The Importance of Ion Homeostasis and Nutrient Status in Seed Development and Germination
Abstract
:1. Introduction
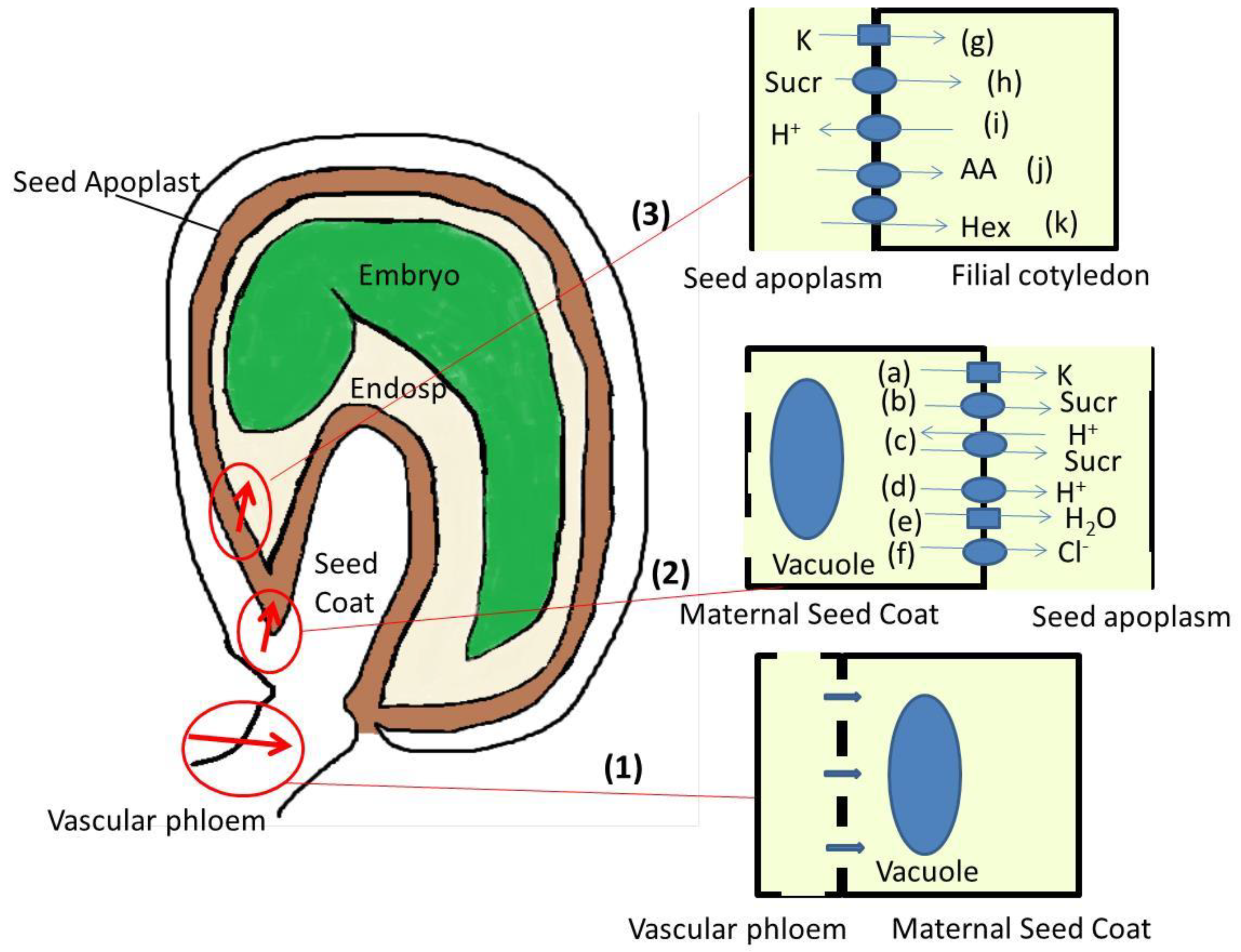
2. Ion Homeostasis during Seed Formation: From Maternal Phloem to Seed Coat
3. Importance of Soil Nutrients in Seed Germination, Development and Seed Pools
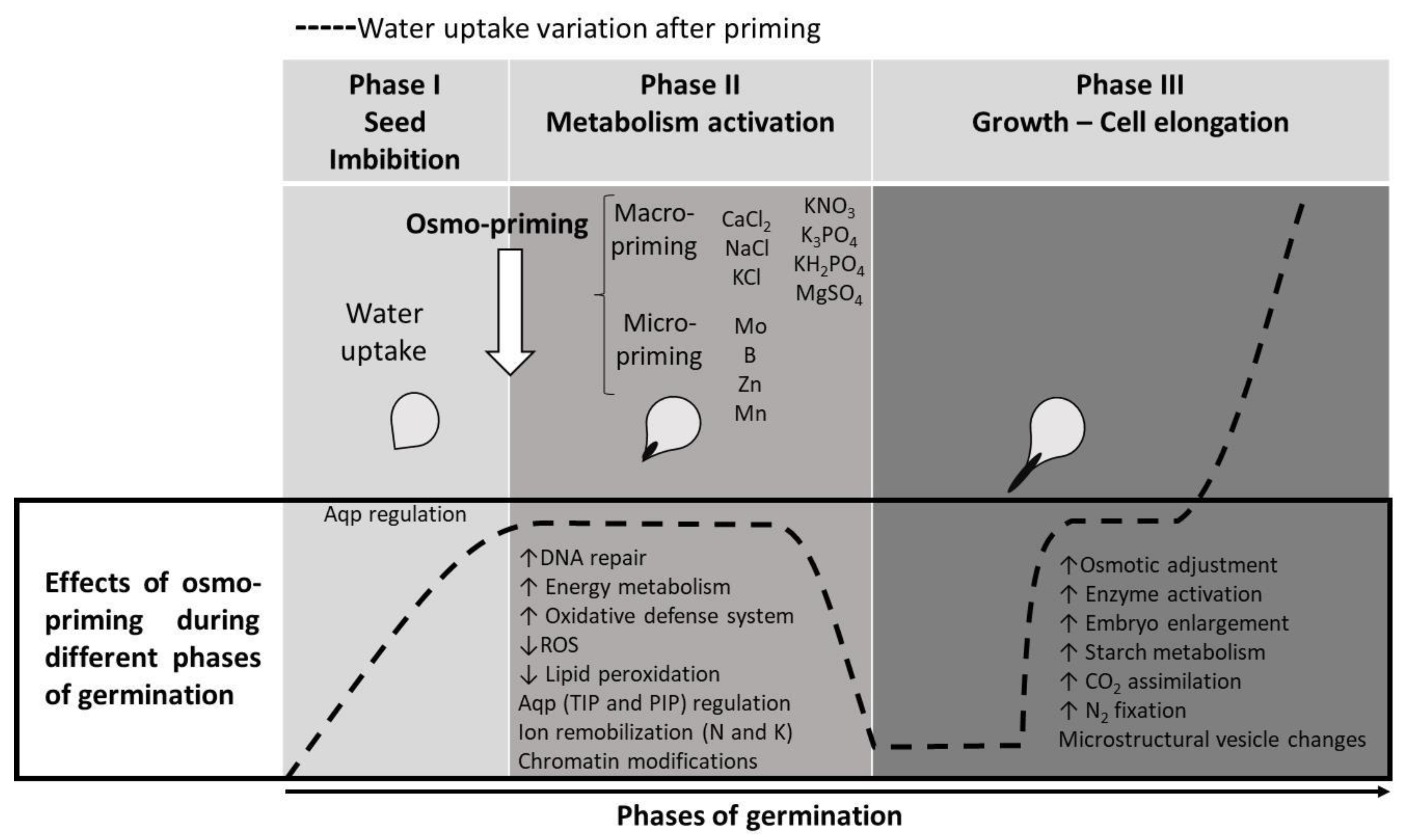
4. Effect of Seed Priming on Ion Homeostasis to Cope with Abiotic Stress
5. Heavy Metals: Effect on Seed Germination and Development
6. Mineral Seed Biofortification to Cope with Nutrient Deficiencies
7. Seed Genes to Cope with Abiotic Stress
8. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Daszkowska-Golec, A. Arabidopsis seed germination under abiotic stress as a concert of action of phytohormones. OMICS J. Integr. Biol. 2011, 15, 763–774. [Google Scholar] [CrossRef]
- Galland, M.; He, D.; Lounifi, I.; Arc, E.; Clément, G.; Balzergue, S.; Huguet, S.; Cueff, G.; Godin, B.; Collet, B.; et al. An Integrated “Multi-Omics” Comparison of Embryo and Endosperm Tissue-Specific Features and Their Impact on Rice Seed Quality. Front. Plant. Sci. 2017, 8, 1984. [Google Scholar] [CrossRef] [Green Version]
- Wen, D.; Xu, H.; Xie, L.; He, H.; Hou, H.; Zhang, C. A loose endosperm structure of wheat seed produced under low nitrogen level promotes early germination by accelerating water uptake. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Eckert, C.; Offenborn, J.N.; Heinz, T.; Armarego-Marriott, T.; Schultke, S.; Zhang, C.; Hillmer, S.; Heilmann, M.; Schumacher, K.; Bock, R.; et al. The vacuolar calcium sensors CBL2 and CBL3 affect seed size and embryonic development in Arabidopsis thaliana. Plant J. 2014, 78, 146–156. [Google Scholar] [CrossRef]
- Gómez-Ramírez, A.; López-Santos, C.; Cantos, M.; García, J.L.; Molina, R.; Cortino, J.; Espinós, J.P.; González-Elipe, A.R. Surface chemistry and germination improvement of Quinoa seeds subjected to plasma activation. Sci. Rep. 2017, 7, 5924. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.G.; Lee, A.K.; Yoon, H.K.; Park, C.M. A membrane-bound NAC transcription factor NTL8 regulates gibberellic acid-mediated salt signaling in Arabidopsis seed germination. Plant J. 2009, 55, 77–88. [Google Scholar] [CrossRef]
- Guo, J.; Wang, J.; Xi, L.; Huang, W.D.; Liang, J.; Chen, J.G. RACK1 is a negative regulator of ABA responses in Arabidopsis. J. Exp. Bot. 2009, 60, 3819–3833. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Zhang, J.; Gao, X.; Tong, J.; Xiao, L.; Li, W.; Zhang, H. The Arabidopsis AP2/ERF transcription factor RAP2.6 participates in ABA, salt and osmotic stress responses. Gene 2010, 457, 1–12. [Google Scholar] [CrossRef]
- Li, W.Y.F.; Wong, F.L.; Tsai, S.N.; Phang, T.H. Tonoplast-located GmCLC1 and GmNHX1 from soybean enhance NaCl tolerance in transgenic bright yellow (BY)-2 cells. Plant Cell Environ. 2006, 29, 1122–1137. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Zhang, N.; Zhang, Q.; Zhou, G.; Tian, H.; Hussain, S.; Ahmed, S.; Wang, T.; Wang, S. Genome editing to integrate seed size and abiotic stress tolerance traits in Arabidopsis reveals a role for DPA4 and SOD7 in the regulation of inflorescence architecture. Int. J. Mol. Sci. 2019, 20, 2695. [Google Scholar] [CrossRef] [Green Version]
- Bihmidine, S.; Hunter, C.T.; Johns, C.E.; Koch, K.E.; Braun, D.M. Regulation of assimilate import into sink organs: Update on molecular drivers of sink strength. Front. Plant. Sci. 2013, 177, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Thiel, J. Development of endosperm transfer cells in barley. Front. Plant. Sci. 2014, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- White, P.J.; Veneklaas, E.J. Nature and nurture: The importance of seed phosphorus content. Plant Soil 2012, 357, 1–8. [Google Scholar] [CrossRef]
- Moon, S.S.; Bhande, M.H.; Gajbhiye, R.P. Effect of Nitrogen and Phosphorus on Seed Quality and Seed Yield of Gaillardia. Int. J. Curr. Microbiol. Appl. Sci. 2018, 6, 1279–1283. [Google Scholar]
- Raboy, V. Approaches and challenges to engineering seed phytate and total phosphorus. Plant Sci. 2009, 177, 281–296. [Google Scholar] [CrossRef]
- Li, Y.T.; Zhang, J.; Zhang, X.; Fan, H.M.; Gu, M.; Qu, H.Y.; Xu, G.H. Phosphate transporter OsPht1; 8 in rice plays an important role in phosphorus redistribution from source to sink organs and allocation between embryo and endosperm of seeds. Plant Sci. 2015, 230, 23–32. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, Y.F.; Pei, W.X.; Jain, A.; Sun, R.; Cao, Y.; Wu, X.; Jiang, T.; Zhang, L.; Fan, X.; et al. Involvement of OsPht1; 4 in phosphate acquisition and mobilization facilitates embryo development in rice. Plant J. 2015, 82, 556–569. [Google Scholar] [CrossRef]
