Abstract
Iodine uptake and translocation was studied in cabbage and tomato cultivated on different soil types (sand, sandy silt, silt) by applying irrigation water containing iodine at concentrations of 0.1 and 0.5 mg/L. Iodine treatment at the concentrations applied did not significantly influence the photosynthetic efficiency and chlorophyll concentration of cabbage and tomato leaves. The growth of cabbage leaves cultivated on sand and sandy silt soil with iodine treatment was slightly stimulated, while, on silt soil, it remained unchanged; for tomato plant parts, independent of the soil-type, the dry mass values remained constant. It can be concluded that iodine treatment had no negative effect on the physiological characteristic of cabbage and tomato plants. Applying 0.5 mg/L in the irrigation water, the highest biofortification with iodine was achieved in plants cultivated in sandy soil and the iodine concentration calculated in the dry matter amounted to 10 and 3.6 mg/kg in the leaves and fruits of cabbage and tomato, respectively. Considering the iodine and moisture content of cabbage leaves and tomato fruits, the consumption of 100 g of fresh vegetable would cover about 80% and 15% of the recommended iodine intake, respectively. The presence of iodine resulted in a reduction in Fe and P concentrations in tomato fruits independent of the soil-type; however, the concentration of Mg, Cu, Mn, Zn, and B remained practically unchanged. However, for cabbage, no similar trend for Fe and P was observed.
1. Introduction
Iodine (I) is an essential element for human health. It is involved in the synthesis of thyroid hormones, which play a dominant role in growth and metabolism [1]. The recommended dietary allowance ranges between 90–250 μg/day, and for a normal adult, it is 150 μg/day. Inadequate iodine intake leads to several issues, termed together as iodine deficiency disorders (IDDs). The typical symptoms of IDDs are goiter and hypothyroidism, but recently iodine deficiency has been found to cause an even broader spectrum of disorders, such as mental retardation, psychomotor defects, and hearing/speech impairment. As IDDs affect a large population, approximately a billion people are estimated to be at risk and thus worldwide prevention of iodine deficiency is of great importance [2,3].
Iodine supplementation by iodized salt is a widely used strategy to eliminate IDDs. On the other hand, many countries have adapted regulations to reduce salt consumption in order to control cardiovascular diseases and hypertension; therefore, it is important to find other options.
Iodine enrichment of fruits and vegetables has been proven to effectively increase the iodine intake (biofortification) of humans. Several agricultural practices (hydroponics, pot, field experiments) have been developed to increase iodine concentration in the edible parts of different cultivated plants (e.g., lettuce [4,5,6,7,8], spinach [9,10,11,12], pakchoi [13], cabbage [14], chinese cabbage [6], tomato [6,15,16], strawberry [17], cucumber [18], carrot [6], celery [19], radish [7], potato [15], rice [20] and bean [21]). Based on the literature, the following observations can be summarized: 1) in the aerial part of plants, the translocation and accumulation of iodide is greater than that of iodate; 2) iodine transport is mainly xylematic; and 3) iodine concentration within the plants decreases from the root to the fruit [13,22,23,24,25]. However, it should be emphasized that, over a certain concentration, iodine has toxic effect on plants, resulting in reduced biomass production [13,14,26].
It is well known that iodine uptake and accumulation in plants is highly dependent on the soil properties [27]. The natural concentration of iodine in different soil varies widely (0.1–150 mg/kg) [27,28], and its chemical form and bioavailability are influenced by several soil parameters, such as pH, microbial activity, and organic matter (OM) or oxide mineral content [27]. The latter plays a dominant role in iodine retention; therefore, a relatively high proportion of iodine in the soil is not accessible to plants due to it being bound to humic and fulvic acids or adsorbed by positively charged iron and aluminum oxides. Iodine exists in the soil in different chemical forms as organic compounds, iodate (IO3−) or iodide (I−). Generally, the latter is the predominant form in acidic conditions, while iodate is dominant in alkaline soils [27]. Microbial activity can also affect the valence state of iodine, e.g., sulphate (SO42−)- and ferric (Fe3+)-reducing bacteria can convert IO3− to I−. Some studies have shown that inorganic iodide compounds are transformed into organoiodine, and this conversion is considerably faster than that of iodate [29,30].
Our selected species, cabbage (Brassica oleracea L. var. capitata L.) and tomato (Solanum lycopersicum L.) belong to the most widely cultivated vegetables. In 2018, their worldwide production was nearly 55 and 100 million tons, and their cultivation area covered more than 2.5 and 3.5 million hectares, respectively [31]. Both species have some nutraceutical properties, for example, cabbage leaves are rich sources of vitamin C and K (consumption of 100 g fresh leaf would cover 44% and 72% of the daily nutritional requirement) [32], and tomato fruits contain a high amount of antioxidants [33]. Due to their widespread production and consumption, as well as the variety of cultivation technologies available, these plants are promising targets for biofortification with iodine.
Recently, a number of biofortification studies have focused on cabbage and tomato plants cultivated under different conditions (i.e., hydroponic, pot, and field experiments). Regarding the biofortification of cabbage, iodized fertilizer in a surface concentration range of 12–150 mg I/m2 resulted in 10–32 mg/kg iodine concentration in the leaves calculated for fresh weight (FW) [34]. In a long-term (10 years) experiment, iodine-containing irrigation water with a concentration of 40–80 μg I/L was sprayed on the cabbage plants, and the edible parts were found to contain iodine in concentration of 50–220 mg/kg FW [35]. These data confirm that cabbage leaves are excellent candidates for biofortification experiments.
