Hybrid Sol–gel Coatings for Corrosion Mitigation: A Critical Review
Abstract
1. Introduction
- 1)
- Adding organic precursors that are soluble in the reactional media (where hydrolysis/condensation reactions take place), although do not take part in the gel formation. The OIH material obtained by this route will display an organic component bonded to the inorganic network by van der Waals forces, or ionic or hydrogen bonds.
- 2)
- Adding organic alkoxides (R’M(OR)x), in which an organic group R’ is bonded to the element M and it is not hydrolysable. In this case, the organic and inorganic components establish covalent bonds.
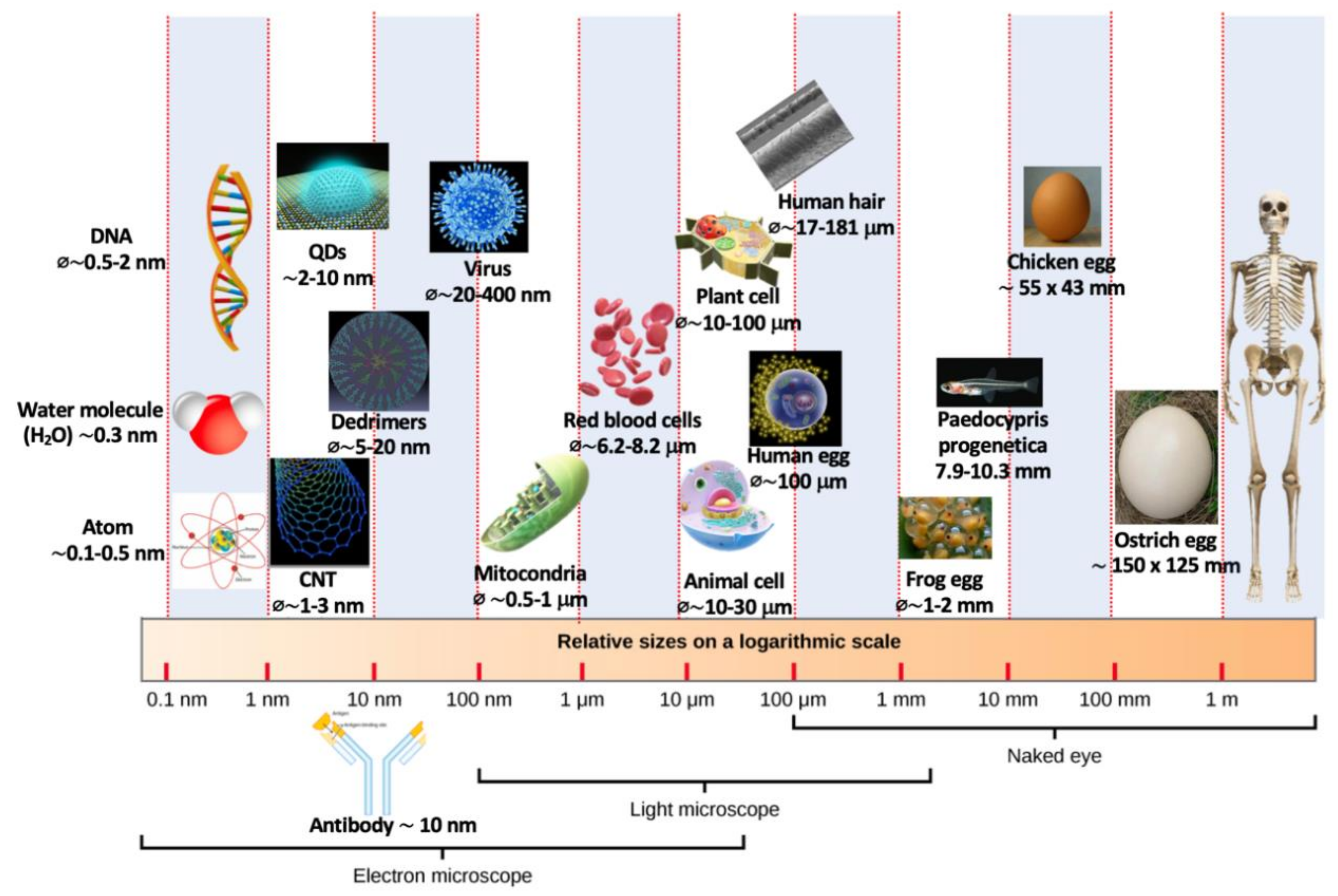
2. Sol–gel Process: A Historical Perspective and Applications
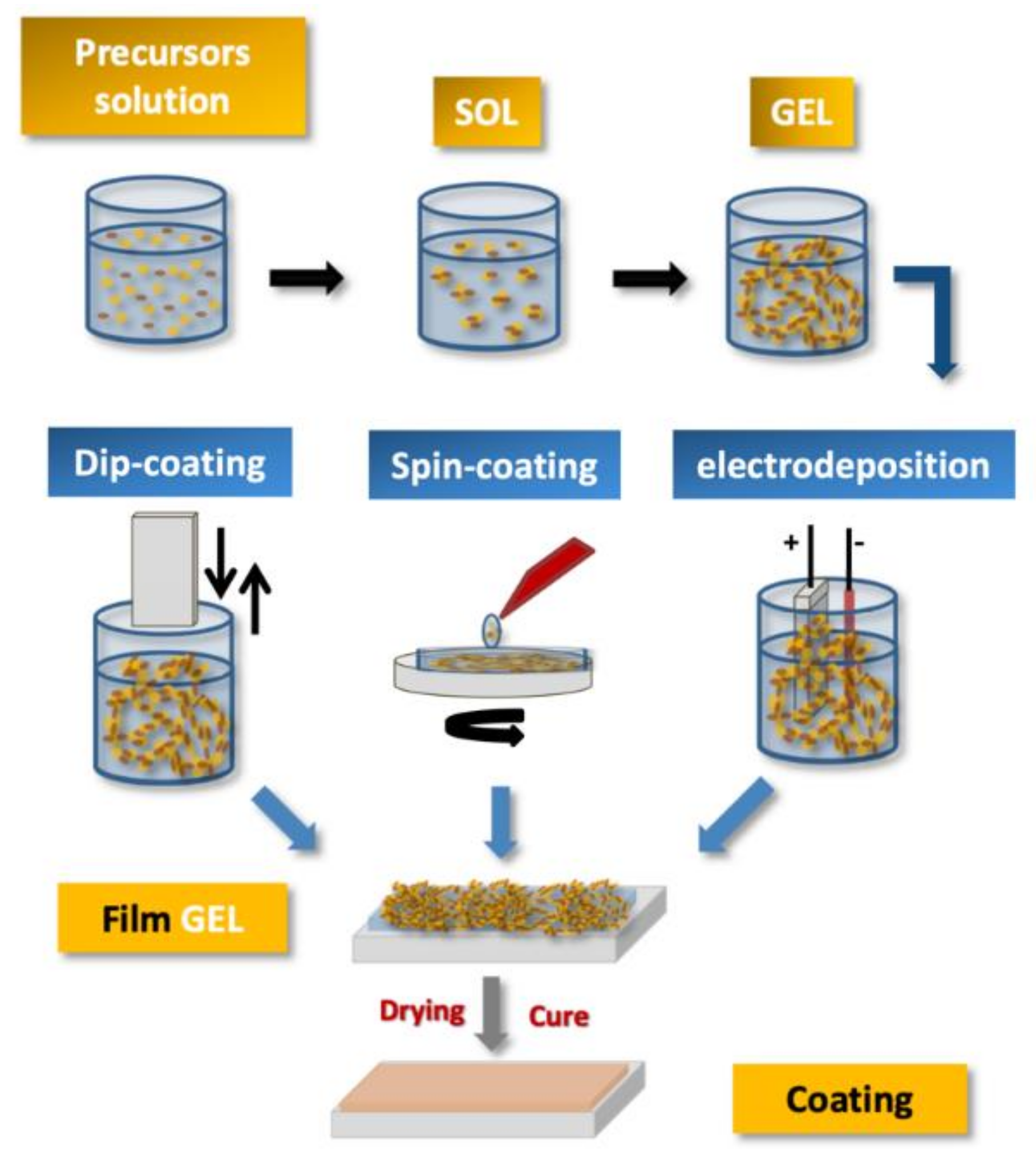
3. General Concepts of the Sol–gel Process.

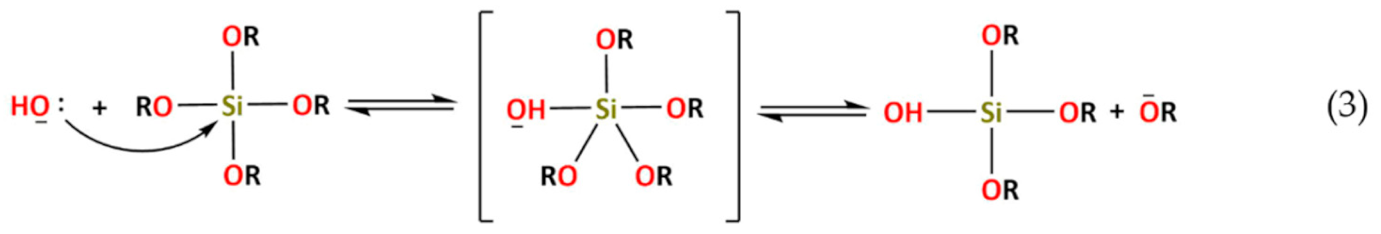
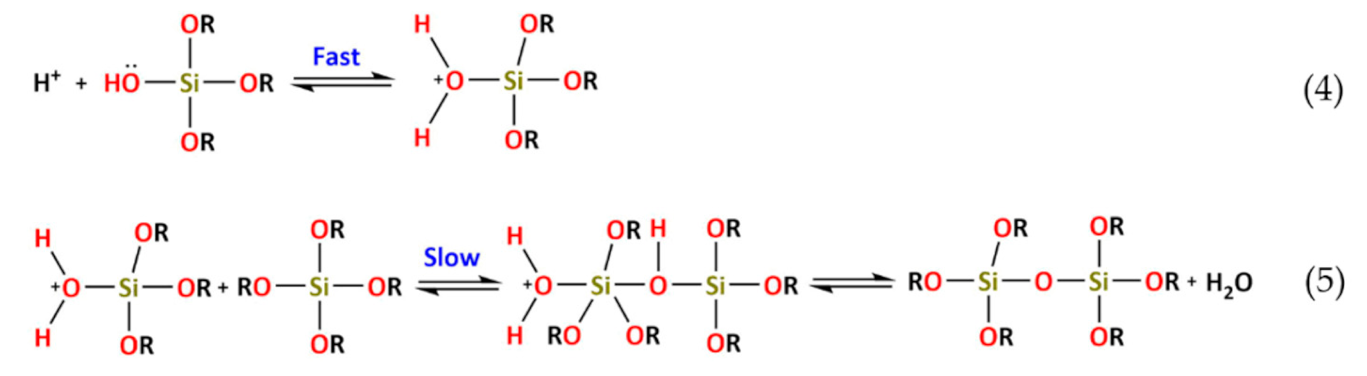

4. Hybrid Sol–gel Coatings for Corrosion Mitigation
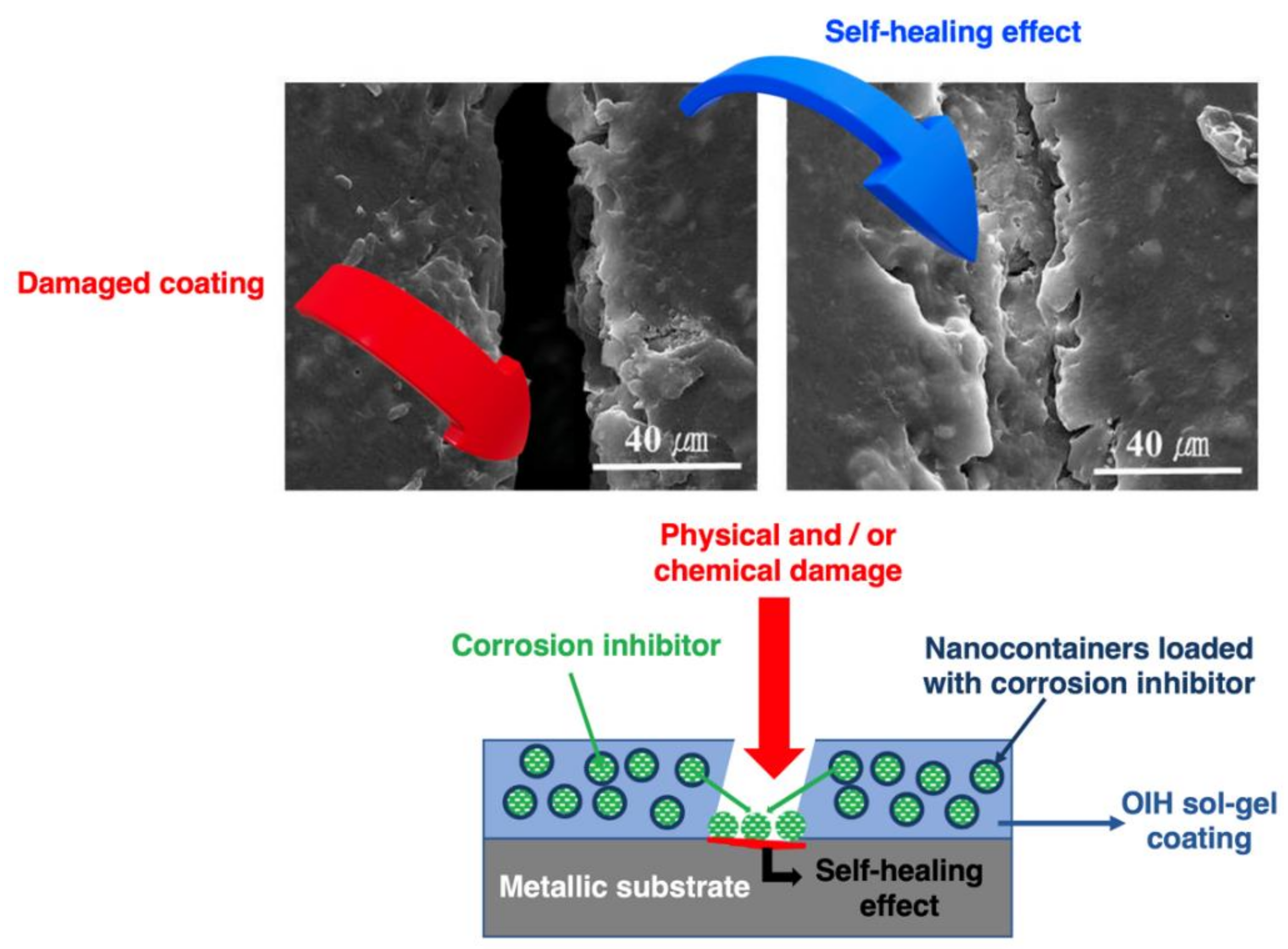
4.1. Sol–gel Coatings with Self-healing Function
4.2. Sol–gel Coatings with Anti-Fouling Function
4.3. Coatings with Superhydrophobic Function
5. Challenges and Prospects for the Future Research
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-D | Three-dimensional |
| EIS | Electrochemical impedance spectroscopy |
| CAH | Contact angle hysteresis |
| CNT | Carbon nanotubes |
| DNA | Deoxyribonucleic acid |
| GDP | Gross domestic product |
| LEIS | Localized electrochemical impedance spectroscopy |
| LTS | Lanthanum triflate salt |
| MIC | Microbiologically influenced corrosion |
| MTES | Methyltriethoxy-silane |
| NPs | Nanoparticles |
| OIH | Organic-inorganic hybrid |
| PDP | Potentiodynamic polarization |
| QDs | Quantum dots |
| SIET | Scanning ion-selective electrode technique |
| SVET | Scanning vibrating electrode technique |
| SKP | Scanning Kelvin probe |
| TEOS | Tetraethoxysilane |
| WCA | Water contact angle |
References
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Boston, MA, USA, 1990; ISBN 0-12-134970-5. [Google Scholar]
- Sanchez, C.; Belleville, P.; Popall, M.; Nicole, L. Hybrid materials themed issue. Chem. Soc. Rev. 2011, 40, 453–1152. [Google Scholar]
- Sanchez, C.; Ribot, F. Design of hybrid organic-inorganic materials synthesized via sol-gel chemistry. New J. Chem. 1994, 18, 1007–1047. [Google Scholar]
- Judeinstein, P.; Sanchez, C. Hybrid organic–inorganic materials: A land of multidisciplinarity. J. Mater. Chem. 1996, 6, 511–525. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- CRC Concise Encyclopedia of Nanotechnology—CRC Press Book. Available online: https://www.crcpress.com/CRC-Concise-Encyclopedia-of-Nanotechnology/Kharisov-Kharissova-Ortiz-Mendez/p/book/9781466580343 (accessed on 4 March 2020).