- Shukla, V.; Kaur, M.; Aggarwal, S.; Bhati, K.K.; Kaur, J.; Mantri, S.; Pandey, A.K. Tissue specific transcript profiling of wheat phosphate transporter genes and its association with phosphate allocation in grains. Sci. Rep. 2016, 6, 39293. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.H.; Zhou, Y.; Dibley, K.E.; Tyerman, S.D.; Furbank, R.T.; Patrick, J.W. Nutrient loading of developing seeds. Funct. Plant Biol. 2007, 34, 314–331. [Google Scholar] [CrossRef]
- Vogiatzaki, E.; Baroux, C.; Jung, J.Y.; Poirier, Y. PHO1 exports phosphate from the chalazal seed coat to the embryo in developing Arabidopsis seeds. Curr. Biol. 2017, 27, 2893–2900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrick, J.W.; Offler, C.E. Compartmentation of transport and transfer events in developing seeds. J. Exp. Bot. 2001, 52, 551–564. [Google Scholar] [CrossRef]
- Agrawal, G.K.; Hajduch, M.; Graham, K.; Thelen, J.J. In-depth investigation of the soybean seed-filling proteome and comparison with a parallel study of rapeseed. Plant Physiol. 2008, 148, 504–518. [Google Scholar] [CrossRef] [Green Version]
- Inoue, H.; Higuchi, K.; Takahashi, M.; Nakanishi, H.; Mori, H.; Nishizawa, N.K. Three rice nicotianamine synthase genes, OsNAS1, OsNAS2, and OsNAS3 are expressed in cells involved in long-distance transport of iron and differentially regulated by iron. Plant J. 2003, 36, 366–381. [Google Scholar] [CrossRef]
- Le Jean, M.; Schikora, A.; Mari, S.; Briat, J.F.; Curie, C. A loss-offunction mutation in AtYSL1 reveals its role in iron and nocotianamine seed loading. Plant J. 2005, 44, 769–782. [Google Scholar] [CrossRef]
- Waters, B.M.; Chu, H.H.; DiDonato, R.J.; Roberts, L.A.; Eisley, R.B.; Lahner, B.; Salt, D.E.; Walker, E.L. Mutations in Arabidopsis yellow stripe-like1 and yellow stripe-like3 reveal their roles in metal homeostasis and loading of metal ions in seeds. Plant Physiol. 2006, 141, 1446–1458. [Google Scholar] [CrossRef] [Green Version]
- Kruger, C.; Berkowitz, O.; Stephan, U.W.; Hell, R. A metal-binding member of the late embryogenesis abundant protein family transports iron in the phloem of Ricinus communis L. J. Biol. Chem. 2002, 277, 25062–25069. [Google Scholar] [CrossRef] [Green Version]
- Tabe, L.M.; Droux, M. Sulfur assimilation in developing lupin cotyledons could contribute significantly to the accumulation of organic sulfur reserves in the seed. Plant. Physiol. 2001, 126, 176–187. [Google Scholar] [CrossRef] [Green Version]
- Awazuhara, M.; Fujiwaa, T.; Hayashi, H.; Watanabe-Takahashi, A.; Takahashi, H.; Saito, K. The function of SULTR2;1 sulfate transporter during seed development in Arabidopsis thaliana. Physiol. Plant. 2005, 125, 95–105. [Google Scholar] [CrossRef]
- Zuber, H.; Poignavent, G.; Le Signor, C.; Aime, D.; Vieren, E.; Tadla, C.; Lugan, R.; Belghazi, M.; Labas, V.; Santoni, A.L.; et al. Legume adaptation to sulfur deficiency revealed by comparing nutrient allocation and seed traits in Medicago truncatula. Plant J. 2013, 76, 982–996. [Google Scholar] [CrossRef]
- Singh, S.P.; Vogel-Mikuš, K.; Arčon, I.; Vavpetič, P.; Jeromel, L.; Pelicon, P.; Kumar, J.; Tul, R. Pattern of iron distribution in maternal and filial tissues in wheat grains with contrasting levels of iron. J. Exp. Bot. 2013, 64, 3249–3260. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.; Chen, S.; Wang, T.; Dai, S. Proteomic insights into seed germination in response to environmental factors. Proteomics 2013, 13, 1850–1870. [Google Scholar] [CrossRef] [PubMed]
- Nagy, R.; Grob, H.; Weder, B.; Green, P.; Klein, M.; Frelet-Barrand, A.; Schjoerring, J.K.; Brarley, C.; Martinoia, E. The Arabidopsis ATP-binding cassette protein AtMRP5/AtABCC5 is a high affinity inositol hexakisphosphate transporter involved in guard cell and phytate storage. J. Biol. Chem. 2009, 284, 33614–33622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, S.; Gillaspy, G.E.; Perera, I.Y. Biosynthesis and possible functions of inositol pyrophosphates in plants. Front. Plant. Sci. 2015, 6, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belmonte, M.F.; Kirkbride, R.C.; Stone, S.L.; Pelletier, J.M.; Bui, A.Q.; Yeung, E.C.; Hashimoto, M.; Fei, J.; Harada, C.M.; Munoz, M.D.; et al. Comprehensive developmental profiles of gene activity in regions and subregions of the Arabidopsis seed. Proc. Natl. Acad. Sci. USA 2013, 110, 435–444. [Google Scholar] [CrossRef] [Green Version]
- Eroglu, S.; Giehl, R.F.H.; Meier, B.; Takahashi, M.; Terada, Y.; Ignatiev, K. Metal tolerance protein 8 mediates manganese homeostasis and iron re-allocation duringseed development and germination. Plant Physiol. 2017, 174, 1633–1647. [Google Scholar] [CrossRef] [Green Version]
