Germination and Emergence Responses of Alfalfa, Triticale and Quinoa Irrigated with Brackish Groundwater and Desalination Concentrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Germination Experiments
2.2. Emergence Experiment
2.3. Germination and Emergence Variables Computation
2.4. Statistical Analysis
3. Results
3.1. Germination Experiments
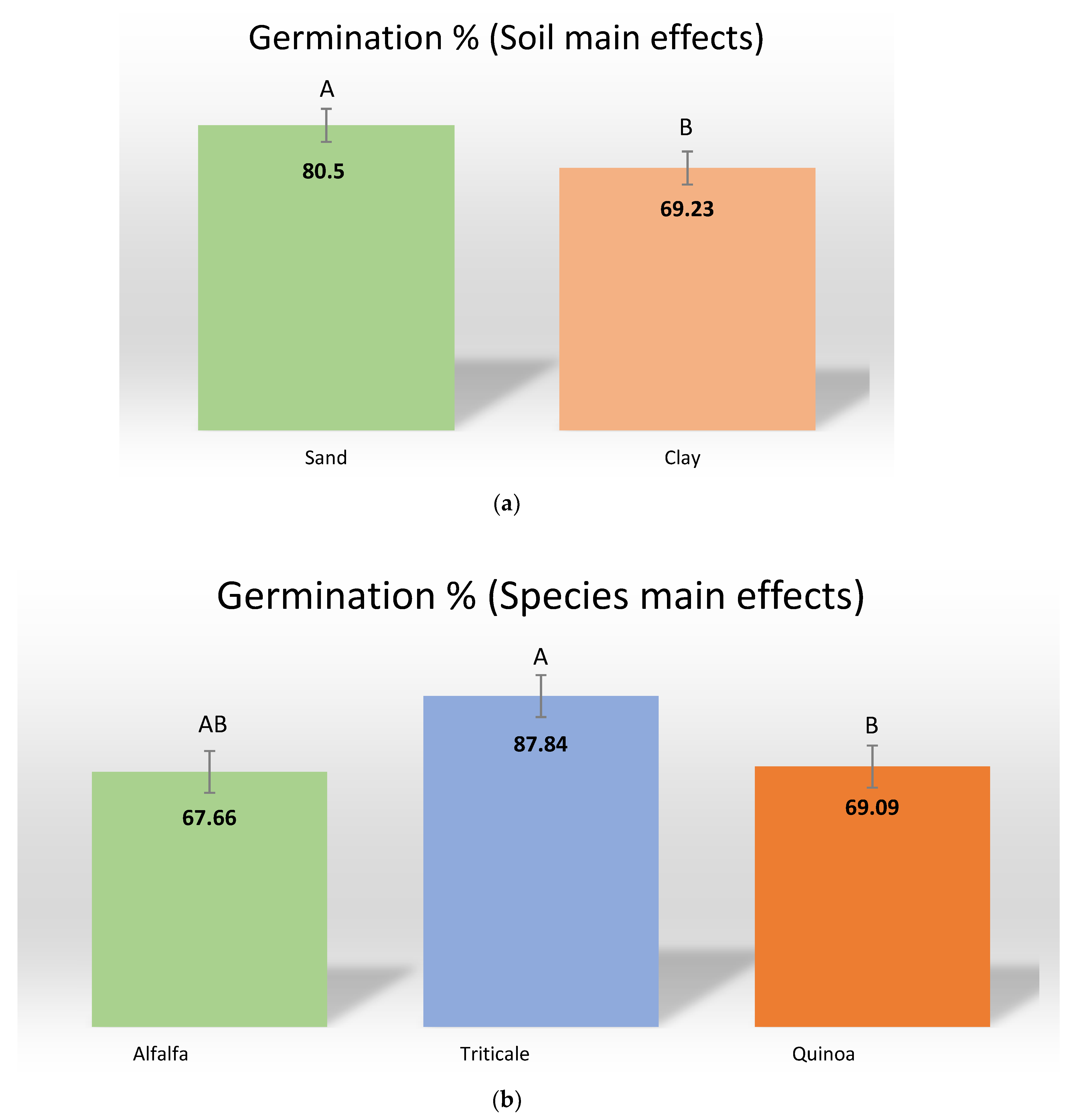
3.1.1. Germination Percent
