Photocatalytic Degradation of Textile Orange 16 Reactive Dye by ZnO Nanoparticles Synthesized via Green Route Using Punica Granatum Leaf Extract
Abstract
1. Introduction
2. Materials and Methodology
2.1. Materials
2.2. Methodology
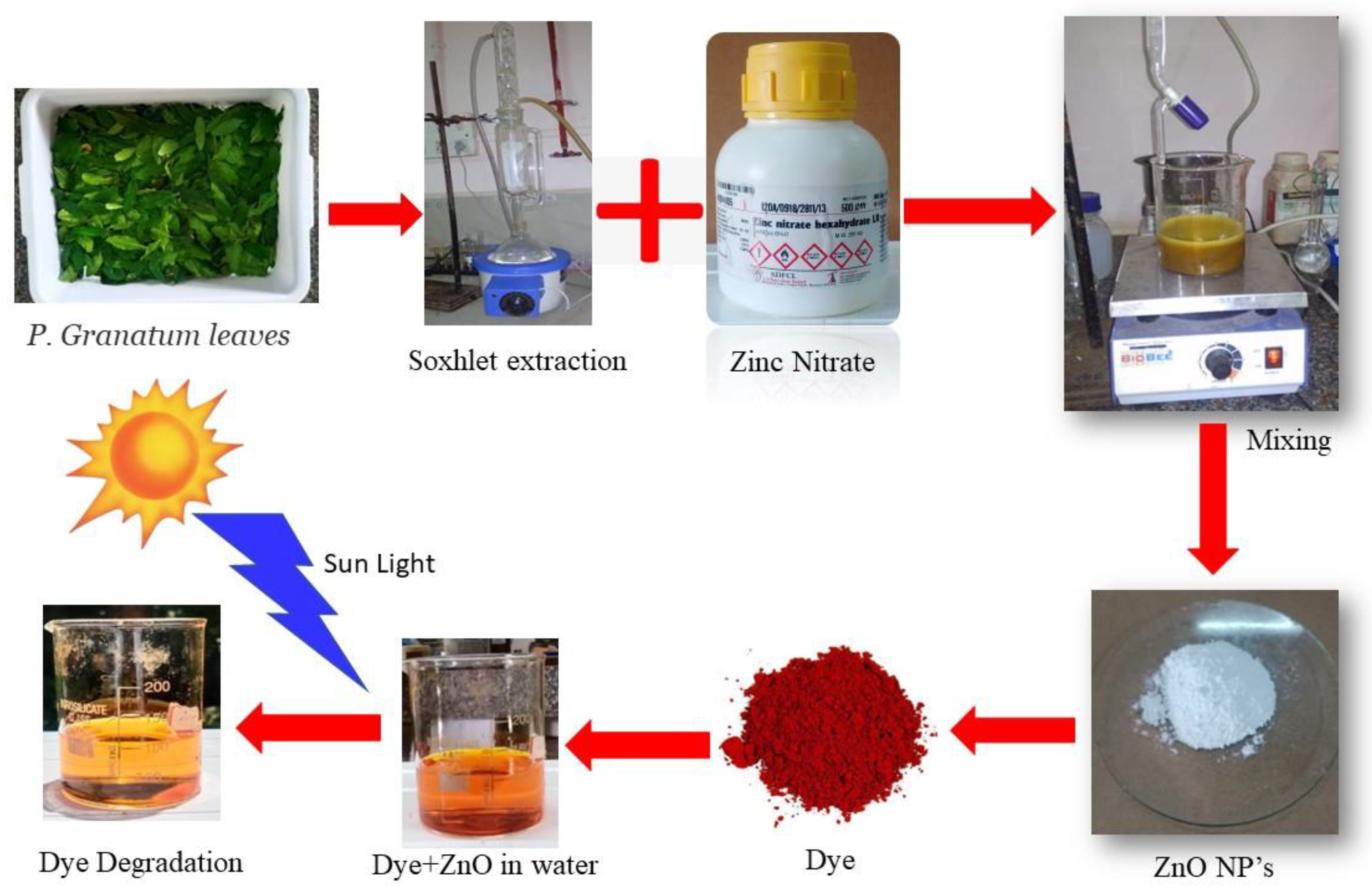
2.2.1. Extraction of Punica granatum Leaf
2.2.2. Green Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs)
2.2.3. Characterization of ZnO-NPs
2.2.4. Photocatalytic Activity of ZnO-NPs
3. Results and Discussion
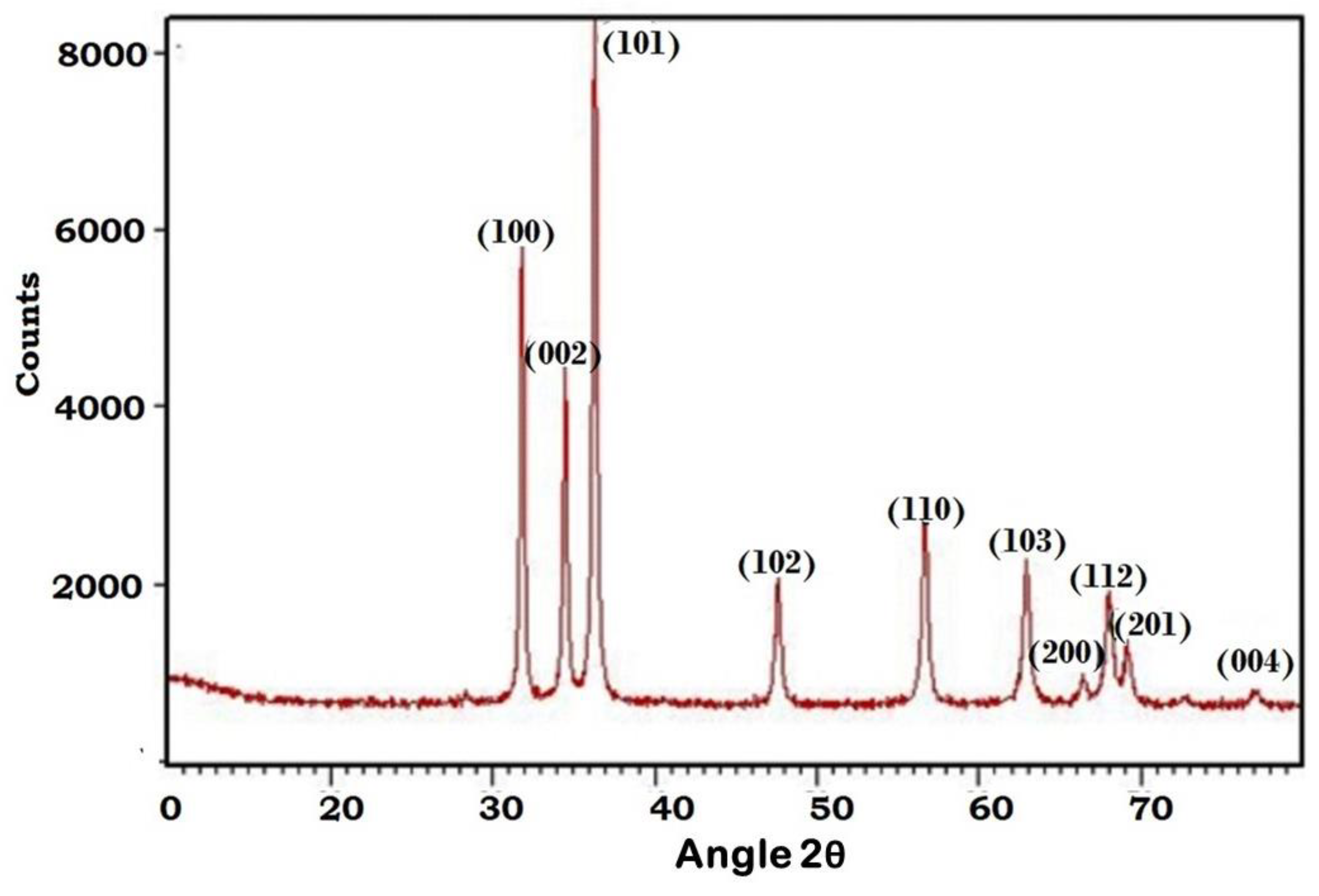
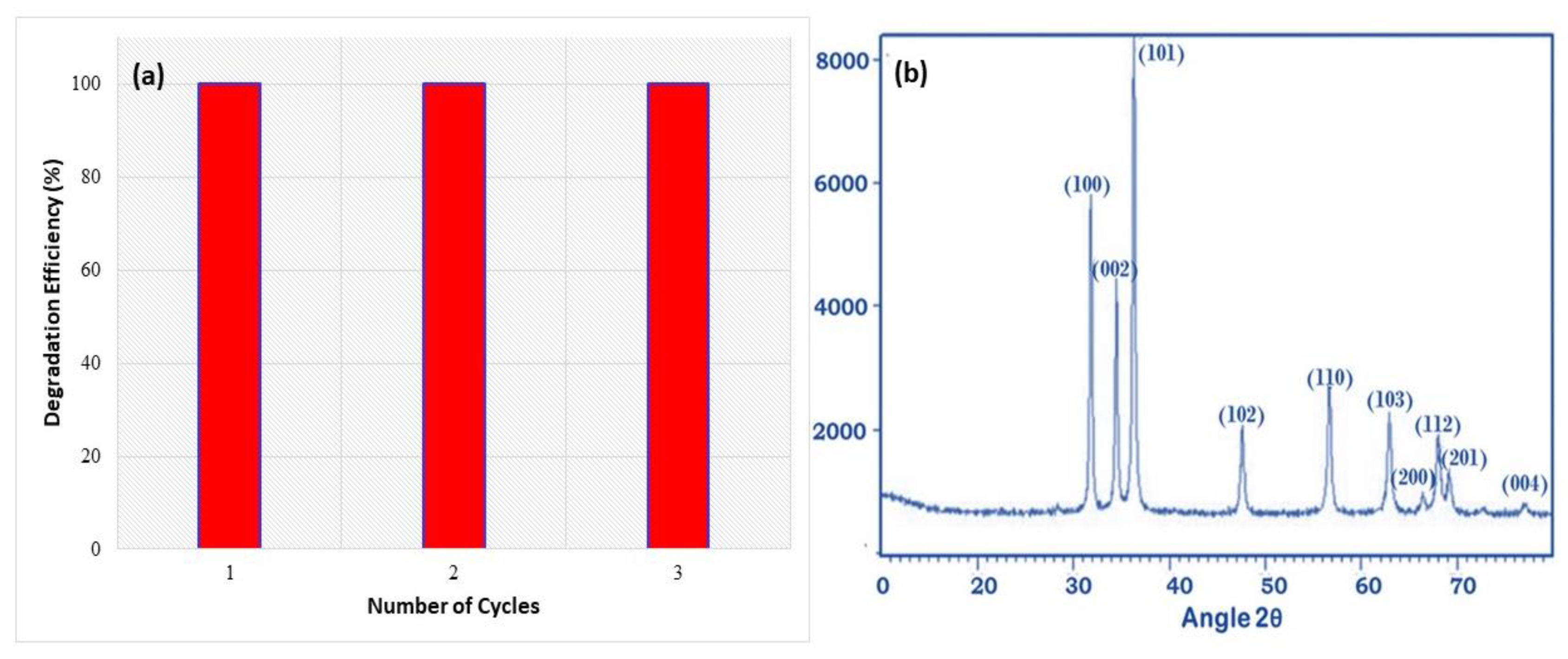
3.1. X-ray Diffraction Analysis
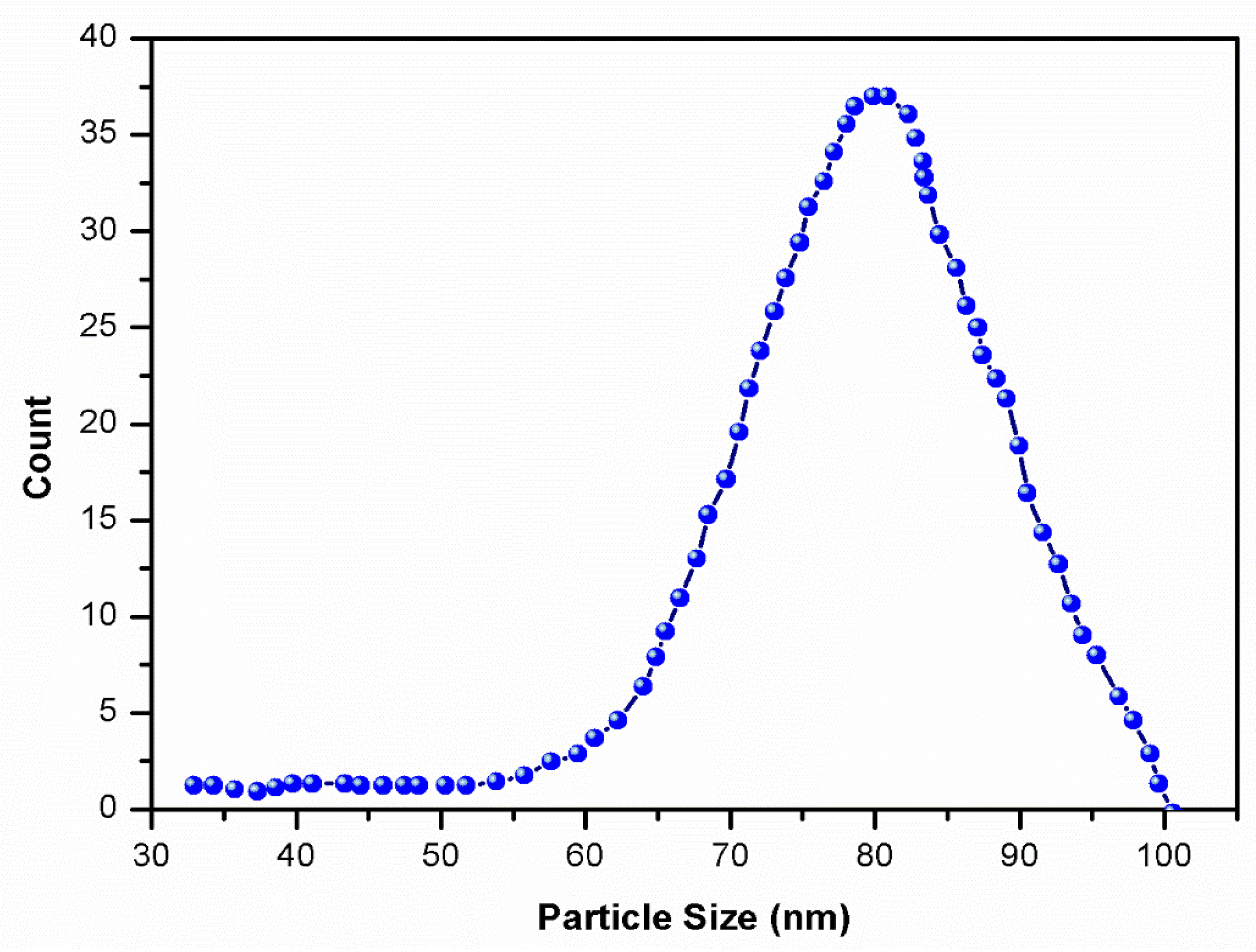
3.2. Particle Size Analysis
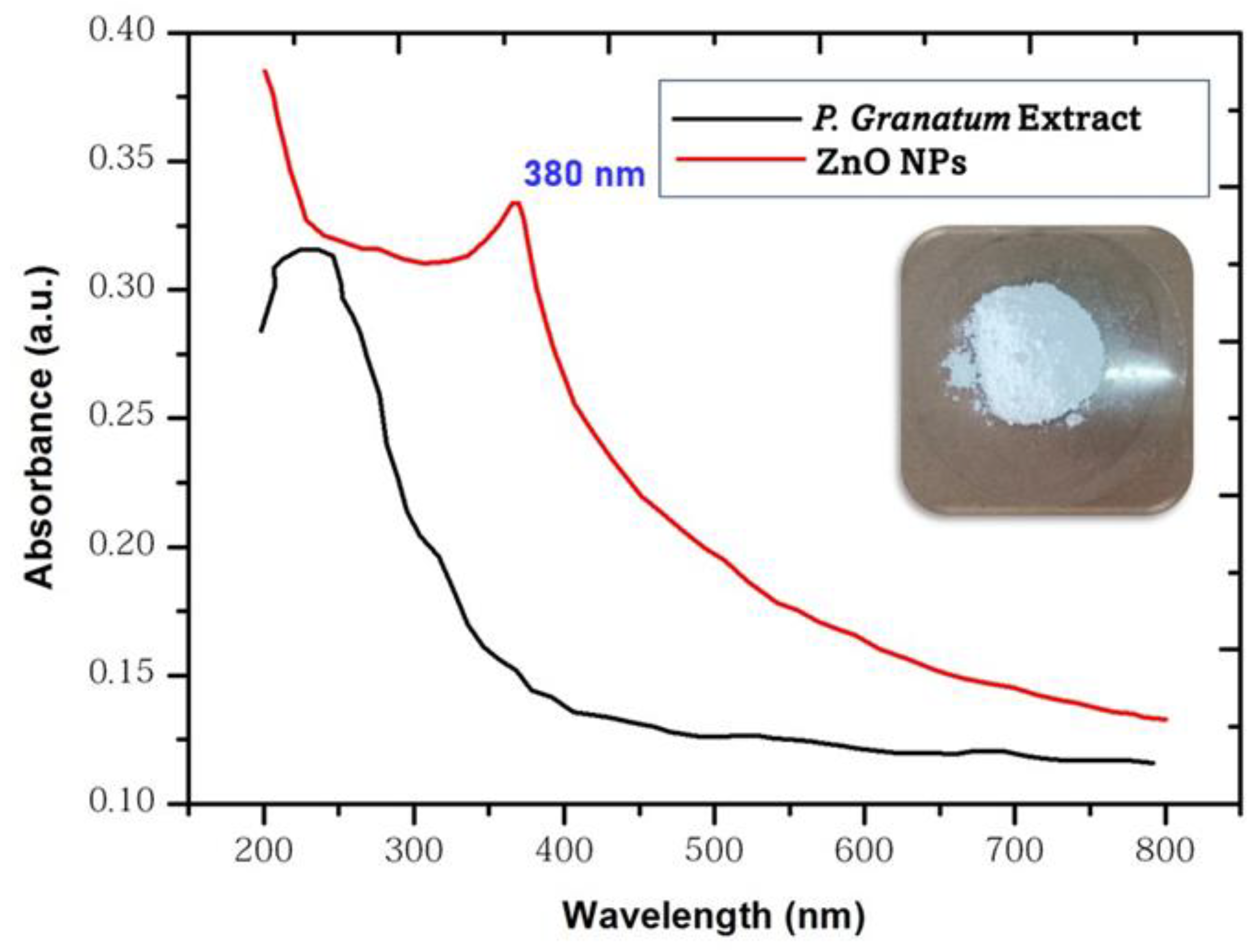
3.3. UV-Visible Spectroscopic Analysis
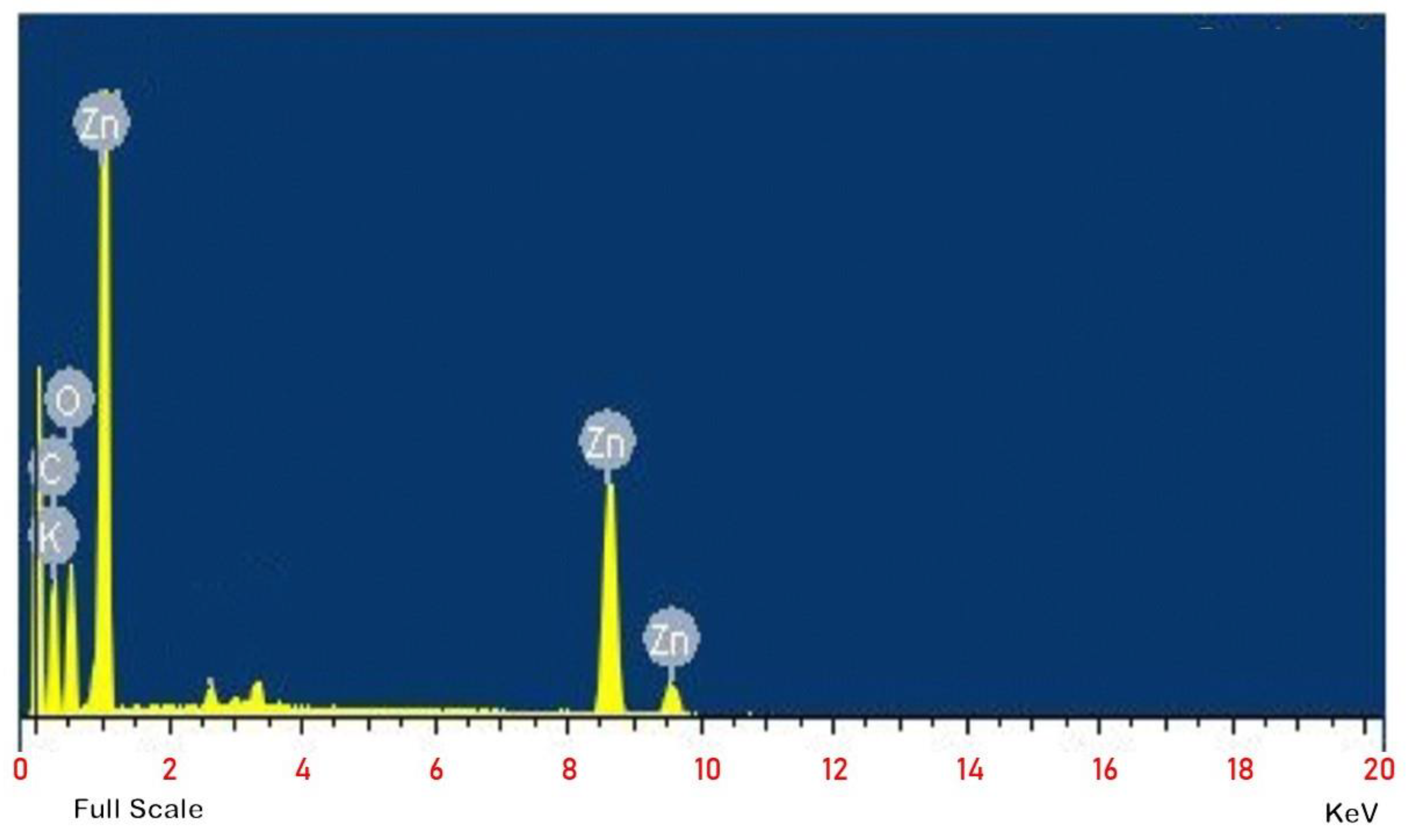
3.4. FESEM and EDX Analysis
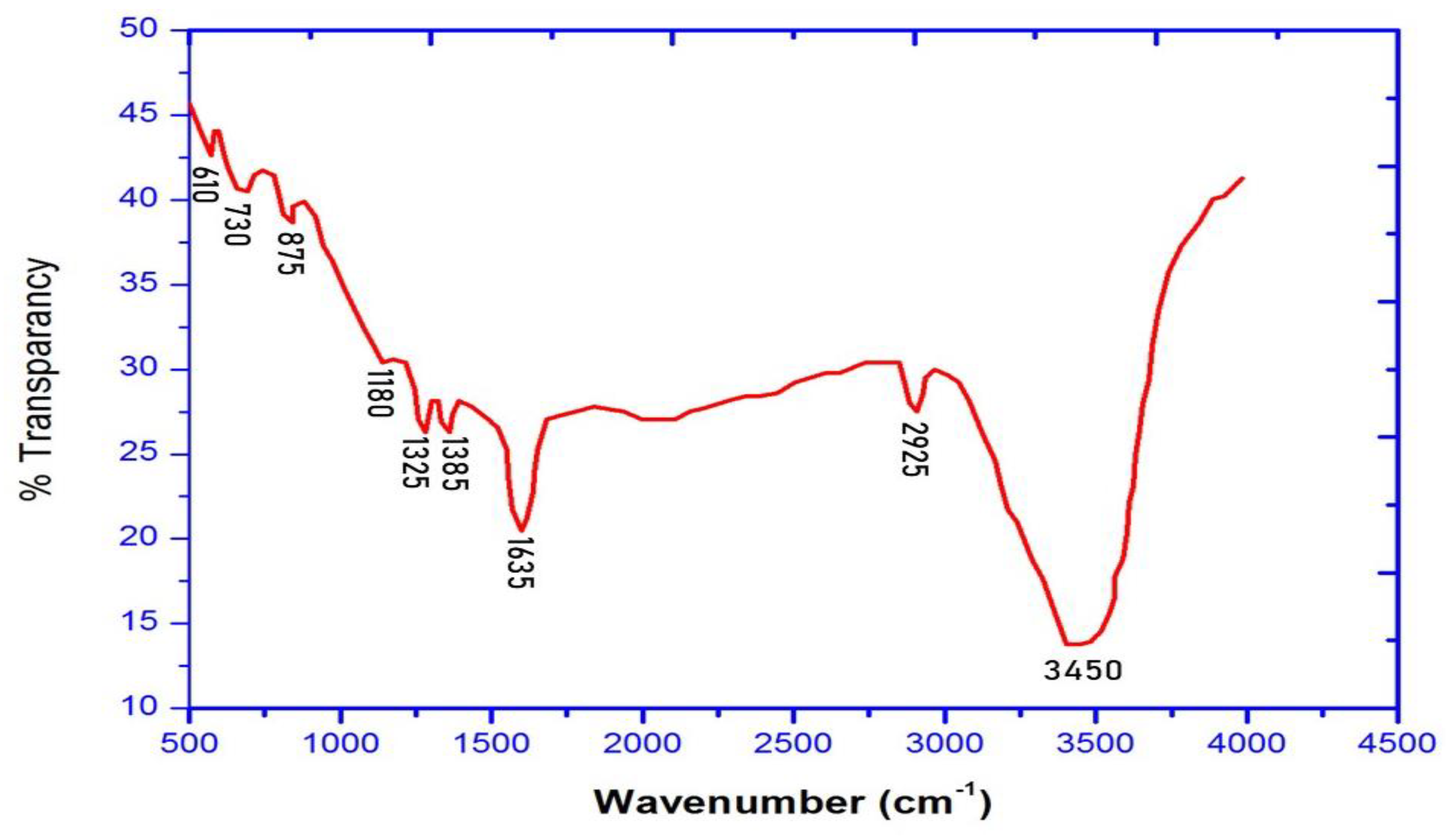
3.5. FT-IR Analysis
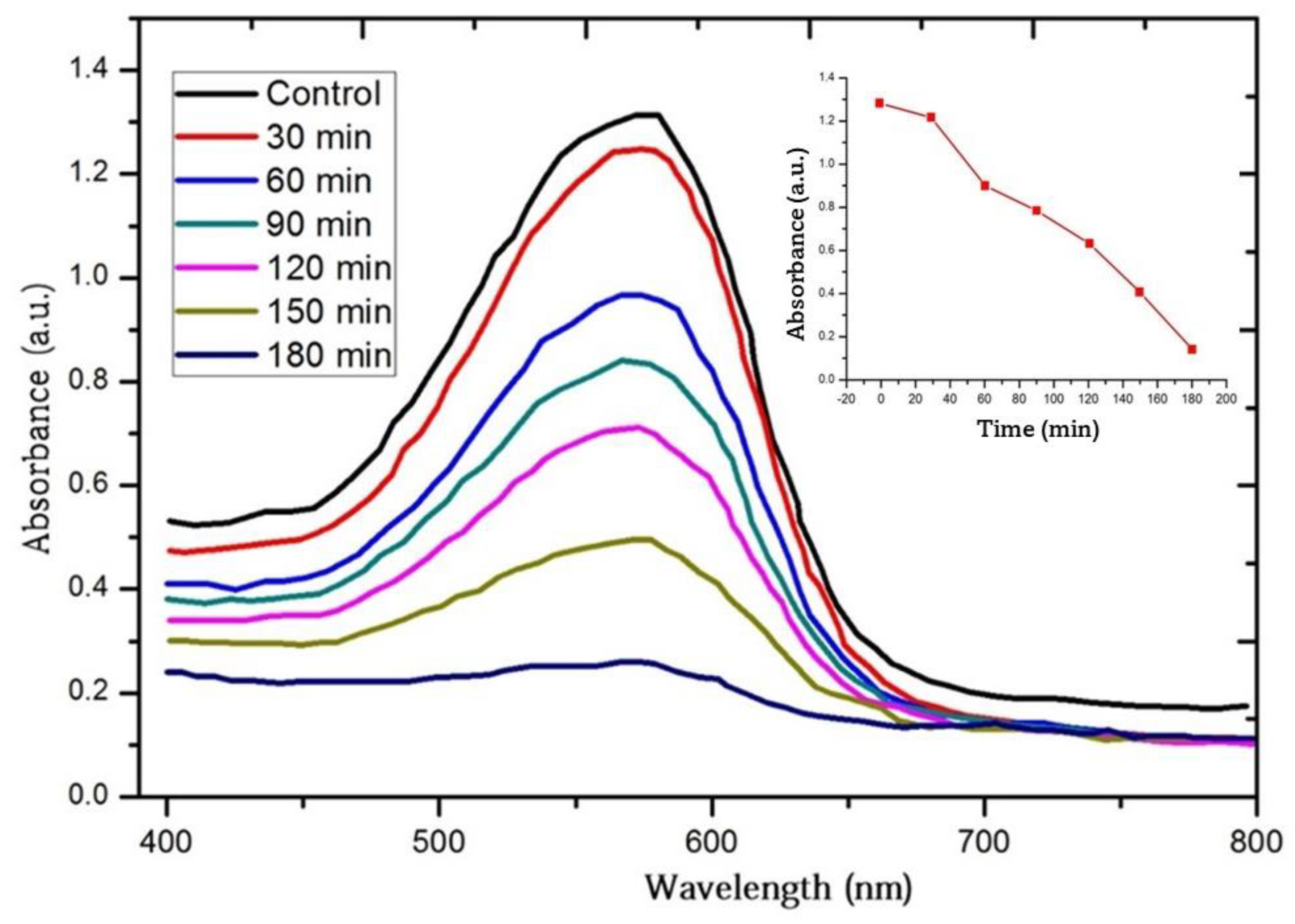
3.6. Photocatalytic Activity
3.7. Recyclability and Photostability
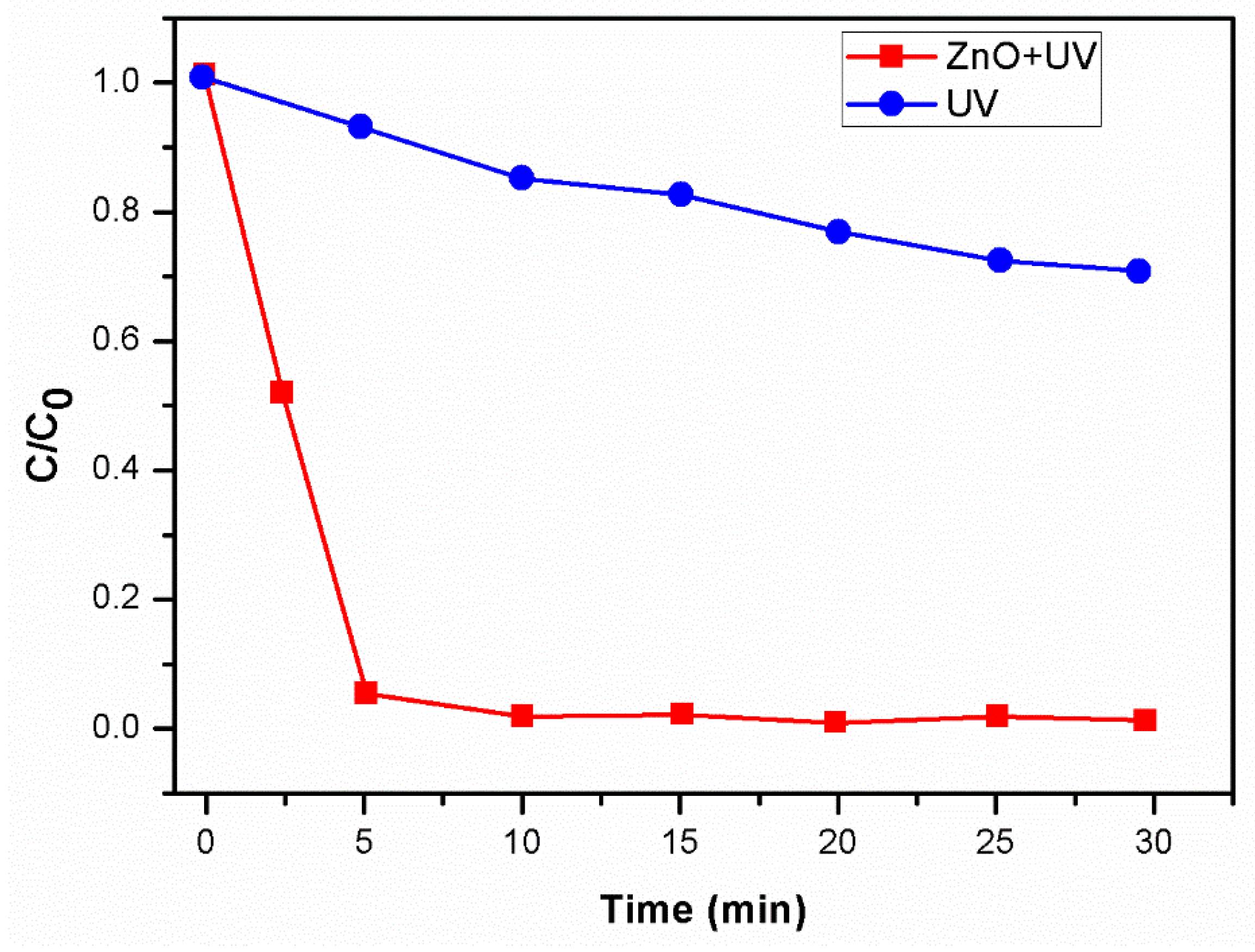
3.8. Photolysis
3.9. Comparison
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saini, R.; Saini, S.; Sharma, S. Nanotechnology: The future medicine. J. Cutan. Aesthetic Surg. 2010, 3, 32. [Google Scholar] [CrossRef]
