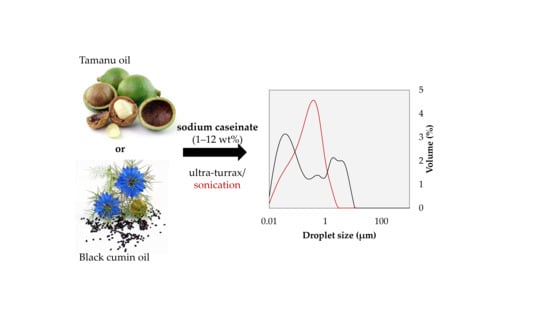
Caseinate-Stabilized Emulsions of Black Cumin and Tamanu Oils: Preparation, Characterization and Antibacterial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Oils
2.2.1. Basic Characteristics
2.2.2. Fatty Acid Composition by Gas Chromatography-Flame Ionization Detector (GC-FID)
2.2.3. Antioxidant Activity
2.2.4. Interfacial Tension
2.3. Preparation of Emulsions
2.4. Characterization of Emulsions
2.4.1. Size of Emulsion Droplets and Zeta Potential
2.4.2. Creaming Index
2.5. Antimicrobial Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Properties and Antioxidant Activity of Oils
3.1.1. Basic Characteristics and Fatty Acid Composition
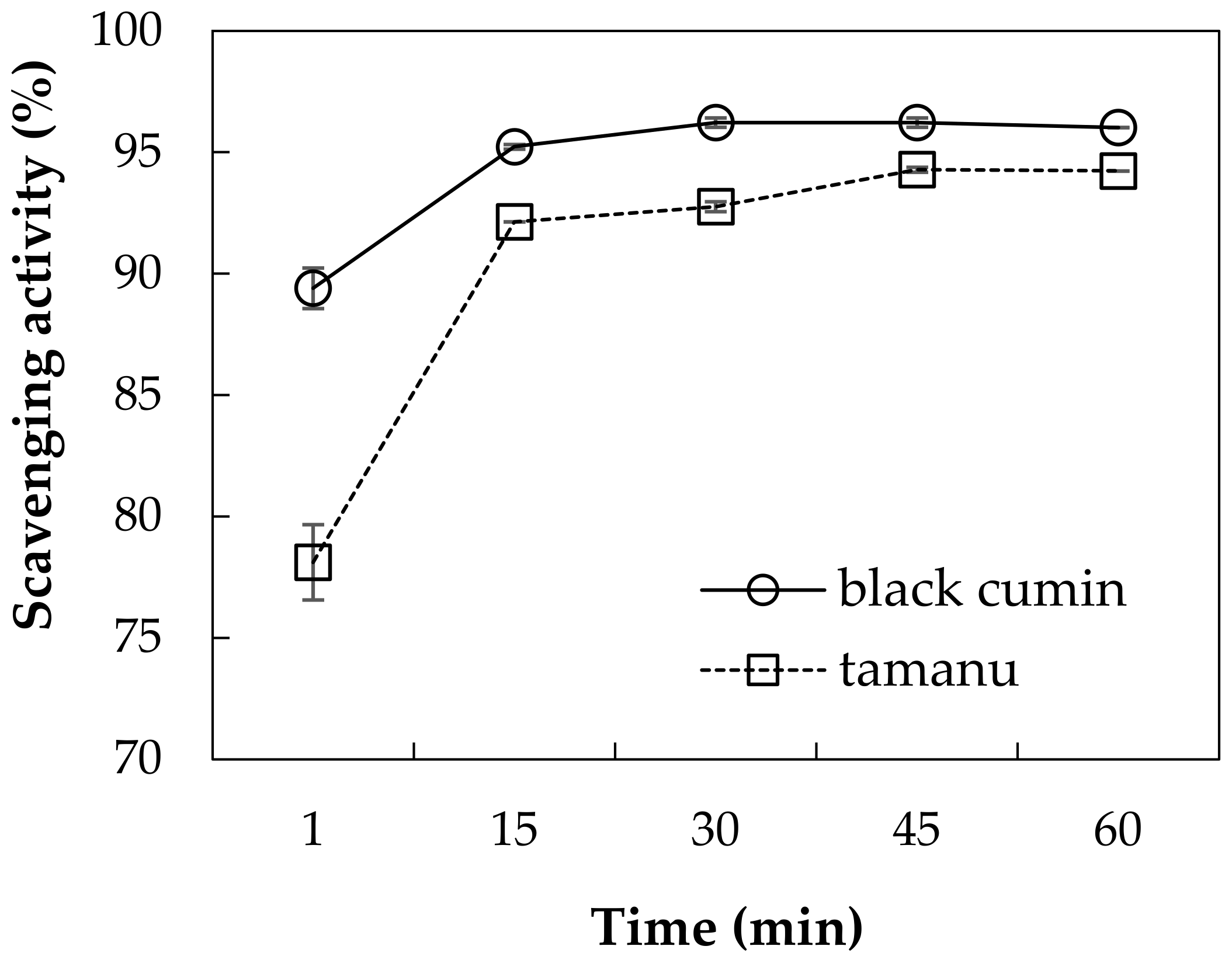
3.1.2. Radical Scavenging Effect of Oils
3.2. Emulsion Properties, Influence of Processing Conditions, and Composition
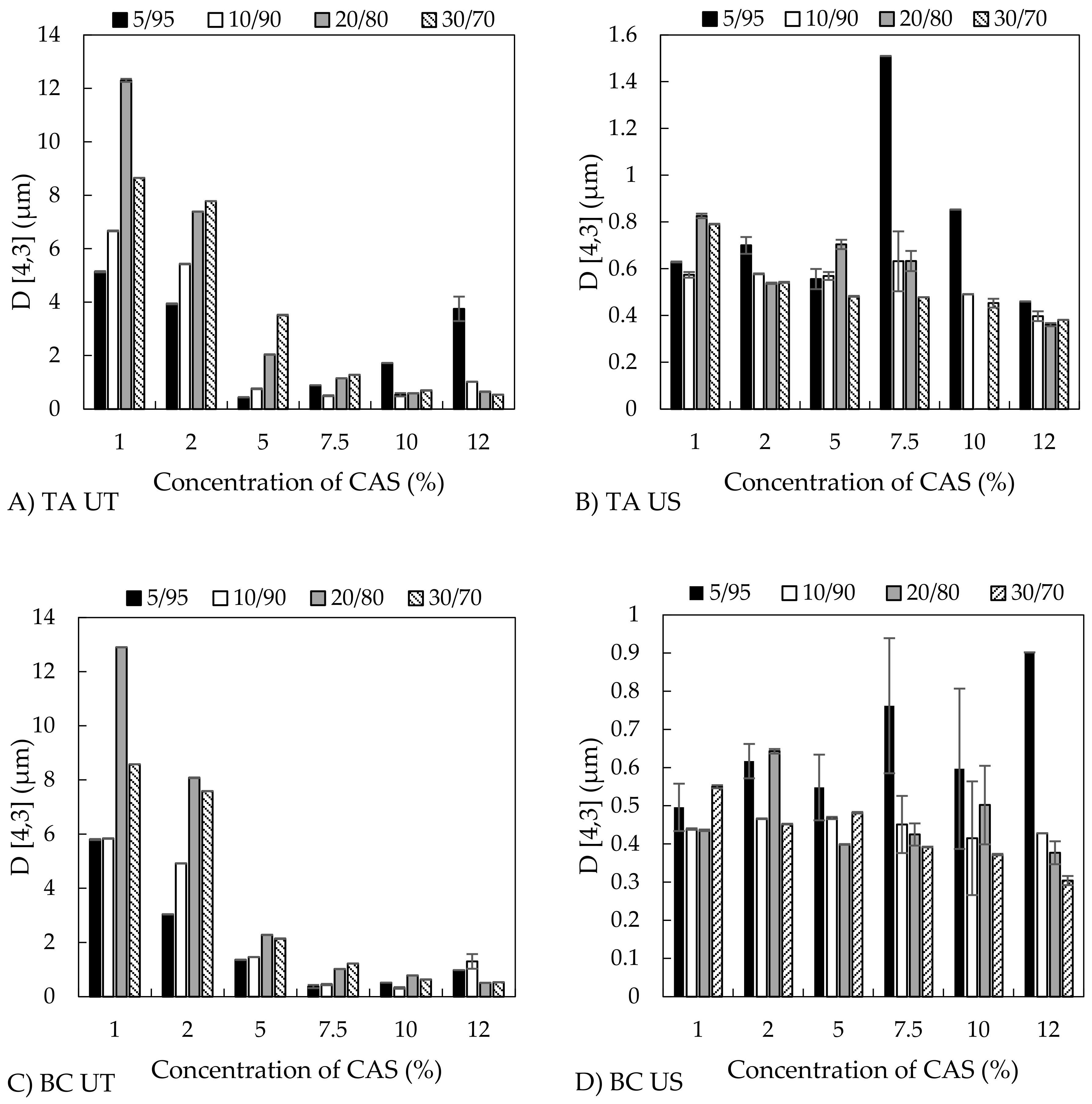
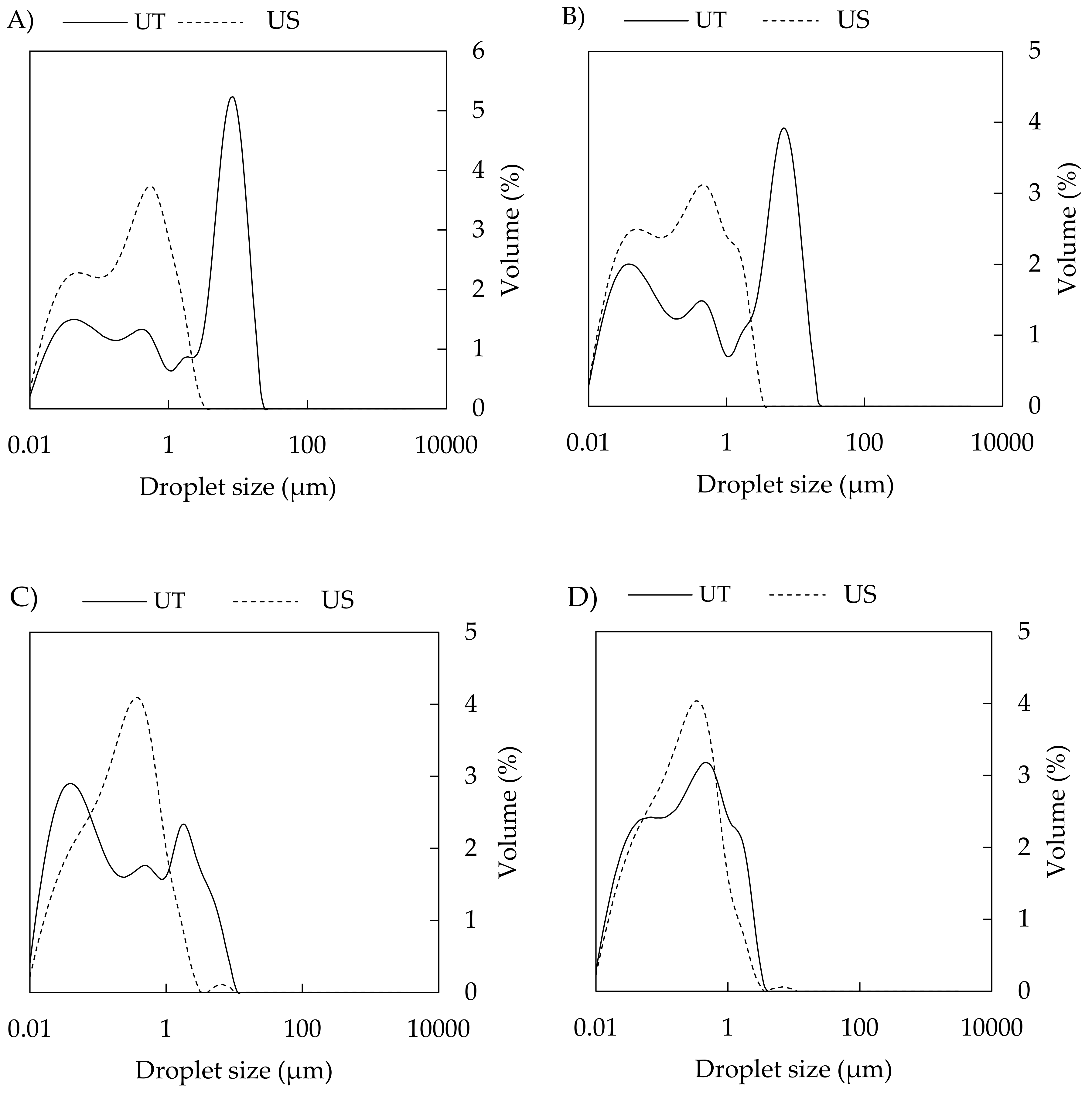
3.2.1. Droplet Size and Distribution
3.2.2. Zeta Potential
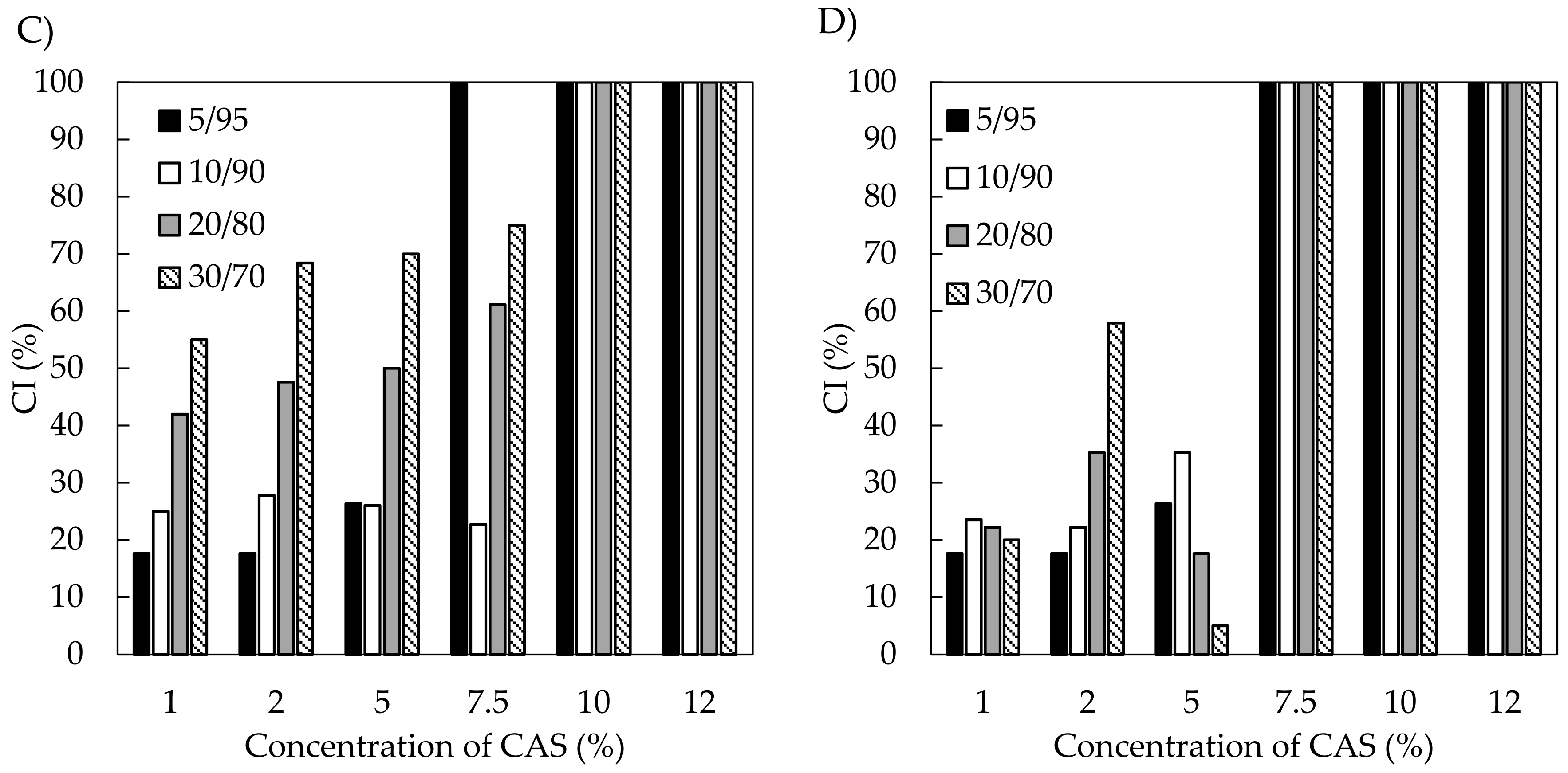
3.2.3. Phase Studies and Emulsion Stability
3.3. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Léguillier, T.; Lecsö-Bornet, M.; Lémus, C.; Rousseau-Ralliard, D.; Lebouvier, N.; Hnawia, E.; Nour, M.; Aalbersberg, W.; Ghazi, K.; Raharivelomanana, P.; et al. The Wound Healing and Antibacterial Activity of Five Ethnomedical Calophyllum Inophyllum Oils: An Alternative Therapeutic Strategy to Treat Infected Wounds. PLoS ONE 2015, 10, e0138602. [Google Scholar] [CrossRef]
- Piras, A.; Rosa, A.; Marongiu, B.; Porcedda, S.; Falconieri, D.; Dessì, M.A.; Ozcelik, B.; Koca, U. Chemical Composition and in Vitro Bioactivity of the Volatile and Fixed Oils of Nigella Sativa, L. Extracted by Supercritical Carbon Dioxide. Ind. Crop. Prod. 2013, 46, 317–323. [Google Scholar] [CrossRef]