3.1.2. Germination Index (GI), Timson’s Index (TI), and Timson’s Modified Index (Tmod)
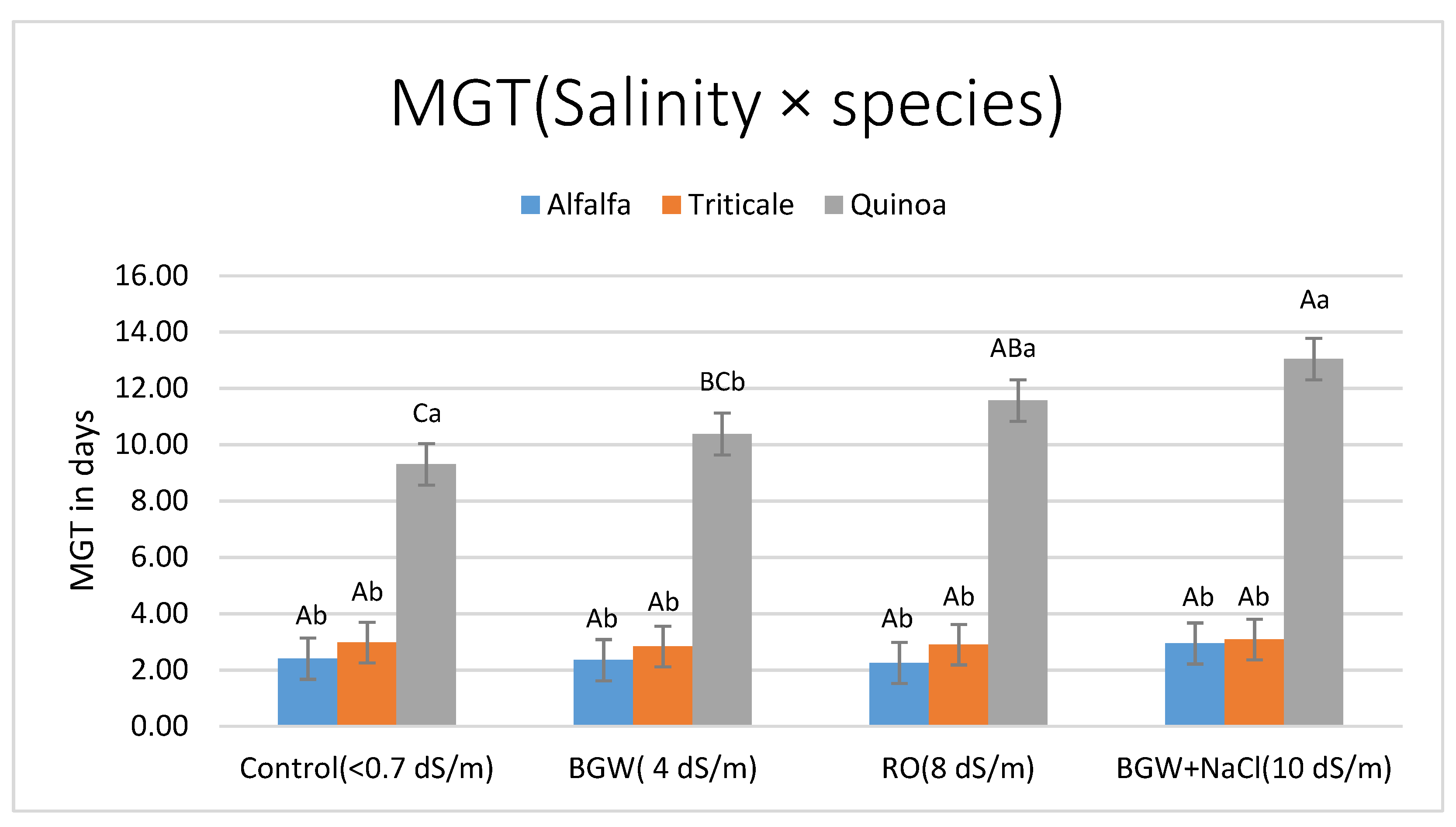
3.1.3. Mean Germination Time (MGT in Days)
3.2. Emergence Experiments
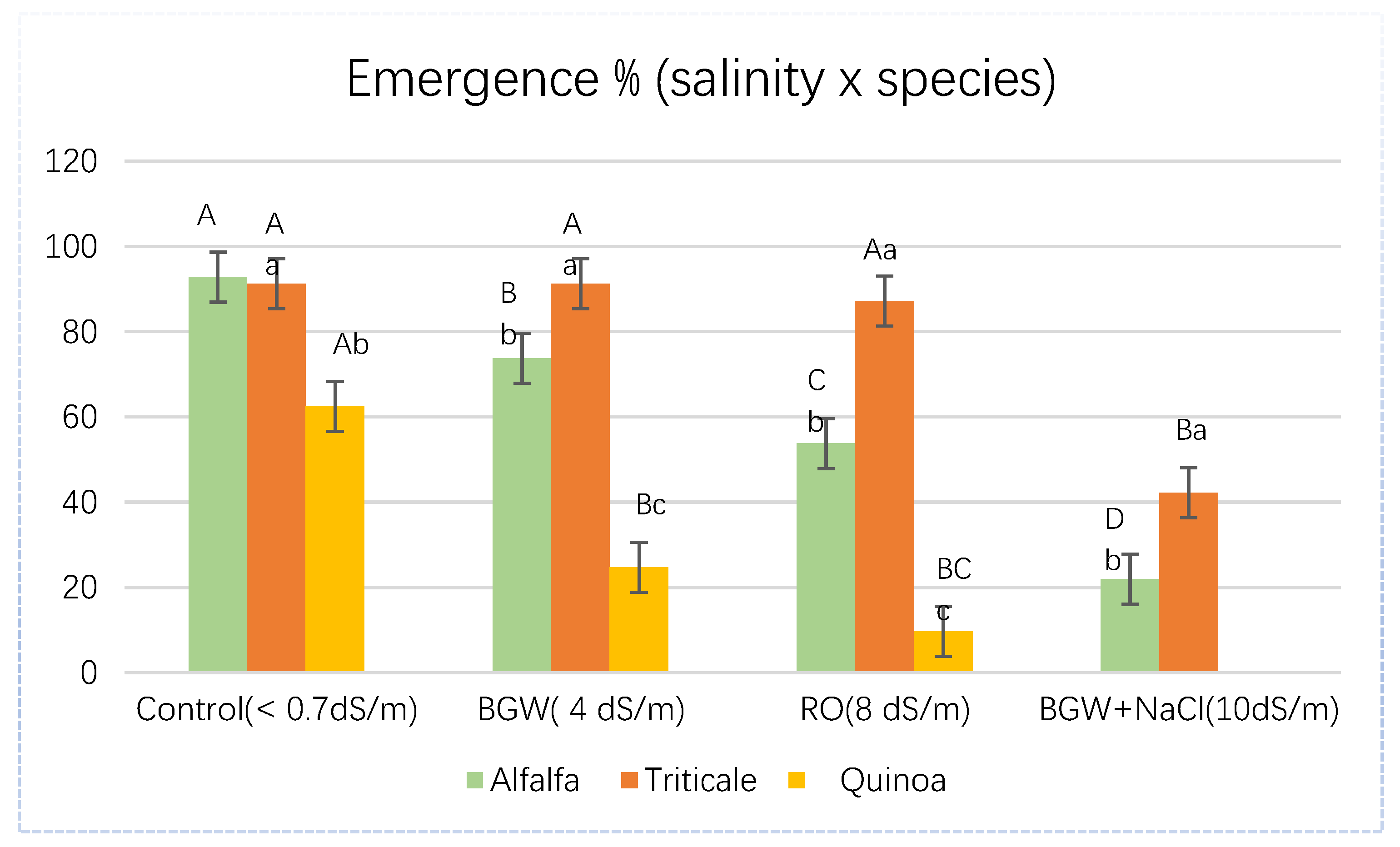
3.2.1. Emergence Percent
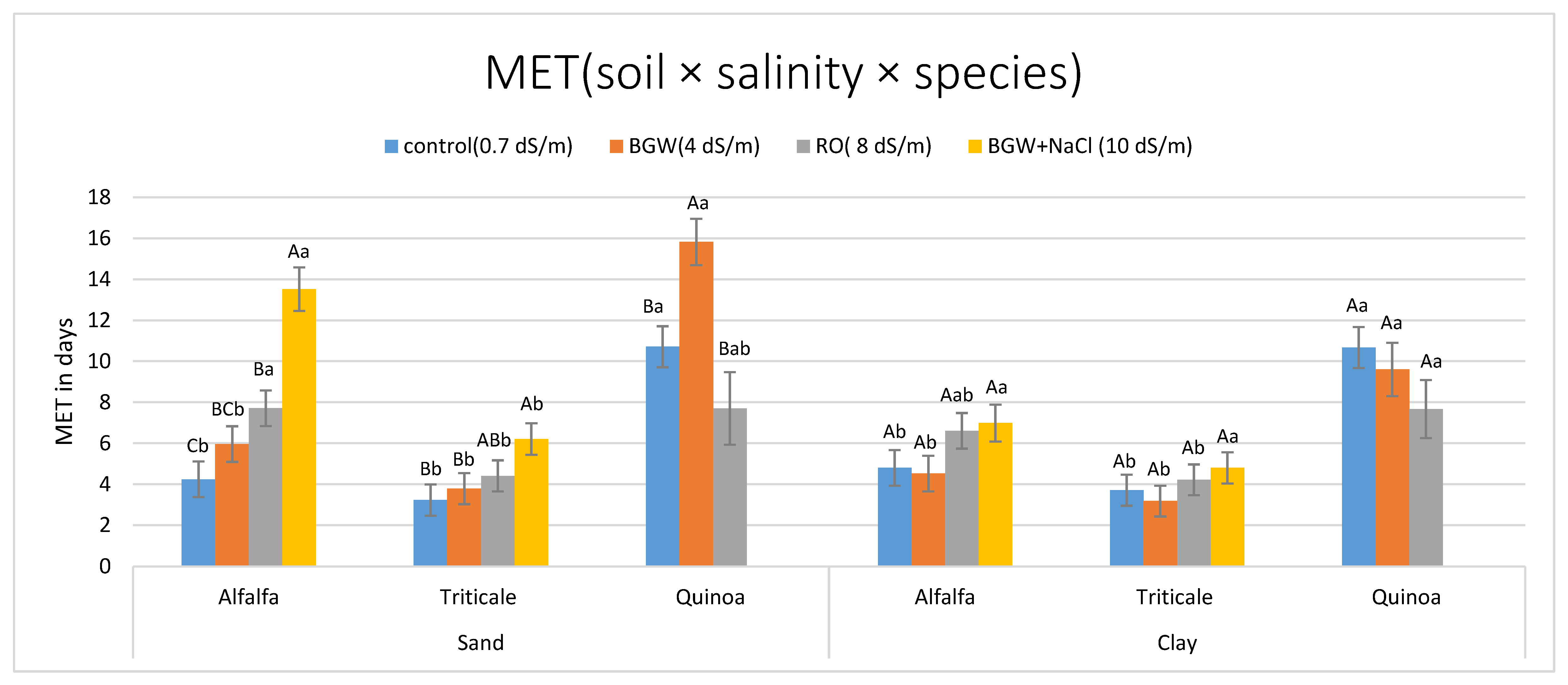
3.2.2. Mean Emergence Time (MET in Days)
3.2.3. Emergence Index (EI), Timson’s Index (TI), and Timsons Modified Index (Tmod)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- D’Antonio, C.; Myerson, L.A. Exotic plant species as problems and solutions in ecological restoration: A Synthesis. Restor. Ecol. 2002, 10, 703–713. [Google Scholar] [CrossRef]
- Herrick, J.E.; Lessard, V.C.; Spaeth, K.E.; Shaver, P.L.; Dayton, R.S.; Pyke, D.A.; Jolley, L.; Goebel, J.J. National ecosystem assessments supported by scientific and local knowledge. Front. Ecol. Environ. 2010, 8, 403–408. [Google Scholar] [CrossRef]
- Lansford, R.; Hernandez, J.; Enis, P.; Truby, D.; Mapel, C. Evaluation of Available Saline Water Resources in New Mexico for the Production of Microalgae; Solar Energy Research Institute: Golden, CO, USA, 1990.
- WRRI (Water Resources Research Institute). Transboundary Aquifers of the El/Ciudad Juarez/Las Cruces Region, VI; Texas Water Development Board and New Mexico Water Resources Research Institute, U.S. Environmental Protection Agency: Region, Philippines, 1997.