- Singh, A. Synthesis, characterization, electrical and sensing properties of ZnO nanoparticles. Adv. Powder Technol. 2010, 21, 609–613. [Google Scholar] [CrossRef]
- Nohavica, D.; Gladkov, P. ZnO nanoparticles and their applications—New achievments. NANOCON 2010, 1, 121–124. [Google Scholar] [CrossRef]
- Azlina, H.N.; Hasnidawani, J.N.; Norita, H.; Surip, S.N. Synthesis of SiO2 nanostructures using sol-gel method. Acta Phys. Pol. A 2016, 129, 842–844. [Google Scholar] [CrossRef]
- Raoufi, D. Synthesis and microstructural properties of ZnO nanoparticles prepared by precipitation method. Renew. Energy 2013, 50, 932–937. [Google Scholar] [CrossRef]
- Ashkarran, A.A.; Irajizad, A.; Mahdavi, S.M.; Ahadian, M.M. ZnO nanoparticles prepared by electrical arc discharge method in water. Mater. Chem. Phys. 2009, 118, 6–8. [Google Scholar] [CrossRef]
- Aneesh, P.M.; Vanaja, K.A.; Jayaraj, M.K. Synthesis of ZnO nanoparticles by hydrothermal method. In Nanophotonic Materials IV; SPIE: Bellingham, WA, USA, 2007. [Google Scholar]