- Amin, B.; Hosseinzadeh, H. Black Cumin (Nigella Sativa) and its Active Constituent, Thymoquinone: An Overview on the Analgesic and Anti-Inflammatory Effects. Planta Med. 2016, 82, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.; Asker, M.; Tadros, M. Antiradical and Antimicrobial Properties of Cold-Pressed Black Cumin and Cumin Oils. Eur. Food Res. Technol. 2012, 234, 833–844. [Google Scholar] [CrossRef]
- Hassanien, M.F.R.; Assiri, A.M.A.; Alzohairy, A.M.; Oraby, H.F. Health-Promoting Value and Food Applications of Black Cumin Essential Oil: An Overview. J. Food Sci. Technol. 2015, 52, 6136–6142. [Google Scholar] [CrossRef] [PubMed]
- Bourgou, S.; Pichette, A.; Marzouk, B.; Legault, J. Bioactivities of Black Cumin Essential Oil and its Main Terpenes from Tunisia. S. Afr. J. Bot. 2010, 76, 210–216. [Google Scholar] [CrossRef]
- Mohammed, N.K.; Tan, C.P.; Abd Manap, M.Y.; Muhialdin, B.J.; Hussin, A.S.M. Production of Functional Non-Dairy Creamer using Nigella Sativa Oil Via Fluidized Bed Coating Technology. Food Bioprocess Technol. 2019, 12, 1352–1365. [Google Scholar] [CrossRef]
- Mazaheri, Y.; Torbati, M.; Azadmard-Damirchi, S.; Savage, G.P. A Comprehensive Review of the Physicochemical, Quality and Nutritional Properties of Nigella Sativa Oil. Food Rev. Int. 2019, 35, 342–362. [Google Scholar] [CrossRef]
- Raharivelomanana, P.; Ansel, J.; Lupo, E.; Mijouin, L.; Guillot, S.; Butaud, J.; Ho, R.; Lecellier, G.; Pichon, C. Tamanu Oil and Skin Active Properties: From Traditional to Modern Cosmetic Uses. OCL 2018, 25, D504. [Google Scholar] [CrossRef]
- Shen, Y.C.; Hung, M.C.; Wang, L.T.; Chen, C.Y. Inocalophyllins A, B and their Methyl Esters from the Seeds of Calophyllum Inophyllum. Chem. Pharm. Bull. 2003, 51, 802–806. [Google Scholar] [CrossRef]
- Itoigawa, M.; Ito, C.; Tan, H.T.-W.; Kuchide, M.; Tokuda, H.; Nishino, H.; Furukawa, H. Cancer Chemopreventive Agents, 4-Phenylcoumarins from Calophyllum Inophyllum. Cancer Lett. 2001, 169, 15–19. [Google Scholar] [CrossRef]
- Bui, C.; Nguyen, B.; Trinh, D.; Vo, N. 1077 Anti-Inflammatory and Wound Healing Activities of Calophyllolide Isolated from Calophyllum Inophyllum Linn. J. Investig. Dermatol. 2018, 138, S183. [Google Scholar] [CrossRef]