- Eroglu, S. Metal transport in the developing plant seed. Adv. Bot. Res. 2018, 87, 91–113. [Google Scholar]
- Mandizvo, T.; Odindo, A.O. Seed mineral reserves and vigour of Bambara groundnut (Vigna subterranean L.) landraces differing in seed coat colour. Heliyon 2019, 5, e01635. [Google Scholar] [CrossRef] [Green Version]
- Brinch-Pedersen, H.; Sørensen, L.D.; Holm, P.B. Engineering crop plants: Getting a handle on phosphate. Trends Plant. Sci. 2002, 7, 118–125. [Google Scholar] [CrossRef]
- Waters, B.M.; Grusak, M.A. Whole-plant mineral partitioning throughout the life cycle in Arabidopsis thaliana ecotypes Columbia, Landsberg erecta, Cape Verde Islands, and the mutant line ysl1ysl3. New Phytol. 2008, 177, 389–405. [Google Scholar] [CrossRef] [Green Version]
- Roschzttardtz, H.; Conéjéro, G.; Curie, C.; Mari, S. Identification of the endodermal vacuole as the iron storage compartment in the Arabidopsis embryo. Plant. Physiol. 2009, 151, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Klein, M.A.; Grusak, M.A. Identification of nutrient and physical seed trait QTL in the model legume Lotus japonicus. Genome 2009, 52, 677–691. [Google Scholar] [CrossRef]
- Kim, S.A.; Punshon, T.; Lanzirotti, A.; Li, L.; Alonso, J.M.; Ecker, J.R.; Kaplan, J.; Guerinot, M.L. Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science 2006, 314, 1295. [Google Scholar] [CrossRef] [PubMed]
- Ariza-Nieto, M.; Blair, M.W.; Welch, R.M.; Glahn, R.P. Screening of iron bioavailability patterns in eight bean (Phaseolus vulgaris L.) genotypes using the Caco-2 cell in vitro model. J. Agric. Food Chem. 2007, 55, 7950–7956. [Google Scholar] [CrossRef] [PubMed]
- Kandari, L.S.; Kulkarni, M.G.; Van Staden, J. Effect of nutrients and smoke solutions on seed germination and seedling growth of tropical soda apple (Solanum viarum). Weed Sci. 2011, 59, 470–475. [Google Scholar] [CrossRef]
- Karimmojeni, H.; Rashidi, B.; Behrozi, D. Effect of different treatments on dormancy-breaking and germination of perennial pepperweed (Lepidium latifolium) (Brassicaceae). Aust. J. Agric. Eng. 2011, 2, 50–55. [Google Scholar]
- Kołodziejek, J. Effect of seed position and soil nutrients on seed mass, germination and seedling growth in Peucedanum oreoselinum (Apiaceae). Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Hendrix, S.D. Variation in seed weight and its effect on germination in Pastinaca sativa L. (Umbelliferae). Am. J. Bot. 1984, 71, 795–802. [Google Scholar] [CrossRef] [Green Version]
- Zampar-Toledo, M.; Arroyo-Garcia, R.; Merlin, A.; Fernandes, D.M. Seed germination and seedling development of white oat affected by silicon and phosphorus fertilization. Sci. Agric. 2011, 68, 18–23. [Google Scholar] [CrossRef]
- Inácio-Cardoso, A.I.; de Toledo, M.; Oliveira, F.; Gomes, P. Phosphate fertilization on production and quality of cauliflower seeds. Ciência Rural 2016, 46, 1337–1343. [Google Scholar] [CrossRef]
- Yang, W. Effect of nitrogen, phosphorus and potassium fertilizer on growth and seed germination of Capsella bursa-pastoris (L.) Medikus. J. Plant Nutr. 2018, 41, 636–644. [Google Scholar] [CrossRef]
- Planes, M.D.; Niñoles, R.; Rubio, L.; Bissoli, G.; Bueso, E.; García-Sánchez, M.J.; Alejandro, S.; Gonzalez-Guzmán, M.; Hedrich, R.; Rodriguez, P.L.; et al. A mechanism of growth inhibition by abscisic acid in germinating seeds of Arabidopsis thaliana based on inhibition of plasma membrane H+-ATPase and decreased cytosolic pH, K+, and anions. J. Exp. Bot. 2015, 66, 813–825. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Yoo, J.H.; Depuydt, S.; Oh, J.W.; Yo, Y.M.; Kim, K.; Brown, M.T.; Han, T. The sensitivity of a hydroponic lettuce root elongation bioassay to metals, phenol and wastewaters. Ecotoxicol. Environ. Saf. 2016, 126, 147. [Google Scholar] [CrossRef] [PubMed]
- Kundrát, J.T.; Gyulai, I.; Simon, E.; Mizsei, E.; Braun, M.; Tóthmérész, B. Study of the effects of high levels of nutrients on seed germination and root elongation. Pol. J. Environ. Stud. 2017, 26, 1–6. [Google Scholar] [CrossRef]
- Suwa, R.; Jayachandran, K.; Nguyen, N.T.; Boulenouar, A.; Fujita, K.; Saneoka, H. Barium toxicity effects in soybean plants. Arch. Environ. Contam. Toxicol. 2008, 55, 397. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Huang, D.; Liu, J. Functions and toxicity of nickel in plants: Recent advances and future prospects. Clean-Soil Air Water 2009, 37, 304. [Google Scholar] [CrossRef]