- Singh, S.C.; Gopal, R. Synthesis of colloidal zinc oxide nanoparticles by pulsed laser ablation in aqueous media. Phys. E Low-Dimens. Syst. Nanostruct. 2008, 40, 724–730. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, S.; Alok, A.; Upadhyay, S.K.; Rawat, M.; Tsang, D.C.; Bolan, N.; Kim, K.H. The potential of green synthesized zinc oxide nanoparticles as nutrient source for plant growth. J. Clean. Prod. 2019, 214, 1061–1070. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Wang, R.; Ding, Y.; Liu, R.; Xiang, L.; Du, L. Pomegranate: Constituents, bioactivities and pharmacokinetics. Fruit Veg. Cereal Sci. Biotechnol. 2010, 4, 77–87. [Google Scholar]
- Hassan, S.S.M.; Azab, W.I.M.E.; Ali, H.R.; Mansour, M.S.M. Green synthesis and characterization of ZnO nanoparticles for photocatalytic degradation of anthracene. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 045012. [Google Scholar] [CrossRef]
- Bhuyan, T.; Mishra, K.; Khanuja, M.; Prasad, R.; Varma, A. Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater. Sci. Semicond. Process. 2015, 32, 55–61. [Google Scholar] [CrossRef]
- Davar, F.; Majedi, A.; Mirzaei, A. Green synthesis of ZnO nanoparticles and its application in the degradation of some dyes. J. Am. Ceram. Soc. 2015, 98, 1739–1746. [Google Scholar] [CrossRef]
