Agarose Hydrogels Enriched by Humic Acids as the Complexation Agent
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Hydrogels
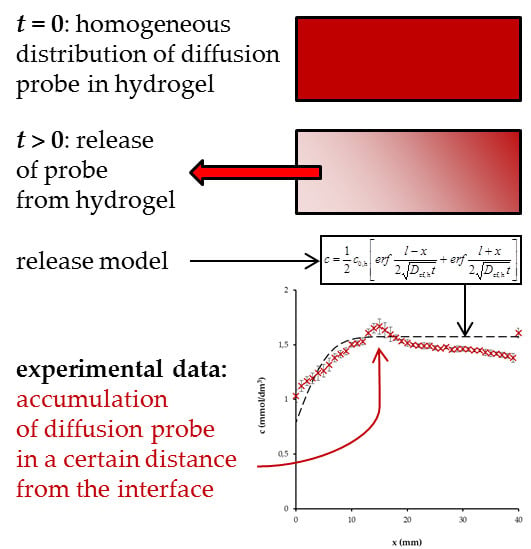
2.3. Diffusion-Release Experiments
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Djabourov, M.; Clark, A.H.; Rowlands, D.W.; Rossmurphy, S.B. Small-angle X-ray-scattering characterization of agarose sols and gels. Macromolecules 1989, 22, 180–188. [Google Scholar] [CrossRef]
- Fatin-Rouge, N.; Milon, A.; Buffle, J.; Goulet, R.R.; Tessier, A. Diffusion and partitioning of solutes in agarose hydrogels: The relative influence of electrostatic and specific interactions. J. Phys. Chem. B 2003, 107, 12126–12137. [Google Scholar] [CrossRef]
- Singh, T.; Meena, R.; Kumar, A. Effect of sodium sulfate on the gelling behavior of agarose and water structure inside the gel networks. J. Phys. Chem. B 2009, 113, 2519–2525. [Google Scholar] [CrossRef]
- Sourbh, T.; Jyoti, C.; Vinod, K.; Vijay, K.T. Progress in pectin based hydrogels for water purification: Trends and challenges. J. Environ. Manag. 2019, 238, 210–223. [Google Scholar]
- Sourbh, T.; Bhawna, S.; Anki, V.; Jyoti, C.; Sigitas, T.; Vijay, K.T. Recent progress in sodium alginate based sustainable hydrogels for environmental applications. J. Clean. Prod. 2018, 198, 143–159. [Google Scholar]
- Sourbh, T.; Penny, P.G.; Messai, A.M.; Sigitas, T.; Yogendra, K.M.; Vijay, K.T. Progress in lignin hydrogels and nanocomposites for water purification: Future perspectives. Vacuum 2017, 146, 342–355. [Google Scholar]
- Fernandez, E.; Lopez, D.; Mijangos, C.; Duskova-Smrckova, M.; Ilavsky, M.; Dusek, K. Rheological and thermal properties of agarose aqueous solutions and hydrogels. J. Polym. Sci. B 2008, 46, 322–328. [Google Scholar] [CrossRef]
- Barrangou, L.M.; Daubert, C.R.; Foegeding, E.A. Textural properties of agarose gels. I. Rheological and fracture properties. Food Hydrocolloid. 2006, 20, 184–195. [Google Scholar] [CrossRef]
- Barrangou, L.M.; Drake, M.; Daubert, C.R.; Foegeding, E.A. Textural properties of agarose gels. II. Relationships between rheological properties and sensory texture. Food Hydrocolloid. 2006, 20, 196–203. [Google Scholar] [CrossRef]
- Aymard, P.; Martin, D.R.; Plucknett, K.; Foster, T.J.; Clark, A.H.; Norton, I.T. Influence of thermal history on the structural and mechanical properties of agarose gels. Biopolymers 2001, 59, 131–144. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.J.; Huh, H.K.; Hwang, H.J.; Lee, S.J. Structural design of a double-layered porous hydrogel for effective mass transport. Biomicrofluidisc 2015, 9, 024104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanan, J.; Xiong, J.Y.; Liu, X.Y. Determination of agarose gel pore size: Absorbance measurements vis a vis other techniques. J. Phys. Conf. Ser. 2006, 28, 83–86. [Google Scholar] [CrossRef]
- Gong, J.P.; Hirota, N.; Kakugo, A.; Narita, T.; Osada, Y. Effect of aspect ratio on protein diffusion in hydrogels. J. Phys. Chem. B 2000, 104, 9904–9908. [Google Scholar] [CrossRef]
- Pluen, A.; Netti, P.A.; Jain, R.K.; Berk, D.A. Diffusion of macromolecules in agarose gels: Comparison of linear and globular configurations. Biophys. J. 1999, 77, 542–552. [Google Scholar] [CrossRef] [Green Version]
- Gutenwik, J.; Nilsson, B.; Axelsson, A. Determination of protein diffusion coefficients in agarose gel with a diffusion cell. Biochem. Eng. J. 2004, 19, 1–7. [Google Scholar] [CrossRef]
- Golmohamadi, M.; Davis, T.A.; Wilkinson, K.J. Diffusion and partitioning of cations in an agarose hydrogel. J. Phys. Chem. A 2012, 116, 6505–6510. [Google Scholar] [CrossRef]