- Said, T.; Dutot, M.; Martin, C.; Beaudeux, J.-L.; Boucher, C.; Enee, E.; Baudouin, C.; Warnet, J.-M.; Rat, P. Cytoprotective Effect Against UV-Induced DNA Damage and Oxidative Stress: Role of New Biological UV Filter. Eur. J. Pharm. Sci. 2007, 30, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Ixtaina, V.Y.; Julio, L.M.; Wagner, J.R.; Nolasco, S.M.; Tomás, M.C. Physicochemical Characterization and Stability of Chia Oil Rnicroencapsulated with Sodium Caseinate and Lactose by Spray-Drying. Powder Technol. 2015, 271, 26–34. [Google Scholar] [CrossRef]
- Komaiko, J.; Sastrosubroto, A.; McClements, D.J. Encapsulation of Omega-3 Fatty Acids in Nanoemulsion-Based Delivery Systems Fabricated from Natural Emulsifiers: Sunflower Phospholipids. Food Chem. 2016, 203, 331–339. [Google Scholar] [CrossRef]
- Day, L.; Xu, M.; Hoobin, P.; Burgar, I.; Augustin, M.A. Characterisation of Fish Oil Emulsions Stabilised by Sodium Caseinate. Food Chem. 2007, 105, 469–479. [Google Scholar] [CrossRef]
- Huck-Iriart, C.; Pizones Ruiz-Henestrosa, V.; Candal, R.; Herrera, M. Effect of Aqueous Phase Composition on Stability of Sodium Caseinate/Sunflower Oil Emulsions. Food Bioprocess Technol. 2013, 6, 2406–2418. [Google Scholar] [CrossRef]
- Keogh, M.K.; O’Kennedy, B.T.; Kelly, J.; Auty, M.A.; Kelly, P.M.; Fureby, A.; Haahr, A.-M. Stability to Oxidation of Spray-Dried Fish Oil Powder Microencapsulated using Milk Ingredients. J. Food Sci. 2001, 66, 217–224. [Google Scholar] [CrossRef]
- Villiere, A.; Viau, M.; Bronnec, I.; Moreau, N.; Genot, C. Oxidative Stability of Bovine Serum Albumin- and Sodium Caseinate-Stabilized Emulsions Depends on Metal Availability. J. Agric. Food Chem. 2005, 53, 1514–1520. [Google Scholar] [CrossRef]
- Hebishy, E.; Buffa, M.; Juan, B.; Blasco-Moreno, A.; Trujillo, A. Ultra High-Pressure Homogenized Emulsions Stabilized by Sodium Caseinate: Effects of Protein Concentration and Pressure on Emulsions Structure and Stability. LWT-Food Sci. Technol. 2017, 76, 57–66. [Google Scholar] [CrossRef]