- Kitture, R.; Koppikar, S.J.; Kaul-Ghanekar, R.; Kale, S.N. Catalyst efficiency, photostability and reusability study of ZnO nanoparticles in visible light for dye degradation. J. Phys. Chem. Solids 2011, 72, 60–66. [Google Scholar] [CrossRef]
- Zainuri, N.Z.; Hairom, N.H.; Sidik, D.A.; Misdan, N.; Yusof, N.; Mohammad, A.W. Reusability performance of zinc oxide nanoparticles for photocatalytic degradation of POME. E3S Web Conf. 2018, 34, 02013. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, K.; Thakur, N.; Chauhan, S.; Chauhan, M.S. The effect of shape and size of ZnO nanoparticles on their antimicrobial and photocatalytic activities: A green approach. Bull. Mater. Sci. 2020, 43, 20. [Google Scholar] [CrossRef]
- Le Pevelen, D.D. (Ed.) Small Molecule X-ray Crystallography, Theory and Workflow. In Encyclopaedia of Spectroscopy and Spectrometry, 2nd ed.; Academic Press: Cambridge, MA, USA, 2010; pp. 2559–2576. [Google Scholar] [CrossRef]
- Zak, K.A.; Abd Majid, W.H.; Abrishami, M.E.; Yousefi, R. X-ray analysis of ZnO nanoparticles by Williamson–Hall and size–strain plot methods. Solid State Sci. 2011, 13, 251. [Google Scholar]
