(Cryo)Transmission Electron Microscopy of Phospholipid Model Membranes Interacting with Amphiphilic and Polyphilic Molecules
Abstract
1. Introduction
2. TEM Preparation Techniques
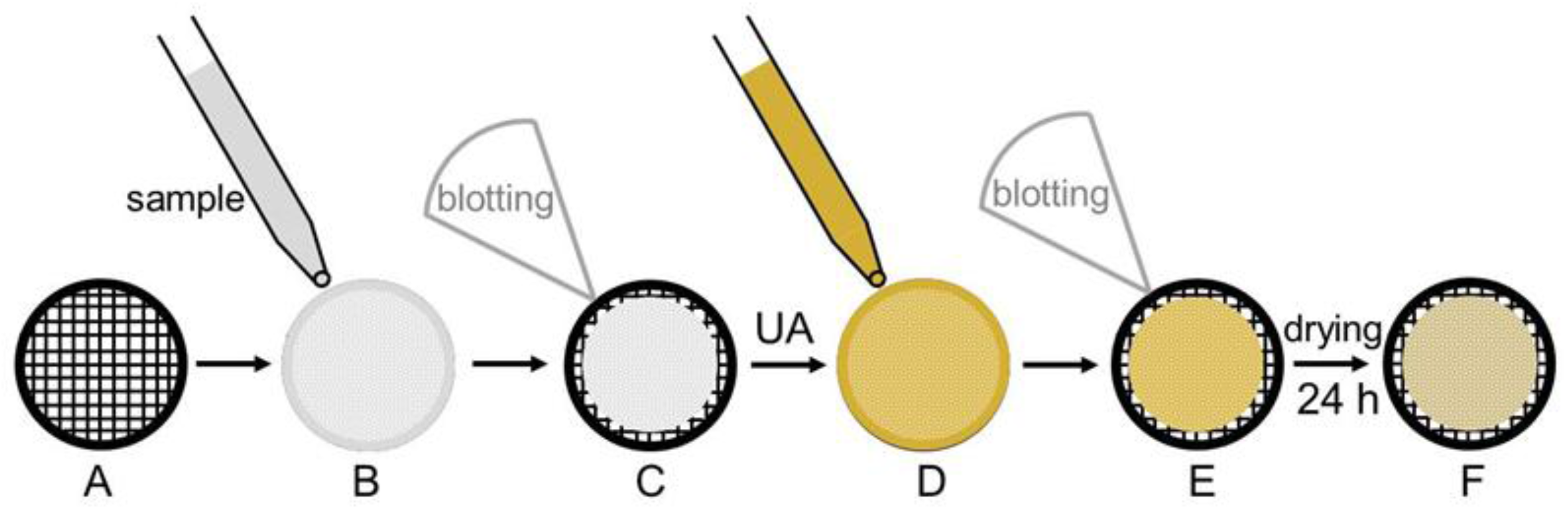
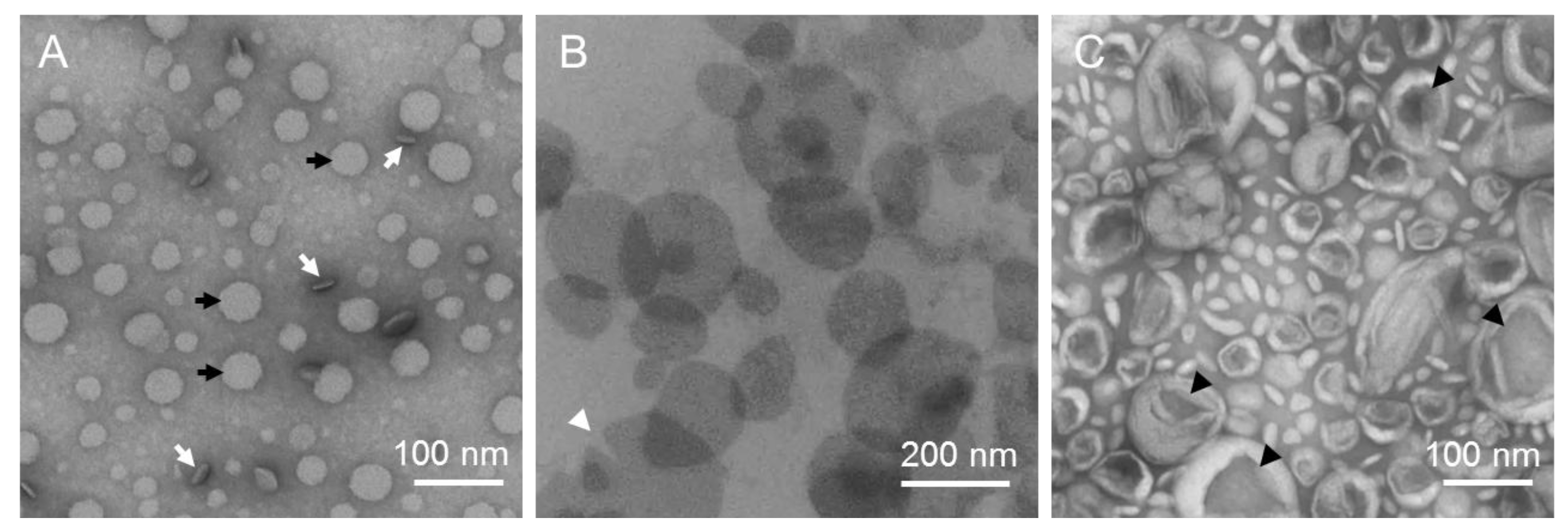
2.1. Preparation of Stained Samples for TEM
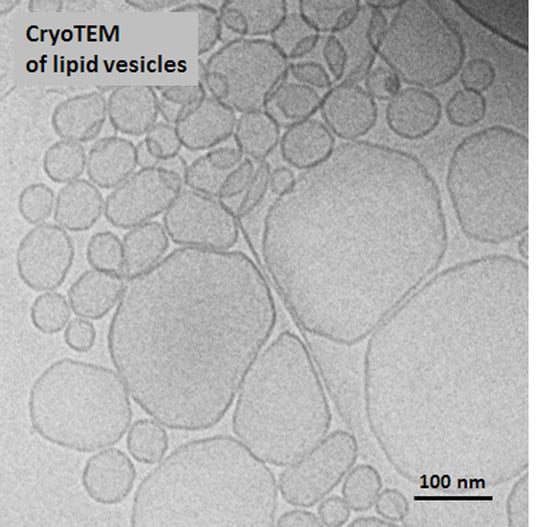
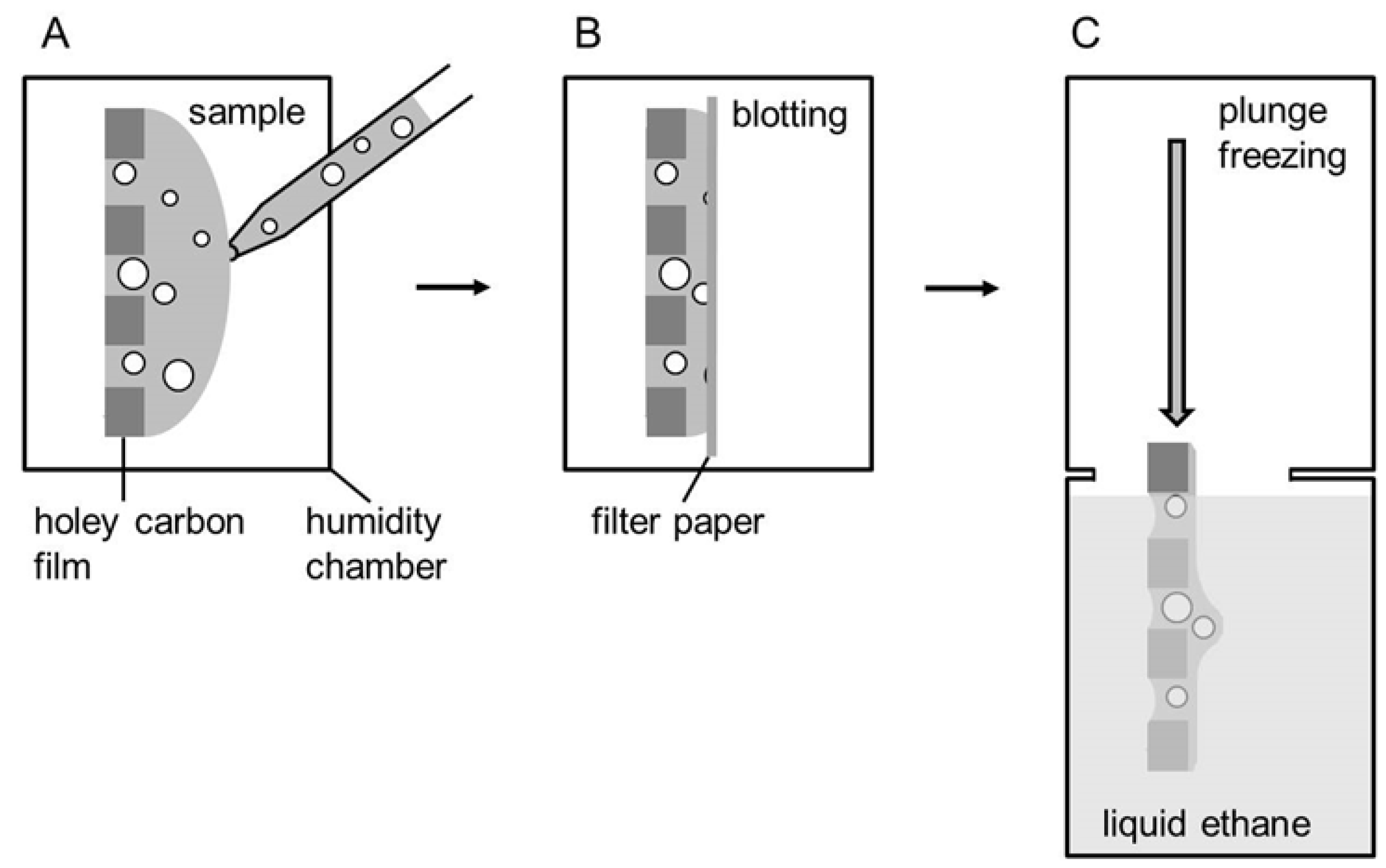
2.2. Preparation of Vitrified Samples for CryoTEM
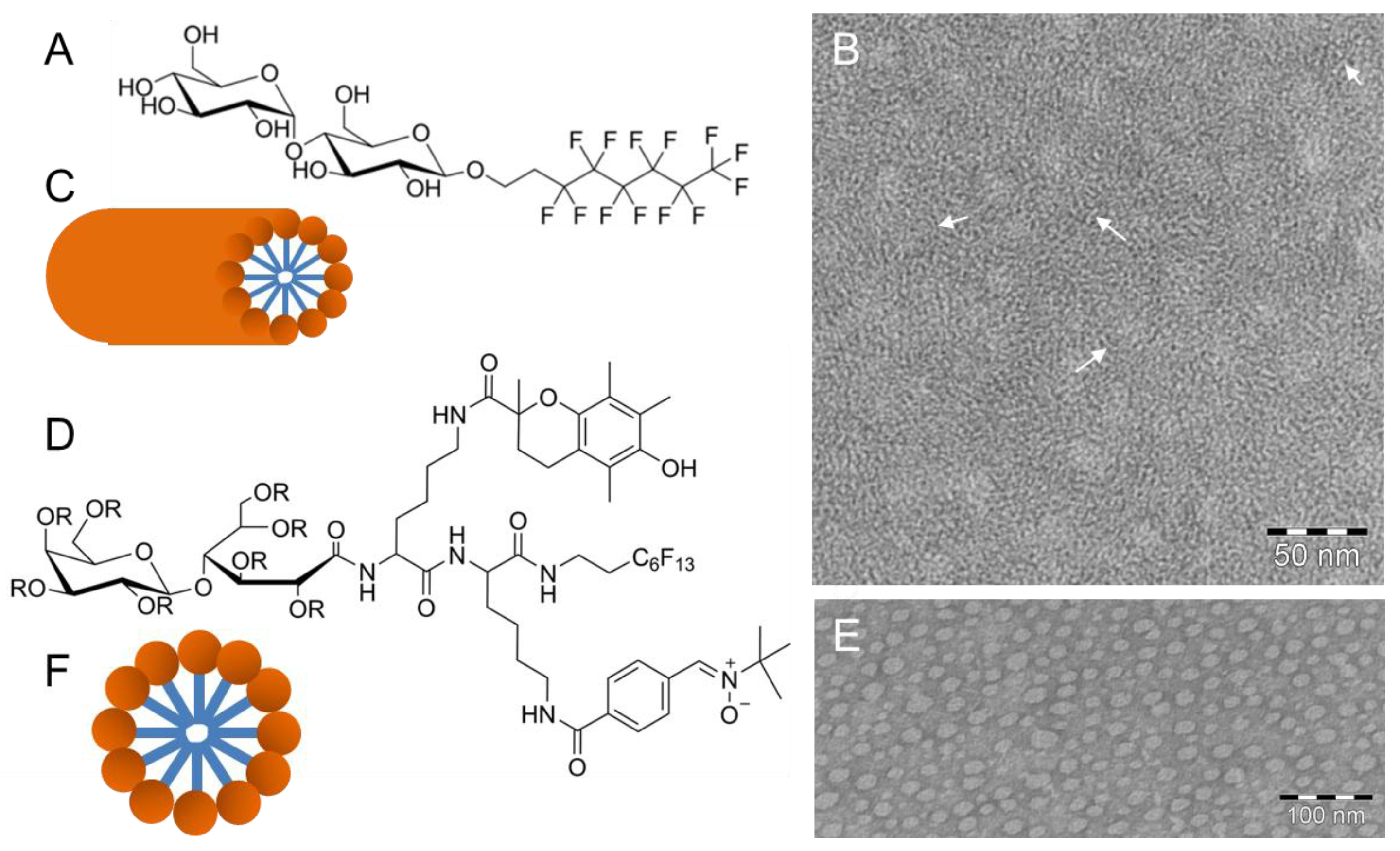
3. (Cryo)TEM of Self-Assembled Fluorinated Amphiphiles
4. (Cryo)TEM of Phospholipid Model Membranes Interacting with Amphiphilic Bolalipids, Amphiphilic T-Shaped Molecules, and X-Shaped Bolapolyphiles
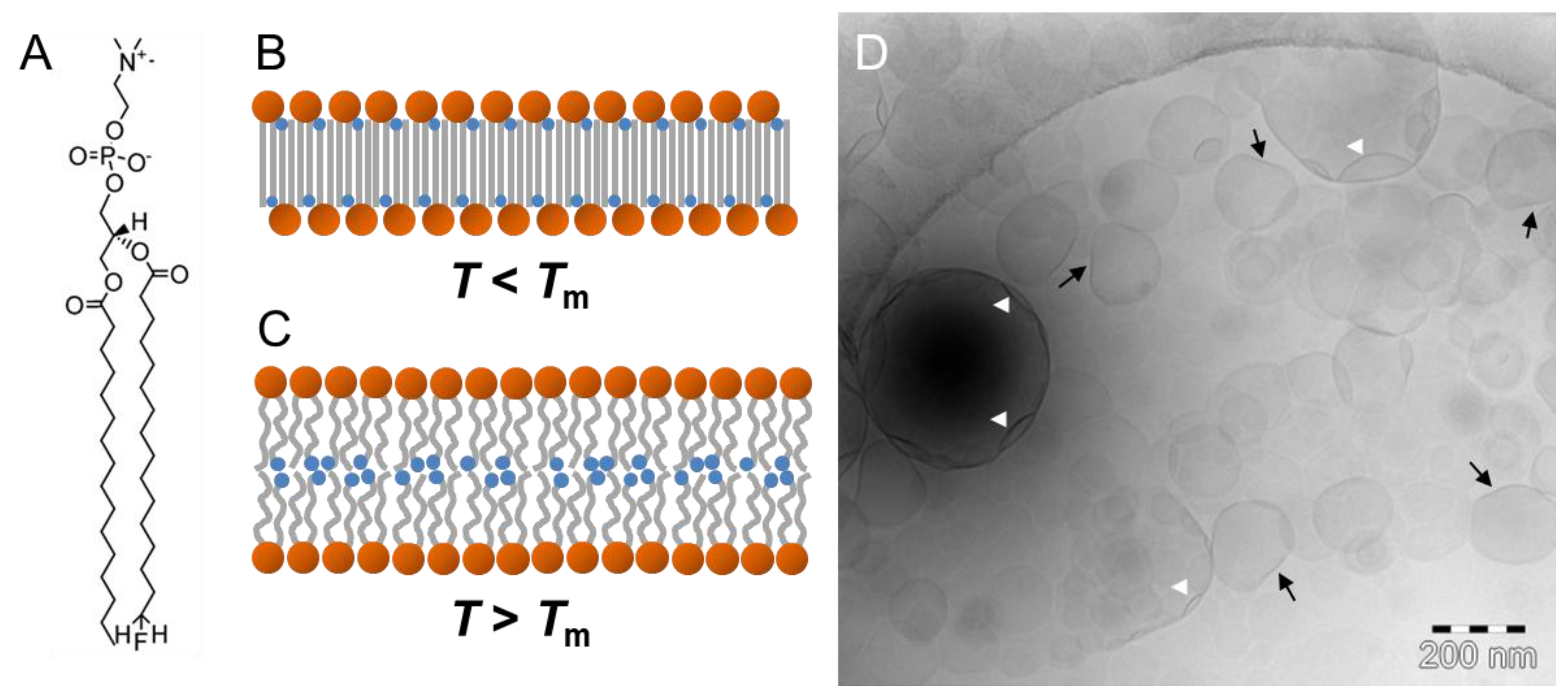
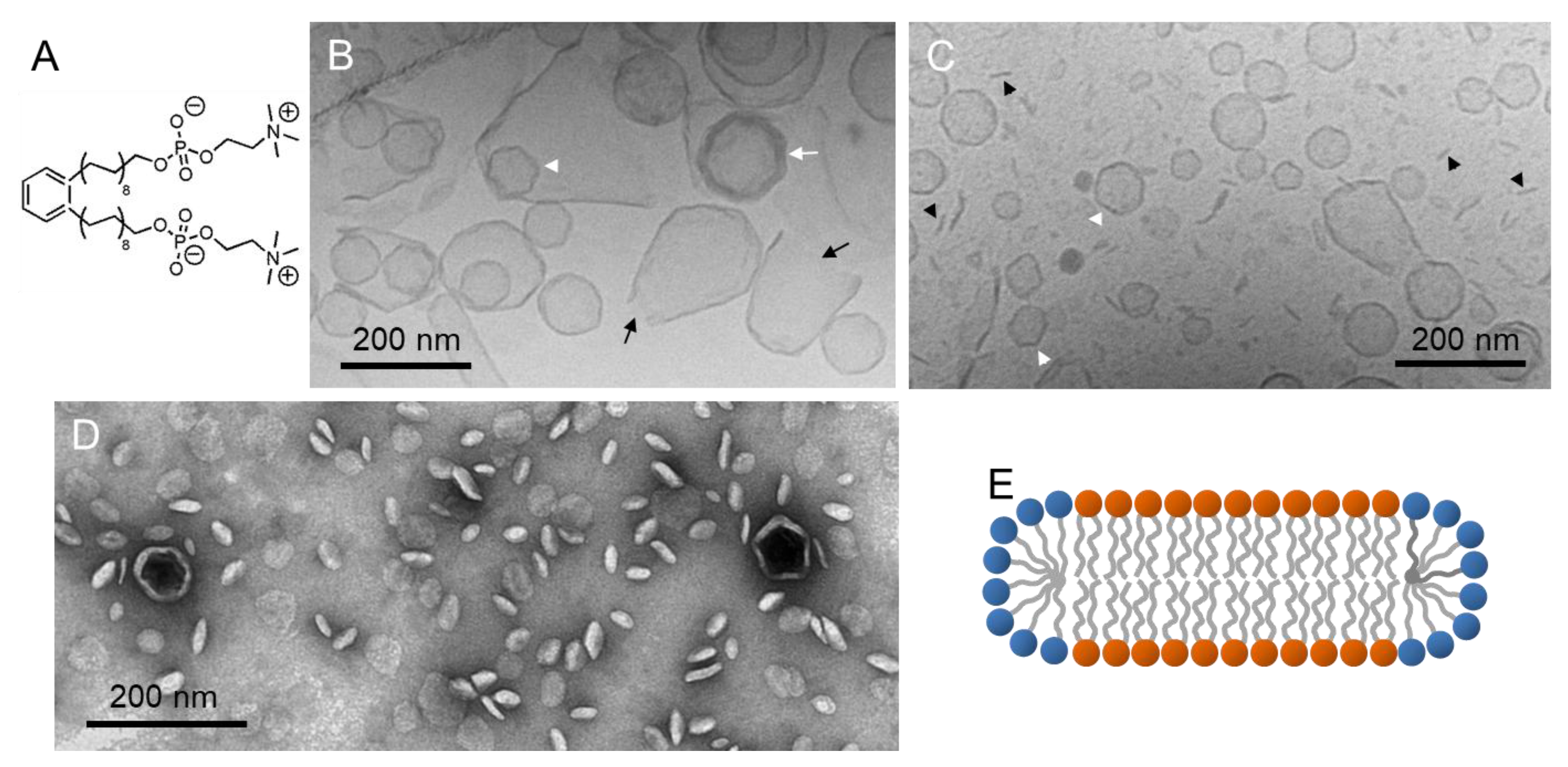
4.1. Phospholipid Membranes and Amphiphilic Bolalipids
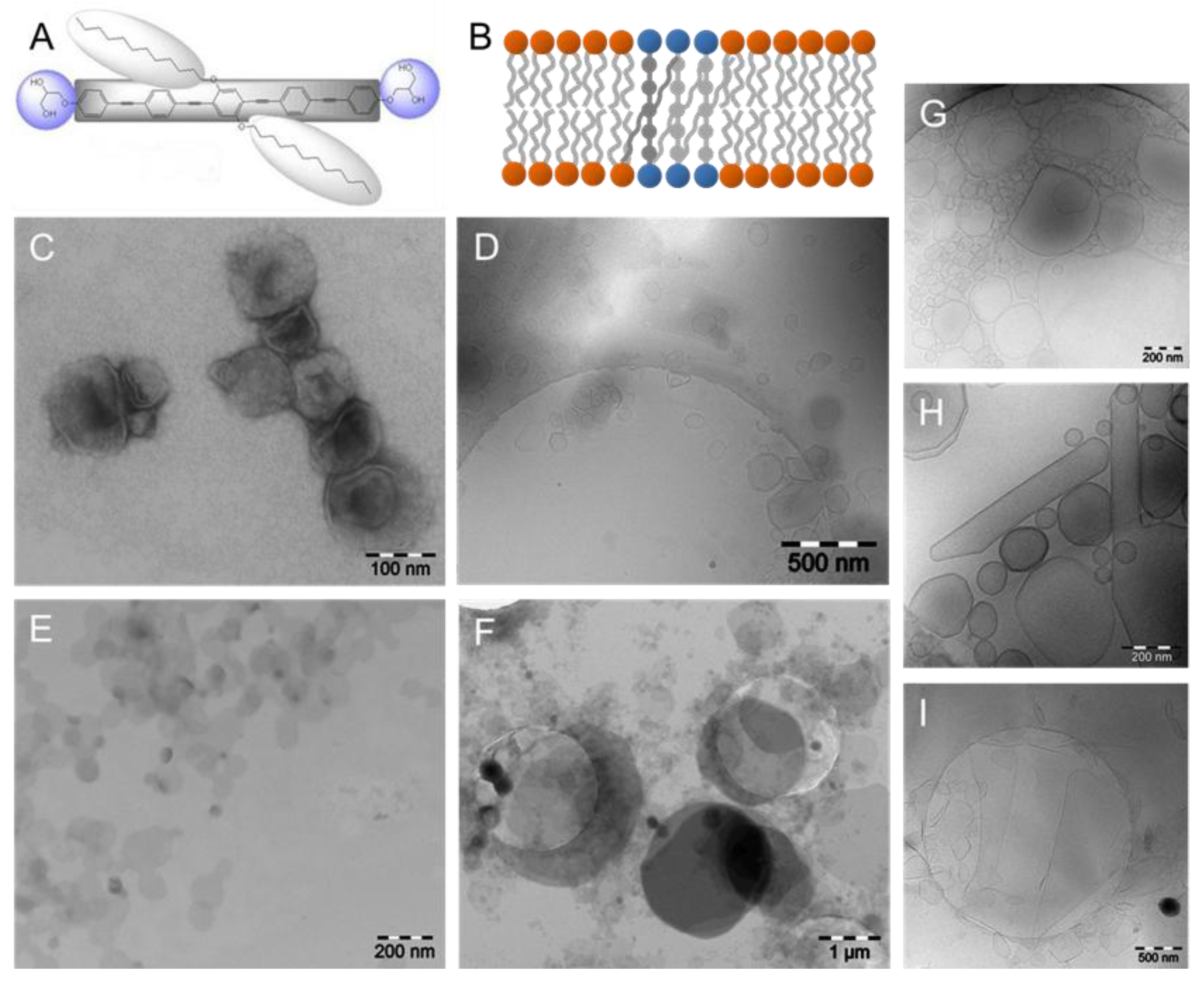
4.2. Phospholipid Membranes and Amphiphilic T-Shaped Molecules
4.3. Phospholipid Membranes and X-Shaped Bolapolyphiles
5. (Cryo)TEM of Phospholipid Model Membranes Interacting with Amphiphilic Macromolecules
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Singer, S.J.; Nicolson, G.L. The fluid mosaic model of the structure of cell membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L. The fluid-mosaic model of membrane structure: Still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochim. Biophys. Acta 2014, 1838, 1451–1466. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, D.; Geier, B.; Pabst, G. Asymmetric lipid membranes: Towards more realistic model systems. Membranes 2015, 5, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Nogales, E.; Scheres, S.H.W. Cryo-EM: A unique tool for the visualization of Macromolecular complexity. Mol. Cell 2015, 58, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Leiro, R.; Scheres, S.H.W. Unravelling biological macromolecules with cryo-electron microscopy. Nature 2016, 537, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Kühlbrandt, W. The resolution revolution. Science 2014, 343, 1443–1444. [Google Scholar] [CrossRef] [PubMed]
- Kühlbrandt, W. Cryo-EM enters a new era. eLife 2014, 3, e03678. [Google Scholar] [CrossRef] [PubMed]
- Binshtein, E.; Ohi, M.D. Cryo-electron microscopy and the amazing race to atomic resolution. Biochemistry 2015, 54, 3133–3141. [Google Scholar] [CrossRef] [PubMed]
- Briggs, J.A.G. Structural biology in situ—The potential of subtomogram averaging. Curr. Opin. Struct. Biol. 2013, 23, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Nogales, E. The development of cryo-EM into a mainstream structural biology technique. Nat. Methods 2016, 13, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Glaeser, B. How good can cryo-EM become? Nat. Methods 2016, 13, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Brilot, A.F.; Chen, J.Z.; Cheng, A.; Pan, J.; Harrison, S.C.; Potter, C.S.; Carragher, B.; Henderson, R.; Grigorieff, N. Beam-induced motion of vitrified specimen on holey carbon film. J. Struct. Biol. 2012, 177, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Harapin, J.; Eibauer, M.; Medalia, O. Structural analysis of supramolecular assemblies by cryo-electron