Abstract
Catalysis fulfills the promise that high-yielding chemical transformations will require little energy and produce no toxic waste. This message is carried by the study of the evolution of molecular catalysis of some of the most important reactions in organic chemistry. After reviewing the conceptual underpinnings of catalysis, we discuss the applications of different catalysts according to the mechanism of the reactions that they catalyze, including acyl group transfers, nucleophilic additions and substitutions, and C–C bond forming reactions that employ umpolung by nucleophilic additions to C=O and C=C double bonds. We highlight the utility of a broad range of organocatalysts other than compounds based on proline, the cinchona alkaloids and binaphthyls, which have been abundantly reviewed elsewhere. The focus is on organocatalysts, although a few examples employing metal complexes and enzymes are also included due to their significance. Classical Brønsted acids have evolved into electrophilic hands, the fingers of which are hydrogen donors (like enzymes) or other electrophilic moieties. Classical Lewis base catalysts have evolved into tridimensional, chiral nucleophiles that are N- (e.g., tertiary amines), P- (e.g., tertiary phosphines) and C-nucleophiles (e.g., N-heterocyclic carbenes). Many efficient organocatalysts bear electrophilic and nucleophilic moieties that interact simultaneously or not with both the electrophilic and nucleophilic reactants. A detailed understanding of the reaction mechanisms permits the design of better catalysts. Their construction represents a molecular science in itself, suggesting that sooner or later chemists will not only imitate Nature but be able to catalyze a much wider range of reactions with high chemo-, regio-, stereo- and enantioselectivity. Man-made organocatalysts are much smaller, cheaper and more stable than enzymes.
Keywords:
acids; amphoteric compounds; asymmetric ion-pair catalysis; asymmetric synthesis; bases; bifunctional catalysts; catalytic enantioselective reactions; conjugate additions; crown ethers; cryptands; cycloadditions; cyclopentenes; electron-rich tertiary amines and phosphines; electrophilic activation; enantioselective electrophilic fluorination; encapsulation; enzymes; N-heterocyclic carbenes; hydrogen-bridging; β-lactams; nucleophilic activation; nucleophilic catalyst; phase transfer catalysis; l-proline; Umpolung 1. Introduction
In 1746, Roebuck developed the lead chamber for sulfuric acid production in which nitrogen oxides catalyze the oxidation of SO2 into SO3. Since then, catalysts have been widely employed in a large portion of the chemical industry. The catalysts can be inorganic, organic, or organometallic compounds, as well as enzymes or microorganisms [1,2,3]. Organocatalysis refers to a form of catalysis, in which the rate of a chemical reaction is increased by an organic compound referred to as an organocatalyst consisting of carbon, hydrogen, nitrogen, oxygen, phosphorus, sulfur, and other nonmetal elements. The most common organocatalysts are acids and bases. In the two last decades, organocatalysis has become a pillar of asymmetric organic synthesis [4,5,6,7,8,9,10]. The term catalyst was coined by Berzelius in 1835 [11,12,13], and the term ‘organocatalyst’ was first used by MacMillan in 2000 [6,14]. A catalyst is a compound, generally used in a substoichiometric amounts, that takes part in a reaction, resulting in an increased rate, but is not consumed. It does not affect the equilibrium constant of the reaction. A catalytic reaction is a reaction that is catalyzed. Some reactions are promoted by an additive. In these cases the additive speeds up the reactions and is converted to another species during the reaction. Most reactions can be catalyzed, usually by changing their mechanism, which is in fact always the case if the catalyst interacts with one or more species of the reaction (reactants, intermediates, transition states, products). Catalysts are classified as heterogeneous (most frequent in the industry, bulk chemicals) or homogeneous. A homogeneous catalyst dissolves in the reaction mixture (completely or partially), while a heterogeneous catalyst does not dissolve but acts with the reaction partners through physisorption (−2 to −10 kcal/mol: binding energies, attraction between permanent or induced dipoles or quadrupoles, and attraction due to van der Waals-London forces), or through chemisorption (covalent/electrostatic forces such as hydrogen-bridging and electron transfer). A catalyst of a given reaction is characterized by its turnover number (TON) which is the maximum number of moles of reactants that one mole of catalyst is able to convert into products before it loses its activity. One defines the lifetime of the catalyst for given reaction conditions and the turnover frequency (TOF; [time−1]), which quantifies the specific activity of the catalyst under the conditions used. For most relevant industrial applications, the TOF is in the range between 10−2 and 102 s−1. For enzymes, the TOF generally lie between 103 and 107 s−1. The term turnover rate is also often used and is synonymous with TOF. In general, any elementary process will be either accelerated (decrease of activation free energy Δ‡G) or retarded (increase of Δ‡G) if the reactants and/or the transition structure (activated complex) are associated (make complexes) with one or more than one (cluster) spectator molecules (solvent, additive, catalyst, inhibitor and combination of them). This is represented in Figure 1 for a bimolecular reaction involving reactants A and B occurring (A) in the gas phase, without any other molecule, (B) in the presence of an additive C that stabilizes the transition structure better than the reactants (Δ‡G((B)) < Δ‡G(A))) and (C) in the presence of an additive D that stabilizes the reactants better than the transition structure (Δ‡G(C)) > Δ‡G((A)). This representation does not involve dynamic effects, i.e., the possibility to have a collection of possible transition structures in the transition state. A catalytic effect results from the combination of an enthalpy effect and an entropy effect.
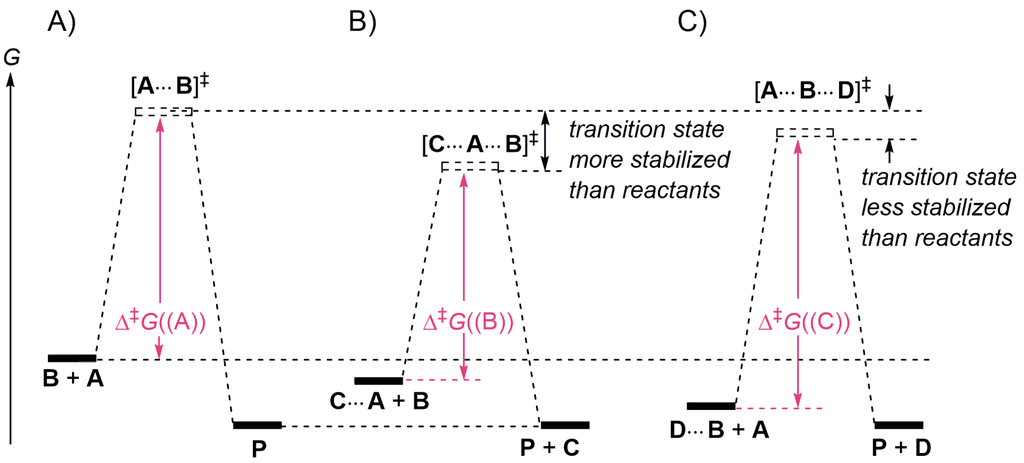
Figure 1.
(A) Free energy diagram for an uncatalyzed gas phase bimolecular reaction converting A + B → P; (B) free energy diagram for a catalyzed (catalyst C) bimolecular reaction; (C) free energy diagram for a bimolecular reaction inhibited by additive D. If D is in substoichiometric concentration, the uncatalyzed reaction (A) will compete with (C). The retardation effect of D increases with its concentration.
Water introduces many unusual features into the energetics of chemical and biological reactions [15,16]. Beyond the properties of bulk water, a single molecule of H2O, or an aggregate may influence the rate of a reaction [17,18]. For instance, water forms complexes with radicals and polar molecules in thermal reactions [19,20] and such complexation alters the reaction activation free energies. Kinetic studies as well as quantum chemical mechanical calculations have shown that one molecule of water accelerates hydrogen abstraction from acetaldehyde by hydroxyl radical, to produce acetyl radical and a water molecule, as represented in Figure 2 [21].
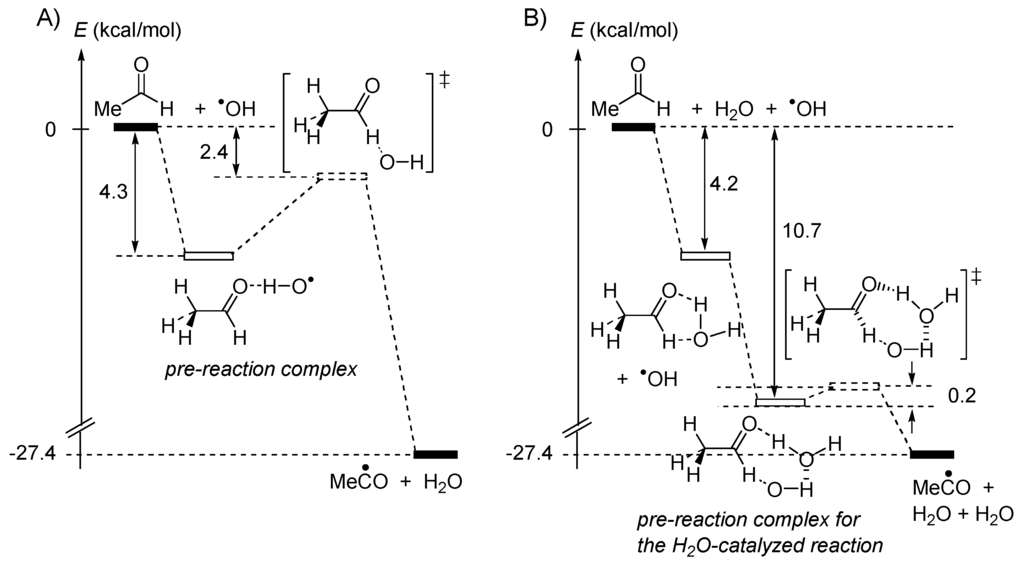
Figure 2.
Relative energies (ΔE ≅ ΔH) for the reaction MeCHO + •OH → MeC• =O + H2O, (A) in the absence of H2O, (B) in the presence of H2O.
A homogeneous catalyst is a molecule that adds intermediates and transition structures on the pathway from reactants to products (Figure 3). A good catalyst first makes a complex with one reactant and then weakens the bonds that must be broken. If the process is bimolecular, the first complex must be able to bind with little activation free energy to the second reactant (template effect) and bring both reactants into a stabilized activated complex (transition state). A good catalyst is a compound that does not bind too strongly to the reactants, does not form intermediates that are more stable than the products, that lowers the activation free energies of all the elementary processes converting the reactants into the initial complex first, and then converts this complex into the next intermediate, and so on. A good catalyst should not bind to the product. Otherwise the product becomes an inhibitor of the catalyzed reaction.
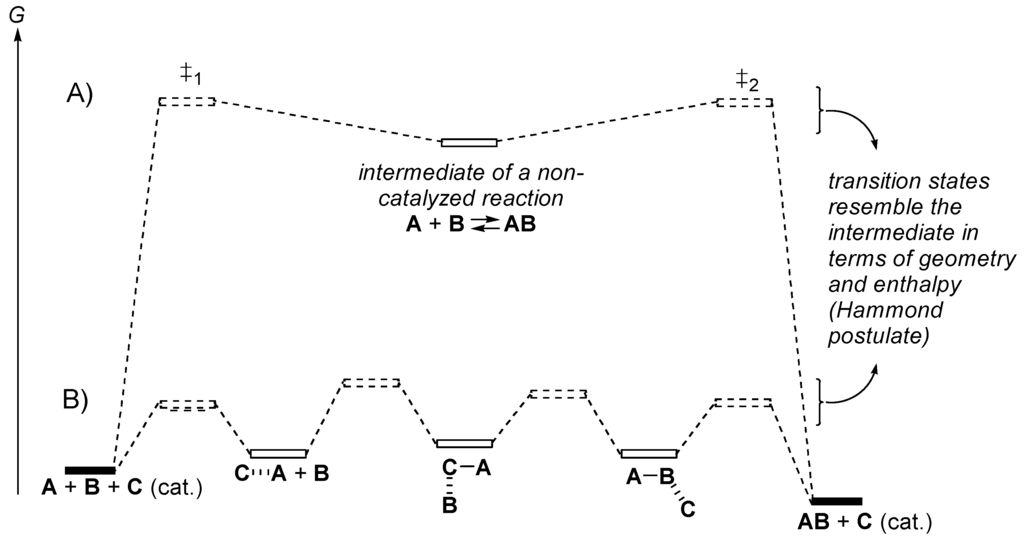
Figure 3.
(A) Free energy diagram for an uncatalyzed reaction converting reactants A + B into product AB; (B) for a reaction catalyzed by catalyst C.
This review is organized by reaction mechanism rather than by the identity of the catalyst, as is usually done in other reviews in the field of organocatalysis. We focus on four major classes of organic reactions: acyl group transfers, nucleophilic additions to the carbonyl group, nucleophilic substitutions, and C–C bond-forming reactions involving umpolung. For each class of reactions, important mechanistic aspects are first reviewed, followed by representative illustrations in catalysis. The focus is on organocatalysts, although a handful of examples involving metal-based and enzyme catalysts have also been included for some reaction classes due to their conceptual significance.
2. Acyl Group Transfers
Acylation (Scheme 1) is one of the most important reactions in preparative chemistry and biochemistry. It exchanges a proton (electrofugal group) from H-Nu for an acyl cation (electrophile). It can also be seen as a nucleophilic displacement (exchange of X− in RCOX for Nu−).
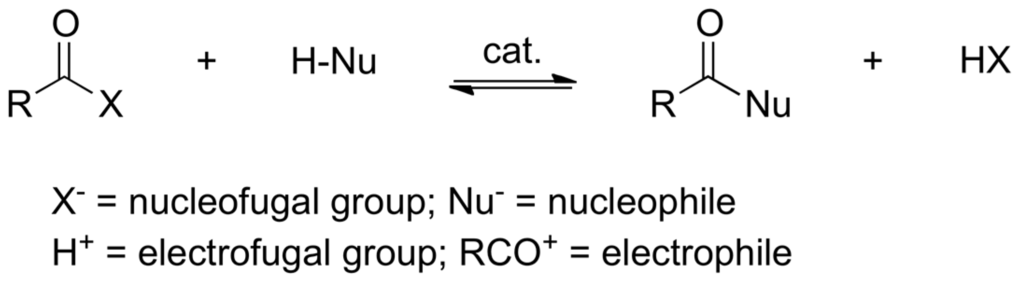
Scheme 1.
The general acylation reaction.
For instance, treatment of acyl chlorides or carboxylic acid anhydrides with alcohols generates esters [22,23] and with amines, the corresponding amides. In industry, these reactions use inorganic acids as catalysts such as H2SO4, zeolites (aluminosilicates that are microporous solids with pore sizes ranging from ca. 3 to 7 Å: e.g., molecular sieves), (CF3SO2)2NH, red phosphorus, or Lewis acids such as ZnCl2, RuCl3, InCl3, Mg(ClO4)2, Cu or Bi triflates (CF3SO3), or p-toluenesulfonates (4-MeC6H4SO3 = TsO). The same reactions are also base-catalyzed (see below). Transesterification (Scheme 2), reactions producing esters by catalytical alcoholysis of other esters, also play an important role in the industry, for instance in the manufacture of glycerol esters in fat chemistry (e.g., biodiesel), the transesterification of dimethyl carbonate to diethyl carbonate, or the production of polyethylene terephthalate from dimethyl terephthalate.

Scheme 2.
The general transesterification reaction.
For these reactions, the usual catalysts are Brønsted mineral acids (H3PO4, H2SO4, HCl) [24,25,26], and organic acids such as MeSO3H and p-toluenesulfonic acid (TsOH) [27], alkoxides such as NaOR, KOR, ROMgBr [28,29,30,31,32], Lewis bases such as 4-dimethylaminopyridine (DMAP) [33,34], 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) [35], Lewis acids such as BX3 [36], or AlCl3 [37], amphoteric compounds (compounds able to react either as acids or bases) such as Bu3SnOR [38,39,40,41], perfluorotin oxides [42], Al(OR)3 [43,44], titanium oxides chlorides [45,46,47,48] or palladium oxides [49,50]. More recently, diaminocarbenes have been introduced as catalysts as well [51,52,53,54]. Mechanisms of the gas-phase acyl group transfers involve tetrahedral adducts as intermediates or transition states, as suggested by quantum calculations [55].
2.1. Esterification and Ester Hydrolysis
Ingold classifies [56] the acid- and base-catalyzed hydrolyses of esters (and the formation of esters, since these are reversible reactions and thus have the same mechanism: principle of microscopic reversibility) into eight possible mechanisms (Table 1), depending on the following criteria: (a) acid- (A) or base- (B) catalyzed; (b) acyl (AC) cleavage or alkyl (AL) cleavage and (c) unimolecular (1) or bimolecular (2). All eight of these are SN1, SN2, or tetrahedral (add/elim) mechanisms. A ninth mechanism implies the base-catalyzed elimination of alcohol and the formation of a ketene intermediate that adds HO− to generate an enolate that is finally protonated into the final carboxylic acid (E1cb pathway [57]). The most common mechanisms are the BAC2 for base-catalyzed acyl transfers, and the AAC2 mechanism for acid-catalyzed acyl transfers. Both involve acyl-oxygen cleavage as evidenced by hydrolysis in H218O that result in the 18O appearing in the acid and not in the alcohol, and for esters with chiral R' groups, alcohols with retention of configuration are observed. Usually two molecules of H2O are required in the transition state of the AAC2 mechanism.

Table 1.
Ingold’s classification of mechanisms for ester hydrolysis and formation.
2.2. Acid or Base-Catalyzed Acyl Transfers
In the absence of any catalyst and in a solvent that cannot play either the role of an acid, nor that of a base (e.g., PhMe), the reactions in Scheme 1 can follow three limiting mechanisms represented in the Jencks-More O’Ferrall type of diagram of Figure 4.
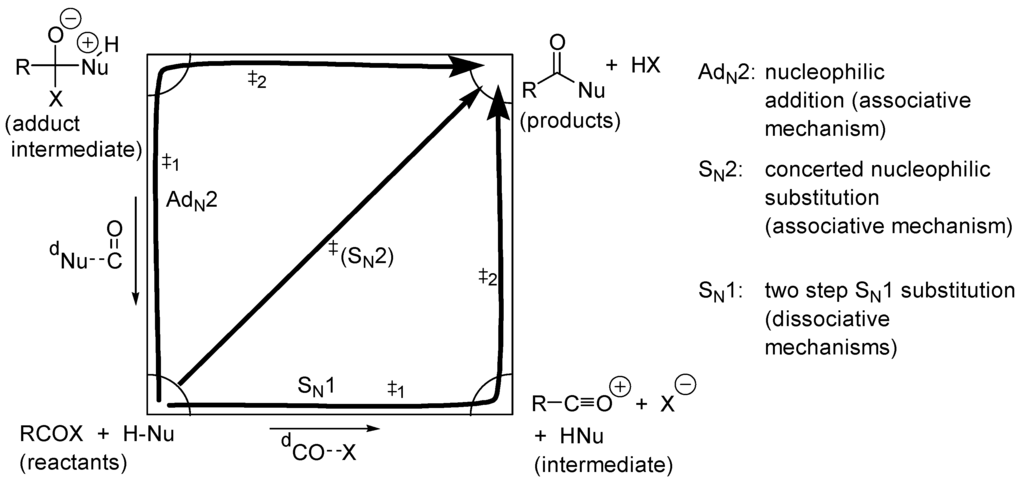
Figure 4.
Limiting mechanisms for an acyl transfer reaction that are neither catalyzed, nor assisted by solvent (x-axis describes distance CO···X, y-axis distance Nu···C=O and the free energy is perpendicular to the plane).
With this hypothetical reaction, one sees that the two-step nucleophilic addition route implies the formation of a zwitterion from uncharged reactants. It is obvious that such a process will be facilitated by polar solvent and by any additive that can take a proton from it (base-catalyzed addition: BAC2) or protonate the alkoxide moiety (acid-catalyzed addition: AAC2). Any compound able to give a proton and take a proton from the zwitterionic, tetrahedral intermediate will catalyze the addition reaction. This is the case with carboxylic acids such as benzoic acid (Scheme 3), which catalyzes the formation of poly(ethylene)terephthalate by transesterification of methyl terephthalate with ethylene glycol [58].
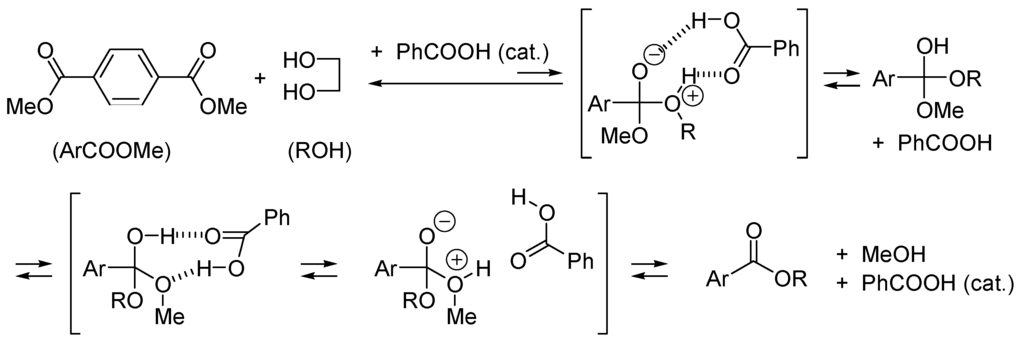
Scheme 3.
Benzoic acid is a bifunctional (acid/base) catalyst for the transesterification. It can give and take a proton, both processes can be concerted.
In an analogous way to benzoic acid that is a bifunctional catalyst (OH: acid; C=O: base), triazabicyclo[4.4.0]dec-5-ene (TBD) is an efficient catalyst of the ring-opening polymerization of cyclic esters (lactones) [59]. The mechanism of the ring-opening reaction of l-lactide [60] and δ-valerolactone [61] (Scheme 4) has been studied computationally. TBD donates a hydrogen bond to the carbonyl group of the lactone, activating it towards nucleophilic attack by the alcohol, which is also activated by TBD through accepting a hydrogen bond to its imine-like nitrogen. TBD then effects the ring opening by hydrogen-bonding to what is formerly the carbonyl oxygen in the tetrahedral intermediate, but transferring the hydrogen (originally from the alcohol) to the ring oxygen adjacent to the former carbonyl group.
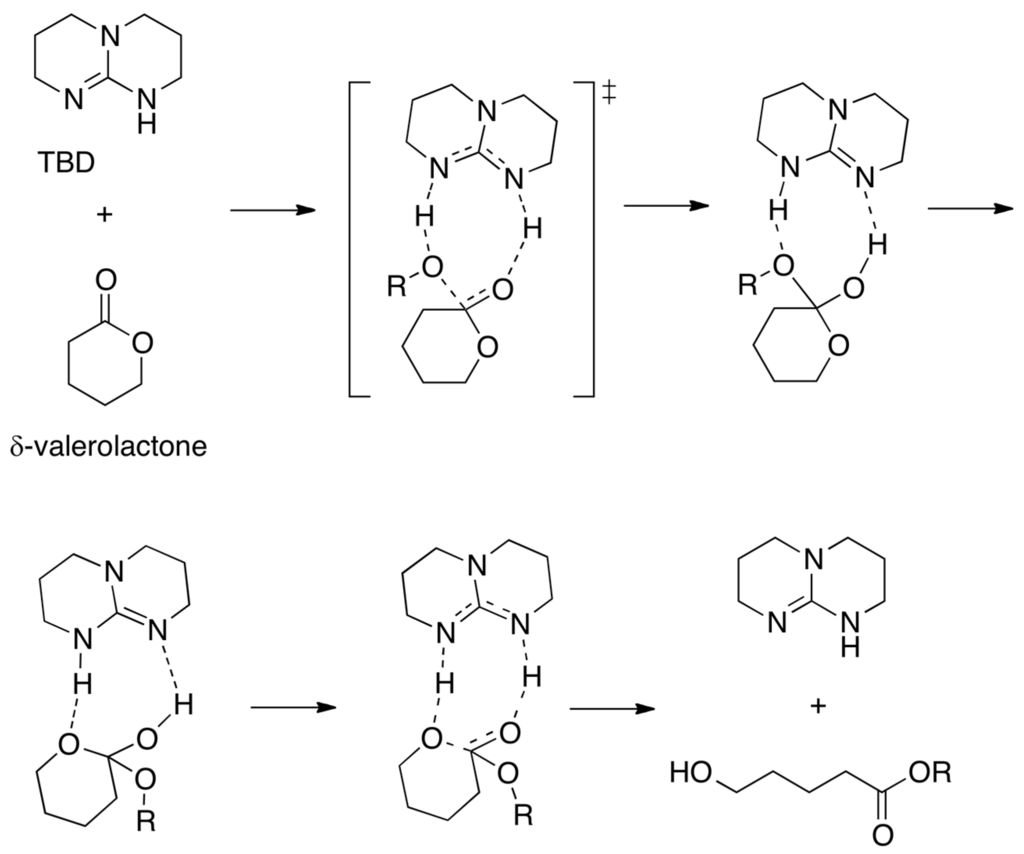
Scheme 4.
Triazabicyclo[4.4.0]dec-5-ene (TBD)-catalyzed alcoholysis of δ-valerolactone.
Stabilization of the alkoxide moiety of the tetrahedral intermediate resulting from the addition H-Nu + RCOX (Figure 4) can be realized by oxyphilic Lewis acids [62]. Thus, acetylation of alcohols with acetic acid can be carried out in the presence of triflates (trifluoromethanesulfonates) such as Yb(OTf)3, La(OTf)3, Ce(OTf)3, Cu(OTf)2, Sc(OTf)3, Er(OTf)3, In(OTf)3, Bi(OTf)3 or ytterbium p-toluenesulfonate (Yb(OTs)3) [63]. These Lewis acids are stable in carboxylic acids, alcohols and water. Direct condensation of carboxylic acids with alcohols has been catalyzed by hafnium(IV) salts such as HfCl4(THF)2, Hf(O-t-Bu)4 [47], by titanium(IV) salts such as Ti(O-t-Bu)4 and by ZrOCl2(H2O)8 or HfOCl2(H2O)8 that are water-tolerant and reusable homogenous catalysts [64]. Synergism in catalytic activity with the combined use of Hf(O-i-Pr)4 or Zr(O-i-Pr)4 and Fe(O-i-Pr)3 has been demonstrated. These catalysts are environmentally benign and can be extracted with ionic liquids for their recovery and reuse [65]. Stabilization of the alkoxide moiety of the tetrahedral intermediate resulting from the addition H-Nu + RCOX can be realized by a neighbouring hydroxy group of the leaving group X. For instance, in aminolyses of adenosine 3′-ester derivatives are faster than aminolyses of the corresponding 3′-esters of 2′-deoxyadenosine [66]. This property is exploited by Nature in the ribosome (peptidyl t-RNA). Esterification and transesterification [67] have been catalyzed by aluminum dodecatungstophosphate (AlPW12O40) [68] and K5CoW12O14(H2O)3 [69] that are water-tolerant and recyclable, non-toxic Lewis acids. Heterogeneous catalysts are preferred in the industry because their recovery requires simple filtration. A large number of these catalysts are available and contain supported Lewis acid or amphoteric compounds (see below). Montmorillonites that are composed of two-dimensional silicate sheets, with alternating layers that are negatively charged and positively charged, are suitable for impregnation of a wide range of catalysts. For instance, TiCl4 + H2O is an efficient catalyst for esterification of carboxylic acids with alcohols [70]. Other heterogeneous catalysts can be silica-supported sulfonic acids [71] or graphite bisulfite [72]. Figure 5 represents the free energy diagrams of four possible mechanisms of the acid (HA)-catalyzed nucleophilic (Nu:−) addition to a carbonyl compound (ester, carboxylic acid, acyl halide, carboxamide, aldehyde, ketone). In the case of Figure 5A the rate-determining step is the one-step addition of Nu:− generating the tetrahedral alkoxide intermediate Int− which is then protonated in a second step. In this case the reaction is not acid-catalyzed. It could be base-catalyzed if the conjugate acid Nu-H were not completed dissociated (weak acid). In the case of Figure 5B, a fast pre-equilibrium exists between reactants and intermediate Int−; the rate-determining step is the proton transfer from HA to Int− generating Int-H and A−. The energy barrier of this process is discussed hereafter. In Figure 5C, a one-step process converts reactants and catalyst HA into a neutral adduct Int-H and the conjugate base A− of the catalyst HA. The addition of the anionic nucleophile Nu:− is assisted by a proton transfer to the forming alkoxide moiety. In a fourth mechanism (Figure 5D), the catalyst protonates the carbonyl compound, but not the anionic nucleophile, in a fast pre-equilibrium that generates a hydroxycarbenium ion intermediate J. The rate-determining step is the nucleophilic addition Nu:− + J → Int-H. This mechanism is followed for reactant H-Nu with pKa lower than pKa (conjugate acid of the carbonyl compound: R(X)C=O+H). The energy barrier of such a reaction arises mostly from solvent reorganization (desolvation of Nu:−, solvation of A−, and desolvation of cationic intermediate J).
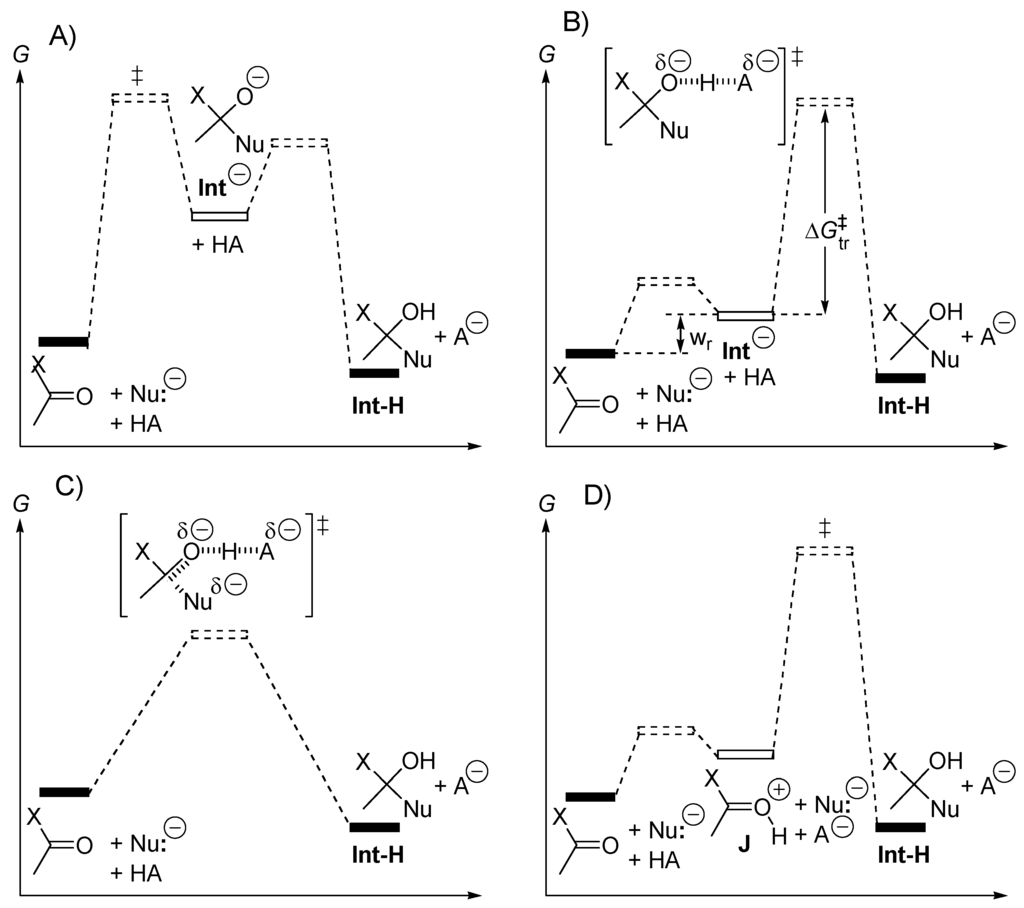
Figure 5.
(A) The nucleophilic addition is the rate-determining step; (B) the proton transfer from the acid catalyst is the rate-determining step; (C) the nucleophile addition and proton transfer are concerted; (D) the nucleophile addition onto the conjugate acid of the carbonyl compound is the rate-determining step.
We now examine mechanism B (Figure 5B). The energy barrier is the sum of the free energy difference Wr between intermediate Int− and reactants, and the activation free enthalpy Δ‡Gtr for the one-step proton transfer Int− + HA → [Int−···H···A]‡ → Int-H + A−. In this reaction, the bond formation Int···H assists the bond breaking A···H. As demonstrated by the Bell-Evans-Polanyi theory for radical transfer reactions, the activation enthalpy (Δ‡H) depends on the exothermicity (ΔrH) and on the polarisability of the reactants (Δ‡H = αΔrH + β, β being the intrinsic activation enthalpy of a thermoneutral reaction). Developed first for electron-transfer reaction, the Marcus theory [73,74,75] proposes relationship (1) for concerted proton-transfer reactions.
where ∆G°r is the free energy difference between Int− + AH and Int-H + A− (−1.36 (pKa(Int-H) − pKa(HA) at 25 °C)) and λ the solvent reorganization term related to solvation changes between Int− + HA and Int-H + A−.