Tomato plants cultivated in a hydroponic solution with iodine concentration of 1–5 mM resulted in 4–24 mg/kg FW iodine concentration in the fruits [36]. In another hydroponic experiment, 5–20 mM iodine concentration was applied during the plant cultivation, and the iodine concentration in the tomato fruit amounted to 10–30 mg/kg FW [16]. Tomato plants were cultivated in pots and irrigated with water containing iodate (295–2950 mg/L) or iodide (380–760 mg/L), and the fruits had iodine concentrations of 5–49 and 39–53 mg/kg FW, respectively [15]. In a greenhouse experiment, KI or KIO3 was added to the irrigation water in the concentration range of 127–635 and 63–252 mg I/L, and the iodide treatment resulted in fruits higher iodine concentration (2–10 mg/kg FW) than the iodate treatment [26]. In field experiments, where tomato plants were cultivated on Bathicalcic Cambisols (pH = 7.80, OM = 0.7%, total iodine was not detailed) and paddy soils (pH = 5.91, OM: 4.09%, total iodine: 2.02 mg/kg) by the application of solid fertilizer to the surface at concentrations of 250–500 g I/ha or 12–150 mg I/m2 the tomato fruits contained iodine in a concentration of 0.006–0.015 mg/kg FW [36] and 0.5–1.5 mg/kg FW [34], respectively. KI-containing fertilizer was applied in the concentration range of 10–150 mg/kg for the cultivation of tomato plants in inceptisol soil (pH = 5.91, OM: 4.09%, total iodine: 2.02 mg/kg), and 1–10 mg/kg FW iodine concentration was measured in the fruits [6]. It can thus be established that, depending on the technology applied, for the biofortification of tomato with iodine, the concentration of this target element in the fruit changes in the range of 0.006–53 mg/kg FW. Although, some positive results in terms of iodine accumulation within the edible parts of these plants have been achieved previously, the accumulation and translocation within the plants, and dependence on the soil features, is still poorly understood.
In our study, iodine accumulation, translocation, and biofortification were investigated in cabbage and tomato plants cultivated in three different soils (sand, sandy silt, and silt) by applying irrigation water containing iodine at concentrations of 0.1 and 0.5 mg/L (as potassium iodide). The iodine concentration of different plant organs, and the distribution of iodine within the plant, was measured. The effects of iodine on the plant growth, uptake, and translocation of essential elements (P, Mg, Mn, Fe, Cu, Zn, B), as well as photosynthetic efficiency, were also investigated. Based on our results, the influence of soil type on the biofortification with iodine will be evaluated, and a recommendation is given to the farmers to select the most appropriate plant–soil system for the production of iodine rich vegetables.
2. Materials and Methods
2.1. Chemicals
All chemicals used during the experiments were of analytical grade. Ultra-pure water (resistivity: 18 MΩ cm−1) was produced by an ELGA Ultra Purelab unit (ELGA LabWater/VWS Ltd., High Wycombe, UK). Iodine stock solution for calibration was prepared using solid KIO3 (Sigma Aldrich Ltd., Missouri, USA), and, for the determination of P, Mg, Mn, Fe, Cu, Zn, and B, a multi-element standard solution (Sigma Aldrich Ltd., Missouri, USA) was applied. The accuracy of the analytical methods was verified using the NIST 1573a tomato leaf (National Institute of Standards and Technology, Gaithersburg, USA) certified reference material.
2.2. Characterization of Soil
The pH was measured in 1:2.5 soil:water after mixing for 12 h [37]. CaCO3 content was measured by applying the Scheibler gas-volumetric method [37]. Organic matter (OM) content was determined using the modified Walkley–Black method [38]. Plant-available P and K concentrations were measured after extraction with ammonium-acetate lactate (AL-P2O5 and AL-K2O) [39]. Total nitrogen (Total-N) content was determined by the Kjeldahl method [40]. The ammonium (NH4-N) and nitrate (NO3-N) concentrations were measured from KCl (Sigma Aldrich Ltd., Missouri, USA) extracts [41]. The cation exchange capacity (CEC) values were measured by applying the modified method of Mehlich [42]. The iodine concentrations were determined by inductively coupled plasma mass spectrometer (ICP-MS) (Plasma Quant MS Elite, Analytik Jena, Germany) following microwave-assisted aqua regia digestion in closed Teflon vessels.
2.3. Plants Cultivation
The effect of irrigation water containing iodine on the target plants was investigated in a pot experiment. The plant cultivation was conducted in a greenhouse at the Experiment Station of the Centre of Agricultural Research in Őrbottyán, Hungary, using 10 L pots with four holes (Ø 0.5 cm) at the bottom. The bottom of the pot was filled with gravel (4–8 mm) in a layer of 1–1.5 cm. The gravel layer was covered with a fine synthetic fiber fabric on which the applied soil was layered in 10 kg of volume. The three investigated soils from different parts of Hungary were: sand (Mollic Umbrisol (Arenic) from Őrbottyán), sandy silt (Luvic Calcic Phaeozem from Gödöllő) and silt (Calcic Chernozem from Hatvan). The soil properties are shown in Table 1. The tomato and cabbage seedlings were planted on 24 May 2018 and 17 July 2018, having a growing period of 88 and 71 days, respectively. The number of pots were 54 (2 plant species x 3 soils x 3 treatments (control + 2 iodine dosages) x 3 replicates) and a single pre-grown seedling was planted in each pot. After planting, the reared seedlings were irrigated with standing drinking water for three weeks. During the growing period, the plants (including control) were watered weekly with Hoagland solution. The irrigation with potassium-iodide-containing solutions started three weeks after planting. The applied iodide concentrations in these solutions were 0.1 and 0.5 mg/L. For the preparation of KI solutions, standing drinking water was also used to eliminate any oxidation effect of free chlorine.