- Setter, T.L.; Waters, I. Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant Soil 2003, 253, 1–34. [Google Scholar] [CrossRef]
- Flores, A.M.; Schutte, B.J.; Shukla, M.K.; Picchioni, G.A.; Ulery, A.L. Time-integrated measurements of seed germination for salt-tolerant plant species. Seed Sci. Technol. 2015, 43, 541–547. [Google Scholar] [CrossRef]
- Flores, A.M.; Shukla, M.K.; Schutte, B.J.; Picchioni, G.; Daniel, D. Daniel Physiologic response of six plant species grown in two contrasting soils and irrigated with brackish groundwater and RO concentrate. J. Arid Land Res. Manag. 2017, 31, 182–230. [Google Scholar] [CrossRef]
- Flowers, T.J.; Läuchli, A. Sodium versus potassium: Substitution and compartmentation. In Inorganic Plant Nutrition. Encyclopedia of Plant Physiology; New Series; Läuchli, A., Bieleski, R.C., Eds.; Springer: New York, NY, USA, 1983; Volume 15B, pp. 651–681. [Google Scholar]
- Subbarao, G.V.; Ito, O.; Berry, W.L.; Wheeler, R.M. Sodium—A functional plant nutrient. Crit. Rev. Plant Sci. 2003, 22, 391–416. [Google Scholar]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Maas, E.V. Crop salt tolerance. In Agricultural Salinity Assessment and Management; ASCE Manuals and Reports on Engineering Practice No. 71; Tanji, K.K., Ed.; American Society of Civil Engineers: New York, NY, USA, 1990; pp. 262–304. [Google Scholar]
- Shalaby, E.E.; Epstein, E.; Qualset, C.O. Variation in salt tolerance among some wheat and triticale genotypes. J. Agron. Crop Sci. 1993, 171, 298–304. [Google Scholar] [CrossRef]
- Almansouri, M.; Kinet, J.M.; Lutts, S. Effect of salt and osmotic stresses on germination in durum wheat (Triticum durum Desf.). Plant Soil 2001, 231, 243–254. [Google Scholar] [CrossRef]
- Ungar, I.A. Effect of salinity on seed germination, growth, and ion accumulation of Atriplex patula (Chenopodiaceae). Am. J. Bot. 1996, 83, 604–607. [Google Scholar] [CrossRef]
- Khan, M.A.; Abdullah, Z. Salinity-sodicity induced changes in reproductive physiology of rice (Oryza sativa) under dense soil conditions. Environ. Exp. Bot. J. 2003, 49, 145–157. [Google Scholar] [CrossRef]
- Ungar, I.A. Seed germination and seed-bank ecology in halophytes. In Seed Development and Germination, Tailor and Francis; Kigel, J., Galili, G., Eds.; Marcel Dekker: New York, NY, USA, 1995; pp. 599–628. [Google Scholar]
- Khan, M.A.; Gul, B.; Weber, D.J. Seed germination in the Great Basin halophyte Salsola iberica. Can. J. Bot. 2002, 80, 650–655. [Google Scholar] [CrossRef][Green Version]
- Gutterman, Y. Seed germination in desert plants. In Adaptations of Desert Organisms; Springer: Berlin, Germany, 1993. [Google Scholar]
- Huang, Z.; Gutterman, Y. Artemisia monosperma achene germination in sand: Effects of sand depth, sand/water content, cyanobacterial sand crust and temperature. J. Arid Environ. 1998, 38, 27–43. [Google Scholar] [CrossRef]
- Huang, Z.; Gutterman, Y. Water absorption by mucilaginous achenes of Artemisia monosperma: Floating and germination affected by salt concentrations. ISR J. Plant Sci. 1999, 47, 27–34. [Google Scholar] [CrossRef]
- Badger, K.S.; Ungar, I.A. The effects of salinity and temperature on the germination of the inland halophyte Hordeum jubatum. Can. J. Bot. 1989, 67, 1420–1425. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Mueller, S.C. Consideration for successful alfalfa stand establishment in the central San Joaquin Valley. In Proceedings of the California Alfalfa and Forage Symposium, San Diego, CA, USA, 12–14 December 2005. [Google Scholar]
