The Mixed-Metal Oxochromates(VI) Cd(HgI2)2(HgII)3O4(CrO4)2, Cd(HgII)4O4(CrO4) and Zn(HgII)4O4(CrO4)—Examples of the Different Crystal Chemistry within the Zinc Triad
Abstract
:1. Introduction
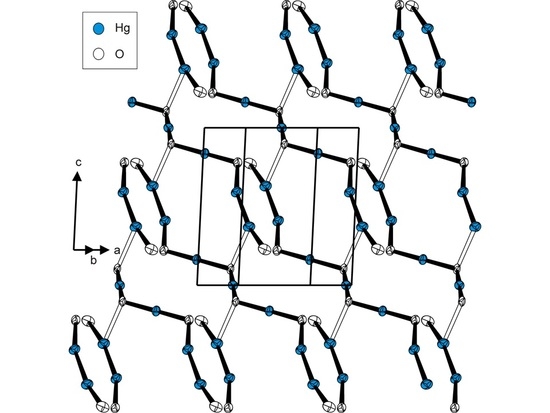
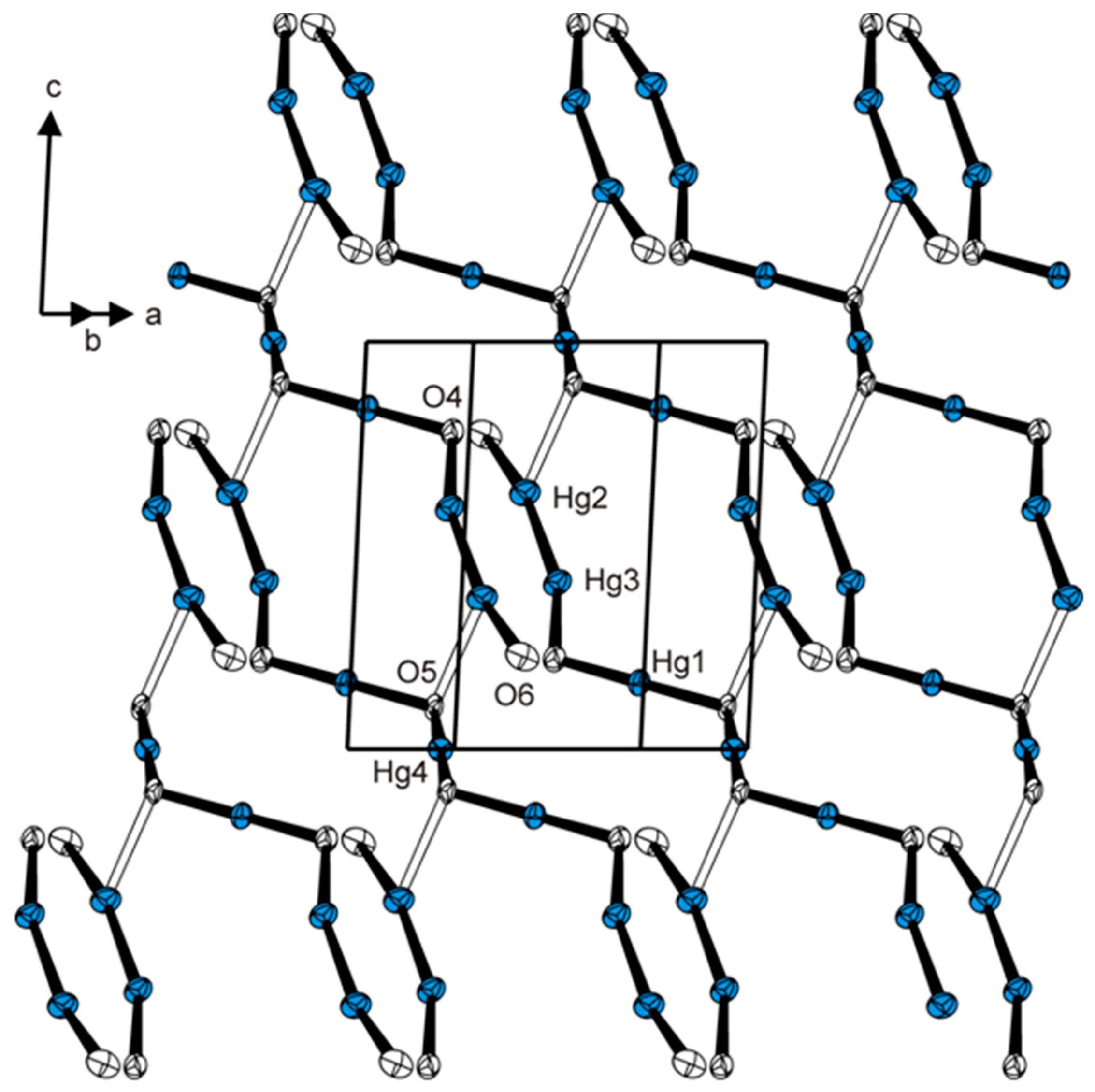
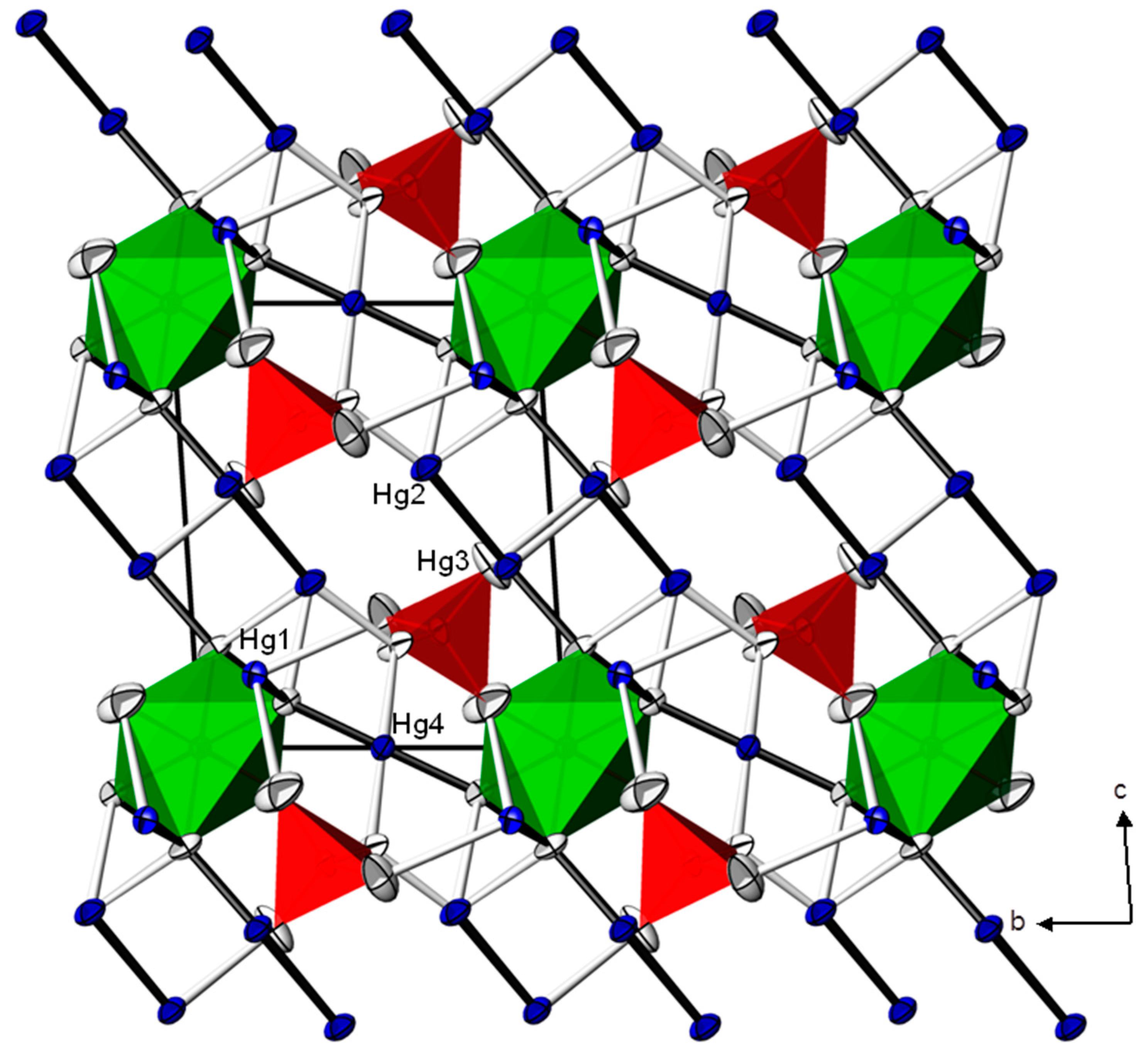
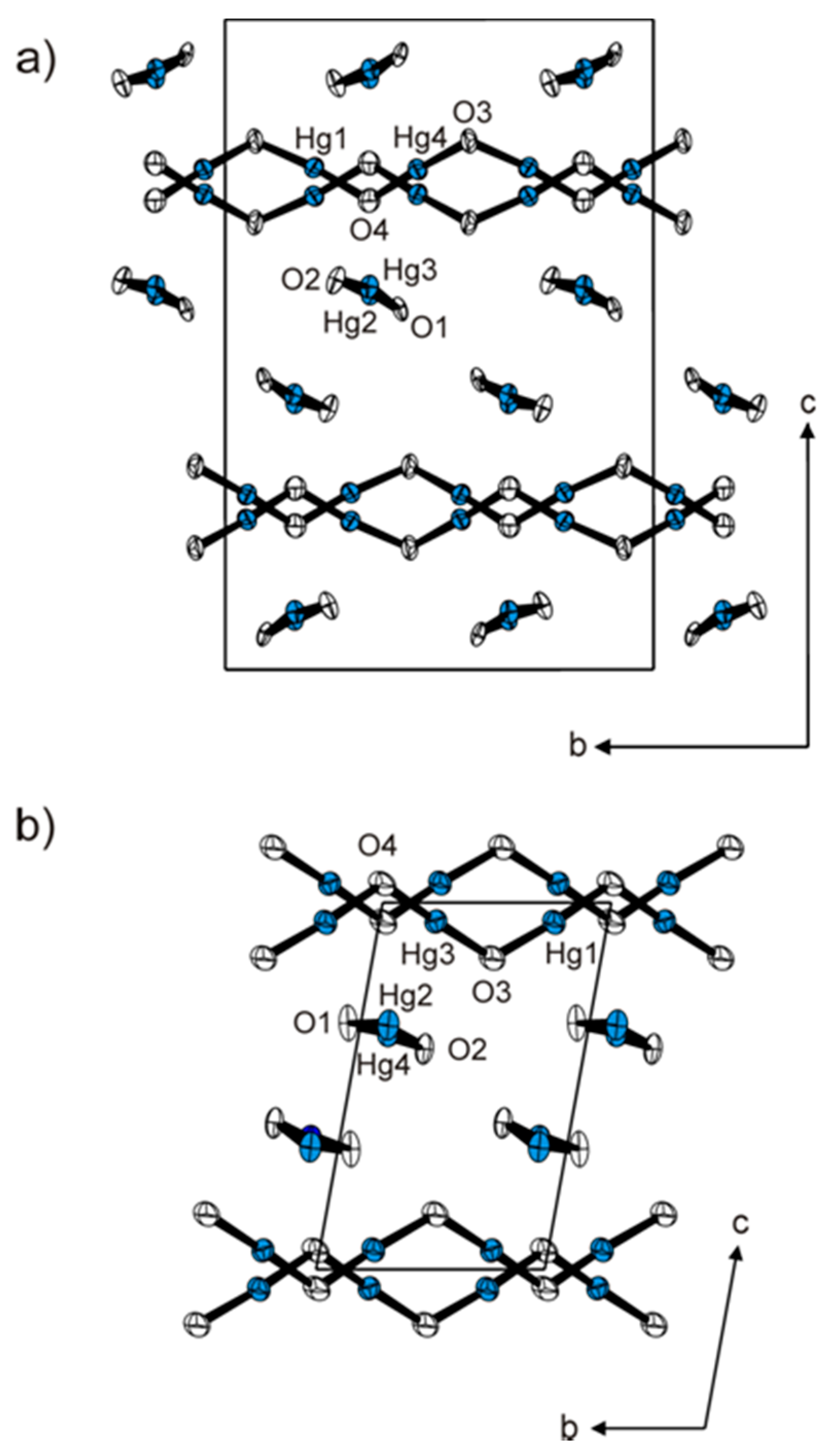
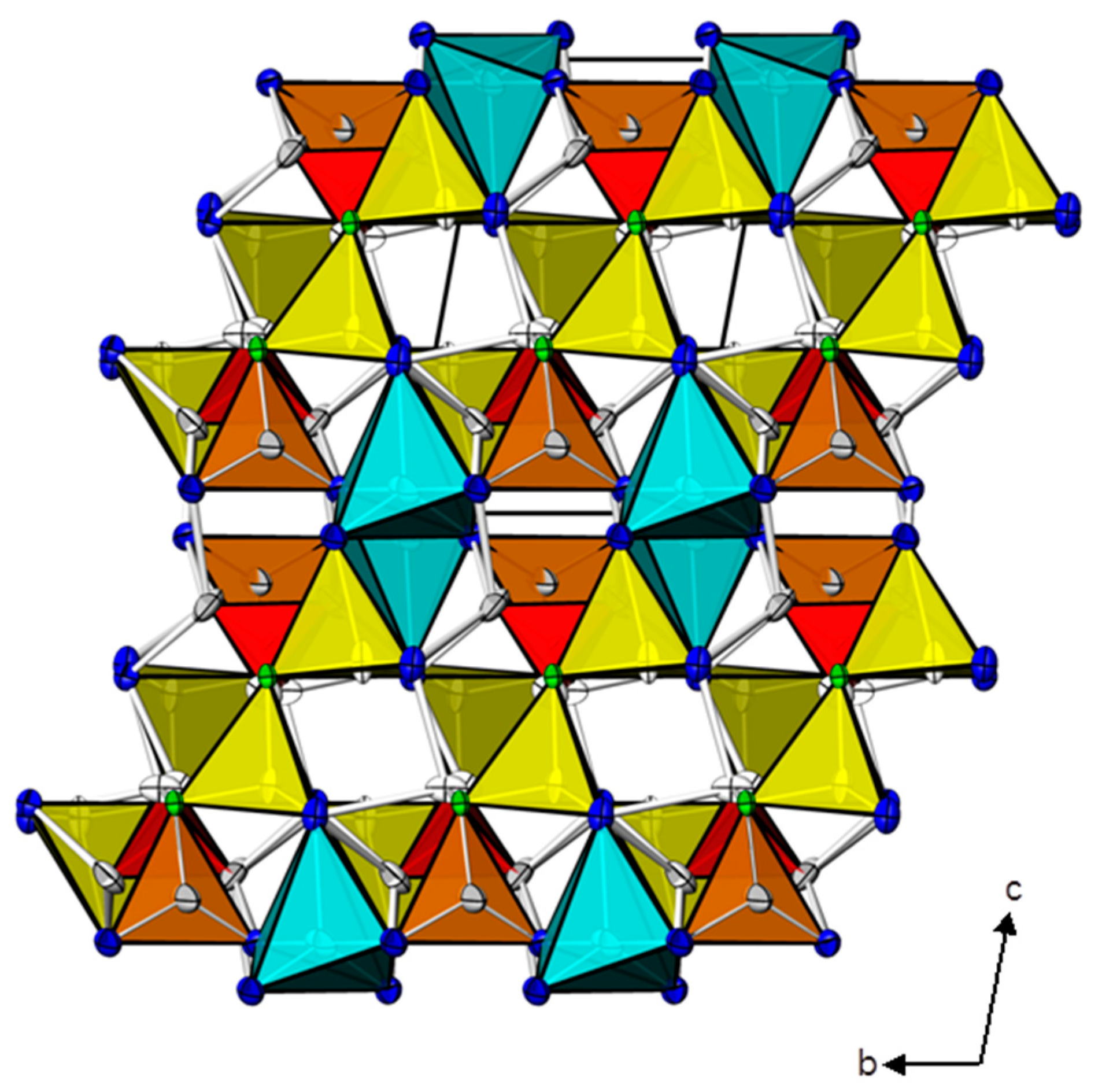
2. Results and Discussion
3. Materials and Methods
3.1. Preparation
3.2. Single Crystal X-ray Diffraction
4. Conclusions
Conflicts of Interest
References and Notes
- Faggiani, R.; Gillespie, R.J.; Vekris, J.E. The Cadmium(I) Ion, (Cd2)2+; X-ray Crystal Structure of Cd2(AlCl4)2. J. Chem. Soc. Chem. Commun. 1986, 7, 517–518. [Google Scholar] [CrossRef]
- Staffel, T.; Meyer, G. Synthesis and crystal structures of Cd(AlCl4)2 and Cd2(AlCl4)2. Z. Anorg. Allg. Chem. 1987, 548, 45–54. [Google Scholar] [CrossRef]
- Pyykkö, P. Relativistic effects in structural chemistry. Chem. Rev. 1988, 88, 563–594. [Google Scholar] [CrossRef]
- Pyykkö, P. Relativistic Effects in Chemistry: More Common Than You Thought. Annu. Rev. Phys. Chem. 2012, 63, 45–64. [Google Scholar] [CrossRef] [PubMed]
- Grdenić, D. The structural chemistry of mercury. Quart. Rev. Chem. Soc. 1965, 19, 303–328. [Google Scholar] [CrossRef]
- Aurivillius, K. The structural chemistry of inorganic mercury(II) compounds. Some aspects of the determination of the positions of “light” atoms in the presence of “heavy” atoms in crystal structures. Ark. Kemi 1965, 24, 151–187. [Google Scholar]
- Breitinger, D.K.; Brodersen, K. Development of and problems in the chemistry of mercury-nitrogen compounds. Angew. Chem. Int. Ed. Eng. 1970, 5, 357–367. [Google Scholar] [CrossRef]