tomography. Structure 2013, 21, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y. Single-particle cryo-EM at crystallographic resolution. Cell 2015, 161, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Scherer, S.; Arheit, M.; Kowal, J.; Zeng, X.; Stahlberg, H. Single particle 3D reconstruction for 2D crystal images of membrane proteins. J. Struct. Biol. 2014, 185, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Rigort, A.; Bäuerlein, F.J.B.; Villa, E.; Eibauer, M.; Laugks, T.; Baumeister, W.; Plitzko, J.M. Focused ion beam micromachining of eukaryotic cells for cryoelectron tomography. Proc. Natl. Acad. Sci. USA 2012, 109, 4449–4454. [Google Scholar] [CrossRef] [PubMed]
- Villa, E.; Schaffer, M.; Plitzko, J.M.; Baumeister, W. Opening windows into the cell: Focused-ion-beam milling for cryo-elctron tomography. Curr. Opin. Struct. Biol. 2013, 23, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Kukulski, W.; Schorb, M.; Welsch, S.; Picco, A.; Kaksonen, M.; Briggs, J.A.G. Correlated fluorescence and 3D electron microscopy with high sensitivity and spatial precision. J. Cell Biol. 2011, 192, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P. Correlative cryo-electron tomography and optical microscopy of cells. Curr. Opin. Struct. Biol. 2013, 23, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Kim, Y.-K.; Zhang, C.; Borshch, V.; Zhou, S.; Park, H.-S.; Jakli, A.; Lavrentovich, O.D.; Tamba, M.-G.; Kohlmeier, A.; et al. Direct observation liquid crystals using cryo-TEM: Specimen preparation and low-dose imaging. Microscopy Res. Tech. 2014, 77, 754–772. [Google Scholar] [CrossRef] [PubMed]
- Kuntsche, J.; Horst, J.C.; Bunjes, H. Cryogenic transmission electron microscopy (cryo-TEM) for studying the morphology of colloidal drug delivery systems. Int. J. Pharm. 2011, 417, 120–137. [Google Scholar] [CrossRef] [PubMed]
- Meister, A.; Finger, S.; Hause, G.; Blume, A. Morphological changes of bacterial model membrane vesicles. Eur. J. Lipid Sci. Technol. 2014, 116, 1228–1233. [Google Scholar] [CrossRef]
- Danino, D. Cryo-TEM of soft molecular assemblies. Curr. Opin. Colloid Interface Sci. 2012, 17, 316–329. [Google Scholar] [CrossRef]
- Blume, A.; Drescher, S.; Graf, G.; Köhler, K.; Meister, A. Self-assembly of different single-chain bolaphospholipids and their miscibility with phospholipids or classical amphiphiles. Adv. Colloid Interface Sci. 2014, 208, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Mason, A.F.; Thordarson, P. Polymersomes with asymmetric membranes based on readily accessible di- and triblock copolymers synthesized via SET-LRP. ACS Macro Lett. 2016, 5, 1172–1175. [Google Scholar] [CrossRef]
- Daum, B.; Auerswald, A.; Gruber, T.; Hause, G.; Balbach, J.; Kühlbrandt, W.; Meister, A. Supramolecular organization of the human N-BAR domain in shaping the sarcolemma membrane. J. Struct. Biol. 2016, 194, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.R. Negative staining of thinly spread biological particulates. In Methods in Molecular Biology; Hajibagheri, N., Ed.; Springer: New York, NY, USA, 1999; Volume 117, pp. 13–30. [Google Scholar]