2.3. Amphoteric Compounds Are Good Catalysts for Acyl Transfers
The tetranuclear zinc cluster Zn4(OCOCF3)6O catalyzes the direct conversion of esters, lactones, and carboxylic acids to oxazolines with remarkable chemoselectivity. The possible mechanism for these reactions is outlined in Scheme 5 for methyl esters 1 reacting with amino alcohols 2 giving oxazolines 3.
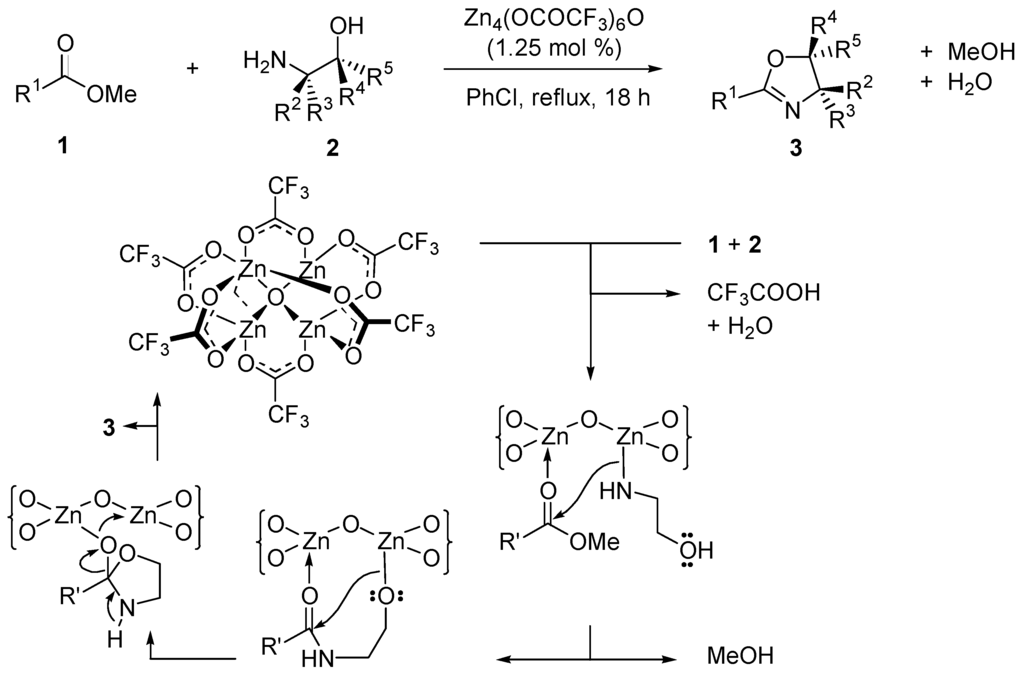
Scheme 5.
Postulated catalytic cycle of the zinc cluster-catalyzed transamination of esters by 2-aminoalcohols yielding oxazolines.
The zinc cluster Zn4(OCOCF3)O is an amphoteric compound (the metallic centers are Lewis acids and the oxygen centers are Lewis bases). This is also the case with distannoxane catalysts of type 4 (Figure 6) [38,39,40,41]. Other oxometallic species catalyze nucleophilic acyl substitution reactions with protic nucleophiles. Examples are TiO(acetylacetonate)2, vanadyl dichloride VOCl2(THF)n, [48] and Y5(O-i-Pr)13O [76], all being water-tolerant catalysts.
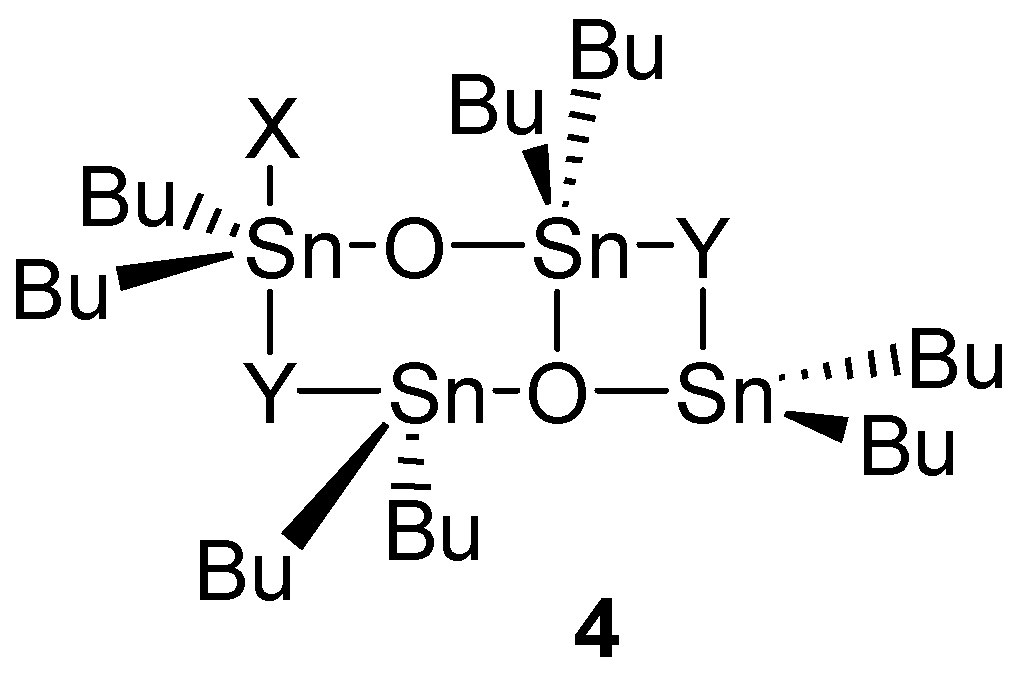
Figure 6.
Distannoxane catalysts.
2.4. Catalysis by Nucleofugal Group Substitution
In 1967, Litvinenko and Kirichenko [77] established that DMAP (4-dimethylaminopyridine) provides a 104-fold rate enhancement versus pyridine in the benzoylation of 3-chloroaniline. In 1969, Steglich and Höfle [78] found that the difficult acetylation of tertiary alcohols [79] with acetic anhydride does not occur in pure pyridine, but only in the presence of DMAP as catalyst [80]. Hassner and Alexanian [81] proposed a room-temperature direct esterification of carboxylic acids with alcohols catalyzed by DMAP or better, by 4-pyrrolidinopyridine (PYP) in the presence of N,N'-dicyclohexylcarbodiimide (DCC: drying agent) (Scheme 6).
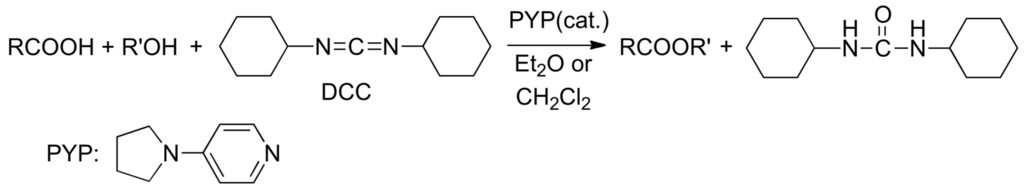
Scheme 6.
Direct esterification of carboxylic acids with alcohols catalyzed by PYP (4-pyrrolidinopyridine).
DMAP and other 4-aminopyridines have been used to catalyze acyl or sulfonyl transfer reactions, including the reactions of (t-BuO)2CO (Boc2O) with amines, alcohols, diols, aminoalcohols, and aminothiols [82] and the chemoselective trifluoroacetylation of anilines using ethyl trifluoroacetate [83]. The catalytical effect of DMAP and analogous 4-aminopyridines results from their high nucleophilicity, favoring a fast addition/elimination process that generates the N-acylpyridinium intermediates 5. In turn, 5 adds rapidly to protic nucleophiles to generate the corresponding products of acyl transfer (Scheme 7).
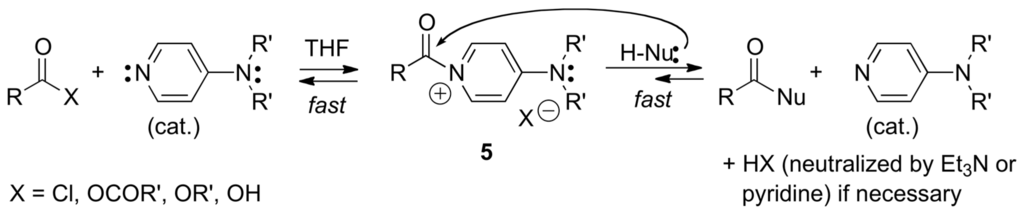
Scheme 7.
DMAP-catalyzed acyl transfer reactions.
Electron-rich pyridines listed below are proposed to be better than DMAP as nucleophilic catalysts (Figure 7) [84,85,86]. In 1996, Vedejs and Chen introduced 6 as the first member of the chiral DMAP family that was used in stoichiometric amounts with two equivalents of a Lewis acid to carry out the resolution of 1-arylethanols [87]. Enantiomerically pure DMAP derivatives such as PPY* and DMAPC5Ph5 (Fu's catalysts also introduced in 1996) [88,89] are commercially available and can be used as chiral catalysts in kinetic resolution of alcohols, amines and to induce asymmetric cycloadditions, protonations, C-acylations, halogenations and Michael additions [90]. A large number of other enantiomerically pure p-dialkylaminopyridines have been proposed for enantioselective acyl transfers [91].
Figure 7.
Electron-rich pyridines as nucleophilic catalysts.
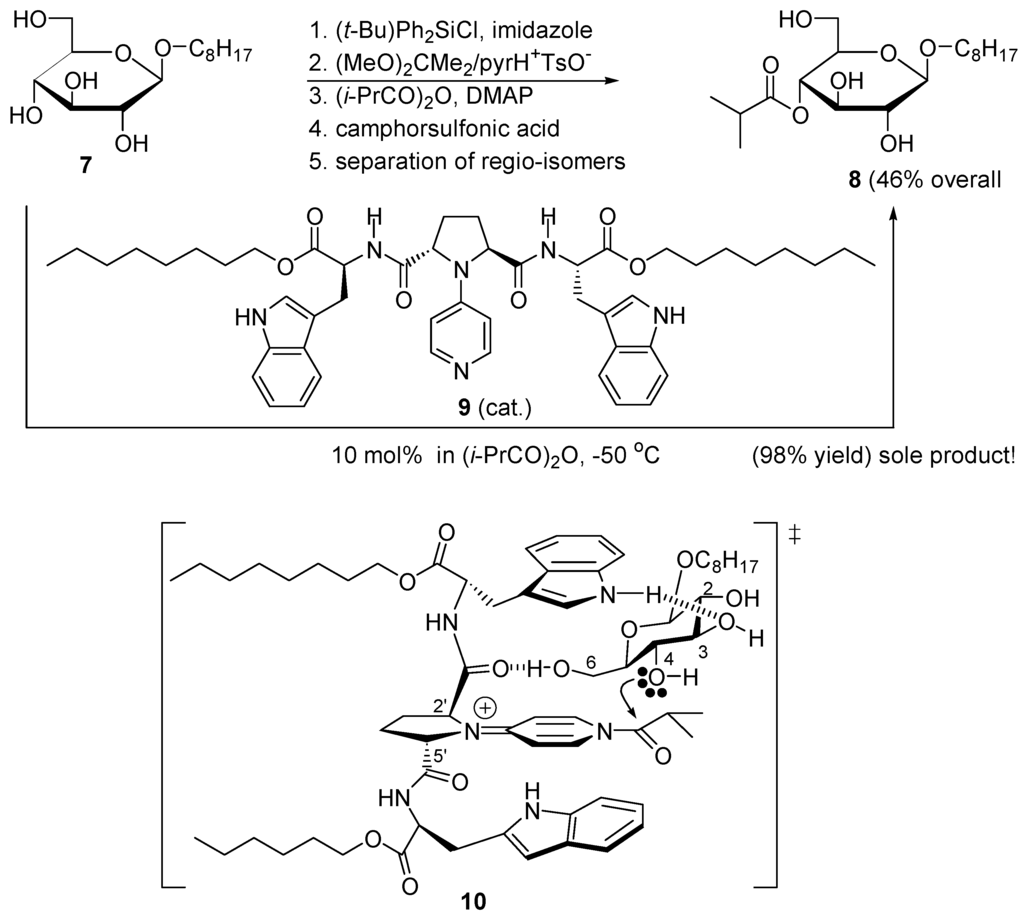
When the nucleophilic catalyst is part of a compound able to recognize a polyfunctional substrate, regioselective acyl transfer can be realized. As shown in Scheme 8, the classical conversion of n-octyl β-D-glucopyranoside (7) into its 4-O-isobutyryl derivative 8 requires five synthetic steps and a final separation of regioisomeric esters. But with 10 mol % catalyst 9, this conversion is accomplished in isobutyryl anhydride at −50 °C giving a sole product in 98% yield. Transition structure 10 has been proposed for this highly regioselective monoacylation [92].
Scheme 8.
Monoacylation of 7 catalyzed by 9 and proposed transition structure for acylation 10.
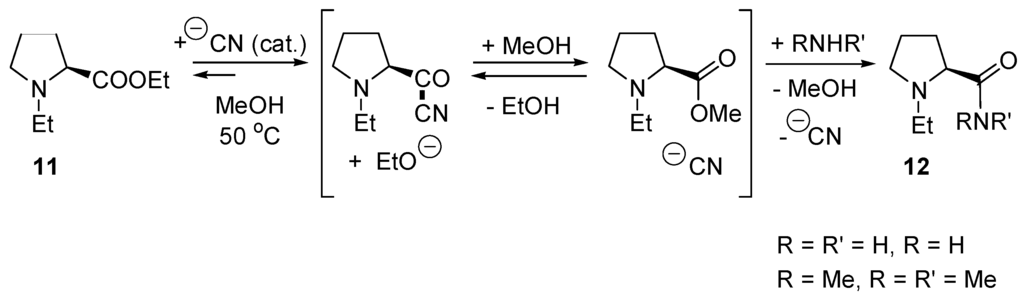
Transesterification of phosphonoacetates catalyzed by DMPA exchanges only the carboxylic esters. Similarly, reactions of phosphonoacetates with primary and secondary amines in the presence of DMAP produce the corresponding phosphonoacetamides in good yields [93]. Instead of DMAP, the cyanide anion can catalyze acyl transfer reactions (Scheme 9). In a study comparing the catalytic activities of DMAP, 2-hydroxypyridine, imidazole, and NaCN in the difficult aminolyses of ethyl (S)-1-ethyl-2-pyrrolidinecarboxylate (11) in MeOH, NaCN proved to be a superior catalyst which does not induce racemization of products 12 [94].
Scheme 9.
Acyl transfer reaction catalyzed by cyanide.
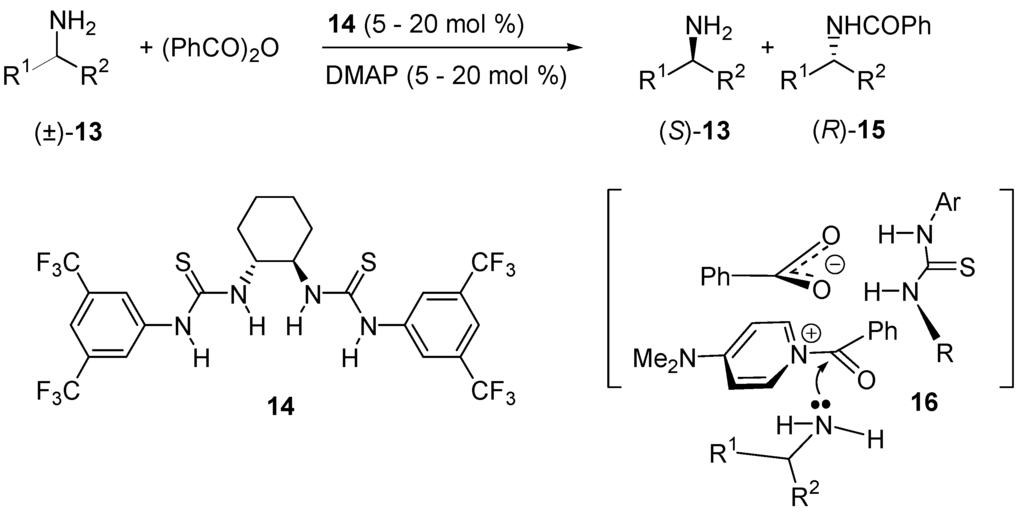
In 1993, Vedejs and co-workers [95,96] found that Bu3P is comparable to DMAP as a nucleophilic catalyst for the acylation of alcohols by anhydrides. The P-arylphosphines are less effective than Bu3P, but diarylphosphines retain sufficient reactivity to activate anhydrides at 25 °C. These observations led to the development of enantiomerically pure phosphines that catalyze enantioselective acyl transfers [97,98,99,100,101]. In 2009, Seidel and co-workers reported the kinetic resolution of racemic secondary amines (±)-13 catalyzed by a combination of DMAP with enantiopure thiourea 14 (up to 77% ee for (S)-13, 82% for (R)-15; Scheme 10) [102]). A possible mechanism is the formation of ternary complex 16 in which the acylium counterion (benzoate anion) binds the chiral thiourea and controls the face-selectivity of the amine addition to the acylium cation [103,104,105].
Scheme 10.
Kinetic resolution of (±)-13 catalyzed by thiourea 14.
Amidines (e.g., 1,8-diazabicyclo[5.4.0]undec-7-ene: DBU, 1,5-diazabicyclo[4.3.0]non-5-ene: DBN) [106,107], isothioureas (e.g., 2,3,6.7-tetrahydro-5H-thiazolo[3,2-a]pyrimidine: THTP, 3,4-dihydro-2H-pyrimido[2,1-b]benzothiazole: DHPH) and guadinines (e.g., 1,5,7-trazabicyclo[4.4.0]dec-5-ene: TBD) can also form acylium salts with carboxylic anhydrides which make them to catalyze acyl transfers through nucleophilic substitution, analogous with electron-rich pyridines (nucleophilic catalysts), not just as bases. Several enantiomerically pure amidine, isothiourea and guadinine derivatives have been prepared and tested for their ability to catalyze enantioselective acyl transfers as shown first by Birman and co-workers in 2004 [108]. Examples are given in Figure 8 [109,110].
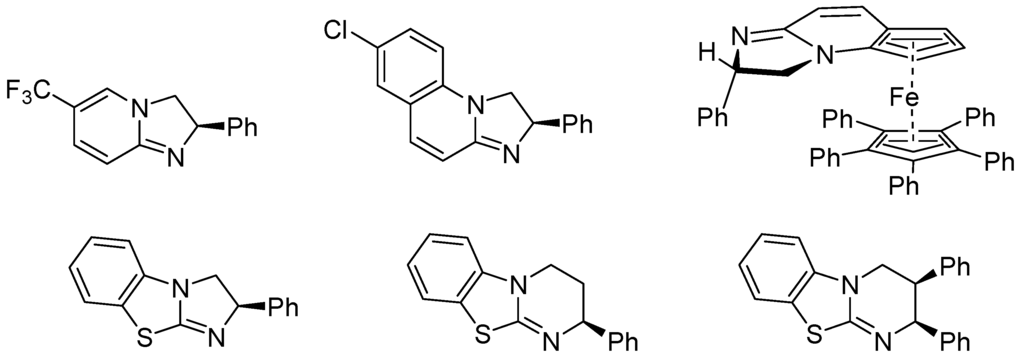
Figure 8.
Catalysts for enantioselective acyl transfers.
2.5. N-Heterocyclic Carbene-Catalyzed Transesterifications
In 1988, Bertrand and co-workers reported the first example of long-lived carbene (Me3SiC(:)P[N(i-Pr)2]2) [111]. The first example of stable, crystalline N-heterocyclic carbenes (N,N'-di(1-adamantyl)imidazol-2-ylidene) was described by Arduengo in 1991 [112]. In 1994, Bakhiar and Smith found that acyl transfers could be promoted by stoichiometric amounts of stable carbenes [113]. In 2002, Nolan's [51] and Hedrick’s [52] research groups reported that N-heterocyclic carbenes (NHC) such as 17 and 18 (imidazol-2-ylidenes) are efficient catalysts in the transesterification of esters and alcohols [53,114], as exemplified by Scheme 11.
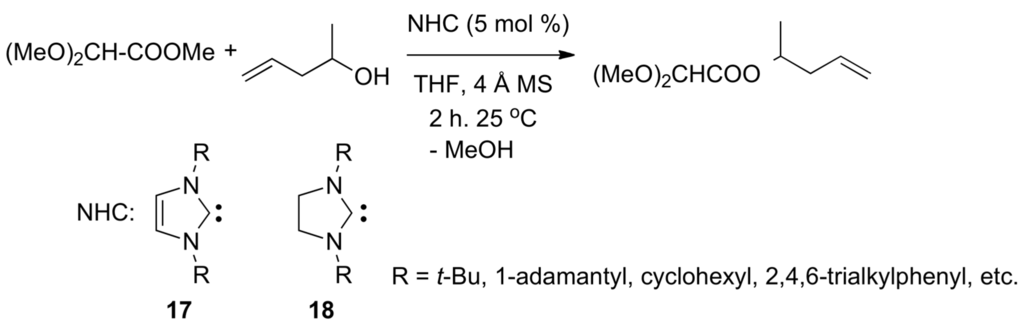
Scheme 11.
NHC-catalyzed (N-heterocyclic carbenes) transesterifications.
N-Heterocyclic carbenes act as catalysts for living ring-opening polymerizations of lactones, generating polyesters [115,116] (see e.g., Scheme 4). The pre-catalyst can be an alcohol adduct of the N-heterocyclic carbene, e.g., 19, which, upon heating, generates 1,3,4-triphenyl-1,4-dihydro-1H-1,2,4-triazol-5-ylidene (20) (Scheme 12). Since these early applications, N-heterocyclic carbenes have been used as organocatalysts for a large variety of reactions [117,118].
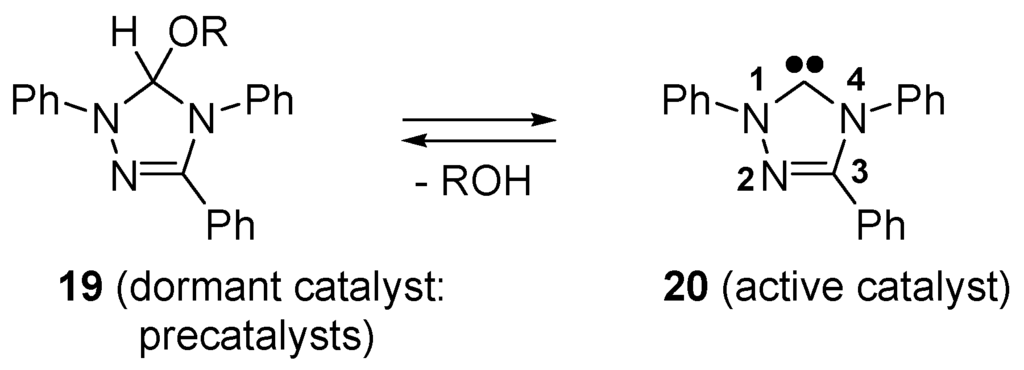
Scheme 12.
Interconversion of pre-catalyst 19 and active catalyst 20 by reversible addition of alcohol.
Applying the B3LYP density functional approach, Hu and co-workers [119] proposed the mechanism in Scheme 13 for the NHC-catalyzed transesterification. The role of NHC is to assist proton transfer from alcohol to the carbonyl group of the ester, forming the tetrahedral intermediate 21, which then decomposes to form the acylated product.
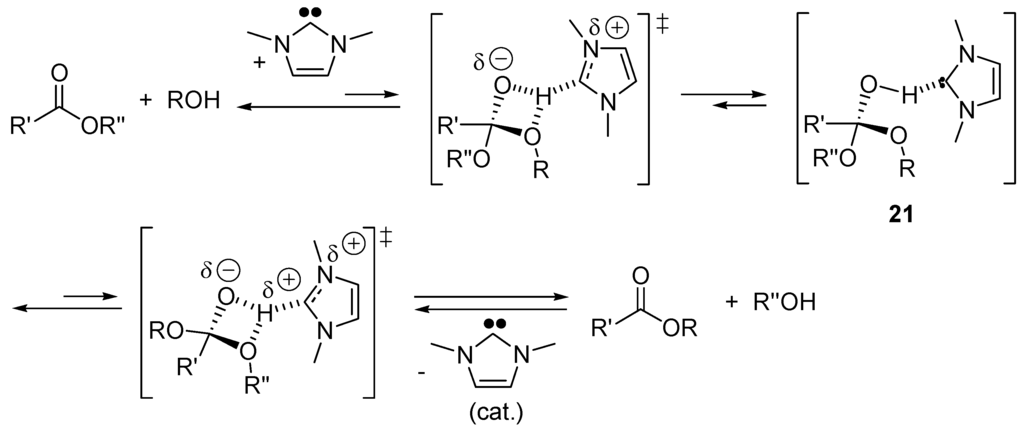
Scheme 13.
One possible mechanism of the N-heterocyclic carbenes (NHC)-catalyzed transesterification.
Singh and Nolan also found that the nucleophilic NHCs can catalyze the transesterification of phosphorous esters under mild conditions using imidazolium salts as pre-catalysts [120]. N-Heterocyclic carbenes catalyze the CO2 fixation as a vinyl carbonate. For instance, the carboxylative cyclization of 2-methylprop-3-yn-2-ol with 5 mol % 1,3-diisopropyl-4,5-dimethylimidazol-2-ylidene (100 °C, 10 MPa, 15 h) gives 5-methylidene-4,4-dimethyl-1,3-dioxolan-2-one in 80% yield (Scheme 14) [121].
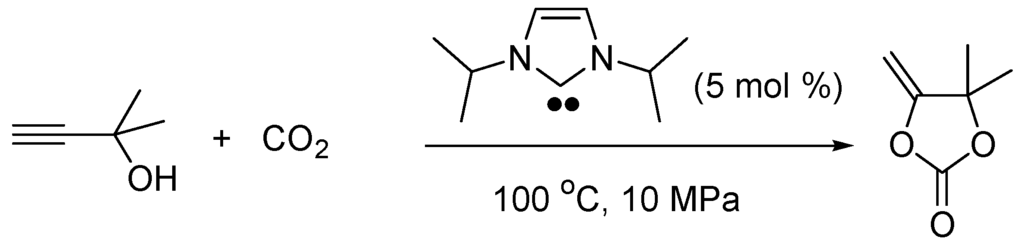
Scheme 14.
NHC-catalyzed fixation of CO2.
In 2004, Suzuki and co-workers reported the first examples of NHCs of type 23 (Scheme 15) as catalysts for enantioselective acyl transfers [122]. They applied them to the kinetic resolution of racemic secondary aryl alkyl alcohols [123]. Further examples were reported by Maruoka’s group [124]. In this case one postulated the formation of 2-acylimidazolium ion intermediates of type 24 (Scheme 15), the NHC acting as a nuleophilic catalyst. The formation of 2-acylimidazolium ion intermediates has been postulated also in the NHC-catalyzed (4+2)-cycloaddition/decarboxylation of silyl dienol ethers with α,β-unsaturated acyl fluorides [125,126] and for several other NHC-catalyzed reactions involving carboxylic moieties [127,128,129].
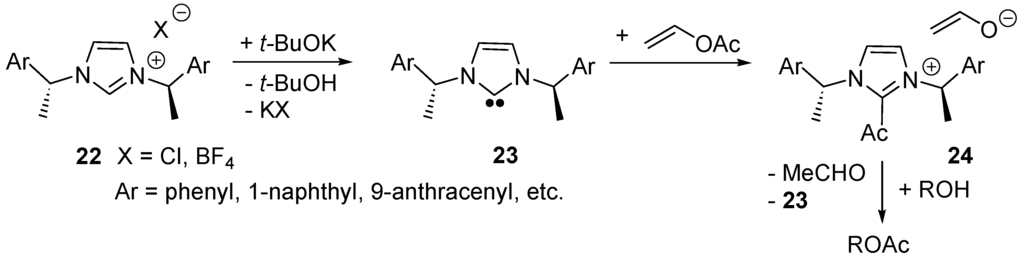
Scheme 15.
NHC-catalyzed enantioselective acyl transfer reactions.
2.6. Enzyme-Catalyzed Acyl Transfers
One important biological acyl transfer reaction is the hydrolysis of the amide linkage in a protein (e.g., 25). Chymotrypsin is one of the most well studied peptidases (enzymes that catalyze the cleavage of peptide bonds) [130,131,132]. Instead of using water as nucleophile, the alcohol side chain of serine-195 is used (Scheme 16). The addition of this alcohol moiety is base-catalyzed by the side chain of histidine-57, an imidazole (step 1). Ion-pairing of the resulting imidazolium ion with the aspartate-102 enhances the basicity of histidine-57. The combination of the serine, histidine and aspartate residues constitutes the catalytic triad. This generates a tetrahedral intermediate 26 (as for mechanism BAC2, Table 1) that is stabilized by hydrogen bonding in a pocket called the oxy-anion hole. Amide NH bond from the enzyme backbone contributes to the hydrogen bonding donors. After reorientation of the tetrahedral intermediate 26, the leaving group departure is acid-catalyzed by the imidazolium ion created in step 1. This forms an acyl-enzyme intermediate 27 (end of step 2) and one of the products, the amine R'NH2. Then water enters the active site and attacks (step 3) the carbonyl group of 28, a base-catalyzed addition promoted, as for the serine-OH addition in step 1, by the imidazole unit of histidine-57. This produces tetrahedral intermediate 29, which then liberates serine-198 and the carboxylic acids RCOOH (30), the second product.
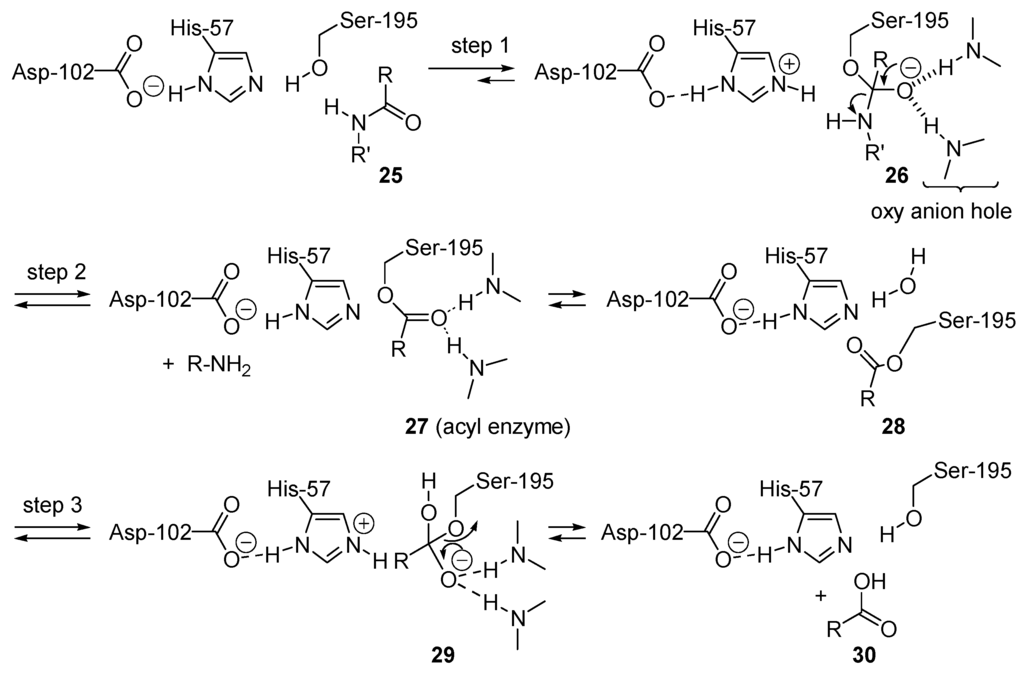
Scheme 16.
Chymotrypsin-catalyzed peptide bond cleavage: an example of covalent catalysis by amide alcoholysis through serine (serine-protease).
Another amide bond cleavage mechanism is followed by the carboxypeptidase A-catalyzed hydrolysis [133,134]. In this case, activation of the nucleophilic addition of water implies coordination of water (the nucleophile) and the carbonyl group (the electrophile) of the amide 21 to a zinc cation (Lewis acid, template effect). Furthermore, the nucleophilicity of water is enhanced by hydrogen bridging with glutamate-270 and by coordination to Zn(II). Its pKa is expected to be decreased significantly because it coordinates Zn(II), as shown for complex 33 [135,136]. Further electrophilic activation of the carbamide is realized by hydrogen bridging of its amino group with the phenolic moiety of tyrosine-248 and of its carbonyl group with arginium cation Arg-127 (step 1, Scheme 17). This leads to the formation of the tetrahedral intermediate 32. In a second step, tyrosine- and glutamic acid-catalyzed elimination of the amine R'NH2 occurs, followed by the liberation of the carboxylic acid, RCOOH.