- Brunel-Muguet, S.; D’Hooghe, P.; Bataillé, M.P.; Larre, C.; Kim, T.H.; Trouverie, J.; Avice, J.C.; Etienne, P.; Dürr, C. Heat stress during seed filling interferes with sulfur restriction on grain composition and seed germination in oilseed rape (Brassica napus L.). Front. Plant Sci. 2015, 6, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, M.; Ashraf, M. Gibberellic acid mediated induction of salt tolerance in wheat plants: Growth, ionic partitioning, photosynthesis, yield and hormonal homeostasis. Environ. Exp. Bot. 2013, 86, 76–85. [Google Scholar] [CrossRef]
- De Oliveira, A.B.; Gomes-Filho, E. How are germination performance and seedling establishment under abiotic stress improved by seed priming? A review. Aust. J. Crop Sci. 2016, 10, 1047–1051. [Google Scholar] [CrossRef]
- Di Girolamo, G.; Barbanti, L. Treatment conditions and biochemical processes influencing seed priming effectiveness. Ital. J. Agron. 2012, 7, 8–18. [Google Scholar] [CrossRef] [Green Version]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant. 2013, 35, 381–1396. [Google Scholar] [CrossRef]
- Lutts, S.; Benincasa, P.; Wojtyla, L.; Kubala, S.; Pace, R.; Lechowska, K.; Quinet, M.; Garnczarska, M. Seed priming: New comprehensive approaches for an old technique. In New Challenges in Seed Biology—Basic and Translational Research Driving Seed Technology; IntechOpen: London, UK, 2016; ISBN 978-953-51-2659-1. [Google Scholar]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Basra, S.M.A.; Hafeez, K. Seed invigoration by osmohardening in coarse and fine rice (Oryza sativa L.). Seed Sci. Technol. 2006, 34, 181–187. [Google Scholar] [CrossRef]
- Zhou, D.; Moxin Xiao, M. Specific ion effects on the seed germination of sunflower. J. Plant Nutr. 2010, 33, 255–266. [Google Scholar] [CrossRef]
- Lechowska, K.; Kubala, S.; Wojtyla, Ł.; Nowaczyk, G.; Quinet, M.; Lutts, S.; Garnczarska, M. New insight on water status in germinating Brassica napus seeds in relation to priming-improved germination. Int. J. Mol. Sci. 2019, 20, 540. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.B.; Prisco, J.T.; Enéas-Filho, J.; Gomes-Filho, E. Salinity effects on germination and establishment of sorghum seedlings from artificially aged and primed seeds. J. New Seeds 2010, 11, 399–411. [Google Scholar] [CrossRef]
- Kubala, S.; Wojtyla, Ł.; Quinet, M.; Lechowska, K.; Lutts, S.; Garnczarska, M. Enhanced expression of the proline synthesis gene P5CSA in relation to seed osmopriming improvement of Brassica napus germination under salinity stress. J. Plant Physiol. 2015, 183, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Tao, Y.; Hussain, S.; Jiang, Q.; Peng, S.; Huang, J.; Cui, K.; Nie, L. Seed priming in dry direct-seeded rice: Consequences for emergence, seedling growth and associated metabolic events under drought stress. Plant. Growth Regul. 2015, 78, 167–178. [Google Scholar] [CrossRef]
- Espanany, A.; Fallah, S.; Tadayyon, A. Seed priming improves seed germination and reduces oxidative stress in black cumin (Nigella sativa) in presence of cadmium. Ind. Crops Prod. 2016, 79, 195–204. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, F.; Cao, W.; Wu, L.; Mingjian Geng, M. Seed Priming Alters the Production and detoxification of reactive oxygen intermediates in rice seedlings grown under sub-optimal temperature and nutrient supply. Front. Plant Sci. 2016, 7, 439. [Google Scholar] [CrossRef] [Green Version]
- Khan, E.; Gupta, M. Arsenic–silicon priming of rice (Oryza sativa L.) seeds influence mineral nutrient uptake and biochemical responses through modulation of Lsi-1, Lsi-2, Lsi-6 and nutrient transporter genes. Sci. Rep. 2018, 8, 10301. [Google Scholar] [CrossRef] [Green Version]
- Chiu, K.Y.; Chuang, S.J.; Sung, J.M. Both anti-oxidation and lipid-carbohydrate conversion enhancements are involved in priming-improved emergence of Echinacea purpurea seeds that differ in size. Sci. Hortic. Amst. 2006, 108, 220–226. [Google Scholar] [CrossRef]
- Conrtah, U. Molecular aspects of defence priming. Trends Plant Sci. 2011, 16, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Shen, Y.; Huan, B. Osmoregulants involved in osmotic adjustment for differential drought tolerance in different Bentgrass genotypes. J. Am. Soc. Hortic. Sci. 2015, 140, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Jafar, M.Z.; Farooq, M.; Cheema, M.A.; Afzal, I.; Basra, S.M.A.; Wahid, M.A.; Azid, T.; Shahid, M. Improving the performance of wheat by seed priming under saline conditions. J. Agric. Crop Sci. 2012, 198, 38–45. [Google Scholar] [CrossRef]