- Drusch, S.; Serfert, Y.; Berger, A.; Shaikh, M.Q.; Raetzke, K.; Zaporojtchenko, V.; Schwarz, K. New Insights into the Microencapsulation Properties of Sodium Caseinate and Hydrolyzed Casein. Food Hydrocoll. 2012, 27, 332–338. [Google Scholar] [CrossRef]
- Amine, C.; Dreher, J.; Helgason, T.; Tadros, T. Investigation of Emulsifying Properties and Emulsion Stability of Plant and Milk Proteins using Interfacial Tension and Interfacial Elasticity. Food Hydrocoll. 2014, 39, 180–186. [Google Scholar] [CrossRef]
- Livney, Y.D. Milk Proteins as Vehicles for Bioactives. Curr. Opin. Colloid Interface Sci. 2010, 15, 73–83. [Google Scholar] [CrossRef]
- Nielsen, N.S.; Jacobsen, C. Methods for Reducing Lipid Oxidation in Fish-Oil-Enriched Energy Bars. Int. J. Food Sci. Technol. 2009, 44, 1536–1546. [Google Scholar] [CrossRef]
- Hu, M.; McClements, D.J.; Decker, E.A. Lipid Oxidation in Corn Oil-in-Water Emulsions Stabilized by Casein, Whey Protein Isolate, and Soy Protein Isolate. J. Agric. Food Chem. 2003, 51, 1696–1700. [Google Scholar] [CrossRef] [PubMed]
- Ansel, J.; Lupo, E.; Mijouin, L.; Guillot, S.; Butaud, J.; Ho, R.; Lecellier, G.; Raharivelomanana, P.; Pichon, C. Biological Activity of Polynesian Calophyllum Inophyllum Oil Extract on Human Skin Cells. Planta Med. 2016, 82, 961–966. [Google Scholar] [CrossRef]
- Mukhtar, H.; Qureshi, A.S.; Anwar, F.; Mumtaz, M.W.; Marcu, M. Nigella Sativa, L. Seed and Seed Oil: Potential Sources of High-Value Components for Development of Functional Foods and Nutraceuticals/Pharmaceuticals. J. Essent. Oil Res. 2019, 31, 171–183. [Google Scholar] [CrossRef]
- Gunstone, F.D.; Harwood, J.L.; Dijkstra, A.J. (Eds.) The Lipid Handbook, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Crane, S.; Aurore, G.; Joseph, H.; Mouloungui, Z.; Bourgeois, P. Composition of Fatty Acids Triacylglycerols and Unsaponifiable Matter in Calophyllum Calaba, L. Oil Guadeloupe Phytochem. 2005, 66, 1825–1831. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Mörsel, J. Characterization of Phospholipid Composition of Black Cumin (Nigella Sativa, L.) Seed Oil. Food Nahr. 2002, 46, 240–244. [Google Scholar] [CrossRef]