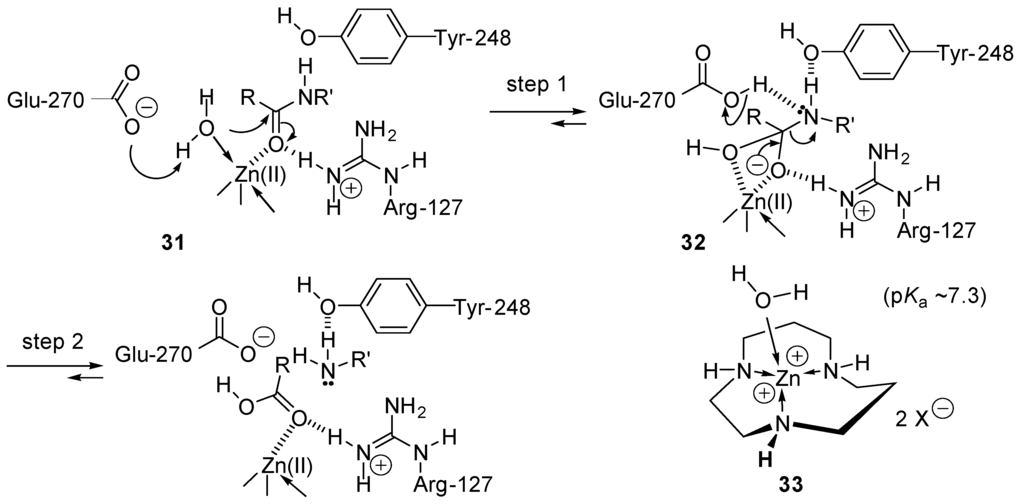
Scheme 17.
Carbopeptidase A (prototypical metalloprotease) catalyzed peptide bond cleavage. Example of zinc(II) electrophilic activation.
2.7. Mimics of Carbopeptidase A
In 1965, Breslow and Chipman [137] reported that zinc pyridinecarboxaldoxime anion complex 34 is an effective catalyst in the hydrolysis of 8-acetoxyquinoline-5-sulfonate (35), a weakly complexing substrate. The reaction occurs in two steps involving acyl transfer within a catalyst-substrate complex (Scheme 18).
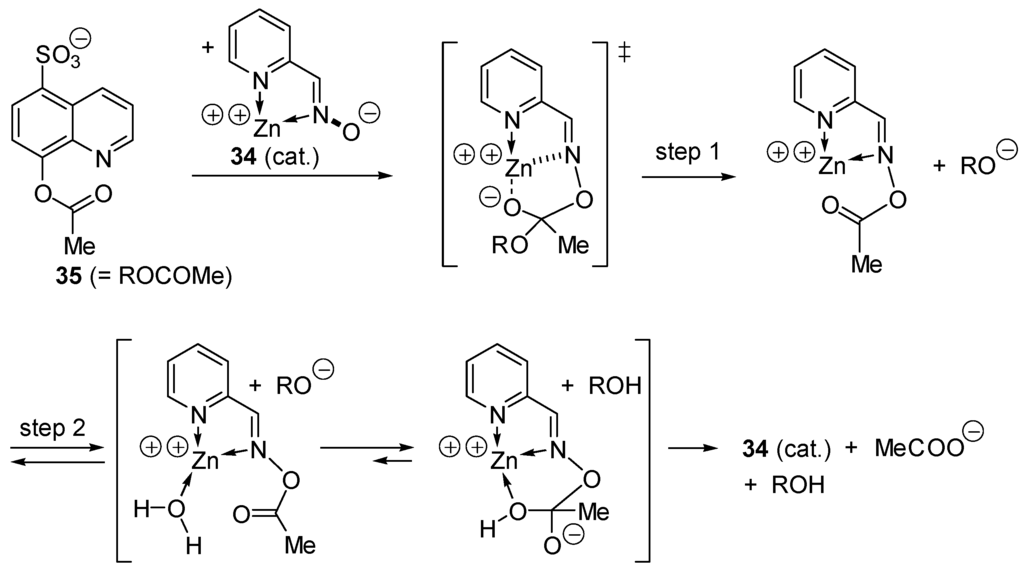
Scheme 18.
Ester hydrolysis catalyzed by a zinc (II) aldoxime complex.
When 34 is coordinated with the β-cyclodextrin derivative 36 containing a linking bipyridyl group, the complex 37 so-obtained catalyzes the hydrolysis of ester 38 with a rate enhancement of 1,700,000-fold over the background reaction (pH 7, 37 °C) [138,139]. The two β-cyclodextrin units offer a cavity to the substrate as an enzyme [140]. The adamantyl moiety of 38 enters the lyophilic cavity of one cyclodextrin, the leaving group (p-nitrophenolate) enters the other cyclodextrin unit, repelling water molecules [141]. The substrate is thus forced to approach the zinc pyridinecarboxaldoxime anion that adds (Scheme 19) onto its carboxylic moiety, generating an acyl-catalyst intermediate that is then hydrolyzed into p-nitrophenol (40) and the carboxylic product (39). Several applications of cyclodextrins as catalysts have been reported [142].
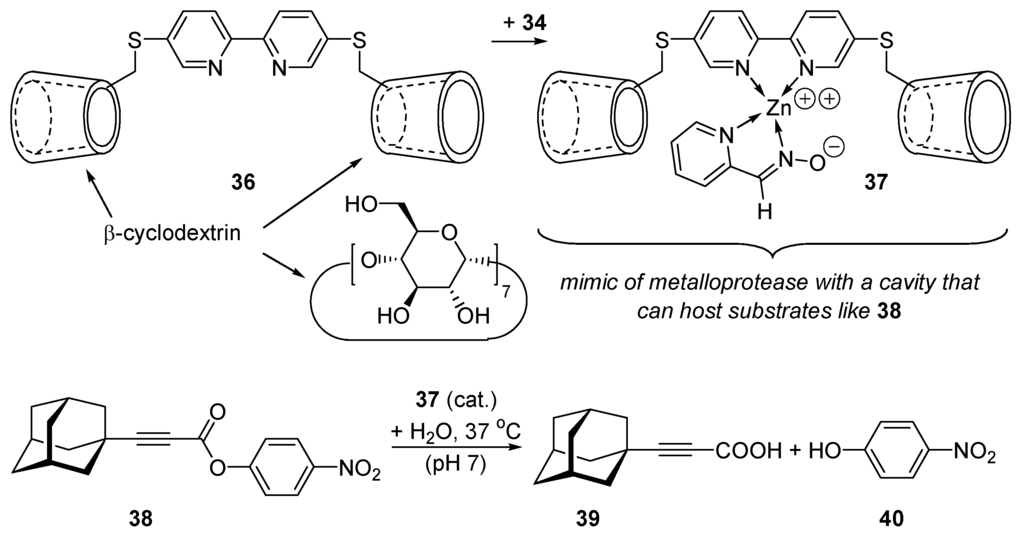
Scheme 19.
The interior of cyclodextrins imitates the lyophilic part of the cavity of enzymes.
2.8. Direct Amide Bond Formation from Amines and Carboxylic Acids
Amides represent one of the most important chemical building blocks found in Nature, in pharmaceuticals [143,144,145], in agrochemicals, and in materials [146,147]. Using gas-phase standard heats of formation [148] one calculates standard heats of reaction ΔH°(AcOH + Me2NH ⇄ AcONMe2 + H2O) = −4.4 kcal/mol and ΔrH°(AcOH + PhNH2 ⇄ AcONHPh + H2O) = −8.0 kcal/mol. In water and at 25 °C equilibrium constant K°(RCOOR + R'NH2 ⇄RCONHR' + H2O) = 103–104.4 for the formation of protected and unprotected di- and tripeptides [149]. Penicillin acylase-catalyzed amidifications of phenylacetic acid with primary amines are exergonic in diluted water for not too basic amines (pKa(RNH3+) < 8.5) [150]. Although the direct formation of amides from amines and carboxylic acids (Scheme 20) has been known for 150 years, this process has little synthetic utility as it requires too harsh conditions (heating that leads to secondary products, epimerization and polymers) [151]. Amide bonds are generally formed from carboxylic acid derivatives such as acyl halides (mostly chlorides and fluorides; the Schotten–Baumann reaction) or anhydrides, including mixed anhydrides, or active esters as those obtained by reaction of the carboxylic acids with coupling reagents such as Castro’s reagent BOP (benzotriazol-1-yl-oxy-tris(dimethylamino)phosphonium hexafluorophosphate) and analoguous phosphonium-based coupling reagents ByBOP, PyAOP, BroP, PyCloP, or the uronium/guanidinum-based coupling reagents BCC, HBTU (O-(1H-benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate), HATU, HAPyCl and TAPipU (Figure 9) [152,153]. The latter reagents are suitable for peptide synthesis requiring smooth conditions to reduce concurrent epimerization of the α-aminoacid moieties. Unfortunately, the one-pot method produces at least three co-products. The amine may also react with HATU and lead to 40 + 41 (Scheme 21). In terms of atomic economy [154,155], these reactions are poorly behaved and thus efficient catalysts permitting the direct amide formation (Scheme 20) under smooth conditions are most welcome [156,157]. Nature makes amides with enzymes as catalysts. In the laboratory mostly lipases (e.g., peptide amidase extracted from orange peel, Candida antartica lipase B, Candida cylindracea lipase, Novozym, porcine pancreas lipase) and microorganism (e.g., Bacillus cereus, Streptomyces halstedii, and Bacillus subtilis) have been used as biocatalysts for direct amidification of carboxylic acids [157]. Since the biocatalysts cannot accept any amines and carboxylic acids as reagents, chemical catalysts are highly desired.
Scheme 20.
Direct formation of amides from carboxylic acids.
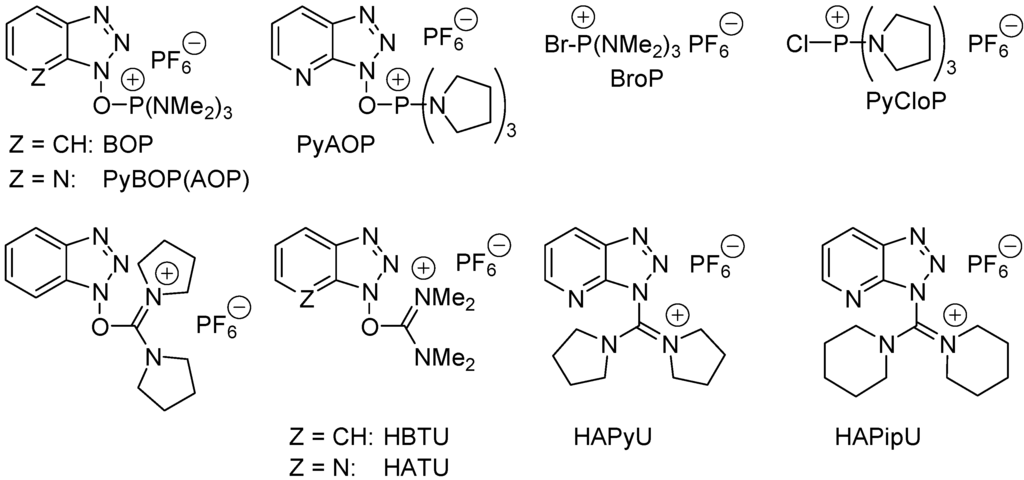
Figure 9.
Examples of coupling-reagents for the one-pot amide (peptide) bond formation from carboxylic acids and amines.
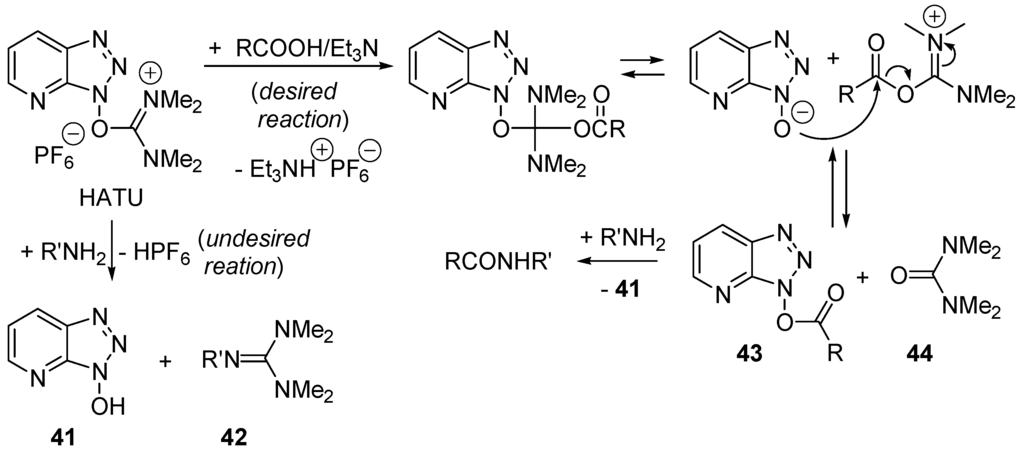
Scheme 21.
One-pot amide bond formation promoted by O-(7-azabenzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HATU) produces several co-products.
Since 1965, stoichiometric amounts of boron-based compounds have been used to promote the direct amidification of carboxylic acids with amines [158]. For instance, in the presence of two equivalents of B(OCH2CF3)2 carboxylic acids and amines are condensed to form the corresponding amides upon heating for several hours at 80–100 °C [159,160]. The boron reagents assist amide formation directly by formation of acyloxy boron intermediates = mixed anhydrides [161,162,163,164,165,166]. In 1996, Yamamoto and co-workers reported that 3,4,5-trifluorobenzeneboronic acid catalyzes (Soxhlet refluxing toluene, xylene or mesitylene, molecular sieve for the drying) the direct amide condensation reactions [163]. They later reported that N-alkyl-4-boronopyridinium halides (45, 46; Scheme 22) are more effective than boric acid and areneboronic acids [167,168]. The proposed mechanism of these catalytic amidifications is shown in Scheme 22 [169].
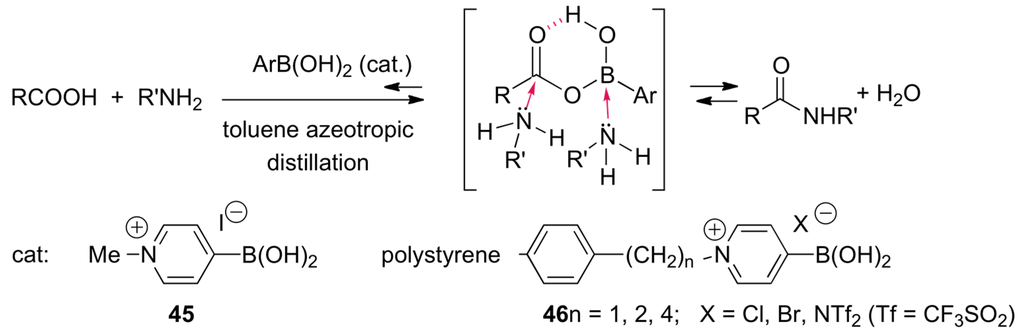
Scheme 22.
Amide formation from carboxylic acids and amines catalyzed by 45.
Recently, Hall and co-workers found that 5-methoxy-2-iodobenzeneborinic acid (MIBA) catalyst leads to higher yields for a wider range of aliphatic and heteroaromatic carboxylic acids and aliphatic amines [170,171]. The methods can be applied to the formation of α-peptides (molecular sieves, boiling CH2Cl2, 48 h) and β-peptides as illustrated in Scheme 23 [172].
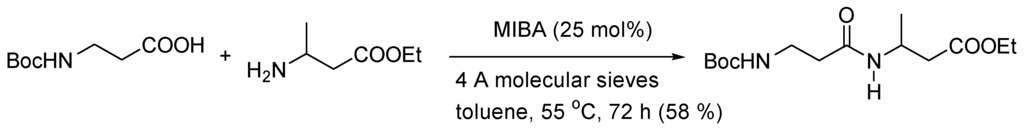
Scheme 23.
MIBA-catalyzed (5-methoxy-2-iodobenzeneborinic acid) formation of β-peptides.
A large number of metallic compounds have been proposed as catalysts for the direct amidification of carboxylic acids with amines. Most of them require heating to above 100 °C such as (cyclopentadienyl)2ZrCl2 [173] and Nb2O5 [174]. The combination of Ph3P + CCl4 has been found to catalyze direct amidifications [175].
3. Catalysis of Nucleophilic Additions
Additions of carbon- and hetero-nucleophiles (E-Nu) to polar unsaturated compounds such as aldehydes (RCH=O), ketones R(R')C=O, imines (R(R')C=NR") and carbonitriles (R–C≡N) (1,2-additions: E–Nu + R2C=Z ⇄ Nu–C(R2)–Z–E), to their α,β-unsaturated derivatives (competition between 1,2- and 1,4-additions E–Nu + R2C=CR'–CR"=Z ⇄ Nu-C(R2)-CR'=CR"-Z-E), to alkenes, alkynes, bearing electron-withdrawing substituents (E-Nu + R2C=CR'-EWG ⇄ Nu-C(R2)-C(R')(E)-EWG, e.g., EWG = SOR, SO2R; CF3) represent the most important reactions of synthetic interest. All these reactions can be catalyzed as illustrated below.
3.1. Catalysis of Nucleophilic Additions to Aldehydes, Ketones and Imines
As for the acyl transfer reactions nucleophilic additions to aldehydes and ketones of reagent E-Nu (Scheme 24) can be catalyzed by a protic (Brønsted) acid (HA), a Lewis base (B:) or a Lewis acid (MX). In the absence of catalyst, the electrofugal moiety of the reagent E-Nu can activate the carbonyl group (mech. A2) and, eventually, auto-catalyze the reaction. In the presence of a protic acid, the carbonyl group is activated by protonation (mech. B1, B3) or/and the nucleophilic reagent is activated as it enters in pre-equilibrium E-Nu + HA ⇄ EA + H+ + Nu:− (mech. B2). Alternatively, the conjugate base of the catalyst HA adds to the carbonyl group according to the pre-equilibrium C=O + HA ⇄ C=A+ + HO−. Then HO− activates the nucleophilic reagent according to equilibrium HO− + E-Nu ⇄ HOE + Nu:− (mech. B3). In the presence of a Lewis base B: the latter activates E-Nu by deprotonation when E = H, or by entering into pre-equilibrium B: + E-Nu ⇄ E-B+ + Nu:− when E ≠ H (mech. C1). Alternatively, the base acts as nucleophilic catalyst by adding first to the carbonyl moiety and equilibrating with an oxycarbenium ion intermediate of type C=O(+)-E, which then adds the negatively charged nucleophile Nu:− rapidly (mech. C2). In the presence of a Lewis acid MX the carbonyl group is activated by forming an ion pair of type C=O(+)–M/X− (mech. D1). Alternatively, E-Nu can be activated by entering into pre-equilibrium E-Nu + MX ⇄ EX + M+/Nu:− (mech. D2). In these mechanistic limits the activation of the carbonyl group implies the formation of ion pairs of type C=O(+)–E/Nu:− (Mech. A2, B2, C=O(+)-H/A:− (mech. B1,B3) or C=O(+)-M/X:− (mech. D1), and the activation of the nucleophilic reagent E-Nu implies the formation of ion pairs of type H+/Nu:− (Mech. B2), E-B+/Nu:− (mech. C1) or M+/Nu:− (mech. D2). As we shall see, in many cases these species do not have to be formed for activation, instead, complexes of types C=O···E–Nu, C=O···H–A (hydrogen bonded carbonyl) or Lewis acid-base complexes C=O···MX react with the nucleophile (E-Nu, Nu:− or Nu:−···H–A). The same mechanistic limits can be retained for the nucleophilic additions of imines (Scheme 24) [176].
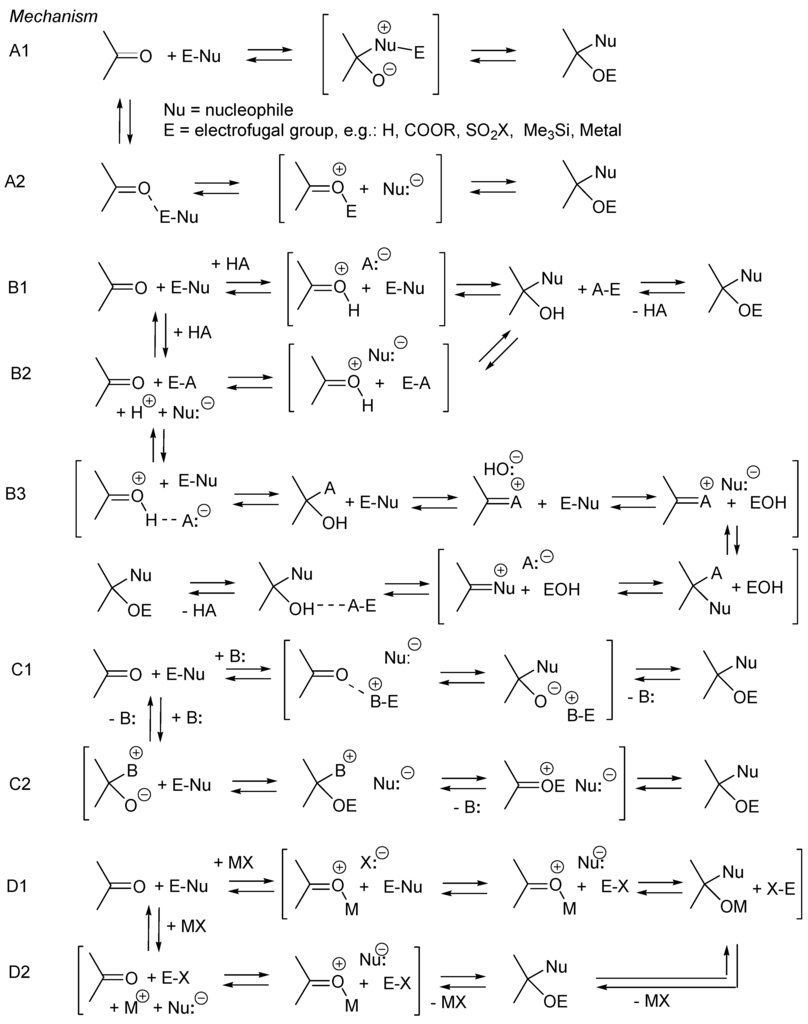
Scheme 24.
Possible mechanism limits for the nucleophilic additions of carbonyl compounds: (A) uncatalyzed reaction; (B) protic acid-catalyzed; (C) Lewis base-catalyzed; (D) Lewis acid-catalyzed.
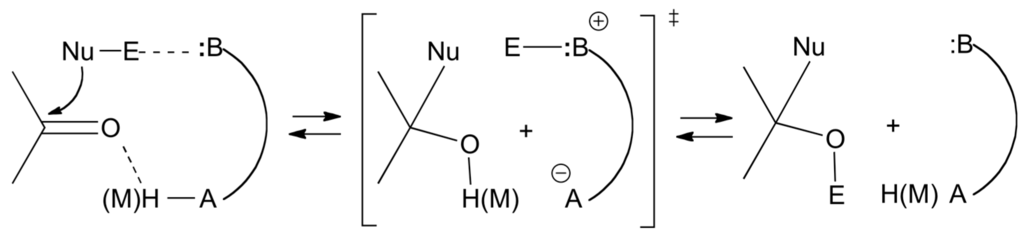
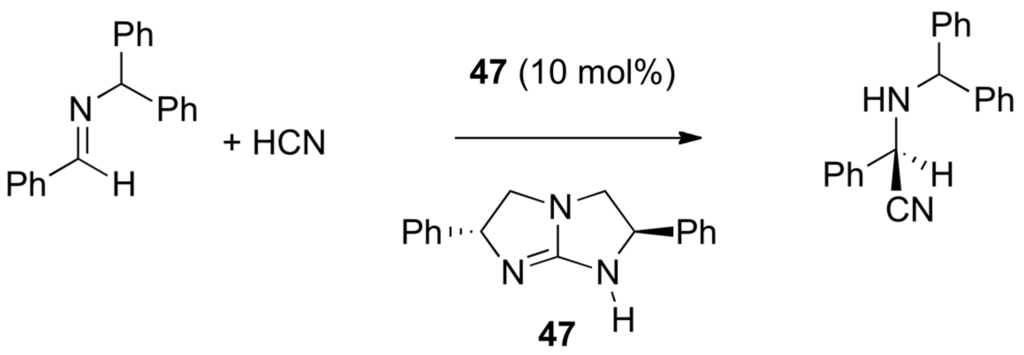
From the preceding discussion, one predicts that the best catalysts of the nucleophilic additions (and of the reverse elimination reaction) possess on the same template functional groups capable of electrophilic activation of the carbonyl or imine moieties and nucleophilic assistance to the dissociation of reagent E-Nu as shown below in Scheme 25 and as illustrated already in Scheme 3 and Scheme 4, where benzoic acid and triazabicyclo[4.4.0]dec-5-ene (TBD) act as bifunctional catalysts (i.e., as proton donor and proton acceptor, both proton transfers being concerted or not). Further illustration is with the asymmetric Strecker reaction (Scheme 26) catalyzed by the chiral, enantiomerically pure bicyclic guanidine 47 reported by Corey and Grogan [177], and the mutarotation of 2,3,4,6-tetra-O-methyl-α-d-glucopyranose catalyzed by 2-pyridinone.
Scheme 25.
Bifunctional catalysts for activation of carbonyl groups towards nucleophilic addition.
Scheme 26.
Asymmetric Strecker reaction catalyzed by chiral guanidine 47.
3.2. Bifunctional Catalysts for Nucleophilic Addition/Elimination
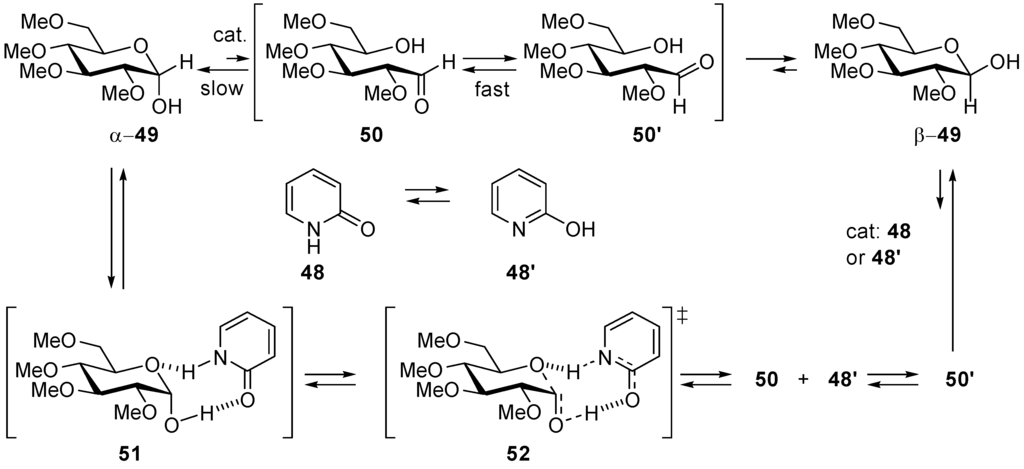
In 1952, Swain and Brown [178,179] reported that neutral 2-pyridinone (48) is a better catalyst of the mutarotation (epimerization) of 2,3,4,6-tetra-O-methyl-α-d-glucopyranose (α-49) into its β-anomer β-49 in benzene than pyridine and phenol alone, and better than a 1:1 mixture of pyridine and phenol. This was taken as a proof that the rate-controlling step involves two-proton transfers coupled with the C(1)···O bond cleavage of the glucose moiety, as shown with transition state 52 (Scheme 27).
Scheme 27.
Mutarotation of tetramethylglucose catalyzed by the bifunctional catalyst α-pyridone ⇄ 2-hydroxypyridine in benzene (apolar solvent).
In the gas phase and in dilute solution of benzene, α-pyridone (48) and its tautomer 2-hydroxypyridine (48') have similar stability [180], each of them may catalyze the mutarotation [181]. The mechanism proposed in Scheme 11 implies that α-pyridone (equilibrates with dimers) forms a precomplex 51 with α-49. The two proton transfers occur in concert with the breaking of the C(1)–O bond leading to the hydroxyaldehyde 50 in one step. Fast rotation about the C(1)–C(2) bond engenders rotameric hydroxyaldehyde 50' that undergoes intramolecular alcohol addition furnishing hemiacetal β-49. This addition is also catalyzed by 48 or 48′ [182,183]. Kinetic deuterium isotope effects determined with (N-D)-2-pyridinone and (1-O-D)-2,3,4,6-tetra-O-methyl-α-d-glucopyranose agree with the above hypothesis, although they could not tell whether the two proton transfers are synchronous or not [184]. Analogues of α-pyridinone such as benzoic acid, formamide/formamidic [185] acid tautomeric couple, or 2-hydroxy-4-methylquinoline, 2-aminopyridine, imidazole [186] and 2′,3′,5′-tri-O-protected RNA nucleosides [187] can also act as bifunctional catalysts. Sodium 2-pyridinolate has been found to catalyze aminolysis of esters [188].
3.3. σ- and π-Nucleophiles as Catalysts for Nucleophilic Additions to Aldehydes and Ketones
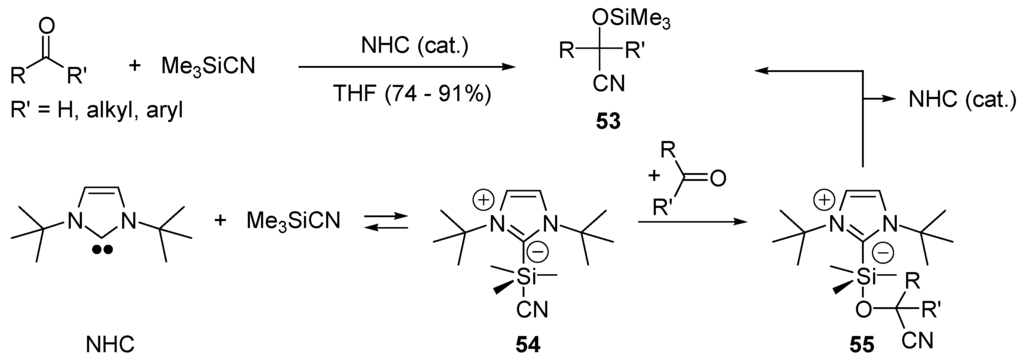
Cyanohydrins are important building blocks for the preparation of α-hydroxy acids, α-hydroxy aldehydes, 1,2-diols, and α-amino alcohols. They can be obtained by cyanosilylation of aldehydes and ketones using Me3SiCN (=E-Nu in Scheme 28 that produces the corresponding silyl ethers 53. The reaction is catalyzed by Lewis acids or bases, metal alkoxides, bifunctional catalysts, iodine, and inorganic salts [189,190]. As for the transesterifications that can be catalyzed by N-heterocyclic carbenes (NHC), the transfer of cyano group from Me3SiCN to a carbonyl compound has been catalyzed by diaminocarbenes [191]. It is proposed (Scheme 28) that the NHC-catalyzed cyanosilylation follows a nucleophilic catalysis pathway (mech C1, Scheme 24) implying intermediate 54 with a pentacoordinated silicon center [192,193,194]. The latter transfers the cyano group to the aldehyde or ketone generating adduct 55 which then dissociates into product 53 and the catalyst (NHC).
Scheme 28.
NHC-catalyzed cyanosilylation of aldehydes and ketones.
N-Heterocyclic carbenes (NHC) are neutral σ-nucleophilic catalysts. According to Wang and Tian [195], electron-rich π-systems should also be catalysts for the cyanosilylation of carbonyl compounds. Indeed, they found that the reaction of PhCHO + Me3SiCN in the presence of Me2C=C(OSiMe3)OMe (10%) is at least 100-fold faster than without catalyst at 25 °C. Aniline acts as a nucleophilic catalyst (mech. B3, Scheme 24) in oxime ligation in aqueous solution through formation of intermediate iminium salt, followed by transimination by the oxime (Scheme 29) [196,197,198].
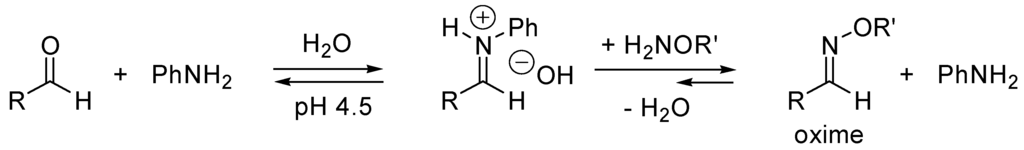
Scheme 29.
Formation of oxime by transamination.
Since the pioneering work of Oguni and co-workers in 1993 that used the chiral Schiff base 56 and Ti(O-i-Pr)4 as the catalyst (Scheme 30) [199], a large number of enantioselective syntheses of cyanohydrins have been developed [200,201,202,203,204]. The transition structure 57 has been proposed to interpret the results (mech. D1, Scheme 24).