- Zhang, Q.; Rue, K. Glycinebetaine seed priming improved osmotic and salinity tolerance in turfgrasses. Hortscience 2012, 47, 1171–1174. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.B.; Alencar, N.L.M.; Prisco, J.T.; Gomes-Filho, E. Accumulation of organic and inorganic solutes in NaCl-stressed sorghum seedlings from aged and primed seeds. Sci. Agrícola 2011, 68, 632–663. [Google Scholar] [CrossRef] [Green Version]
- Bonilla, I.; El-Hamdaoui, A.; Bolaños, L. Boron and calcium increase Pisum sativum seed germination and seedling development under salt stress. Plant Soil 2004, 267, 97–107. [Google Scholar] [CrossRef]
- Zaman, B.U.; Ali, A.; Hyder, S.I.; Arshadullah, M.; Bhatti, S.U. Potassium chloride as a nutrient seed primer to enhance salt-tolerance in maize. Pesqui. Agropecuária Bras. 2012, 47, 1181–1184. [Google Scholar] [CrossRef] [Green Version]
- Majda, C.; Khalid, D.; Aziz, A.; Rachid, B.; Badr, A.S.; Lotfi, A.; Mohamed, B. Nutri-priming as an efficient means to improve the agronomic performance of molybdenum in common bean (Phaseolus vulgaris L.). Sci. Total Environ. 2019, 661, 654–663. [Google Scholar] [CrossRef]
- Imran, M.; Volker, R.; Neumann, G. Accumulation and distribution of Zn and Mn in soybean seeds after nutrient seed priming and its contribution to plant growth under Zn and Mn deficient conditions. J. Plant Nutr. 2017, 40, 695–708. [Google Scholar]
- Rehman, A.; Farooq, M.; Naveed, M.; Nawaz, A.; Shahzad, B. Seed priming of Zn with endophytic bacteria improves the productivity and grain biofortification of bread wheat. Eur. J. Agron. 2018, 94, 98–107. [Google Scholar] [CrossRef]
- Ghiyasi, S.; Moghaddam, S.S.; Amirnia, R.; Damalas, A.C. Chemical priming with salt and urea improves germination and seedling growth of black cumin (Nigella sativa L.) under osmotic stress. J. Plant Growth Reg. 2019. [Google Scholar] [CrossRef]
- Sethy, S.K.; Ghosh, S. Effect of heavy metals on germination of seeds. J. Nat. Sci. Biol. Med. 2013, 4, 272–275. [Google Scholar] [PubMed] [Green Version]
- Divol, F.; Couch, D.; Conéjéro, G.; Roschzttardtz, H.; Mari, S.; Curie, C. The Arabidopsis YELLOW STRIPE LIKE4 and 6 transporters control iron release from the chloroplast. Plant Cell 2013, 25, 1040–1055. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Khan, M.A.; Yamaguchi, S.; Kamiya, Y. Effects of heavy metals on seed germination and early seedling growth of Arabidopsis thaliana. Plant Growth Reg. 2005, 46, 45–50. [Google Scholar] [CrossRef]
- Seregin, I.V.; Kozhevnikova, A.D. Distribution of cadmium, lead, nickel, and strontium in imbibing maize Caryopses. Russ. J. Plant Physlol. 2005, 52, 565–569. [Google Scholar] [CrossRef]
- Samantaray, S.; Rout, G.R.; Das, P. Role of chromium on plant growth and metabolism. Acta Physiol. Plant. 1998, 20, 201–212. [Google Scholar] [CrossRef]
- Ahsan, N.; Lee, D.G.; Lee, S.H.; Kang, K.Y.; Lee, J.J.; Kim, P.J.; Yoon, H.S.; Kim, J.S.; Lee, B.H. Excess copper induced physiological and proteomic changes in germinating rice seeds. Chemosphere 2007, 67, 1182–1193. [Google Scholar] [CrossRef]
- Ahsan, N.; Lee, S.H.; Lee, D.G.; Lee, H.; Lee, S.W.; Bahk, J.D.; Lee, B.H. Physiological and protein profiles alternation of germinating rice seedlings exposed to acute cadmium toxicity. Comptes Rendus Biol. 2007, 330, 735–746. [Google Scholar] [CrossRef]
- Karmous, I.; Ferjani, E.E.; Chaoui, A. Copper excess impairs mobilization of storage proteins in bean cotyledons. Biol. Trace Elem. Res. 2011, 144, 1251–1259. [Google Scholar] [CrossRef]
- Jaouani, K.; Chaoui, A.; Ferjani, E.E. Alteration of cotyledonary globulins and albumins mobilization in pea exposed to cadmium. Sci. Res. Essays 2012, 7, 1273–1279. [Google Scholar]
- Ahmad, M.S.; Ashraf, M. Essential roles and hazardous effects of nickel in plants. Rev. Environ. Contam. Toxicol. 2011, 214, 125–167. [Google Scholar]
- Singh, H.P.; Kaur, G.; Batish, D.R.; Kohli, R.K. Lead (Pb)-inhibited radicle emergence in Brassica campestris involves alterations in starch-metabolizing enzymes. Biol. Trace Elem. Res. 2011, 144, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Kalai, T.; Khamassi, K.; Teixeira da Silva, J.A.; Gouia, H.; Bettaieb Ben-Kaab, L. Cadmium and copper stress affect seedling growth and enzymatic activities in germinating barley seeds. Arch. Agron. Soil Sci. 2013, 60, 765–783. [Google Scholar] [CrossRef]
- Nath, K.; Singh, D.H.; Shyam, S.; Sharma, Y.K. Effect of chromium and tannery effluent toxicity on metabolism and growth in cowpea (Vigna sinensis L. Saviex Hassk) seedling. Res. Environ. Life Sci. 2008, 1, 91–94. [Google Scholar]
