi, P.; Franken, P. Impact of dark septate endophytes on tomato growth and nutrient uptake. Plant Ecol. Divers. 2018, 11, 637–648. [Google Scholar] [CrossRef]
- Douds, D.D.; Schenck, N.C. Relationship of colonization and sporulation by VA mycorrhizal fungi to plant nutrient and carbohydrate contents. New Phytol. 1990, 116, 621–627. [Google Scholar] [CrossRef]
- Vance, C.P. Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources. Plant Phys. 2001, 127, 390–397. [Google Scholar] [CrossRef]
- Römer, W.; Schilling, G. Phosphorus requirements of the wheat plant in various stages of its life cycle. Plant Soil 1986, 91, 221–229. [Google Scholar] [CrossRef]
- Makádi, M.; Tomócsik, A.; Orosz, V. Digestate: A new nutrient source—Review. In Biogas; Kumar, S., Ed.; IntechOpen: London, UK, 2012; pp. 295–310. [Google Scholar] [CrossRef]
| Crop Species | P Source | Shoot Biomass, g/plant | Shoot P Content, g/kg DM | P Uptake, mg/shoo | SPAD Value |
|---|---|---|---|---|---|
| Buckwheat | No fertilizer | 0.18 ef | 6.09 h | 1.06 fg | 18 a |
| Synthetic fertilizer | 0.29 gh | 4.71 f | 1.41 gh | 32 cde | |
| Digestate | 0.26 gh | 4.47 f | 1.16 g | 31 cde | |
| Meat bone meal | 0.31 hi | 5.42 g | 1.66 h | 29 cd | |
| White lupine | No fertilizer | 0.32 hi | 4.80 f | 1.52 gh | 46 f |
| Synthetic fertilizer | 0.23 gh | 4.98 f | 1.09 fg | 50 f | |
| Digestate | 0.24 gh | 4.67 f | 1.12 fg | 45 f | |
| Meat bone meal | 0.23 gh | 4.78 f | 1.08 fg | 47 f | |
| Maize | No fertilizer | 0.29 gh | 3.43 cd | 0.95 f | 18 a |
| Synthetic fertilizer | 0.39 j | 2.13 ab | 0.82 e | 21 ab | |
| Digestate | 0.36 ij | 1.75 a | 0.62 d | 22 ab | |
| Meat bone meal | 0.37 ij | 2.07 ab | 0.76 de | 22 ab | |
| Oilseed rape | No fertilizer | 0.03 a | 3.61 e | 0.11 a | 29 cd |
| Synthetic fertilizer | 0.20 fg | 3.95 ef | 0.79 de | 37 e | |
| Digestate | 0.15 cd | 3.47 cd | 0.50 d | 34 de | |
| Meat bone meal | 0.20 fg | 3.88 ef | 0.76 de | 35 de | |
| Spelt wheat | No fertilizer | 0.08 ab | 2.62 bc | 0.20 ab | 16 a |
| Synthetic fertilizer | 0.10 bc | 2.72 bc | 0.27 b | 28 bc | |
| Digestate | 0.11 bc | 2.30 ab | 0.25 b | 28 bc | |
| Meat bone meal | 0.15 cd | 2.12 ab | 0.31 bc | 31 cde | |
| Significance (p) | Crop species (C) | <0.001 | <0.001 | <0.001 | <0.001 |
| P source (P) | <0.001 | <0.01 | <0.05 | <0.001 | |
| C x P | <0.001 | <0.05 | <0.001 | <0.001 |
| Crop Species | P Source | Soil pH | Mycorrhizal Spores, No | Mycorrhizal Colonization, % | |||
|---|---|---|---|---|---|---|---|
| Bulk | Rhizosphere | Type 1 | Type 2 | Total | |||
| Buckwheat | No fertilizer | 4.88 ab | 4.90 | 416 ab | 58 ab | 474 a | 11.5 |
| Synthetic fertilizer | 4.84 ab | 4.56 | 319 a | 200 abc | 519 ab | 7.5 | |
| Digestate | 5.07 ab | 5.03 | 350 ab | 184 abc | 533 ab | 17.6 | |
| Meat bone meal | 5.39 ab | 5.20 | 416 ab | 177 abc | 592 ab | 26.2 | |
| White lupine | No fertilizer | 5.54 b | 5.70 | 424 ab | 41 ab | 465 a | 10.5 |
| Synthetic fertilizer | 5.84 b | 5.37 | 330 a | 403 bc | 733 ab | 8.3 | |
| Digestate | 5.97 b | 5.64 | 383 ab | 382 abc | 764 ab | 5.6 | |
| Meat bone meal | 5.84 b | 5.63 | 357 ab | 113 ab | 470 a | 12.7 | |
| Maize | No fertilizer | 5.52 ab | 5.63 | 417 ab | 27 a | 444 a | 8.8 |
| Synthetic fertilizer | 4.89 ab | 5.13 | 405 ab | 516 c | 921 b | 21.4 | |
| Digestate | 5.87 b | 5.69 | 559 b | 89 ab | 647 ab | 29.2 | |
| Meat bone meal | 5.34 ab | 5.50 | 293 a | 74 ab | 367 a | 27.8 | |
| Oilseed rape | No fertilizer | 5.97 b | 5.80 | 335 a | 64 ab | 399 a | 14.4 |
| Synthetic fertilizer | 4.75 ab | 5.39 | 277 a | 95 ab | 372 a | 17.5 | |
| Digestate | 5.85 b | 5.98 | 301 a | 89 ab | 389 a | 8.9 | |
| Meat bone meal | 5.89 b | 5.71 | 322 a | 89 ab | 411 a | 23.2 | |
| Spelt wheat | No fertilizer | 5.30 ab | 5.70 | 440 ab | 37 ab | 477 ab | 16.7 |
| Synthetic fertilizer | 4.26 a | 4.78 | 319 a | 230 abc | 549 ab | 31.8 | |
| Digestate | 6.01 b | 5.17 | 438 ab | 86 ab | 524 ab | 32.9 | |
| Meat bone meal | 5.46 ab | 5.34 | 282 a | 106 ab | 388 a | 36.7 | |
| Significance (p) | Crop species (C) | <0.01 | <0.001 | <0.05 | <0.05 | <0.01 | NA |
| P source (P) | <0.001 | <0.01 | <0.01 | <0.001 | <0.01 | NA | |
| C x P | <0.05 | 0.898 | <0.05 | <0.05 | <0.05 | NA | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mäkelä, P.S.A.; Wasonga, D.O.; Solano Hernandez, A.; Santanen, A. Seedling Growth and Phosphorus Uptake in Response to Different Phosphorus Sources. Agronomy 2020, 10, 1089. https://doi.org/10.3390/agronomy10081089
Mäkelä PSA, Wasonga DO, Solano Hernandez A, Santanen A. Seedling Growth and Phosphorus Uptake in Response to Different Phosphorus Sources. Agronomy. 2020; 10(8):1089. https://doi.org/10.3390/agronomy10081089
Chicago/Turabian StyleMäkelä, Pirjo S. A., Daniel O. Wasonga, Ainhoa Solano Hernandez, and Arja Santanen. 2020. "Seedling Growth and Phosphorus Uptake in Response to Different Phosphorus Sources" Agronomy 10, no. 8: 1089. https://doi.org/10.3390/agronomy10081089
APA StyleMäkelä, P. S. A., Wasonga, D. O., Solano Hernandez, A., & Santanen, A. (2020). Seedling Growth and Phosphorus Uptake in Response to Different Phosphorus Sources. Agronomy, 10(8), 1089. https://doi.org/10.3390/agronomy10081089