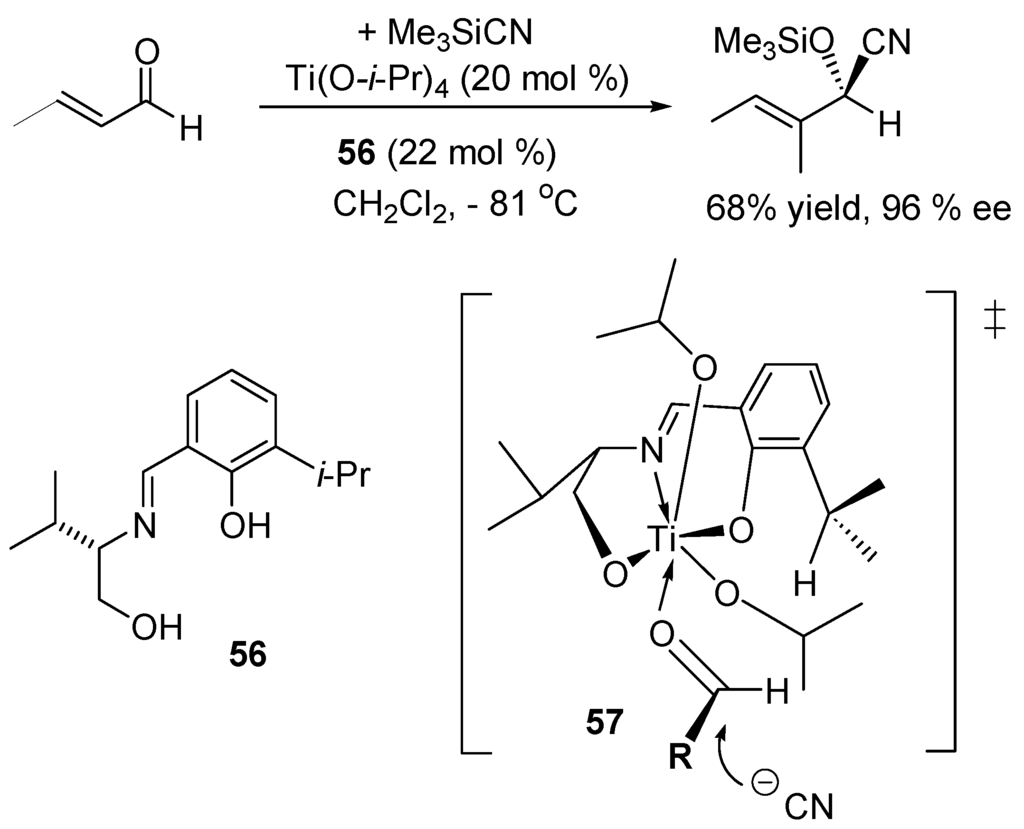
Scheme 30.
Enantioselective synthesis by cyanohydrins.
An example of enantioselective cyanation of an aldimine with the quinine-derived thiourea catalyst 58 is given in Scheme 31 for which transition structure 59 is proposed to explain the high yield and enantioselectivity (mech. B1, Scheme 24).
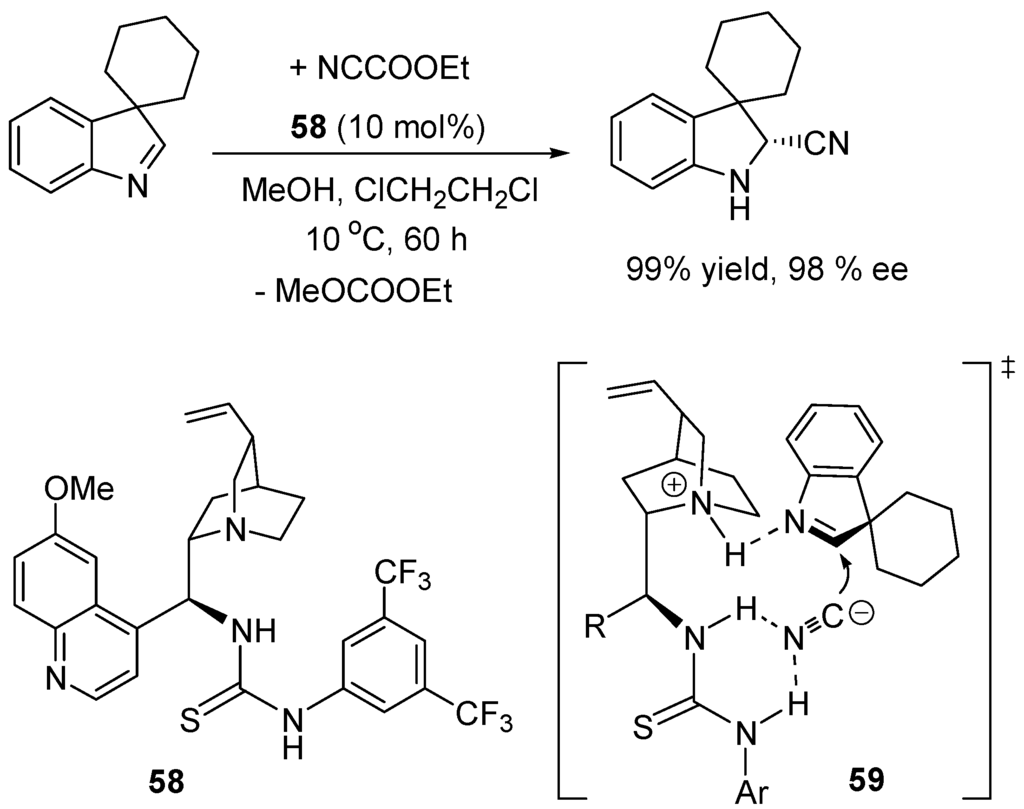
Scheme 31.
Quinine–thiourea catalyzed cyanation of an aldimine.
3.4. Catalysis by Self-Assembled Encapsulation
The self-assembly of six equivalents of ligand 60 (L) with four equivalents of Gd(acac)3 in water generates the negatively charged supramolecular assembly Gd4L612− that can host a wide variety of small monocationic guests in its cavity (Figure 10). The naphthalene walls render the interior hydrophobic and are thus able to promote acid-catalyzed reactions upon encapsulation. This was suggested by the observation that N,N,N′,N′-tetramethyl-1,4-diaminobutane or N,N,N′,N′-tetramethyl-1,2-diaminoethane are doubly protonated when encapsulated with Gd4L612−. Orthoesters normally require acidic conditions to be hydrolyzed (acid-catalyzed elimination, reverse of nucleophilic addition to a carbonyl compound for one step of the overall process). Under neutral or alkaline conditions their hydrolysis is very slow. However, when orthoesters HC(OR)3 (R = Me, Et, Pr, i-Pr, Bu, i-Bu) are mixed in D2O (22 °C, pH = 11, K2CO3) with small amounts of Gd4L612−, hydrolysis occurs. The phenomenon is not observed with larger orthoesters (e.g., with R = pentyl). Thus, upon encapsulation, the orthoesters are protonated into 62 and then dissociate into two alcohol moieties and the conjugate acids 63 of the corresponding formates. The latter diffuse out the host and are saponified by the alkaline medium (Figure 10). Importantly, the catalysis by the host of type Ga4L612− obeys Michaelis-Menten kinetics and exhibits competitive inhibition. When compared to the background hydrolysis reactions under the same conditions, rate accelerations (kcat/kuncat) for triethyl orthoformate and triisopropyl formate are 560 and 890, respectively (pH 11, 22 °C, D2O) [205,206].
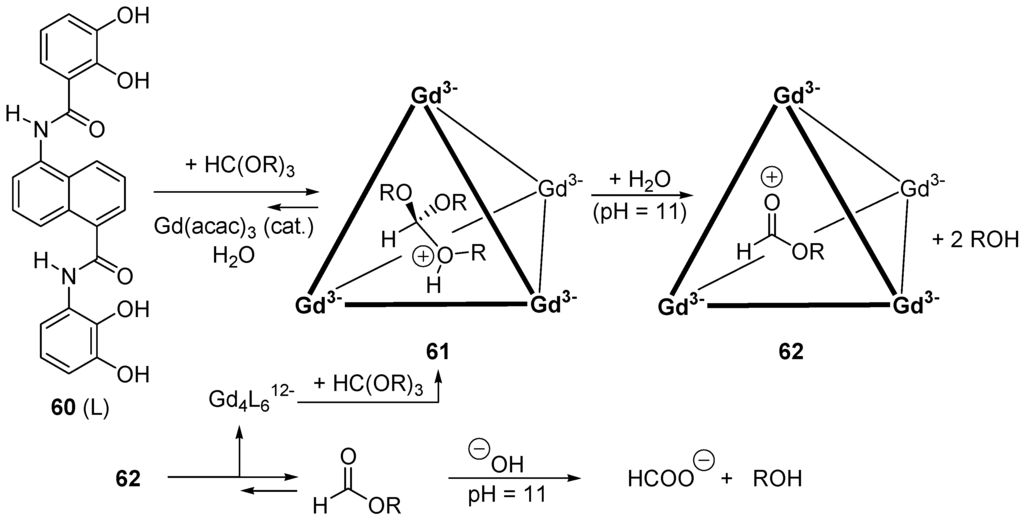
Figure 10.
Catalysis by supramolecular assembly. Hydrolysis of orthoesters under alkaline conditions.
3.5. Catalysis of 1,4- (Conjugate) Additions
Conjugate nucleophilic additions to electron-deficient alkenes [207] and dienes [208] is one of the most useful tools in organic synthesis. In 1977, Wynberg and co-workers [209] reported that cinchona alkaloids catalyze the asymmetric conjugate addition of aromatic thiols to cycloalkenones (Scheme 32) [210]. Quantum mechanical calculations proposed transition state of type 63 for these reactions [211].
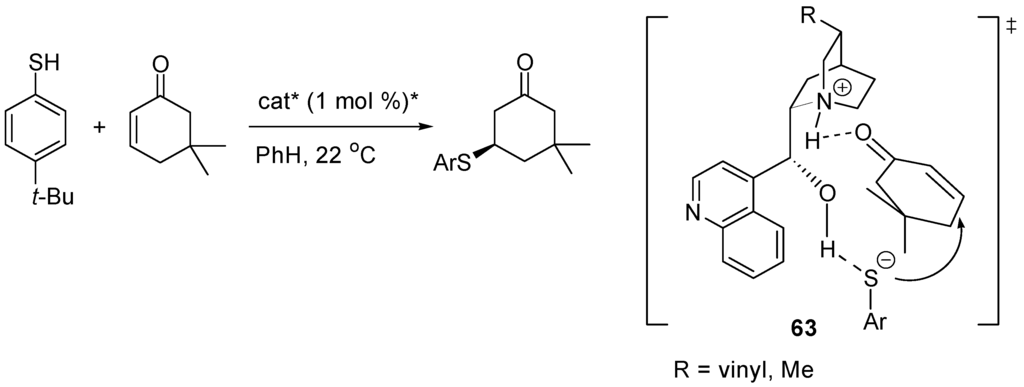
Scheme 32.
Cinchona alkaloid-catalyzed conjugate addition and transition state 63.
This has been the starting point of several enantioselective conjugate addition using chiral catalysts [212,213,214,215,216]. For instance, the enantioslective Michael addition of dimethyl malonate to cyclohex-2-enone is carried out on the kilogram scale (Scheme 33). It uses the bifunctional Lewis acid/Brønsted base catalyst 64 developed by Shibazaki and co-workers [190,217,218,219]. Adduct 65 is the starting material in the synthesis of (−)-strychnine.
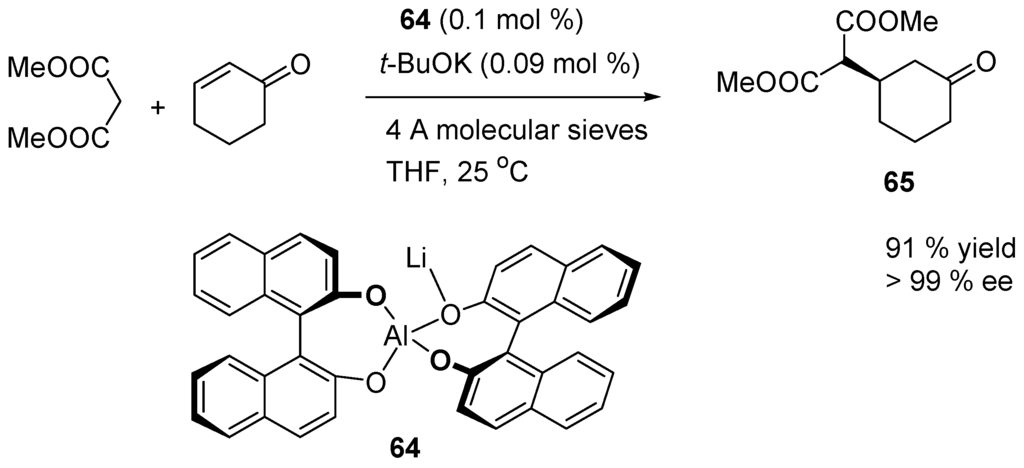
Scheme 33.
Enantioselective Michael addition of dimethyl malonate to cyclohex-2-enone.
The bifunctional catalyst 66 developed by Takemoto and co-workers (Scheme 34) [220] activates both the nucleophile and the carbonyl group of the Michael acceptor as illustrated below [221].
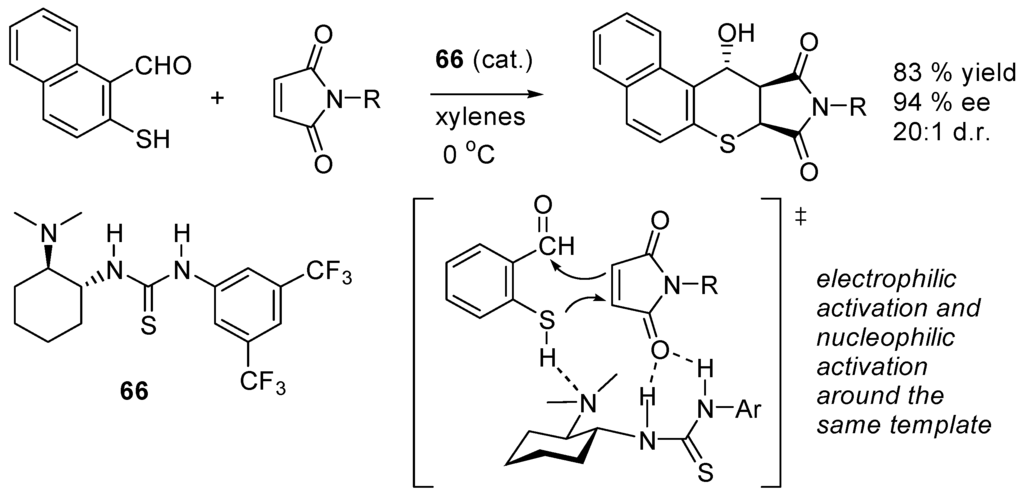
Scheme 34.
Michael addition catalyzed by Takemoto’s bifunctional catalyst 66.
4. Anionic Nucleophilic Displacement Reactions
Nucleophilic substitution is one of the most important class of reactions that generate C–C and C–heteroatom bonds. There are four classes of nucleophilic displacement reactions (Scheme 35): reaction (a) involves neutral electrophile R–X and nucleophile Nu: and generates a salt (e.g., Menschutkin reaction: quaternization of amines, of phosphines, formation of oxonium and sulfonium ions); reaction (b) is similar to the reverse reaction of equilibrium (a) in which a positively charged electrophile is substituted by a negatively charged nucleophile Y:−; reaction (c) engages a neutral electrophile R-X and an anionic nucleophile Y:− with a proton or cation as counter-ion and finally, in reaction (d) a neutral nucleophile attacks a cationic electrophile. Very important for organic synthesis is the SN2 reaction (c) and we discuss now how it can be accelerated or/and catalyzed.
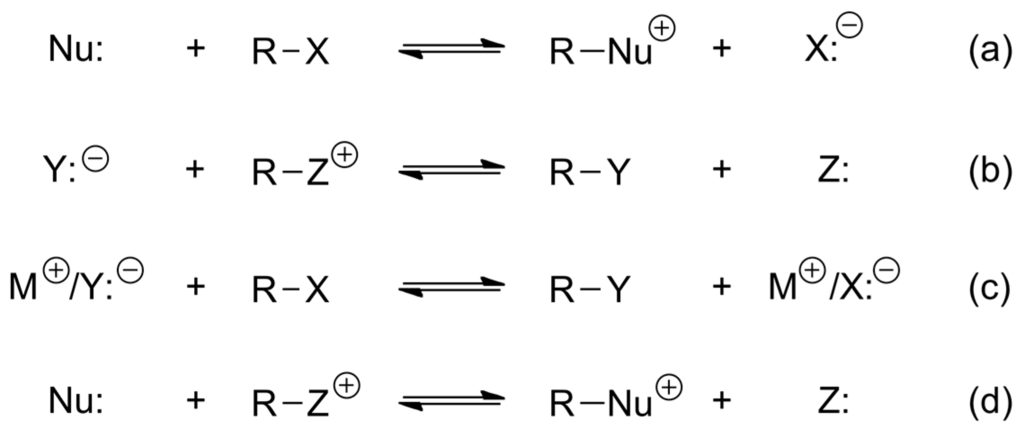
Scheme 35.
Four classes of nucleophilic displacement reactions.
4.1. Displacement Reactions in the Gas Phase
Reactions between anions and molecules occurring in the Fourier Transform Ion Cyclotron Resonance (FT-ICR) of Flowing Afterglow (AF) spectrometer should be exothermic or thermoneutral. In both types of instruments, the concentration of ions is about 104 times lower than the concentration of neutral molecules. A simple pseudo-first order kinetic law is thus observed for the ion/molecule reactions, and these proceed much faster in the gas phase than in solution. For the non-solvated hydroxide anion HO−, the rate constant for substitution HO− + MeBr → HO-Me + Br− is about 1016 times faster in the gas phase than in solution [222]. The SN2 reactions of superoxide anion water clusters (O2•−(H2O)n (n = 0–5)) with Me–Cl and Me–Br have rates that decrease when the number of water molecules in the cluster increases [223]. In general, the rate constants of gas-phase SN2 displacements (Figure 11) can be explained on the basis of the double potential-well model represented in Figure 7, and pioneered in the work of Brauman in the 1970s and after [224,225,226,227].
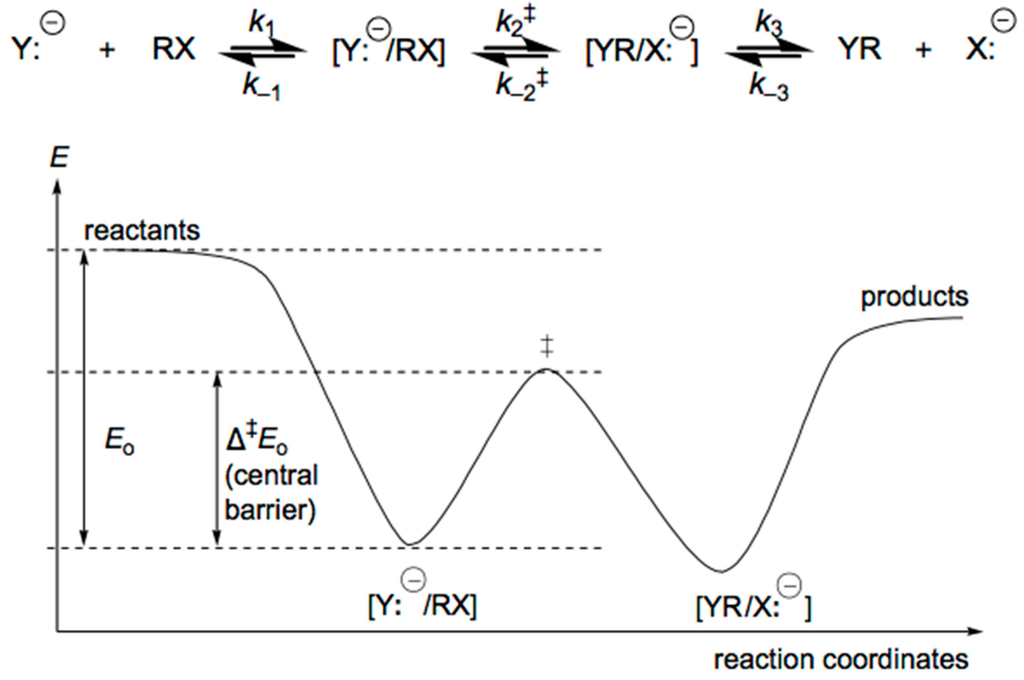
Figure 11.
Potential energy hypersurface for gas phase SN2 substitutions.
In this model, first a ion/molecule complex [Y:−/RX] is formed, which corresponds to a minimum in potential energy and is relatively long-lived. The free anion Y:− is thus “solvated” by the molecule RX, with a binding energy ΔEo (Figure 11) that can be as large as 25 kcal/mol. This binding is due mostly to electrostatic interactions that are long-range ion-induced dipole and ion-dipole interactions between Y:− and RX. These electrostatic interactions can be estimated by relationship (2), where ΔE(r) is the drop in potential energy upon approach of ion and molecular from infinity to a distance r, α is the polarizability of RX, q the charge of Y:− and μD the permanent dipole of RX.
For the substitution reaction to occur, the complex [Y:−/RX] must adopt the configuration of the activated complex. In potential energy terms, this corresponds to the maximum of the central barrier with height Δ‡Eo. Passing over this barrier will lead to a second ion/molecule complex [X:−/RY], which in turn corresponds to the potential minimum on the products side and will eventually separate (low pressure, high temperature). The height of the barrier Δ‡Eo depends on the type of ion/molecule reaction and on the nature of the reactant ion, product ion and neutral species involved, as predicted by the Bell-Evans-Polanyi theory [228,229] for concerted one-step reactions (Δ‡H = αΔrH + β). The larger the exothermicity ΔrH for [Y:−/RX] ⇄ [X:−/RY], the smaller is Δ‡Eo. Higher temperature, or greater collision energy of reactants, causes the dissociation of the complex to accelerate more than the reaction accelerates. This corresponds to the negative temperature dependence of gas-phase exothermic ion/molecule reactions. In solution, however, the reactants are more highly solvated than the complex, and only the central barrier of Figure 11 remains! In the latter case, the reactants and ion/molecule complex must acquire energy or become activated by increasing the temperature, or by collisions with the surrounding molecules to overcome the local energy barrier Δ‡Eo. Nevertheless, ion/molecule reactions in the gas phase and solution both correspond in the same way to an increasing height of this local energy barrier. In both cases the overall rate constants for reaction then become smaller. Quantum mechanical calculations give the following central barriers for Y:− + MeX ⇄ MeY + X:− (Scheme 36) [230].
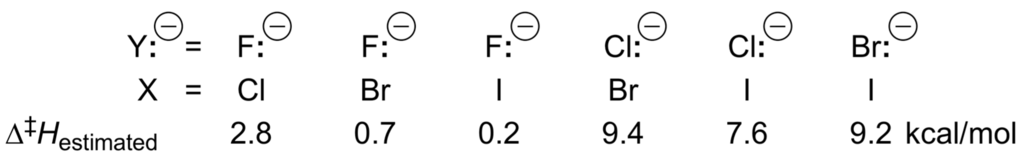
Scheme 36.
Central barriers for the nucleophilic displacements Y:− + MeX ⇄ MeY + X:−.
The overall rate constant kobs for Y:− + RX ⇄ X:− + RY is given by Equation (3) when the system is treated according to the steady state approximation. Here k1 is the collision rate constant for Y:− + RX ⇄ [Y:−/RX] and k−1 is the rate constant for the reverse reaction. The ratio between kobs and k1 is termed the reaction efficiency, which gives an estimate of the number of collisions resulting in product formation. For an exothermic reaction it can be assumed that k−2 << k3; kobs will then be given by Equation (4).
The Marcus Equation (5) originally developed to model electron transfer reactions [73,74,75] has been found to apply also to methyl transfer (SN2) reactions in the gas phase [15,231].
Δ‡G = (ΔrG°)2/(16Δ‡Go) + Δ‡Go + 0.5ΔrG°
In this theory, ΔrG°, Δ‡G and Δ‡Go are the free energy of reaction, activation free energy, and “intrinsic” free energy of activation, respectively. This treatment parallels the observations of Brønsted made in the 1920s who found that strongly exothermic acid- and base-catalyzed reactions have low activation energy [232]. It does not differ significantly from the Bell-Evans-Polanyi equation for activation enthalpies Δ‡H = αΔrH° + β [228,229,233] which considers that the energy barrier of an one-step reaction depends on the exothermicity (the more exothermic, the faster the reaction; Dimroth principle [234]), on an “intrinsic” energy barrier β which relates to the type of reaction and reactants (steric factors, solvation, desolvation, bond deformation) and the polarizability of the reactants. In the gas phase, a naked anion Y:− and a neutral electrophile RX equilibrate without activation barrier with complex [Y:···R–X]−. The substrate RX solvates the anionic nucleophile. A subsequent bond reorganization gives another complex [Y–R···X:]− with an energy barrier that depends on the exothermicity of the reaction and the polarizability of the reactants. Polarizability depends above all on the ionization energy (IE) of Y:− (the easier it can lose an electron, the more nucleophilic it is) and on the electron affinity (-EA) of RX (the easier RX can accept an electron the more stabilized is the corresponding radical-anion RX•−, the more electrophilic is RX, the faster is the nucleophilic substitution). Shaik and Pross, and others, have described the SN2 reaction in terms of valence bond theory [235,236,237,238]. This led to the transition state model (Figure 12) for the SN2 reactions of type (c) in Scheme 35 [239,240,241,242].
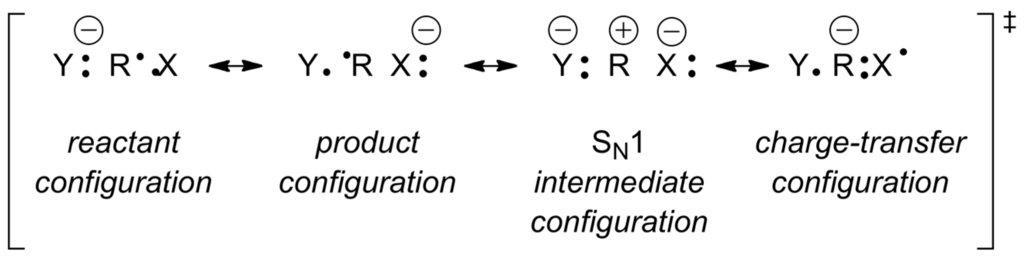
Figure 12.
An SN2 transition state model for the reactions of type c in Scheme 35.
With this model one predicts that any additive that stabilizes one or several of the above configurations will be a catalyst of the SN2 reaction. One stabilizes the reactant configuration by dissociating the nucleophile Y:− to form its counter-cation. One accelerates the reaction by an additive that can bind to the leaving (nucleofugal) group X: by increasing the exothermicity of the reaction and by increasing the electron affinity of R–X. Electrophilic agents that can interact with R (form a complex) that increases the electron affinity of R–X (stabilizing the charge configuration) will catalyze the reaction.
4.2. Pulling on the Leaving Group
The electron affinity of substrates R–X can be enhanced by coordination to an electrophilic catalyst, such as a metallic cation M'+. For it to act as a catalyst of the SN2 reaction, the binding energy M–Y must be lower than that in M′–X [243]. This is the case with soluble Ti+ and Ag+ salts (e.g., AgNO3, AgClO4, AgOSO2Cl) in MeCN and alkyl halides as reactants (Scheme 37) [244,245].
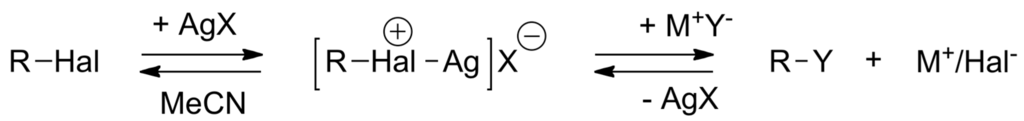
Scheme 37.
Use of Ag+ salts in the catalysis of SN2 reactions.
In the case of SN2 reactions of sulfonic esters, the counter-cation M+ of M+/Y:– can assist the departure of the nucleofugal group (sulfonate anion) by coordinating with the S=O groups [246,247]. Alternatively, the displacement of a sulfonic ester can be activated through hydrogen-donors making strong hydrogen bonds with the S=O moieties. Similarly, a sulfone is an electrophilic reagent when the departure of the nucleofugal group (sulfinate anion) is activated by hydrogen bridging of the SO2 moiety. Applying this feature, an asymmetric Mannich-type reaction of aromatic α-amido sulfones with malonate using guanidine–thiourea bifunctional organocatalysts 67 has been proposed (Scheme 38) [248].
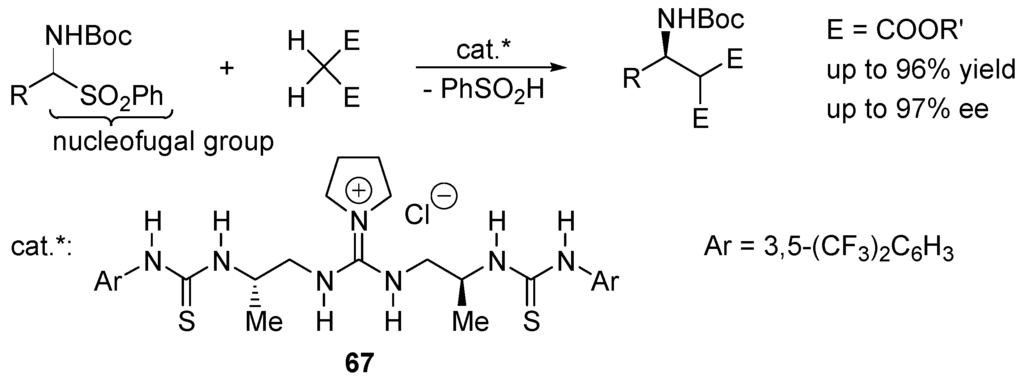
Scheme 38.
Asymmetric Mannich-type reaction catalyzed by guanidine–thiourea 67.
4.3. Phase Transfer Catalysis
In solution, a large extent of the energy barrier of SN2 reaction of type (c) in Scheme 35 implies dissociation of M+/Y:− and de-solvation of the anionic nucleophile Y:−. It is thus obvious that such a reaction will be faster in an aprotic, polar solvent such as MeCN, DMSO, DMF, N-methylpyrrolidone or sulfolane, than in a protic polar solvent such as H2O, alcohols, AcOH, and CF3COOH. The latter solvents stabilize the nucleophile by specific solvation, by hydrogen-bridging, thus making it less nucleophilic. A “naked” anionic nucleophile Y:− will be much more reactive than a nucleophile dressed up by the solvent. The nature of the counter ion M+ is very important for the rate of the substitution reaction. The larger it is, the better it dissociates from Y:− and allows for equilibrium with free ion pairs to form. When Y:− is strongly associated with M+ (formation of tight ion-pair), it is less reactive as nucleophile than as free ion, completely dissociated from M+. Any additive to the reaction mixture that takes away M+ and leaves Y:− “naked” will catalyze SN2 displacement reactions of type (c) in Scheme 35. In some solvent Y:− is more “naked” (less solvated) than in others. Non-polar, aprotic solvents such as hydrocarbons (pentane, toluene, chlorobenzene) should be chosen. Alternatively, the organic phase can be the reactant RX itself. However, in most cases salts M+/Y:− are not soluble in such a medium. The salts dissolve better in water. Thus, under such conditions we have a two-phase system (R-X in the organic phase, or realizing the organic phase, and MY a solid phase, or as an aqueous, non-miscible solution). In order to bring the anionic nucleophile Y:− in contact with the electrophilic reagent R-X it must be transported into the organic phase. As pioneered by Makosza [249,250,251,252], this can be done using a phase-transfer catalyst (PTC) [253,254,255,256] that is a large ammonium or phosphonium ion (with large alkyl groups making them soluble in the organic phase) [243,244,245,246,247,248,249,250,251,252,253,254,255,256,257], or by encapsulation (formation of stable complexes or crytaplexes) of M+ with a suitable crown ether [244,245,246] or cryptand. For example, the displacement reaction of 1-chlorooctane with aqueous NaCN is accelerated many thousand fold by the addition of hexadecyltributylphosphonium bromide (68) as a phase-transfer catalyst (Scheme 39). The high rate of displacement is mainly due to the high lipophilicity of the phosphonium salt Q+/CN− and the large ionic radius of Q+, which reduces the electrostatic interaction between Q+ and CN− and thus leaves the anionic nucleophile dissociated. The use of enantiomerically pure ammonium or phosphonium salts as phase-transfer catalysts allow enantioselective C–C bond formation [247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268], in particular for the industrial synthesis of amino acids [269].