Table 1.
Physical-chemical properties of soils.
2.4. In Situ Measurements of Photosynthetic Efficiency and Chlorophyll Concentration
The photosynthetic efficiency of photosystem (PS) II of leaves was characterized by in situ measurements of Chlorophyll-a applying an Os30p handheld, pulse modulated fluorometer (Opti-Sciences, Hudson, USA). To indicate the potential plant stress of iodine, the Fv/Fm ratios were calculated, where Fv = variable fluorescence level from dark-adapted leaves, and Fm = maximal fluorescence level from dark-adapted leaves [43]. Before the measurements, plants were dark-adapted for 30 min. The Chlorophyll Content Index (CCI) of leaves was monitored by using a CCM-200 plus Chlorophyll Content Meter (Opti-Sciences, Hudson, USA). The Fv/Fm and CCI data were measured on all plants at the harvesting stage.
2.5. Sample Preparation and Elemental Analysis of Plants
The plants were harvested (tomato: 21 Aug 2018; cabbage: 25 Sept 2018), cleaned with deionized water, and then the roots, aerial parts (stem + leaves), and fruits were separated. The aerial part and root samples were dried at 40 °C for two days in a laboratory dryer, while tomato fruit samples were freeze-dried at −70 °C in an Alpha 1 (Christ) equipment (at 200 Pa for 72 h), after which the dry mass of plant organs were determined. All samples were homogenized with a household blending machine, equipped with plastic housing and a stainless-steel blend. The dried and homogenized samples were mineralized in a microwave-assisted acid digestion system (TopWave, Analytik Jena, Germany). Twelve Teflon bombs were used, one for the blank and eleven for the samples. Blanks were measured every time. In total, 400–500 mg of dried plant samples was digested in a mixture of 7 cm3 67% nitric acid (VWR International, Pennsylvania, USA) and 3 cm3 30% hydrogen-peroxide (VWR International, Pennsylvania, USA). After digestion, the internal standards were added to the solutions and filled up to 15 cm3 with deionized water. The concentration of iodine, macro-, and micro-elements was measured by ICP-MS.
2.6. Statistical Analysis
Data visualization and statistical analysis were made with R statistical software [44]. The line plots visualizing the mean and standard deviations of the data were made with the ggpubr package [45]. Linear regression models were used to compare the impact of treatment dosages, soil types and their interactions on the plants’ iodine, selected macro- and microelement concentration, dry mass and photosynthetic efficiency. Post hoc pair-wise comparisons were made by Tukey multiple comparisons of means, using the multcomp package [46].
3. Results
3.1. Photosynthetic Efficiency and Chlorophyll Content Index
The photosynthetic efficiency of photosystem II, as measured by the ratio of Fv/Fm of dark-adapted cabbage and tomato leaves, as well as the CCI values, are listed in Table 2. The presence of iodine in irrigation water affected significantly neither the photosynthetic efficiency of chlorophyll-a, nor the CCI values, and its effect was independent of soil type. The Fv/Fm values of the tomato plants cultivated on the sand or sandy silt soils were higher than on the silt soil, and the lowest photosynthetic efficiency of PS II was measured in both plant species at the 0.5 mg/L iodine treatments (except tomato grown on silt). Values of Fv/Fm were similar (around 0.8) in all treatments; however, they were significantly higher for cabbage (0.817–0.843) than tomato plants (0.745–0.810). Iodine treatment (especially using 0.1 mg/L) had a slight, but not significant positive effect on the CCI (except on silt soil), and CCI also showed significantly higher values in tomato plants than in cabbage.

Table 2.
Effect of iodine concentrations in irrigation water on the photosynthetic efficiency (Fv/Fm) of photosystem (PS) II and chlorophyll content index of leaves (CCI). The values indicate the means of three replicates (RSD%), while different letters indicate statistically significant differences between treatments (p < 0.05; linear regression and Tukey’s test).
3.2. Biomass Yield of Plants
Comparison of the biomass data of control samples leads to the following observations on the effect of soil types (Table 3):

Table 3.
Effect of iodine concentrations in the irrigation water on dry mass of the investigated plants. Values indicate means of three replicates (RSD%), while different letters indicate statistically significant differences between the treatments (p < 0.05, linear regression, Tukey’s test).
- The root mass of cabbage increased in the order of sandy silt < silt < sand (showing significant difference between sandy silt and sand), maybe due to increasing pH of the soil (Table 1). However, the tomato showed different pattern. Here, the root mass increased in the order of sandy silt < sand < silt, showing significant differences between silt and the other two soil types.
- The dry mass of aerial parts did not show significant difference between the soils for tomato, but for cabbage, significantly lower mass was measured on sandy silt than on the other two soils. The highest dry mass values of aerial parts were measured on silt for both species.
- The effect of soil type on the fruit production in tomato was negligible (no statistical difference), and the fruit mass increased in the order of sandy silt < silt < sand.