- Booth, D.S.; Avila-Sakar, A.; Cheng, Y. Visualizing proteins and macromolecular complexes by negative stain EM: From grid preparation to image acquisition. J. Vis. Exp. 2011, 58, e3227. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.R.; Roos, C.; Djalali, R.; Theingans, O.; Maskos, M.; Schmidt, M. Application of the negative staining technique to both aqueous and organic solvent solutions of polymer particles. Micron 1999, 30, 289–298. [Google Scholar] [CrossRef]
- Bremer, A.; Henn, C.; Engel, A.; Baumeister, W.; Aebi, U. Has negative staining still a place in biomacromolecular electron microscopy? Ultramicroscopy 1992, 46, 85–111. [Google Scholar] [CrossRef]
- De Carlo, S.; Harris, J.R. Negative staining and cryo-negative staining of macromolecules and viruses for TEM. Micron 2011, 42, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Ohi, M.; Li, Y.; Cheng, Y.; Walz, T. Negative staining and image classification—Powerful tools in modern electron microscopy. Biol. Proced. Online 2004, 6, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Orlova, E.V.; Saibil, H.R. Structural analysis of macromolecular assemblies by electron microscopy. Chem. Rev. 2011, 111, 7710–7748. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.R.; Horne, R.W. Negative staining: A brief assessment of current technical benefits, limitations and future possibilities. Micron 1994, 25, 5–13. [Google Scholar] [CrossRef]
- Drescher, S.; Garamus, V.M.; Garvey, C.J.; Meister, A.; Blume, A. Aggregation behaviour of a single-chain, phenylene-modified bolalipid and its miscibility with classical phospholipids. Beilstein J. Org. Chem. 2017, 13, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Lechner, B.-D. Wechselwirkungen X-Förmiger Polyphiler Moleküle Mit Phospholipiden in Modellmembranen. Ph.D. Thesis, MLU Halle-Wittenberg, Halle, Germany, 2015. [Google Scholar]
- Adrian, M.; Dubochet, J.; Lepault, J.; McDowall, A.W. Cryo-electron microscopy of viruses. Nature 1984, 308, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Almgren, M.; Edwards, K.; Karlsson, G. Cryo transmission electron microscopy of liposomes and related structures. Colloids Surf. A 2000, 174, 3–21. [Google Scholar] [CrossRef]
- Kourkoutis, L.F.; Plitzko, J.M.; Baumeister, W. Electron microscopy of biological materials at the nanometer scale. Annu. Rev. Mater. Res. 2012, 42, 33–58. [Google Scholar] [CrossRef]
- Frederik, P.M.; Hubert, D.H.W. Cryoelectron microscopy of liposomes. Methods Enzymol. 2005, 391, 431–448. [Google Scholar] [PubMed]
- Resch, G.P.; Brandstetter, M.; Pickl-Herk, A.M.; Königsmaier, L.; Wonesch, V.I.; Urban, E. Immersion freezing of biological specimens: Rationale, principles, and instrumentation. Cold Spring Harb. Protoc. 2011, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Resch, G.P.; Brandstetter, M.; Königsmaier, L.; Urban, E.; Pickl-Herk, A.M. Immersion freezing of suspended particles and cells for cryo-electron microscopy. Cold Spring Harb. Protoc. 2011, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Dobro, M.J.; Melanson, L.A.; Jensen, G.J.; McDowall, A.W. Plunge freezing for electron cryomicroscopy. Methods Enzymol. 2010, 481, 63–82. [Google Scholar] [PubMed]