- Zheludkevich, M.L.; Salvado, I.M.; Ferreira, M.G.S. Sol–gel coatings for corrosion protection of metals. J. Mater. Chem. 2005, 15, 5099. [Google Scholar] [CrossRef]
- Pierre, A.C. Applications of Sol-Gel Processing. In Introduction to Sol-Gel Processing; The Kluwer International Series in Sol-Gel Processing: Technology and Applications; Springer: New York, NY, USA, 1998; pp. 347–386. ISBN 978-0-7923-8121-1. [Google Scholar]
- Benvenutti, E.V.; Moro, C.C.; Costa, T.M.H.; Gallas, M.R. Materiais híbridos à base de sílica obtidos pelo método sol-gel. Química Nova 2009, 32, 1926–1933. [Google Scholar] [CrossRef]
- José, N.M.; de Prado, L.A.S.A. Materiais Híbridos Orgânico-Inorgânicos: Preparação e Algumas Aplicações. Quimica Nova 2005, 28, 281–288. [Google Scholar] [CrossRef]
- Nedeljko, P.; Turel, M.; Lobnik, A. Hybrid sol-gel based sensor layers for optical determination of biogenic amines. Sens. Actuators B Chem. 2017, 246, 1066–1073. [Google Scholar] [CrossRef]
- Mensing, J.P.; Wisitsoraat, A.; Tuantranont, A.; Kerdcharoen, T. Inkjet-printed sol–gel films containing metal phthalocyanines/porphyrins for opto-electronic nose applications. Sens. Actuators B Chem. 2013, 176, 428–436. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R. Hybrid Sol-Gel Coatings for Surface Protection. In World Scientific Reference of Hybrid Materials; World Scientific Series in Nanoscience and Nanotechnology; World Scientific: Singapore, 2019; pp. 145–192. ISBN 978-981-327-055-8. [Google Scholar]
- Figueira, R.B.; Silva, C.J.; Pereira, E.V.; Salta, M.M. Ureasilicate Hybrid Coatings for Corrosion Protection of Galvanized Steel in Cementitious Media. J. Electrochem. Soc. 2013, 160, C467–C479. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.; Pereira, E.V.; Salta, M.M. Alcohol-Aminosilicate Hybrid Coatings for Corrosion Protection of Galvanized Steel in Mortar. J. Electrochem. Soc. 2014, 161, C349–C362. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Hot-dip galvanized steel dip-coated with ureasilicate hybrid in simulated concrete pore solution: Assessment of coating morphology and corrosion protection efficiency. Prog. Org. Coat. 2015, 88, 245–255. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.; Pereira, E.V. Ureasilicate Hybrid Coatings for Corrosion Protection of Galvanized Steel in Chloride-Contaminated Simulated Concrete Pore Solution. J. Electrochem. Soc. 2015, 162, C666–C676. [Google Scholar] [CrossRef]
- Ismail, W.N.W. Sol–gel technology for innovative fabric finishing—A Review. J. Sol.-Gel Sci. Technol 2016, 78, 698–707. [Google Scholar] [CrossRef]
- Aklalouch, M.; Calleja, A.; Granados, X.; Ricart, S.; Boffa, V.; Ricci, F.; Puig, T.; Obradors, X. Hybrid sol–gel layers containing CeO2 nanoparticles as UV-protection of plastic lenses for concentrated photovoltaics. Sol. Energy Mater. Sol. Cells 2014, 120 Pt. A, 175–182. [Google Scholar] [CrossRef]
- Rathinamala, I.; Jeyakumaran, N.; Prithivikumaran, N. Sol-gel assisted spin coated CdS/PS electrode based glucose biosensor. Vacuum 2019, 161, 291–296. [Google Scholar] [CrossRef]
- Wu, J.; Fu, Z.; Yan, F.; Ju, H. Biomedical and clinical applications of immunoassays and immunosensors for tumor markers. TrAC Trends Anal. Chem. 2007, 26, 679–688. [Google Scholar] [CrossRef]
- Chen, Y. Synthesis of Hollow Mesoporous Silica Nanoparticles by Silica-Etching Chemistry for Biomedical Applications. In Design, Synthesis, Multifunctionalization and Biomedical Applications of Multifunctional Mesoporous Silica-Based Drug Delivery Nanosystems; Springer Theses; Springer: Berlin/Heidelberg, Germany, 2016; pp. 31–46. ISBN 978-3-662-48620-7. [Google Scholar]
- Razo-Medina, D.A.; Trejo-Durán, M.; Alvarado-Méndez, E. Cholesterol biosensor based on a plastic optical fibre with sol–gel: Structural analysis and sensing properties. J. Mod. Opt. 2018, 65, 348–352. [Google Scholar] [CrossRef]
- Panneerselvam, S.; Choi, S. Nanoinformatics: Emerging Databases and Available Tools. Int. J. Mol. Sci. 2014, 15, 7158–7182. [Google Scholar] [CrossRef] [PubMed]
- Le Bail, N.; Benayoun, S.; Toury, B. Mechanical properties of sol–gel coatings on polycarbonate: A review. J. Sol.-Gel Sci. Technol. 2015, 75, 710–719. [Google Scholar] [CrossRef]
- Lionti, K.; Toury, B.; Boissière, C.; Benayoun, S.; Miele, P. Hybrid silica coatings on polycarbonate: Enhanced properties. J. Sol.-Gel Sci. Technol. 2013, 65, 52–60. [Google Scholar] [CrossRef]
- Çakır, M. Investigation of Coating Performance of UV-Curable Hybrid Polymers Containing 1H,1H,2H,2H-Perfluorooctyltriethoxysilane Coated on Aluminum Substrates. Coatings 2017, 7, 37. [Google Scholar] [CrossRef]
- Rivero, P.J.; Maeztu, J.D.; Berlanga, C.; Miguel, A.; Palacio, J.F.; Rodriguez, R. Hydrophobic and Corrosion Behavior of Sol-Gel Hybrid Coatings Based on the Combination of TiO2 NPs and Fluorinated Chains for Aluminum Alloys Protection. Metals 2018, 8, 1076. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Organic–inorganic hybrid sol–gel coatings for metal corrosion protection: A review of recent progress. J. Coat. Technol. Res. 2015, 12, 1–35. [Google Scholar] [CrossRef]
- Lei, L.; Cao, Z.; Xie, Q.; Fu, Y.; Tan, Y.; Ma, M.; Yao, S. One-pot electrodeposition of 3-aminopropyltriethoxysilane–chitosan hybrid gel film to immobilize glucose oxidase for biosensing. Sens. Actuators B Chem. 2011, 157, 282–289. [Google Scholar] [CrossRef]
- Yu, M.; Xue, B.; Liu, J.; Li, S.; Zhang, Y. Electrophoretic deposition of hybrid coatings on aluminum alloy by combining 3-aminopropyltrimethoxysilan to silicon–zirconium sol solutions for corrosion protection. Thin Solid Film. 2015, 590, 33–39. [Google Scholar] [CrossRef]
- Kim, E.K.; Won, J.; Do, J.; Kim, S.D.; Kang, Y.S. Effects of silica nanoparticle and GPTMS addition on TEOS-based stone consolidants. J. Cult. Herit. 2009, 10, 214–221. [Google Scholar] [CrossRef]
- Mrad, M.; Montemor, M.F.; Dhouibi, L.; Triki, E. Deposition of hybrid 3-GPTMS’s film on AA2024-T3: Dependence of film morphology and protectiveness performance on coating conditions. Prog. Org. Coat. 2012, 73, 264–271. [Google Scholar] [CrossRef]
- Sanchez, C.; Rozes, L.; Ribot, F.; Laberty-Robert, C.; Grosso, D.; Sassoye, C.; Boissiere, C.; Nicole, L. “Chimie douce”: A land of opportunities for the designed construction of functional inorganic and hybrid organic-inorganic nanomaterials. Comptes Rendus Chim. 2010, 13, 3–39. [Google Scholar] [CrossRef]
- Figueira, R.B.; Fontinha, I.R.; Silva, C.J.R.; Pereira, E.V. Hybrid Sol-Gel Coatings: Smart and Green Materials for Corrosion Mitigation. Coatings 2016, 6, 12. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R. Application of Sol–Gel Method to Synthesize Organic–Inorganic Hybrid Coatings to Minimize Corrosion in Metallic Substrates. In Hybrid Organic-Inorganic Interfaces; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 355–412. ISBN 978-3-527-80713-0. [Google Scholar]
- Van Ooij, W.J.; Sabata, A. Characterization of films of organofunctional silanes by TOFSIMS and XPS. J. Adhes. Sci. Technol. 1991, 5, 843–863. [Google Scholar] [CrossRef]
- Verma, a.R.B.; van Ooij, W.J. High-temperature batch hot-dip galvanizing. Part 1. General description of coatings formed at 560 °C. Surf. Coat. Technol. 1997, 89, 132–142. [Google Scholar] [CrossRef]
- Fedrizzi, L.; Terryn, H.; Simoes, A. Innovative Pre-Treatment Techniques to Prevent Corrosion of Metallic Surfaces; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-1-84569-368-8. [Google Scholar]
- Pehkonen, S.O.; Yuan, S. Tailored Thin Coatings for Corrosion Inhibition Using a Molecular Approach; Academic Press: Cambridge, MA, USA, 2018; ISBN 978-0-12-813585-3. [Google Scholar]
- Ferreira, M.G.S.; Duarte, R.G.; Montemor, M.F.; Simões, A.M.P. Silanes and rare earth salts as chromate replacers for pre-treatments on galvanised steel. Electrochim. Acta 2004, 49, 2927–2935. [Google Scholar] [CrossRef]
- Figueira, R.B. Hybrid Sol-Gel Coatings: Erosion-Corrosion Protection. Prod. Prop. Appl. High. Temp. Coat. 2018, 334–380. [Google Scholar]
- Bhandari, J.; Khan, F.; Abbassi, R.; Garaniya, V.; Ojeda, R. Modelling of pitting corrosion in marine and offshore steel structures—A technical review. J. Loss Prev. Process. Ind. 2015, 37, 39–62. [Google Scholar] [CrossRef]
- Price, S.; Figueira, R. Corrosion Protection Systems and Fatigue Corrosion in Offshore Wind Structures: Current Status and Future Perspectives. Coatings 2017, 7, 25. [Google Scholar] [CrossRef]
- The Global Cost and Impact of Corrosion. Available online: https://inspectioneering.com/news/2016-03-08/5202/nace-study-estimates-global-cost-of-corrosion-at-25-trillion-ann (accessed on 28 January 2020).
- Market Research Reports, Industry Analysis, Business Overview & Trends—360 Market Updates. Available online: https://www.360marketupdates.com/aboutus (accessed on 27 January 2020).
