Eco-Friendly Biocomposites from Chestnut Waste: Production, Optimization, Characterization, and Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Material Characterization
FTIR
2.3. Production and Optimization of Biocomposites
2.4. Shellac Gum Preparation and Application
2.5. Biocomposite Characterization
2.5.1. Mechanical Properties
2.5.2. Morphology
2.5.3. Wettability
2.5.4. Water Absorption
2.5.5. Thermal Conductivity
2.5.6. TGA
2.5.7. DSC
2.6. Candle Holder Application
3. Results and Discussion
3.1. Characterization by FTIR
3.2. Optimization of Mechanical Properties
3.3. Validation of the Optimal Composition Predicted by a BBD
3.4. Biocomposite Characterization
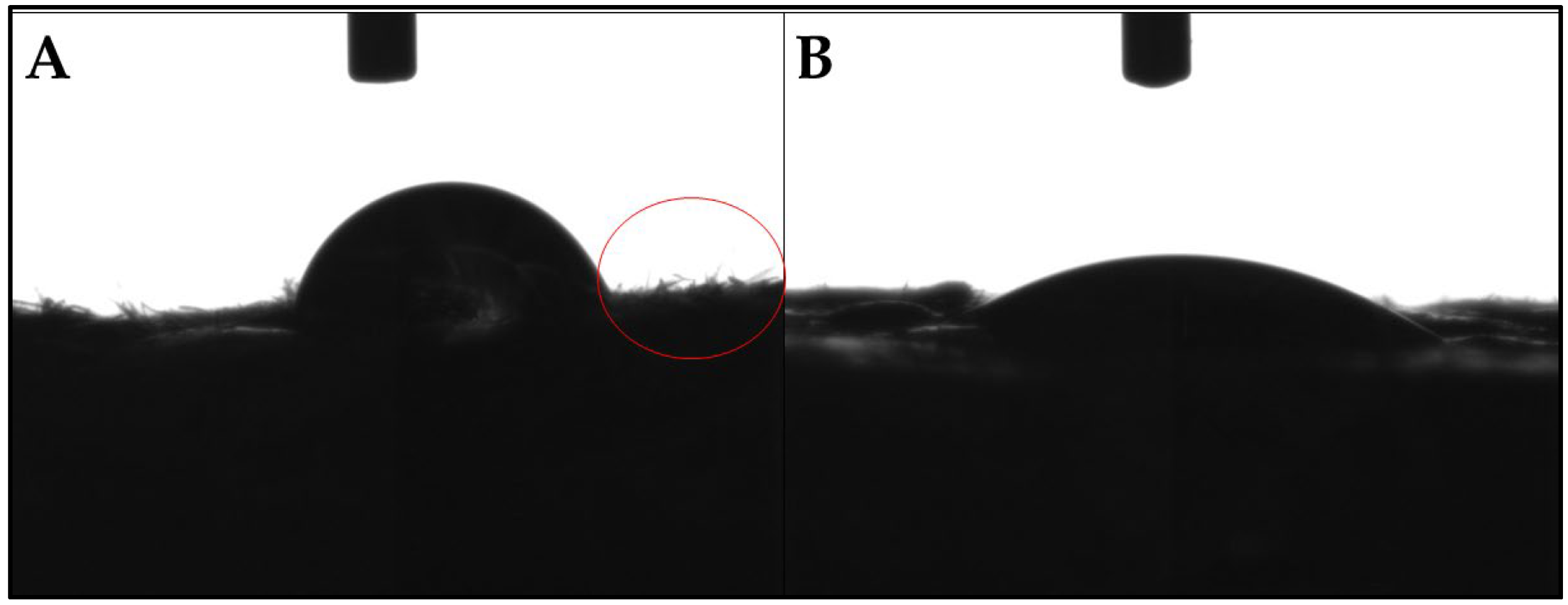
3.4.1. Wettability
3.4.2. Water Absorption
3.4.3. Thermal Conductivity
3.4.4. TGA and DSC
3.5. Application: Candle Holder
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iroegbu, A.O.C.; Ray, S.S.; Mbarane, V.; Bordado, J.C.; Sardinha, J.P. Plastic Pollution: A Perspective on Matters Arising: Challenges and Opportunities. ACS Omega 2021, 6, 19343–19355. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Natural Fibers, Biopolymers, and Biocomposites; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Dicker, M.P.; Duckworth, P.F.; Baker, A.B.; Francois, G.; Hazzard, M.K.; Weaver, P.M. Green Composites: A Review of Material Attributes and Complementary Applications. Compos. Part A Appl. Sci. Manuf. 2014, 56, 280–289. [Google Scholar] [CrossRef]
- FAOSTAT. Crops and Livestock Products; Ministry of Agriculture: Prague, Czech Republic, 2022. [Google Scholar]
- Fang, M.; Lizotte, E.; Malone, T. A Hard Nut to Crack: Identifying Factors Relevant to Chestnut Consumption. J. Food Distrib. Res. 2019, 50, 27–47. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Q.; Pan, S.; Xu, C.; Xiong, Y.L. Chemical Composition and Quality Traits of Chinese Chestnuts (Castanea mollissima) Produced in Different Ecological Regions. Food Biosci. 2015, 11, 33–42. [Google Scholar] [CrossRef]
- Hu, M.; Yang, X.; Chang, X. Bioactive Phenolic Components and Potential Health Effects of Chestnut Shell: A Review. J. Food Biochem. 2021, 45, e13696. [Google Scholar] [CrossRef]
- Çitlacifci, H.; Pekgözlü, A.K.; Gülsoy, S.K. Characterization of Cheestnut Shell. Bartın Univ. Int. J. Nat. Appl. Sci. 2022, 5, 145–150. [Google Scholar] [CrossRef]
- EuroStat Data of Crop Chestnut Production in EU Standard Humidity. Available online: https://ec.europa.eu/eurostat/databrowser/view/apro_cpsh1/default/table?lang=en (accessed on 4 November 2024).
- Mota, M.; Pinto, T.; Vilela, A.; Marques, T.; Borges, A.; Caço, J.; Ferreira-Cardoso, J.; Raimundo, F.; Gomes-Laranjo, J. Irrigation Positively Affects the Chestnut’s Quality: The Chemical Composition, Fruit Size and Sensory Attributes. Sci. Hortic. 2018, 238, 177–186. [Google Scholar] [CrossRef]
- Vázquez, G.; Fernández-Agulló, A.; Gómez-Castro, C.; Freire, M.; Antorrena, G.; González-Álvarez, J. Response Surface Optimization of Antioxidants Extraction from Chestnut (Castanea sativa) Bur. Ind. Crop. Prod. 2012, 35, 126–134. [Google Scholar] [CrossRef]