In general, the iodine treatments did not have a significant effect on the mass of any plant organs, except the leaf production of cabbage. The response of cabbage leaves depended on the soil; on sandy (sand and sandy silt) soils, a significant increase of dry mass was measured by increasing the iodine concentration of the irrigation water, while the dry mass of cabbage plants grown in silt soil did not change significantly as compared to the control samples. The observed increase on sandy soils was significantly higher on the sandy silt soil than on sand.
3.3. Uptake and Translocation of Iodine
In general, the edible parts of plants showed less iodine concentration than the other plant organs (Figure 1), varying between 0.27–0.30 mg/kg dry weight (DW) for control cabbage leaves and 0.07–0.08 mg/kg DW for control tomato fruits. Without iodine treatment, the soil type had no significant effect on iodine concentration (p > 0.09, linear regression). The addition of iodine to the irrigation water resulted in higher iodine concentration in all investigated plant tissues, especially when the dosage was 0.5 mg/L. In cabbage, the iodine accumulation was significantly higher in the roots than in the leaves, while, in tomato, it was similar in the two plant organs. However, the edible parts of both plants showed the least response to the treatment. Investigating the iodine translocation from the root to the edible parts, it can be established that cabbage plants have higher transfer factor (0.09–0.17) than tomato (0.01–0.08). Silty soils significantly reduced the treatment effects on all plant organs. The highest iodine concentrations in cabbage leaves and tomato fruits were measured in plants cultivated on sand soil and the concentration values amounted to 10 mg/kg and 3.6 mg/kg dry weight (DW), respectively. These values were about twice as high as for sandy silt soil, while, in silt soil, a reduction in iodine concentration was observed, compared to those on sand soil.
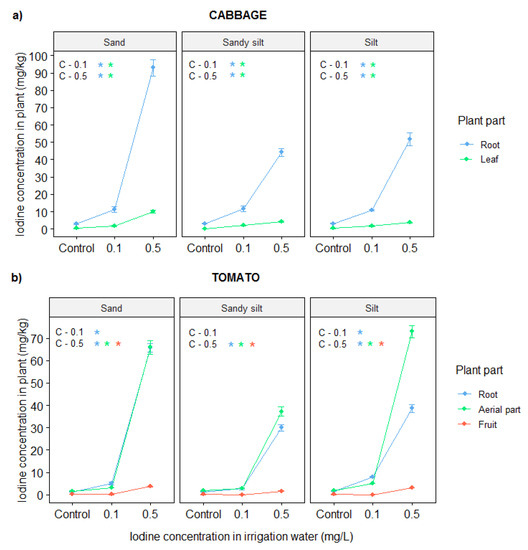
Figure 1.
Iodine concentration (dry weight (DW)) in different parts of cabbage (a) and tomato (b) plants, cultivated in different soils (sand, sandy silt, silt), and irrigated with iodine containing (0.1 and 0.5 mg/L) irrigation water. Significant treatment effects are signed on the top left corner, * means p < 0.05 based on linear regression.
The average iodine distribution among the different plant organs was calculated on the basis of the dry biomass and iodine concentration values (Table 4.) For tomato, the highest iodine content was measured in the aerial parts and the lowest in the fruits, while, in cabbage, the picture was different, i.e., the highest proportion of iodine was partitioned to the edible leaf. The soil type did not influence the distribution of iodine among the plant organs and the partitioning of iodine in edible parts of cabbage and tomato varied in the range of 57%–83% and 1%–4%, respectively.

Table 4.
Iodine distribution in cabbage and tomato plants cultivated in different soils.
Application of 0.5 mg/L iodine concentration in the irrigation water and cultivation of plants in sandy soil seems to be the most effective way to achieve biofortification with iodine. Based on the maximum accumulated iodine content in the edible parts, and the moisture content of the plants, it can be calculated that consuming 100 g of fresh vegetables would be enough to cover about 80% (cabbage) and 15% (tomato) of the recommended dietary allowance.
3.4. Transport of Selected Macro- and Micro Elements
The measured macro- and microelement concentrations in different plant organs of cabbage and tomato are presented in Table 5. On the basis of these results focusing on the edible parts of the investigated plants, it can be established that the presence of iodine can partly influence the uptake and translocation of macro- and microelements. Inhibitory effects were observed for iron in cabbage leaf and tomato fruit, and for boron in cabbage leaf, independent of the soil type. In cabbage leaves and tomato fruits, the concentrations of Fe decreased, and the Cu concentrations remained about at the same level as in the control plants. A positive effect was observed for Zn and Mg concentrations in tomato fruits. The concentration of P in cabbage leaves was practically the same as in the control plants, while, in tomato fruits, a significant decrease was observed.

Table 5.
Effect of iodine concentrations in the irrigation water on the concentration (n = 3) of macro and micro-nutrients in the cases of cabbage and tomato plants. Different letters indicate significant differences (p < 0.05, Tukey’s test).
4. Discussion
Iodine at the applied concentrations had no significant effect on the photosynthetic efficiency, (Fv/Fm) and CCI values cabbage and tomato leaves. This is an important pre-requisite to avoid any inhibition of plant growth. To the best of our knowledge, experiments related to cabbage and tomato have not been reported in the literature so far. However, there are experimental data for two other plants. Buckwheat microgreens were soaked in water containing iodine at concentration of 1000 mg/L (as I− or IO3−). The Fv/Fm ratios changed within a small range from 0.78 to 0.80, while the chlorophyll-a concentration was moderately stimulated [47]. In lettuce plants cultivated in hydroponic solutions containing I− and IO3− at doses of 2.5–10 mg/L, the iodate treatment enhanced the chlorophyll-a concentration of the leaves, while, in the presence of iodide, no effect was detectable as compared to the control plants [48]. In addition, it should be noted that the iodide concentrations applied in our experiments were considerably lower than in the cited papers.