- Tedim, J.; Poznyak, S.K.; Kuznetsova, A.; Raps, D.; Hack, T.; Zheludkevich, M.L.; Ferreira, M.G.S. Enhancement of Active Corrosion Protection via Combination of Inhibitor-Loaded Nanocontainers. ACS Appl. Mater. Interfaces 2010, 2, 1528–1535. [Google Scholar] [CrossRef]
- Lakshmi, R.V.; Aruna, S.T.; Sampath, S. Ceria nanoparticles vis-à-vis cerium nitrate as corrosion inhibitors for silica-alumina hybrid sol-gel coating. Appl. Surf. Sci. 2017, 393, 397–404. [Google Scholar]
- Yasakau, K.A.; Ferreira, M.G.S.; Zheludkevich, M.L. Sol-Gel Coatings with Nanocontainers of Corrosion Inhibitors for Active Corrosion Protection of Metallic Materials. In Handbook of Sol-Gel Science and Technology; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–37. ISBN 978-3-319-19454-7. [Google Scholar]
- Varma, P.C.R.; Colreavy, J.; Cassidy, J.; Oubaha, M.; McDonagh, C.; Duffy, B. Corrosion protection of AA 2024-T3 aluminium alloys using 3, 4-diaminobenzoic acid chelated zirconium–silane hybrid sol–gels. Thin Solid Film. 2010, 518, 5753–5761. [Google Scholar] [CrossRef]
- Nourani-Vatani, M.; Ganjali, M.; Solati-Hashtjin, M.; Zarrintaj, P.; Reza Saeb, M. Zirconium-based hybrid coatings: A versatile strategy for biomedical engineering applications. Mater. Today Proc. 2018, 5, 15524–15531. [Google Scholar] [CrossRef]
- Adraider, Y.; Pang, Y.X.; Nabhani, F.; Hodgson, S.N.; Sharp, M.C.; Al-Waidh, A. Fabrication of zirconium oxide coatings on stainless steel by a combined laser/sol–gel technique. Ceram. Int. 2013, 39, 9665–9670. [Google Scholar] [CrossRef]
- Yoganandan, G.; Pradeep Premkumar, K.; Balaraju, J.N. Evaluation of corrosion resistance and self-healing behavior of zirconium–cerium conversion coating developed on AA2024 alloy. Surf. Coat. Technol. 2015, 270, 249–258. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F.; Giovanardi, R.; Veronesi, P. Corrosion behavior and mechanical properties of bioactive sol-gel coatings on titanium implants. Mater. Sci. Eng. C 2014, 43, 375–382. [Google Scholar] [CrossRef]
- Wen, C.E.; Xu, W.; Hu, W.Y.; Hodgson, P.D. Hydroxyapatite/titania sol-gel coatings on titanium-zirconium alloy for biomedical applications. Acta Biomater. 2007, 3, 403–410. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Giovanardi, R.; Veronesi, P. Modification of Ti6Al4V implant surfaces by biocompatible TiO2/PCL hybrid layers prepared via sol-gel dip coating: Structural characterization, mechanical and corrosion behavior. Mater. Sci. Eng. C 2017, 74, 501–507. [Google Scholar] [CrossRef]
- Thai, T.T.; Trinh, A.T.; Olivier, M.-G. Hybrid sol–gel coatings doped with cerium nanocontainers for active corrosion protection of AA2024. Prog. Org. Coat. 2020, 138, 105428. [Google Scholar] [CrossRef]
- Zhang, D.; Yuan, T.; Wei, G.; Wang, H.; Gao, L.; Lin, T. Preparation of self-healing hydrophobic coating on AA6061 alloy surface and its anti-corrosion property. J. Alloy. Compd. 2019, 774, 495–501. [Google Scholar] [CrossRef]
- Stankiewicz, A. 14—Self-healing nanocoatings for protection against steel corrosion. In Nanotechnology in Eco-Efficient Construction, 2nd ed.; Pacheco-Torgal, F., Diamanti, M.V., Nazari, A., Granqvist, C.G., Pruna, A., Amirkhanian, S., Eds.; Woodhead Publishing Series in Civil and Structural Engineering; Woodhead Publishing: Sawston, UK; Cambridge, UK, 2019; pp. 303–335. ISBN 978-0-08-102641-0. [Google Scholar]
- Habib, S.; Khan, A.; Nawaz, M.; Sliem, M.H.; Shakoor, R.A.; Kahraman, R.; Abdullah, A.M.; Zekri, A. Self-Healing Performance of Multifunctional Polymeric Smart Coatings. Polymers 2019, 11, 1519. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Kuznetsova, A.; Kallip, S.; Starykevich, M.; Tedim, J.; Ferreira, M.G.S.; Zheludkevich, M.L. A novel bilayer system comprising LDH conversion layer and sol-gel coating for active corrosion protection of AA2024. Corros. Sci. 2018, 143, 299–313. [Google Scholar] [CrossRef]
- Montemor, M.F.; Vicente, C. Functional Self-Healing Coatings: A New Trend in Corrosion Protection by Organic Coatings. In Encyclopedia of Interfacial Chemistry; Wandelt, K., Ed.; Elsevier: Oxford, UK, 2018; pp. 236–249. ISBN 978-0-12-809894-3. [Google Scholar]
- Calado, L.M.; Taryba, M.G.; Carmezim, M.J.; Montemor, M.F. Self-healing ceria-modified coating for corrosion protection of AZ31 magnesium alloy. Corros. Sci. 2018, 142, 12–21. [Google Scholar] [CrossRef]
- Adsul, S.H.; Soma Raju, K.R.C.; Sarada, B.V.; Sonawane, S.H.; Subasri, R. Evaluation of self-healing properties of inhibitor loaded nanoclay-based anticorrosive coatings on magnesium alloy AZ91D. J. Magnes. Alloy. 2018, 6, 299–308. [Google Scholar] [CrossRef]
- Manasa, S.; Jyothirmayi, A.; Siva, T.; Sathiyanarayanan, S.; Gobi, K.V.; Subasri, R. Effect of inhibitor loading into nanocontainer additives of self-healing corrosion protection coatings on aluminum alloy A356.0. J. Alloy. Compd. 2017, 726, 969–977. [Google Scholar] [CrossRef]
- Lutz, A.; van den Berg, O.; Wielant, J.; De Graeve, I.; Terryn, H. A Multiple-Action Self-Healing Coating. Front. Mater. 2016, 2, 73. [Google Scholar] [CrossRef]
- Lutz, A.; Mol, J.M.C.; De Graeve, I.; Terryn, H. 6—Smart corrosion protection by multi-action self-healing polymeric coatings. In Smart Composite Coatings and Membranes; Montemor, M.F., Ed.; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Sawston, UK; Cambridge, UK, 2016; pp. 157–181. ISBN 978-1-78242-283-9. [Google Scholar]
- Scharf, S.; Noeske, M.; Cavalcanti, W.L.; Schiffels, P. 4—Multi-functional, self-healing coatings for corrosion protection: Materials, design and processing. In Handbook of Smart Coatings for Materials Protection; Makhlouf, A.S.H., Ed.; Woodhead Publishing: Sawston, UK; Cambridge, UK, 2014; pp. 75–104. ISBN 978-0-85709-680-7. [Google Scholar]
- Mittal, V. 8—Self-healing anti-corrosion coatings for applications in structural and petrochemical engineering A2—Makhlouf, Abdel Salam Hamdy. In Handbook of Smart Coatings for Materials Protection; Woodhead Publishing: Sawston, UK; Cambridge, UK, 2014; pp. 183–197. ISBN 978-0-85709-680-7. [Google Scholar]
- Shchukin, D.G.; Grigoriev, D.O. 10—The use of nanoreservoirs in corrosion protection coatings. In Corrosion Protection and Control Using Nanomaterials; Saji, V.S., Cook, R., Eds.; Woodhead Publishing Series in Metals and Surface Engineering; Woodhead Publishing: Sawston, UK; Cambridge, UK, 2012; pp. 264–282. ISBN 978-1-84569-949-9. [Google Scholar]
- Abdolah Zadeh, M.; van der Zwaag, S.; Garcia, S.J. Routes to extrinsic and intrinsic self-healing corrosion protective sol-gel coatings: A review. Self-Heal. Mater. 2013, 1, 1–18. [Google Scholar] [CrossRef]
- Tedim, J.; Zheludkevich, M.L.; Salak, A.N.; Lisenkov, A.; Ferreira, M.G.S. Nanostructured LDH-container layer with active protection functionality. J. Mater. Chem. 2011, 21, 15464–15470. [Google Scholar] [CrossRef]
- Yan, T.; Xu, S.; Peng, Q.; Zhao, L.; Zhao, X.; Lei, X.; Zhang, F. Self-Healing of Layered Double Hydroxide Film by Dissolution/Recrystallization for Corrosion Protection of Aluminum. J. Electrochem. Soc. 2013, 160, C480–C486. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Tedim, J.; Montemor, M.F.; Salak, A.N.; Zheludkevich, M.L.; Ferreira, M.G.S. Mechanisms of Localized Corrosion Inhibition of AA2024 by Cerium Molybdate Nanowires. J. Phys. Chem. C 2013, 117, 5811–5823. [Google Scholar] [CrossRef]
- Figueira, R.B.; Sousa, R.; Silva, C.J.R. Chapter 3—Multifunctional and smart organic–inorganic hybrid sol–gel coatings for corrosion protection applications. In Advances in Smart Coatings and Thin Films for Future Industrial and Biomedical Engineering Applications; Makhlouf, A.S.H., Abu-Thabit, N.Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 57–97. ISBN 978-0-12-849870-5. [Google Scholar]
- Faustini, M.; Nicole, L.; Ruiz-Hitzky, E.; Sanchez, C. History of Organic–Inorganic Hybrid Materials: Prehistory, Art, Science, and Advanced Applications. Adv. Funct. Mater. 2018, 28, 1704158. [Google Scholar] [CrossRef]
- Zub, Y.L.; Kessler, V.G. Sol.-Gel Methods for Materials Processing: Focusing on Materials for Pollution Control., Water Purification, and Soil Remediation; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; ISBN 978-1-4020-8514-7. [Google Scholar]
- Reisfeld, R.; Jorgensen, C.K. (Eds.) Chemistry, Spectroscopy and Applications of Sol.-Gel Glasses; Springer: Berlin/Heidelberg, Germany, 1992; ISBN 3-540-54374-0. [Google Scholar]
- Bergna, H.E.; Roberts, W.O. Colloidal Silica: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2005; ISBN 978-1-4200-2870-6. [Google Scholar]
- Park, J.; Joo, J.; Kwon, S.G.; Jang, Y.; Hyeon, T. Synthesis of Monodisperse Spherical Nanocrystals. Angew. Chem. Int. Ed. 2007, 46, 4630–4660. [Google Scholar] [CrossRef]
- Manez. The Supramolecular Chemistry of Organic Inorganic Hybrid. Mater. by Manez; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Zarzycki, J. Past and present of sol-gel science and technology. J. Sol.-Gel Sci. Technol. 1997, 22, 17–22. [Google Scholar] [CrossRef]
- Gupta, R.; Chaudhury, N.K. Entrapment of biomolecules in sol–gel matrix for applications in biosensors: Problems and future prospects. Biosens. Bioelectron. 2007, 22, 2387–2399. [Google Scholar] [CrossRef]
- Leite, F.R.F.; Santos, W.d.J.R.; Kubota, L.T. Selective determination of caffeic acid in wines with electrochemical sensor based on molecularly imprinted siloxanes. Sens. Actuators B Chem. 2014, 193, 238–246. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Voeroes, J.; Reimhult, E. Electrochemical biosensors-Sensor principles and architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Pauliukaite, R.; Schoenleber, M.; Vadgama, P.; Brett, C.M.A. Development of electrochemical biosensors based on sol-gel enzyme encapsulation and protective polymer membranes. Anal. Bioanal. Chem. 2008, 390, 1121–1131. [Google Scholar] [CrossRef]
- Privett, B.J.; Shin, J.H.; Schoenfisch, M.H. Electrochemical Sensors. Anal. Chem. 2010, 82, 4723–4741. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, X.; Peng, Y.; Hong, X.; Liu, Y.; Jiang, S.; Ning, B.; Gao, Z. Ultrasensitive sensing of diethylstilbestrol based on AuNPs/MWCNTs-CS composites coupling with sol-gel molecularly imprinted polymer as a recognition element of an electrochemical sensor. Sens. Actuators B Chem. 2017, 238, 420–426. [Google Scholar] [CrossRef]
- Deiminiat, B.; Razavipanah, I.; Rounaghi, G.H.; Arbab-Zavar, M.H. A novel electrochemical imprinted sensor for acetylsalicylic acid based on polypyrrole, sol-gel and SiO2@Au core-shell nanoparticles. Sens. Actuators B Chem. 2017, 244, 785–795. [Google Scholar] [CrossRef]
- Choi, M.M.F. Progress in Enzyme-Based Biosensors Using Optical Transducers. Microchim. Acta 2004, 148, 107–132. [Google Scholar] [CrossRef]
- Jerónimo, P.C.A.; Araújo, A.N.; Conceição, B.S.M.; Montenegro, M. Optical sensors and biosensors based on sol–gel films. Talanta 2007, 72, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Borisov, S.M.; Wolfbeis, O.S. Optical Biosensors. Chem. Rev. 2008, 108, 423–461. [Google Scholar] [CrossRef] [PubMed]
- Monton, M.R.N.; Forsberg, E.M.; Brennan, J.D. Tailoring Sol–Gel-Derived Silica Materials for Optical Biosensing. Chem. Mater. 2012, 24, 796–811. [Google Scholar] [CrossRef]
- Wang, Y.; Tu, S.; Pinchuk, A.N.; Xiong, M.P. Active drug encapsulation and release kinetics from hydrogel-in-liposome nanoparticles. J. Colloid Interface Sci. 2013, 406, 247–255. [Google Scholar] [CrossRef][Green Version]
- Cho, H.; Gao, J.; Kwon, G.S. PEG-b-PLA micelles and PLGA-b-PEG-b-PLGA sol–gels for drug delivery. J. Control. Release 2016, 240, 191–201. [Google Scholar] [CrossRef]
- Khamsehashari, N.; Hassanzadeh-Tabrizi, S.A.; Bigham, A. Effects of strontium adding on the drug delivery behavior of silica nanoparticles synthesized by P123-assisted sol-gel method. Mater. Chem. Phys. 2018, 205, 283–291. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Kim, H.-E.; Jeong, S.-H. One-pot synthesis of silane-modified hyaluronic acid hydrogels for effective antibacterial drug delivery via sol–gel stabilization. Colloids Surf. B Biointerfaces 2019, 174, 308–315. [Google Scholar] [CrossRef]
- Hernández-Abad, V.J.; Sánchez-González, E.G.; Espinosa-Contreras, C.; Marroquín-Segura, R.; Mora-Guevara, J.L.A.; Flores-Cabrera, Y. Controlled release of glibenclamide from monolithic silica subdermal implants produced by the sol-gel process and its use for hyperglycaemia treatment in a murine model. Mater. Sci. Eng. C 2019, 94, 1009–1019. [Google Scholar] [CrossRef]
- Catauro, M.; Papale, F.; Bollino, F. Coatings of titanium substrates with xCaO(1−x)SiO2 sol–gel materials: Characterization, bioactivity and biocompatibility evaluation. Mater. Sci. Eng. C 2016, 58, 846–851. [Google Scholar] [CrossRef]