- Cheikh-Rouhou, S.; Besbes, S.; Hentati, B.; Blecker, C.; Deroanne, C.; Attia, H. Nigella Sativa, L.: Chemical Composition and Physicochemical Characteristics of Lipid Fraction. Food Chem. 2007, 101, 673–681. [Google Scholar] [CrossRef]
- Singh, S.; Das, S.S.; Singh, G.; Schuff, C.; de Lampasona, M.P.; Catalán, C.A.N. Composition, in Vitro Antioxidant and Antimicrobial Activities of Essential Oil and Oleoresins obtained from Black Cumin Seeds (Nigella Sativa, L.). BioMed Res. Int. 2014, 2014, 918209. [Google Scholar] [CrossRef] [PubMed]
- Perrechil, F.A.; Cunha, R.L. Oil-in-Water Emulsions Stabilized by Sodium Caseinate: Influence of pH, High-Pressure Homogenization and Locust Bean Gum Addition. J. Food Eng. 2010, 97, 441–448. [Google Scholar] [CrossRef]
- Amalia Kartika, I.; Cerny, M.; Vandenbossche, V.; Rigal, L.; Sablayrolles, C.; Vialle, C.; Suparno, O.; Ariono, D.; Evon, P. Direct Calophyllum Oil Extraction and Resin Separation with a Binary Solvent of N-Hexane and Methanol Mixture. Fuel 2018, 221, 159–164. [Google Scholar] [CrossRef]
- Mohammed, N.K.; Abd Manap, M.Y.; Tan, C.P.; Muhialdin, B.J.; Alhelli, A.M.; Meor Hussin, A.S. The Effects of Different Extraction Methods on Antioxidant Properties, Chemical Composition, and Thermal Behavior of Black Seed (Nigella sativa, L.) Oil. Evid. Based Complement. Altern. Med. eCAM 2016, 2016, 6273817. [Google Scholar] [CrossRef] [PubMed]
- Wooster, T.J.; Golding, M.; Sanguansri, P. Impact of Oil Type on Nanoemulsion Formation and Ostwald Ripening Stability. Langmuir 2008, 24, 12758–12765. [Google Scholar] [CrossRef] [PubMed]
- Álvarez Cerimedo, M.S.; Iriart, C.H.; Candal, R.J.; Herrera, M.L. Stability of Emulsions Formulated with High Concentrations of Sodium Caseinate and Trehalose. Food Res. Int. 2010, 43, 1482–1493. [Google Scholar] [CrossRef]