The measured changes in biomass production in our study, caused by iodine administration do not harmonize with literature data. However, it has to be emphasized that the treatment technologies, and the iodine concentrations were different, and, in previous studies, the influence of soil type was not investigated. The cultivation of cabbage plants in a pot experiment established that on using 150 mg KI/kg soil fertilizer, the plant growth was reduced by 35% [14]. The biofortification of cabbage was studied by applying a solid fertilizer with surface concentration of 0.59 kg I/ha, and the resultant dry mass decreased by 46% [49]. Studies focused on tomato plants reported a 45% or 70% decrease in the biomass production by applying solid fertilizer containing 150 mg KI/kg soil (pH = 5.91, OM = 4.09%, total iodine = 2.02 mg/kg) [6] or irrigation water having 760 mg/L iodide concentration [15]. When the iodine concentration applied for biofortification was considerably reduced to 6.3 mg I−/L or 1–5 mM, it was found that the biomass production of edible parts did not change significantly as compared to the control plants [26,50]. The differences between our experimental and literature data can be explained by the fact that the applied iodine concentrations in our experiments were considerably lower, and the treatment technologies were also different. It can be concluded that the iodine concentrations used in the present study did not cause any negative effect on the physiological characteristics of cabbage and tomato plants.
Based on the results of the present study, it can be summarized, that increasing iodine concentration of irrigation water resulted in increasing iodine concentration in all investigated plant organs. These observations agree with several other studies [6,16,26,34]. According to our results, the maximum iodine concentration in the edible part of cabbage and tomato amounted to 10 and 3.6 mg/kg (DW), respectively. These favorable concentration values were measured in plants cultivated in sandy soil, which had the lowest organic matter content among the three soils investigated (Table 1). These results can be explained by the fact that iodine amended to the soil by irrigation water partially bound to organic compounds, resulting in a distribution between the solid phase and soil solution. It means that the ratio of the “free” and bound iodine compounds strongly depends on the soil properties and first of all on the organic matter content [27]. Unfortunately, in the literature, there are only a few biofortification studies in which the influence of soil quality has been assessed Solid iodine-containing fertilizer at 0.59 kg/ha was applied for biofortification of cabbage plants grown on loess soil (pH = 8.16, OM = 1.36%, total iodine = 1.7 mg/kg), and, in the leaf samples, 1.1 mg I/kg DW iodine concentration was detected [49]. Cabbage plants were also grown in sandy loam (pH = 5.60, OM = 3.10%, total iodine = 5.6 mg/kg), and clay (pH = 4.50, OM = 4.70%, total iodine = 1.3 mg/kg) soils using the same 5–15 g I−/ha foliar spray technology. The effect of soil on the iodine concentration of leaves was not investigated as the iodine concentrations obtained on the two soils were averaged (105 mg/kg DW) [51]. Fertilizer containing iodine (10–590 mg/kg) was applied for biofortification, and iodine concentrations of 30 mg/kg FW [34] and 160 mg/kg FW [14] were achieved in the cabbage leaves. By using irrigation water containing iodine (0.04–0.08 mg I−/L) the maximum iodine concentration in the edible part of cabbage was 0.2 mg/kg FW [35]. Tomato plants were biofortified by applying fertilizer technologies with iodine in a concentration range of 10–150 mg I−/kg and cultivated in paddy soil type (pH = 5.91, OM = 4.09%, total iodine = 2.0 mg/kg), and the edible parts contained 1.5 mg I/kg [6,34]. In another study, a 0.25 kg/ha dosage was applied on Bathicalcic Cambisols (pH = 7.80, OM = 0.7%, total iodine was not detailed) soil type and the iodine concentration of the fruit was found to be 15 g/kg FW [36]. When tomato plants were watered with irrigation water having 127–760 mg I−/L, the maximum iodine concentration of the fruits amounted to 2–53 mg/kg FW [15,26].
While the uptake and translocation of iodine and its effects on the growth of different vegetables have been widely studied both in laboratory and field experiments, only a few studies investigated the interaction of iodine with other macro or micro elements. Tomato plants were irrigated with water containing iodine (as KI) in a concentration of 10−6−10−5 M, and the concentration of Mg and Cu in leaf organs was moderately stimulated [52]. In lettuce, it was found that the soil application of KI (0.5–2.0 kg I/ha) and foliar spraying with KIO3 (0.02–2.0 kg I/ha) in a two-year-long investigation on silt loam soil (pH, 6.99–6.73, OM = 3.41–3.76%) had a positive correlation between the concentration of iodine and K, Mg, Ca, Mn [53]. However, in another long-term experiment (3 years), the same group investigated the same target plants cultivated in heavy soils (pH = 6.92–7.45, OM = 2.33%–2.56%), using solid fertilizer containing KI or KIO3 at a dose of 5 kg I/ha, and, in both treatments, negative correlations were found between the concentration of iodine and K, Mg, Ca, S, Na, B, Fe, Mn, Cd [54]. Due to the different plant–soil systems, the chemical form and concentration of the added iodine, as well as the various cultivation technologies, a clear trend on the iodine effect cannot be established. Therefore, before the application of biofortification processes in a selected plant–soil system, a thorough analysis is necessary to characterize the chemical composition of the edible parts for the optimization of plant production, and, in addition, the effect of iodine on the microflora of soil should also be clarified.