- Asri, R.I.M.; Harun, W.S.W.; Hassan, M.A.; Ghani, S.A.C.; Buyong, Z. A review of hydroxyapatite-based coating techniques: Sol–gel and electrochemical depositions on biocompatible metals. J. Mech. Behav. Biomed. Mater. 2016, 57, 95–108. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F. Response of SAOS-2 cells to simulated microgravity and effect of biocompatible sol–gel hybrid coatings. Acta Astronaut. 2016, 122, 237–242. [Google Scholar] [CrossRef]
- Rashti, A.; Yahyaei, H.; Firoozi, S.; Ramezani, S.; Rahiminejad, A.; Karimi, R.; Farzaneh, K.; Mohseni, M.; Ghanbari, H. Development of novel biocompatible hybrid nanocomposites based on polyurethane-silica prepared by sol gel process. Mater. Sci. Eng. C 2016, 69, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Sidane, D.; Rammal, H.; Beljebbar, A.; Gangloff, S.C.; Chicot, D.; Velard, F.; Khireddine, H.; Montagne, A.; Kerdjoudj, H. Biocompatibility of sol-gel hydroxyapatite-titania composite and bilayer coatings. Mater. Sci. Eng. C 2017, 72, 650–658. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Xie, Y.; Xing, Q.; Ni, P.; Han, Y.; Dai, H. Sol-gel synthesis of biocompatible Eu3+/Gd3+ co-doped calcium phosphate nanocrystals for cell bioimaging. J. Lumin. 2017, 192, 902–909. [Google Scholar] [CrossRef]
- Shunzhi, Y.; Zhonghai, L.; Liwei, H.; Yantao, Z.; Tao, F. Biocompatible MgO Film on Titanium Substrate Prepared by Sol-gel Method. Rare Met. Mater. Eng. 2018, 47, 2663–2667. [Google Scholar] [CrossRef]
- Alcázar, J.C.B.; Lemos, R.M.J.; Conde, M.C.M.; Chisini, L.A.; Salas, M.M.S.; Noremberg, B.S.; Motta, F.V.d.; Demarco, F.F.; Tarquinio, S.B.C.; Carreño, N.L.V. Preparation, characterization, and biocompatibility of different metal oxide/PEG-based hybrid coating synthesized by sol–gel dip coating method for surface modification of titanium. Prog. Org. Coat. 2019, 130, 206–213. [Google Scholar] [CrossRef]
- Barceló, D.; Hansen, P.-D.A.J. Biosensors for Environmental Monitoring of Aquatic Systems Bioanalytical and Chemical Methods for Endocrine Disruptors; Springer: Dordrecht, The Netherlands; New York, NY, USA, 2009; ISBN 978-3-540-36253-1. [Google Scholar]
- Buskens, P.; Wouters, M.; Rentrop, C.; Vroon, Z. A brief review of environmentally benign antifouling and foul-release coatings for marine applications. J. Coat. Technol. Res. 2013, 10, 29–36. [Google Scholar] [CrossRef]
- Maleki, H. Recent advances in aerogels for environmental remediation applications: A review. Chem. Eng. J. 2016, 300, 98–118. [Google Scholar] [CrossRef]
- Attia, S.M.; Wang, J.; Wu, G.; Shen, J.; Ma, J. Review on sol-gel derived coatings: Process, techniques and Optical applications. J. Mater. Sci. Technol. 2002, 18, 211–218. [Google Scholar]
- Chu, C.-S.; Lin, C.-A. Optical fiber sensor for dual sensing of temperature and oxygen based on PtTFPP/CF embedded in sol–gel matrix. Sens. Actuators B Chem. 2014, 195, 259–265. [Google Scholar] [CrossRef]
- Choi, Y.-E.; Kwak, J.-W.; Park, J.W. Nanotechnology for Early Cancer Detection. Sensors 2010, 10, 428–455. [Google Scholar] [CrossRef] [PubMed]
- Mousa, S. Biosensors: The new wave in cancer diagnosis. Nanotechnol. Sci. Appl. 2010, 4, 1. [Google Scholar] [CrossRef]
- Simon, E. Biological and chemical sensors for cancer diagnosis. Meas. Sci. Technol. 2010, 21, 112002. [Google Scholar] [CrossRef]
- Arya, S.K.; Bhansali, S. Lung Cancer and Its Early Detection Using Biomarker-Based Biosensors. Chem. Rev. 2011, 111, 6783–6809. [Google Scholar] [CrossRef] [PubMed]
- Baer, D.R.; Burrows, P.E.; El-Azab, A.A. Enhancing coating functionality using nanoscience and nanotechnology. Prog. Org. Coat. 2003, 47, 342–356. [Google Scholar] [CrossRef]
- Almeida, R.M.; Fortes, L.M.; Clara Gonçalves, M. Sol–gel derived photonic bandgap coatings for solar control. Opt. Mater. 2011, 33, 1867–1871. [Google Scholar] [CrossRef]
- Bansal, N.P.; Wise, B. Sol–gel synthesis of La0.6Sr0.4CoO3−x and Sm0.5Sr0.5CoO3−x cathode nanopowders for solid oxide fuel cells. Ceram. Int. 2012, 38, 5535–5541. [Google Scholar] [CrossRef][Green Version]
- Choi, Y.-G.; Park, J.-Y.; Son, J.-W.; Lee, J.-H.; Je, H.-J.; Kim, B.-K.; Lee, H.-W.; Yoon, K.J. Ceria-based electrolyte reinforced by sol–gel technique for intermediate-temperature solid oxide fuel cells. Int. J. Hydrog. Energy 2013, 38, 9867–9872. [Google Scholar] [CrossRef]
- Tseng, C.-J.; Chang, J.-K.; Hung, I.-M.; Lee, K.-R.; Lee, S.-W. BaZr0.2Ce0.8−xYxO3−δ solid oxide fuel cell electrolyte synthesized by sol–gel combined with composition-exchange method. Int. J. Hydrog. Energy 2014, 39, 14434–14440. [Google Scholar] [CrossRef]
- Chen, M.; Chu, W.; Zhu, J.; Dong, L. Plasma assisted preparation of cobalt catalysts by sol–gel method for methane combustion. J. Sol.-Gel Sci. Technol. 2008, 47, 354–359. [Google Scholar] [CrossRef]
- Tichit, D.; Coq, B.; Armendariz, H. One-step sol-gel synthesis of sulfated-zirconia catalysts. Catal. Lett. 1996, 38, 109–113. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Mennig, M. Sol.-Gel Technologies for Glass Producers and Users; Kluwer Academic Publishers: Boston, MA, USA, 2004; ISBN 1-4020-7938-9. [Google Scholar]
- Dimitriev, Y.; Ivanova, Y.; Iordanova, R. History of sol-gel science and technology. J. Univ. Chem. Technol. Matallurgy 2008, 43, 181–192. [Google Scholar]
- Ciriminna, R.; Fidalgo, A.; Pandarus, V.; Béland, F.; Ilharco, L.M.; Pagliaro, M. The Sol-Gel Route to Advanced Silica-Based Materials and Recent Applications. Chem. Rev. 2013. [Google Scholar] [CrossRef] [PubMed]
- Gilberts, J.; Tinnemans, A.H.A.; Hogerheide, M.P.; Koster, T.P.M. UV Curable Hard Transparent Hybrid Coating Materials on Polycarbonate Prepared by the Sol-Gel Method. J. Sol.-Gel Sci. Technol. 1998, 11, 153–159. [Google Scholar] [CrossRef]
- Kiruthika, P.; Subasri, R.; Jyothirmayi, A.; Sarvani, K.; Hebalkar, N.Y. Effect of plasma surface treatment on mechanical and corrosion protection properties of UV-curable sol-gel based GPTS-ZrO2 coatings on mild steel. Surf. Coat. Technol. 2010, 204, 1270–1276. [Google Scholar] [CrossRef]
- Senani, S.; Campazzi, E.; Villatte, M.; Druez, C. Potentiality of UV-cured hybrid sol–gel coatings for aeronautical metallic substrate protection. Surf. Coat. Technol. 2013, 227, 32–37. [Google Scholar] [CrossRef]
- Detty, M.R.; Ciriminna, R.; Bright, F.V.; Pagliaro, M. Xerogel Coatings Produced by the Sol–Gel Process as Anti-Fouling, Fouling-Release Surfaces: From Lab Bench to Commercial Reality. ChemNanoMat 2015, 1, 148–154. [Google Scholar] [CrossRef]
- Oldani, V.; Sergi, G.; Pirola, C.; Sacchi, B.; Bianchi, C.L. Sol-gel hybrid coatings containing silica and a perfluoropolyether derivative with high resistance and anti-fouling properties in liquid media. J. Fluor. Chem. 2016, 188, 43–49. [Google Scholar] [CrossRef]
- Chen, D. Anti-reflection (AR) coatings made by sol–gel processes: A review. Sol. Energy Mater. Sol. Cells 2001, 68, 313–336. [Google Scholar] [CrossRef]
- Mahadik, D.B.; Lakshmi, R.V.; Barshilia, H.C. High performance single layer nano-porous antireflection coatings on glass by sol–gel process for solar energy applications. Sol. Energy Mater. Sol. Cells 2015, 140, 61–68. [Google Scholar] [CrossRef]
- Avci, N.; Cimieri, I.; Smet, P.F.; Poelman, D. Stability improvement of moisture sensitive CaS:Eu2+ micro-particles by coating with sol–gel alumina. Opt. Mater. 2011, 33, 1032–1035. [Google Scholar] [CrossRef]
- Bera, S.; Rout, T.K.; Udayabhanu, G.; Narayan, R. Comparative Study of Corrosion Protection of Sol–Gel Coatings with Different Organic Functionality on Al-2024 substrate. Prog. Org. Coat. 2015, 88, 293–303. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Liu, R. Organic–inorganic hybrid sol–gel coatings for corrosion protection of aluminum alloys. Surf. Innov. 2016, 4, 51–69. [Google Scholar] [CrossRef]
- Gąsiorek, J.; Szczurek, A.; Babiarczuk, B.; Kaleta, J.; Jones, W.; Krzak, J. Functionalizable Sol-Gel Silica Coatings for Corrosion Mitigation. Materials 2018, 11, 197. [Google Scholar] [CrossRef]
- Doong, R.; Lee, P.-S.; Anitha, K. Simultaneous determination of biomarkers for Alzheimer’s disease using sol-gel-derived optical array biosensor. Biosens. Bioelectron. 2010, 25, 2464–2469. [Google Scholar] [CrossRef]
- Fang, W.; Linder, M.B.; Laaksonen, P. Modification of carbon nanotubes by amphiphilic glycosylated proteins. J. Colloid Interface Sci. 2018, 512, 318–324. [Google Scholar] [CrossRef]
- Kristensen, J.B.; Meyer, R.L.; Poulsen, C.H.; Kragh, K.M.; Besenbacher, F.; Laursen, B.S. Biomimetic silica encapsulation of enzymes for replacement of biocides in antifouling coatings. Green Chem. 2010, 12, 387–394. [Google Scholar] [CrossRef]
- Choi, J.-W.; Kang, D.-Y.; Jang, Y.-H.; Kim, H.-H.; Min, J.; Oh, B.-K. Ultra-sensitive surface plasmon resonance based immunosensor for prostate-specific antigen using gold nanoparticle–antibody complex. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313–314, 655–659. [Google Scholar] [CrossRef]
- DeLisa, M.P.; Zhang, Z.; Shiloach, M.; Pilevar, S.; Davis, C.C.; Sirkis, J.S.; Bentley, W.E. Evanescent wave long-period fiber bragg grating as an immobilized antibody biosensor. Anal. Chem. 2000, 72, 2895–2900. [Google Scholar] [CrossRef]
- Mujahid, A.; Dickert, F.L. Chemical Sensors Based on Molecularly Imprinted Sol-Gel Materials. Materials 2010, 3, 2196–2217. [Google Scholar] [CrossRef]
- Shrivastava, S.; Jadon, N.; Jain, R. Next-generation polymer nanocomposite-based electrochemical sensors and biosensors: A review. TrAC Trends Anal. Chem. 2016, 82, 55–67. [Google Scholar] [CrossRef]
- Suginta, W.; Khunkaewla, P.; Schulte, A. Electrochemical Biosensor Applications of Polysaccharides Chitin and Chitosan. Chem. Rev. 2013, 113, 5458–5479. [Google Scholar] [CrossRef] [PubMed]
- Matsuhisa, H.; Tsuchiya, M.; Hasebe, Y. Protein and polysaccharide-composite sol–gel silicate film for an interference-free amperometric glucose biosensor. Colloids Surf. B Biointerfaces 2013, 111, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.A.; Maddux, B.L.S.; Hutchison, J.E. Toward greener nanosynthesis. Chem. Rev. 2007, 107, 2228–2269. [Google Scholar] [CrossRef] [PubMed]
- Zheludkevich, M.L.; Tedim, J.; Ferreira, M.G.S. “Smart” coatings for active corrosion protection based on multi-functional micro and nanocontainers. Electrochim. Acta 2012, 82, 314–323. [Google Scholar] [CrossRef]
- Carneiro, J.; Tedim, J.; Fernandes, S.C.M.; Freire, C.S.R.; Gandini, A.; Ferreira, M.G.S.; Zheludkevich, M.L. Chitosan as a Smart Coating for Controlled Release of Corrosion Inhibitor 2-Mercaptobenzothiazole. ECS Electrochem. Lett. 2013, 2, C19–C22. [Google Scholar] [CrossRef]
- Abu-Thabit, N.Y.; Hamdy, A.S. Stimuli-responsive Polyelectrolyte Multilayers for fabrication of self-healing coatings—A review. Surf. Coat. Technol. 2016, 303, 406–424. [Google Scholar] [CrossRef]
- Alaneme, K.K.; Bodunrin, M.O. Self-healing using metallic material systems—A review. Appl. Mater. Today 2017, 6, 9–15. [Google Scholar] [CrossRef]
- Samiee, R.; Ramezanzadeh, B.; Mahdavian, M.; Alibakhshi, E. Assessment of the smart self-healing corrosion protection properties of a water-base hybrid organo-silane film combined with non-toxic organic/inorganic environmentally friendly corrosion inhibitors on mild steel. J. Clean. Prod. 2019, 220, 340–356. [Google Scholar] [CrossRef]
- Innocenzi, P.; Malfatti, L. Processing of Sol–Gel Films from a Top-Down Route. In The Sol-Gel Handbook; Levy, D., Zayat, R., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 165–194. ISBN 978-3-527-67081-9. [Google Scholar]
- Zayat, M.; Almendro, D.; Vadillo, V.; Levy, D. Sol–Gel Optical and Electro-Optical Materials. In The Sol-Gel Handbook; Levy, D., Zayat, R., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 1239–1280. ISBN 978-3-527-67081-9. [Google Scholar]
- Durán, A.; Castro, Y.; Conde, A.; de Damborenea, J.J. Sol–Gel Protective Coatings for Metals. In Handbook of Sol-Gel Science and Technology; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–65. ISBN 978-3-319-19454-7. [Google Scholar]
- Wang, D.; Bierwagen, G.P. Sol–gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Cushing, B.L.; Kolesnichenko, V.L.; O’Connor, C.J. Recent Advances in the Liquid-Phase Syntheses of Inorganic Nanoparticles. Chem. Rev. 2004, 104, 3893–3946. [Google Scholar] [CrossRef]
- Yamasaki, S.; Sakuma, W.; Yasui, H.; Daicho, K.; Saito, T.; Fujisawa, S.; Isogai, A.; Kanamori, K. Frontiers Nanocellulose Xerogels With High Porosities and Large Specific Surface Areas Chemistry. Available online: https://www.frontiersin.org/articles/10.3389/fchem.2019.00316/full (accessed on 4 March 2020).
