Effect of the Activator B(OCH3)3 on the Microstructure and Mechanical Properties of Cu-Mn-Al Alloy Coating via CMT Cladding
Abstract
1. Introduction
2. Experimental Design
2.1. Experimental Materials
2.2. Cladding Procedure and Sample Preparation
2.3. Microstructure and Performance Analysis
3. Results and Discussion
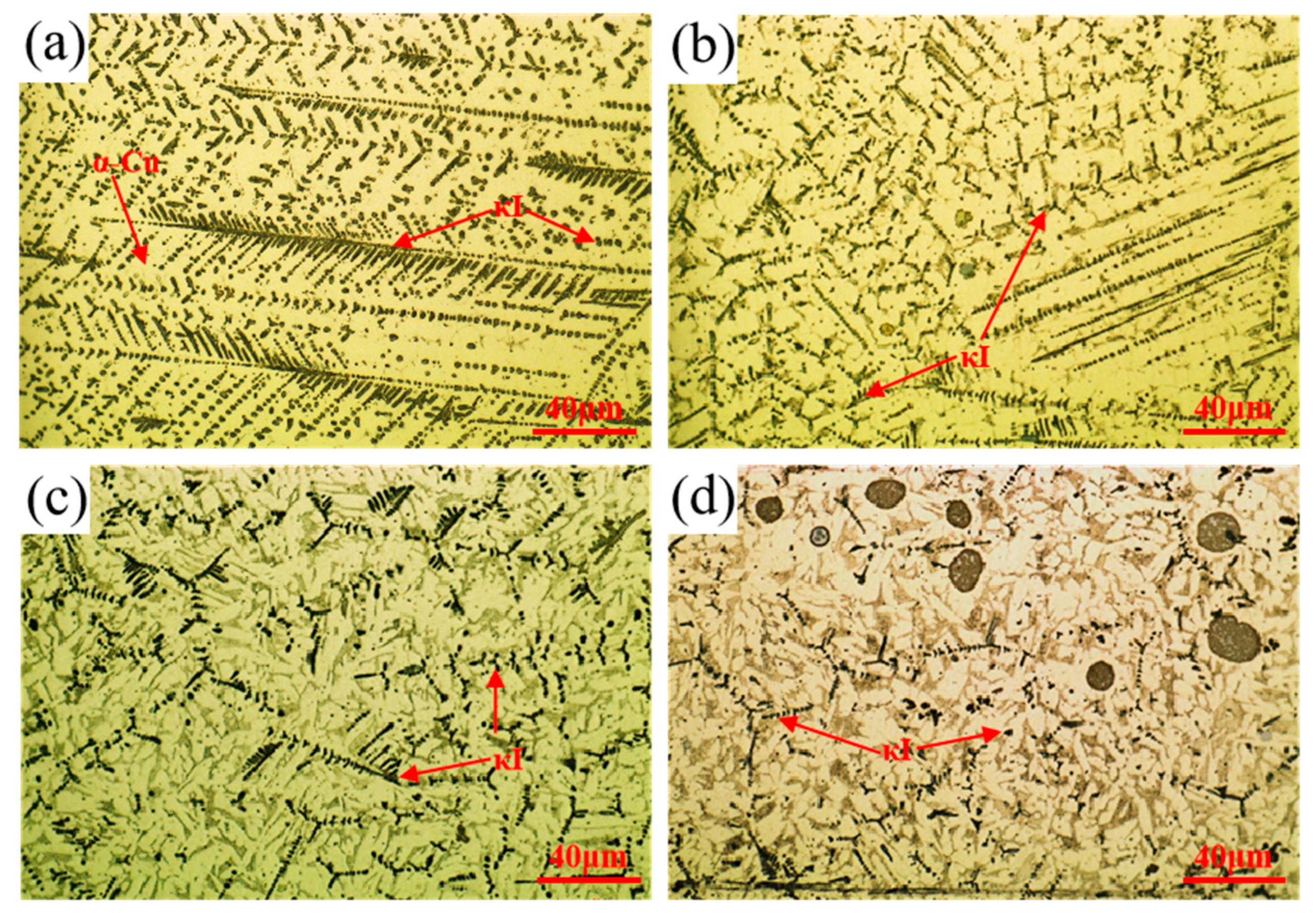
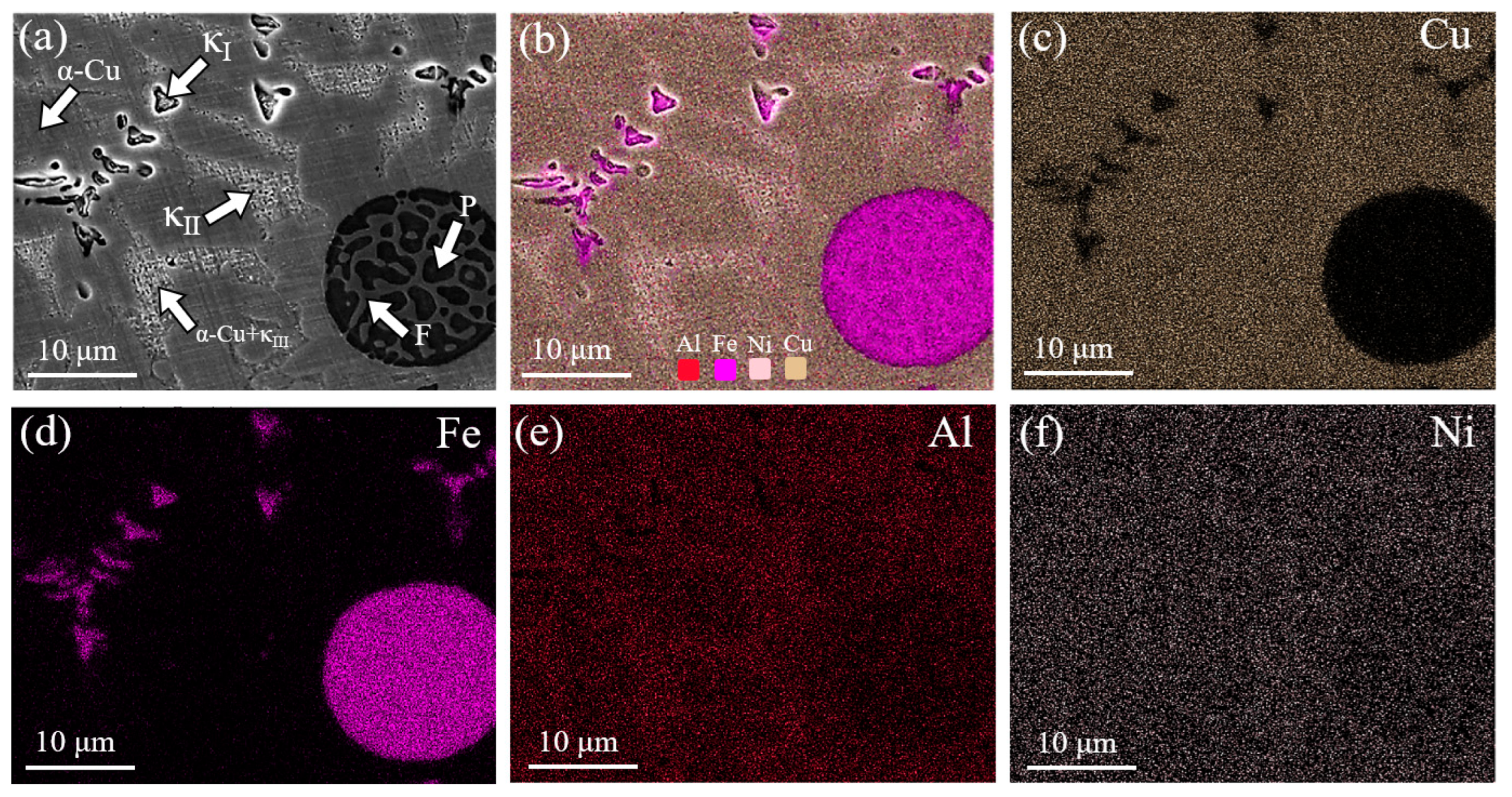
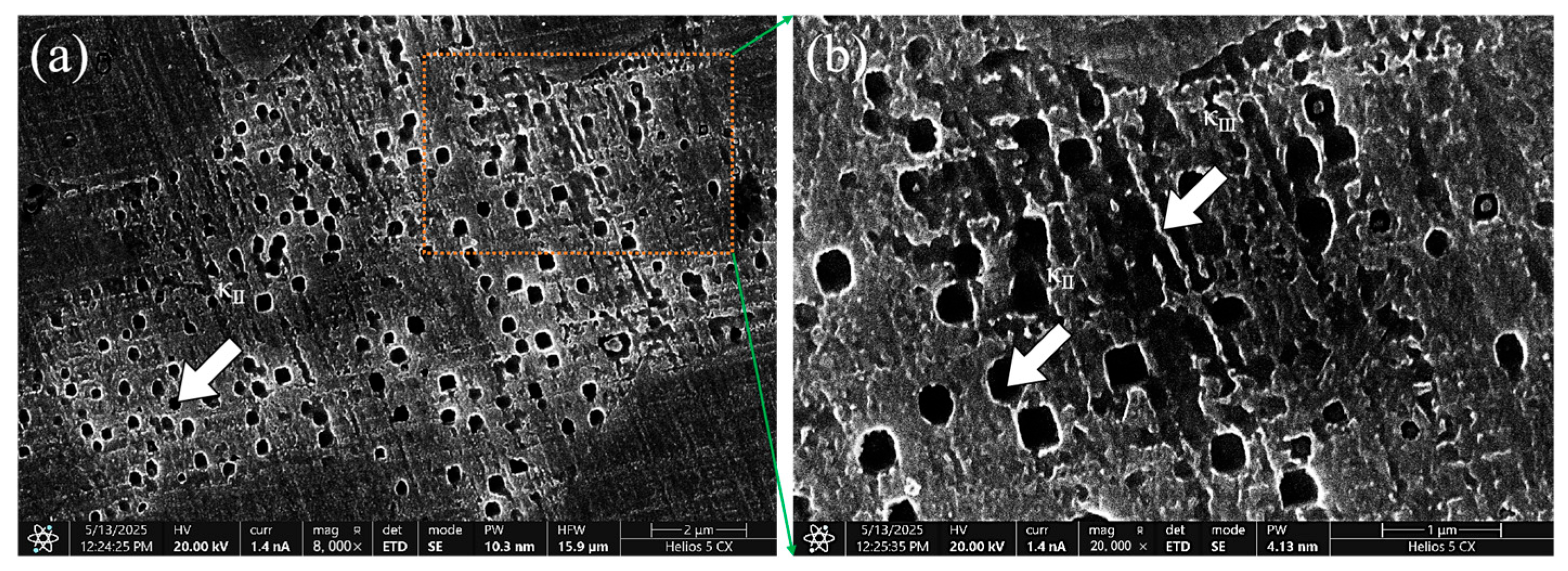
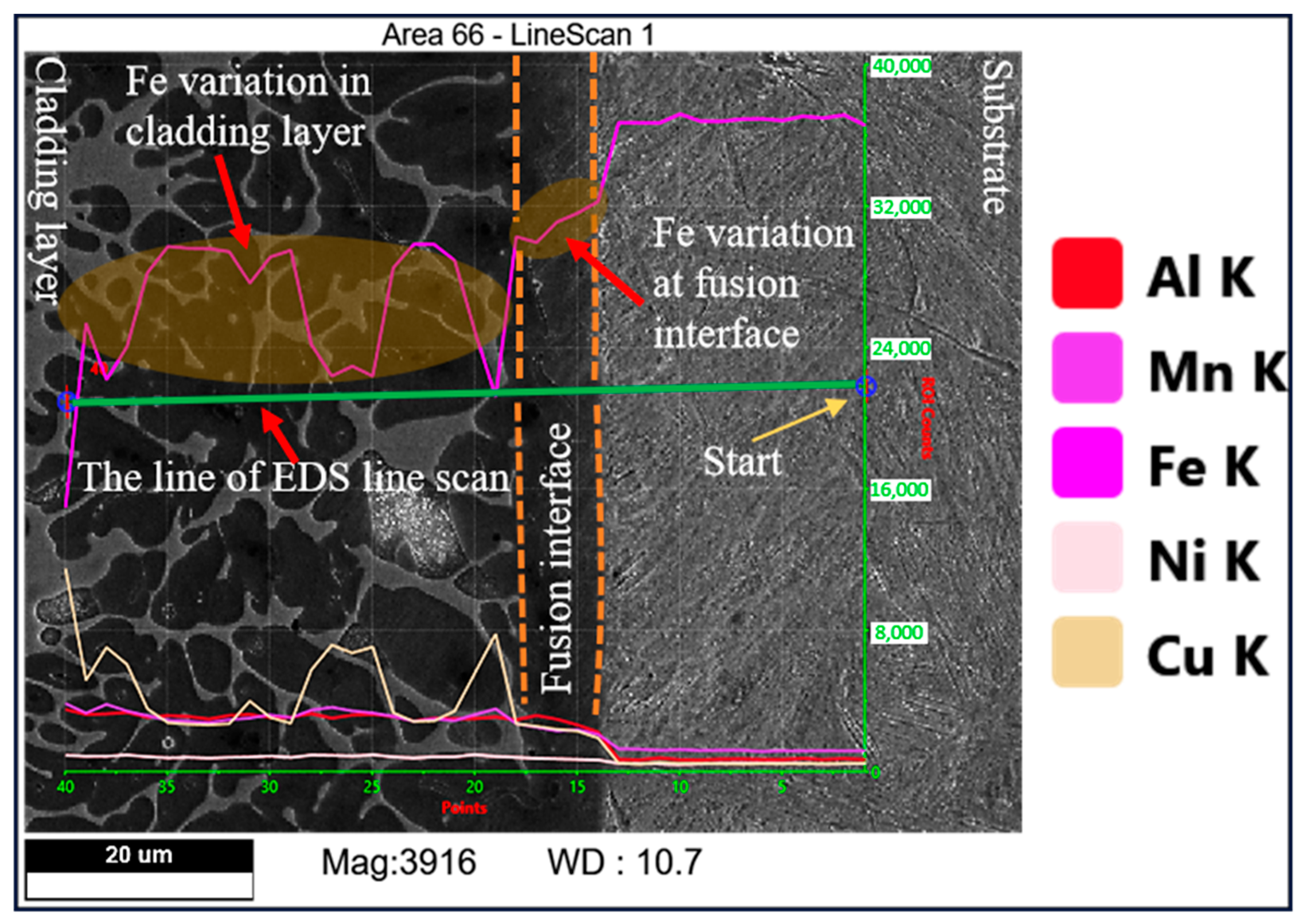
3.1. Effect of Mixed Gas Flow Rate on the Microstructure of the Cladding Layer