- Müller-Buschbaum, H. On the crystal chemistry of oxomercurates(II). J. Alloys Compd. 1995, 229, 107–122. [Google Scholar] [CrossRef]
- Pervukhina, N.V.; Magarill, S.A.; Borisov, S.V.; Romanenko, G.V.; Pal’chik, N.A. Crystal chemistry of compounds containing mercury in low oxidation states. Russ. Chem. Rev. 1999, 68, 615–636. [Google Scholar] [CrossRef]
- Borisov, S.V.; Magarill, S.A.; Pervukhina, N.V.; Peresypkina, E.V. Crystal chemistry of mercury oxo- and chalcohalides. Cryst. Rev. 2005, 11, 87–123. [Google Scholar] [CrossRef]
- Breitinger, D.K. Cadmium and Mercury. In Comprehensive Coordination Chemistry II; McCleverty, J.A., Meyer, T.J., Eds.; Elsevier: Oxford, UK, 2004; pp. 1253–1292. [Google Scholar]
- Archibald, S.J. Zinc. In Comprehensive Coordination Chemistry II; McCleverty, J.A., Meyer, T.J., Eds.; Elsevier: Oxford, UK, 2004; pp. 1147–1251. [Google Scholar]
- Weil, M. Preparation and crystal structures of the isotypic compounds CdXO4·2HgO (X = S, Se). Z. Naturforsch. 2004, 59b, 281–285. [Google Scholar] [CrossRef]
- Weil, M. Preparation and crystal structure analyses of compounds in the systems HgO/MXO4/H2O (M = Co, Zn, Cd; X = S, Se). Z. Anorg. Allg. Chem. 2004, 630, 921–927. [Google Scholar] [CrossRef]
- Weil, M.; Stöger, B. Dimorphism im mercurous chromate—The crystal structures of α- and β-Hg2CrO4. Z. Anorg. Allg. Chem. 2006, 632, 2131. [Google Scholar] [CrossRef]
- Weil, M.; Stöger, B. The mercury chromates Hg6Cr2O9 and Hg6Cr2O10—Preparation and crystal structures, and thermal behaviour of Hg6Cr2O9. J. Solid State Chem. 2006, 179, 2479–2486. [Google Scholar] [CrossRef]
- Stålhandske, C. Mercury(II) chromate. Acta Crystallogr. 1978, B34, 1968–1969. [Google Scholar] [CrossRef]
- Stöger, B.; Weil, M. Hydrothermal crystal growth and crystal structures of the mercury(II) chromates(VI) α-HgCrO4, β-HgCrO4 and HgCrO4(H2O). Z. Naturforsch. 2006, 61, 708–714. [Google Scholar] [CrossRef]