- Smiri, M.; Chaoui, A.; Ferjani, E.E. Respiratory metabolism in the embryonic axis of germinating pea seed exposed to cadmium. J. Plant Physiol. 2009, 166, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zong, K.; Jiang, L.; Sun, J.; Ren, Y.; Sun, Z.; We, C.; Chen, X.; Cao, S. Characterization of an Arabidopsis cadmium-resistant mutant cdr3-1D reveals a link between heavy metal resistance as well as seed development and flowering. Planta 2011, 233, 697–706. [Google Scholar] [CrossRef]
- Ryan, J.; Miyamoto, S.; Stroehlein, J.L. Salt and specific ion effects on germination of four grass. J. Range Manag. 1975, 28, 61–64. [Google Scholar] [CrossRef]
- Guan, B.; Zhou, D.; Zhang, H.; Tian, Y.; Japhet, W.; Wang, P. Germination responses of Medicago ruthenica seeds to salinity, alkalinity, and temperature. J. Arid Environ. 2009, 73, 135–138. [Google Scholar] [CrossRef]
- Bosker, T.; Bouwman, L.J.; Brun, N.R.; Behrens, P.; Vijver, M.G. Microplastics accumulate on pores in seed capsule and delay germination and root growth of the terrestrial vascular plant Lepidium sativum. Chemosphere 2019, 226, 774–781. [Google Scholar] [CrossRef]
- Garg, M.; Sherma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified crops generated by breeding, agronomy, and transgenic approaches are improving lives of millions of people around the world. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E.; Allen, L.H.; Bhutta, Z.A.; Caulfield, L.E.; de Onis, M.; Ezzati, M.; Mathers, C.; Rivera, J. Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: Global and regional exposures and health consequence. Lancet 2008, 371, 243–260. [Google Scholar] [CrossRef]
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves Junior, A.C.; Nacke, H.; Marengoni, N.G.; Carvalho, E.A.; Coelho, G.F. Produtividade e componentes de produção da soja adubada com diferentes doses de fósforo, potássio e zinco. Ciência e Agrotecnologia 2010, 34, 660–666. [Google Scholar] [CrossRef]
- Smith, M.R.; Golden, C.D.; Myers, S.S. Potential rise in iron deficiency due to future anthropogenic carbon dioxide emissions. GeoHealth 2017, 1, 248–257. [Google Scholar] [CrossRef]
- Rengel, Z.; Graham, R.D. Wheat genotypes differ in Zn efficiency when grown in chelate-buffered nutrient solution. I. Growth. Plant Soil 1995, 176, 307–316. [Google Scholar] [CrossRef]
- Rengel, Z.; Graham, R.D. Wheat genotypes differ in Zn efficiency when grown in chelate-buffered nutrient solution. II. Nutrient uptake. Plant Soil 1995, 176, 317–324. [Google Scholar] [CrossRef]
- Cakmak, I.; Kalaycı, M.; Ekiz, H.; Braun, H.J.; Kılınç, Y.; Yılmaz, A. Zinc deficiency as a practical problem in plant and human nutrition in Turkey: A NATO-science for stability project. Field Crops Res. 1999, 60, 175–188. [Google Scholar] [CrossRef] [Green Version]
- Uauy, C.; Distelfeld, A.; Fahima, T.; Blechl, A.; Dubcovsky, J.A. NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 2006, 314, 1298–1301. [Google Scholar] [CrossRef] [Green Version]
- Toklu, F.; Ozkan, H.; Karayoy, T.; Coyne, C.J. Evaluation of advanced lentil lines for diversity in seed mineral concentration, grain yield and yield components. J. Agric. Sci. 2017, 23, 213–222. [Google Scholar]
- Waters, B.M.; Sankaran, R.P. Moving micronutrients from the soil to the seeds: Genes and physiological processes from a biofortification perspective. Plant Sci. 2011, 180, 562–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, M.; Nozoye, T.; Kitajima, N.; Fukuda, N.; Hokura, A.; Terada, Y.; Nakai, I.; Ishimaru, Y.; Kobayashi, T.; Nakanishi, H.; et al. In vivo analysis of metal distribution and expression of metal transporters in rice seed during germination process by microarray and X-ray Fluorescence Imaging of Fe, Zn, Mn, and Cu. Plant Soil 2009, 325, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Welch, R.M.; Graham, R.D. Breeding for micronutrients in staple food crops from a human nutrition perspective. J. Exp. Bot. 2004, 55, 353–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slamet-Loedin, I.H.; Johnson-Beebout, S.E.; Impa, S.; Tsakirpaloglou, N. Enriching rice with Zn and Fe while minimizing Cd risk. Front. Plant. Sci. 2015, 6, 121. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, L.; Slamet, I.H. Genetic biofortification to enrich rice and wheat grain iron: From genes to product. Front. Plant Sci. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Kim, S.; Tsukamoto, T.; Oki, H.; Kobayashi, T.; Watanabe, S.; Matsuhashi, S.; Takahashi, M.; Nakanishi, H.; Mori†, S.; et al. Mutational reconstructed ferric chelate reductase confers enhanced tolerance in rice to iron deficiency in calcareous soil. Proc. Natl. Acad. Sci. USA 2007, 104, 7373–7378. [Google Scholar] [CrossRef] [Green Version]