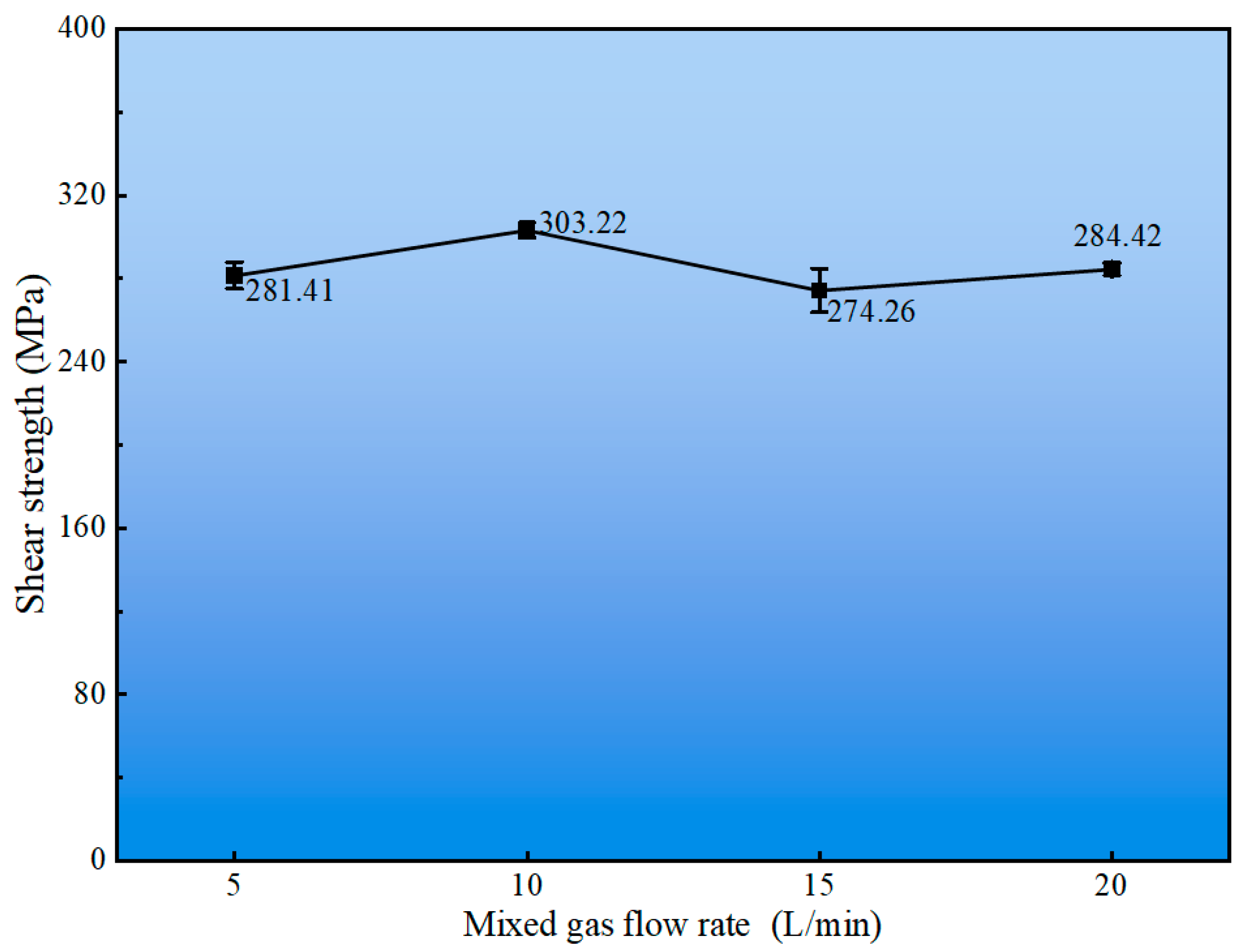
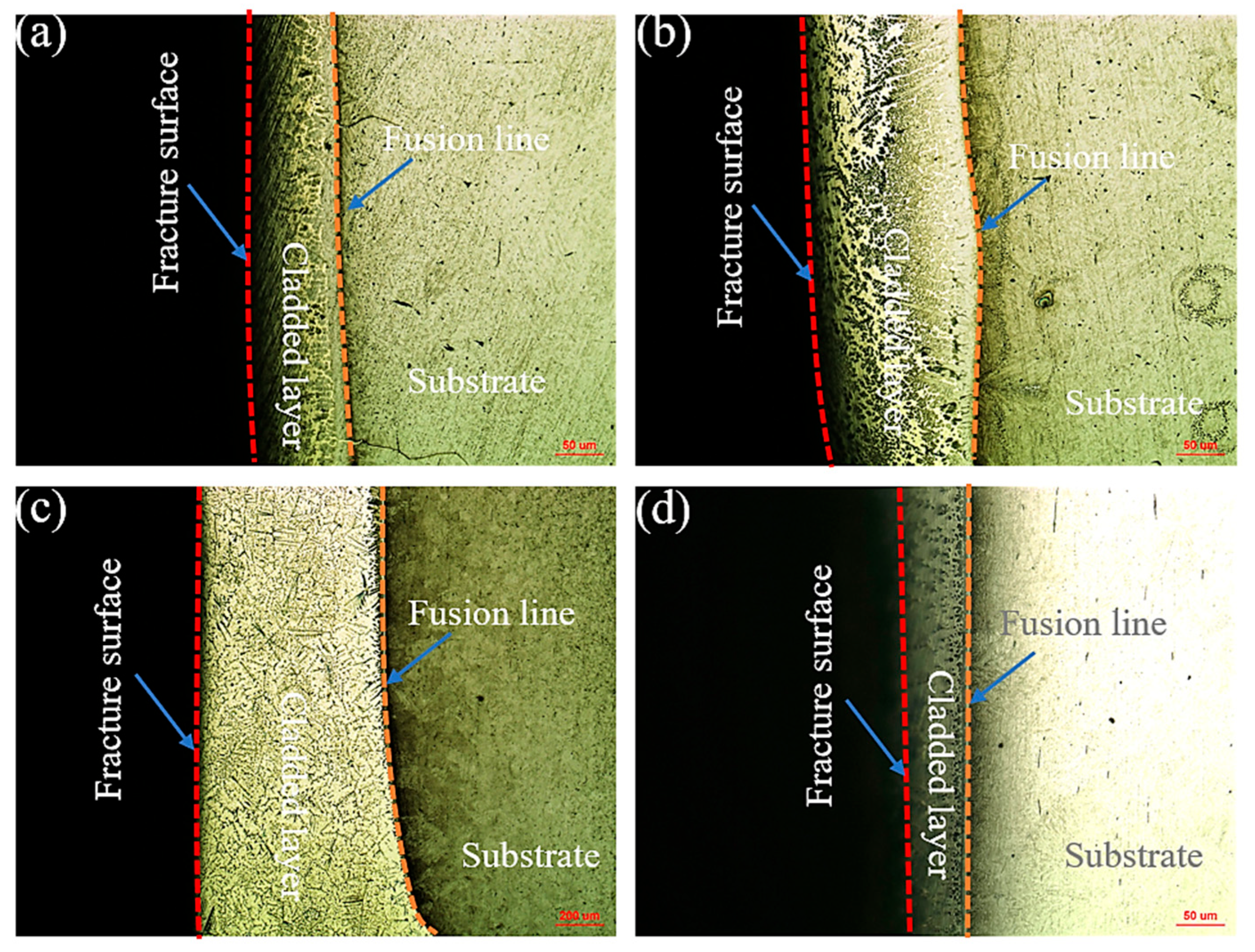
3.2. Effect of Mixed Gas Flow Rate on Cladding Layer Properties
4. Conclusions
- (1)
- The mixed gas flow rate critically governs the microstructure evolution. As the flow rate increases from 5 to 20 L/min, the morphology of the primary κI phase evolves from dendritic and granular to petal-like and rod-like configurations. At higher flow rates (15–20 L/min), the cladding layer develops light gray eutectoid structures (α-Cu + κIII phase) and spherical κII phases. Notably, distinct dark spherical Fe-rich phases, originating from the intensified stirring and incorporation of the steel substrate, are prominently observed at 20 L/min.
- (2)
- An optimal gas flow rate of 10 L/min was identified, achieving a balance between effective shielding and molten pool stability. Under this condition, the cladding layer attains its peak shear strength of 303.22 MPa. The consistent occurrence of shear fractures within the cladding layer itself, characterized by dimpled ductile fracture surfaces, unequivocally demonstrates that the interfacial bonding strength surpasses the cohesive strength of the Cu-Mn-Al alloy.
- (3)
- The cladding layer deposited at 10 L/min also exhibits superior corrosion resistance in a 3.5 wt% NaCl solution, demonstrating the lowest corrosion current density (3.2842 × 10−6 A·cm−2) and corrosion rate (0.02935 mm/y). Furthermore, all cladding layers provided significantly better corrosion protection than the uncoated 27SiMn steel substrate.