- Hansen, T.; Müller-Buschbaum, H.; Walz, L. Einkristallröntgenstrukturanalyse an Quecksilberchromat(VI): Hg3O2CrO4. Z. Naturforsch. 1995, 50, 47–50. [Google Scholar]
- Weil, M.; Stöger, B.; Zobetz, E.; Baran, E.J. Crystal structure and characterisation of mercury(II) dichromate(VI). Monatsh. Chem. 2006, 137, 987–996. [Google Scholar] [CrossRef]
- Aurivillius, K.; Stålhandske, C. Neutron diffraction study of mercury(II) chromate hemihydrate, HgCrO4(H2O)0.5. Z. Kristallogr. 1975, 142, 129–141. [Google Scholar]
- Groat, L.A.; Roberts, A.C.; le Page, Y. The crystal structure of wattersite, Hg41+Hg2+Cr6+O6. Can. Mineral. 1995, 33, 41–46. [Google Scholar]
- Klein, W.; Curda, J.; Friese, K.; Jansen, M. Dilead mercury chromate(VI), Pb2HgCrO6. Acta Crystallogr. 2002, C58, i23–i24. [Google Scholar] [CrossRef]
- Klein, W.; Curda, J.; Jansen, M. Dilead trimercury chromate(VI), Pb2(Hg3O4)(CrO4). Acta Crystallogr. 2005, C61, i63–i64. [Google Scholar]
- Brandt, K. X-ray Analysis of CrVO4 and Isomorphous Compounds. Ark. Kemi Mineral. Geol. 1943, 17, 1–13. [Google Scholar]
- Riou, A.; Lecerf, A. Les hydroxychromates M2(OH)2CrO4 (M = Mg2+, Ni2+, Zn2+). C. R. Acad. Sci. Paris 1970, C270, 1109–1112. [Google Scholar]
- Muller, O.; White, W.B.; Roy, R. X-ray diffraction study of the chromates of nickel, magnesium and cadmium. Z. Kristallogr. 1969, 130, 112–120. [Google Scholar] [CrossRef]
- Weil, M. The crystal structures of Hg7Se3O13H2 and Hg8Se4O17H2—Two mixed-valent mercury oxoselenium compounds with a multifarious crystal chemistry. Z. Kristallogr. 2004, 219, 621–629. [Google Scholar] [CrossRef]
- Weil, M. The Mixed-valent Mercury(I/II) Compounds Hg3(HAsO4)2 and Hg6As2O10. Z. Naturforsch. 2014, 69, 665–673. [Google Scholar] [CrossRef]