- Dubochet, J.; Lepault, J.; Freeman, R.; Berriman, J.A.; Homo, J.-C. Electron microscopy of frozen water and aqueous solutions. J. Microsc. 1982, 128, 219–237. [Google Scholar] [CrossRef]
- Friedrich, H.; Frederik, P.M.; de With, G.; Sommerdijk, N.A.J.M. Imaging of self-assembled structures: Interpretation of TEM and cryo-TEM images. Angew. Chem. Int. Ed. 2010, 49, 7850–7858. [Google Scholar] [CrossRef] [PubMed]
- Hope, M.J.; Bally, M.B.; Webb, G.; Cullis, P.R. Production of large unilamellar vesicles by a rapid extrusion procedure. Characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim. Biophys. Acta 1985, 812, 55–65. [Google Scholar] [CrossRef]
- Andersson, M.; Hammarström, L.; Edwards, K. Effect of bilayer phase transitions on vesicle structure and its influence on the kinetics of viologen reduction. J. Phys. Chem. 1995, 99, 14531–14538. [Google Scholar] [CrossRef]
- Almgren, M.; Edwards, K.; Gustafsson, J. Cryotransmission electron microscopy of thin vitrified samples. Curr. Opin. Colloid Interface Sci. 1996, 1, 270–278. [Google Scholar] [CrossRef]
- Kunitake, T.; Okahata, Y.; Yasunami, S. Formation and enhanced stability of fluoroalkyl bilayer membranes. J. Am. Chem. Soc. 1982, 104, 5547–5549. [Google Scholar] [CrossRef]
- Krafft, M.P. Controlling phospholipid self-assembly and film properties using highly fluorinated components—Fluorinated monolayers, vesicles, emulsions and microbubbles. Biochimie 2012, 94, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Motegi, T.; Morita, K.; Takagi, T.; Amii, H.; Kanamori, T.; Sonoyama, M.; Tero, R. Lateral diffusion and molecular interaction in a bilayer membrane consisting of partially fluorinated phospholipids. Langmuir 2016, 32, 10712–10718. [Google Scholar] [CrossRef] [PubMed]
- Mahrhauser, D.-S.; Reznicek, G.; Kotisch, H.; Brandstetter, M.; Nagelreiter, C.; Kwizda, K.; Valenta, C. Semi-solid fluorinated-DPPC liposomes: Morphological, rheological and thermic properties as well as examination of the influence of a model drug on their skin permeation. Int. J. Pharm. 2015, 486, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Krafft, M.P.; Schiedknecht, L.; Marie, P.; Giulieri, F.; Schmutz, M.; Poulain, N.; Nakache, E. Fluorinated vesicles allow intrabilayer polymerization of a hydrophobic monomer, yielding polymerized microcapsules. Langmuir 2001, 17, 2872–2877. [Google Scholar] [CrossRef]
- Santaella, C.; Vierling, P.; Riess, J.G.; Gulik-Krzywicki, T.; Gulik, A.; Monasse, G. Polymeric phase behavior of perfluoroalkylated phosphatidylcholines. Biochim. Biophys. Acta 1994, 1190, 25–39. [Google Scholar] [CrossRef]
- Guedj, C.; Pucci, B.; Zarif, L.; Coulomb, C.; Riess, J.G.; Pavia, A.A. Vesicles and other supramolecular systems from biocompatible synthetic glycolipids with hydrocarbon and/or fluorocarbon chains. Chem. Phys. Lipids 1994, 72, 153–173. [Google Scholar] [CrossRef]
- Hirsh, D.J.; Lazaro, N.; Wright, L.R.; Boggs, J.M.; McIntosh, T.J.; Schaefer, J.; Blazyk, J. A new monofluorinated phosphatidylcholine forms interdigitated bilayers. Biophys. J. 1998, 75, 1858–1868. [Google Scholar] [CrossRef]