- Doi, M.; Edwards, S.F. The Theory of Polymer Dynamics; Oxford University Press: Oxford, UK, 1986. [Google Scholar]
- De Gennes, P.G. Sealing Concepts in Polymer Physics; Cornell University Press: Ithac, NY, USA, 1979. [Google Scholar]
- Wang, Y.; Ding, S.; Gong, M.; Xu, S.; Xu, W.; Zhang, C. Diffusion characteristics of agarose hydrogel used in diffusive gradients in thin films for measurements of cations and anions. Anal. Chim. Acta 2016, 945, 47–56. [Google Scholar] [CrossRef]
- Urík, J.; Vrána, B. An improved design of a passive sampler for polar organic compounds based on diffusion in agarose hydrogel. Environ. Sci. Pollut. Res. 2019, 26, 15273–15284. [Google Scholar] [CrossRef]
- Sedláček, P.; Smilek, J.; Klučáková, M. How interactions with polyelectrolytes affect mobility of low molecular ions - Results from diffusion cells. React. Funct. Polym. 2013, 73, 1500–1509. [Google Scholar] [CrossRef]
- Sedláček, P.; Smilek, J.; Klučáková, M. How interactions with polyelectrolytes affect mobility of low molecular ions – 2. Non-stationary diffusion experiments. React. Funct. Polym. 2014, 75, 41–50. [Google Scholar] [CrossRef]
- Smilek, J.; Sedláček, P.; Kalina, M.; Klučáková, M. On the role of humic acids’ carboxyl groups in the binding of charged organic compounds. Chemosphere 2015, 138, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Klučáková, M.; Smilek, J.; Sedláček, P. How humic acids affect the rheological and transport properties of hydrogels. Molecules 2019, 24, 1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klučáková, M.; Pekař, M. Transport of copper (II) ions in humic gel—New results from diffusion couple. Colloid. Surf. A 2009, 349, 96–101. [Google Scholar] [CrossRef]
- Klučáková, M.; Jarábková, S.; Velcer, T.; Kalina, M.; Pekař, M. Transport of a model diffusion probe in polyelectrolyte-surfactant hydrogels. Colloid. Surf. A 2019, 573, 73–79. [Google Scholar] [CrossRef]
- Klučáková, M.; Pekař, M. Study of Structure and properties of humic and fulvic acids. III. Study of complexation of Cu2+ ions with humic acid in sols. J. Polym. Mater. 2003, 20, 145–154. [Google Scholar]
- Klučáková, M.; Pekař, M. Study of structure and properties of humic and fulvic acids. IV. Study of interactions of Cu2+ ions with humic gels and final comparison. J. Polym. Mater. 2003, 20, 155–162. [Google Scholar]
- Klučáková, M.; Kalina, M.; Smilek, J.; Laštůvková, M. The transport of metal ions in hydrogels containing humic acids as active complexation agent. Colloid. Surf. A 2018, 557, 116–122. [Google Scholar] [CrossRef]
- Manceau, A.; Matynia, A. The nature of Cu bonding to natural organic matter. Geochim. Cosmochim. Acta 2010, 74, 2556–2580. [Google Scholar] [CrossRef]
- Xu, J.; Tan, W.; Xiong, J.; Wang, M.; Fang, L.; Koopal, L.K. Copper binding to soil fulvic and humic acids: NICA-Donnan modeling and conditional affinity spectra. J. Colloid Interface Sci. 2016, 473, 141–151. [Google Scholar] [CrossRef]
- Sierra, J.; Roig, N.; Gimenez Papiol, G.; Perez-Gallego, E.; Schuhmacher, M. Prediction of the bioavailability of potentially toxic elements in freshwaters. Comparison between speciation models and passive samplers. Sci. Total Environ. 2017, 605–606, 211–218. [Google Scholar] [CrossRef]
- Baek, K.; Yang, J.-W. Humic-substance-enhanced ultrafiltration for removal of heavy metals. Sep. Sci. Technol. 2005, 40, 699–708. [Google Scholar] [CrossRef]
- Humic-Substances.Org. Available online: https://www.humic-substances.org (accessed on 24 February 2020).