- Weil, M.; Tillmanns, E.; Pushcharovsky, D.Y. Hydrothermal Single-Crystal Growth in the Systems Ag/Hg/X/O (X = VV, AsV): Crystal Structures of (Ag3Hg)VO4, (Ag2Hg2)3(VO4)4, and (Ag2Hg2)2(HgO2)(AsO4)2 with the Unusual Tetrahedral Cluster Cations (Ag3Hg)3+ and (Ag2Hg2)4+ and Crystal Structure of AgHgVO4. Inorg. Chem. 2005, 44, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Pressprich, M.R.; Willett, R.D.; Poshusta, R.D.; Saunders, S.C.; Davis, H.B.; Gard, H.B. Preparation and crystal structure of dipyrazinium trichromate and bond length correlation for chromate anions of the form CrnO3n + 12−. Inorg. Chem. 1988, 27, 260–264. [Google Scholar] [CrossRef]
- Siidra, O.I.; Krivovichev, S.V.; Filatov, S.K. Minerals and synthetic Pb(II) compounds with oxocentered tetrahedra: Review and classification. Z. Kristallogr. 2008, 223, 114–125. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Mentre, O.; Siidra, O.I.; Colmont, M.; Filatov, S.K. Anion-centered tetrahedra in inorganic compounds. Chem. Rev. 2013, 113, 6459–6535. [Google Scholar] [CrossRef] [PubMed]
- Addison, A.W.; Nageswara Rao, T.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Extreme values of τ5 for a five-coordinate atom are 0 for a square-pyramidal arrangement and 1 for a trigonal-bipyramidal arrangement.
- Brown, I.D. The Chemical Bond in Inorganic Chemistry: The Bond Valence Model. Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Crystallogr. 1991, B47, 192–197. [Google Scholar] [CrossRef]
- Values of GII < 0.10 v.u. suggest that little or no strain is present in the crystal structure while values between 0.10 and 0.20 v.u. indicate a significant lattice-induced strain. Values > 0.20 v.u. point to an instability of the structure due to too much strain.