- Toimil, P.; Davina, R.; Sabin, J.; Prieto, G.; Sarmiento, F. Influence of temperature on the colloidal stability of the F-DPPC and DPPC liposomes induced by lanthanum ions. J. Colloid Interface Sci. 2012, 367, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.W.H. Investigations of the Potential of Synthetic Phospholipids as Membrane Mimics: Interactions with Amphiphilic and Polyphilic Block Copolymers. Ph.D. Thesis, MLU Halle-Wittenberg, Halle, Germany, 2016. [Google Scholar]
- Sanii, B.; Szmodis, A.W.; Bricarello, D.A.; Oliver, A.E.; Parikh, A.N. Frustrated phase transformations in supported, interdigitating lipid bilayers. J. Phys. Chem. B 2010, 114, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Krafft, M.P.; Riess, J.G. Highly fluorinated amphiphiles and colloidal systems, and their applications in the biomedical field. A contribution. Biochimie 1998, 80, 489–514. [Google Scholar] [CrossRef]
- Krafft, M.P. Strasbourg’s SOFFT team—Soft functional systems self-assembled from perfluoroalkylated molecular components. J. Fluorine Chem. 2012, 134, 90–102. [Google Scholar] [CrossRef]
- Kovalchuk, N.M.; Trybala, A.; Starov, V.; Matar, O.; Ivanova, N. Fluoro- vs. hydrocarbon sufactants: Why do they differ in wetting performance? Adv. Colloid Interface Sci. 2014, 210, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Chabaud, E.; Barthélémy, P.; Mora, N.; Popot, J.-L.; Pucci, B. Stabilization of integral membrane proteins in aqueous solution using fluorinated surfactants. Biochimie 1998, 80, 515–530. [Google Scholar] [CrossRef]
- Polidori, A.; Presset, M.; Lebaupain, F.; Améduri, B.; Popot, J.-L.; Breyton, C.; Pucci, B. Fluorinated and hemifluorinated surfactants derived from maltose: Synthesis and application to handling membrane proteins in aqueous solution. Bioorg. Med. Chem. Lett. 2006, 16, 5827–5831. [Google Scholar] [CrossRef] [PubMed]
- Abla, M.; Unger, S.; Keller, S.; Bonneté, F.; Ebel, C.; Pucci, B.; Breyton, C.; Durand, G. Micellar and biochemical properties of a propyl-ended fluorinated surfactant designed for membrane-protein study. J. Colloid Interface Sci. 2015, 445, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Polidori, A.; Raynal, S.; Barret, L.-A.; Dahani, M.; Barrot-Ivolot, C.; Jungas, C.; Frotscher, E.; Keller, S.; Ebel, C.; Breyton, C.; et al. Sparingly fluorinated maltoside-based surfactants for membrane-protein stabilization. New J. Chem. 2016, 40, 5364–5378. [Google Scholar] [CrossRef]
- Breyton, C.; Pucci, B.; Popot, J.-L. Amphiopols and fluorinated surfactants: Two alternatives to detergents for studying membrane proteins in vivo. In Methods in Molecular Biology; Mus-Veteau, I., Ed.; Springer: New York, NY, USA, 2010; Volume 601, pp. 219–245. [Google Scholar]
- Frotscher, E.; Danielczak, B.; Vargas, C.; Meister, A.; Durand, G.; Keller, S. A fluorinated detergent for membrane-protein applications. Angew. Chem. Int. Ed. 2015, 54, 5069–5073. [Google Scholar] [CrossRef] [PubMed]