- Amaral, L.; Rodrigues, F.; Silva, A.; Costa, P.; Delerue-Matos, C.; Vieira, E.F. Reinforcement of Starch Film with Castanea sativa Shells Polysaccharides: Optimized Formulation and Characterization. Food Chem. 2022, 396, 133609. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Freitas, O.; Vieira, E.; Delerue-Matos, C.; Domingues, V. A Comprehensive Review on Valorization of Chestnut Processing Wastes into Bio-Based Composites and Bioplastics. Polym. Compos. 2025, 1–20. [Google Scholar] [CrossRef]
- Liang, J.; Wu, J.; Xu, J. Low-Formaldehyde Emission Composite Particleboard Manufactured from Waste Chestnut Bur. J. Wood Sci. 2021, 67, 21. [Google Scholar] [CrossRef]
- Torun, S.B.; Pesman, E.; Cavdar, A.D.A. Effect of Alkali Treatment on Composites Made from Recycled Polyethylene and Chestnut Cupula. Polym. Compos. 2019, 40, 4442–4451. [Google Scholar] [CrossRef]
- Torres, F.G.; Mayorga, J.P.; Vilca, C.; Arroyo, J.; Castro, P.; Rodriguez, L. Preparation and Characterization of a Novel Starch–Chestnut Husk Biocomposite. SN Appl. Sci. 2019, 1, 1158. [Google Scholar] [CrossRef]
- Ribeiro, J.E.; Rocha, J.; Queijo, L. The Influence of Manufacturing Factors in the Short-Fiber Non-Woven Chestnut Hedgehog Spine-Reinforced Polyester Composite Performance. J. Nat. Fibers 2019, 18, 1307–1319. [Google Scholar] [CrossRef]
- Borazan, A.A.; Adiguzel, G. Influence of the Addition Chestnut Shell to Kaolin/Polyester Composites. J. Eng. Res. Appl. Sci. 2018, 7, 937–943. [Google Scholar]
- Wu, C.-S.; Liao, H.-T. The Mechanical Properties, Biocompatibility and Biodegradability of Chestnut Shell Fibre and Polyhydroxyalkanoate Composites. Polym. Degrad. Stab. 2014, 99, 274–282. [Google Scholar] [CrossRef]
- Wu, C.-S.; Hsu, Y.-C.; Liao, H.-T.; Yen, F.-S.; Wang, C.-Y.; Hsu, C.-T. Characterization and Biocompatibility of Chestnut Shell Fiber-Based Composites with Polyester. J. Appl. Polym. Sci. 2014, 131, 40730. [Google Scholar] [CrossRef]
- Rafeeq, S.N.; Hussein, S.M. Characteristic of Hybrid Chestnut Shell Fillers/ Epoxy Composite. Eng. Technol. J. 2013, 31, 368–380. [Google Scholar] [CrossRef]
- Kaymakci, A.; Ayrilmis, N. Waste Chestnut Shell as a Source of Reinforcing Fillers for Polypropylene Composites. J. Thermoplast. Compos. Mater. 2012, 27, 1054–1064. [Google Scholar] [CrossRef]
- Kartal, I.; Nayci, G.; Demirer, H. The Effect of Chestnut Wood Flour Size on the Mechanical Properties of Vinyl Ester Composites. Emerg. Mater. Res. 2020, 9, 960–965. [Google Scholar] [CrossRef]
- Mert, C.; Ertürk, Ü. Chemical Chemical Compositions and Sugar Profiles of Consumed Chestnut Cultivars in the Marmara Region, Turkey. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 203–207. [Google Scholar] [CrossRef]
- Cruz-Lopes, L.P.; Domingos, I.; Ferreira, J.; Esteves, B. Chemical Composition and Study on Liquefaction Optimization of Chestnut Shells. Open Agric. 2020, 5, 905–911. [Google Scholar] [CrossRef]