- Herrendorf, W.H. Program for Optimization of the Crystal Shape for Numerical Absorption Correction; Universities of Karlsruhe: Gießen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. Phase annealing in SHELX-90: Direct methods for larger structures. Acta Cryst. 1990, A46, 467–473. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Dowty, E. ATOMS; Shape Software: Kingsport, TN, USA, 2006. [Google Scholar]
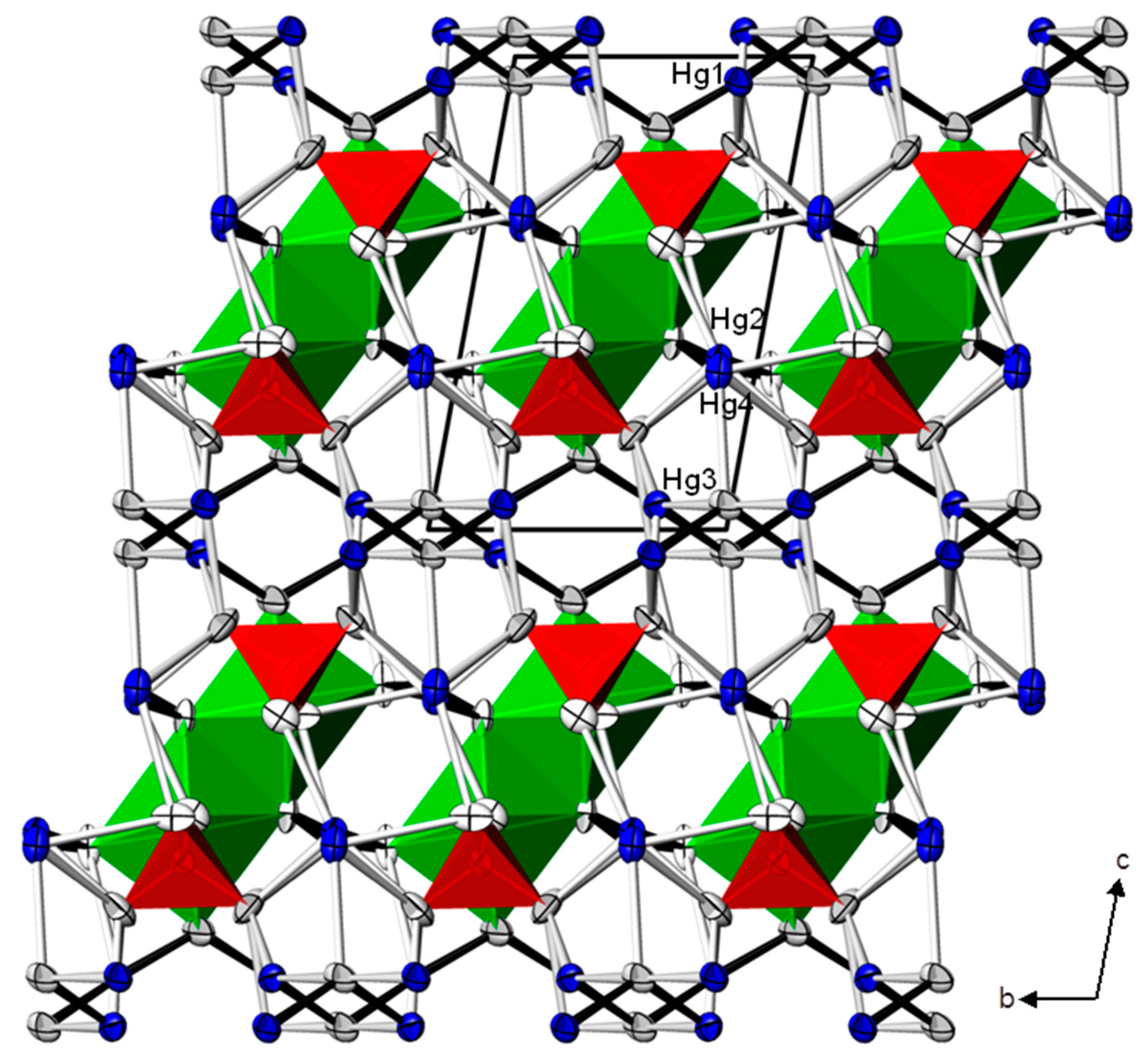
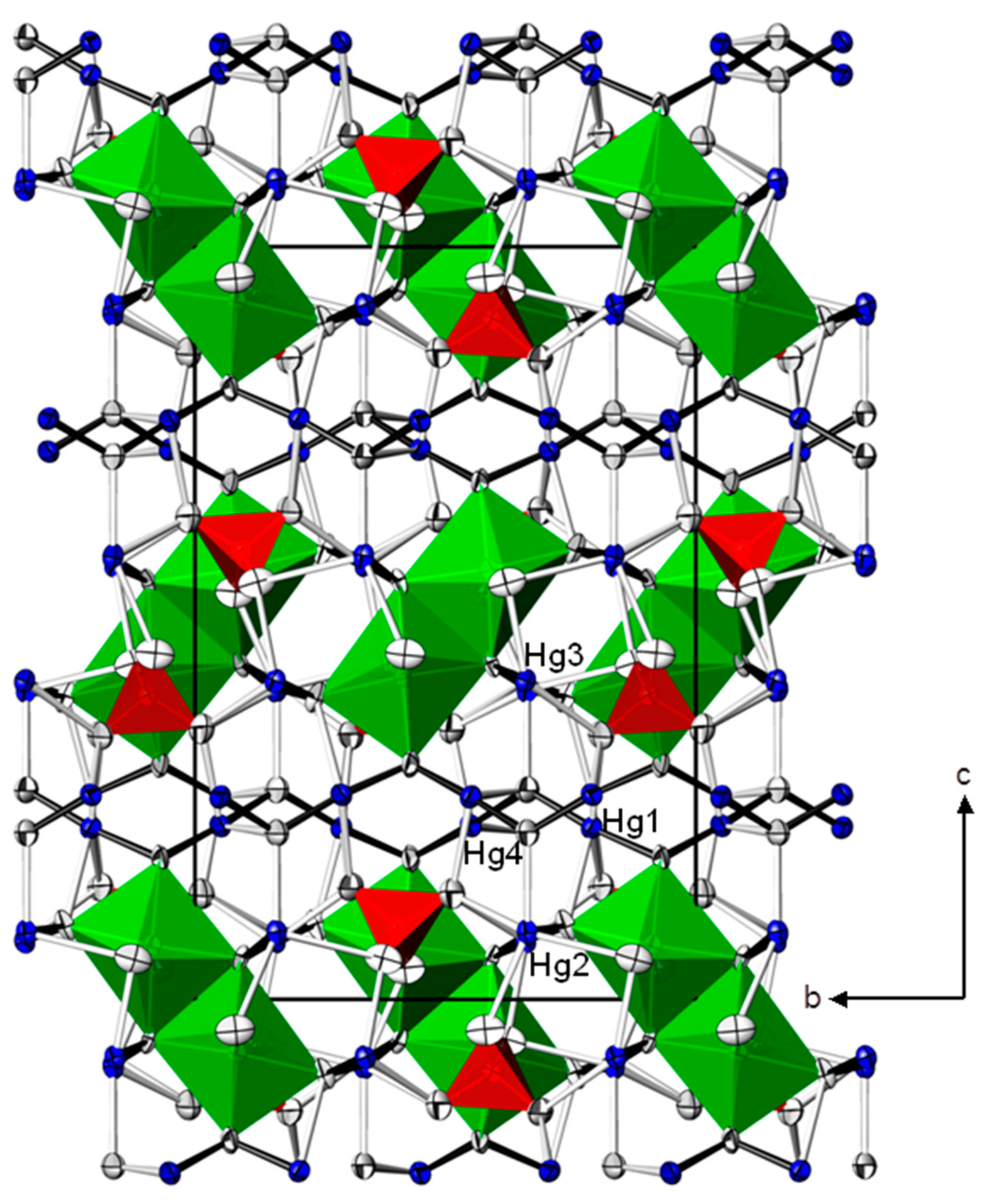
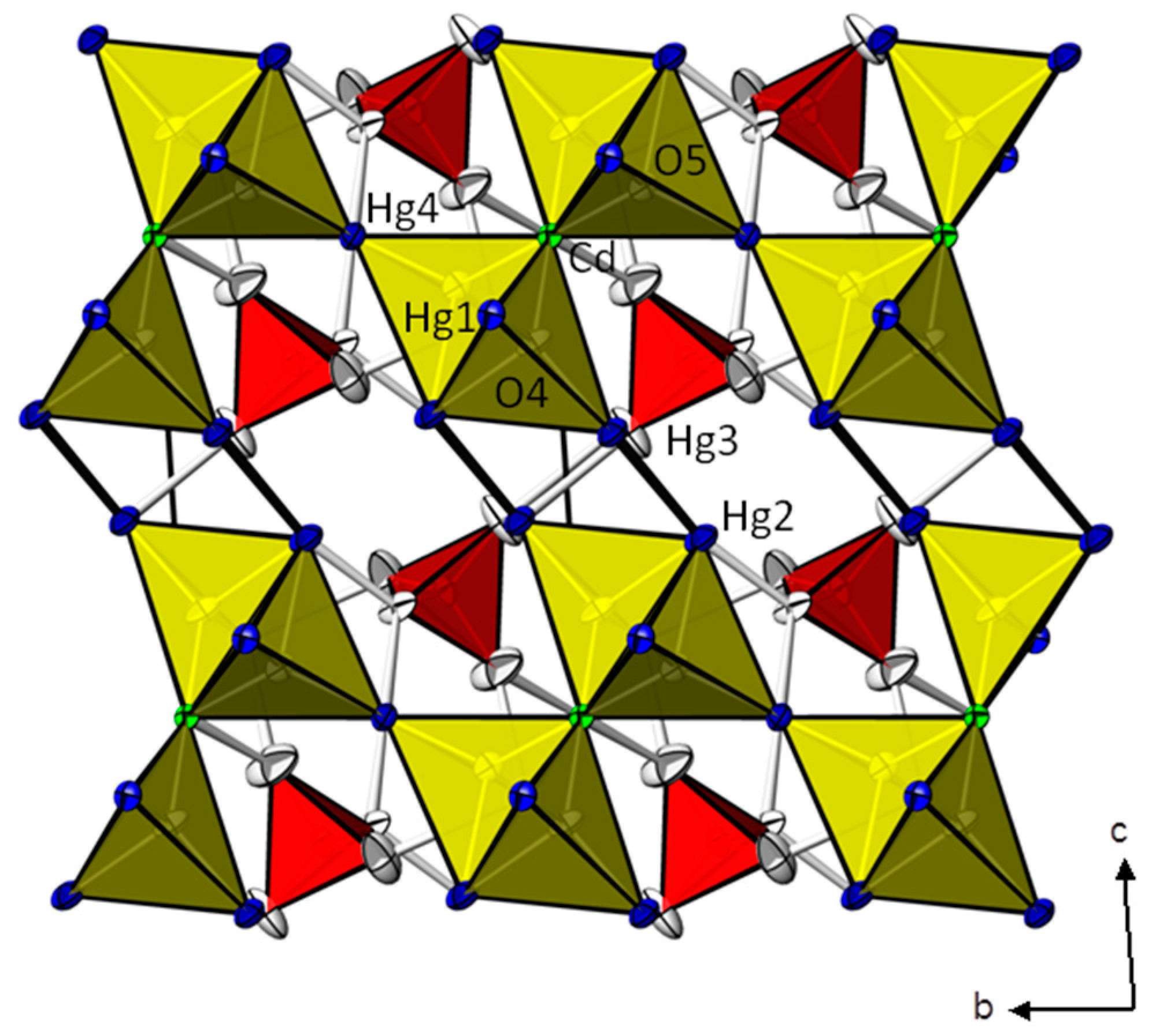
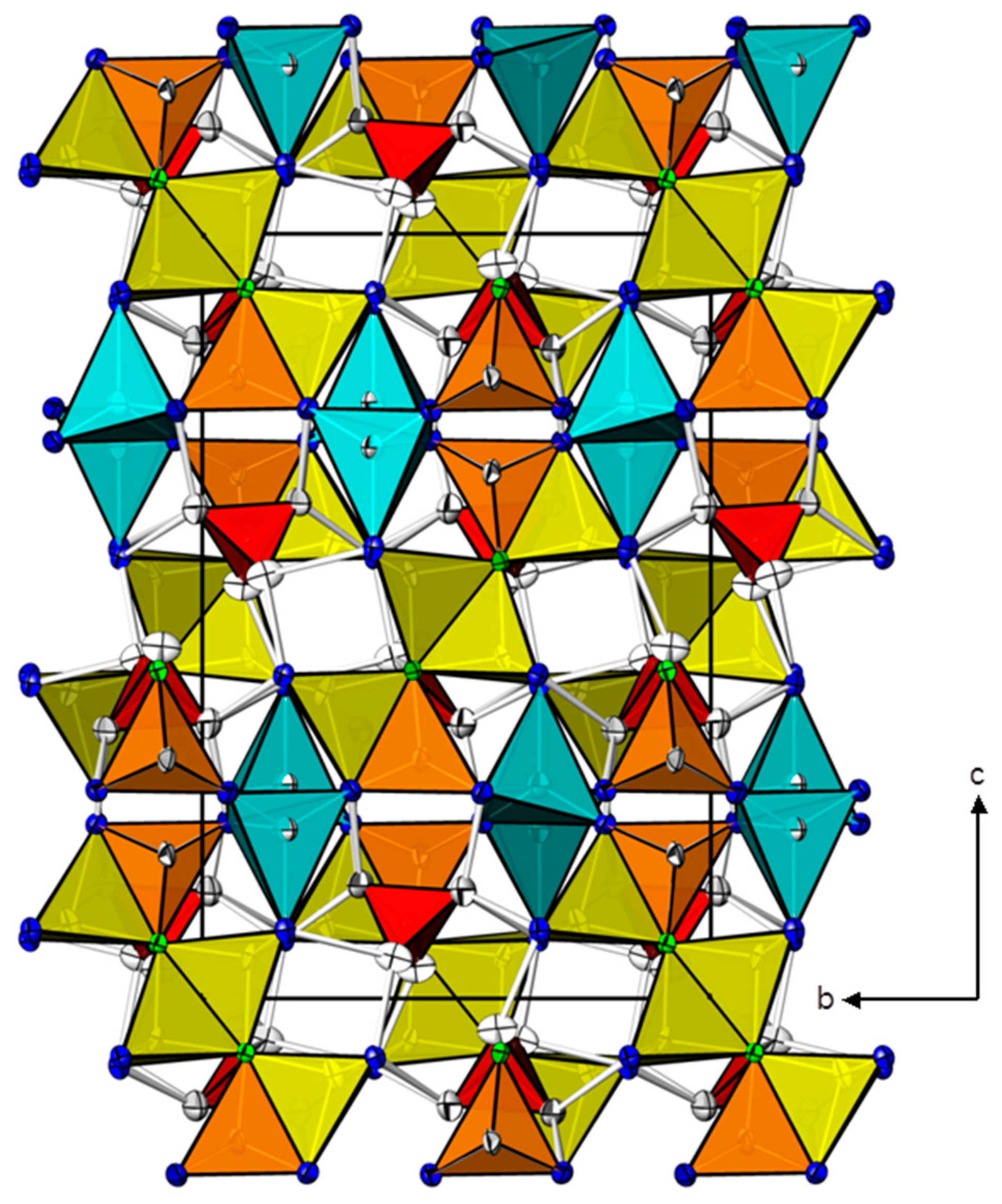
| Cd(HgI2)2(HgII)3O4(CrO4)2 | Zn(HgII)4O4(CrO4) | ||||||
|---|---|---|---|---|---|---|---|
| Hg1 | O4 | 2.002(8) | Hg1 | O3 | 2.030(7) | ||
| Hg1 | O5 | 2.016(8) | Hg1 | O4 | 2.045(6) | ||
| Hg1 | O3 | 2.732(11) | Hg1 | O8 | 2.703(7) | ||
| Hg1 | O2 | 2.734(13) | Hg1 | O1 | 2.805(8) | ||
| Hg2 | O6 | 2.192(8) | Hg1 | O4 | 2.819(7) | ||
| Hg2 | O5 | 2.528(8) | Hg1 | O7 | 2.840(7) | ||
| Hg2 | Hg3 | 2.5301(6) | Hg2 | O1 | 2.043(6) | ||
| Hg2 | O4 | 2.692(9) | Hg2 | O2 | 2.069(6) | ||
| Hg3 | O4 | 2.098(8) | Hg2 | O4 | 2.728(7) | ||
| Hg3 | O1 | 2.734(10) | Hg2 | O5 | 2.776(7) | ||
| Hg3 | O1 | 2.803(11) | Hg2 | O5 | 2.896(7) | ||
| Hg4 | O5 | 2.037(8) | 2x | Hg2 | O8 | 2.903(7) | |
| Hg4 | O6 | 2.600(9) | 2x | Hg3 | O3 | 2.015(6) | |
| Cd | O5 | 2.252(7) | 2x | Hg3 | O4 | 2.024(6) | |
| Cd | O4 | 2.293(9) | 2x | Hg3 | O7 | 2.610(7) | |
| Cd | O2 | 2.322(11) | 2x | Hg3 | O8 | 2.838(8) | |
| Cr | O1 | 1.611(11) | Hg3 | O4 | 2.932(7) | ||
| Cr | O3 | 1.615(10) | Hg4 | O1 | 2.009(6) | ||
| Cr | O2 | 1.665(12) | Hg4 | O2 | 2.027(6) | ||
| Cr | O6 | 1.697(8) | Hg4 | O6 | 2.625(7) | ||
| Hg4 | O8 | 2.731(8) | |||||
| O4 | Hg1 | O5 | 175.2(3) | Hg4 | O7 | 2.746(7) | |