- Brar, G.S.; Gomez, J.F.; Matches, A.G.; Taylor, H.M.; McMichael, B.L. Root development of 12 forage legumes as affected by temperature. Agron. J. 1990, 82, 1024–1026. [Google Scholar] [CrossRef]
- Brar, G.S.; Gomez, J.F.; McMichael, B.L.; Matches, A.G.; Taylor, H.M. Tailor Germination of twenty forage legumes as influenced by temperature. Agron. J. 1991, 83, 173–175. [Google Scholar] [CrossRef]
- Klos, K.L.E. Variation in Alfalfa (Medicago sativa L.) for Germination and Seedling Vigor at Suboptimal Temperatures; and Laboratory and Field Responses to Selection within Six Alfalfa Populations. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 1999. [Google Scholar]
- Lacefield, G.D.; Henning, J.C.; Rasnake, M.; Collins, M. Alfalfa, the Queen of Forage Crops; Agr-76; Cooperative Extention Service, University Kentucky: Lexington, Kentucky, 1997. [Google Scholar]
- Lehman, W.F.; Robinson, F.E. Progress in developing salt tolerance in alfalfa. In Proceedings of the 9th California Alfalfa Symp, Fresco, CA, USA, 12–13 December 1979; University of California Cooperative Extension Service: Davis, CA, USA, 1979; pp. 73–75. [Google Scholar]
- Stone, J.E.; Marx, D.B.; Dobrenz, A.K. Interaction of sodium chloride and temperature on germination of two alfalfa cultivars. Agron. J. 1979, 75, 425–427. [Google Scholar] [CrossRef]
- Cornacchione, M.V.; Suarez, D.L.; Cornacchione, M.V.; San, I.-E.E.A. Emergence, Forage Production, and Ion Relations of Alfalfa in Response to Saline Waters, (february). Crop Sci. 2015, 55, 444–457. [Google Scholar] [CrossRef]
- Cantale, C.; Petrazzuolo, F.; Correnti, A.; Farneti, A.; Felici, F.; Latini, A.; Galeffi, P. Triticale for Bioenergy Production. Agric. Agric. Sci. Procedia 2016, 8, 609–616. [Google Scholar] [CrossRef]
- Hill, G.M. Quality: Triticale in animal nutrition. In Proceedings of the 2nd Int. Triticale Symp, Passo Fundo, Rio Grande do Sul, Brazil, 1–5 October 1990; pp. 422–427. [Google Scholar]
- Jacobsen, S.-E.; Mujica, A.; Jensen, C.R. The resistance of quinoa (Chenopodium quinoa Willd.) to adverse abiotic factors. Food Rev. Int. 2003, 19, 99–109. [Google Scholar] [CrossRef]
- Jacobsen, S.-E.; Monteros, C.; Corcuera, L.J.; Bravo, L.A.; Christiansen, J.L.; Mujica, A. Frost resistance mechanisms in quinoa (Chenopodium quinoa Willd.). Eur. J. Agron. 2007, 26, 471–475. [Google Scholar] [CrossRef]
- Jacobsen, S.-E.; Liu, F.; Jensen, C.R. Does root-sourced ABA play a role for regulation of stomata under drought in quinoa (Chenopodium quinoa Willd). Sci. Hortic. J. Elsevier 2009, 122, 281–287. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, F.; Bendevis, M.; Shabala, S.; Jacobsen, S.-E. Sensitivity of two quinoa (Chenopodium quinoa Willd.) varieties to progressive drought stress. J. Agron. Crop Sci. 2014, 200, 12–23. [Google Scholar] [CrossRef]
- Hargreaves, H.G.; Merkley, P.G. Irrigation Fundamental; Water Resources Publications, LLC: Colorado, CO, USA, 1998; pp. 90–91. [Google Scholar]
- Ranal, M.A.; Santana, D.G. How and why to measure the germination process? Rev. Bras. Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef]
- Goodchild, N.A.; Walker, M.G. A method of measuring seed germination in physiological studies. Ann. Bot. 1971, 35, 615–621. [Google Scholar] [CrossRef]
- Shannon, M.C. Adaptation of plants to salinity. In Advances in Agronomy; Donald, L.S., Ed.; Academic Press: Cambridge, MA, USA, 1997; pp. 75–120. [Google Scholar]
- Smith, S.E. Salinity and the production of alfalfa (Medicago sativa L.). In Handbook of Plant and Crop Stress; Pessarakli, M., Ed.; Marcel Dekker: New York, NY, USA, 1994; pp. 431–448. [Google Scholar]