- Holme, I.B.; Dionisio, G.; Brinch-Pedersen, H.; Wendt, T.; Madsen, C.K.; Vincze, E.; Holm, P.B. Cisgenic barley with improved phytase activity. Plant Biotechnol. J. 2012, 10, 237–247. [Google Scholar] [CrossRef]
- Guttieri, M.; Bowen, D.; Dorsch, J.A.; Raboy, V.; Souza, E. Identification and characterization of a low phytic acid wheat. Crop Sci. 2004, 44, 418–424. [Google Scholar] [CrossRef]
- Pilu, R.; Panzeri, G.; Gavazzi, G.; Rasmussen, S.; Consonni, G.; Nielsen, E. Phenotypic, genetic and molecular characterization of a maize low phytic acid mutant (lpa241). Theor. Appl. Genet. 2003, 107, 980–987. [Google Scholar] [CrossRef]
- Oltmans, S.E.; Fehr, W.R.; Welke, G.A.; Raboy, V.; Peterson, K.L. Agronomic and seeds traits of soybean lines with low-phytate phosphorus. Crop Sci. 2005, 45, 593–598. [Google Scholar] [CrossRef]
- Conte, S.S.; Walker, E.L. Transporters contributing to iron trafficking in plants. Mol. Plant 2011, 4, 464–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regvar, M.; Eichert, D.; Kaulich, B.; Gianoncelli, A.; Pongrac, P.; Vogel-Mikuš, K.; Kreft, I. New insights into globoids of protein storage vacuoles in wheat aleurone using synchrotron soft X-ray microscopy. J. Exp. Bot. 2011, 62, 3929–3939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Yang, M.; Li, H.; Li, D.; Shi, X.; Zhang, Y. Genetic processes of iron and zinc accumulation in edible portion of crops and their agro-biofortification: A review. Am. J. Agric. For. 2017, 5, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.Y.; Gruissem, W.; Bhullar, N.K. Targeting intracellular transport combined with efficient uptake and storage significantly increases grain iron and zinc levels in rice. Plant Biotechnol. J. 2019, 17, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Bashir, K.; Takahashi, R.; Akhtar, S.; Ishimaru, Y.; Nakanishi, H.; Nishizawa, N.K. The knockdown of OsVIT2 and MIT affects iron localization in rice seed. Rice 2013, 6, 31. [Google Scholar] [CrossRef] [Green Version]
- Subramanyam, K.; Laing, G.D.; Els, J.M.; Van Damme, E.J.M. Sodium selenate treatment using a combination of seed priming and foliar spray alleviates salinity stress in rice Front. Plant. Sci. 2019, 10, 116. [Google Scholar]
- Iqbal, M.; Hussain, I.; Liaqat, H.; Ashraf, M.A.; Rasheed, R.; Rehman, A.U. Exogenously applied selenium reduces oxidative stress and induces heat tolerance in spring wheat Plant Physiol. Biochem. 2015, 94, 95–103. [Google Scholar]
- Pazurkiewicz-Kocot, K.; Galas, W.; Kita, A. The effect of selenium on the accumulation of some metals in Zea mays L. plants treated with indole-3-acetic acid. Cell. Mol. Biol. Lett. 2003, 8, 97–103. [Google Scholar]
- Sohindji, F.S.; Sogbohossou, D.E.O.; Zohoungbogbo, H.P.E.; Houdegbe, C.A.; Achigan-Dako, E.G. Understanding molecular mechanisms of seed dormancy for improved germination in traditional leafy vegetables: An overview. Agronomy 2020, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Vishal, B.; Kumar, P.P. Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A.; Collin, A.; Marzec, M.; Slota, M.; Kurowska, M.; Gajecka, M.; Gajewska, P.; Płociniczak, T.; Sitko, K.; Pacak, A.; et al. Mutation in HvCBP20 (cap binding protein 20) adapts barley to drought stress at phenotypic and transcriptomic levels. Front. Plant Sci. 2017, 8, 942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Zhu, H.; Pan, Y.; Yu, Y.; Luan, S.; Li, L. A DTX/MATE-type transporter facilitates abscisic acid efflux and modulates ABA sensitivity and drought tolerance in Arabidopsis. Mol. Plant 2014, 7, 1522–1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, D.-E.; Hou, P.; Xiao, F.; Liu, Y. Overexpression of Arabidopsis XERICO gene confers enhanced drought and salt stress tolerance in rice (Oryza sativa L.). J. Plant Biochem. Biotechnol. 2015, 24, 56–64. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, P.; Verma, V.; Stamm, P.; Kumar, P.P. A novel RGL2-DOF6 complex contributes to primary seed dormancy in Arabidopsis thaliana by regulating a GATA transcription factor. Mol. Plant. 2017, 10, 1307–1320. [Google Scholar] [CrossRef] [Green Version]
- Yan, A.; Wu, M.; Yan, L.; Hu, R.; Ali, I.; Gan, Y. AtEXP2 is involved in seed germination and abiotic stress response in Arabidopsis. PLoS ONE 2014, 9, e85208. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Parkhey, S.; Naithani, S.C.; Keshavkant, S. ROS production and lipid catabolism in desiccating Shorea robusta seeds during aging. Plant Physiol. Biochem. 2012, 57, 261–267. [Google Scholar] [CrossRef]
- Kanwischer, M.; Porfirova, S.; Bergmuller, E.; Dormann, P. Alterations in tocopherol cyclase activity in transgenic and mutant plants of Arabidopsis affect tocopherol content, tocopherol composition, and oxidative stress. Plant Physiol. 2005, 137, 713–723. [Google Scholar] [CrossRef] [Green Version]