| O5 | Hg4 | O5 | 180.0 | Zn | O3 | 2.045(8) | |
| O6 | Hg2 | Hg3 | 165.6(2) | Zn | O1 | 2.055(6) | |
| Hg3 | Hg2 | O4 | 94.91(17) | Zn | O2 | 2.075(6) | |
| Zn | O5 | 2.097(6) | |||||
| Cd(HgII)4O4(CrO4) | Zn | O6 | 2.146(6) | ||||
| Hg1 | O4 | 2.016(7) | Zn | O2 | 2.325(7) | ||
| Hg1 | O3 | 2.049(6) | Cr | O8 | 1.634(7) | ||
| Hg1 | O7 | 2.638(7) | Cr | O7 | 1.643(7) | ||
| Hg1 | O2 | 2.667(6) | Cr | O6 | 1.652(7) | ||
| Hg1 | O7 | 2.790(7) | Cr | O5 | 1.657(6) | ||
| Hg2 | O2 | 2.012(6) | |||||
| Hg2 | O1 | 2.045(7) | O3 | Hg1 | O4 | 172.8(3) | |
| Hg2 | O5 | 2.584(7) | O1 | Hg2 | O2 | 163.3(3) | |
| Hg2 | O7 | 2.740(7) | O3 | Hg3 | O4 | 176.6(3) | |
| Hg2 | O8 | 2.882(8) | O1 | Hg4 | O2 | 175.7(3) | |
| Hg3 | O1 | 2.057(6) | Hg4 | O1 | Hg2 | 115.1(3) | |
| Hg3 | O2 | 2.062(6) | Hg4 | O2 | Hg2 | 116.0(3) | |
| Hg3 | O4 | 2.577(6) | Hg3 | O3 | Hg1 | 123.2(4) | |
| Hg3 | O8 | 2.725(7) | Hg3 | O4 | Hg1 | 120.2(3) | |
| Hg3 | O6 | 2.752(7) | |||||
| Hg3 | O4 | 2.838(8) | |||||
| Hg4 | O4 | 2.014(7) | |||||
| Hg4 | O3 | 2.026(6) | |||||
| Hg4 | O8 | 2.700(7) | |||||
| Hg4 | O4 | 2.838(8) | |||||
| Cd | O3 | 2.237(6) | |||||
| Cd | O5 | 2.251(7) | |||||
| Cd | O2 | 2.273(6) | |||||
| Cd | O6 | 2.283(7) | |||||
| Cd | O1 | 2.299(6) | |||||
| Cd | O1 | 2.421(6) | |||||
| Cr | O8 | 1.620(7) | |||||
| Cr | O7 | 1.627(7) | |||||
| Cr | O6 | 1.633(7) | |||||
| Cr | O5 | 1.658(7) | |||||
| O4 | Hg1 | O3 | 173.6(3) | ||||
| O2 | Hg2 | O1 | 174.0(3) | ||||
| O1 | Hg3 | O2 | 166.4(2) | ||||
| O4 | Hg4 | O3 | 176.6(3) | ||||
| Hg2 | O1 | Hg3 | 118.6(3) | ||||
| Hg2 | O2 | Hg3 | 117.0(3) | ||||
| Hg4 | O3 | Hg1 | 109.3(3) | ||||
| Hg4 | O4 | Hg1 | 122.2(3) | ||||
| Cd(HgI2)2(HgII)3O4(CrO4)2 |