5. Conclusions
The responses of cabbage and tomato plants cultivated in sand, silty sand and silt soil, in the same environmental conditions and irrigated with water containing 0.1 and 0.5 mg/L iodine, were investigated by means of physiological and chemical analysis. These species can be adequately biofortified with iodine without any observable physiological stress symptoms or significant changes to their photosynthetic activity, independent of the soil types on which they were investigated. However, the cultivation in sandy soil resulted in the best yield and the highest iodine concentration in the edible parts of both species. In addition, the iodine effect on the concentration of essential macro and micro elements in these plants were relatively low, therefore their nutrition value did not change considerably due to the iodine treatment. Considering the accumulated iodine concentration of cabbage leaves and tomato fruits, as well as the moisture content of these plant organs, it can be calculated that consuming 100 g of fresh vegetables would cover about 80% (cabbage) and 15% (tomato) of the recommended dietary allowance (150 μg).
Author Contributions
P.D. was responsible for original draft preparation, writing, analytical experimental work, and data evaluation; V.V., M.Ó. participated in analytical experimental work and data visualization; K.K., S.S. were responsible for the sample-preparation; A.E. conducted the statistical data analyses and data visualization; M.R. carried out the plant growing, T.T. was responsible for the photosynthetic efficiency and chlorophyll content measurements, G.Z. contributed to the conceptualization, editing, writing and reviewing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Development and Innovation Office (Grant No. NVKP_16-1-2016-0044).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Velasco, I.; Bath, S.C.; Rayman, M.P. Iodine as essential nutrient during the first 1,000 days of life. Nutrients 2018, 10, 290. [Google Scholar] [CrossRef] [PubMed]
- Koukkou, E.G.; Roupas, N.D.; Markou, K.B. Effect of excess iodine intake on thyroid on human health. Minerva Med. 2017, 108, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Hays, S.M.; Poddalgoda, D.; Macey, K.; Aylward, L.; Nong, A. Biomonitoring equivalents for interpretation of urinary iodine. Regul. Toxicol. Pharmacol. 2018, 94, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Blasco, B.; Rios, J.J.; Cervilla, L.M.; Sánchez-Rodrigez, E.; Ruiz, J.M.; Romero, L. Iodine biofortification and antioxidant capacity of lettuce: Potential benefits for cultivation and human health. Ann. App. Biol. 2008, 152, 289–299. [Google Scholar] [CrossRef]
- Blasco, B.; Ríos, J.J.; Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.M.; Leyva, R.; Romero, L.; Ruiz, M.J. Study of the interactions between iodine and mineral nutrients in lettuce plants. J. Plant Nutr. 2012, 35, 1958–1969. [Google Scholar] [CrossRef]
- Hong, C.L.; Weng, H.X.; Qin, Y.C.; Yan, A.L.; Xie, L.L. Transfer of iodine from soil to vegetables by applying exogenous iodine. Agron. Sustain. Dev. 2008, 28, 575–583. [Google Scholar] [CrossRef]
- Lawson, P.G.; Daum, D.; Czauderna, R.; Meuser, H.; Härtling, J.W. Soil versus foliar iodine fertilization as a biofortification strategy for field-grown vegetables. Front. Plant Sci. 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Voogt, W.; Holwerda, H.T.; Khodabaks, R. Biofortification of lettuce (Lactuca sativa L.) with iodine: The effect of iodine form and concentration in the nutrient solution on growth, development and iodine uptake of lettuce grown in water culture. J. Sci. Food Agric. 2010, 90, 906–913. [Google Scholar] [CrossRef]
- Dai, J.L.; Zhu, Y.G.; Huang, Y.Z.; Zhang, M.; Song, J.L. Availability of iodide and iodate to spinach (Spinacia oleracea L.) in relation to total iodine in soil solution. Plant Soil 2006, 289, 301–308. [Google Scholar] [CrossRef]
- Weng, H.X.; Yan, A.L.; Hong, C.L.; Xie, L.L.; Qin, Y.C.; Cheng, C.Q. Uptake of different species of iodine by water spinach and its effect to growth. Biol. Trace Elem. Res. 2008, 125, 184–194. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Huang, Y.Z.; Hu, Y.; Liu, Y.X. Iodine uptake by spinach (Spinacia oleracea L.) plants grown in solution culture: Effects of iodine species and solution concentrations. Environ. Int. 2003, 29, 33–37. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Huang, Y.; Hu, Y.; Liu, Y.; Christie, P. Interactions between selenium and iodine uptake by spinach (Spinacia oleracea L.) in solution culture. Plant Soil 2004, 261, 99–105. [Google Scholar] [CrossRef]