- Polyurethane/Ionic Silica Xerogel Composites for CO2 Capture. Available online: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1516-14392019000700228 (accessed on 4 March 2020).
- Pierre, A.C.; Pajonk, G.M. Chemistry of Aerogels and Their Applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef] [PubMed]
- Boev, V.I.; Soloviev, A.; Silva, C.J.R.; Gomes, M.J.M.; Barber, D.J. Highly transparent sol-gel derived ureasilicate monoliths exhibiting long-term optical stability. J. Sol.-Gel Sci. Technol. 2006, 41, 223–229. [Google Scholar] [CrossRef]
- Zhang, G.; Dass, A.; Rawashdeh, A.-M.M.; Thomas, J.; Counsil, J.A.; Sotiriou-Leventis, C.; Fabrizio, E.F.; Ilhan, F.; Vassilaras, P.; Scheiman, D.A.; et al. Isocyanate-crosslinked silica aerogel monoliths: Preparation and characterization. J. Non-Cryst. Solids 2004, 350, 152–164. [Google Scholar]
- Moreira, S.D.F.C.; Silva, C.J.R.; Prado, L.A.S.A.; Costa, M.F.M.; Boev, V.I.; Martín-Sánchez, J.; Gomes, M.J.M. Development of new high transparent hybrid organic-inorganic monoliths with surface engraved diffraction pattern. J. Polym. Sci. Part. B Polym. Phys. 2012, 50, 492–499. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Du, W.; Lei, W.; Si, X.; Zhou, T.; Lin, J.; Peng, L. Superhydrophobic silica antireflective coatings with high transmittance via one-step sol-gel process. Thin Solid Film. 2017, 631, 193–199. [Google Scholar] [CrossRef]
- Esposito, S. “Traditional” Sol-Gel Chemistry as a Powerful Tool for the Preparation of Supported Metal and Metal Oxide Catalysts. Materials 2019, 12, 668. [Google Scholar] [CrossRef] [PubMed]
- Bescher, E.; Hoshino, Y.; Nishizawa, Y.; Cooley, K.; Mackenzie, J.D. The Role of Fe in the Thermal Stabilization of Ormosils. J. Sol.-Gel Sci. Technol. 2003, 26, 297–301. [Google Scholar] [CrossRef]
- Shilova, O. Synthesis and structure features of composite silicate and hybrid TEOS-derived thin films doped by inorganic and organic additives. J. Sol.-Gel Sci. Technol. 2013, 68, 387–410. [Google Scholar] [CrossRef]
- Argente-García, A.; Muñoz-Ortuño, M.; Molins-Legua, C.; Moliner-Martínez, Y.; Campíns-Falcó, P. A solid device based on doped hybrid composites for controlling the dosage of the biocide N-(3-aminopropyl)-N-dodecyl-1,3-propanediamine in industrial formulations. Talanta 2016, 147, 147–154. [Google Scholar] [CrossRef]
- Subbiah, K.; Han-Seung, L.; Yun Su, L.; Jitendra Kumar, S.; Seung-Jun, K.; Rethinam, N. Fabrication of a cerium-doped nickel ferrite solid-state reference electrode and its performance evaluation in concrete environment. Sens. Actuators B Chem. 2017, 251, 509–523. [Google Scholar] [CrossRef]
- Wojcik, A.; Klein, L.C. Transparent organic/inorganic hybrid gels: A classification scheme. Appl. Organomet. Chem. 1997, 11, 129–135. [Google Scholar] [CrossRef]
- Xing, W.; You, B.; Wu, L. Chemical and anticorrosion characterization of polysilsesquioxane coatings catalyzed by different acids. J. Coat. Technol. Res. 2008, 5, 65–72. [Google Scholar] [CrossRef]
- Loy, D.A.; Obrey-DeFriend, K.A.; Wilson, K.V.; Minke, M.; Baugher, B.M.; Baugher, C.R.; Schneider, D.A.; Jamison, G.M.; Shea, K.J. Influence of the alkoxide group, solvent, catalyst, and concentration on the gelation and porosity of hexylene-bridged polysilsesquioxanes. J. Non-Crystalline Solids 2013, 362, 82–94. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, R.; Mu, Y.; Sun, C.; Ji, C.; Zhang, Y.; An, K.; Jia, X.; Zhang, Y. Amino- and Thiol-Polysilsesquioxane Simultaneously Coating on Poly(p-Phenylenetherephthal Amide) Fibers: Bifunctional Adsorbents for Hg(II). Front. Chem. 2019, 7. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanide-Based Luminescent Hybrid Materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [PubMed]
- Yabuta, T.; Bescher, E.P.; Mackenzie, J.D.; Tsuru, K.; Hayakawa, S.; Osaka, A. Synthesis of PDMS-Based Porous Materials for Biomedical Applications. J. Sol.-Gel Sci. Technol. 2003, 26, 1219–1222. [Google Scholar] [CrossRef]
- Mazo, M.A.; Nistal, A.; Caballero, A.C.; Rubio, F.; Rubio, J.; Oteo, J.L. Influence of processing conditions in TEOS/PDMS derived silicon oxycarbide materials. Part 1: Microstructure and properties. J. Eur. Ceram. Soc. 2013, 33, 1195–1205. [Google Scholar] [CrossRef]
- Sanchez, C.; Soler-Illia, G.J.D.A.A.; Ribot, F.; Lalot, T.; Mayer, C.R.; Cabuil, V. Designed Hybrid Organic−Inorganic Nanocomposites from Functional Nanobuilding Blocks. Chem. Mater. 2001, 13, 3061–3083. [Google Scholar] [CrossRef]
- Mammeri, F.; Bourhis, E.L.; Rozes, L.; Sanchez, C. Mechanical properties of hybrid organic–inorganic materials. J. Mater. Chem. 2005, 15, 3787. [Google Scholar] [CrossRef]
- Dash, S.; Mishra, S.; Patel, S.; Mishra, B.K. Organically modified silica: Synthesis and applications due to its surface interaction with organic molecules. Adv. Colloid Interface Sci. 2008, 140, 77–94. [Google Scholar] [CrossRef]
- Lebedev, E. Hybrid organo-inorganic polymer systems: Synthesis, structure, and properties. Theor. Exp. Chem. 2011, 46, 391–396. [Google Scholar] [CrossRef]
- Brinker, C.; Hurd, A.; Schunk, P.; Frye, G. Review of sol-gel thin film formation. J. Non-Cryst. Solids 1992, 148, 424–436. [Google Scholar] [CrossRef]
- Wazarkar, K.; Patil, D.; Rane, A.; Balgude, D.; Kathalewar, M.; Sabnis, A. Microencapsulation: An emerging technique in the modern coating industry. RSC Adv. 2016, 6, 106964–106979. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Q.; Ding, X.; Shen, Q.; Wu, C.; Zhang, L.; Yang, H. Synthesis and application of several sol–gel-derived materials via sol–gel process combining with other technologies: A review. J. Sol.-Gel Sci. Technol. 2016, 79, 328–358. [Google Scholar] [CrossRef]
- Zvonkina, I.; Soucek, M. Inorganic–organic hybrid coatings: Common and new approaches. Curr. Opin. Chem. Eng. 2016, 11, 123–127. [Google Scholar] [CrossRef]
- Ulaeto, S.B.; Rajan, R.; Pancrecious, J.K.; Rajan, T.P.D.; Pai, B.C. Developments in smart anticorrosive coatings with multifunctional characteristics. Prog. Org. Coat. 2017, 111, 294–314. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Fihri, A.; Bovero, E.; Al-Shahrani, A.; Al-Ghamdi, A.; Alabedi, G. Recent progress in superhydrophobic coatings used for steel protection: A review. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 378–390. [Google Scholar] [CrossRef]
- Aparicio, M.; Mosa, J. Electrochemical characterization of sol–gel coatings for corrosion protection of metal substrates. J. Sol.-Gel. Sci. Technol. 2018, 88, 77–89. [Google Scholar] [CrossRef]
- Barroso, G.; Li, Q.; Bordia, R.K.; Motz, G. Polymeric and ceramic silicon-based coatings—a review. J. Mater. Chem. A 2019, 7, 1936–1963. [Google Scholar] [CrossRef]
- Chou, T.; Chandrasekaran, C.; Cao, G.Z. Sol-gel-derived hybrid coatings for corrosion protection. J. Sol.-Gel Sci. Technol. 2003, 26, 321–327. [Google Scholar] [CrossRef]
- Hammer, P.; Santos, F.C.; dos Cerrutti, B.M.; Pulcinelli, S.H.; Santilli, C.V. Highly corrosion resistant siloxane-polymethyl methacrylate hybrid coatings. J. Sol.-Gel Sci. Technol. 2012, 63, 266–274. [Google Scholar] [CrossRef]
- Balan, P.; Ng, A.; Beng Siang, C.; Singh Raman, R.K.; Chan, E.S. Effect of Nanoparticle Addition in Hybrid Sol-Gel Silane Coating on Corrosion Resistance of Low Carbon Steel. Adv. Mater. Res. 2013, 686, 244–249. [Google Scholar] [CrossRef]
- Fedel, M.; Callone, E.; Diré, S.; Deflorian, F.; Olivier, M.-G.; Poelman, M. Effect of Na-Montmorillonite sonication on the protective properties of hybrid silica coatings. Electrochim. Acta 2014, 124, 92–99. [Google Scholar] [CrossRef]
- Habazaki, H.; Kimura, T.; Aoki, Y.; Tsuji, E.; Yano, T. Highly Enhanced Corrosion Resistance of Stainless Steel by Sol-Gel Layer-by-Layer Aluminosilicate Thin Coatings. J. Electrochem. Soc. 2013, 161, C57–C61. [Google Scholar] [CrossRef]
- Hernandez, M.; Barba, A.; Genesca, J.; Covelo, A.; Bucio, E.; Torres, V. Characterization of Hybrid Sol-Gel Coatings Doped with Hydrotalcite-like Compounds on Steel and Stainless Steel Alloys. ECS Trans. 2013, 47, 195–206. [Google Scholar] [CrossRef]
- Claire, L.; Marie, G.; Julien, G.; Jean-Michel, S.; Jean, R.; Marie-Jolle, M.; Stefano, R.; Michele, F. New architectured hybrid sol-gel coatings for wear and corrosion protection of low-carbon steel. Prog. Org. Coat. 2016, 99, 337–345. [Google Scholar] [CrossRef]
- Maia, F.; Yasakau, K.A.; Carneiro, J.; Kallip, S.; Tedim, J.; Henriques, T.; Cabral, A.; Venâncio, J.; Zheludkevich, M.L.; Ferreira, M.G.S. Corrosion protection of AA2024 by sol–gel coatings modified with MBT-loaded polyurea microcapsules. Chem. Eng. J. 2016, 283, 1108–1117. [Google Scholar] [CrossRef]
- Sarmento, V.H.V.; Schiavetto, M.G.; Hammer, P.; Benedetti, a.V.; Fugivara, C.S.; Suegama, P.H.; Pulcinelli, S.H.; Santilli, C.V. Corrosion protection of stainless steel by polysiloxane hybrid coatings prepared using the sol–gel process. Surf. Coat. Technol. 2010, 204, 2689–2701. [Google Scholar] [CrossRef]
- Cambon, J.-B.; Ansart, F.; Bonino, J.-P.; Turq, V. Effect of cerium concentration on corrosion resistance and polymerization of hybrid sol–gel coating on martensitic stainless steel. Prog. Org. Coat. 2012, 75, 486–493. [Google Scholar] [CrossRef]
- Hanetho, S.M.; Kaus, I.; Bouzga, A.; Simon, C.; Grande, T.; Einarsrud, M.-A. Synthesis and characterization of hybrid aminopropyl silane-based coatings on stainless steel substrates. Surf. Coat. Technol. 2014, 238, 1–8. [Google Scholar] [CrossRef]
- Eliziane, M.; Souza, P.D.; Ariza, E.; Ballester, M.; Valéria, I.; Yoshida, P. Characterization of Organic-inorganic Hybrid Coatings for Corrosion Protection of Galvanized Steel and Electroplated ZnFe Steel 2. Experimental Procedure. Mater. Res. 2006, 9, 59–64. [Google Scholar]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Hybrid sol–gel coatings for corrosion protection of hot-dip galvanized steel in alkaline medium. Surf. Coat. Technol. 2015, 265, 191–204. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Hybrid sol–gel coatings for corrosion protection of galvanized steel in simulated concrete pore solution. J. Coat. Technol. Res. 2016, 13, 355–373. [Google Scholar] [CrossRef]