- (4)
- The incorporation of B(OCH3)3 as an active agent in the shielding gas fundamentally improves the deposition process. It significantly enhances the wettability of the Cu-Mn-Al alloy, resulting in a smaller wetting angle and a wider, more uniform cladding layer compared to using pure argon.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.; Kang, J.; Shu, L.; Xing, Z.; Wang, H. Study on the Low-Cycle Fatigue Performance and Failure Behavior of IN718 Coatings Laser Cladded on the Surface of 27SiMn Steel. Eng. Fail. Anal. 2025, 174, 109537. [Google Scholar] [CrossRef]
- Li, C.; Ni, Y.; Wang, M.; Lu, Z.; Zhang, P. Analysis of Microstructure and Performance of Laser Cladding WC-Fe316L Alloy on the Surface of 27SiMn Steel. J. Phys. Conf. Ser. 2022, 2185, 012083. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, S.; Liu, B.; Gutsev, D.M.; Wu, J.; Levchenko, V.; Wang, H.; Ma, D. Tribological Properties of Corrosion-Resistant GLC/CrCN Multilayer Coatings. Surf. Coat. Technol. 2025, 501, 131946. [Google Scholar] [CrossRef]
- Singh, I.J.; Murtaza, Q.; Kumar, P. A Comprehensive Review on Effect of Cold Metal Transfer Welding Parameters on Dissimilar and Similar Metal Welding. J. Eng. Res. 2025, 13, 1184–1201. [Google Scholar] [CrossRef]
- Li, C.; Quan, G.; Zhang, Q.; Wang, X.; Li, X.; Kou, S. A Novel Amorphous Alloy Coating for Elevating Corrosion Resistance of X70 Pipeline Steel. J. Therm. Spray Technol. 2024, 33, 1612–1629. [Google Scholar] [CrossRef]
- Ding, Y.; Bi, W.; Zhong, C.; Wu, T.; Gui, W. A Comparative Study on Microstructure and Properties of Ultra-High-Speed Laser Cladding and Traditional Laser Cladding of Inconel625 Coatings. Materials 2022, 15, 6400. [Google Scholar] [CrossRef]
- Rajeev, G.P.; Kamaraj, M.; Bakshi, S.R. Al-Si-Mn Alloy Coating on Aluminum Substrate Using Cold Metal Transfer (CMT) Welding Technique. JOM 2014, 66, 1061–1067. [Google Scholar] [CrossRef]
- Das, B.; Panda, B.N.; Sharma, F.; Dixit, U.S. Recent Developments in Cladding and Coating Using Cold Metal Transfer Technology. J. Mater. Eng. Perform. 2024, 33, 3130–3147. [Google Scholar] [CrossRef]