- Ritchie, J.D.; Perdue, E.M. Proton-binding study of standard and reference fulvic acids, humic acids, and natural organic matter. Geochim. Cosmochim. Acta 2003, 67, 85–96. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarendon Press: Oxford, UK, 1975. [Google Scholar]
- Cussler, E.L. Diffusion: Mass Transfer in Fluid Systems, 2nd ed.; Cambridge University Press: Cambridge, UK, 1984. [Google Scholar]
- Haynes, W.M. Handbook of Chemistry and Physics, 93rd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Leaist, D.G. The effect of aggregation, counterion binding, and added NaCl on diffusion of aqueous methylene blue. Can. J. Chem. 1988, 66, 2452–2456. [Google Scholar] [CrossRef]
- Samprovalaki, K.; Robbins, P.T.; Fryer, P.J. Investigation of the diffusion of dyes in agar gels. J. Food Eng. 2012, 111, 537–545. [Google Scholar] [CrossRef]
- Gendron, P.O.; Avaltroni, F.; Wilkinson, K.J. Diffusion coefficients of several rhodamine derivatives as determined by pulsed field gradient-nuclear magnetic resonance and fluorescence correlation spectroscopy. J. Fluoresc. 2008, 18, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Manek, A.; Nizogi, S.; Hudson, J. Determination of dynamic metal complexes and their diffusion coefficients in the presence of different humic substances by combining two analytical techniques. Anal. Lett. 2014, 47, 1224–1241. [Google Scholar] [CrossRef]
- Zhang, H.; Davison, W. Diffusional characteristics of hydrogels used in DGT and DET techniques. Anal. Chim. Acta 1999, 398, 329–340. [Google Scholar] [CrossRef]
- Majer, G.; Melchior, J.P. Characterization of the fluorescence correlation spectroscopy (FCS) standard Rhodamine 6G and calibration of its diffusion coefficient in aqueous solutions. J. Chem. Phys. 2014, 140, 094201. [Google Scholar] [CrossRef]
- Klucakova, M.; Veznikova, K. The role of concentration and solvent character in the molecular organization of humic acids. Molecules 2016, 21, 1410. [Google Scholar] [CrossRef] [Green Version]
- Klucakova, M.; Veznikova, K. Micro-organization of humic acids in aqueous solutions. J. Mol. Struct. 2017, 1144, 33–40. [Google Scholar] [CrossRef]
- Piccolo, A. The supramolecular structure of humic substances. Soil Sci. 2001, 166, 810–832. [Google Scholar] [CrossRef] [Green Version]
- Ramirez Coutino, V.A.; Torres Bustillos, L.G.; Godinez Mora Tovar, L.A.; Guerra Sanchez, R.J.; Rodriguez Valadez, F.J. pH effect on surfactant properties and supramolecular structure of humic substances obtained from sewage sludge composting. Rev. Int. Contam. Ambie. 2013, 29, 191–199. [Google Scholar]
- Fischer, T. Humic supramolecular structures have polar surfaces and unpolar cores in native soil. Chemosphere 2017, 183, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Tarasevich, Y.I.; Dolenko, S.A.; Trifonova, M.Y.; Alekseenko, E.Y. Association and colloid-chemical properties of humic acids in aqueous solutions. Colloid J. 2013, 75, 207–213. [Google Scholar] [CrossRef]
- Baalousha, M.; Motelica-Heino, M.; Galaup, S.; Le Coustumer, P. Supramolecular structure of humic acids by TEM with improved sample preparation and staining. Microsc. Res. Technol. 2005, 66, 299–306. [Google Scholar] [CrossRef]