- Tan, A.L.K.; Soutar, A.M. Hybrid sol-gel coatings for corrosion protection of copper. Thin Solid Film. 2008, 516, 5706–5709. [Google Scholar] [CrossRef]
- Li, Y.-S.; Lu, W.; Wang, Y.; Tran, T. Studies of (3-mercaptopropyl)trimethoxylsilane and bis(trimethoxysilyl)ethane sol-gel coating on copper and aluminum. Spectrochim. Acta. Part. A Mol. Biomol. Spectrosc. 2009, 73, 922–928. [Google Scholar] [CrossRef]
- Karthik, N.; Lee, Y.R.; Sethuraman, M.G. Hybrid sol-gel/thiourea binary coating for the mitigation of copper corrosion in neutral medium. Prog. Org. Coat. 2017, 102, 259–267. [Google Scholar] [CrossRef]
- Khramov, A.N.; Balbyshev, V.N.; Kasten, L.S.; Mantz, R.A. Sol–gel coatings with phosphonate functionalities for surface modification of magnesium alloys. Thin Solid Films 2006, 514, 174–181. [Google Scholar] [CrossRef]
- Montemor, M.F.; Ferreira, M.G.S. Electrochemical study of modified bis-(triethoxysilylpropyl) tetrasulfide silane films applied on the AZ31 Mg alloy. Electrochim. Acta 2007, 52, 7486–7495. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Montemor, M.F.; Galio, A.F.; Zheludkevich, M.L.; Trindade, C.; Dick, L.F.; Ferreira, M.G.S. Novel hybrid sol–gel coatings for corrosion protection of AZ31B magnesium alloy. Electrochim. Acta 2008, 53, 4773–4783. [Google Scholar] [CrossRef]
- Khramov, A.N.; Johnson, J.A. Phosphonate-functionalized ORMOSIL coatings for magnesium alloys. Prog. Org. Coat. 2009, 65, 381–385. [Google Scholar] [CrossRef]
- Galio, A.F.; Lamaka, S.V.; Zheludkevich, M.L.; Dick, L.F.P.; Müller, I.L.; Ferreira, M.G.S. Inhibitor-doped sol–gel coatings for corrosion protection of magnesium alloy AZ31. Surf. Coat. Technol. 2010, 204, 1479–1486. [Google Scholar] [CrossRef]
- Adsul, S.H.; Siva, T.; Sathiyanarayanan, S.; Sonawane, S.H.; Subasri, R. Self-healing ability of nanoclay-based hybrid sol-gel coatings on magnesium alloy AZ91D. Surf. Coat. Technol. 2017, 309, 609–620. [Google Scholar] [CrossRef]
- Nezamdoust, S.; Seifzadeh, D.; Rajabalizadeh, Z. PTMS/OH-MWCNT sol-gel nanocomposite for corrosion protection of magnesium alloy. Surf. Coat. Technol. 2018, 335, 228–240. [Google Scholar] [CrossRef]
- Rodič, P.; Milošev, I. Corrosion Inhibition of Pure Aluminium and Alloys AA2024-T3 and AA7075-T6 by Cerium(III) and Cerium(IV) Salts. J. Electrochem. Soc. 2016, 163, C85–C93. [Google Scholar] [CrossRef]
- Snihirova, D.; Lamaka, S.V.; Montemor, M.F. Smart composite coatings for corrosion protection of aluminium alloys in aerospace applications. In Smart Composite Coatings and Membranes; Elsevier: Amsterdam, The Netherlands, 2016; pp. 85–121. ISBN 978-1-78242-283-9. [Google Scholar]
- Vignesh, R.B.; Balaji, J.; Sethuraman, M.G. Surface modification, characterization and corrosion protection of 1,3-diphenylthiourea doped sol-gel coating on aluminium. Prog. Org. Coat. 2017, 111, 112–123. [Google Scholar] [CrossRef]
- Khandanjou, S.; Ghoranneviss, M.; Saviz, S. The detailed analysis of the spray time effects of the aluminium coating using self-generated atmospheric plasma spray system on the microstructure and corrosion behaviour. Results Phys. 2017, 7, 1440–1445. [Google Scholar] [CrossRef]
- Rodič, P.; Milošev, I.; Lekka, M.; Andreatta, F.; Fedrizzi, L. Corrosion behaviour and chemical stability of transparent hybrid sol-gel coatings deposited on aluminium in acidic and alkaline solutions. Prog. Org. Coat. 2018, 124, 286–295. [Google Scholar] [CrossRef]
- Tiringer, U.; Milošev, I.; Durán, A.; Castro, Y. Hybrid sol–gel coatings based on GPTMS/TEOS containing colloidal SiO2 and cerium nitrate for increasing corrosion protection of aluminium alloy 7075-T6. J. Sol.-Gel Sci. Technol. 2018, 945, 546–557. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Möhwald, H. Surface-Engineered Nanocontainers for Entrapment of Corrosion Inhibitors. Adv. Funct. Mater. 2007, 17, 1451–1458. [Google Scholar] [CrossRef]
- Balaskas, A.C.; Kartsonakis, I.A.; Tziveleka, L.A.; Kordas, G.C. Improvement of anti-corrosive properties of epoxy-coated AA 2024-T3 with TiO2 nanocontainers loaded with 8-hydroxyquinoline. Prog. Org. Coat. 2012, 74, 418–426. [Google Scholar] [CrossRef]
- Borisova, D.; Möhwald, H.; Shchukin, D.G. Influence of embedded nanocontainers on the efficiency of active anticorrosive coatings for aluminum alloys part II: Influence of nanocontainer position. ACS Appl. Mater. Interfaces 2013, 5, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Alibakhshi, E.; Ghasemi, E.; Mahdavian, M.; Ramezanzadeh, B. A comparative study on corrosion inhibitive effect of nitrate and phosphate intercalated Zn-Al- layered double hydroxides (LDHs) nanocontainers incorporated into a hybrid silane layer and their effect on cathodic delamination of epoxy topcoat. Corros. Sci. 2017, 115, 159–174. [Google Scholar] [CrossRef]
- Caldarelli, A.; Raimondo, M.; Veronesi, F.; Boveri, G.; Guarini, G. Sol–gel route for the building up of superhydrophobic nanostructured hybrid-coatings on copper surfaces. Surf. Coat. Technol. 2015, 276, 408–415. [Google Scholar] [CrossRef]
- Wei, H.; Wang, Y.; Guo, J.; Shen, N.Z.; Jiang, D.; Zhang, X.; Yan, X.; Zhu, J.; Wang, Q.; Shao, L.; et al. Advanced micro/nanocapsules for self-healing smart anticorrosion coatings. J. Mater. Chem. A 2014, 3, 469–480. [Google Scholar] [CrossRef]
- Thakur, V.K.; Kessler, M.R. Self-healing polymer nanocomposite materials: A review. Polymer 2015, 69, 369–383. [Google Scholar] [CrossRef]
- Zahidah, K.A.; Kakooei, S.; Ismail, M.C.; Bothi Raja, P. Halloysite nanotubes as nanocontainer for smart coating application: A review. Prog. Org. Coat. 2017, 111, 175–185. [Google Scholar] [CrossRef]
- Zhang, F.; Ju, P.; Pan, M.; Zhang, D.; Huang, Y.; Li, G.; Li, X. Self-healing mechanisms in smart protective coatings: A review. Corros. Sci. 2018, 144, 74–88. [Google Scholar] [CrossRef]
- Vijayan, P.P.; Al-Maadeed, M. Self-Repairing Composites for Corrosion Protection: A Review on Recent Strategies and Evaluation Methods. Materials 2019, 12, 2754. [Google Scholar]
- Montemor, M.F. Functional and smart coatings for corrosion protection: A review of recent advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Hia, I.L.; Vahedi, V.; Pasbakhsh, P. Self-Healing Polymer Composites: Prospects, Challenges, and Applications. Polym. Rev. 2016, 56, 225–261. [Google Scholar] [CrossRef]
- Kim, D.-M.; Song, I.-H.; Choi, J.-Y.; Jin, S.-W.; Nam, K.-N.; Chung, C.-M. Self-Healing Coatings Based on Linseed-Oil-Loaded Microcapsules for Protection of Cementitious Materials. Coatings 2018, 8, 404. [Google Scholar] [CrossRef]
- Snihirova, D.; Lamaka, S.V.; Montemor, M.F. “SMART” protective ability of water based epoxy coatings loaded with CaCO3 microbeads impregnated with corrosion inhibitors applied on AA2024 substrates. Electrochim. Acta 2012, 83, 439–447. [Google Scholar] [CrossRef]
- Aramaki, K. Self-healing mechanism of an organosiloxane polymer film containing sodium silicate and cerium(III) nitrate for corrosion of scratched zinc surface in 0.5 M NaCl. Corros. Sci. 2002, 44, 1621–1632. [Google Scholar] [CrossRef]
- Montemor, M.F.; Ferreira, M.G.S. Analytical characterization of silane films modified with cerium activated nanoparticles and its relation with the corrosion protection of galvanised steel substrates. Prog. Org. Coat. 2008, 63, 330–337. [Google Scholar] [CrossRef]
- Tavandashti, N.P.; Sanjabi, S. Corrosion study of hybrid sol–gel coatings containing boehmite nanoparticles loaded with cerium nitrate corrosion inhibitor. Prog. Org. Coat. 2010, 69, 384–391. [Google Scholar] [CrossRef]
- Tiringer, U.; Mušič, B.; Zimerl, D.; Šekularac, G.; Stavber, S.; Milošev, I. The effects of cerium ions on the curing, polymerisation and condensation of hybrid sol-gel coatings. J. Non-Cryst. Solids 2019, 510, 93–100. [Google Scholar] [CrossRef]
- Abuín, M.; Serrano, A.; Llopis, J.; García, M.A.; Carmona, N. Silica doped with lanthanum sol–gel thin films for corrosion protection. Thin Solid Film. 2012, 520, 5267–5271. [Google Scholar] [CrossRef]
- Balan, P.; Raman, R.K.S.; Chan, E.-S.; Harun, M.K.; Swamy, V. Effectiveness of lanthanum triflate activated silica nanoparticles as fillers in silane films for corrosion protection of low carbon steel. Prog. Org. Coat. 2016, 90, 222–234. [Google Scholar] [CrossRef]
- Peña-Poza, J.; Agua, F.; Gil, C.; Villegas, M.-Á.; García-Heras, M. Lanthanum-Silica Sol-Gel Coatings for Protecting Metallic Materials in Museums: Approaches to Copper, Bronze, Lead and Steel. Coatings 2018, 8, 138. [Google Scholar] [CrossRef]
- Jiang, X.; Guo, R.; Jiang, S. Evaluation of self-healing ability of Ce–V conversion coating on AZ31 magnesium alloy. J. Magnes. Alloy. 2016, 4, 230–241. [Google Scholar] [CrossRef]
- Song, J.; Cui, X.; Liu, Z.; Jin, G.; Liu, E.; Zhang, D.; Gao, Z. Advanced microcapsules for self-healing conversion coating on magnesium alloy in Ce(NO3)3 solution with microcapsules containing La(NO3)3. Surf. Coat. Technol. 2016, 307, 500–505. [Google Scholar] [CrossRef]
- Aramaki, K. Preparation of chromate-free, self-healing polymer films containing sodium silicate on zinc pretreated in a cerium(III) nitrate solution for preventing zinc corrosion at scratches in 0.5 M NaCl. Corros. Sci. 2002, 44, 1375–1389. [Google Scholar] [CrossRef]
- Zheludkevich, M.; Serra, R.; Montemor, M.; Yasakau, K.; Salvado, I.; Ferreira, M. Nanostructured sol–gel coatings doped with cerium nitrate as pre-treatments for AA2024-T3Corrosion protection performance. Electrochim. Acta 2005, 51, 208–217. [Google Scholar] [CrossRef]
- Dry, C.M.; Sottos, N.R. Passive smart self-repair in polymer matrix composite materials. In Proceedings of the Smart Structures and Materials 1993: Smart Materials, International Society for Optics and Photonics, Albuquerque, NM, USA, 23 July 1993; pp. 438–445. [Google Scholar]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef]