- Selvi, S.; Vishvaksenan, A.; Rajasekar, E. Cold Metal Transfer (CMT) Technology—An Overview. Def. Technol. 2018, 14, 28–44. [Google Scholar] [CrossRef]
- Bellamkonda, P.N.; Dwivedy, M.; Addanki, R. Cold Metal Transfer Technology—A Review of Recent Research Developments. Results Eng. 2024, 23, 102423. [Google Scholar] [CrossRef]
- Qi, L.; Chai, L.; Li, Z.; Yang, T.; Zhou, J.; Cheng, R.; Zhang, K. Microstructure and Corrosion Resistance of an Al-Bronze Coating Prepared by Cold Metal Transfer on 27SiMn Steel. Surf. Coat. Technol. 2024, 478, 130493. [Google Scholar] [CrossRef]
- Liu, F.; Gao, X.; Zeng, Z.; You, Q.; Liu, F.; Wang, Z.; Xu, Y.; Zheng, H.; Liu, Q.; Lin, X.; et al. Comparative Study on Microstructure and Oxidation Resistance of Inconel 625 Superalloy Coatings on 12CrMoV Steel Surfaces Prepared by CMT and MIG Cladding. J. Mater. Res. Technol. 2024, 29, 3910–3922. [Google Scholar] [CrossRef]
- He, K.; Dong, L.; Wang, Q.; Zhang, H.; Li, Y.; Liu, L.; Zhang, Z. Comparison on the Microstructure and Corrosion Behavior of Inconel 625 Cladding Deposited by Tungsten Inert Gas and Cold Metal Transfer Process. Surf. Coat. Technol. 2022, 435, 128245. [Google Scholar] [CrossRef]
- Meng, X.; Zhu, L.; Li, Y.; Hu, P.; Cai, G.; Liu, J.; Zhang, Q.; Dong, Z.; Zhang, X. The Influence of Ultrasonic Vibration on Micro-Arc Oxidation Behaviour of Manganese Aluminium Bronze. J. Mater. Res. Technol. 2024, 33, 758–772. [Google Scholar] [CrossRef]
- Song, Q.N.; Zhang, H.N.; Li, H.L.; Hong, H.; Sun, S.Y.; Xu, N.; Zhang, G.Y.; Bao, Y.F.; Qiao, Y.X. Corrosion and Cavitation Erosion Behaviors of the Manganese-Aluminum-Bronze Cladding Layer Prepared by MIG in 3.5% NaCl Solution. Mater. Today Commun. 2022, 31, 103566. [Google Scholar] [CrossRef]
- Song, Q.N.; Wang, Y.; Jin, Z.T.; Zhang, Y.C.; Xu, N.; Bao, Y.F.; Jiang, Y.F.; Lu, Q.Q.; Zhao, J.H.; Gao, Y.; et al. Comparison of the Corrosion and Cavitation Erosion Behaviors of the Cast and Surface-Modified Manganese-Aluminum Bronzes in Sodium Chloride Solution. J. Mater. Res. Technol. 2024, 30, 4310–4321. [Google Scholar] [CrossRef]