- Rosselin, M.; Meyer, G.; Guillet, P.; Cheviet, T.; Walther, G.; Meister, A.; Hadjipavlou-Litina, D.; Durand, G. Divalent amino-acid-based amphiphilic antioxidants: Synthesis, self-assembling properties, and biological evaluation. Bioconjugate Chem. 2016, 27, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Choteau, F.; Durand, G.; Ranchon-Cole, I.; Cercy, C.; Pucci, B. Cholesterol-based a-phenyl-N-tert-butyl nitrone derivatives as antioxidants against light-induced retinal degeneration. Bioorg. Med. Chem. Lett. 2010, 20, 7405–7409. [Google Scholar] [CrossRef] [PubMed]
- Der Mardirossian, C.; Krafft, M.P.; Gulik-Krzywicki, T.; le Maire, M.; Lederer, F. Perfluoroalkylphosphocholines are poor protein-solubilizing surfactants, as tested with neutrophil plasma membrane. Biochimie 1998, 80, 531–541. [Google Scholar] [CrossRef]
- Gabriel, J.L.; Chong, P.L. Molecular modeling of archaebacterial bipolar tetraether lipid membranes. Chem. Phys. Lipids 2000, 105, 193–200. [Google Scholar] [CrossRef]
- Benvegnu, T.; Réthoré, G.; Brard, M.; Richter, W.; Plusquellec, D. Archaeosomes based on novel synthetic tetraether-type lipids for the development of oral delivery systems. Chem. Commun. 2005, 5536–5538. [Google Scholar] [CrossRef] [PubMed]
- Jacquemet, A.; Barbeau, J.; Lemiègre, L.; Benvegnu, T. Archaeal tetraether bipolar lipids: Structures, functions and applications. Biochimie 2009, 91, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Drescher, S.; Lechner, B.-D.; Garamus, V.M.; Almásy, L.; Meister, A.; Blume, A. The headgroup (a)symmetry strongly determines the aggregation behavior of single-chain phenylene-modified bolalipids and their miscibility with classical phospholipids. Langmuir 2014, 30, 9273–9284. [Google Scholar] [CrossRef] [PubMed]
- Meister, A.; Blume, A. Single-chain bolaphospholipids: Temperature-dependent self-assembly and mixing behavior with phospholipids. Adv. Planar Lip. Bilayers Liposomes 2012, 16, 93–128. [Google Scholar]
- Meister, A.; Drescher, S.; Garamus, V.M.; Karlsson, G.; Graf, G.; Dobner, B.; Blume, A. Temperature-dependent self-assembly and mixing behavior of symmetrical single-chain bolaamphiphiles. Langmuir 2008, 24, 6238–6246. [Google Scholar] [CrossRef] [PubMed]
- Drescher, S.; Meister, A.; Garamus, V.M.; Hause, G.; Garvey, C.J.; Dobner, B.; Blume, A. Phenylene bolaamphiphiles: Influence of the substitution pattern on the aggregation behavior and the miscibility with classical phospholipids. Eur. J. Lipid Sci. Technol. 2014, 116, 1205–1216. [Google Scholar] [CrossRef]
- Tschierske, K. Liquid crystal engineering—New complex mesophase structures and their relations to polymer morphologies, nanoscale patterning and crystal engineering. Chem. Soc. Rev. 2007, 36, 1930–1970. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Baumeister, U.; Pelzl, G.; Das, M.K.; Zeng, X.B.; Ungar, G.; Tschierske, C. Carbohydrate rod conjugates: Ternary rod-coil molecules forming complex liquid crystal structures. J. Am. Chem. Soc. 2005, 127, 16578–16591. [Google Scholar] [CrossRef] [PubMed]
- Scholtysek, P.; Achilles, A.; Hoffmann, C.-V.; Lechner, B.-D.; Meister, A.; Tschierske, C.; Saalwächter, K.; Edwards, K.; Blume, A. A T-shaped amphiphilic molecule forms closed vesicles in water and bicelles in mixtures with a membrane lipid. J. Phys. Chem. B 2012, 116, 4871–4878. [Google Scholar] [CrossRef] [PubMed]