- Hong, C.L.; Weng, H.X.; Yan, A.L.; Xie, L.L. Dynamic characterization of iodine uptake in vegetable plants. Acta Ecol. Sin. 2009, 29, 1438–1447. [Google Scholar]
- Weng, H.X.; Hong, C.L.; Yan, A.L.; Pan, L.H.; Qin, Y.C.; Bao, L.T.; Xie, L.L. Mechanism of iodine uptake by cabbage: Effects of iodine species and where it is stored. Biol. Trace Elem. Res. 2008, 125, 59–71. [Google Scholar] [CrossRef]
- Caffagni, A.; Arru, L.; Meriggi, P.; Milc, J.; Perata, P.; Pecchioni, N. Iodine fortification plant screening process and accumulation in tomato fruits and potato tubers. Commun. Soil Sci. Plant. 2011, 42, 706–718. [Google Scholar] [CrossRef]
- Landini, M.; Gonzali, S.; Perata, P. Iodine biofortification in tomato. J. Plant Nutr. Soil Sci. 2011, 174, 480–486. [Google Scholar] [CrossRef]
- Li, R.; Liu, H.P.; Hong, C.L.; Dai, Z.X.; Liu, J.W.; Zhou, J.; Hua, C.Q.; Weng, H.X. Iodide and iodate effects on the growth and fruit quality of strawberry. J. Sci. Food Agric. 2016, 1, 231–235. [Google Scholar] [CrossRef]
- Voogt, W.; Steenhuizen, J.; Eveleens, B. Uptake and distribution of iodine in cucumber, sweet pepper, round, and cherry tomato. Rep. Wagening. UR Greenh. Horicult. 2014, 1329, 1–72. [Google Scholar]
- Hong, C.L.; Weng, H.X.; Yan, A.L.; Islam, E.U. The fate of exogenous iodine in pot soil cultivated with vegetable. Environ. Geochem. Hlth. 2009, 31, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Wachi, T.; Yoshihira, K.; Nakagawa, T.; Ishikawa, A.; Takagi, D.; Tezuka, A.; Yoshida, H.; Yoshida, S.; Sekimoto, H.; et al. Rice (Oryza sativa L.) roots have iodate reduction activity in response to iodine. Front. Plant Sci. 2013, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dobosy, P.; Kröpfl, K.; Óvá, M.; Sandil, S.; Németh, K.; Engloner, A.; Tünde, T.; Záray, G.Y. Biofortification of green bean (Phaseolus vulgaris L.) and lettuce (Lactuca sativa L.) with iodine in a plant-calcareous sandy soil system irrigated with water containing KI. J. Food Compos. Anal. 2020, 88, 103434. [Google Scholar] [CrossRef]
- Hong, C.L.; Weng, H.X.; Yan, A.L.; Xie, L.L. Characteristics of iodine uptake and accumulation by vegetables. Chin. J. App. Ecol. 2007, 18, 2313–2318. [Google Scholar]
- Umaly, R.C.; Poel, L.W. Effects of iodine in various formulations on the growth of barley and pea plants in nutrient solution culture. Ann. Bot. 1971, 35, 127–131. [Google Scholar] [CrossRef]
- Xie, L.L.; Weng, H.X.; Hong, C.L.; Yan, A.L. Uptake of bok-choy and Ipomoea aquatica Forsk to iodine species. Plant Nutr. Fert. Sci. 2007, 13, 123–128. [Google Scholar]
- Yu, W.J.; Yao, Y.; Wei, H.M.; Long, M.H.; Tang, X.F. Absorption of exogenous iodine in rhizosphere and its effects on physiological parameters of cherry tomato plants. Guihaia 2011, 31, 513–519. [Google Scholar]
- Kiferle, C.; Gonzali, S.; Holwerda, H.T.; Ibaceta, R.R.; Perata, P. Tomato fruits: A good target for iodine biofortification. Front. Plant Sci. 2013, 4, 1–10. [Google Scholar] [CrossRef]
- Medrano-Macías, J.; Leija-Martínez, P.; González-Morales, S.; Juárez-Maldonado, A.; Benavides-Mendoza, A. Use of iodine to biofortify and promote growth and stress tolerance in crops. Front. Plant Sci. 2016, 7, 1146. [Google Scholar] [CrossRef]
- Johnson, C.C. The geochemistry of iodine and its application to environmental strategies for reducing the risks from iodine deficiency disorders. In British Geological Survey Commissioned Report Number CR/03/057; British Geological Survey: Nottingham, UK, 2003; pp. 1–48. [Google Scholar]
- Seki, M.; Oikawa, J.; Taguchi, T.; Ohnuki, T.; Muramatsu, Y.; Sakamoto, K.; Amachi, S. Laccase-catalyzed oxidation of iodide and formation of organically bound iodine in soils. Environ. Sci. Technol. 2013, 47, 390–397. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Nakano, M.; Takamatsuc, R.; Tanida, H. Inorganic iodine incorporation into soil organic matter: Evidence from iodine K-edge X-ray absorption near-edge structure. J. Environ. Radioact. 2010, 101, 451–457. [Google Scholar] [CrossRef]
- UN Food and Agriculture Organization, Corporate Statistical Database (FAOSTAT); Food and Agriculture Organization: Rome, Italy, 2019.