- Ashraf, M.; Foolad, M.R. Pre-sowing seed treatment—A shotgun approach to improve germination, plant growth, and crop yield under saline and non-saline conditions. Adv. Agron. 2005, 88, 223–271. [Google Scholar] [CrossRef]
- Ungar, I.A. An ecological study of the vegetation of the Big Salt Marsh, Stafford County, Kansas. Univ. Kansas Sci. Bull. 1965, 46, 1–98. [Google Scholar]
- Ungar, I.A. Vegetation-soil relationships on saline soils in northern Kansas. Am. Midl. Nat. 1966, 78, 98–120. [Google Scholar] [CrossRef]
- Scasta, J.D.; Trostle, C.L.; Foster, M.A. Evaluating alfalfa (Medicago sativa L.) cultivars for salt tolerance using laboratory, greenhouse and field methods. J. Agric. Sci. 2012, 4, 90. [Google Scholar] [CrossRef]
- Ries, R.E.; Hofmann, L. Effect of sodium and magnesium sulfate on forage seed germination. J. Range Manag. 1983, 36, 658–662. [Google Scholar] [CrossRef]
- Wilson, C.; Read, J.J.; Abo-Kassem, E. Effect of mixed-salt salinity on growth and ion relations of a quinoa and a wheat variety. J. Plant Nutr. 2002, 25, 2689–2704. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Jacobsen, S.E.; Akhtar, S.S.; Muscolo, A. Effect of saline water on seed germination and early seedling growth of the halophyte quinoa. AoB Plants 2014, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, O.F.; Shukla, M.K.; Stringam, B.; Picchioni, G.A.; Gard, C. Irrigation with brackish water changes evapotranspiration, growth and ion uptake of halophytes. Agric. Water Manag. 2018, 195, 142–153. [Google Scholar] [CrossRef]
- Miyamoto, S.; Piela, K.; Davis, J.; Petticrew, J. Salt effects on germination and seedling emergence of several vegetable crops and guayule. Irrig. Sci. 1985, 6, 159–170. [Google Scholar] [CrossRef]
- Assadian, N.W.; Miyamoto, S. Salt Effects on Alfalfa Seedling Emergence. Agron. J. 1987, 14, 710–714. [Google Scholar] [CrossRef]
| Soils (dSm−1) | Sand (%) | Silt (%) | Clay (%) | BD (gcm−3) | Ca (meq L−1) | Mg (meq L−1) | Na (meq L−1) | K (meq L−1) | SAR | pH | EC (dSm−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sand | 83 | 5 | 12 | 1.17 | 0.46 | 0.34 | 8.37 | 2.1 | 13.18 | 6.67 | 1.43 |
| Clay | 38.96 | 29.60 | 31.44 | 1.27 | 3.04 | 2.58 | 15.3 | 26.1 | 9.13 | 7.18 | 0.73 |
| Ion Concentration | ||||||
|---|---|---|---|---|---|---|
| dSm−1 | meq L−1 | meq L−1 | meq L−1 | mg L−1 | mg L−1 | |
| Salinity | Na+ | Ca2+ | Mg2+ | K+ | Cl− | SAR |
| < 0.7 | 2.53 | 2.59 | 0.79 | 5.33 | 57.2 | 1.95 |
| 4 | 15.87 | 20.4 | 16.54 | 6.74 | 697.7 | 3.69 |
| 8 | 30.09 | 34.88 | 30.12 | 14.0 | 892.7 | 5.28 |
| 10 | 84.35 | 19.81 | 16.05 | 19.1 | 3024.3 | 19.92 |
| p-Value | |||||
|---|---|---|---|---|---|
| Effect | Germination | MGT | GI | TI | Tmod |
| Soils | 0.0279 | 0.0513 | 0.0199 | 0.0201 | 0.0910 |
| Salinity | 0.3441 | 0.0108 | 0.2136 | 0.2192 | 0.0404 |
| Soils × Salinity | 0.8631 | 0.9966 | 0.9794 | 0.9761 | 0.9973 |
| Species | 0.0465 | 0.0001 | 0.0002 | 0.0003 | 0.0026 |
| Soils × Species | 0.6657 | 0.4511 | 0.7682 | 0.7733 | 0.5827 |
| Salinity × Species | 0.3782 | 0.0343 | 0.2660 | 0.2709 | 0.2528 |
| Soils × Salinity × Species | 0.8796 | 0.9909 | 0.9057 | 0.9057 | 0.9868 |
| Lots(Species) | 0.4217 | 0.0026 | 0.3606 | 0.3616 | 0.0026 |
| Lots × Soils(Species) | 0.0500 | 0.0185 | 0.0485 | 0.0481 | 0.0188 |
| Lots × Salinity(Species) | 0.8655 | 0.1089 | 0.8782 | 0.8766 | 0.1072 |
| Lots × Soil × Salinity(Species) | 0.8890 | 0.6619 | 0.7970 | 0.8025 | 0.6610 |
| GI | TI | |
|---|---|---|
| Soils main effects | ||
| Sand | 12.03 (A) | 320.78 (A) |
| Clay | 10.28 (B) | 274.45 (B) |
| Species main effects | ||
| Alfalfa | 11.88 ± 0.79 (B) | 313.83 ± 20.98 (B) |
| Triticale | 14.99 ± 0.77 (A) | 396.73 ± 20.60 (A) |