- Thombare, N.; Kumar, S.; Kumari, U.; Sakare, P.; Yogi, R.K.; Prasad, N.; Sharma, K.K. Shellac as a Multifunctional Biopolymer: A Review on Properties, Applications and Future Potential. Int. J. Biol. Macromol. 2022, 215, 203–223. [Google Scholar] [CrossRef] [PubMed]
- Loreto, A. Goma Laca-Fácil de Preparar. Available online: https://www.artesanatocomamemeia.com/2013/09/goma-laca-facil-de-preparar.html (accessed on 23 August 2024).
- ISO 14125; Fibre-Reinforced Plastic Composites—Determination of Flexural Properties. British Standard: Geneva, Switzerland, 1998.
- ASTM D570; Standard Test Method for Water Absorption of Plastics. ASTM International: Conshohocken, PA, USA, 2022.
- Moreira, D.C. Desenvolvimento e Conceção de Protótipo de Pequenas Dimensões Para Processo de Compressão a Quente; Polytechnic of Porto-School of Engineering: Porto, Portugal, 2024. [Google Scholar]
- Irimia-Vladu, M.; Głowacki, E.D.; Schwabegger, G.; Leonat, L.; Akpinar, H.Z.; Sitter, H.; Bauer, S.; Sariciftci, N.S. Natural Resin Shellac as a Substrate and a Dielectric Layer for Organic Field-Effect Transistors. Green Chem. 2013, 15, 1473–1476. [Google Scholar] [CrossRef]
- Yan, G.; Cao, Z.; Devine, D.; Penning, M.; Gately, N.M. Physical Properties of Shellac Material Used for Hot Melt Extrusion with Potential Application in the Pharmaceutical Industry. Polymers 2021, 13, 3723. [Google Scholar] [CrossRef]
- Baranović, G.; Šegota, S. Infrared Spectroscopy of Flavones and Flavonols. Reexamination of the Hydroxyl and Carbonyl Vibrations in Relation to the Interactions of Flavonoids with Membrane Lipids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 192, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Boeriu, C.G.; Bravo, D.; Gosselink, R.J.; van Dam, J.E. Characterisation of Structure-Dependent Functional Properties of Lignin with Infrared Spectroscopy. Ind. Crop. Prod. 2004, 20, 205–218. [Google Scholar] [CrossRef]
- Garside, P.; Wyeth, P. Identification of Cellulosic Fibres by FTIR Spectroscopy. Stud. Conserv. 2003, 48, 269–275. [Google Scholar] [CrossRef]
- Jaafar, J.; Siregar, J.P.; Tezara, C.; Hamdan, M.H.M.; Rihayat, T. A Review of Important Considerations in the Compression Molding Process of Short Natural Fiber Composites. Int. J. Adv. Manuf. Technol. 2019, 105, 3437–3450. [Google Scholar] [CrossRef]
- Teixeira, S.C.; Silva, R.R.A.; de Oliveira, T.V.; Stringheta, P.C.; Pinto, M.R.M.R.; Soares, N.d.F.F. Glycerol and Triethyl Citrate Plasticizer Effects on Molecular, Thermal, Mechanical, and Barrier Properties of Cellulose Acetate Films. Food Biosci. 2021, 42, 101202. [Google Scholar] [CrossRef]
- Asfaw, W.A.; Tafa, K.D.; Satheesh, N. Optimization of Citron Peel Pectin and Glycerol Concentration in the Production of Edible Film Using Response Surface Methodology. Heliyon 2023, 9, e13724. [Google Scholar] [CrossRef]