- Cho, S.H.; White, S.R.; Braun, P.V. Self-Healing Polymer Coatings. Adv. Mater. 2009, 21, 645–649. [Google Scholar] [CrossRef]
- García, S.J.; Fischer, H.R.; White, P.A.; Mardel, J.; González-García, Y.; Mol, J.M.C.; Hughes, A.E. Self-healing anticorrosive organic coating based on an encapsulated water reactive silyl ester: Synthesis and proof of concept. Prog. Org. Coat. 2011, 70, 142–149. [Google Scholar] [CrossRef]
- Yang, S.; Liu, J.; Pan, F.; Yin, X.; Wang, L.; Chen, D.; Zhou, Y.; Xiong, C.; Wang, H. Fabrication of self-healing and hydrophilic coatings from liquid-like graphene@SiO2 hybrids. Compos. Sci. Technol. 2016, 136, 133–144. [Google Scholar] [CrossRef]
- Snihirova, D.; Lamaka, S.V.; Taryba, M.; Salak, A.N.; Kallip, S.; Zheludkevich, M.L.; Ferreira, M.G.S.; Montemor, M.F. Hydroxyapatite Microparticles as Feedback-Active Reservoirs of Corrosion Inhibitors. ACS Appl. Mater. Interfaces 2010, 2, 3011–3022. [Google Scholar] [CrossRef]
- Carneiro, J.; Tedim, J.; Fernandes, S.C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Gandini, A.; Ferreira, M.G.S.; Zheludkevich, M.L. Chitosan-based self-healing protective coatings doped with cerium nitrate for corrosion protection of aluminum alloy 2024. Prog. Org. Coat. 2012, 75, 8–13. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Shchukin, D.G.; Yasakau, K.A.; Möhwald, H.; Ferreira, M.G.S. Anticorrosion Coatings with Self-Healing Effect Based on Nanocontainers Impregnated with Corrosion Inhibitor. Chem. Mater. 2007, 19, 402–411. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Poznyak, S.K.; Rodrigues, L.M.; Raps, D.; Hack, T.; Dick, L.F.; Nunes, T.; Ferreira, M.G.S. Active protection coatings with layered double hydroxide nanocontainers of corrosion inhibitor. Corrosion Sci. 2010, 52, 602–611. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Tedim, J.; Freire, C.S.R.; Fernandes, S.C.M.; Kallip, S.; Lisenkov, A.; Gandini, A.; Ferreira, M.G.S. Self-healing protective coatings with “green” chitosan based pre-layer reservoir of corrosion inhibitor. J. Mater. Chem. 2011, 21, 4805–4812. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Zheludkevich, M.L.; Karavai, O.V.; Ferreira, M.G.S. Influence of inhibitor addition on the corrosion protection performance of sol–gel coatings on AA2024. Prog. Org. Coat. 2008, 63, 352–361. [Google Scholar] [CrossRef]
- Maia, F.; Tedim, J.; Lisenkov, A.D.; Salak, A.N.; Zheludkevich, M.L.; Ferreira, M.G.S. Silica nanocontainers for active corrosion protection. Nanoscale 2012, 4, 1287–1298. [Google Scholar] [CrossRef]
- Montemor, M.F.; Snihirova, D.V.; Taryba, M.G.; Lamaka, S.V.; Kartsonakis, I.A.; Balaskas, A.C.; Kordas, G.C.; Tedim, J.; Kuznetsova, A.; Zheludkevich, M.L.; et al. Evaluation of self-healing ability in protective coatings modified with combinations of layered double hydroxides and cerium molibdate nanocontainers filled with corrosion inhibitors. Electrochim. Acta 2012, 60, 31–40. [Google Scholar] [CrossRef]
- Adsul, S.H.; Siva, T.; Sathiyanarayanan, S.; Sonawane, S.H.; Subasri, R. Aluminum pillared montmorillonite clay-based self-healing coatings for corrosion protection of magnesium alloy AZ91D. Surf. Coat. Technol. 2018, 352, 445–461. [Google Scholar] [CrossRef]
- Kim, B.-M.; Saravanan, M.; Lee, D.-H.; Kang, J.-H.; Kim, M.; Jung, J.-H.; Rhee, J.-S. Exposure to sublethal concentrations of tributyltin reduced survival, growth, and 20-hydroxyecdysone levels in a marine mysid. Mar. Environ. Res. 2018, 140, 96–103. [Google Scholar] [CrossRef]
- Barletta, M.; Aversa, C.; Pizzi, E.; Puopolo, M.; Vesco, S. Design, development and first validation of “biocide-free” anti-fouling coatings. Prog. Org. Coat. 2018, 123, 35–46. [Google Scholar] [CrossRef]
- Detty, M.R.; Ciriminna, R.; Bright, F.V.; Pagliaro, M. Environmentally benign sol-gel antifouling and foul-releasing coatings. Acc. Chem. Res. 2014, 47, 678–687. [Google Scholar] [CrossRef]
- Wouters, M.; Rentrop, C.; Willemsen, P. Surface structuring and coating performance: Novel biocidefree nanocomposite coatings with anti-fouling and fouling-release properties. Prog. Org. Coat. 2010, 68, 4–11. [Google Scholar] [CrossRef]
- Wallström, E.; Jespersen, H.T.; Schaumburg, K. A new concept for anti-fouling paint for Yachts. Prog. Org. Coat. 2011, 72, 109–114. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, X.; Schenderlein, M.; Borisova, D.; Cao, R.; Möhwald, H.; Shchukin, D. Self-Healing and Antifouling Multifunctional Coatings Based on pH and Sulfide Ion Sensitive Nanocontainers. Adv. Funct. Mater. 2013, 23, 3307–3314. [Google Scholar] [CrossRef]
- Akuzov, D.; Vladkova, T.; Zamfirova, G.; Gaydarov, V.; Nascimento, M.V.; Szeglat, N.; Grunwald, I. Polydimethyl siloxane coatings with superior antibiofouling efficiency in laboratory and marine conditions. Prog. Org. Coat. 2017, 103, 126–134. [Google Scholar] [CrossRef]
- Trentin, I.; Romairone, V.; Marcenaro, G.; De Carolis, G. Quick test methods for marine antifouling paints. Prog. Org. Coat. 2001, 42, 15–19. [Google Scholar] [CrossRef]
- Almeida, E.; Diamantino, T.C.; de Sousa, O. Marine paints: The particular case of antifouling paints. Prog. Org. Coat. 2007, 59, 2–20. [Google Scholar] [CrossRef]
- Faÿ, F.; Linossier, I.; Peron, J.J.; Langlois, V.; Vallée-Rehel, K. Antifouling activity of marine paints: Study of erosion. Prog. Org. Coat. 2007, 60, 194–206. [Google Scholar] [CrossRef]
- Faÿ, F.; Linossier, I.; Legendre, G.; Vallée-Réhel, K. Micro-Encapsulation and Antifouling Coatings: Development of Poly(lactic acid) Microspheres Containing Bioactive Molecules. Macromol. Symp. 2008, 272, 45–51. [Google Scholar] [CrossRef]
- Lejars, M.; Margaillan, A.; Bressy, C. Fouling Release Coatings: A Nontoxic Alternative to Biocidal Antifouling Coatings. Chem. Rev. 2012, 112, 4347–4390. [Google Scholar] [CrossRef]
- Carteau, D.; Vallée-Réhel, K.; Linossier, I.; Quiniou, F.; Davy, R.; Compère, C.; Delbury, M.; Faÿ, F. Development of environmentally friendly antifouling paints using biodegradable polymer and lower toxic substances. Prog. Org. Coat. 2014, 77, 485–493. [Google Scholar] [CrossRef]
- Telegdi, J.; Trif, L.; Románszki, L. 5—Smart anti-biofouling composite coatings for naval applications A2—Montemor, M.F. In Smart Composite Coatings and Membranes; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Sawston, UK; Cambridge, UK, 2016; pp. 123–155. ISBN 978-1-78242-283-9. [Google Scholar]
- Palanichamy, S.; Subramanian, G. Antifouling properties of marine bacteriocin incorporated epoxy based paint. Prog. Org. Coat. 2017, 103, 33–39. [Google Scholar] [CrossRef]
- Oldani, V.; del Negro, R.; Bianchi, C.L.; Suriano, R.; Turri, S.; Pirola, C.; Sacchi, B. Surface properties and anti-fouling assessment of coatings obtained from perfluoropolyethers and ceramic oxides nanopowders deposited on stainless steel. J. Fluor. Chem. 2015, 180, 7–14. [Google Scholar] [CrossRef]
- Junaidi, M.U.M.; Ahmad, N.N.R.; Leo, C.P.; Yee, H.M. Near superhydrophobic coating synthesized from rice husk ash: Anti-fouling evaluation. Prog. Org. Coat. 2016, 99, 140–146. [Google Scholar] [CrossRef]
- Movahedi, A.; Zhang, J.; Kann, N.; Moth-Poulsen, K.; Nydén, M. Copper-coordinating polymers for marine anti-fouling coatings: A physicochemical and electrochemical study of ternary system of copper, PMMA and poly(TBTA). Prog. Org. Coat. 2016, 97, 216–221. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, W.; Liu, Y.; Hu, H.; Pei, X.; Wu, Y.; Zhou, F. The effect of wetting property on anti-fouling/foul-release performance under quasi-static/hydrodynamic conditions. Prog. Org. Coat. 2016, 95, 64–71. [Google Scholar] [CrossRef]
- Barletta, M.; Aversa, C.; Pizzi, E.; Puopolo, M.; Vesco, S. Design, manufacturing and testing of anti-fouling/foul-release (AF/FR) amphiphilic coatings. Prog. Org. Coat. 2018, 123, 267–281. [Google Scholar] [CrossRef]
- Li, Y.; Ning, C. Latest research progress of marine microbiological corrosion and bio-fouling, and new approaches of marine anti-corrosion and anti-fouling. Bioact. Mater. 2019, 4, 189–195. [Google Scholar] [CrossRef]
- Olsen, S.M.; Kristensen, J.B.; Laursen, B.S.; Pedersen, L.T.; Dam-Johansen, K.; Kiil, S. Antifouling effect of hydrogen peroxide release from enzymatic marine coatings: Exposure testing under equatorial and Mediterranean conditions. Prog. Org. Coat. 2010, 68, 248–257. [Google Scholar] [CrossRef]
- Zanaroli, G.; Negroni, A.; Calisti, C.; Ruzzi, M.; Fava, F. Selection of commercial hydrolytic enzymes with potential antifouling activity in marine environments. Enzym. Microb. Technol. 2011, 49, 574–579. [Google Scholar] [CrossRef]
- Meißler, M.; Taden, A.; Börner, H.G. Enzyme-Triggered Antifouling Coatings: Switching Bioconjugate Adsorption via Proteolytically Cleavable Interfering Domains. Acs Macro Lett. 2016, 5, 583–587. [Google Scholar] [CrossRef]
- Krupa, A.N.D.; Vimala, R. Evaluation of tetraethoxysilane (TEOS) sol–gel coatings, modified with green synthesized zinc oxide nanoparticles for combating microfouling. Mater. Sci. Eng. C 2016, 61, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Holberg, S.; Losada, R.; Blaikie, F.H.; Hansen, H.H.W.B.; Soreau, S.; Onderwater, R.C.A. Hydrophilic silicone coatings as fouling release: Simple synthesis, comparison to commercial, marine coatings and application on fresh water-cooled heat exchangers. Mater. Today Commun. 2020, 22, 100750. [Google Scholar] [CrossRef]
- Arukalam, I.O.; Oguzie, E.E.; Li, Y. Fabrication of FDTS-modified PDMS-ZnO nanocomposite hydrophobic coating with anti-fouling capability for corrosion protection of Q235 steel. J. Colloid Interface Sci. 2016, 484, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Nuraini, L.; Prifiharni, S.; Priyotomo, G.; Sundjono, Gunawan, H. Evaluation of anticorrosion and antifouling paint performance after exposure under seawater Surabaya–Madura (Suramadu) bridge. Aip Conf. Proc. 2017, 1823, 020101. [Google Scholar]
- Superhydrophobic Surfaces. Available online: https://www.crcpress.com/Superhydrophobic-Surfaces/Carre-Mittal/p/book/9789004165939 (accessed on 4 March 2019).