- Rahni, M.R.M.; Beidokhti, B.; Haddad-Sabzevar, M. Effect of Filler Metal on Microstructure and Mechanical Properties of Manganese–Aluminum Bronze Repair Welds. Trans. Nonferrous Met. Soc. China 2017, 27, 507–513. [Google Scholar] [CrossRef]
- Ouyang, C.; Bai, Q.; Yan, X.; Chen, Z.; Han, B.; Liu, Y. Microstructure and Corrosion Properties of Laser Cladding Fe-Based Alloy Coating on 27SiMn Steel Surface. Coatings 2021, 11, 552. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Choudhary, S.; Singh, A. Trimethyl Borate-Catalyzed, Solvent-Free Reductive Amination. J. Org. Chem. 2021, 86, 4274–4280. [Google Scholar] [CrossRef]
- Shestakov, V.A.; Kosyakov, V.I.; Kosinova, M.L. Thermodynamic Modeling of Decomposition Processes of Trimethyl Borate in Different Gas Mixtures. Mater. Today Proc. 2019, 16, 88–94. [Google Scholar] [CrossRef]
- Kutlubay, A.; Gültekin, E. Investigation of TMB and TEB as Fuel Additives for an Aerojet Engine. Fuel 2025, 402, 135970. [Google Scholar] [CrossRef]
- Chang, C.-C.; Lee, K.-Y.; Lee, H.-Y.; Su, Y.-H.; Her, L.-J. Trimethyl Borate and Triphenyl Borate as Electrolyte Additives for LiFePO4 Cathode with Enhanced High Temperature Performance. J. Power Sources 2012, 217, 524–529. [Google Scholar] [CrossRef]
- Makuch, N.; Dziarski, P. Importance of Trimethyl Borate Temperature Used during Gas Boriding for Microstructure, Nanomechanical Properties and Residual Stresses Distribution on the Cross-Section of the Produced Layer. Surf. Coat. Technol. 2021, 405, 126508. [Google Scholar] [CrossRef]
- Lei, X.; Wang, L.; Shen, B.; Sun, F.; Zhang, Z. Effect of Boron-Doped Diamond Interlayer on Cutting Performance of Diamond Coated Micro Drills for Graphite Machining. Materials 2013, 6, 3128–3138. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Peng, Y.; Chen, L.-Y.; Zhang, T.-Y.; He, S.; Wang, K.-H. Corrosion Behavior of Wire-Arc Additive Manufactured and as-Cast Ni-Al Bronze in 3.5 wt% NaCl Solution. Corros. Sci. 2023, 215, 111048. [Google Scholar] [CrossRef]
- Dharmendra, C.; Hadadzadeh, A.; Amirkhiz, B.S.; Janaki Ram, G.D.; Mohammadi, M. Microstructural Evolution and Mechanical Behavior of Nickel Aluminum Bronze Cu-9Al-4Fe-4Ni-1Mn Fabricated through Wire-Arc Additive Manufacturing. Addit. Manuf. 2019, 30, 100872. [Google Scholar] [CrossRef]