- Prashanth, B.H.M.; Gouda, P.S.S.; Manjunatha, T.S.; Navaneeth, I.M.; Chethan, K.M. Effect of Glycerin on Mechanical Properties of a Hybrid Kenaf-Jute Polyester Composite. Eng. Res. Express 2023, 5, 025034. [Google Scholar] [CrossRef]
- Lan, H.; Venkatesh, T. On the Relationships between Hardness and the Elastic and Plastic Properties of Isotropic Power-Law Hardening Materials. Philos. Mag. 2013, 94, 35–55. [Google Scholar] [CrossRef]
- Borba, M.; Della Bona, Á.; Cecchetti, D. Flexural Strength and Hardness of Direct and Indirect Composites. Braz. Oral Res. 2009, 23, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Castelló, M.L.; Dweck, J.; Aranda, D.A.G. Thermal Stability and Water Content Determination of Glycerol by Thermogravimetry. J. Therm. Anal. Calorim. 2009, 97, 627–630. [Google Scholar] [CrossRef]
- de Kergariou, C.; Le Duigou, A.; Popineau, V.; Gager, V.; Kervoelen, A.; Perriman, A.; Saidani-Scott, H.; Allegri, G.; Panzera, T.H.; Scarpa, F. Measure of Porosity in Flax Fibres Reinforced Polylactic Acid Biocomposites. Compos. Part A Appl. Sci. Manuf. 2020, 141, 106183. [Google Scholar] [CrossRef]
- Chen, L.; Bonaccurso, E. Electrowetting—From Statics to Dynamics. Adv. Colloid Interface Sci. 2014, 210, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ma, X.; Wang, W.; Gong, X.; Zhao, Y.; Mu, Q.; Xue, Z.; Liu, X.; Zheng, H.; Xu, W. Recent Advances in Gel Materials with Special Wettability: A Review. J. Mater. Sci. 2022, 57, 13179–13201. [Google Scholar] [CrossRef]
- Ouarhim, W.; Bensalah, M.-O.; Rodrigue, D.; Essabir, H.; Bouhfid, R.; Qaiss, A.e.K. Production and Characterization of High Density Polyethylene Reinforced by Eucalyptus Capsule Fibers. J. Bionic Eng. 2018, 15, 558–566. [Google Scholar] [CrossRef]
- Chanut, J.; Wang, Y.; Cin, I.D.; Ferret, E.; Gougeon, R.D.; Bellat, J.-P.; Karbowiak, T. Surface Properties of Cork: Is Cork a Hydrophobic Material? J. Colloid Interface Sci. 2021, 608, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-Based Biodegradable Materials: Challenges and Opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]
- Karimah, A.; Ridho, M.R.; Munawar, S.S.; Adi, D.S.; Ismadi; Damayanti, R.; Subiyanto, B.; Fatriasari, W.; Fudholi, A. A Review on Natural Fibers for Development of Eco-Friendly Bio-Composite: Characteristics, and Utilizations. J. Mater. Res. Technol. 2021, 13, 2442–2458. [Google Scholar] [CrossRef]
- Ben, Z.Y.; Samsudin, H.; Yhaya, M.F. Glycerol: Its Properties, Polymer Synthesis, and Applications in Starch Based Films. Eur. Polym. J. 2022, 175, 111377. [Google Scholar] [CrossRef]
- Bioucas, F.E.B.; Koller, T.M.; Fröba, A.P. Thermal Conductivity of Glycerol at Atmospheric Pressure Between 268 K and 363 K by Using a Steady-State Parallel-Plate Instrument. Int. J. Thermophys. 2024, 45, 52. [Google Scholar] [CrossRef]
- Onur, N. Appendix: Thermophysical Properties of Matter. In Introduction to Convective Heat Transfer; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- Shen, T.; Zhang, F.; Yang, S.; Wang, Y.; Liu, H.; Wang, H.; Hu, J. Comprehensive Study on the Pyrolysis Process of Chestnut Processing Waste (Chestnut Shells): Kinetic Triplet, Thermodynamic, in-Situ Monitoring of Evolved Gasses and Analysis Biochar. Fuel 2022, 331, 125944. [Google Scholar] [CrossRef]