- Sharma, I.; Pati, P.K.; Bhardwaj, R. Effect of 28-homobrassinolide on antioxidant defence system in Raphanus sativus L. under chromium toxicity. Ecotoxicology 2011, 20, 862–874. [Google Scholar] [CrossRef]
- Zhang, J.; Shu, W.S. Mechanisms of heavy metal cadmium tolerance in plants. Physiological and transcriptomic responses in the seed coat of field-grown soybean (Glycine max L. Merr.) to abiotic stress. J. Plant Physiol. Mol. Biol. 2006, 32, 1–8. [Google Scholar] [PubMed]
- Leisner, C.P.; Craig, R.; Yendrek, C.R.; Ainsworth, E.A. Physiological and transcriptomic responses in the seed coat of field-grown soybean (Glycine max L. Merr.) to abiotic stress. BMC Plant Biol. 2017, 17, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimotho, R.J.; Baillo, E.H.; Zhan, Z. Transcription factors involved in abiotic stress responses in Maize (Zea mays L.) and their roles in enhanced productivity in the post genomics era. PeerJ 2019, 1–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sujeeth, N.; Mehterov, N.; Gupta, S.; Qureshi, M.K.; Fischer, A.; Proost, S.; Omidbakhshfard, M.A.; Obata, T.; Benina, M.; Staykov, N.; et al. A novel seed plants gene regulates oxidative stress tolerance in Arabidopsis thaliana. Cell. Mol. Life Sci. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, D.M.; Yang, G.D.; Wu, C.A.; Zheng, C.C. Seed-specific overexpression of antioxidant genes in Arabidopsis enhances oxidative stress tolerance during germination and early seedling growth. Plant. Biotechnol. J. 2010, 8, 796–806. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, K.L.; Li, T.D.; Zhang, Y.; Wang, Y.P.; Zhao, Q.; Liu, J.X.; Zhang, H.W.; Liu, C.M.; Ran, Y.D.; et al. Efficient DNA-free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nat. Commun. 2017, 8, 14261. [Google Scholar] [CrossRef]
- Zhu, J.; Song, N.; Sun, S.; Yang, W.; Zhao, H.; Song, W.; Lai, J. Efficiency and inheritance of targeted mutagenesis in maize using CRISPR-Cas9. J. Genet. Genom. 2016, 43, 25–36. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.B.; Xing, A.; Moon, B.P.; Koellhoffer, J.P.; Huang, L.; Ward, R.T.; Clifton, E.; Falco, S.C.; Cigan, A.M. Cas9-guide RNA directed genome editing in soybean. Plant Physiol. 2015, 169, 960–970. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Chen, L.; Liu, X.; Sun, S.; Wu, C.; Jiang, B.; Han, T.; Hou, W. CRISPR/Cas9-mediated genome editing in soybean hairy roots. PLoS ONE 2015, 10, e0136064. [Google Scholar] [CrossRef]
- Shao, G.N.; Xie, L.H.; Jiao, G.A.; Wei, X.J.; Sheng, Z.H.; Tang, S.; Hu, P.S. CRISPR/CAS9-mediated editing of the fragrant gene Badh2 in rice. Chin. J. Rice Sci. 2017, 31, 216–222. [Google Scholar]
- Zhang, A.; Feiming, Y.L.; Li, W.T.; Chen, Z.; Kong, D.; Bi, J.; Zhang, F.; Luo, X.; Wang, J.; Tang, J.; et al. Enhanced rice salinity tolerance via CRISPR/Cas9-targeted mutagenesis of the OsRR22 gene. Mol. Breed. 2019, e39, 47. [Google Scholar] [CrossRef] [Green Version]
- Bláha, L.; Kadlec, P.; Kohout, L.; Gottwaldová, P.; Čepl, J.; Macháčkova, I.; Hnilička, F. Vigour of seeds, quality of seed and influence of these traits on the selected crops, minor crops and potato for plant breeding, seed production ant plant production. Úroda 2008, 12, 53–60. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Ballesta, M.d.C.; Egea-Gilabert, C.; Conesa, E.; Ochoa, J.; Vicente, M.J.; Franco, J.A.; Bañon, S.; Martínez, J.J.; Fernández, J.A. The Importance of Ion Homeostasis and Nutrient Status in Seed Development and Germination. Agronomy 2020, 10, 504. https://doi.org/10.3390/agronomy10040504
Martínez-Ballesta MdC, Egea-Gilabert C, Conesa E, Ochoa J, Vicente MJ, Franco JA, Bañon S, Martínez JJ, Fernández JA. The Importance of Ion Homeostasis and Nutrient Status in Seed Development and Germination. Agronomy. 2020; 10(4):504. https://doi.org/10.3390/agronomy10040504
Chicago/Turabian StyleMartínez-Ballesta, María del Carmen, Catalina Egea-Gilabert, Encarnación Conesa, Jesús Ochoa, María José Vicente, Jose A. Franco, Sebastián Bañon, Juan J. Martínez, and Juan A. Fernández. 2020. "The Importance of Ion Homeostasis and Nutrient Status in Seed Development and Germination" Agronomy 10, no. 4: 504. https://doi.org/10.3390/agronomy10040504
APA StyleMartínez-Ballesta, M. d. C., Egea-Gilabert, C., Conesa, E., Ochoa, J., Vicente, M. J., Franco, J. A., Bañon, S., Martínez, J. J., & Fernández, J. A. (2020). The Importance of Ion Homeostasis and Nutrient Status in Seed Development and Germination. Agronomy, 10(4), 504. https://doi.org/10.3390/agronomy10040504