- Klucakova, M.; Kalina, M. Diffusivity of Cu (II) ions in humic gels—influence of reactive functional groups of humic acids. Colloid. Surface. A 2015, 483, 162–170. [Google Scholar] [CrossRef]
- Terdale, S.; Tantray, A. Spectroscopic study of the dimerization of rhodamine 6G in water and different organic solvents. J. Mol. Liq. 2017, 225, 662–671. [Google Scholar] [CrossRef]
- Talap, P.D. Self-aggregation of Rhodamine—6G in aqueous medium and aqueous solution of Bu4NBr. Arch. Appl. Sci. Res. 2014, 6, 183–187. [Google Scholar]
- Florence, N.; Naorem, H. Study on the effect of an electrolyte on the self-aggregation and the geometry of the dye aggregates of methylene blue in aqueous media. J. Surf. Sci. Technol. 2016, 32, 28–34. [Google Scholar] [CrossRef]
- Moreno-Vasilda, I.; Torres-Gallegos, C.; Araya-Hermosilla, R.; Nishide, H. Influence of the linear aromatic density on methylene blue aggregation around polyanions containing sulfonate groups. J. Phys. Chem. B 2010, 114, 4151–4158. [Google Scholar] [CrossRef]
- Marras-Marquez, T.; Pena, J.; Veiga-Ochoa, M.D. Agarose drug delivery systems upgraded by surfactants inclusion: Critical role of the pore architecture. Carbohydr. Polym. 2014, 103, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Chen, C.; Zhao, D.; Wang, X.; Zhao, L.; Shi, H.; Ma, G.; Su, Z. Pore size analysis from low field NMR spin–spin relaxation measurements of porous microspheres. J. Porous Mater. 2015, 22, 11–20. [Google Scholar] [CrossRef]



| Hydrogel | Cu: c0,h (mmol.dm−3) | MB: c0,h (mmol.dm−3) | RH: c0,h (mmol.dm−3) |
|---|---|---|---|
| AG | 85.3 ± 7.1 | 7.6 ± 0.4 | 6.4 ± 0.1 |
| AG–ESHA | 91.2 ± 7.6 | 6.9 ± 0.6 | 6.0 ± 0.2 |
| AG–PPHA | 90.8 ± 5.9 | 5.7 ± 0.3 | 4.6 ± 0.1 |
| AG–SRHA | 88.6 ± 6.6 | 5.2 ± 0.2 | 4.4 ± 0.2 |
| AG–LEHA | 96.2 ± 8.2 | 7.3 ± 0.2 | 5.7 ± 0.1 |
| Hydrogel | Cu: Def,h (10−10 m2·s−1) | MB: Def,h (10−11 m2·s-1) | RH: Def,h (10−11 m2·s−1) |
|---|---|---|---|
| AG | 12.21 ± 0.72 | 4.16 ± 0.13 | 5.21 ± 0.16 |
| AG–ESHA | 6.40 ± 0.30 | 1.25 ± 0.10 | 1.33 ± 0.06 |
| AG–PPHA | 7.34 ± 0.36 | 1.86 ± 0.21 | 3.30 ± 0.25 |
| AG–SRHA | 8.39 ± 0.30 | 0.93 ± 0.04 | 3.76 ± 0.25 |
| AG–LEHA | 8.76 ± 0.38 | 1.26 ± 0.06 | 1.49 ± 0.07 |
| Hydrogel | Cu: K (-) | MB: K (-) | RH: K (-) |
|---|---|---|---|
| AG–ESHA | 0.64 ± 0.03 | 2.32 ± 0.19 | 2.90 ± 0.13 |
| AG–PPHA | 0.22 ± 0.01 | 1.23 ± 0.07 | 0.58 ± 0.04 |
| AG–SRHA | 0.37 ± 0.01 | 3.45 ± 0.15 | 0.38 ± 0.02 |
| AG–LEHA | 0.89 ± 0.01 | 2.29 ± 0.11 | 2.49 ± 0.12 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klučáková, M. Agarose Hydrogels Enriched by Humic Acids as the Complexation Agent. Polymers 2020, 12, 687. https://doi.org/10.3390/polym12030687
Klučáková M. Agarose Hydrogels Enriched by Humic Acids as the Complexation Agent. Polymers. 2020; 12(3):687. https://doi.org/10.3390/polym12030687
Chicago/Turabian StyleKlučáková, Martina. 2020. "Agarose Hydrogels Enriched by Humic Acids as the Complexation Agent" Polymers 12, no. 3: 687. https://doi.org/10.3390/polym12030687