- Nishimoto, S.; Bhushan, B. Bioinspired self-cleaning surfaces with superhydrophobicity, superoleophobicity, and superhydrophilicity. RSC Adv. 2012, 3, 671–690. [Google Scholar] [CrossRef]
- Montazer, M.; Harifi, T. 12—Water-repellent textile nanofinishes. In Nanofinishing of Textile Materials; Montazer, M., Harifi, T., Eds.; The Textile Institute Book Series; Woodhead Publishing: Sawston, UK; Cambridge, UK, 2018; pp. 183–195. ISBN 978-0-08-101214-7. [Google Scholar]
- Varanasi, K.K.; Deng, T.; Hsu, M.F.; Bhate, N. Design of Superhydrophobic Surfaces for Optimum Roll-Off. and Droplet Impact Resistance; American Society of Mechanical Engineers: New York, NY, USA, 2008; pp. 637–645. [Google Scholar]
- Zhang, D.; Wang, L.; Qian, H.; Li, X. Superhydrophobic surfaces for corrosion protection: A review of recent progresses and future directions. J. Coat. Technol. Res. 2015, 13, 11–29. [Google Scholar] [CrossRef]
- Barkhudarov, P.M.; Shah, P.B.; Watkins, E.B.; Doshi, D.A.; Brinker, C.J.; Majewski, J. Corrosion inhibition using superhydrophobic films. Corros. Sci. 2008, 50, 897–902. [Google Scholar] [CrossRef]
- Maeztu, J.D.; Rivero, P.J.; Berlanga, C.; Bastidas, D.M.; Palacio, J.F.; Rodriguez, R. Effect of graphene oxide and fluorinated polymeric chains incorporated in a multilayered sol-gel nanocoating for the design of corrosion resistant and hydrophobic surfaces. Appl. Surf. Sci. 2017, 419, 138–149. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Caprì, A.; Proverbio, E. Adhesion aspects of hydrophobic silane zeolite coatings for corrosion protection of aluminium substrate. Prog. Org. Coat. 2014, 77, 1341–1350. [Google Scholar] [CrossRef]
- Bescher, E.P.; Noori, A.; Mackenzie, J.D. Fluorinated Copolymer-Oxide Hybrids. J. Sol.-Gel Sci. Technol. 2004, 32, 69–72. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Abdullah, A.M.; Younan, N.A. Corrosion behavior of superhydrophobic surfaces: A review. Arab. J. Chem. 2015, 8, 749–765. [Google Scholar] [CrossRef]
- Matin, A.; Merah, N.; Ibrahim, A. Superhydrophobic and self-cleaning surfaces prepared from a commercial silane using a single-step drop-coating method. Prog. Org. Coat. 2016, 99, 322–329. [Google Scholar] [CrossRef]
- Vasiljević, J.; Tomšič, B.; Jerman, I.; Orel, B.; Jakša, G.; Kovač, J.; Simončič, B. Multifunctional superhydrophobic/oleophobic and flame-retardant cellulose fibres with improved ice-releasing properties and passive antibacterial activity prepared via the sol–gel method. J. Sol.-Gel Sci. Technol. 2014, 70, 385–399. [Google Scholar]
- Latthe, S.S.; Sudhagar, P.; Devadoss, A.; Kumar, A.M.; Liu, S.; Terashima, C.; Nakata, K.; Fujishima, A. A mechanically bendable superhydrophobic steel surface with self-cleaning and corrosion-resistant properties. J. Mater. Chem. A 2015, 3, 14263–14271. [Google Scholar] [CrossRef]
- Zhang, L.; Zuo, W.; Li, T. Controlled synthesis of bifunctional PbWO4 dendrites via a facile solution method at room temperature: Photoluminescence and superhydrophobic property. Mater. Sci. Semicond. Process. 2015, 39, 188–191. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Z.; Mai, W.; Min, C.; Zhou, B.; Shan, M.; Li, Y.; Yang, C.; Wang, Z.; Qian, X. Improved hydrophilicity, permeability, antifouling and mechanical performance of PVDF composite ultrafiltration membranes tailored by oxidized low-dimensional carbon nanomaterials. J. Mater. Chem. A 2013, 1, 3101–3111. [Google Scholar] [CrossRef]
- Jurgis Philipavičius, I.K. Hydrophobic Antireflective Silica Coatings via Sol-gel Process. Medziagotyra 2008, 14, 283–287. [Google Scholar]
- Lakshmi, R.V.; Bharathidasan, T.; Basu, B.J. Superhydrophobic sol–gel nanocomposite coatings with enhanced hardness. Appl. Surf. Sci. 2011, 257, 10421–10426. [Google Scholar] [CrossRef]
- Meera, K.M.S.; Sankar, R.M.; Murali, A.; Jaisankar, S.N.; Mandal, A.B. Sol-gel network silica/modified montmorillonite clay hybrid nanocomposites for hydrophobic surface coatings. Colloids Surf. B Biointerfaces 2012, 90, 204–210. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, Y.; Zhang, H.; Yan, H.; Lv, H.; Jiang, B. Sol-gel preparation of hydrophobic silica antireflective coatings with low refractive index by base/acid two-step catalysis. ACS Appl. Mater. Interfaces 2014, 6, 11470–11475. [Google Scholar] [CrossRef]
- Oldani, V.; Sergi, G.; Pirola, C.; Bianchi, C.L. Use of a sol-gel hybrid coating composed by a fluoropolymer and silica for the mitigation of mineral fouling in heat exchangers. Appl. Therm. Eng. 2016, 106, 427–431. [Google Scholar] [CrossRef]
- Sharma, V.; Sharma, V.; Goyat, M.S.; Hooda, A.; Pandey, J.K.; Kumar, A.; Gupta, R.; Upadhyay, A.K.; Prakash, R.; Kirabira, J.B.; et al. Recent progress in nano-oxides and CNTs based corrosion resistant superhydrophobic coatings: A critical review. Prog. Org. Coat. 2020, 140, 105512. [Google Scholar] [CrossRef]
- Huang, X.; Tepylo, N.; Pommier-Budinger, V.; Budinger, M.; Bonaccurso, E.; Villedieu, P.; Bennani, L. A survey of icephobic coatings and their potential use in a hybrid coating/active ice protection system for aerospace applications. Prog. Aerosp. Sci. 2019, 105, 74–97. [Google Scholar] [CrossRef]
- Xu, L.; He, J. Fabrication of Highly Transparent Superhydrophobic Coatings from Hollow Silica Nanoparticles. Langmuir 2012, 28, 7512–7518. [Google Scholar] [CrossRef]
- Wankhede, R.G.; Morey, S.; Khanna, A.S.; Birbilis, N. Development of water-repellent organic–inorganic hybrid sol–gel coatings on aluminum using short chain perfluoro polymer emulsion. Appl. Surf. Sci. 2013, 283, 1051–1059. [Google Scholar] [CrossRef]
- Shang, Q.; Wang, M.; Liu, H.; Gao, L.; Xiao, G. Facile fabrication of water repellent coatings from vinyl functionalized SiO2 spheres. J. Coat. Technol. Res. 2013, 10, 465–473. [Google Scholar] [CrossRef]
- Mokhtari, S.; Karimzadeh, F.; Abbasi, M.H.; Raeissi, K. Development of super-hydrophobic surface on Al 6061 by anodizing and the evaluation of its corrosion behavior. Surf. Coat. Technol. 2017, 324, 99–105. [Google Scholar] [CrossRef]
- Lee, J.-W.; Hwang, W. Exploiting the silicon content of aluminum alloys to create a superhydrophobic surface using the sol–gel process. Mater. Lett. 2016, 168, 83–85. [Google Scholar] [CrossRef]
- Liang, J.; Hu, Y.; Wu, Y.; Chen, H. Facile formation of superhydrophobic silica-based surface on aluminum substrate with tetraethylorthosilicate and vinyltriethoxysilane as co-precursor and its corrosion resistant performance in corrosive NaCl aqueous solution. Surf. Coat. Technol. 2014, 240, 145–153. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Guo, Z.; Shi, T.; Yu, J.; Tang, M.; Huang, X. Fabrication of superhydrophobic surface with improved corrosion inhibition on 6061 aluminum alloy substrate. Appl. Surf. Sci. 2015, 342, 76–83. [Google Scholar] [CrossRef]
- Zheng, S.; Li, C.; Fu, Q.; Hu, W.; Xiang, T.; Wang, Q.; Du, M.; Liu, X.; Chen, Z. Development of stable superhydrophobic coatings on aluminum surface for corrosion-resistant, self-cleaning, and anti-icing applications. Mater. Des. 2016, 93, 261–270. [Google Scholar] [CrossRef]
- Forooshani, H.M.; Aliofkhazraei, M.; Rouhaghdam, A.S. Superhydrophobic aluminum surfaces by mechanical/chemical combined method and its corrosion behavior. J. Taiwan Inst. Chem. Eng. 2017, 72, 220–235. [Google Scholar] [CrossRef]
- Rao, A.V.; Latthe, S.S.; Mahadik, S.A.; Kappenstein, C. Mechanically stable and corrosion resistant superhydrophobic sol–gel coatings on copper substrate. Appl. Surf. Sci. 2011, 257, 5772–5776. [Google Scholar] [CrossRef]
- Wang, S.; Guo, X.; Xie, Y.; Liu, L.; Yang, H.; Zhu, R.; Gong, J.; Peng, L.; Ding, W. Preparation of superhydrophobic silica film on Mg–Nd–Zn–Zr magnesium alloy with enhanced corrosion resistance by combining micro-arc oxidation and sol–gel method. Surf. Coat. Technol. 2012, 213, 192–201. [Google Scholar] [CrossRef]
- Wu, L.-K.; Zhang, X.-F.; Hu, J.-M. Corrosion protection of mild steel by one-step electrodeposition of superhydrophobic silica film. Corros. Sci. 2014, 85, 482–487. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Chen, R.-J.; Hu, J.-M. Superhydrophobic surface constructed on electrodeposited silica films by two-step method for corrosion protection of mild steel. Corros. Sci. 2016, 104, 336–343. [Google Scholar] [CrossRef]
- Valipour Motlagh, N.; Birjandi, F.C.; Sargolzaei, J.; Shahtahmassebi, N. Durable, superhydrophobic, superoleophobic and corrosion resistant coating on the stainless steel surface using a scalable method. Appl. Surf. Sci. 2013, 283, 636–647. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Zhang, C.; Qu, X.; Liu, J.; Yang, Z. Regenerative superhydrophobic coating from microcapsules. J. Mater. Chem. 2010, 20, 3211–3215. [Google Scholar] [CrossRef]
- Deng, X.; Mammen, L.; Zhao, Y.; Lellig, P.; Müllen, K.; Li, C.; Butt, H.-J.; Vollmer, D. Transparent, Thermally Stable and Mechanically Robust Superhydrophobic Surfaces Made from Porous Silica Capsules. Adv. Mater. 2011, 23, 2962–2965. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.; Li, B.; Hu, T.; Yang, Y.; Li, L.; Zhang, J. Environmentally benign and durable superhydrophobic coatings based on SiO2 nanoparticles and silanes. J. Colloid Interface Sci. 2019, 542, 8–14. [Google Scholar] [CrossRef]
- Milionis, A.; Loth, E.; Bayer, I.S. Recent advances in the mechanical durability of superhydrophobic materials. Adv. Colloid Interface Sci. 2016, 229, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Ellinas, K.; Tserepi, A.; Gogolides, E. Durable superhydrophobic and superamphiphobic polymeric surfaces and their applications: A review. Adv. Colloid Interface Sci. 2017, 250, 132–157. [Google Scholar] [CrossRef] [PubMed]

| Chemical Name | Abbreviation | Chemical Structures |
|---|---|---|
| Tetraethoxysilane | TEOS |  |
| Tetramethylorthosilicate | TMOS |  |
| Methyltriethoxysilane | MTES |  |
| Methyltrimethoxysilane | MTMS |  |
| Vinyltrimethoxysilane | VTMS |  |
| Phenyltrimethoxysilane | PTMS | 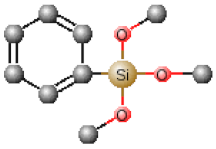 |
| 3-Aminopropyltrimethoxysilane | APTMS | 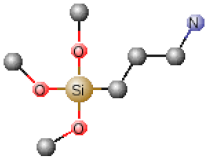 |
| Aminopropyltriethoxysilane | APTES | 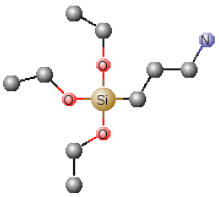 |
| N-(2-aminoethyl) 3-aminopropyltrimethoxysilane | AEAPS | 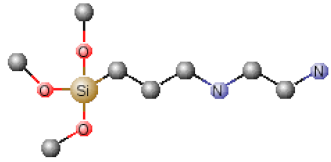 |
| 3 - Glycidoxypropyltrimethoxysilane | GPTMS |  |
| 3-Methacryloxypropyltrimethoxysilane | MAPTS |  |
| Year | Reference | Discussed subject matter |
|---|---|---|
| 2016 | [183] | Principles and morphologies of microcapsules, purpose of microencapsulation,physical, and chemical techniques and healing mechanisms for the coating industry were debated. |
| [184] | The progress made in the synthesis of several sol–gel-derived materials was reviewed. The main achievements in the field of anticorrosion coatings were also debated. | |
| [37] | Summary of the main research achievements in the development, of OIH sol–gel coatings for corrosion mitigation of steel and aluminum substrates. | |
| [185] | The general features of OIH coatings, and recent developments were summarized. | |
| [137] | Summary of the latest achievements and strategies for the sol–gel process parameters and other factors that influence the corrosion properties of the OIH coatings for corrosion protection of aluminum-based alloys. | |
| [20] | Overview of sol–gel technology where the fabric functions that can be achieved by this technology are debated including anticorrosion coatings. | |
| 2017 | [186] | Advances in smart coatings, including OIH coatings, with response to different stimuli and damage modes were reviewed. Emphasis was on corrosion sensing, self-cleaning, anti-fouling, and self-healing polymeric coating systems. |
| [187] | Recent applications using PDMS polymers for anticorrosion, anti-biofouling, anti-icing, flame-resistant and self-cleaning, anti-reflection were reviewed. | |
| [188] | Overview of the superhydrophobic coatings (including OIH sol–gel) reported in literature for steel protection and their performances. | |
| 2018 | [138] | Different solutions for slow down the corrosion processes of metallic substrates by using the oxides and doped oxides obtained by the sol–gel method were discussed. |
| [189] | Analysis of some of the most representative examples of the application of electrochemical techniques such as EIS, PDP; SVET, SIET, SKP and LEIS to determine the exact mechanism of protection offered by sol–gel coatings on metallic substrates. | |
| [78] | Description of the historical perception of OIH material science. The major periods linked to the genesis of OIH materials were discussed. | |
| 2019 | [190] | The main aspects of the use of silicon polymers for coatings were debated. The advantages and disadvantages of these materials, and the processing methods developed were discussed. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueira, R.B. Hybrid Sol–gel Coatings for Corrosion Mitigation: A Critical Review. Polymers 2020, 12, 689. https://doi.org/10.3390/polym12030689
Figueira RB. Hybrid Sol–gel Coatings for Corrosion Mitigation: A Critical Review. Polymers. 2020; 12(3):689. https://doi.org/10.3390/polym12030689
Chicago/Turabian StyleFigueira, Rita B. 2020. "Hybrid Sol–gel Coatings for Corrosion Mitigation: A Critical Review" Polymers 12, no. 3: 689. https://doi.org/10.3390/polym12030689
APA StyleFigueira, R. B. (2020). Hybrid Sol–gel Coatings for Corrosion Mitigation: A Critical Review. Polymers, 12(3), 689. https://doi.org/10.3390/polym12030689