- Schwieger, C.; Achilles, A.; Scholz, S.; Rüger, J.; Bacia, K.; Saalwächter, K.; Kressler, J.; Blume, A. Binding of amphiphilic and triphilic block copolymers to lipid model membranes: The role of perfluorinated moieties. Soft Matter 2014, 10, 6147–6160. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, P.; Prehm, M.; Glettner, B.; Pelz, K.; Baumeister, U.; Liu, F.; Zeng, X.; Ungar, G.; Tschierske, C. X-shaped polyphilics: Liquid crystal honeycombs with single-molecule walls. Chem. Commun. 2008, 3861–3863. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Ebert, H.; Lechner, B.D.; Lange, F.; Achilles, A.; Bärenwald, R.; Poppe, S.; Blume, A.; Saalwächter, K.; Tschierske, C.; et al. Dendritic domains with hexagonal symmetry formed by X-shaped bolapolyphiles in lipid membranes. Chem. Eur. J. 2015, 21, 8840–8850. [Google Scholar] [CrossRef] [PubMed]
- Lechner, B.-D.; Ebert, H.; Prehm, M.; Werner, S.; Meister, A.; Hause, G.; Beerlink, A.; Saalwächter, K.; Bacia, K.; Tschierske, C.; et al. Temperature-dependent in-plane structure formation of an X-shaped bolapolyphile within lipid bilayers. Langmuir 2015, 31, 2839–2850. [Google Scholar] [CrossRef] [PubMed]
- Hädicke, A.; Blume, A. Interactions of pluronic block copolymers with lipid vesicles depend on lipid phase and pluronic aggregation state. Colloid Polym. Sci. 2014, 293, 267–276. [Google Scholar] [CrossRef]
- Tribet, C.; Vial, F. Flexible macromolecules attached to lipid bilayers: Impact on fluidity, curvature, permeability and stability of the membranes. Soft Matter 2008, 4, 68–81. [Google Scholar] [CrossRef]
- Mansfeld, U.; Hoeppener, S.; Kempe, K.; Schumers, J.-M.; Gohy, J.-F.; Schubert, U.S. Tuning the morphology of triblock terpoly(2-oxazoline)s containing a 2-phenyl-2-oxazoline block with varying fluorine content. Soft Matter 2013, 9, 5966–5974. [Google Scholar] [CrossRef]
- Kaberov, L.I.; Verbraeken, B.; Hruby, M.; Riabtseva, A.; Kovacik, L.; Kereiche, S.; Brus, J.; Stepanek, P.; Hoogenboom, R.; Filippov, S.K. Novel triphilic block copolymers based on poly(2-methyl-2-oxazoline)-block-poly(2-octyl-2-oxazoline) with different terminal perfluoroalkyl fragments: Synthesis and self-assembly behaviour. Eur. Polym. J. 2017, 88, 645–655. [Google Scholar] [CrossRef]
- Kyeremateng, S.O.; Amado, E.; Blume, A.; Kressler, J. Synthesis of ABC and CABAC triphilic block copolymers by ATRP Combined with ‘click’ chemistry. Macromol. Rapid Commun. 2008, 29, 1140–1146. [Google Scholar] [CrossRef]
- Kissa, E. Fluorinated surfactants. In Surface Sience Series; No. 50; Marcel Dekker: New York, NY, USA, 1994. [Google Scholar]
- Rossi, S.; Karlsson, G.; Ristori, S.; Martini, G.; Edwards, K. Aggregate structures in a dilute aqueous dispersion of a fluorinated/hydrogenated surfactant system. A cryo-transmission electron microscopy study. Langmuir 2001, 17, 2340–2345. [Google Scholar] [CrossRef]
- Scholtysek, P.; Li, Z.; Kressler, J.; Blume, A. Interactions of DPPC with semitelechelic poly(glycerol methacrylate)s with perfluoroalkyl end groups. Langmuir 2012, 28, 15651–15662. [Google Scholar] [CrossRef] [PubMed]