| Hg1 2.07, Hg2 1.03, Hg3 1.04, Hg4 2.05, Cd1 2.13, Cr1 5.99, O1 1.82 [2 Hg, 1 Cr], O2 1.87 [1 Hg, 1 Cd, 1 Cr], O3 1.75 [1 Hg, 1 Cr], O4 1.94 [3 Hg, 1 Cd], O5 2.29 [3 Hg, 1 Cd], O6 1.91 [1 Cr, 2 Hg]. |
| Cd(HgII)4O4(CrO4) |
| Hg1 2.13, Hg2 2.21, Hg3 2.13, Hg4 2.18, Cd1 2.12, Cr1 6.16, O1 2.21 [2 Hg, 2 Cd], O2 2.20 [3 Hg, Cd], O3 2.08 [2 Hg, Cd], O4 2.07 [4 Hg], O5 2.12 [Cr, Cd, 2 Hg], O6 2.02 [Cr, Cd, Hg], O7 1.97 [Cr, 3 Hg], O8 1.96 [Cr, 3 Hg]. |
| Zn(HgII)4O4(CrO4) |
| Hg1 2.12, Hg2 2.00, Hg3 2.11, Hg4 2.19, Zn1 1.99, Cr1 5.96, O1 2.20 [3 Hg, Zn], O2 2.18 [2 Hg, 2 Zn], O3 2.15 [2 Hg, Zn], O4 1.98 [5 Hg], O5 1.99 [Cr, Zn, 2 Hg], O6 1.94 [Cr, Zn, Hg], O7 1.98 [Cr, 4 Hg], O8 1.98 [Cr, 4 Hg]. |
| Compound | Cd(HgI2)2(HgII)3O4(CrO4)2 | Cd(HgII)4O4(CrO4) | Zn(HgII)4O4(CrO4) | |
|---|---|---|---|---|
| Diffractometer | Siemens SMART | Nonius CAD4 | Siemens SMART | |
| Formula weight | 1812.53 | 1094.76 | 1047.73 | |
| Crystal dimensions/mm3 | 0.08 × 0.10 × 0.25 | 0.04 × 0.04 × 0.23 | 0.01 × 0.05 × 0.10 | |
| Crystal description | red, irregular fragment | orange, plate | yellow, plate | |
| Space group | P | Pbca | P | |
| Formula units Z | 1 | 8 | 2 | |
| a/Å | 6.1852(5) | 6.9848(10) | 6.873(3) | |
| b/Å | 7.3160(6) | 12.8019(15) | 6.928(3) | |
| c/Å | 8.5038(7) | 19.227(3) | 10.413(4) | |
| α/° | 85.5840(10) | 90 | 89.725(7) | |
| β/° | 87.2820(10) | 90 | 70.903(7) | |
| γ/° | 72.0160(10) | 90 | 61.694(7) | |
| V/Å3 | 364.80(5) | 1719.3(4) | 405.7(3) | |
| μ/mm−1 | 76.241 | 74.832 | 79.606 | |
| X-ray density/g·cm−3 | 8.250 | 8.459 | 8.576 | |
| Range θmin–θmax/° | 2.40–30.47 | 3.18–29.99 | 3.40–30.58 | |
| Range | h | −8 → 7 | −9 → 9 | −9 → 9 |
| k | −10 → 9 | −17 → 17 | −9 → 9 | |
| l | −12 → 12 | −27 → 27 | −14 → 12 | |