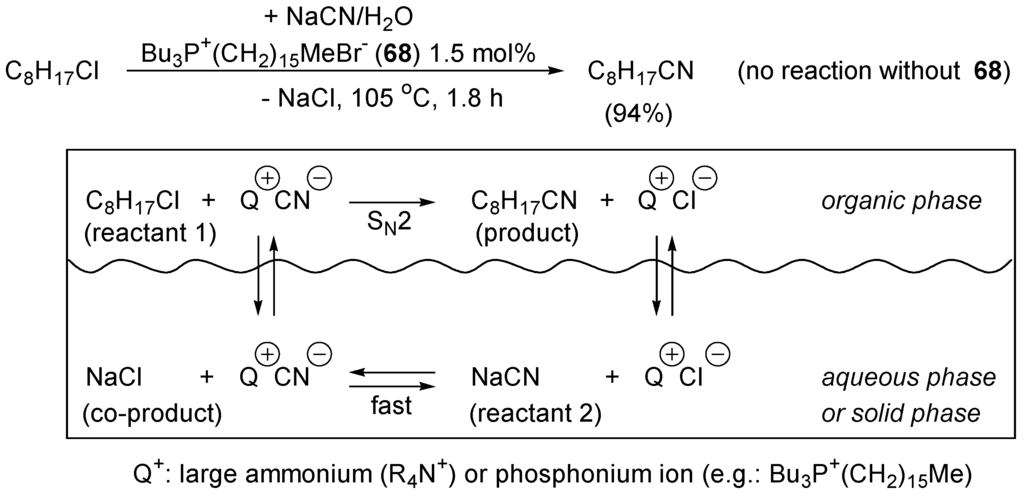
Scheme 39.
Example of phase-transfer catalysis (PTC).
Nicolas and Lacour [270] have prepared the triazatriangulenium cations 70 from trityl salt 69 and shown that they can be used as phase-transfer catalysts for several organic reactions, thanks to their intrinsic stability in strongly basic and nucleophilic conditions (pKR+ > 20) (Scheme 40).
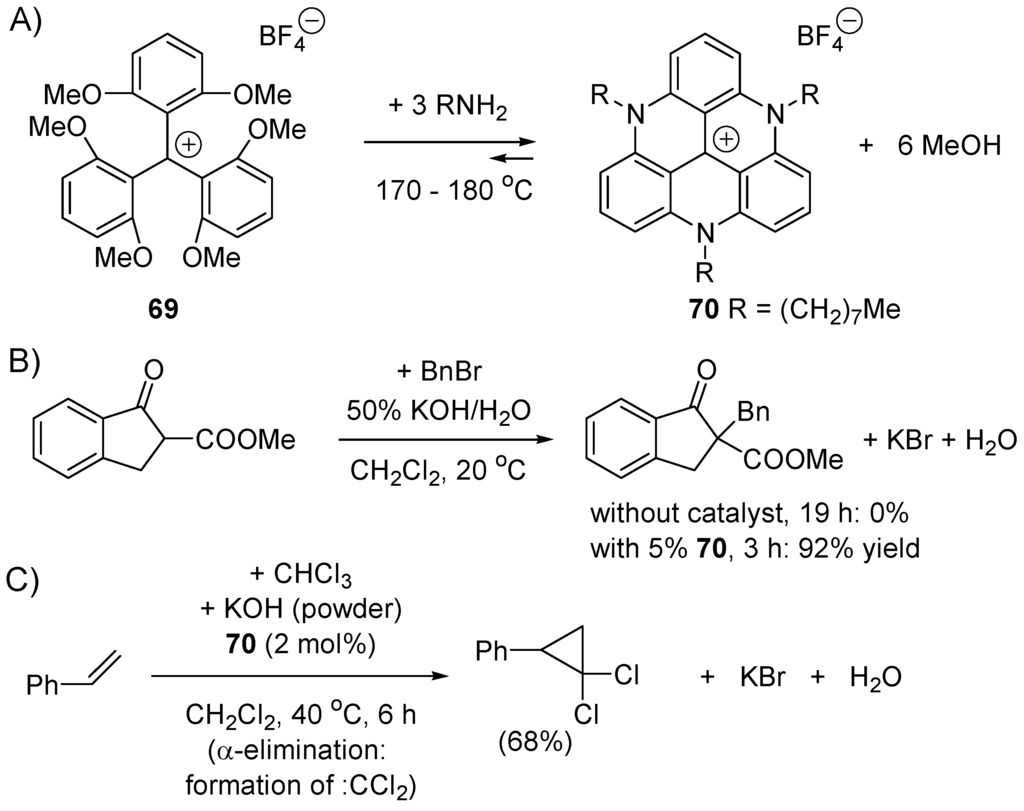
Scheme 40.
Lacour’s transfer catalyst (trityl cation) (A) preparation; (B) examples of nucleophilic displacement; (C) example of 1,1-elimination and cyclopropanation.
Crown ethers and cryptands make stable complexes with metal ions M+, and thus increase the dissociation of MY into ions pairs and provide highly reactive, unsolvated (“naked”) anions Y:− [271,272,273,274]. The nature of the crown ether or cryptand can be chosen to fit M+ the best and for an optimal catalytic effect. For instance, 12-crown-4 binds Li+ but not K+ whereas 18-crown-6 binds K+ not Li+ [275]. Crown ethers having aliphatic chains, e.g., dicyclohexyl-18-crown-6 (71), can be used as phase-transfer catalysts in anion-promoted two-phase reactions [276,277]. An example of such processes is shown in Scheme 41. n-Octyl methanesulfonate is converted to n-C8H17–I with an aqueous solution of NaI containing 5% equivalent of 71. Crown ether 71 also catalyzes the oxidation of 1-octene in benzene (insoluble in H2O) with an aqueous solution of KMnO4 into n-heptanoic acid. Similarly alkyl-substituted aza-macrobicyclic polyethers such as 72 are highly efficient catalysts for SN2 displacement reactions of type (c) in Scheme 35 forming C–C or C–heteroatom bonds [278]. Crown ethers derived from monosaccharides also allow asymmetric phase transfer reactions [279,280,281,282].
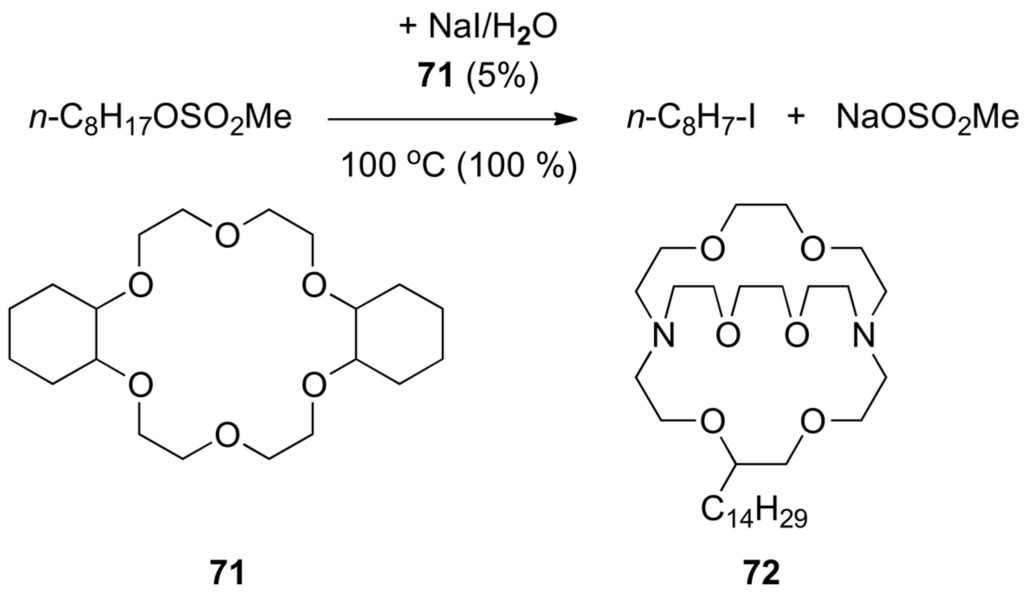
Scheme 41.
Catalysis of SN2 reactions by crown ethers.
Organofluorine compounds are very important in material sciences, in crop protection and in medicine [283,284,285,286]. One of the most economically viable methods for their preparation is nucleophilic fluorination of alkyl halides or sulfonate esters using alkaline fluorides. Because the fluoride anion makes strong hydrogen bonds and is a strong base in the gas phase [287,288,289,290,291,292,293,294,295,296,297,298,299] elimination through E2H mechanism competes with the desired SN2 displacement for reactions in polar aprotic solvent or in ionic liquids [290,291,292]. Liotta and Harris [293] have reported the use the of 18-crown-6 ether for solubilizing KF in acetonitrile and benzene solutions. For benzene solution at 90 °C, 2-bromooctane reacts to give 32% of 2-fluorooctane and 68% of octenes. With 1-bromooctane the proportion of products of elimination is reduced to 8%. Cox and co-workers [294] found that Bu4NF containing 0.1–0.3 equivalents of H2O reacts at 25 °C with 2-bromooctane without solvent giving a 1:9 mixture of 2-fluorooctane and alkenes. For the reaction of 1-bromooctane under the same conditions the product of SN2 substitution, 1-fluorooctane, is formed in 48% yield, the other products being 1-octene and 1-octanol (see also: [295]). Competition between the SN2 and E2H processes can be affected by temperature, solvent and the amount of water present in the reaction mixture. Although water makes stable clusters F−(H2O)n with much less reactivity than naked F− [296] the proportion of SN2 versus E2H product might increase [297,298]. Transition metal-free procedures for the Sonogashira reaction have been performed in water as solvent, polyethyleneglycol (PEG) as a phase-transfer agent, and NaOH as a base (Scheme 42). The reaction is a SNAr (addition/elimination) process that requires microwave heating [299].
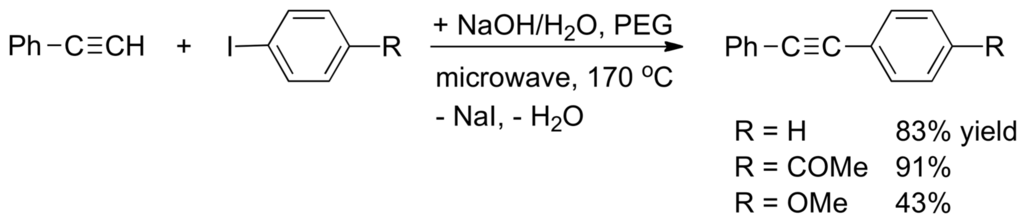
Scheme 42.
Transition-metal free Sonogashira reaction.
By using insoluble phase-transfer catalysts that are fixed to either on insoluble resin [300] or an inorganic support the problem of waste can be resolved as the catalyst can be recovered by simple filtration after the reaction. Unfortunately, most of these insoluble catalysts are less efficient than the conventionally used soluble phase-transfer catalysts. Fluorous tetraalkylphosphonium iodides catalyze the synthesis of propylene carbonate in supercritical CO2 [301] and recyclable fluorous chiral ammonium salts [302] have been used to promote the asymmetric alkylation of a protected glycine derivative. The N,N'-dialkyl-4,13-diaza-18-crown-6 lariat ethers 73 and 74 with polyfluorinated side-arms are efficient phase-transfer catalysts under solid-liquid conditions. In the case of the reaction shown in Scheme 43 and using 73, the catalyst has been recycled several times by fluorous solid-phase extraction without loss in activity [303].
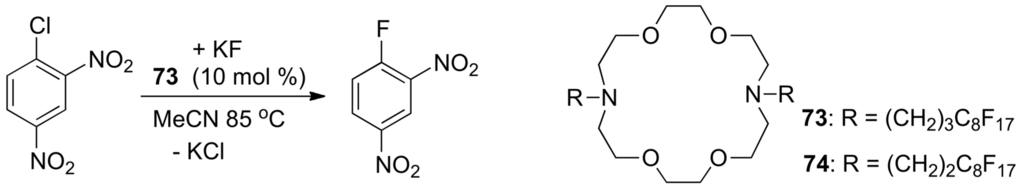
Scheme 43.
Catalysis by fluorous lariat crown ethers.
4.4. Asymmetric Ion-Pairing Catalysis
When chiral ammonium ions, phosphonium ions, or crown ethers are used for enantioselective reactions, the nucleophilic reagents are associated with chiral cationic counter-ions (asymmetric ion-pairing catalysis) [304]. Asymmetric electrophilic reactions have been realized in which the electrophile is associated with a chiral anion responsible for the phase transfer catalyzed process (chiral anion phase-transfer (CAPT) catalysis) [305,306,307]. An example is given with the enantioselective electrophilic fluorination of alkenes developed by Toste and co-workers (Scheme 44) [308,309]. Chiral anion transfer of arenediazonium cations has permitted the enantioselective synthesis of C(3)-diazenated pyrroloindolines in a similar way [310].
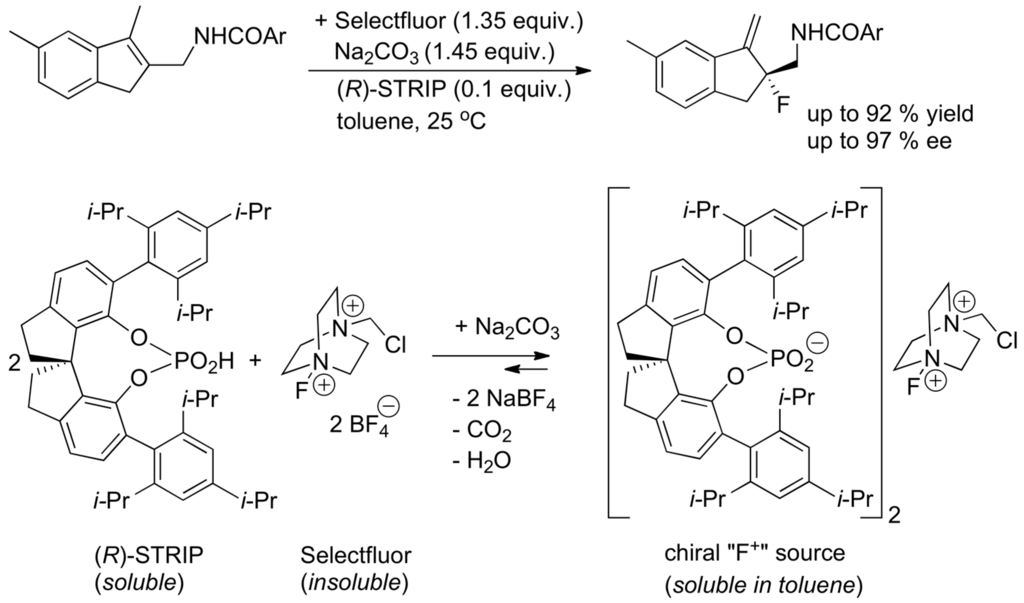
Scheme 44.
Asymmetric electrophilic fluorination of alkenes under chiral anion phase-transfer catalysis.
5. Catalytic Umpolung C–C Bond Forming Reactions
When an alkyl halide, which has an electrophilic carbon center, is converted to a polar alkylmetal species, a nucleophilic carbon reagent is realized (Scheme 45A). One speaks of Umpolung (inversion of polarity) of the carbon center. Unsaturated compounds such as aldehydes, alkenes and alkynes conjugated with aldehyde, ketone, ester, carbonitrile, carboxamide, sulfonyl, phosphonyl moieties are electrophiles undergoing 1,2 or/and 1,4-additions with nucleophiles. They can be converted to nucleophilic carbon reagents upon suitable modifications. A well-known case is the conversion of aldehydes into dithianes (Scheme 45B) that can be metallated by hydrogen/metal exchange to generate nucleophilic carbon reagents that are acyl anion equivalents [311]. Alternatively, an electron-poor (electrophilic) π-system can be converted to a nucleophile by addition of a nucleophilic species or by capture of an electron (SET). Similarly, an electron-rich (nucleophilic) π-system can be converted to an electrophilic reagent by protonation, by addition of an electrophile (Scheme 45C), or by oxidation (SET) into a radical-cation. In this chapter we shall be concerned by C–C bond forming cross-coupling reactions involving catalytical nucleophilic Umpolung. The other possible modes of catalytic Umpolung (e.g., via SET) will not be not treated here.
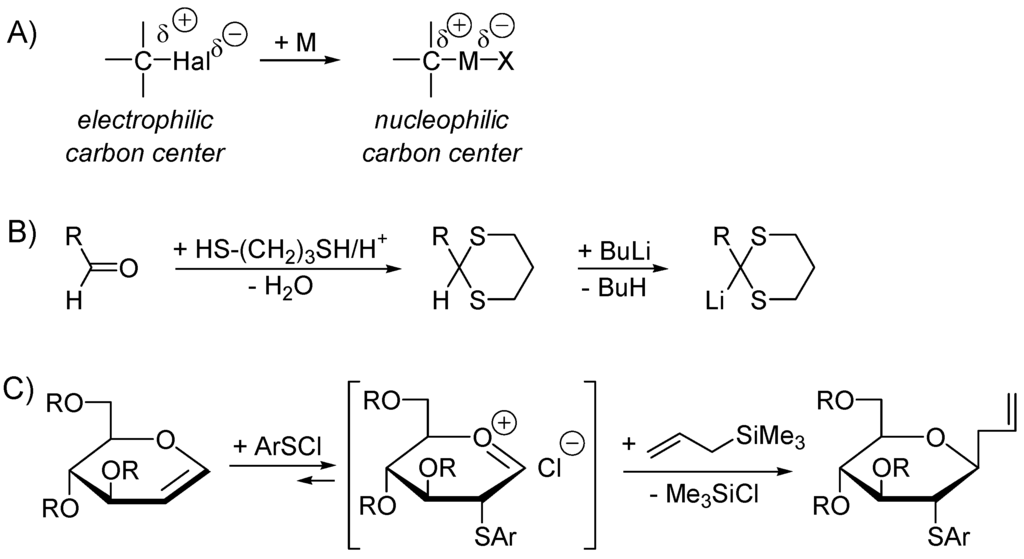
Scheme 45.
Examples of stoichiometric Umpolung. (A) Halogen/metal exchange; (B) metallation of dithianes derivatives of aldehydes [311]; (C) Smoliakova’s C-glycosidation using glycals [312,313].
5.1. Benzoin Condensation: Umpolung of Aldehydes
In 1832, Wöhler and Liebig discovered the so-called benzoin condensation catalyzed by cyanide anion (Scheme 46) [314]. In this reaction, one aldehyde molecule does the hydrocarbation of another aldehyde molecule. In 1903, Lapworth proposed the mechanism given below for this reaction [315] (see however [316]).
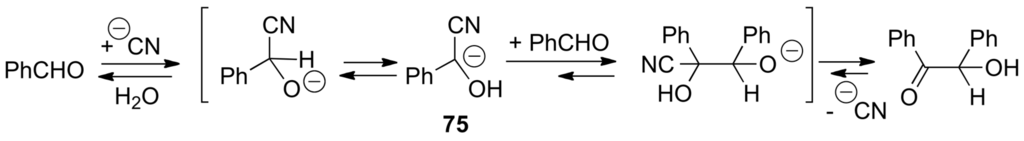
Scheme 46.
The benzoin condensation discovered by Wöhler and Liebig.
An intermediate carbanion 75 represents an active aldehyde with inverted reactivity of the carbonyl carbon atom (i.e., nucleophilic) [317]. In 1943, Ukai and co-workers found that thiazolium salts could be used as catalysts for the benzoin condensation as well as cyanide ion [318]. A few years later, Mizuhara and co-workers described the catalytic activity of natural thiamine (76) which, in the form of its pyrophosphate (77), is the coenzyme for a number of important biochemical reactions, including decarboxylation of pyruvate into acetaldehyde, the transketolase-catalyzed reaction and the oxidative decarboxylation of pyruvic acid into active acetate [319]. In 1958, Breslow presented the mechanism shown in Scheme 47 for the thiazolium ion-catalyzed benzoin condensation. Deuterium exchange studies showed the easy deprotonation of thiazolium ions 78 to form the corresponding zwitterions 65′ (α-elimination). They can be seen as thiazol-2-ylidenes 79 that are relatively stable carbenes with a strong σ-nucleophilic character. Intermediate carbenes 79 are the catalytically active species [320,321].
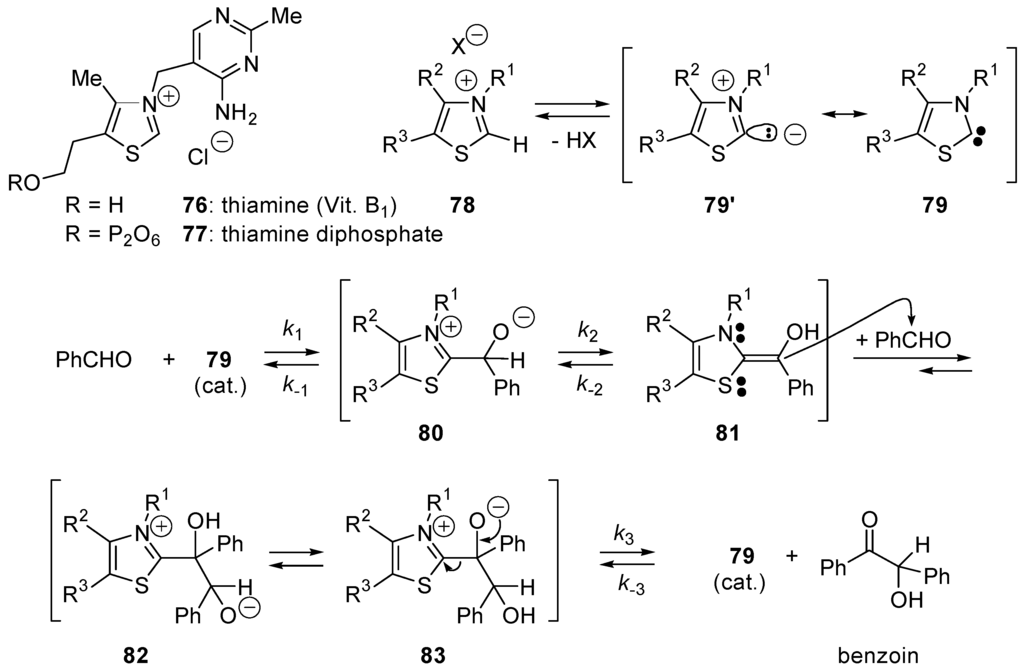
Scheme 47.
The thiazolium ion-catalyzed benzoin condensation: the Breslow's mechanism involving thiazol-2-ylidenes 79.
They add to the carbonyl group of benzaldehyde giving adducts 80 that equilibrate (probably by protonation/deprotonation) with the electron-rich alkenes 81. The latter adds onto benzaldehyde giving zwitterions 82 that equilibrate with 83. Finally, β-elimination furnishes benzoin and regenerates the catalyst 79. In the case of benzoin condensation catalyzed by 3-benzyl-5-(2-hydroxyethyl)-4-methylthiazolium bromide in MeOH buffered with Et3N/Et3NH+Cl−, White and Leeper found that all three steps k1, k2 and k3 are partially rate-determining. A normal kinetic deuterium isotope effect for the overall reaction kH/kD ≅ 3.4 is observed using PhCDO, and a large inverse solvent isotope effect kD/kH ≅ 5.9 is observed using CD3OD, consistent with the mechanism shown in Scheme 47 [321,322,323]. Thiazol-2-ylidenes 79, 1,3,5-triphenyl-1,3,4-triazol-2-ylidene (20) also catalyze the formoin condensation converting formaldehyde into glycoaldehyde (84) (Scheme 48) [324,325]. This reaction is the first step of the formose reaction discovered in 1861 by Butlerow [326] and which generates mixtures of racemic aldoses and ketoses (monosaccharides) by oligomerization of formaldehyde in the presence of Ca(OH)2. When carried out in 8:1 dimethylformamide (DMF)/water with Et3N and thiamine hydrochloride (catalyst) at 75 °C for 1 h, DL-2-C-hydroxymethyl-3-pentulose (85) is obtained in 28% yield (Scheme 48) [327,328].
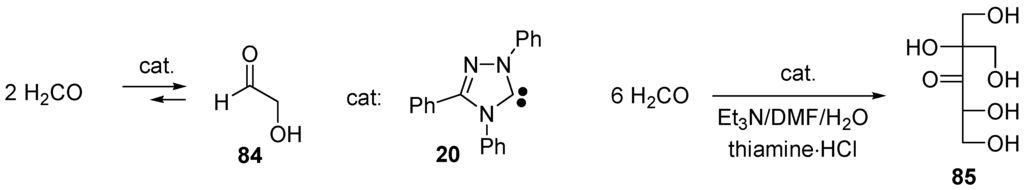
Scheme 48.
The formose reaction and the oligomerization of formaldehyde catalyzed by NHC 20.
In the presence of secondary amines such as piperidine and morpholine, which combine with benzaldehyde to give iminium ion intermediates, modest yields of α-aminoketones (PhCOCH(NR2)Ph) have been obtained in boiling EtOH in the presence of 3-methylthiazolium tetrafluoroborate and Et3N [329]. However, aromatic aldehydes or benzoins react with unactivated imines in the presence of NHCs affording α-aminoketones in good yields [330]. Benzoin condensations have been catalyzed by diaminocarbenes resulting from the Wanzlick equilibrium [331] and by NHC obtained by α-elimination of benzimidazonium salts [332]. NaCN-promotes the intramolecular cyclization of dialdimines in DMF, MeOH or CH2Cl2/H2O (phase-transfer conditions) yielding various heterocyclic systems [333]. On attaching thiazolium salts to carbon center C(6) of a d-glucose unit of γ-cyclodextrin (cyclomaltooctaose: cycloamylose containing eight α-d-glucopyronose units), as in 86 (Figure 13), Breslow and Kool realized artificial enzymes that catalyze the benzoin condensation of benzaldehyde more efficiently than the corresponding free thiazolium ions. This is explained by invoking that two benzaldehyde molecules can reside simultaneously in the hydrophobic interior of γ-cyclodextrin. Analogues of 86 made of β-cyclodextrin (cyclomaltoheptaose) do not catalyze the benzoin condensation as well [334,335].
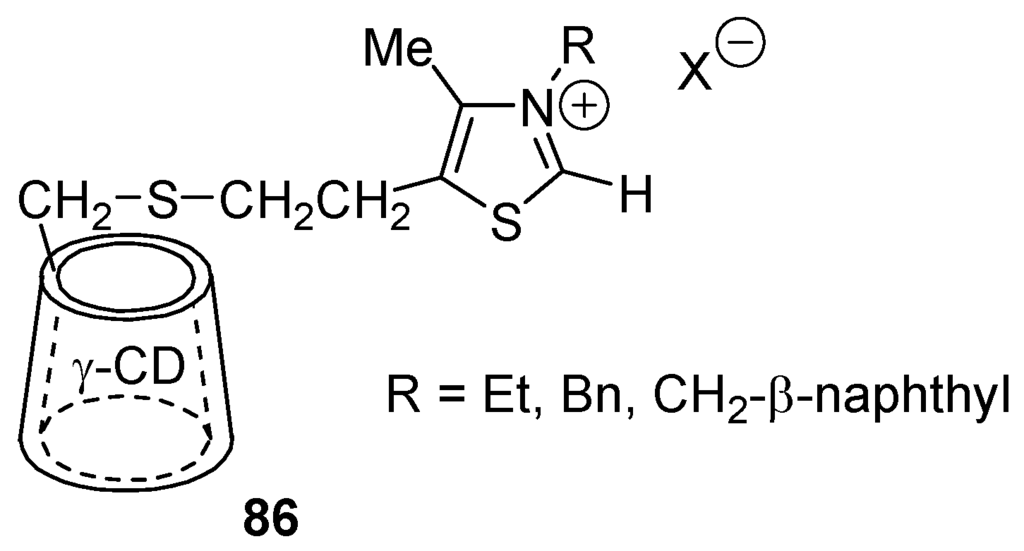
Figure 13.
Structure of 86.
The α-hydroxycarbonyl compounds are valuable synthetic intermediates. Cross-condensation of two different aldehydes through the benzoin reaction represents the most direct route to these systems. Since all of the steps in the benzoin condensation are reversible, selectivity for the product of cross-coupling depends on their relative stability with respect to the products of homocoupling. In most cases it remains small, thus selectivity in the product of cross-coupling can be obtained only by using an excess of one of the two aldehydes. Using cyanide-catalyzed benzoin reaction involving mixtures of acylsilanes 87 and aldehydes 90, Johnson and co-workers found conditions under which high yields of products of cross-coupling 94 are obtained. Using La(CN)3 as catalyst, the scope of the cross silyl benzoin addition can be extended to aryl, heteroaryl, alkyl and α,β-unsaturated aldehydes [336]. The proposed mechanism (Scheme 49) implies a cyanide-promoted [1,2]-Brook rearrangement 88 → 89 [337,338]. Acyl phosphonates also undergo cyanide-catalyzed additions to aldehydes [339]. A strategy utilizing N-heterocyclic carbenes (NHCs) derived from thiazolium salts has been developed for the generation of carbonyl anions from acylsilanes [340].
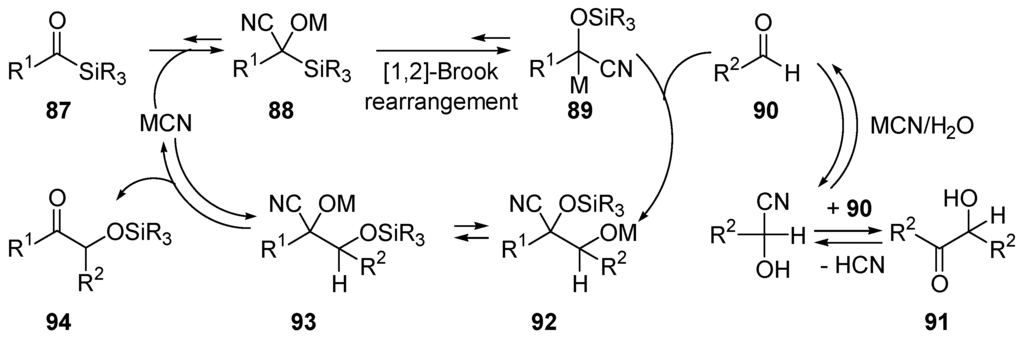
Scheme 49.
Proposed mechanism for the cyanide-catalyzed cross-silyl benzoin addition. In this case (MCN = La(CN)3) homo-coupling 90 → 91 is slower than cross-coupling 87 + 90 → 94.
Enantioselective benzoin syntheses have been realized using enantiomerically pure, chiral N-heterocyclic carbenes as catalysts [118,341]. An example is given in Scheme 50A. Enantioselective cross-benzoin reactions of aliphatic aldehydes and α-ketoesters (Scheme 50B) and aza-benzoin reactions of enals with activated ketimines (Scheme 50C) have also been realized. In these reactions α-eliminations of HBF4 from the pre-catalysts 95, 96 and 97 generate the corresponding N-heterocyclic carbenes that are chiral nucleophiles. They add to the carbonyl groups as cyanide anion or thiazole-2-ilydenes (carbenes of Breslow), leading to the Umpolung of the aldehydes.
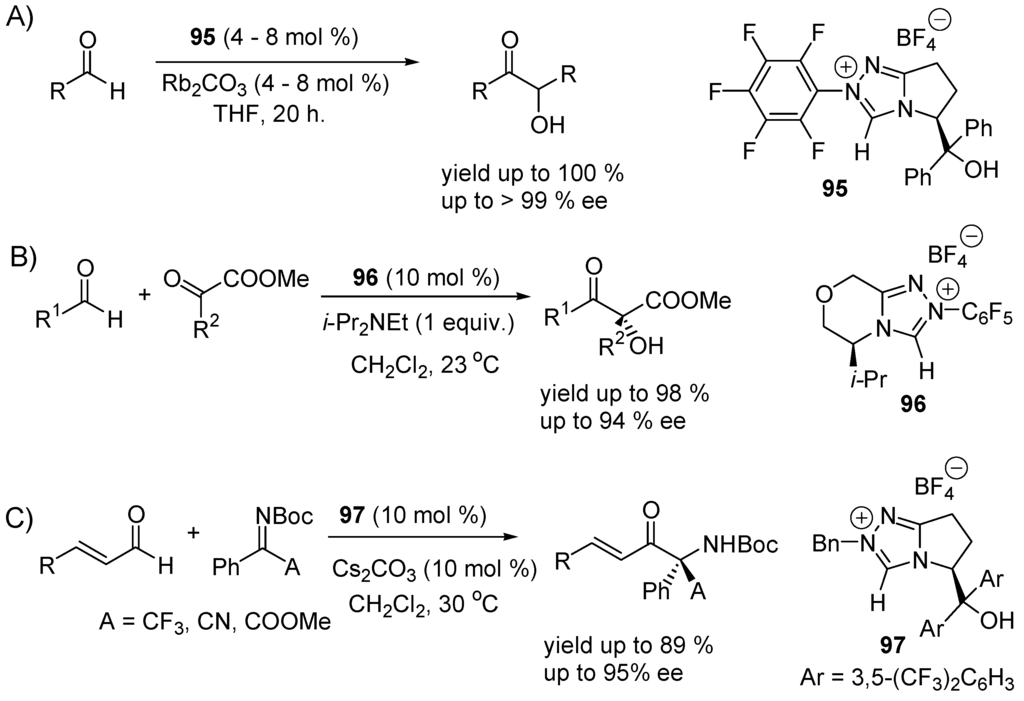
Scheme 50.