- Lizarraga, M.S.; Pan, L.G.; Añon, M.C.; Santiago, L.G. Stability of Concentrated Emulsions Measured by Optical and Rheological Methods. Effect of Processing Conditions-I. Whey Protein Concentrate. Food Hydrocoll. 2008, 22, 868–878. [Google Scholar] [CrossRef]
- Jafari, S.M.; He, Y.; Bhandari, B. Production of Sub-Micron Emulsions by Ultrasound and Microfluidization Techniques. J. Food Eng. 2007, 82, 478–488. [Google Scholar] [CrossRef]
- Dickinson, E.; Golding, M. Depletion Flocculation of Emulsions Containing Unadsorbed Sodium Caseinate. Food Hydrocoll. 1997, 11, 13–18. [Google Scholar] [CrossRef]
- Montes de Oca-Ávalos, J.M.; Candal, R.J.; Herrera, M.L. Colloidal Properties of Sodium Caseinate-Stabilized Nanoemulsions Prepared by a Combination of a High-Energy Homogenization and Evaporative Ripening Methods. Food Res. Int. 2017, 100, 143–150. [Google Scholar] [CrossRef]
- Srinivasan, M.; Singh, H.; Munro, P.A. Formation and Stability of Sodium Caseinate Emulsions: Influence of Retorting (121 °C for 15 Min) before or After Emulsification. Food Hydrocoll. 2002, 16, 153–160. [Google Scholar] [CrossRef]
- Dickinson, E.; Radford, S.J.; Golding, M. Stability and Rheology of Emulsions Containing Sodium Caseinate: Combined Effects of Ionic Calcium and Non-Ionic Surfactant. Food Hydrocoll. 2003, 17, 211–220. [Google Scholar] [CrossRef]
- Allen, K.E.; Dickinson, E.; Murray, B. Acidified Sodium Caseinate Emulsion Foams Containing Liquid Fat: A Comparison with Whipped Cream. LWT-Food Sci. Technol. 2006, 39, 225–234. [Google Scholar] [CrossRef]
- Barreto, P.; Roeder, J.; Crespo, J.S.; Maciel, G.R.; Terenzi, H.; Pires, A.; Soldi, V. Effect of Concentration, Temperature and Plasticizer Content on Rheological Properties of Sodium Caseinate and Sodium Caseinate/Sorbitol Solutions and Glass Transition of their Films. Food Chem. 2003, 82, 425–431. [Google Scholar] [CrossRef]