- Mao, L.; Imam, S.; Gordon, S.; Cinelli, P.; Chiellini, E. Extruded Cornstarch-Glycerol-Polyvinyl Alcohol Blends: Mechanical Properties, Morphology, and Biodegradability. J. Polym. Environ. 2000, 8, 205–211. [Google Scholar] [CrossRef]






| Run | Independent Variables | Dependent Variables | |||||
|---|---|---|---|---|---|---|---|
| X1 a (%) | X2 (%) | X3 (°C) | Y1 (MPa) | Y2 (MPa) | |||
| Exp b | Pred c | Exp b | Pred c | ||||
| 1 | 65 | 15 | 120 | 5.9 ± 1.3 | 6.1 | 350 ± 120 | 343 |
| 2 | 65 | 30 | 100 | 0.55 ± 0.14 | 0.9 | 17.3 ± 3.8 | −3 |
| 3 | 75 | 15 | 100 | 3.21 ± 0.51 | 3.9 | 160 ± 17 | 160 |
| 4 | 75 | 0 | 80 | 3.9 ± 1.7 | 4.7 | 390 ± 120 | 470 |
| 5 | 75 | 30 | 80 | 0.177 ± 0.020 | −1.3 | 4.31 ± 0.65 | 3.8 |
| 6 | 85 | 15 | 120 | 4.17 ± 0.36 | 6.1 | 192 ± 17 | 225 |
| 7 | 75 | 0 | 120 | 9.90 ± 0.14 | 9.1 | 850 ± 280 | 910 |
| 8 | 65 | 0 | 100 | 8.7 ± 1.0 | 6.9 | 940 ± 230 | 840 |
| 9 | 75 | 15 | 100 | 4.3 ± 2.4 | 3.9 | 130 ± 20 | 160 |
| 10 | 65 | 15 | 80 | 0.44 ± 0.15 | 1.8 | 20 ± 11 | 90 |
| 11 | 75 | 30 | 120 | 3.6 ± 2.9 | 3.1 | 64 ± 18 | 54 |
| 12 | 85 | 30 | 100 | 1.04 ± 0.20 | 0.9 | 30.8 ± 5.3 | 61 |
| 13 | 85 | 15 | 80 | 1.40 ± 0.46 | 1.7 | 41 ± 11 | −22 |
| 14 | 75 | 15 | 100 | 2.46 ± 0.39 | 3.9 | 110 ± 19 | 160 |
| 15 | 85 | 0 | 100 | 6.83 ± 0.78 | 6.9 | 590 ± 130 | 540 |
| 16 | 75 | 15 | 100 | 5.42 ± 0.53 | 3.9 | 270 ± 73 | 160 |
| 17 | 75 | 15 | 100 | 4.27 ± 0.17 | 3.9 | 180 ± 41 | 160 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, S.B.; Freitas, O.M.; Vieira, E.F.; Gomes, A.; Carreiras, A.R.; Moreira, D.C.; Esfandiari, P.; Silva, J.F.; Delerue-Matos, C.; Domingues, V.F. Eco-Friendly Biocomposites from Chestnut Waste: Production, Optimization, Characterization, and Application. Polymers 2025, 17, 616. https://doi.org/10.3390/polym17050616
Silva SB, Freitas OM, Vieira EF, Gomes A, Carreiras AR, Moreira DC, Esfandiari P, Silva JF, Delerue-Matos C, Domingues VF. Eco-Friendly Biocomposites from Chestnut Waste: Production, Optimization, Characterization, and Application. Polymers. 2025; 17(5):616. https://doi.org/10.3390/polym17050616
Chicago/Turabian StyleSilva, Simão B., Olga M. Freitas, Elsa F. Vieira, Amália Gomes, Ana R. Carreiras, Diogo C. Moreira, Púria Esfandiari, João F. Silva, Cristina Delerue-Matos, and Valentina F. Domingues. 2025. "Eco-Friendly Biocomposites from Chestnut Waste: Production, Optimization, Characterization, and Application" Polymers 17, no. 5: 616. https://doi.org/10.3390/polym17050616
APA StyleSilva, S. B., Freitas, O. M., Vieira, E. F., Gomes, A., Carreiras, A. R., Moreira, D. C., Esfandiari, P., Silva, J. F., Delerue-Matos, C., & Domingues, V. F. (2025). Eco-Friendly Biocomposites from Chestnut Waste: Production, Optimization, Characterization, and Application. Polymers, 17(5), 616. https://doi.org/10.3390/polym17050616