Examples of enantioselective, intermolecular benzoin condensations. (A) Homobenzoin reaction; (B) cross-benzoin reaction; (C) aza-benzoin reaction.
5.2. The Stetter Reaction: Umpolung of Aldehyde
Since its discovery in 1973 [342], the Stetter reaction has become one of the most important Umpolung procedures for the synthesis of 1,4-dicarbonyl compounds (Scheme 51) [343,344]. In the presence of Et3N, thiazolium salts of type 78 generate the corresponding electron-rich carbenes 79 that react with aldehydes to generate enol intermediates 81 that, in turn, add to α,β-unsaturated carbonyl compounds 98 in a 1,4-fashion producing the final 1,4-dicarbonyl compounds 99.
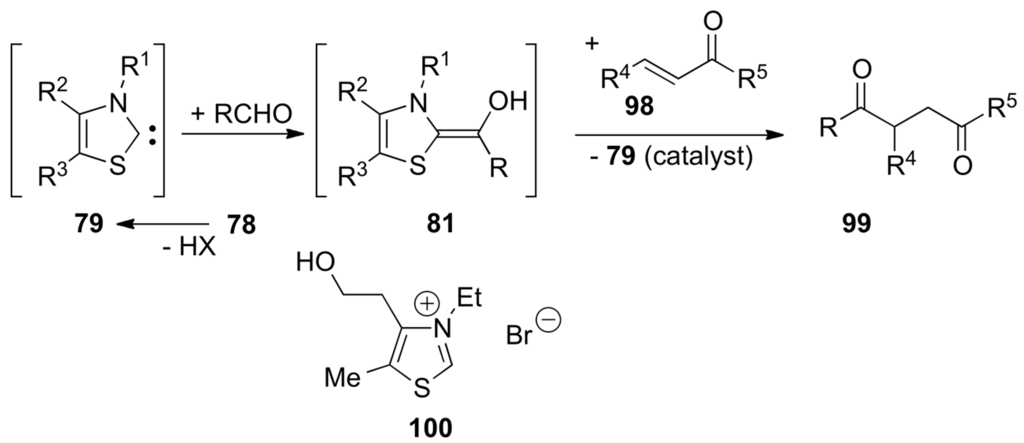
Scheme 51.
The Stetter reaction.
Because aldehydes RCHO can also react with intermediates 81 and give products of benzoin homo-coupling, 81 must be more reactive toward 98 than RCHO for successful intermolecular Stetter reactions. Thus, intramolecular Stetter reactions have found a faster development than intermolecular Stetter reactions [345,346,347], including asymmetric versions [348,349,350]. Nevertheless, using acylsilanes 87 (Scheme 16) and enones of type 98 in the presence of DBU (base), THF (solvent) and thiazolium salt 100 (catalyst), good yields are obtained for the intermolecular Stetter reaction [351]. An example of catalytic enantioselective intermolecular Stetter reaction is given in Scheme 52 [352,353].
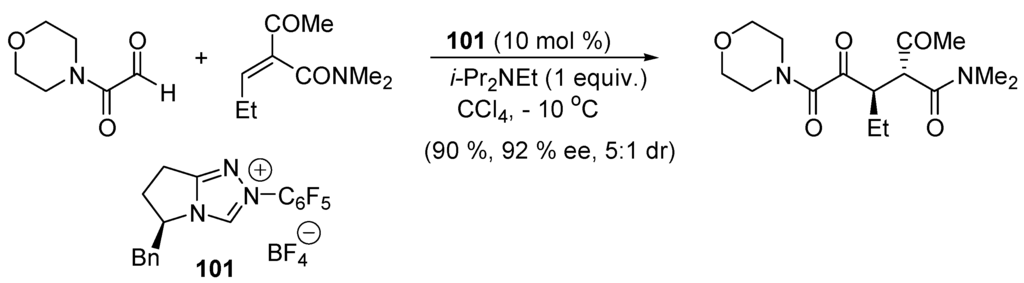
Scheme 52.
An enantioselective intermolecular Stetter reaction.
5.3. Umpolung of Enals
In 2004, the groups of Glorius [354] and Bode [355] reported the first examples of NHC-catalyzed annulations of enals and aldehydes into lactones. Combining α,β-unsaturated aldehydes 102 with 2,2,2-trifluoro-1-phenylethanone (103) in the presence of 5 mol·% of (IMes·HCl) and 10 mol % t-BuOK in THF, a mixture of cis/trans γ-butyrolactones 107 was obtained (Scheme 53). In this case, the aldehyde reacts with the diaminocarbene (IMes: Herrmann’s diaminocarbene [356]) generated in situ giving the nucleophilic dienol 104, which can be seen as a homoenolate. It adds to ketone 103 producing intermediates 105 ⇄ 106 that generate product 107 and liberate the catalyst (IMes). Enantioselective lactonization of cinnamaldehyde with PhCOCF3 has been realized by applying a chiral diaminocarbene catalyst [357].
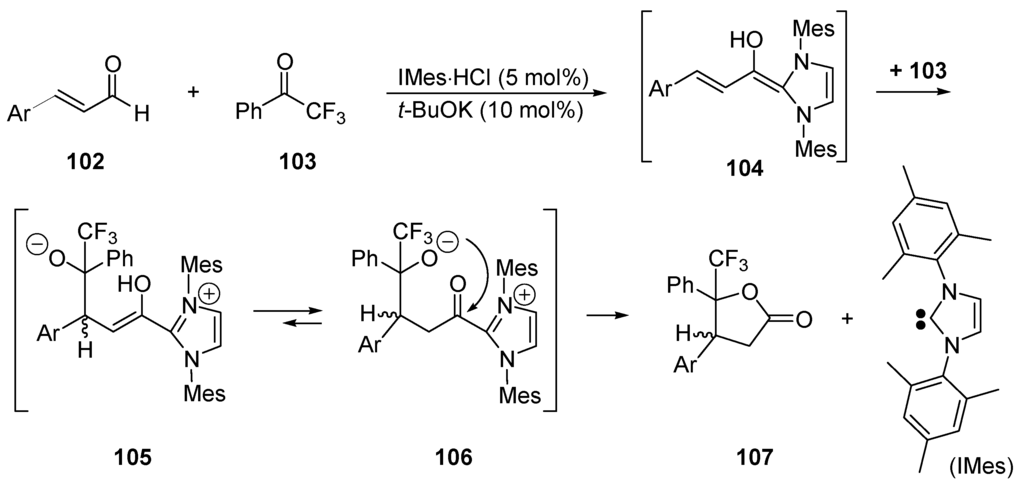
Scheme 53.
Synthesis of γ-butyrolactones by Umpolung of an enal with a diaminocarbene catalyst (IMes).
Nair and co-workers [358] uncovered a IMes-catalyzed cross-condensation of enals and enones producing 1,3,4-trisubstituted cyclopentenes (Scheme 54A). The diaminocarbene does the Umpolung of the enal into the corresponding homoenol which undergoes conjugate addition to the enone. The intermediate is cyclized into the corresponding β-lactones 108 that undergo (2+2)-cycloreversions with liberation of CO2. When the enones are replaced by α,β-unsaturated imines the corresponding lactams 109 form, as shown first by Bode and He. Using a chiral diaminocarbene precursor such as 110, catalytic enantioselective lactamizations have been realized (Scheme 54B) [359].
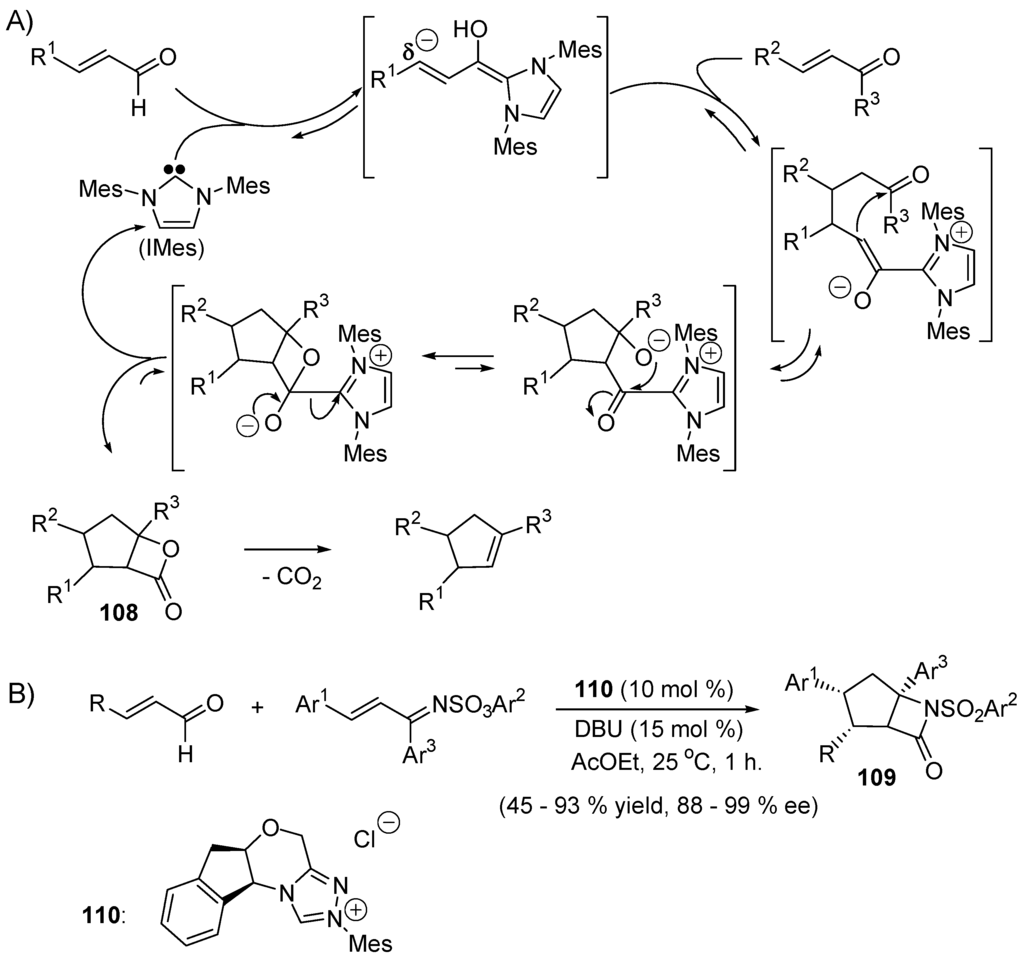
Scheme 54.
(A) Nair’s synthesis of 1,3,4-trisubstituted cyclopentenes; (B) Bode’s asymmetric synthesis of β-lactams.
5.4. Umpolung of Michael Acceptors
Huisgen and co-workers showed that dimethyl azodicarboxylate adds to triphenylphosphine forming zwitterion 111 (E = COOMe). The latter can be trapped with 1,2-diketones as illustrated in Scheme 55 [360].
Scheme 55.
Reactions of azodicarboxylates with triphenylphosphine and subsequent trapping with benzyl.
In this process, the electrophilic nitrogen center of the azo compound becomes nucleophilic after addition of the phosphine catalyst giving zwitterion 111 that adds to electrophilic carbonyl moieties. In 1973, White and Baizer at Monsanto reported a phosphine-catalyzed Michael addition of 2-nitropropane to ethyl acrylate, acrylonitrile and methyl vinyl ketone (CH2=CHCOOMe + Me2CHNO2 → Me2C(NO2)CH2CH2COOMe) The low basicity of the catalysts, Bu3P, Ph3P, PhMe2P and Ph2MeP, suggested a mechanism in which the phosphines behave as nucleophiles rather than as bases. In this hypothesis, zwitterionic intermediates of type R3P(+)CH2CH(−)COOMe3 are formed that deprotonate the carbon acid (Me2CHNO2) generating the corresponding conjugate base Me2C(−)NO2. The latter adds to the activated alkenes [361]. The reaction has been extended to other carbon acids than 2-nitropropane [362,363]. In 1992, Trost and Kazmaier [364] reported that Ph3P catalyzes the isomerization of conjugated ynones, ynoates and ynecarboxamides (113) into conjugated dienes 121 (Scheme 56).
Scheme 56.
Isomerization of conjugated alkynes into conjugated dienes.
The proposed mechanism implies the formation of carbanionic intermediates (e.g., 114 ⇄ 115; 117 ⇄ 118 ↔ 119 ⇄ 120) resulting from protonation and deprotonation of zwitterionic phosphonium intermediates [99]. Methyl but-2-ynoate (122) adds to Ph3P generating zwitterion 123. In the presence of carbon acids such as malonic esters and analogues with pKa < 16, proton transfer occurs giving ion pair 124 (AcOH/AcONa). Collapse of ion pair 124 produces intermediate 125 that isomerizes into its tautomer 126. β-Elimination of Ph3P furnishes product of cross-coupling 127. This can be viewed as Umpolung of the γ-position of activated alkyne 122 from nucleophilic to electrophilic because of its acidity, as demonstrated by Trost and Li in 1994 (Scheme 57) [365].
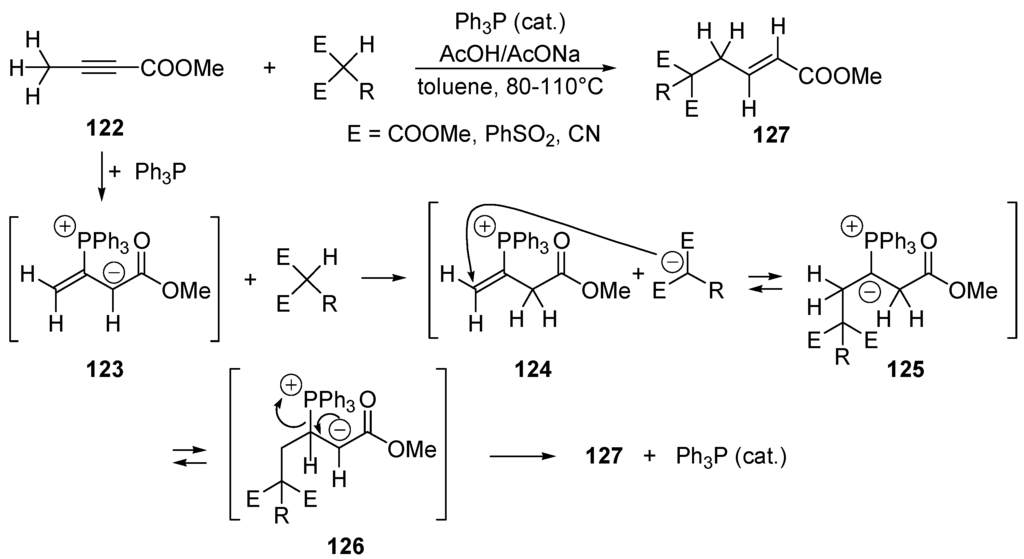
Scheme 57.
Trost’s Umpolung γ-alkylation of an ynoate by triphenylphosphine.
Oxygen nucleophiles (alcohols [366], carboxylates [367]) and acidic nitrogen nucleophiles such as p-toluenesulfonamides have also been utilized as Umpolung catalysts in the nucleophilic γ-additions of conjugated yne-ketones and yne-esters [368]. Fu and co-workers [369] have optimized the carbene-catalyzed mode of β-alkylation of enolates, as shown in Scheme 58. Ph3P has been used as nucleophilic catalyst for the Umpolung addition of azoles to electron-deficient allenes [370].
Scheme 58.
Umpolung of Michael acceptors catalyzed by NHCs.
5.5. The Rauhut–Currier Reaction
In 1963, Rauhut and Currier reported phosphine-catalyzed dimerizations of acrylonitrile and acrylic esters (Scheme 59A) [371]. These transformations were then investigated by McClure [372] and Baizer and Anderesen [373]. They are believed to involve reversible phosphine conjugate additions to electron-poor alkenes giving zwitterions 128, followed by Michael additions of the intermediate enolates to a second alkene moiety producing 129. A prototropic shift gives zwitterion 130. Finally, elimination forms the dimeric compound 131. The Rauhut–Currier reaction is a hydrocarbation of an activated alkene with the α-C–H moiety of an activated alkene. Small trialkylphosphines are superior to amine nucleophiles such as DABCO, DBU, Et2NH and DMAP (4-Me2N-pyridine) [374,375,376]. The Rauhut–Currier reaction applied to allene esters and 2-phenylethylene-1,1-dicarbonitrile generates cyclohexene derivatives. For instance, the reaction of alleneoate 132 and electron-poor alkene 133 in the presence of P(NMe2)3 catalyst produces 98% yield of 134, whereas in the presence of (4-ClC6H4)3P as catalyst leads to 92% yield of isomeric cyclohexene 135 (Scheme 59B) [377]. Related to the Rauhut–Currier reaction is the formal (4+1)-cycloaddition of sulfur (S8) with two equivalents of acetylenedicarboxylic esters producing tetraalkyl thiophene-2,3,4,5-tetracarboxylate, a reaction catalyzed by nucleophilic bases such as triethyl phosphite, isoquinoline, pyridine, and N-methylimidazole [378]. An example of catalytic enantioselective intermolecular cross-Rauhut–Currier reaction is given in Scheme 59C [379].
Scheme 59.
The addition of phosphine to Michael acceptors engenders nucleophilic intermediates. (A) The Rauhut–Currier dimerization of acrylic derivatives; (B) proposed mechanisms for the phosphine-catalyzed (4+2)-annulations of alleneoate and electron-poor alkenes; (C) example of catalytic enantioslective cross-Rauhut–Currier reaction.
5.6. The Morita–Baylis–Hillman Reaction
The Morita–Baylis–Hillman (MBH) reaction is an atom-economical coupling reaction of activated alkenes 136 and aldehydes 137 in the presence of a nucleophilic catalyst (Scheme 60A). It corresponds to the hydrocarbation of the aldehyde with the α-C–H bond of the activated alkene. The reaction involves formally a sequence of Michael addition, aldol reaction, and β-elimination. The reaction was first reported by Morita and co-workers in 1968 [380] and in 1972 by Baylis and Hillman [381]. The nucleophilic catalyst can be phosphines or tertiary amines such as DABCO [382,383]. Activated alkenes include acrylic esters, acrylonitriles, vinyl ketones, phenyl vinyl sulfones, phenyl vinyl sulfonates, vinyl phosphates and acrolein. β-Substituted alkenes require more forcing conditions due to the slow addition of the nucleophilic catalyst. A variety of electrophiles can be used such as aldehydes, α-ketoesters, 1,2-diketones, aldimines, methyl α-bromoesters [384,385] and arenes [386].
Scheme 60.
The Morita–Baylis–Hillman (MBH) reaction: (A) an intermolecular version; (B) an intramolecular version.
The generally accepted mechanism of the MBH reaction involves three steps: (1) nucleophilic additions to the activated alkene 136 generating a zwitterionic 139 that adds to aldehyde 137 (or other electrophiles) to give zwitterion 140; (2) a protonation and deprotonation step 140 → 141 → 142; and (3) β-elimination with recovery of the nucleophilic catalyst (Scheme 60A). DFT calculations reported by Aggarwal and Harvey for a reaction in aprotic solvents suggest that deprotonation of the α-position 140 → 142 is the rate-determining step and occurs though a cyclic transition state 141, with proton transfer to a hemiacetal alkoxide formed by addition of a second molecule of aldehyde to intermediate 140. This mechanism is supported by the observation of second-order kinetics with respect to RCHO concentration in the absence of protic solvent. In contrast, in MeOH, Aggarwal and Harvey proposed that the alcohol serves as a shuttle to transfer the proton from carbon to oxygen (140 → 142) [387]. However, this proposal was refuted by Singleton and Prata, who favored a simple, albeit computationally intractable, acid–base mechanism on the basis of a combination of experimental mechanistic probes [388]. In the case of the intramolecular MBH alkylation of 143 → 146, intermediate 145 has been isolated and characterized (Scheme 60B) [389]. The original MBH reaction of Scheme 23 represents a hydrocarbation of an aldehyde by the α-C-H moiety of an activated alkenes. Reaction 143 → 146 corresponds to a δ-elimination of HBr involving the α-C-H moiety of the conjugated enone 143, HBr being neutralized by KOH/H2O buffered by BnEt3N+Cl−. Treatment of acetates 148 (resulting from the MBH reactions 147 + Ph3P) with α-silyloxyfuran leads to an efficient and stereoselective synthesis of γ-butenolides 150 (Scheme 61) [390].
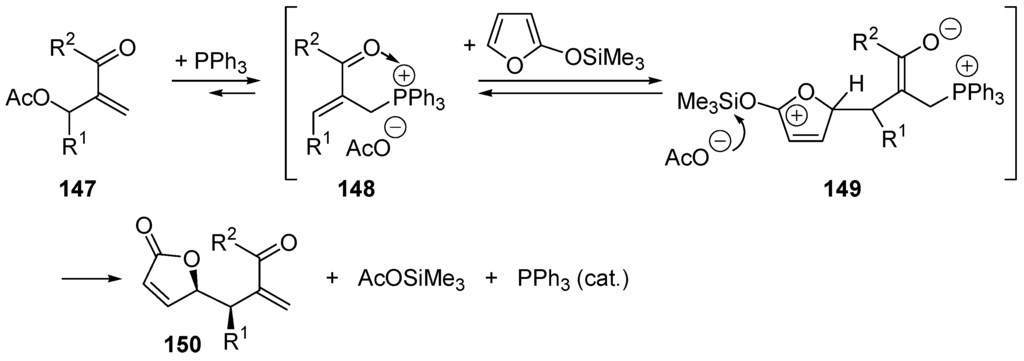
Scheme 61.
Stereoselective synthesis of γ-butenolides 150.
Much effort have been devoted to the development of catalytic enantioselective MBH reactions [391,392,393,394]. As shown in Figure 14, the catalyst can be a chiral Lewis base (e.g., 151 [395], 152 [396]), a chiral tertiary phosphine (e.g., 153 [397,398], 154 [399]), a chiral Lewis acid (e.g., 155 [217,400]) or a chiral Brønsted acid (e.g., 156) [401]. An example of aza-Morita–Baylis–Hillman (aza-MBH) reaction is given in Scheme 62, which applies the P-chirogenic organocatalyst 157 [402].
Figure 14.
Chiral catalysts for enantioselective Morita–Baylis–Hillman reactions.
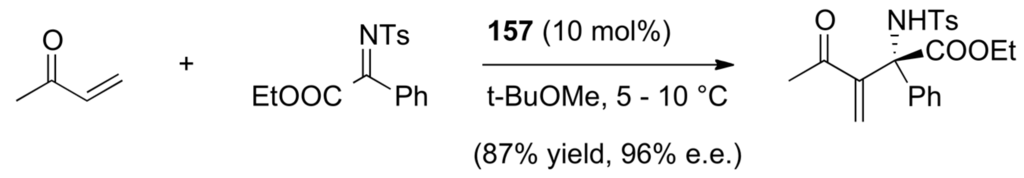
Scheme 62.
An enantioselective aza-Morita–Baylis–Hillman reaction.
5.7. Nucleophilic Catalysis of Cycloadditions
Mixing 2-alkyl-2,3-butadienoates 158 with N-tosylimines 159 in the presence of 20 mol % Bu3P results in the diastereoselective formation of tetrahydropyridines 160. The butadienoates 158 act as 1,4-dipole synthons (and not as 1,3-dipole synthons like 97), giving products of (4+2)-cycloaddition 160 (Scheme 63). A γ-addition to imine 159 by the zwitterionic phosphonium adduct 161 gives 162. The two successive proton-transfer steps giving 163 sets up for an N-tosyl Michael addition to close the six-membered ring [403].
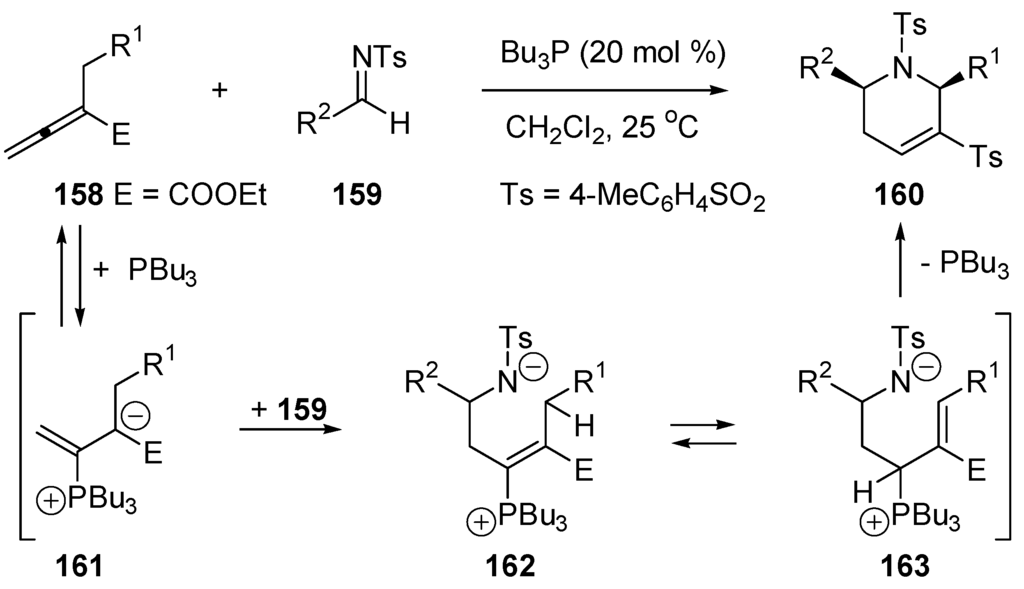
Scheme 63.
A phosphine-catalyzed (4+2)-annulation.
l-Proline catalyses the enantioselective aza-Diels–Alder reaction of arylimines with conjugated enones as illustrated in Scheme 64. l-Proline and the enone equilibrate with the corresponding 2-aminodiene 164 that adds to the imine equilibrating with the arylamine and formaldehyde. The reaction is assumed to involve the formation of the zwitterionic intermediate 165 that cyclizes quickly and irreversibly into the corresponding Diels–Alder cycloadduct [404]. For other examples of catalyzed enantioselective aza-Diels–Alder reactions, see [405,406,407,408,409].
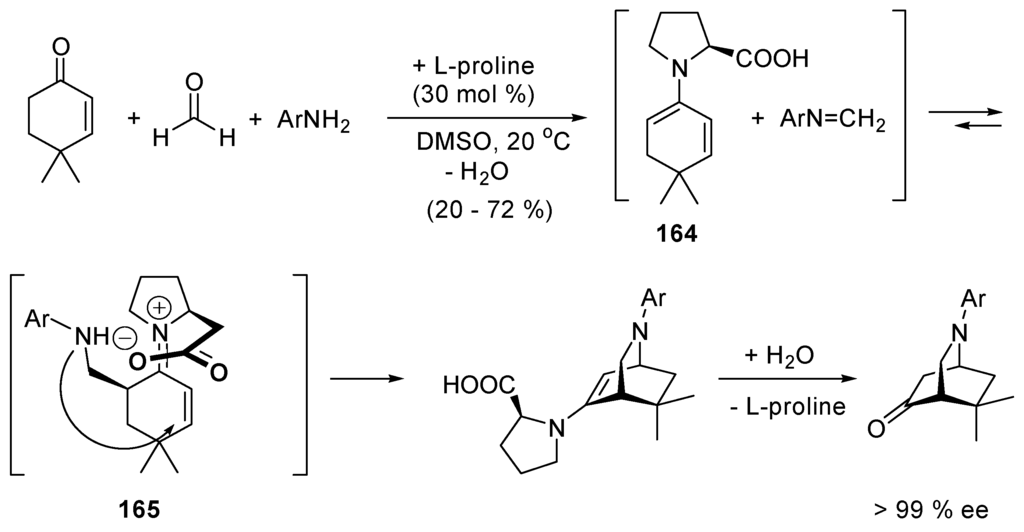
Scheme 64.
Enantioselective aza-Diels–Alder reaction catalyzed by l-proline.
In 2006, Bode and co-workers reported the stereoselective (4+2)-hetero-Diels–Alder reaction proceeding via a NHC-bound azolium enolate intermediate 168 derived from ethyl 4-oxobut-2-enoate and enantiomerically pure diaminocarbene 166 (Scheme 65) [410]. Enolate 168 is the dienophile that adds to the azadiene giving zwitterion 169. The latter undergoes facile β-elimination providing the cycloadduct 167 and the starting catalyst (166). Yields up to 90% with 97%–99% ee were obtained. In the meatime, more examples of nucleophilic catalyzed (4+2)-cycloadditions have been reported [411,412].
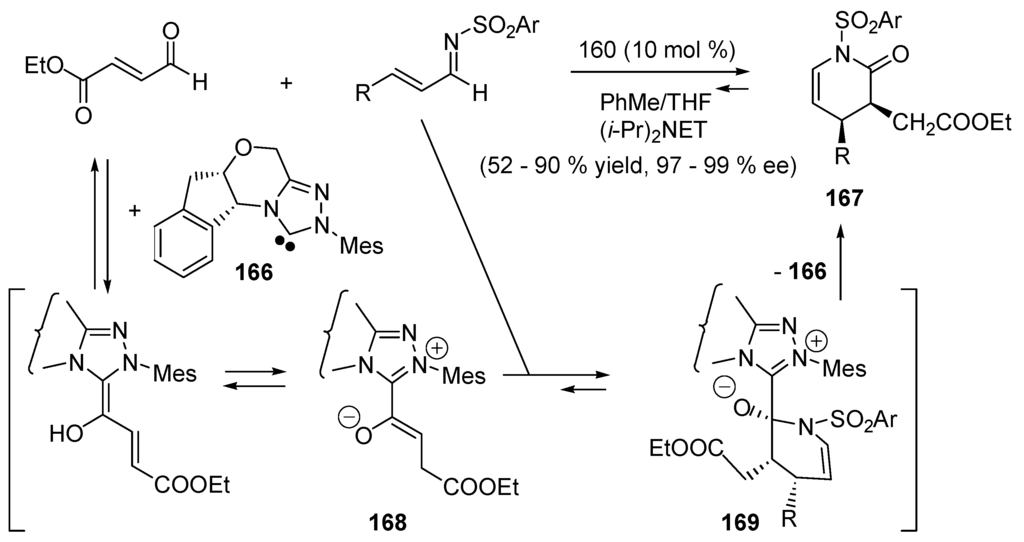
Scheme 65.
Bode’s enantioselective hetero-Diels–Alder reaction.
Ketenes react with tertiary amines giving enolates of type 170 that react as nucleophiles onto electrophilic imines giving zwitterion intermediates 171, which then undergo intramolecular displacement reactions to produce the corresponding β-lactams, liberating the nucleophilic catalyst. The ketene-enolates 170 can also be formed by reaction of the tertiary amine with acyl chlorides (Scheme 66A). When an enantiomerically pure tertiary amine is used, it can induce asymmetry in the formation of β-lactam [413]. Instead of a tertiary amine, one can employ an enantiomerically pure chiral N-heterocyclic carbene as the nucleophilic catalyst (Scheme 66B) [414].
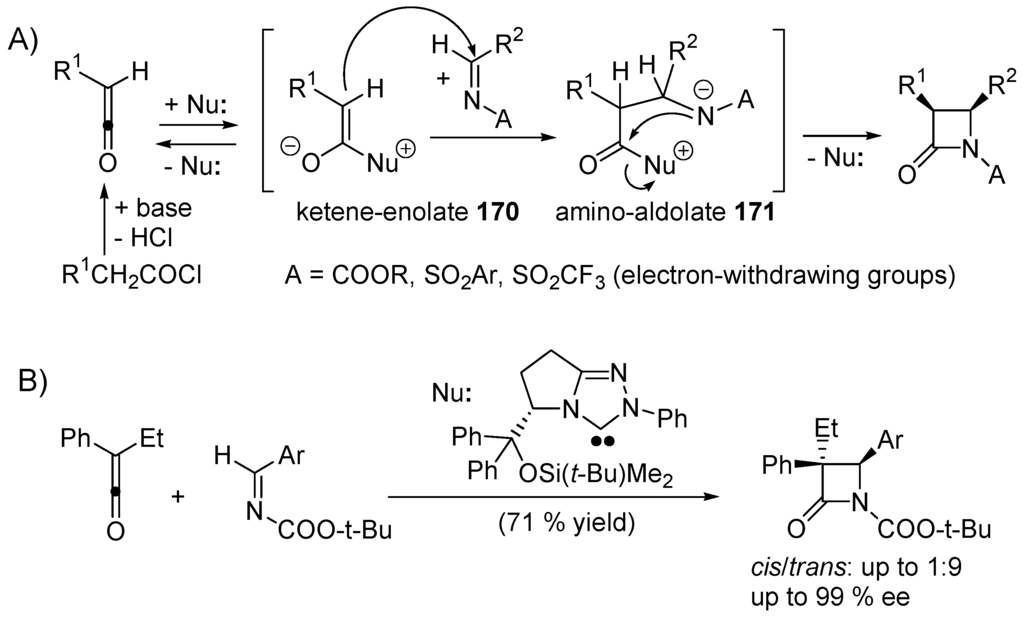
Scheme 66.