- De Figueiredo Furtado, G.; Mantovani, R.A.; Consoli, L.; Hubinger, M.D.; da Cunha, R.L. Structural and Emulsifying Properties of Sodium Caseinate and Lactoferrin Influenced by Ultrasound Process. Food Hydrocoll. 2017, 63, 178–188. [Google Scholar] [CrossRef]
- Taha, A.; Hu, T.; Hu, H.; Zhang, Z.; Bakry, A.M.; Khalifa, I.; Pan, S. Effect of Different Oils and Ultrasound Emulsification Conditions on the Physicochemical Properties of Emulsions Stabilized by Soy Protein Isolate. Ultrason. Sonochem. 2018, 49, 283–293. [Google Scholar] [CrossRef]
- Liang, Y.; Gillies, G.; Patel, H.; Matia-Merino, L.; Ye, A.; Golding, M. Physical Stability, Microstructure and Rheology of Sodium-Caseinate-Stabilized Emulsions as Influenced by Protein Concentration and Non-Adsorbing Polysaccharides. Food Hydrocoll. 2014, 36, 245–255. [Google Scholar] [CrossRef]
- Yerramilli, M.; Ghosh, S. Long-Term Stability of Sodium Caseinate-Stabilized Nanoemulsions. J. Food Sci. Technol. 2017, 54, 82–92. [Google Scholar] [CrossRef]
- Dickinson, E. Structure Formation in Casein-Based Gels, Foams, and Emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2006, 288, 3–11. [Google Scholar] [CrossRef]
- Silva, E.K.; Gomes, M.T.M.S.; Hubinger, M.D.; Cunha, R.L.; Meireles, M.A.A. Ultrasound-Assisted Formation of Annatto Seed Oil Emulsions Stabilized by Biopolymers. Food Hydrocoll. 2015, 47, 1–13. [Google Scholar] [CrossRef]
- Desrumaux, A.; Marcand, J. Formation of Sunflower Oil Emulsions Stabilized by Whey Proteins with High-Pressure Homogenization (Up to 350 MPa): Effect of Pressure on Emulsion Characteristics. Int. J. Food Sci. Technol. 2002, 37, 263–269. [Google Scholar] [CrossRef]

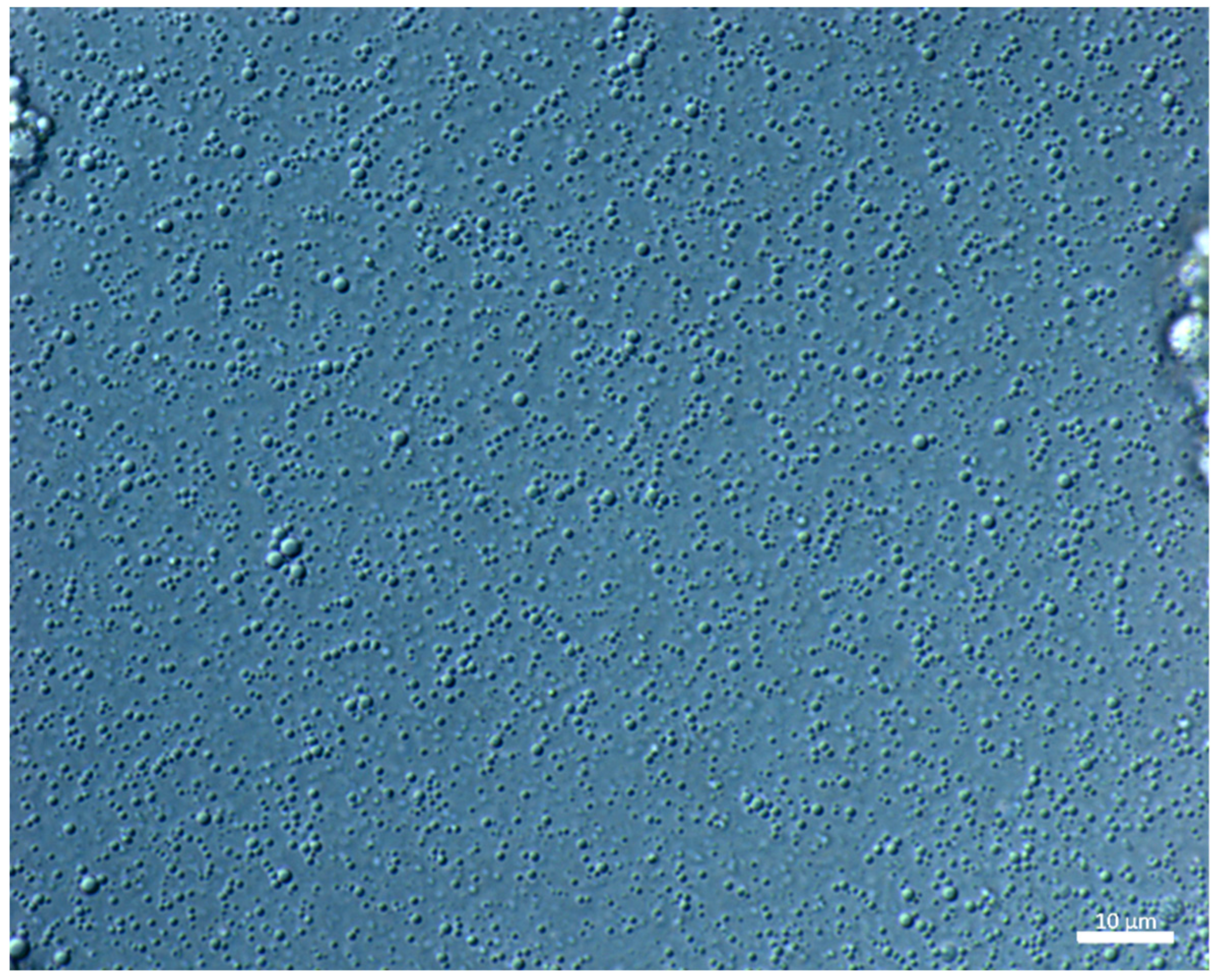




| Oil | IV (g I2/100 g) | SV (mg KOH·g−1) | AV (mg KOH·g−1) | PV (µval·g−1) |
|---|---|---|---|---|
| TA | 83.3 ± 0.5 | 200.8 ± 1.9 | 43.6 ± 0.6 | 1.23 ± 0.0 |
| BC | 116.0 ± 0.9 | 197.2 ± 0.5 | 8.4 ± 0.7 | 1.05 ± 0.0 |
| Fatty Acid (% of Total Content) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Oil | C16:0 | C16:1 | C17:0 | C18:0 | C18:1 | C18:2 | C20:0 | C22:0 |