- Scholtysek, P. Chirale und Achirale Polymere in Wechselwirkung mit Phospholipid-Monolayern und -Bilayern. Ph.D. Thesis, MLU Halle-Wittenberg, Halle, Germany, 2014. [Google Scholar]
- Knowles, T.J.; Finka, R.; Smith, C.; Lin, Y.-P.; Dafforn, T.; Overduin, M. Membrane proteins solublilized intact in lipid containing nanoparticles bounded by styrene maleic acid copolymer. J. Am. Chem. Soc. 2009, 131, 7484–7485. [Google Scholar] [CrossRef] [PubMed]
- Dörr, J.M.; Scheidelaar, S.; Koorengevel, M.C.; Dominguez, J.J.; Schaefer, M.; van Walree, C.A.; Killian, J.A. The styrene-maleic acid copolymer: A versatile tool in membrane research. Eur. Biophys. J. 2016, 45, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Grethen, A.; Oluwole, A.O.; Danielczak, B.; Vargas, C.; Keller, S. Thermodynamics of nanodisc formation mediated by styrene/maleic acid (2:1) copolymer. Sci. Rep. 2017, 7, 11517. [Google Scholar] [CrossRef] [PubMed]
- Cuevas Arenas, R.; Danielczak, B.; Martel, A.; Porcar, L.; Bryton, C.; Ebel, C.; Keller, S. Fast collisional lipid transfer among polymer-bounded nanodiscs. Sci. Rep. 2017, 7, 45875. [Google Scholar] [CrossRef] [PubMed]
- Scheidelaar, S.; Koorengevel, M.C.; Pardo, J.D.; Meeldijk, J.D.; Breukink, E.; Killian, J.A. Molecular model for the solubilization of membranes into nanodisks by styrene maleic acid copolymers. Biophys. J. 2015, 108, 279–290. [Google Scholar]
- Vargas, C.; Cuevas Arenas, R.; Frotscher, E.; Keller, S. Nanoparticle self-assembly in mixtures of phospholipids with styrene/maleic acid copolymers or fluorinated surfactants. Nanoscale 2015, 7, 20685. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, A.O.; Danielszak, B.; Meister, A.; Babalola, J.O.; Vargas, C.; Keller, S. Solubilization of membrane proteins into functional lipid-bilayer nanodiscs using a diisobutylene/maleic acid copolymer. Angew. Chem. Int. Ed. 2017, 56, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- Ravula, T.; Ramadugu, S.K.; Di Mauro, G.; Ramamoorthy, A. Bioinspired, size-tunable self-assembly of polymer-lipid bilayer nanodiscs. Angew. Chem. Int. Ed. 2017, 56, 11466–11470. [Google Scholar] [CrossRef] [PubMed]

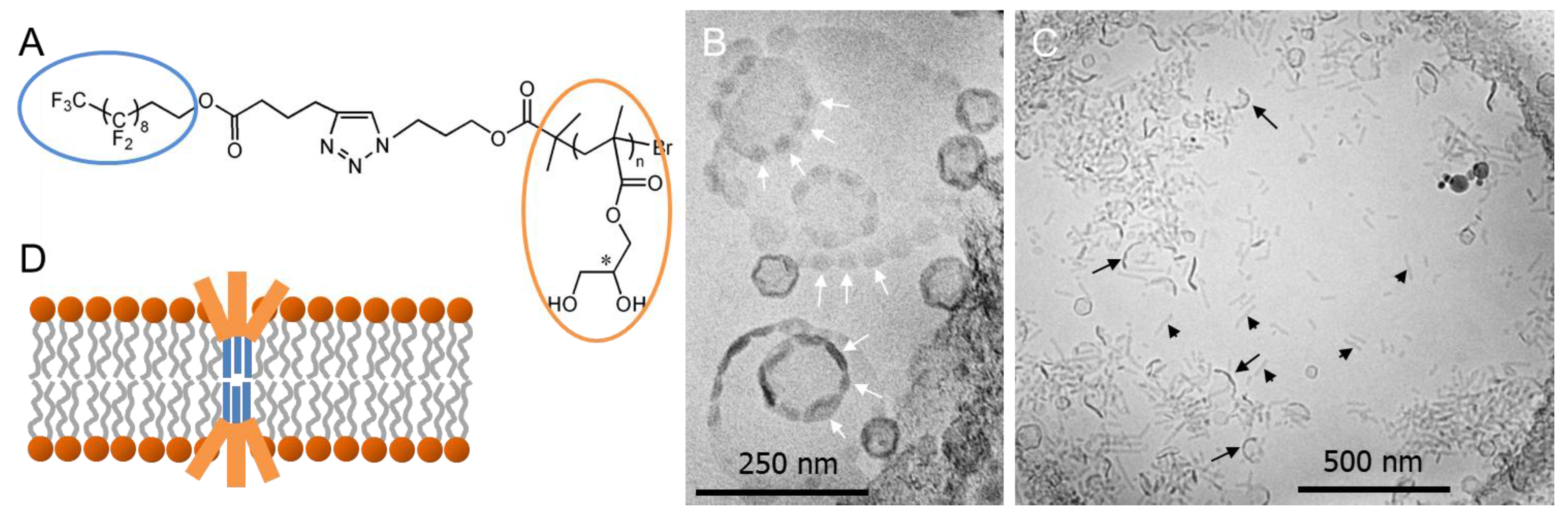
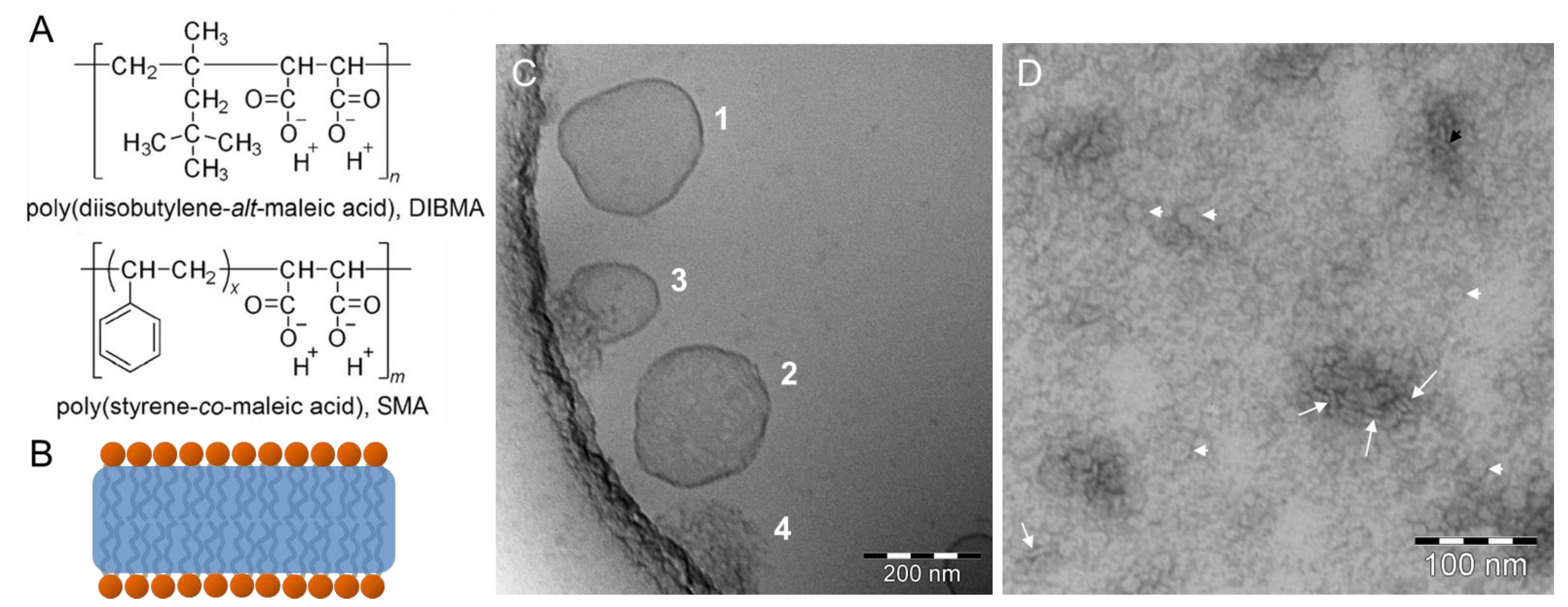
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meister, A.; Blume, A. (Cryo)Transmission Electron Microscopy of Phospholipid Model Membranes Interacting with Amphiphilic and Polyphilic Molecules. Polymers 2017, 9, 521. https://doi.org/10.3390/polym9100521
Meister A, Blume A. (Cryo)Transmission Electron Microscopy of Phospholipid Model Membranes Interacting with Amphiphilic and Polyphilic Molecules. Polymers. 2017; 9(10):521. https://doi.org/10.3390/polym9100521
Chicago/Turabian StyleMeister, Annette, and Alfred Blume. 2017. "(Cryo)Transmission Electron Microscopy of Phospholipid Model Membranes Interacting with Amphiphilic and Polyphilic Molecules" Polymers 9, no. 10: 521. https://doi.org/10.3390/polym9100521
APA StyleMeister, A., & Blume, A. (2017). (Cryo)Transmission Electron Microscopy of Phospholipid Model Membranes Interacting with Amphiphilic and Polyphilic Molecules. Polymers, 9(10), 521. https://doi.org/10.3390/polym9100521