(A) Nucleophile catalyzed (2+2)-cycloaddition; (B) Asymmetric synthesis of β-lactams via enantiomerically pure ketene-enolate intermediates (NHC-catalyzed Staudinger reaction).
The allylic anions of type 161 (Scheme 63) resulting from the nucleophilic addition of triphenylphosphine to conjugated yne-esters, or allene carboxylic esters, can be reacted with activated alkenes to give products of (3+2)-annulation (Scheme 67) [415,416,417]. The (3+2)-cycloaddition of 172 gives ylide intermediates 173. Protonation/deprotonation gives zwitterions 174 that eliminate triphenylphosphine (catalyst) producing the corresponding cyclopentenes 175.
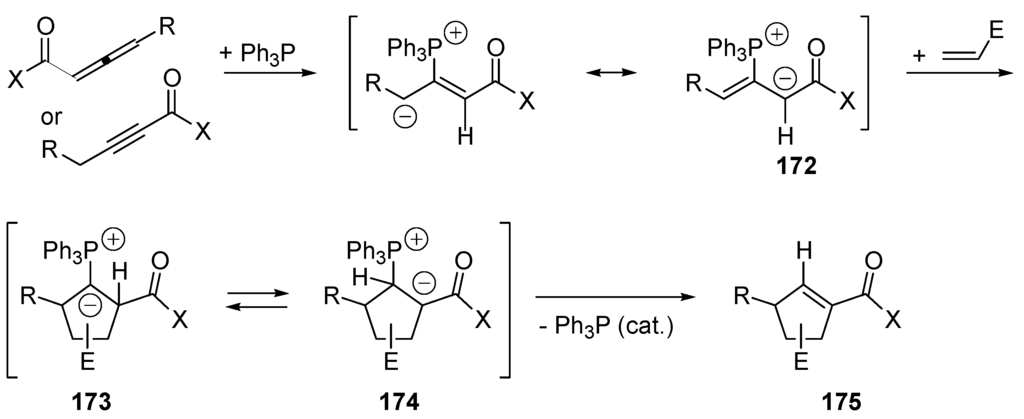
Scheme 67.
Phosphine-catalyzed (3+2)-annulation.
In 2010, Ye and co-workers reported a formal (3+2)-cycloaddition of ketenes 176 to oxaziridine 177 giving the corresponding oxyzolin-4-ones 179. Using the diaminocarbene precursor 178 they obtained cycloadducts with yields varying from 38%–78%, with high diastereoselectivity (dr up to 15:1) and enantioselectivity (up to 95%). The proposed reaction mechanism is shown in Scheme 68 [418].
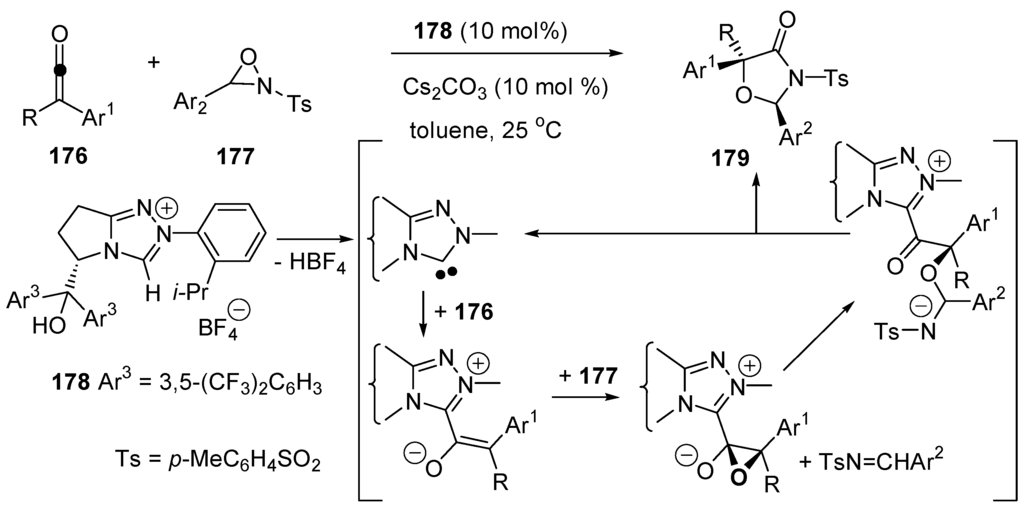
Scheme 68.
NHC-catalyzed (3+2)-cycloaddition of ketenes and oxariridines.
6. Conclusions
For a long time, catalysts were “magical” additives that make chemical reactions to be accelerated. In ca. 4 billion years, Nature generated life on Earth and during that time invented enzymes that are very efficient catalysts for specialized reactions necessary for living species. In 1746, the lead chamber for sulfuric acid production was invented. In the 1800s, acids and bases were recognized to catalyze many organic reactions. Over the last century, the study of reaction mechanisms has made the design of catalysts a molecular science in itself, especially when homogenous catalysis is concerned. Heterogenous and homogenous catalysts made of transition metal salts or complexes have permitted miracles for chemists and the chemical industry. Because of limiting resources on Earth and the necessity to protect our environment, more sustainable processes have to be developed and this is associated with the search for catalysts that are less costly and less toxic than transition metals. Nature utilizes organic molecules and non-polluting metallic cations. In less than 100 years, chemists have learned to do almost the same, even for reactions Nature does not utilize. Moreover, typical organic catalysts are relatively small molecules (not like enzymes); they are relatively cheap, less toxic, and environmentally benign. This overview illustrates the chemical evolution that has started from a knowledge of the reaction mechanisms to the invention of homogenous molecular catalysts for the most important organic reactions. Importantly, enantioselective catalysis is now possible for these reactions and can often be applied to a large variety of substrates and reagents. Thus, man-made molecular catalysts are less limited than enzymes and can be used in a much wider range of reaction conditions (solvent, acidity) and temperature than enzymes. We have chosen to discuss acyl transfers, the nucleophilic additions and substitutions that represent the most important organic reactions. The best catalysts are multifunctional compounds that interact with both the nucleophilic reagent and the electrophilic partner to make them more reactive. They have an electrophilic (e.g., hydrogen donor, oxyphilic cation) and a nucleophilic function (O-, N-, C- (carbene), P-nucleophile).
Acknowledgments
We thank the Swiss Institute of Technology in Lausanne (EPFL), the European Health HP7 Panacreas program, and the University of California, Los Angeles for financial support. We thank also Annelis Carrupt (EPFL) for her help in typesetting and drawing.
Author Contributions
P.V. was the primary author of this review. Y.-h.L. supplied additional references. Y.-h.L. and A.S. provided assistance in typesetting and revision of this review in response to referees' comments. K.N.H. edited this review.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Cp | cyclopentadienyl |
| DCC | N,N'-dicyclohexycarbodiimide |
| DABCO | 1,4-diazabicyclo[2.2.2]octane |
| DBU | 1,8-diazabicyclo[5.4.0]undec-7-ene |
| DMF | dimethylformamide |
| DHP | 3,4-dihydro-2H-pyrimido[2,1-b]benzothiazole |
| DMAO | 4-dimethylaminopyridine |
| MBH | Morita–Baylis–Hillman reaction |
| MIBA | 4-methoxy-2-iodobenzeneboronic acid |
| NHC | N-heterocyclic carbene |
| THF | tetrahydrofuran |
| OTf | triflates |
| OTs | p-toluenesulfonate |
| PTC | phase transfer catalyst |
| PYP | 4-pyrrolidinopyridine |
| pyr | pyridine |
| SET | single electron transfer |
| TBD | triazabicyclo[4.4.0]dec-5-ene |
| THTP | 2,3,6,7-tetrahydro-5H-thiazolo[3,2-a]pyrimidine |
| TON | turnover number |
| TOF | turnover frequency |
References
- Haber, J. Catalysis—Where Science and Industry Meet. Pure Appl. Chem. 1994, 66, 1597–1620. [Google Scholar] [CrossRef]
- Hagen, J. Industrial Catalysis: A Practical Approach, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; pp. 1–507. [Google Scholar]
- Llyod, L. Handbook of Industrial Catalysts. In Fundamental & Applied Catalysis; Springer: New York, NY, USA, 2011; pp. 1–490. [Google Scholar]
- Dalko, P.I.; Moisan, L. In the golden age of organocatalysis. Angew. Chem. Int. Ed. 2004, 43, 5138–5175. [Google Scholar] [CrossRef] [PubMed]
- List, B. Introduction: Organocatalysis. Chem. Rev. 2007, 107, 5413–5415. [Google Scholar] [CrossRef]
- MacMillan, D.W.C. The advent and development of organocatalysis. Nature 2008, 455, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, S.; Jørgensen, K.A. Organocatalysis-after the gold rush. Chem. Soc. Rev. 2009, 38, 2178–2189. [Google Scholar] [CrossRef] [PubMed]
- List, B. Emil Knoevenagel and the Roots of Aminocatalysis. Angew. Chem. Int. Ed. 2010, 49, 1730–1734. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, L.; Jiang, H.; Jørgensen, K.A. A Simple Recipe for Sophisticated Cocktails: Organocatalytic One-Pot Reactions-Concept, Nomenclature, and Future Perspectives. Angew. Chem. Int. Ed. 2011, 50, 8492–8509. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.F. Total synthesis of natural and pharmaceutical products powered by organocatalytic reactions. Tetrahedron Lett. 2015, 56, 2133–2140. [Google Scholar] [CrossRef]
- Berzelius, J. Jahres-Bericht über Fortschritte der Physischen Wissenschaften; Laupp: Tübingen, Germany, 1836; Volume 15. [Google Scholar]
- Robertson, A.J.B. The Early History of Catalysis. Platinum Met. Rev. 1975, 19, 64–69. [Google Scholar]
- Roberts, M. M. Birth of the catalytic concept (1800–1900). Catal. Lett. 2000, 67, 1–4. [Google Scholar] [CrossRef]
- Ahrendt, K.A.; Borths, C.J.; MacMillan, D.W.C. New strategies for organic catalysis: The first highly enantioselective organocatalytic Diels–Alder reaction. J. Am. Chem. Soc. 2000, 122, 4243–4244. [Google Scholar] [CrossRef]
- Garrett, B.C. Ions at the air/water interface. Science 2004, 303, 1146–1147. [Google Scholar] [CrossRef] [PubMed]
- Tait, M.J.; Franks, F. Water in Biological Systems. Nature 1971, 230, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Chabinyc, M.L.; Craig, S.L.; Regan, C.K.; Brauman, J.I. Gas-phase ionic reactions: Dynamics and mechanism of nucleophilic displacements. Science 1998, 279, 1882–1886. [Google Scholar] [CrossRef] [PubMed]
- Aloisio, S.; Francisco, J.S. Radical-water complexes in Earth’s atmosphere. Acc. Chem. Res. 2000, 33, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.W.M.; Ravishankara, A.R. Role of hydrogen-bonded intermediates in the bimolecular reactions of the hydroxyl radical. J. Phys. Chem. A 2002, 106, 4798–4807. [Google Scholar] [CrossRef]
- Hansen, J.C.; Francisco, J.S. Radical-molecule complexes: Changing our perspective on the molecular mechanisms of radical-molecule reactions and their impact on atmospheric chemistry. ChemPhysChem 2002, 3, 833–840. [Google Scholar] [CrossRef]
- Voehringer-Martinez, E.; Hansmann, B.; Hernandez-Soto, H.; Francisco, J.S.; Troe, J.; Abel, B. Water catalysis of a radical-molecule gas-phase reaction. Science 2007, 315, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Otera, J. Transesterification. Chem. Rev. 1993, 93, 1449–1470. [Google Scholar] [CrossRef]
- Otera, J. Esterification: Methods, Reactions and Applications; John Wiley & Sons: Chichester, UK, 2003; p. 450. [Google Scholar]
- Rehberg, C.E.; Fisher, C.H. Preparation and properties of the n-alkyl acrylates. J. Am. Chem. Soc. 1944, 66, 1203–1207. [Google Scholar] [CrossRef]
- Rehberg, C.E. N-Butyl-acrylate. Org. Synth. 1955, 3, 146–147. [Google Scholar]
- Dewolfe, R.H. Synthesis of Carboxylic and Carbonic Ortho Esters. Synthesis 1974, 153–172. [Google Scholar] [CrossRef]
- Rothman, E.S.; Silbert, L.S.; Pfeffer, P.E.; Hecht, S.S. Enol esters 15. Synthesis of highly hindered esters via isopropenyl ester intermediates. J. Org. Chem. 1972, 37, 3551–3552. [Google Scholar] [CrossRef]
- Taft, R.W., Jr.; Newman, M.S.; Verhoek, F.H. The Kinetics of the Base-Catalyzed Methanolysis of Ortho-Substituted, Meta-Substituted and Para-Substituted l-Menthyl Benzoates. J. Am. Chem. Soc. 1950, 72, 4511–4519. [Google Scholar] [CrossRef]
- Billman, J.H.; Smith, W.T.; Rendall, J.L. Anticonvulsants 5. Esters of ɣ-Diethylamino-α-Phenylbutyric Acid. J. Am. Chem. Soc. 1947, 69, 2058–2059. [Google Scholar] [CrossRef] [PubMed]
- Reimer, M.; Downes, H.R. Preparation of esters by direct replacement of alkoxyl groups. J. Am. Chem. Soc. 1921, 43, 945–951. [Google Scholar] [CrossRef]
- Rossi, R.A.; De Rossi, R.H. Preparation of Benzoate Esters of Tertiary Alcohols by Transesterification. J. Org. Chem. 1974, 39, 855–856. [Google Scholar] [CrossRef]
- Frank, R.L.; Davis, H.R.; Drake, S.S.; McPherson, J.B. Ester interchange by means of the Grignard complex. J. Am. Chem. Soc. 1944, 66, 1509–1510. [Google Scholar] [CrossRef]
- Taber, D.F.; Amedio, J.C.; Patel, Y.K. Preparation of Beta-Keto-Esters by 4-DMAP-Catalyzed Ester Exchange. J. Org. Chem. 1985, 50, 3618–3619. [Google Scholar] [CrossRef]
- Mottet, C.; Hamelin, O.; Garavel, G.; Depres, J.P.; Greene, A.E. A simple and efficient preparation of propargylic β-keto esters through transesterification. J. Org. Chem. 1999, 64, 1380–1382. [Google Scholar] [CrossRef]
- Seebach, D.; Thaler, A.; Blaser, D.; Ko, S.Y. Transesterifications with 1,8-Diazabicyclo[5.4.0]Undec-7-Ene/Lithium Bromide (DBU/LiBr)—Also Applicable to Cleavage of Peptides from Resins in Merrifield Syntheses. Helv. Chim. Acta 1991, 74, 1102–1118. [Google Scholar] [CrossRef]
- Yazawa, H.; Tanaka, K.; Kariyone, K. Reaction of Carboxylic Esters with Boron Tribromide—Convenient Method for Synthesis of Amides and Transesterification. Tetrahedron Lett. 1974, 3995–3996. [Google Scholar] [CrossRef]
- Blossey, E.C.; Turner, L.M.; Neckers, D.C. Polymer Protected Reagents 2. Esterifications with P-AlCl3. Tetrahedron Lett. 1973, 1823–1826. [Google Scholar] [CrossRef]
- Otera, J.; Yano, T.; Kawabata, A.; Nozaki, H. Novel Distannoxane-Catalyzed Transesterification and A New Entry to α,β-Unsaturated Carboxylic-Acids. Tetrahedron Lett. 1986, 27, 2383–2386. [Google Scholar] [CrossRef]
- Otera, J.; Dan-oh, N.; Nozaki, H. Unique Template Effects of Distannoxane Catalysts in Transesterification of Diol Esters. Tetrahedron 1993, 49, 3065–3074. [Google Scholar] [CrossRef]
- Pereyre, M.; Colin, G.; Delvigne, J.P. Alkoxytrialkyltin compounds as transesterification reaction catalysts. Bull. Soc. Chim. Fr. 1969, 262–263. [Google Scholar]
- Otera, J.; Dan-oh, N.; Nozaki, H. Novel Template Effects of Distannoxane Catalysts in Highly Efficient Transesterification and Esterification. J. Org. Chem. 1991, 56, 5307–5311. [Google Scholar] [CrossRef]
- Xiang, J.N.; Toyoshima, S.; Orita, A.; Otera, J. A practical and green chemical process: Fluoroalkyldistannoxane-catalyzed biphasic transesterification. Angew. Chem. Int. Ed. 2001, 40, 3670–3672. [Google Scholar] [CrossRef]
- Kunz, H.; Waldmann, H. 1,3-Dithian-2-yl Methyl-Ester as 2-Step Protecting Group for the Carboxy Function in Peptide-Synthesis. Angew. Chem. Int. Ed. Engl. 1983, 22, 62. [Google Scholar] [CrossRef]
- Waldmann, H.; Kunz, H. 1,3-Dithian-2-Ylmethyl Esters as 2-Step Carboxy-Protecting Groups in the Synthesis of N-Glycopeptides. J. Org. Chem. 1988, 53, 4172–4175. [Google Scholar] [CrossRef]
- Seebach, D.; Hungerbühler, E.; Näf, R.; Schnurrenberger, P.; Weidmann, B.; Zuger, M. Titanate-Mediated Transesterifications with Functionalized Substrates. Synthesis 1982, 138–141. [Google Scholar] [CrossRef]
- Imwinkelried, R.; Schiess, M.; Seebach, D. Diisopropyl (2S,3S)-2,3-O-isopropylidenetartrate [1,3-dioxolane-4,5-dicarboxylic acid, 2,2-dimethyl-, bis(1-methylethyl)ester, (4R-trans)-]. Org. Synth. 1987, 65, 230–235. [Google Scholar]
- Ishihara, K.; Ohara, S.; Yamamoto, H. Direct condensation of carboxylic acids with alcohols catalyzed by hafnium(IV) salts. Science 2000, 290, 1140–1142. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Kuo, J.H.; Ku, C.H.; Weng, S.S.; Liu, C.Y. Nucleophilic acyl substitutions of esters with protic nucleophiles mediated by amphoteric, oxotitanium, and vanadyl species. J. Org. Chem. 2005, 70, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Kubota, M.; Yamamoto, T.; Yamamoto, A. Trans-Esterification Reaction Catalyzed by Alkoxo(triphenylphosphine)copper(I) Complexes. Bull. Chem. Soc. Jpn. 1979, 52, 146–150. [Google Scholar] [CrossRef]
- Kim, Y.J.; Osakada, K.; Takenaka, A.; Yamamoto, A. Alkylnickel and Alklpalladium Alkoxides Associated with Alcohols through Hydrogen-Bonding. J. Am. Chem. Soc. 1990, 112, 1096–1104. [Google Scholar] [CrossRef]
- Grasa, G.A.; Kissling, R.M.; Nolan, S.P. N-heterocyclic carbenes as versatile nucleophilic catalysts for transesterification/acylation reactions. Org. Lett. 2002, 4, 3583–3586. [Google Scholar] [CrossRef] [PubMed]
- Nyce, G.W.; Lamboy, J.A.; Connor, E.F.; Waymouth, R.M.; Hedrick, J.L. Expanding the catalytic activity of nucleophilic N-heterocyclic carbenes for transesterification reactions. Org. Lett. 2002, 4, 3587–3590. [Google Scholar] [CrossRef] [PubMed]
- Grasa, G.A.; Gueveli, T.; Singh, R.; Nolan, S.P. Efficient transesterification/acylation reactions mediated by N-heterocyclic carbene catalysts. J. Org. Chem. 2003, 68, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kissling, R.M.; Letellier, M.A.; Nolan, S.P. Transesterification/acylation of secondary alcohols mediated by N-heterocyclic carbene catalysts. J. Org. Chem. 2004, 69, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Kim, C.K.; Li, H.G.; Sohn, C.K.; Kim, C.K.; Lee, H.W.; Lee, B.S. Acyl-transfer mechanisms involving various acyl functional groups: > X-Y with X = C, S, P and Y = O, S. J. Am. Chem. Soc. 2000, 122, 11162–11172. [Google Scholar] [CrossRef]
- Ingold, C.K. Structure and Mechanism in Organic Chemistry; Cornell Univ. Press: Ithaca NY, USA, 1969; p. 1266. [Google Scholar]
- Rao, G.V.; Balakrishnan, M.; Venkatasubramanian, N. E1Cb Pathway of Acyl Transfer. J. Sci. Ind. Res. 1978, 37, 547–557. [Google Scholar]
- Otton, J.; Ratton, S. Investigation of the Formation of Poly(Ethylene-Terephthalate) with Model Molecules—Kinetics and Mechanism of the Catalytic Esterification and Alcoholysis Reactions 1. Carboxylic-Acid Catalysis (Monofunctional Reactants). J. Polym. Sci. Part A Polym. Chem. 1988, 26, 2183–2197. [Google Scholar] [CrossRef]
- Pratt, R.C.; Lohmeijer, B.G.G.; Long, D.A.; Waymouth, R.M.; Hedrick, J.L. Triazabicyclodecene: A simple bifunctional organocatalyst for acyl transfer and ring-opening polymerization of cyclic esters. J. Am. Chem. Soc. 2006, 128, 4556–4557. [Google Scholar] [CrossRef] [PubMed]
- Chuma, A.; Horn, H.W.; Swope, W.C.; Pratt, R.C.; Zhang, L.; Lohmeijer, B.G.G.; Wade, C.G.; Waymouth, R.M.; Hedrick, J.L.; Rice, J.E. The Reaction Mechanism for the Organocatalytic Ring-Opening Polymerization of L-Lactide Using a Guanidine-Based Catalyst: Hydrogen-Bonded or Covalently Bound? J. Am. Chem. Soc. 2008, 130, 6749–6754. [Google Scholar] [CrossRef] [PubMed]
- Simón, L.; Goodman, J.M. The Mechanism of TBD-Catalyzed Ring-Opening Polymerization of Cyclic Esters. J. Org. Chem. 2007, 72, 9656–9662. [Google Scholar] [CrossRef] [PubMed]
- Yatsimirsky, A.K. Metal ion catalysis in acyl and phosphoryl transfer: Transition states as ligands. Coord. Chem. Rev. 2005, 249, 1997–2011. [Google Scholar] [CrossRef]
- Parac-Vogt, T.N.; Deleersnyder, K.; Binnemans, K. Lanthanide(III) tosylates as new acylation catalysts. Eur. J. Org. Chem. 2005, 1810–1815. [Google Scholar] [CrossRef]
- Nakayama, M.; Sato, A.; Ishihara, K.; Yamamoto, H. Water-tolerant and reusable catalysts for direct ester condensation between equimolar amounts of carboxylic acids and alcohols. Adv. Synth. Catal. 2004, 346, 1275–1279. [Google Scholar] [CrossRef]
- Sato, A.; Nakamura, Y.; Maki, T.; Ishihara, K.; Yamamoto, H. Zr(IV)-Fe(III), -Ga(III), and -Sn(IV) binary metal complexes as synergistic and reusable esterification catalysts. Adv. Synth. Catal. 2005, 347, 1337–1340. [Google Scholar] [CrossRef]
- Bayryamov, S.G.; Rangelov, M.A.; Mladjova, A.P.; Yomtova, V.; Petkov, D.D. Unambiguous evidence for efficient chemical catalysis of adenosine ester aminolysis by its 2′/3′-OH. J. Am. Chem. Soc. 2007, 129, 5790–5791. [Google Scholar] [CrossRef] [PubMed]
- Hoydonckx, H.E.; De Vos, D.E.; Chavan, S.A.; Jacobs, P.A. Esterification and transesterification of renewable chemicals. Top. Catal. 2004, 27, 83–96. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Iranpoo, N.; Jafari, A.A. Facile and selective preparation of esters from carboxylic acids catalyzed by aluminum dodecatungstophosphate (AlPW12O40) as a versatile, recyclable and a highly water tolerant green Lewis acid catalyst. Lett. Org. Chem. 2006, 3, 25–28. [Google Scholar] [CrossRef]
- Bose, D.S.; Satyender, A.; Das, A.P.R.; Mereyala, H.B. A facile, catalytic and environmentally benign method for esterification of carboxylic acids and transesterification of carboxylic esters with nearly equimolar amounts of alcohols. Synthesis 2006, 2392–2396. [Google Scholar] [CrossRef]
- Kawabata, T.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Highly efficient esterification of carboxylic acids with alcohols by montmorillonite-enwrapped titanium as a heterogeneous acid catalyst. Tetrahedron Lett. 2003, 44, 9205–9208. [Google Scholar] [CrossRef]
- Bossaert, W.D.; De Vos, D.E.; Van Rhijn, W.M.; Bullen, J.; Grobet, P.J.; Jacobs, P.A. Mesoporous sulfonic acids as selective heterogeneous catalysts for the synthesis of monoglycerides. J. Catal. 1999, 182, 156–164. [Google Scholar] [CrossRef]
- Bertin, J.; Kagan, H.B.; Luche, J.L.; Setton, R. Graphite Electrolytic Lamellar Reagents in Organic-Chemistry-Esterifications in Presence of Graphite Bisulfate. J. Am. Chem. Soc. 1974, 96, 8113–8115. [Google Scholar] [CrossRef]
- Marcus, R.A. Chemical + Electrochemical Electron-Transfer Theory. Ann. Rev. Phys. Chem. 1964, 15, 155–196. [Google Scholar] [CrossRef]
- Marcus, R.A. Electron-Transfer Reactions in Chemistry—Theory and Experiment (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1993, 32, 1111–1121. [Google Scholar] [CrossRef]
- Albery, W.J. The Application of the Marcus Relation to Reactions in Solution. Ann. Rev. Phys. Chem. 1980, 31, 227–263. [Google Scholar] [CrossRef]
- Lin, M.H.; Rajanbabu, T.V. Metal-catalyzed acyl transfer reactions of enol esters: Role of Y5(OiPr13O and (thd)2Y(OiPr) as transesterification catalysts. Org. Lett. 2000, 2, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Litvinenko, L.M.; Kirichenko, A.I. Basicity and Stereospecificity in Nucleophile Catalysis by Tertiary Amines. Dokl. Akad. Nauk SSSR 1967, 176, 97–100. [Google Scholar]
- Steglich, W.; Höfle, G. N,N-Dimethyl-4-Pyridinamine, A Very Effective Acylation Catalyst. Angew. Chem. Int. Ed. Engl. 1969, 8, 981. [Google Scholar] [CrossRef]
- Haslam, E. Recent Developments in Methods for the Esterification and Protection of the Carboxyl Group. Tetrahedron 1980, 36, 2409–2433. [Google Scholar] [CrossRef]
- Hoefle, G.; Steglich, W.; Vorbrüggen, H. 4-Dialkylaminopyridines as Acylation Catalysts 4. 4-Dialkylaminopyridines as Highly Active Acylation Catalysts. Angew. Chem. Int. Ed. Engl. 1978, 17, 569–583. [Google Scholar] [CrossRef]
- Hassner, A.; Alexanian, V. Synthetic Methods 12. Direct Room-Temperature Esterification of Carboxylic-Acids. Tetrahedron Lett. 1978, 4475–4478. [Google Scholar] [CrossRef]
- Basel, Y.; Hassner, A. Di-tert-butyl dicarbonate and 4-(dimethylamino)pyridine revisited. Their reactions with amines and alcohols. J. Org. Chem. 2000, 65, 6368–6380. [Google Scholar] [CrossRef] [PubMed]
- Prashad, M.; Hu, B.; Har, D.; Repic, O.; Blacklock, T.J. A new, convenient and selective 4-dimethylaminopyridine-catalyzed trifluoroacetylation of anilines with ethyl trifluoroacetate. Tetrahedron Lett. 2000, 41, 9957–9961. [Google Scholar] [CrossRef]
- Singh, S.; Das, G.; Singh, O.V.; Han, H. Development of more potent 4-dimethylaminopyridine analogues. Org. Lett. 2007, 9, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Held, I.; Xu, S.J.; Zipse, H. Modular design of pyridine-based acyl-transfer catalysts. Synthesis 2007, 1185–1196. [Google Scholar]
- Baidya, M.; Kobayashi, S.; Brotzel, F.; Schmidhammer, U.; Riedle, E.; Mayr, H. DABCO and DMAP—Why are they different in organocatalysis? Angew. Chem. Int. Ed. 2007, 46, 6176–6179. [Google Scholar] [CrossRef] [PubMed]
- Vedejs, E.; Chen, X.H. Kinetic resolution of secondary alcohols. Enantioselective acylation mediated by a chiral (dimethylamino)pyridine derivative. J. Am. Chem. Soc. 1996, 118, 1809–1810. [Google Scholar] [CrossRef]
- Ruble, J.C.; Fu, G.C. Chiral π-complexes of heterocycles with transition metals: A versatile new family of nucleophilic catalysts. J. Org. Chem. 1996, 61, 7230–7231. [Google Scholar] [CrossRef] [PubMed]
- Wurz, R.P.; Lee, E.C.; Ruble, J.C.; Fu, G.C. Synthesis and resolution of planar-chiral derivatives of 4-(Dimethylamino)pyridine. Adv. Synth. Catal. 2007, 349, 2345–2352. [Google Scholar] [CrossRef]
- Wurz, R.P. Chiral dialkylaminopyridine catalysts in asymmetric synthesis. Chem. Rev. 2007, 107, 5570–5595. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.E.; Schreiner, P.R. Organocatalytic Enantioselective Acyl Transfer onto Racemic as well as meso Alcohols, Amines, and Thiols. Angew. Chem. Int. Ed. 2011, 50, 6012–6042. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, T.; Muramatsu, W.; Nishio, T.; Shibata, T.; Schedel, H. A catalytic one-step process for the chemo- and regioselective acylation of Monosaccharides. J. Am. Chem. Soc. 2007, 129, 12890–12895. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, S.; Satoh, K.; Sakurai, K.; Takano, S. Efficient Ester Exchange-Reaction of Phosphonoacetates. Tetrahedron Lett. 1987, 28, 2713–2716. [Google Scholar] [CrossRef]
- Hoegberg, T.; Strom, P.; Ebner, M.; Raemsby, S. Cyanide as an Efficient and Mild Catalyst in the Aminolysis of Esters. J. Org. Chem. 1987, 52, 2033–2036. [Google Scholar] [CrossRef]
- Vedejs, E.; Diver, S.T. Tributylphosphine—A Remarkable Acylation Catalyst. J. Am. Chem. Soc. 1993, 115, 3358–3359. [Google Scholar] [CrossRef]
- Vedejs, E.; Bennett, N.S.; Conn, L.M.; Diver, S.T.; Gingras, M.; Lin, S.; Oliver, P.A.; Peterson, M.J. Tributylphosphine-Catalyzed Acylations of Alcohols—Scope and Related Reactions. J. Org. Chem. 1993, 58, 7286–7288. [Google Scholar] [CrossRef]
- Vedejs, E.; Daugulis, O.; Mackay, J.A.; Rozners, E. Enantioselective acyl transfer using chiral phosphine catalysts. Synlett 2001, 1499–1505. [Google Scholar] [CrossRef]
- Mackay, J.A.; Vedejs, E. Synthesis and reactivity of new chiral bicyclic phospholanes as acyl-transfer catalysts. J. Org. Chem. 2006, 71, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Methot, J.L.; Roush, W.R. Nucleophilic phosphine organocatalysis. Adv. Synth. Catal 2004, 346, 1035–1050. [Google Scholar] [CrossRef]
- Holz, J.; Gensow, M.; Zayas, O.; Boerner, A. Synthesis of chiral heterocyclic phosphines for application in asymmetric catalysis. Curr. Org. Chem. 2007, 11, 61–106. [Google Scholar] [CrossRef]
- Spivey, A.C.; Arseniyadis, S. Amine, Alcohol and Phosphine Catalysts for Acyl Transfer Reactions. Top. Curr. Chem. 2009, 291, 233–280. [Google Scholar]
- De Kanta, C.; Klauber, E.G.; Seidel, D. Merging Nucleophilic and Hydrogen Bonding Catalysis: An Anion Binding Approach to the Kinetic Resolution of Amines. J. Am. Chem. Soc. 2009, 131, 17060–17061. [Google Scholar]
- Klauber, E.G.; de Kanta, C.; Shah, T.K.; Seidel, D. Merging Nucleophilic and Hydrogen Bonding Catalysis: An Anion Binding Approach to the Kinetic Resolution of Propargylic Amines. J. Am. Chem. Soc. 2010, 132, 13624–13626. [Google Scholar] [CrossRef] [PubMed]
- Klauber, E.G.; Mittal, N.; Shah, T.K.; Seidel, D. A Dual-Catalysis/Anion-Binding Approach to the Kinetic Resolution of Allylic Amines. Org. Lett. 2011, 13, 2464–2467. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Lippert, K.M.; De, C.K.; Klauber, E.G.; Emge, T.J.; Schreiner, P.R.; Seidel, D. A Dual-Catalysis Anion-Binding Approach to the Kinetic Resolution of Amines: Insights into the Mechanism via a Combined Experimental and Computational Study. J. Am. Chem. Soc. 2015, 137, 5748–5758. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.; Reau, R.; Dahan, F.; Bertrand, G. DBU and DBN Are Strong Nucleophiles—X-ray Crystal-Structures of Onio-Substituted and Dionio-Substituted Phosphanes. Angew. Chem. Int. Ed. Engl. 1993, 32, 399–401. [Google Scholar] [CrossRef]