- Lingaraju, K.; Raja Naika, H.; Manjunath, K.; Basavaraj, R.B.; Nagabhushana, H.; Nagaraju, G.; Suresh, D. Biogenic synthesis of zinc oxide nanoparticles using Ruta graveolens (L.) and their antibacterial and antioxidant activities. Appl. Nanosci. 2016, 6, 703. [Google Scholar] [CrossRef]
- Fuku, X.; Diallo, A.; Maaza, M. Nanoscaled electrocatalytic optically modulated ZnO nanoparticles through green process of Punica granatum L. and their antibacterial activities. Int. J. Electrochem. 2016, 2016, 4682967. [Google Scholar] [CrossRef]
- Fuku, X.; Kaviyarasu, K.; Matinise, N.; Maaza, M. Punicalagin green functionalized Cu/Cu2O/ZnO/CuO nanocomposite for potential electrochemical transducer and catalyst. Nanoscale Res. Lett. 2016, 11, 386. [Google Scholar] [CrossRef]
- Matinise, N.; Fuku, X.G.; Kaviyarasu, K.; Mayedwa, N.; Maaza, M.J. ZnO nanoparticles via Moringa oleifera green synthesis: Physical properties & mechanism of formation. Appl. Surf. Sci. 2017, 406, 339–347. [Google Scholar] [CrossRef]
- Choudhary, M.K.; Kataria, J.; Sharma, S. Novel Green Biomimetic Approach for Preparation of Highly Stable Au-ZnO Heterojunctions with Enhanced Photocatalytic Activity. Appl. Nano Mater. 2018, 1, 1870. [Google Scholar] [CrossRef]