| Quinoa | 6.60 ± 0.76 (C) | 182.28 ± 20.25 (C) |
| Tmod Values for Salinity × Species Interactions | ||||
|---|---|---|---|---|
| Salinity (dSm−1) | Alfalfa | Triticale | Quinoa | |
| < 0.7(control) | 18.60 | 18.03 | 11.70 | 16.11(A) |
| 4 | 18.65 | 18.17 | 10.62 | 15.81(A) |
| 8 | 18.75 | 18.10 | 9.42 | 15.42(AB) |
| 10 | 18.06 | 17.92 | 7.96 | 14.65(B) |
| 18.51 ± 0.44(a) | 18.05 ± 0.38(a) | 9.93 ± 0.57(b) | ||
| Soils | Species | < 0.7 dSm−1 | 4 dSm−1 | 8 dSm−1 | 10 dSm−1 |
|---|---|---|---|---|---|
| Sand | Alfalfa | 100.00 | 100.00 | 100.00 | 50.00 |
| Triticale | 100.00 | 100.00 | 100.00 | 87.50 | |
| Quinoa | 100.00 | 68.75 | 25.00 | 0.00 | |
| Clay | Alfalfa | 100.00 | 100.00 | 100.00 | 87.50 |
| Triticale | 100.00 | 100.00 | 100.00 | 93.75 | |
| Quinoa | 100.00 | 56.25 | 50.00 | 0.00 |
| Emergence Index (EI) for Salinity × Species Interaction | |||
|---|---|---|---|
| Salinity (dSm−1) | Alfalfa | Triticale | Quinoa |
| < 0.7(control) | 23.66 ± 1.24 (A) (a) | 24.22 ± 1.17 (A) (a) | 12.28 ± 1.24 (A) (b) |
| 4 | 18.15 ± 1.24 (B) (b) | 24.21 ± 1.17 (A) (a) | 6.50 ± 1.48 (B) (c) |
| 8 | 12.34 ± 1.24 (C) (b) | 22.43 ± 1.17 (A) (a) | 5.19 ± 1.98(B) (c) |
| 10 | 6.09 ± 1.42 (D) (b) | 11.46 ± 1.20 (B) (a) | - |
| Timson’s Index (TI) for Salinity × Species Interaction | |||
|---|---|---|---|
| Salinity (dSm−1) | Alfalfa | Triticale | Quinoa |
| < 0.7(control) | 245.91 ± 12.83(A) (a) | 251.31 ± 12.14 (A) (a) | 129.06 ± 12.95 (A) (b) |
| 4 | 188.87 ± 12.83(B) (b) | 251.25 ± 12.14 (A) (a) | 68.80 ± 15.46 (B) (c) |
| 8 | 128.81 ± 12.83(C) (b) | 233.00 ± 12.14 (A) (a) | 54.28 ± 20.57(B) (c) |
| 10 | 63.85 ± 14.72 (D) (b)) | 119.32 ± 12.40 (B) (a) | - |
| Tmod for Soils × Salinity × Species Interactions | ||||
|---|---|---|---|---|
| Salinity (dSm−1) | Alfalfa | Triticale | Quinoa | |
| < 0.7(control) | Sand | 26.76 ±0.87 (A)(a) | 27.77 ±0.76 (A)(a) | 20.29 ±1.00 (A)(b) |
| 4 | 25.04 ±0.87 (AB)(a) | 27.21 ±0.76 (A)(a) | 15.18 ±1.13 (B)(b) | |
| 8 | 23.29 ±0.87 (B)(b) | 26.59 ±0.76 (AB)(a) | 23.30±1.77 (A)(ab) | |
| 10 | 17.48±1.06 (C)(b) | 24.79 ±0.77 (B)(a) | - | |
| < 0.7(control) | Clay | 26.20 ±0.87 (A)(a) | 27.29 ±0.76 (A)(a) | 20.33 ±1.00 (A)(b) |
| 4 | 26.48 ±0.87 (A)(a) | 27.81 ±0.76(A)(a) | 21.40 ±1.30 (A)(b) | |
| 8 | 24.39 ±0.87 (A)(ab) | 26.77 ±0.76(A)(a) | 23.33 ±1.42 (A)(b) | |
| 10 | 24.01 ±0.90 (A)(a) | 26.20 ±0.76(A)(a) | - | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kankarla, V.; Shukla, M.K.; Picchioni, G.A.; VanLeeuwen, D.; Schutte, B.J. Germination and Emergence Responses of Alfalfa, Triticale and Quinoa Irrigated with Brackish Groundwater and Desalination Concentrate. Agronomy 2020, 10, 549. https://doi.org/10.3390/agronomy10040549
Kankarla V, Shukla MK, Picchioni GA, VanLeeuwen D, Schutte BJ. Germination and Emergence Responses of Alfalfa, Triticale and Quinoa Irrigated with Brackish Groundwater and Desalination Concentrate. Agronomy. 2020; 10(4):549. https://doi.org/10.3390/agronomy10040549
Chicago/Turabian StyleKankarla, Vanaja, Manoj K. Shukla, Geno A. Picchioni, Dawn VanLeeuwen, and Brian J. Schutte. 2020. "Germination and Emergence Responses of Alfalfa, Triticale and Quinoa Irrigated with Brackish Groundwater and Desalination Concentrate" Agronomy 10, no. 4: 549. https://doi.org/10.3390/agronomy10040549
APA StyleKankarla, V., Shukla, M. K., Picchioni, G. A., VanLeeuwen, D., & Schutte, B. J. (2020). Germination and Emergence Responses of Alfalfa, Triticale and Quinoa Irrigated with Brackish Groundwater and Desalination Concentrate. Agronomy, 10(4), 549. https://doi.org/10.3390/agronomy10040549