| TA | 14.1 ± 0.3 | 0.3 ± 0.1 | 0.1 ± 0 | 15.7 ± 0.5 | 41.2 ± 0.2 | 27.6 ± 0.5 | 0.2 ± 0 | 0.2 ± 0 |
| BC | 12.6 ± 0.0 | n.d. | n.d. | 3.4 ± 0.6 | 24.9 ± 1.9 | 57.1 ± 2.6 | n.d. | n.d. |
| Size of Zone ± SD (mm) | ||||||
|---|---|---|---|---|---|---|
| B. cereus | S. aureus | M. luteus | E. faecalis | |||
| Inhibition | Halo | Inhibition | Halo | Halo | Halo | |
| Tamanu | ||||||
| Oil | 8.2 ± 0.4 | 14.8 ± 1.3 | 6.0 ± 1.2 | 9.5 ± 1.3 | 9.3 ± 1.2 | 7.7 ± 0.7 |
| Emulsion 2 % CAS | 7.8 ± 1.6 | 14.3 ± 0.7 | 6.0 ± 0.6 | 8.8 ± 0.4 | 14.3 ± 3.7 | 8.3 ± 1.4 |
| Emulsion 7.5 % CAS | 6.3 ± 0.7 | 11.2 ± 1.3 | 4.7 ± 0.5 | 7.3 ± 0.5 | 9.0 ± 0.8 | 11.0 ± 3.1 |
| Black Cumin | ||||||
| Oil | 5.7 ± 1.5 | 8.7 ± 0.9 | 4.2 ± 0.7 | 6.7 ± 0.9 | n.d | n.d |
| Emulsion 2 % CAS | 2.0 ± 0.8 | n.d. | 3.3 ± 0.9 | n.d | n.d | n.d |
| Emulsion 7.5 % CAS | 1.7 ± 0.5 | n.d. | 2.0 ± 0.6 | n.d | n.d | n.d |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbánková, L.; Kašpárková, V.; Egner, P.; Rudolf, O.; Korábková, E. Caseinate-Stabilized Emulsions of Black Cumin and Tamanu Oils: Preparation, Characterization and Antibacterial Activity. Polymers 2019, 11, 1951. https://doi.org/10.3390/polym11121951
Urbánková L, Kašpárková V, Egner P, Rudolf O, Korábková E. Caseinate-Stabilized Emulsions of Black Cumin and Tamanu Oils: Preparation, Characterization and Antibacterial Activity. Polymers. 2019; 11(12):1951. https://doi.org/10.3390/polym11121951
Chicago/Turabian StyleUrbánková, Lucie, Věra Kašpárková, Pavlína Egner, Ondřej Rudolf, and Eva Korábková. 2019. "Caseinate-Stabilized Emulsions of Black Cumin and Tamanu Oils: Preparation, Characterization and Antibacterial Activity" Polymers 11, no. 12: 1951. https://doi.org/10.3390/polym11121951
APA StyleUrbánková, L., Kašpárková, V., Egner, P., Rudolf, O., & Korábková, E. (2019). Caseinate-Stabilized Emulsions of Black Cumin and Tamanu Oils: Preparation, Characterization and Antibacterial Activity. Polymers, 11(12), 1951. https://doi.org/10.3390/polym11121951