- Suresh, D.; Nethravathi, P.C.; Lingaraju, K.; Rajanaika, H.; Sharma, S.C.; Nagabhushana, H. EGCG assisted green synthesis of ZnO nanopowders: Photodegradative, antimicrobial and antioxidant activities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136 Pt C, 1467–1474. [Google Scholar] [CrossRef]
- Hancock, J.M.; Rankin, W.M.; Hammad, T.M.; Salem, J.S.; Chesnel KHarrison, R.G. Optical and Magnetic Properties of ZnO Nanoparticles Doped with Co, Ni and Mn and Synthesized at Low Temperature. J. Nanosci. Nanotechnol. 2015, 15, 3809. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, M.; Mahjoub, S.; Elahi, S.M.; Zabihi, E.; Ebrahim Zabihi, E.; Tashakkorian, H. Green synthesis, formulation and biological evaluation of a novel ZnO nanocarrier loaded with paclitaxel as drug delivery system on MCF-7 cell line. Colloids Surf. B Biointerfaces 2020, 186, 110686. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Singh, J.; Rawat, M. Green synthesis of zinc oxide nanoparticles using Punica Granatum leaf extract and its application towards photocatalytic degradation of Coomassie brilliant blue R-250 dye. SN Appl. Sci. 2019, 1, 624. [Google Scholar] [CrossRef]