- Capurso, A.; Gaetano, C.; Capurso, C. Benefits of the Mediterranean Diet in the Elderly Patient, 1st ed.; Springer: Berlin, Germany, 2018; p. 185. [Google Scholar]
- Basu, A.; Imrhan, V. Tomatoes versus lycopene in oxidative stress and carcinogenesis: Conclusions from clinical trials. Eur. J. Clin. Nutr. 2007, 61, 295–303. [Google Scholar] [CrossRef]
- Weng, H.X.; Hong, C.L.; Xia, T.H.; Bao, L.T.; Liu, H.P.; Li, D.W. Iodine biofortification of vegetable plants—An innovative method for iodine supplementation. Chinese Sci. Bull. 2013, 58, 2066–2072. [Google Scholar] [CrossRef]
- Ren, Q.; Fan, J.; Zhang, Z.; Zheng, X.; Delong, G.R. An environmental approach to correcting iodine deficiency: Supplementing iodine in soil by iodination of irrigation water in remote areas. J. Trace Elem. Med. Biol. 2008, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Caffagni, A.; Pecchioni, N.; Meriggi, P.; Bucci, V.; Sabatini, E.; Acciarri, N.; Ciriaci, T.; Pulcini, L.; Felicioni, N.; Beretta, M.; et al. Iodine uptake and distribution in horticultural and fruit tree species. Ital. J. Agron. 2012, 7, 229–236. [Google Scholar] [CrossRef]
- MSZ-08-0206/2:1978 Evaluation of Some Chemical Properties of the Soil. Laboratory Tests. (pH Value, Phenolphtalein Alkalinity Expressed in Soda, Total Water Soluble Salt Content, Hydrolytic (y1 Value) and Exchangeable Acidity (y2 Value)); Hungarian Standard Association Budapest: Budapest, Hungary, 1978. [Google Scholar]
- MSZ-08-0452:1980 Use of high-capacity analyser systems for soils analyses. Quantitative Determination of the Organic Carbon Content of the Soil on Contiflo Analyzer System; Hungarian Standard Association Budapest: Budapest, Hungary, 1980. [Google Scholar]
- Egnér, H.; Riehm, H.; Domingo, W.R. Investigations on chemical soil analysis as the basis for estimating soil fertility. II. Chemical extraction methods for phosphorus and potassium determination. K. Lantbr. Hogsk. Annlr. 1960, 26, 199–215. [Google Scholar]
- ISO 11261:1995 Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method; ISO: Geneva, Switzerland, 1995.
- MSZ 20135:1999 Determination of the Soluble Nutrient Element Content of the Soil; Hungarian Standard Association Budapest: Budapest, Hungary, 1999.
- MSZ-08-0215:1978 Determination of the Cation Adsorption Capacity of the Soil; Modified Mechlich Technique; Hungarian Standard Association Budapest: Budapest, Hungary, 1978.
- Tsimilli-Michael, M.; Strasser, R.J. In vivo assessment of stress impact on plant’s vitality: Applications in detecting and evaluating the beneficial role of mycorrhization on host plants. Mycorrhiza 2008, 3, 679–703. [Google Scholar]
- R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2019. Available online: https://www.R-project.org/ (accessed on 5 April 2020).
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. R package version 0.2.4. 2019. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 5 April 2020).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biometrical J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Germ, M.; Stibilj, V.; Šircelj, H.; Jerše, A.; Kroflič, A.; Golob, A.; Maršić, N.K. Biofortification of common buckwheat microgreens and seeds with different forms of selenium and iodine. J. Sci. Food Agric. 2019, 99, 4353–4362. [Google Scholar] [CrossRef]
- Blasco, B.; Rios, J.J.; Leyva, R.; Melgarejo, R.; Constán-Aguilar, C.; Sanchez-Rodriguez, E.; Rubio-Wilhelmi, M.M.; Romero, L.; Ruiz, J.M. Photosynthesis and metabolism of sugars from lettuce plants (Lactuca sativa L. var. longifolia) subjected to biofortification with iodine. Plant Growth Regul. 2011, 65, 137–143. [Google Scholar] [CrossRef]
- Mao, H.; Wang, J.; Wang, Z.; Zan, Y.; Lyons, G.; Zou, C. Using agronomic biofortification to boost zinc, selenium, and iodine concentrations of food crops grown on the loess plateau in China. J. Soil Sci. Plant Nut. 2014, 14, 459–470. [Google Scholar] [CrossRef]
- Halka, M.; Smoleń, S.; Ledwożyw-Smoleń, I.; Sady, W. Comparison of effects of potassium iodide and iodosalicylates on the antioxidant potential and iodine accumulation in young tomato plants. J. Plant. Growth Reg. 2020, 39, 282–295. [Google Scholar] [CrossRef]
- Ojok, J.; Omara, P.; Opolot, E.; Odongo, W.; Olum, S. Iodine agronomic biofortification of cabbage (Brassica oleracea var. capitata) and cowpea (Vigna unguiculata L.) is effective under farmer field conditions. Agronomy 2019, 9, 797. [Google Scholar] [CrossRef]
- Hageman, R.H.; Hodge, E.S.; McHargue, J.S. Effect of potassium iodide on the ascorbic acid content and growth of tomato plants. Plant Physiol. 1942, 17, 465–472. [Google Scholar] [CrossRef]
- Smoleń, S.; Rożek, S.; Ledwożyw-Smoleń, I.; Strzetelski, P. Preliminary evaluation of the influence of soil fertilization and foliar nutrition with iodine on the efficiency of iodine biofortification and chemical composition of lettuce. J. Elem. 2011, 16, 613–622. [Google Scholar] [CrossRef]
- Smoleń, S.; Skoczylas, L.; Rakoczy, R.; Ledwożyw-Smoleń, I.; Kopeć, A.; Piątkowska, E.; Bieżanowska-Kopeć, R.; Pysz, M.; Koronowicz, A.; Kapusta-Duch, J.; et al. Mineral composition of field-grown lettuce (Lactuca sativa L.) depending on the diversified fertilization with iodine and selenium compounds. Acta Sci. Pol. Hortorum Cultus 2015, 14, 97–114. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).