- Zangene, D.; Kayvandarian, F.; Khodabakhshi, F.; Malekan, M.; Hájovská, Z. Nickel-Aluminum Bronze (NAB) Alloy Design under Two-Steps Casting and Submerged Friction Stir Processing. Mater. Sci. Eng. A 2024, 890, 145960. [Google Scholar] [CrossRef]

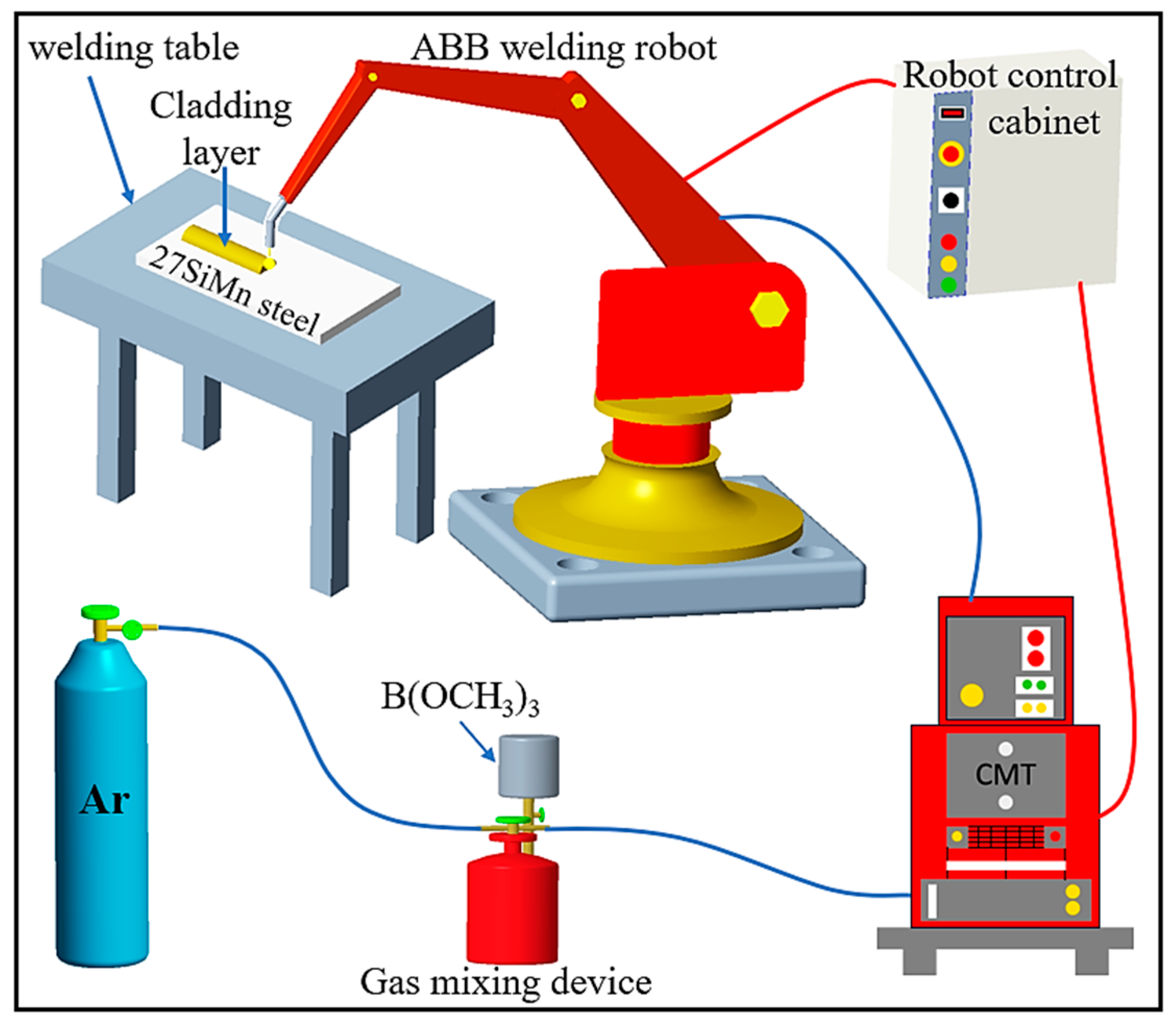
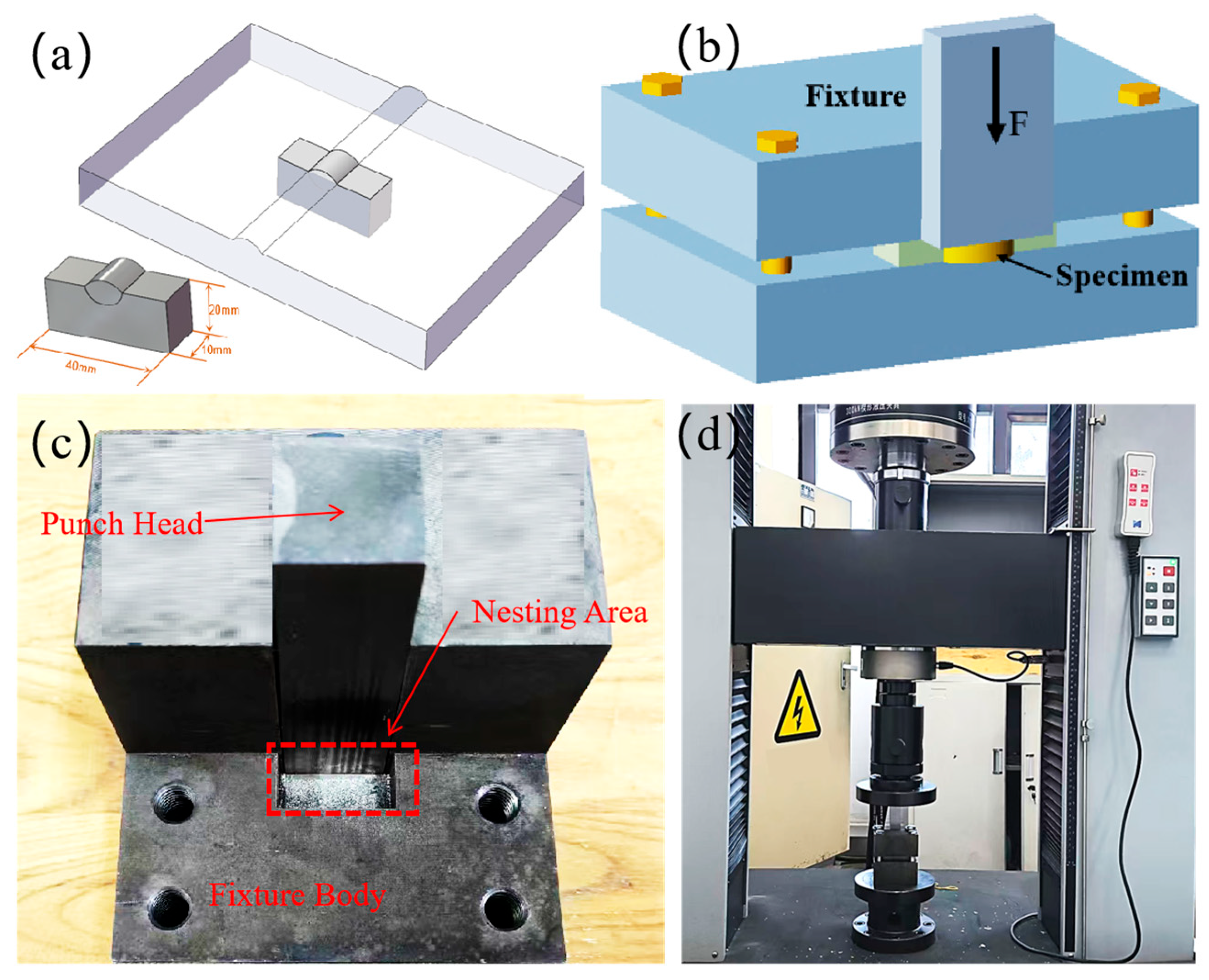
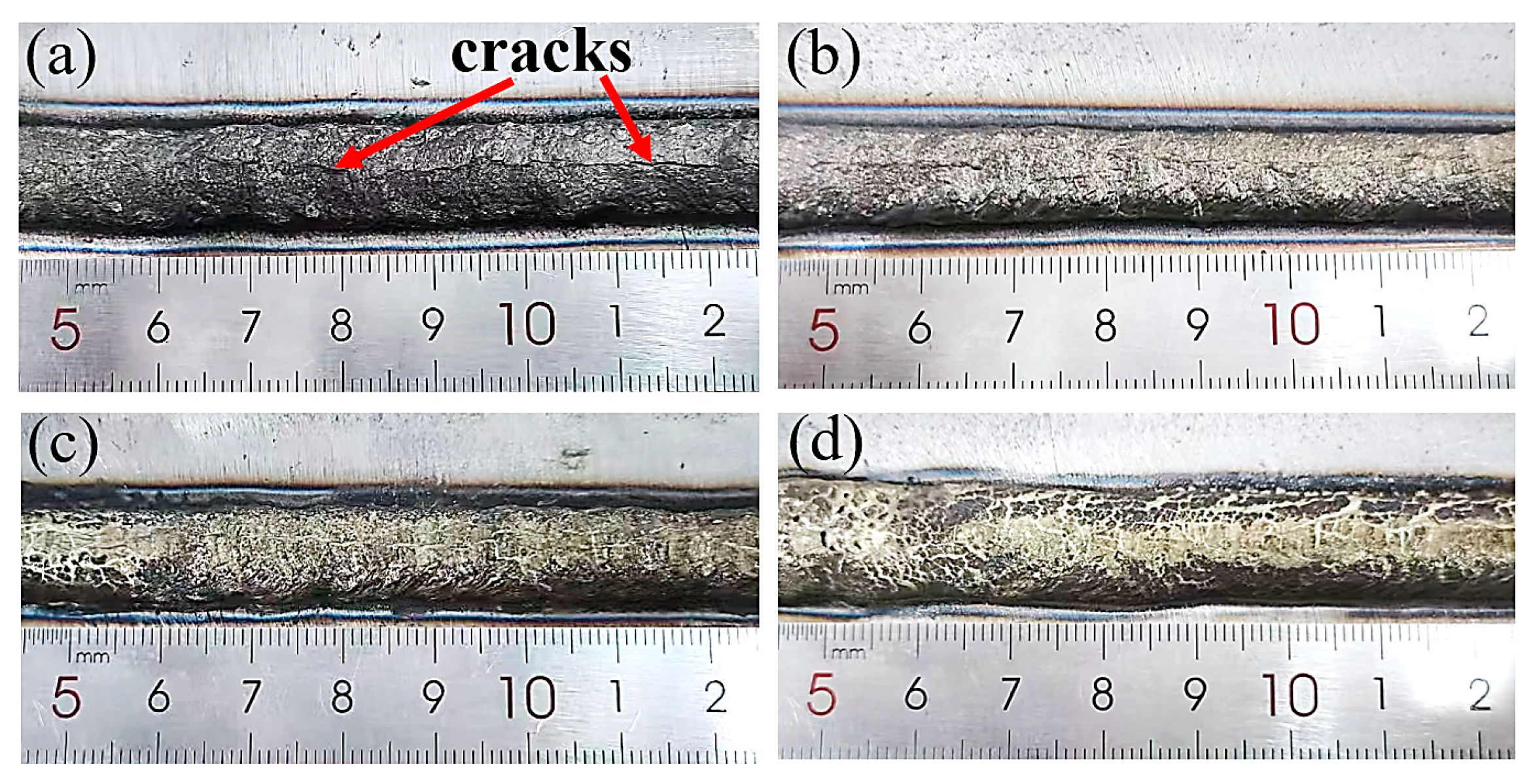

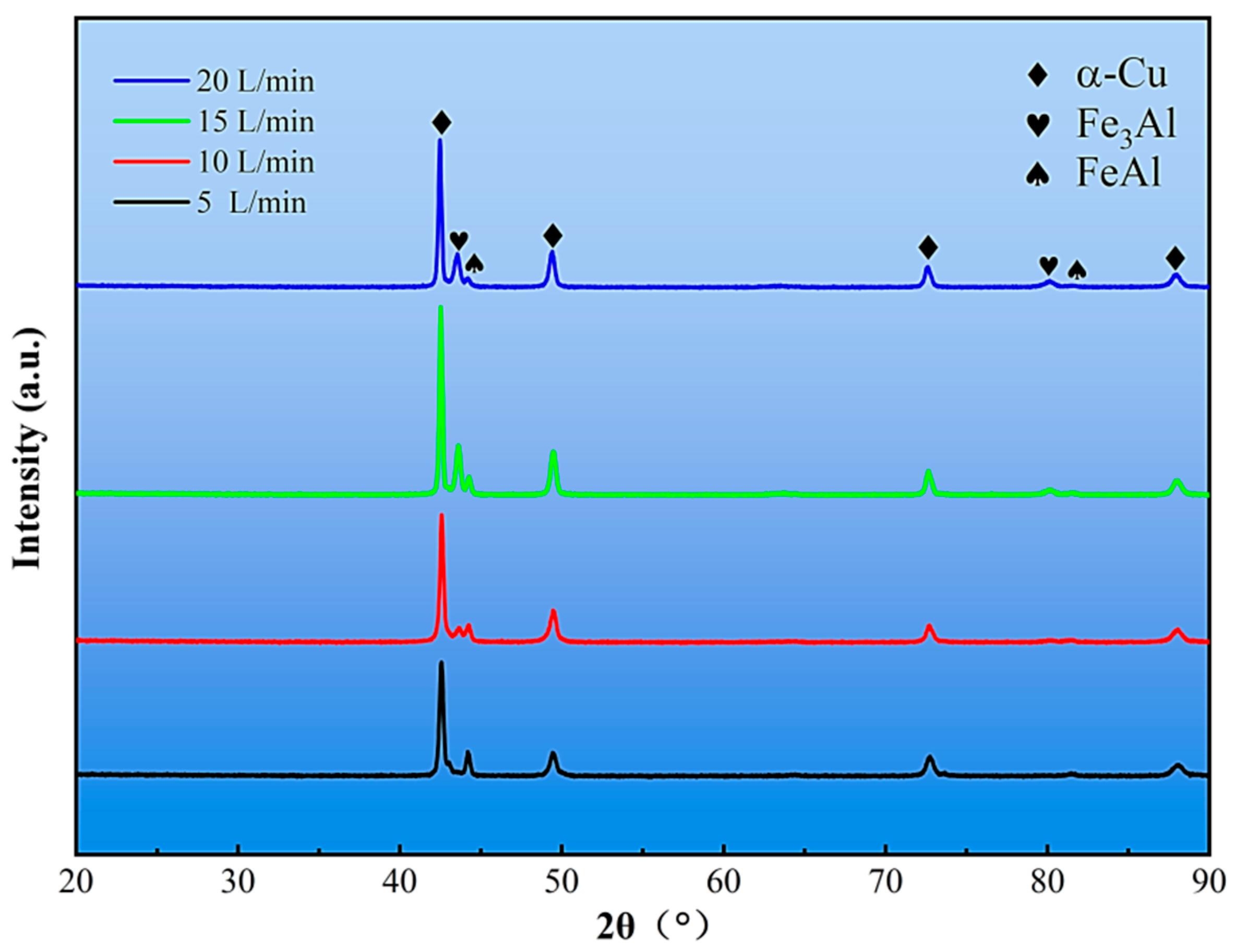



| Element | C | Si | Mn | V | Ni | Cu | Fe | Al | Zn |
|---|---|---|---|---|---|---|---|---|---|
| 27SiMn | 0.24~0.32 | 1.10~1.40 | 1.10~1.40 | 0.07~0.12 | ≤0.30 | ≤0.30 | Bal. | - | - |
| BCu74MnAlFeNi | - | ≤0.1 | 11.0~14.0 | - | 2.5~3.0 | Bal. | 2.0~4.0 | 7.0~8.5 | ≤0.15 |
| Parameter | Value |
|---|---|
| Welding current (A) | 180 |
| Welding speed (m/min) | 0.36 |
| Wire feed speed (m/min) | 6.9 |
| Arc voltage (V) | 14–18 |
| Shielding gas (Mixed Gas) (L/min) | 5, 10, 15, 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, J.; Xie, S.; Xia, J.; Wang, X.; Ni, Z.; Wang, P.; Chen, N. Effect of the Activator B(OCH3)3 on the Microstructure and Mechanical Properties of Cu-Mn-Al Alloy Coating via CMT Cladding. Crystals 2025, 15, 881. https://doi.org/10.3390/cryst15100881
Peng J, Xie S, Xia J, Wang X, Ni Z, Wang P, Chen N. Effect of the Activator B(OCH3)3 on the Microstructure and Mechanical Properties of Cu-Mn-Al Alloy Coating via CMT Cladding. Crystals. 2025; 15(10):881. https://doi.org/10.3390/cryst15100881
Chicago/Turabian StylePeng, Jin, Shihua Xie, Junhai Xia, Xingxing Wang, Zenglei Ni, Pei Wang, and Nannan Chen. 2025. "Effect of the Activator B(OCH3)3 on the Microstructure and Mechanical Properties of Cu-Mn-Al Alloy Coating via CMT Cladding" Crystals 15, no. 10: 881. https://doi.org/10.3390/cryst15100881
APA StylePeng, J., Xie, S., Xia, J., Wang, X., Ni, Z., Wang, P., & Chen, N. (2025). Effect of the Activator B(OCH3)3 on the Microstructure and Mechanical Properties of Cu-Mn-Al Alloy Coating via CMT Cladding. Crystals, 15(10), 881. https://doi.org/10.3390/cryst15100881