- Otaibi, A.A.; Patil, M.B.; Rajamani, S.B.; Mathad, S.N.; Patil, A.Y.; Amshumali, M.K.; Shaik, J.P.; Asiri, A.M.; Khan, A. Development and Testing of Zinc Oxide Embedded Sulfonated Poly (Vinyl Alcohol) Nanocomposite Membranes for Fuel Cells. Crystals 2022, 12, 1739. [Google Scholar] [CrossRef]


| am (mmol/kg) | D (nm) | d (nm) | a = b (nm) | c (nm) | V (nm3) |
|---|---|---|---|---|---|
| 50 | 31.158 | 0.256 | 0.325 | 0.527 | 4.721 |
| am (mmpl/kg) | λmax (nm) | Eg (eV) |
|---|---|---|
| 5 | 381 | 3.436 ± 0.3 |
| 10 | 386 | 3.369 ± 0.4 |
| 50 | 390 | 3.361 ± 0.3 |
| Sl. No | Photocatalyst | Time (min) | % Photocatalytic Degradation of Dye | Reference |
|---|---|---|---|---|
| 1 | ZnO | 180 | 94 | Present work |
| 2 | ZnO | 180 | 95 | Commercial ZnO |
| 3 | ZnO | 160 | 86.9 | [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Zahrani, S.A.; Patil, M.B.; Mathad, S.N.; Patil, A.Y.; Otaibi, A.A.; Masood, N.; Mansour, D.; Khan, A.; Manikandan, A.; Syafri, E. Photocatalytic Degradation of Textile Orange 16 Reactive Dye by ZnO Nanoparticles Synthesized via Green Route Using Punica Granatum Leaf Extract. Crystals 2023, 13, 172. https://doi.org/10.3390/cryst13020172