| Measured reflections | 4245 | 18,439 | 4655 | |
| Independent reflections | 2177 | 2486 | 2408 | |
| Obs. reflections [I > 2σ(I)] | 2153 | 1772 | 1996 | |
| Ri | 0.0450 | 0.0898 | 0.0431 | |
| Absorption correction | ?HABITUS? | |||
| Trans. coeff. Tmin/Tmax | 0.004/0.055 | 0.1393/0.2185 | 0.0222/0.5407 | |
| Ext. coef. (SHELXL97) | 0.0057(2) | 0.000177(9) | 0.00054(7) | |
| Number of parameters | 104 | 128 | 128 | |
| Δemax; Δemax/e−·Å−3 | 2.10; −1.78 | 1.94, −1.94 | 2.50; −2.24 | |
| R[F2 > 2σ(F2)] | 0.0336 | 0.0244 | 0.0284 | |
| wR2(F2 all) | 0.0727 | 0.0446 | 0.0570 | |
| Goof | 1.304 | 1.021 | 0.925 | |
| CSD number | 433,656 | 433,657 | 433,658 | |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weil, M. The Mixed-Metal Oxochromates(VI) Cd(HgI2)2(HgII)3O4(CrO4)2, Cd(HgII)4O4(CrO4) and Zn(HgII)4O4(CrO4)—Examples of the Different Crystal Chemistry within the Zinc Triad. Crystals 2017, 7, 340. https://doi.org/10.3390/cryst7110340
Weil M. The Mixed-Metal Oxochromates(VI) Cd(HgI2)2(HgII)3O4(CrO4)2, Cd(HgII)4O4(CrO4) and Zn(HgII)4O4(CrO4)—Examples of the Different Crystal Chemistry within the Zinc Triad. Crystals. 2017; 7(11):340. https://doi.org/10.3390/cryst7110340
Chicago/Turabian StyleWeil, Matthias. 2017. "The Mixed-Metal Oxochromates(VI) Cd(HgI2)2(HgII)3O4(CrO4)2, Cd(HgII)4O4(CrO4) and Zn(HgII)4O4(CrO4)—Examples of the Different Crystal Chemistry within the Zinc Triad" Crystals 7, no. 11: 340. https://doi.org/10.3390/cryst7110340
APA StyleWeil, M. (2017). The Mixed-Metal Oxochromates(VI) Cd(HgI2)2(HgII)3O4(CrO4)2, Cd(HgII)4O4(CrO4) and Zn(HgII)4O4(CrO4)—Examples of the Different Crystal Chemistry within the Zinc Triad. Crystals, 7(11), 340. https://doi.org/10.3390/cryst7110340