Al-Zahrani SA, Patil MB, Mathad SN, Patil AY, Otaibi AA, Masood N, Mansour D, Khan A, Manikandan A, Syafri E. Photocatalytic Degradation of Textile Orange 16 Reactive Dye by ZnO Nanoparticles Synthesized via Green Route Using Punica Granatum Leaf Extract. Crystals. 2023; 13(2):172. https://doi.org/10.3390/cryst13020172
Chicago/Turabian StyleAl-Zahrani, Salma A., Mallikarjunagouda B. Patil, Shridhar N. Mathad, Arun Y. Patil, Ahmed A. Otaibi, Najat Masood, Dorsaf Mansour, Anish Khan, A. Manikandan, and Edi Syafri. 2023. "Photocatalytic Degradation of Textile Orange 16 Reactive Dye by ZnO Nanoparticles Synthesized via Green Route Using Punica Granatum Leaf Extract" Crystals 13, no. 2: 172. https://doi.org/10.3390/cryst13020172
APA StyleAl-Zahrani, S. A., Patil, M. B., Mathad, S. N., Patil, A. Y., Otaibi, A. A., Masood, N., Mansour, D., Khan, A., Manikandan, A., & Syafri, E. (2023). Photocatalytic Degradation of Textile Orange 16 Reactive Dye by ZnO Nanoparticles Synthesized via Green Route Using Punica Granatum Leaf Extract. Crystals, 13(2), 172. https://doi.org/10.3390/cryst13020172