- Birman, V.B.; Li, X.; Han, Z. Nonaromatic amidine derivatives as acylation catalysts. Org. Lett. 2007, 9, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Birman, V.B.; Uffman, E.W.; Hui, J.; Li, X.M.; Kilbane, C.J. 2,3-dihydroimidazo[1,2-a]pyridines: A new class of enantioselective acyl transfer catalysts and their use in kinetic resolution of alcohols. J. Am. Chem. Soc. 2004, 126, 12226–12227. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Bumbu, V.D.; Liu, P.; Li, X.; Jiang, H.; Uffman, E.W.; Guo, L.; Zhang, W.; Jiang, X.; Houk, K.; Birman, V.B. Catalytic, Enantioselective N-Acylation of Lactams and Thiolactams Using Amidine-Based Catalysts. J. Am. Chem. Soc. 2012, 134, 17605–17612. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.E.; Bull, S.D.; Williams, J.M. Amidines, isothioureas, and guanidines as nucleophilic catalysts. Chem. Soc. Rev. 2012, 41, 2109–2121. [Google Scholar] [CrossRef] [PubMed]
- Igau, A.; Grützmacher, H.; Baceiredo, A.; Bertrand, G. Analogous α,α-Bis-Carbenoid Triply Bonded Species—Synthesis of A Stable λ-3-Phosphinocarbene λ-5-Phosphaacetylene. J. Am. Chem. Soc. 1988, 110, 6463–6466. [Google Scholar] [CrossRef]
- Arduengo, A.J.; Harlow, R.L.; Kline, M. A Stable Crystalline Carbene. J. Am. Chem. Soc. 1991, 113, 361–363. [Google Scholar] [CrossRef]
- Bakhtiar, C.; Smith, E.H. Transfer of Alkoxycarbonyl from Alkyl Imidazolium-2-Carboxylates to Benzyl Alcohol, A Cyclohexanone Enamine and Diethylamine. J. Chem. Soc. Perkin Trans. 1 1994, 3, 239–243. [Google Scholar] [CrossRef]
- Grasa, G.A.; Singh, R.; Nolan, S.P. Transesterification/acylation reactions catalyzed by molecular catalysts. Synthesis 2004, 971–985. [Google Scholar] [CrossRef]
- Connor, E.F.; Nyce, G.W.; Myers, M.; Moeck, A.; Hedrick, J.L. First example of N-heterocyclic carbenes as catalysts for living polymerization: Organocatalytic ring-opening polymerization of cyclic esters. J. Am. Chem. Soc. 2002, 124, 914–915. [Google Scholar] [CrossRef] [PubMed]
- Coulembier, O.; Lohmeijer, B.G.G.; Dove, A.P.; Pratt, R.C.; Mespouille, L.; Culkin, D.A.; Benight, S.J.; Dubois, P.; Waymouth, R.M.; Hedrick, J.L. Alcohol adducts of N-heterocyclic carbenes: Latent catalysts for the thermally-controlled living polymerization of cyclic esters. Macromolecules 2006, 39, 5617–5628. [Google Scholar] [CrossRef]
- Enders, D.; Niemeier, O.; Henseler, A. Organocatalysis by N-heterocyclic, carbenes. Chem. Rev. 2007, 107, 5606–5655. [Google Scholar] [CrossRef] [PubMed]
- Flanigan, D.M.; Michailidis, F.R.; White, N.A.; Rovis, T. Organocatalytic Reactions Enabled by N-Heterocyclic Carbenes. Chem. Rev. 2015, 115, 9307–9387. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.L.; Lee, H.M.; Hu, C.H. Theoretical study on the mechanism of N-heterocyclic carbene catalyzed transesterification reactions. Tetrahedron Lett. 2005, 46, 6265–6270. [Google Scholar] [CrossRef]
- Singh, R.; Nolan, S.P. Synthesis of phosphorus esters by transesterification mediated by N-heterocyclic carbenes (NHCs). Chem. Commun. 2005, 5456–5458. [Google Scholar] [CrossRef] [PubMed]
- Kayaki, Y.; Yamamoto, M.; Ikariya, T. N-Heterocyclic Carbenes as Efficient Organocatalysts for CO2 Fixation Reactions. Angew. Chem. Int. Ed. 2009, 48, 4194–4197. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Yamauchi, K.; Muramatsu, K.; Sato, M. First example of chiral N-heterocyclic carbenes as catalysts for kinetic resolution. Chem. Commun. 2004, 2770–2771. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Muramatsu, K.; Yamauchi, K.; Morie, Y.; Sato, M. Chiral N-heterocyclic carbenes as asymmetric acylation catalysts. Tetrahedron 2006, 62, 302–310. [Google Scholar] [CrossRef]
- Kano, T.; Sasaki, K.; Maruoka, K. Enantioselective acylation of secondary alcohols catalyzed by chiral N-heterocyclic carbenes. Org. Lett. 2005, 7, 1347–1349. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.J.; Candish, L.; Lupton, D.W. N-Heterocyclic Carbene-Catalyzed (4+2) Cycloaddition/Decarboxylation of Silyl Dienol Ethers with α,β-Unsaturated Acid Fluorides. J. Am. Chem. Soc. 2011, 133, 4694–4697. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.J.; Stasch, A.; Paddon-Row, M.N.; Lupton, D.W. Synthetic and Quantum Mechanical Studies into the N-Heterocyclic Carbene Catalyzed (4+2) Cycloaddition. J. Org. Chem. 2012, 77, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Candish, L.; Forsyth, C.M.; Lupton, D.W. N-tert-Butyl Triazolylidenes: Catalysts for the Enantioselective (3+2) Annulation of α,β-Unsaturated Acyl Azoliums. Angew. Chem. Int. Ed. 2013, 52, 9149–9152. [Google Scholar] [CrossRef] [PubMed]
- Candish, L.; Levens, A.; Lupton, D.W. N-Heterocyclic carbene catalysed redox isomerisation of esters to functionalised benzaldehydes. Chem. Sci. 2015, 6, 2366–2370. [Google Scholar] [CrossRef]
- Levens, A.; Zhang, C.; Candish, L.; Forsyth, C.M.; Lupton, D.W. Enantioselective N-Heterocyclic Carbene Catalyzed Diene Regenerative (4+2) Annulation. Org. Lett. 2015, 17, 5332–5335. [Google Scholar] [CrossRef] [PubMed]
- Sigler, P.B.; Blow, D.M.; Matthews, B.W.; Henderso, R. Structure of Crystalline α-Chymotrypsin 2. A Preliminary Report Including A Hypothesis for Activation Mechanism. J. Mol. Biol. 1968, 35, 143–164. [Google Scholar] [CrossRef]
- Kasserra, H.P.; Laidler, K.J. Mechanisms of Action of Trypsin and Chymotrypsin. Can. J. Chem. 1969, 47, 4031–4039. [Google Scholar] [CrossRef]
- Hedstrom, L. Serine protease mechanism and specificity. Chem. Rev. 2002, 102, 4501–4523. [Google Scholar] [CrossRef] [PubMed]
- Quiocho, F.A.; Lipsomb, W.N. Carboxypeptidase A: A protein and an enzyme. Adv. Protein Chem. 1971, 25, 1–78. [Google Scholar] [PubMed]
- Christianson, D.W.; Lipscomb, W.N. Carboxypeptidase-A. Acc. Chem. Res. 1989, 22, 62–69. [Google Scholar] [CrossRef]
- Satoh, T.; Imai, T.; Kitajyo, Y.; Kakuchi, T. Novel synthetic method for preparing artificial carbohydrate polymers. Curr. Top. Polym. Res. 2005, 195–231. [Google Scholar]
- Kimura, E.; Shiota, T.; Koike, T.; Shiro, M.; Kodama, M. A Zinc(II) Complex of 1,5,9-Triazacyclododecane ([12]aneN3) as A Model for Carbonic Anhydrase. J. Am. Chem. Soc. 1990, 112, 5805–5811. [Google Scholar] [CrossRef]
- Breslow, R.; Chipman, D. Mixed metal complexes as enzyme models I. Intracomplex nucleophilic catalysis by an oxime anion. J. Am. Chem. Soc. 1965, 87, 4195–4196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.L.; Breslow, R. Ester hydrolysis by a catalytic cyclodextrin dimer enzyme mimic with a metallobipyridyl linking group. J. Am. Chem. Soc. 1997, 119, 1676–1681. [Google Scholar] [CrossRef]
- Yan, J.M.; Breslow, R. An enzyme mimic that hydrolyzes an unactivated ester with catalytic turnover. Tetrahedron Lett. 2000, 41, 2059–2062. [Google Scholar] [CrossRef]
- Luzhkov, V.; Aqvist, J. Free-energy perturbation calculations of binding and transition-state energies: Hydrolysis of phenyl esters by β-cyclodextrin. Chem. Phys. Lett. 1999, 302, 267–272. [Google Scholar] [CrossRef]
- Breslow, R. The hydrophobic effect in reaction mechanism studies and in catalysis by artificial enzymes. J. Phys. Org. Chem. 2006, 19, 813–822. [Google Scholar] [CrossRef]
- Bhosale, S.V.; Bhosale, S.V. β-Cyclodextrin as a catalyst in organic synthesis. Mini-Rev. Org. Chem. 2007, 4, 231–242. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Carey, J.S.; Laffan, D.; Thomson, C.; Williams, M.T. Analysis of the reactions used for the preparation of drug candidate molecules. Org. Biomol. Chem. 2006, 4, 2337–2347. [Google Scholar] [CrossRef] [PubMed]
- Roughley, S.D.; Jordan, A.M. The Medicinal Chemist’s Toolbox: An Analysis of Reactions Used in the Pursuit of Drug Candidates. J. Med. Chem. 2011, 54, 3451–3479. [Google Scholar] [CrossRef] [PubMed]
- Deming, T.J. Synthetic polypeptides for biomedical applications. Prog. Polym. Sci. 2007, 32, 858–875. [Google Scholar] [CrossRef]
- Holowka, E.P.; Sun, V.Z.; Kamei, D.T.; Deming, T.J. Polyarginine segments in block copolypeptides drive both vesicular assembly and intracellular delivery. Nat. Mater. 2007, 6, 52–57. [Google Scholar] [CrossRef] [PubMed]
- NIST Chemistry WebBook. NIST Standard Reference Database; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2009.
- Ulijn, R.V.; Moore, B.D.; Janssen, A.E.M.; Halling, P.J. A single aqueous reference equilibrium constant for amide synthesis-hydrolysis. J. Chem. Soc. Perkin Trans. 2 2002, 5, 1024–1028. [Google Scholar] [CrossRef]
- Guranda, D.T.; Ushakov, G.A.; Yolkin, P.G.; Svedas, V.K. Thermodynamics of phenylacetamides synthesis: Linear free energy relationship with the pK of amine. J. Mol. Cat. B: Enzym. 2012, 74, 48–53. [Google Scholar] [CrossRef]
- Perreux, L.; Loupy, A.; Volatron, F. Solvent-free preparation of amides from acids and primary amines under microwave irradiation. Tetrahedron 2002, 58, 2155–2162. [Google Scholar] [CrossRef]
- Montalbetti, C.A.G.N.; Falque, V. Amide bond formation and peptide coupling. Tetrahedron 2005, 61, 10827–10852. [Google Scholar] [CrossRef]
- Bode, J.W. Emerging methods in amide- and peptide-bond formation. Curr. Opin. Drug Discov. Dev. 2006, 9, 765–775. [Google Scholar] [CrossRef]
- Trost, B.M. The Atom Economy—A Search for Synthetic Efficiency. Science 1991, 254, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M. Atom Economy—A Challenge for Organic-Synthesis—Homogeneous Catalysis Leads the Way. Angew. Chem. Int. Ed. Engl. 1995, 34, 259–281. [Google Scholar] [CrossRef]
- Pattabiraman, V.R.; Bode, J.W. Rethinking amide bond synthesis. Nature 2011, 480, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, H.; Tinnis, F.; Selander, N.; Adolfsson, H. Catalytic amide formation from non-activated carboxylic acids and amines. Chem. Soc. Rev. 2014, 43, 2714–2742. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.; Pelter, A. Trisdialkylaminoboranes—New Reagents for Synthesis of Enamines and Amides. J. Chem. Soc. 1965, 5142–5144. [Google Scholar] [CrossRef]
- Lanigan, R.M.; Starkov, P.; Sheppard, T.D. Direct Synthesis of Amides from Carboxylic Acids and Amines Using B(OCH2CF3)3. J. Org. Chem. 2013, 78, 4512–4523. [Google Scholar] [CrossRef] [PubMed]
- Karaluka, V.; Lanigan, R.M.; Murray, P.M.; Badland, M.; Sheppard, T.D. B(OCH2CF3)3-mediated direct amidation of pharmaceutically relevant building blocks in cyclopentyl methyl ether. Org. Biomol. Chem. 2015, 13, 10888–10894. [Google Scholar] [CrossRef] [PubMed]
- Pelter, A.; Levitt, T.E.; Nelson, P. Some Amide Forming Reactions Involving Boron Reagents. Tetrahedron 1970, 26, 1539–1544. [Google Scholar] [CrossRef]
- Latta, R.; Springsteen, G.; Wang, B.H. Development and synthesis of an arylboronic acid-based solid-phase amidation catalyst. Synthesis 2001, 1611–1613. [Google Scholar] [CrossRef]
- Ishihara, K.; Ohara, S.; Yamamoto, H. 3,4,5-Trifluorobenzeneboronic acid as an extremely active amidation catalyst. J. Org. Chem. 1996, 61, 4196–4197. [Google Scholar] [CrossRef] [PubMed]
- Arnold, K.; Davies, B.; Giles, R.L.; Grosjean, C.; Smith, G.E.; Whiting, A. To catalyze or not to catalyze? Insight into direct amide bond formation from amines and carboxylic acids under thermal and catalyzed conditions. Adv. Synth. Catal. 2006, 348, 813–820. [Google Scholar] [CrossRef]
- Marcelli, T. Mechanistic Insights into Direct Amide Bond Formation Catalyzed by Boronic Acids: Halogens as Lewis Bases. Angew. Chem. Int. Ed. 2010, 49, 6840–6843. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, H.Z.; Fu, Y.; Guo, Q.X. Mechanism of arylboronic acid-catalyzed amidation reaction between carboxylic acids and amines. Org. Biomol. Chem. 2013, 11, 2140–2146. [Google Scholar] [CrossRef] [PubMed]
- Maki, T.; Ishihara, K.; Yamamoto, H. N-Alkyl-4-boronopyridinium salts as thermally stable and reusable amide condensation catalysts. Org. Lett. 2005, 7, 5043–5046. [Google Scholar] [CrossRef] [PubMed]
- Maki, T.; Ishihara, K.; Yamamoto, H. N-Alkyl-4-boronopyridinium halides versus boric acid as catalysts for the esterification of α-hydroxycarboxylic acids. Org. Lett. 2005, 7, 5047–5050. [Google Scholar] [CrossRef] [PubMed]
- Maki, T.; Ishihara, K.; Yamamoto, H. New boron(III)-catalyzed amide and ester condensation reactions. Tetrahedron 2007, 63, 8645–8657. [Google Scholar] [CrossRef]
- Gernigon, N.; Al-Zoubi, R.M.; Hall, D.G. Direct Amidation of Carboxylic Acids Catalyzed by ortho-Iodo Arylboronic Acids: Catalyst Optimization, Scope, and Preliminary Mechanistic Study Supporting a Peculiar Halogen Acceleration Effect. J. Org. Chem. 2012, 77, 8386–8400. [Google Scholar] [CrossRef] [PubMed]
- Gernigon, N.; Zheng, H.; Hall, D.G. Solid-supported ortho-iodoarylboronic acid catalyst for direct amidation of carboxylic acids. Tetrahedron Lett. 2013, 54, 4475–4478. [Google Scholar] [CrossRef]
- Fatemi, S.; Gernigon, N.; Hall, D.G. A multigram-scale lower E-factor procedure for MIBA-catalyzed direct amidation and its application to the coupling of α and β aminoacids. Green Chem. 2015, 17, 4016–4028. [Google Scholar] [CrossRef]
- Lenstra, D.C.; Thao Nguyen, D.; Mecinovic, J. Zirconium-catalyzed direct amide bond formation between carboxylic esters and amines. Tetrahedron 2015, 71, 5547–5553. [Google Scholar] [CrossRef]
- Ali, M.A.; Siddiki, S.M.A.H.; Onodera, W.; Kon, K.; Shimizu, K.-i. Amidation of Carboxylic Acids with Amines by Nb2O5 as a Reusable Lewis Acid Catalyst. ChemCatChem 2015, 7, 3555–3561. [Google Scholar] [CrossRef]
- Lenstra, D.C.; Rutjes, F.P.; Mecinovic, J. Triphenylphosphine-catalysed amide bond formation between carboxylic acids and amines. Chem. Commun. 2014, 50, 5763–5766. [Google Scholar] [CrossRef] [PubMed]
- Miyabe, H.; Takemoto, Y. Carbonyl and Imine Activation. In Comprehensive Organic Synthesis, 2nd ed.; Knochel, P., Molander, G.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 1, pp. 751–769. [Google Scholar]
- Corey, E.J.; Grogan, M.J. Enantioselective synthesis of α-amino nitriles from N-benzhydryl imines and HCN with a chiral bicyclic guanidine as catalyst. Org. Lett. 1999, 1, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Swain, C.G.; Brown, J.F. Concerted Displacement Reactions 7. The Mechanism of Acid Base Catalysis in Non-Aqueous Solvents. J. Am. Chem. Soc. 1952, 74, 2534–2537. [Google Scholar] [CrossRef]
- Swain, C.G.; Brown, J.F. Concerted Displacement Reactions 8. Polyfunctional Catalysis. J. Am. Chem. Soc. 1952, 74, 2538–2543. [Google Scholar] [CrossRef]
- Beak, P. Energies and Alkylations of Tautomeric Heterocyclic-Compounds—Old Problems New Answers. Acc. Chem. Res. 1977, 10, 186–192. [Google Scholar] [CrossRef]
- Morpurgo, S.; Bossa, M. The epimerisation of 2-tetrahydropyranol catalysed by the tautomeric couples 2-pyridone/2-hydroxypyridine and formamide/formamidic acid as a model for the sugar’s mutarotation: A theoretical study. Phys. Chem. Chem. Phys. 2003, 5, 1181–1189. [Google Scholar] [CrossRef]
- Rony, P.R. Polyfunctional Catalysis I. Activation Parameters for Mutarotation of Tetramethyl-d-Glucose in Benzene. J. Am. Chem. Soc. 1968, 90, 2824–2831. [Google Scholar] [CrossRef]
- Arai, K. Preparation of Poly(4-Vinyl-2-Hydroxypyridine) and Its Catalytic Activity on Mutarotation of 2,3,4,6-Tetramethyl-α-d-Glucose. J. Polym. Sci. Part A: Polym. Chem. 1993, 31, 193–197. [Google Scholar] [CrossRef]
- Engdahl, K.A.; Bivehed, H.; Ahlberg, P.; Saunders, W.H., Jr. Rate-Controlling 2-Proton Transfer Coupled with Heavy-Atom Motion in the 2-Pyridinone-Catalyzed Mutarotation of Tetramethylglucose—Experimental and Calculated Deuterium-Isotope Effects. J. Am. Chem. Soc. 1983, 105, 4767–4774. [Google Scholar] [CrossRef]
- Kergomard, A.; Renard, M. Mutarotation of Tetramethylglucose Catalysed by Carboxylic Acids in Benzene Solution. Tetrahedron 1968, 24, 6643–6651. [Google Scholar] [CrossRef]
- Breslow, R. Bifunctional Acid-Base Catalysis by Imidazole Groups in Enzyme Mimics. J. Mol. Catal. 1994, 91, 161–174. [Google Scholar] [CrossRef]
- Melander, C.; Horne, D.A. Mutarotation of tetramethylglucose catalyzed by ribonucleosides. Tetrahedron Lett. 1997, 38, 713–716. [Google Scholar] [CrossRef]
- Nakamizo, N. Catalysis in Peptide Synthesis with Active Esters 3. Prominent Catalysis of Sodium 2-Pyridinolate Or 4-Pyridinolate in Aminolysis of Esters. Bull. Chem. Soc. Jpn. 1971, 44, 2006. [Google Scholar] [CrossRef]
- Chen, F.X.; Feng, X.M. Synthesis of racemic tertiary cyanohydrins. Synlett 2005, 892–899. [Google Scholar] [CrossRef]
- Kanai, M.; Kato, N.; Ichikawa, E.; Shibasaki, M. Power of cooperativity: Lewis acid-Lewis base bifunctional asymmetric catalysis. Synlett 2005, 1491–1508. [Google Scholar] [CrossRef]
- Song, J.J.; Gallou, F.; Reeves, J.T.; Tan, Z.L.; Yee, N.K.; Senanayake, C.H. Activation of TMSCN by N-heterocyclic carbenes for facile cyanosilylation of carbonyl compounds. J. Org. Chem. 2006, 71, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Brefort, J.L.; Corriu, R.; Guérin, C.; Henner, B. Hypervalent Silicon Hydrides—Evidence of Their Intermediacy in the Reaction of Optically-Active 1-NpPhMeSiH(D) with Hydrides. J. Organomet. Chem. 1989, 370, 9–15. [Google Scholar] [CrossRef]
- Gutsev, G.L. A Theoretical Investigation on the Structure of the Hypervalent Carbon and Silicon Pentahalogenides as Well as Their Singly Charged Anions. Chem. Phys. 1992, 166, 57–68. [Google Scholar] [CrossRef]
- Murthy, V.S.; Miller, J.M. Formation of Pentavalent Silicon Anions, (CH3O)2Si(OEt)3–and CH3OSiH(OEt)3–, in the Gas-Phase. Rapid Commun. Mass Spectrom. 1994, 8, 698–700. [Google Scholar] [CrossRef]
- Wang, X.; Tian, S.K. Neutral π-nucleophile-catalyzed cyanation of aldehydes and ketones. Synlett 2007, 1416–1420. [Google Scholar] [CrossRef]
- Dirksen, A.; Dirksen, S.; Hackeng, T.M.; Dawson, P.E. Nucleophilic catalysis of hydrazone formation and transimination: Implications for dynamic covalent chemistry. J. Am. Chem. Soc. 2006, 128, 15602–15603. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, A.; Hackeng, T.M.; Dawson, P.E. Nucleophilic catalysis of oxime ligation. Angew. Chem. Int. Ed. 2006, 45, 7581–7584. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, A.; Dawson, P.E. Rapid Oxime and Hydrazone Ligations with Aromatic Aldehydes for Biomolecular Labeling. Bioconjugate Chem. 2008, 19, 2543–2548. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Miyamoto, Y.; Inoue, T.; Oguni, N. Enantioselective Trimethylsilylcyanation of Some Aldehydes Catalyzed by Chiral Schiff-Base Titanium Alkoxide Complexes. J. Org. Chem. 1993, 58, 1515–1522. [Google Scholar] [CrossRef]
- Groger, H. Catalytic enantioselective Strecker reactions and analogous syntheses. Chem. Rev. 2003, 103, 2795–2828. [Google Scholar] [CrossRef] [PubMed]
- North, M.; Usanov, D.L.; Young, C. Lewis Acid Catalyzed Asymmetric Cyanohydrin Synthesis. Chem. Rev. 2008, 108, 5146–5226. [Google Scholar] [CrossRef] [PubMed]
- Gawronski, J.; Wascinska, N.; Gajewy, J. Recent Progress in Lewis Base Activation and Control of Stereoselectivity in the Additions of Trimethylsilyl Nucleophiles. Chem. Rev. 2008, 108, 5227–5252. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, X.; Lin, L.; Feng, X. Recent Progress in the Chemically Catalyzed Enantioselective Synthesis of Cyanohydrins. Eur. J. Org. Chem. 2010, 4751–4769. [Google Scholar] [CrossRef]
- Kurono, N.; Ohkuma, T. Catalytic Asymmetric Cyanation Reactions. ACS Catal. 2016, 6, 989–1023. [Google Scholar] [CrossRef]
- Pluth, M.D.; Bergman, R.G.; Raymond, K.N. Acid catalysis in basic solution: A supramolecular host promotes orthoformate hydrolysis. Science 2007, 316, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Pluth, M.D.; Bergman, R.G.; Raymond, K.N. Proton-Mediated Chemistry and Catalysis in a Self-Assembled Supramolecular Host. Acc. Chem. Res. 2009, 42, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.B. The Michael Reaction in March’s Adanced Organic Chemistry; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2013; Chapter 15.24 to 15.27; pp. 943–959. [Google Scholar]
- Csaky, A.G.; de la Herran, G.; Murcia, M.C. Conjugate addition reactions of carbon nucleophiles to electron-deficient dienes. Chem. Soc. Rev. 2010, 39, 4080–4102. [Google Scholar] [CrossRef] [PubMed]
- Helder, R.; Arends, R.; Bolt, W.; Hiemstra, H.; Wynberg, H. Alkaloid Catalyzed Asymmetric Synthesis 3. Addition of Mercaptans to 2-Cyclohexene-1-one—Determination of Enantiomeric Excess Using C-13 NMR. Tetrahedron Lett. 1977, 2181–2182. [Google Scholar] [CrossRef]
- Hiemstra, H.; Wynberg, H. Addition of Aromatic Thiols to Conjugated Cycloalkenones, Catalyzed by Chiral β-Hydroxy Amines—A Mechanistic Study on Homogeneous Catalytic Asymmetric-Synthesis. J. Am. Chem. Soc. 1981, 103, 417–430. [Google Scholar] [CrossRef]
- Grayson, M.N.; Houk, K.N. Cinchona Alkaloid-Catalyzed Asymmetric Conjugate Additions: The Bifunctional Brønsted Acid-Hydrogen Bonding Model. J. Am. Chem. Soc. 2016, 138, 1170–1173. [Google Scholar] [CrossRef] [PubMed]
- Sibi, M.P.; Manyem, S. Enantioselective conjugate additions. Tetrahedron 2000, 56, 8033–8061. [Google Scholar] [CrossRef]
- Maudit, M.; Basle, O.; Clavier, H.; Crevisy, C.; Denicourt-Nowicki, A. Metal-Catalyzed Asymmetric Nucleophilic Addition to Electron-Deficient Alkenes. In Comprehensive Organic Synthesis; Knochel, P., Molander, G.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Chapter 4; pp. 189–341. [Google Scholar]
- Vicario, J.L.; Reyes, E.; Carrillo, L.; Uria, U. Organocatalytic Asymmetric Nucleophilic Addition to Electron-Deficient Alkenes in Comprehensive Organic Synthesis; Knochel, P., Molander, G.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Chapter 4; pp. 119–188. [Google Scholar]
- Howell, G.P. Asymmetric and Diastereoselective Conjugate Addition Reactions: C–C Bond Formation at Large Scale. Org. Process Res. Dev. 2012, 16, 1258–1272. [Google Scholar] [CrossRef]
- Melchiorre, P. Cinchona-based Primary Amine Catalysis in the Asymmetric Functionalization of Carbonyl Compounds. Angew. Chem. Int. Ed. 2012, 51, 9748–9770. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, M.; Sasai, H.; Arai, T. Asymmetric catalysis with heterobimetallic compounds. Angew. Chem. Int. Ed. Engl. 1997, 36, 1237–1256. [Google Scholar] [CrossRef]
- Shibasaki, M.; Kanai, M.; Matsunaga, S.; Kumagai, N. Recent Progress in Asymmetric Bifunctional Catalysis Using Multimetallic Systems. Acc. Chem. Res. 2009, 42, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Takada, H.; Lin, S.; Kumagai, N.; Shibasaki, M. Direct Catalytic Asymmetric Vinylogous Conjugate Addition of Unsaturated Butyrolactones to α,β-Unsaturated Thioamides. Angew. Chem. Int. Ed. 2014, 53, 5327–5331. [Google Scholar] [CrossRef] [PubMed]
- Hoashi, Y.; Okino, T.; Takemoto, Y. Enantioselective Michael addition to α,β-unsaturated imides catalyzed by a bifunctional organocatalyst. Angew. Chem. Int. Ed. 2005, 44, 4032–4035. [Google Scholar] [CrossRef] [PubMed]
- Zu, L.-S.; Xie, H.; Li, H.; Wang, H.; Jiang, W.; Wang, W. Chiral amine thiourea-promoted enantioselective domino Michael-Aldol reactions between 2-mercaptobenzaldehydes and maleimides. Adv. Synth. Catal. 2007, 349, 1882–1886. [Google Scholar] [CrossRef]
- Bohme, D.K.; Mackay, G.I. Bridging the Gap between the Gas-Phase and Solution—Transition in the Kinetics of Nucleophilic Displacement-Reactions. J. Am. Chem. Soc. 1981, 103, 978–979. [Google Scholar] [CrossRef]
- Ryding, M.J.; Debnarova, A.; Fernandez, I.; Uggerud, E. Nucleophilic Substitution in Reactions between Partially Hydrated Superoxide Anions and Alkyl Halides. J. Org. Chem. 2015, 80, 6133–6142. [Google Scholar] [CrossRef] [PubMed]
- Gronert, S. Mass spectrometric studies of organic ion/molecule reactions. Chem. Rev. 2001, 101, 329–360. [Google Scholar] [CrossRef] [PubMed]
- Brauman, J.I.; Olmstead, W.N.; Lieder, C.A. Gas-Phase Nucleophilic Displacement-Reactions. J. Am. Chem. Soc. 1974, 96, 4030–4031. [Google Scholar] [CrossRef]
- Wladkowski, B.D.; Lim, K.F.; Allen, W.D.; Brauman, J.I. The SN2 Identity Exchange-Reaction ClCH2CN + Cl- →Cl– + ClCH2CN—Experiment and Theory. J. Am. Chem. Soc. 1992, 114, 9136–9153. [Google Scholar] [CrossRef]
- Vayner, G.; Houk, K.N.; Jorgensen, W.L.; Brauman, J.I. Steric retardation of SN2 reactions in the gas phase and solution. J. Am. Chem. Soc. 2004, 126, 9054–9058. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.G.; Polanyi, M. Further considerations on the thermodynamics of chemical equilibria and reaction rates. Trans. Faraday Soc. 1936, 32, 1333–1359. [Google Scholar] [CrossRef]
- Evans, M.G.; Polanyi, M. Inertia and driving force of chemical reactions. Trans. Faraday Soc. 1938, 34, 11–24. [Google Scholar] [CrossRef]
- Glukhovtsev, M.N.; Pross, A.; Radom, L. Gas-phase non-identity SN2 reactions of halide anions with methyl halides: A high-level computational study. J. Am. Chem. Soc. 1996, 118, 6273–6284. [Google Scholar] [CrossRef]
- Dodd, J.A.; Brauman, J.I. On the Application of the Marcus Equation to Methyl Transfer (SN2) Reactions. J. Am. Chem. Soc. 1984, 106, 5356–5357. [Google Scholar] [CrossRef]
- Brønsted, J.N. Acid and basic catalysis. Chem. Rev. 1928, 5, 231–238. [Google Scholar] [CrossRef]
- Bell, R.P. The kinetics of proton transfer reactions. Trans. Faraday Soc. 1938, 34, 0229–0236. [Google Scholar] [CrossRef]
- Dimroth, O. Relationships between affinity and reactions speed. Angew. Chem. 1933, 46, 571–576. [Google Scholar] [CrossRef]
- Shaik, S.S.; Pross, A. SN2 Reactivity of CH3X Derivatives—A Valence Bond Approach. J. Am. Chem. Soc. 1982, 104, 2708–2719. [Google Scholar] [CrossRef]
- Pross, A. Relationship Between Rates and Equilibria and the Mechanistic Significance of the Bronsted Parameter—A Qualitative Valence-Bond Approach. J. Org. Chem. 1984, 49, 1811–1818. [Google Scholar] [CrossRef]
- Shaik, S.S.; Schlegel, H.B.; Wolfe, S. Theoretical Aspects of Physical Organic Chemistry. The SN2 Mechanism; Wiley: New York, NY, USA, 1992. [Google Scholar]
- Shaik, S.S.; Ioffe, A.; Reddy, A.C.; Pross, A. Is the Avoided Crossing State a Good Approximation for the Transition-State of A Chemical-Reaction—An Analysis of Menschutkin and Ionic SN2 Reactions. J. Am. Chem. Soc. 1994, 116, 262–273. [Google Scholar] [CrossRef]
- Anglada, J.M.; Besalú, E.; Bofill, J.M.; Crehuet, R. Prediction of approximate transition states by Bell-Evans-Polanyi principle: I. J. Comput. Chem. 1999, 20, 1112–1129. [Google Scholar] [CrossRef]
- Bofill, J.M.; Anglada, J.M.; Besalú, E.; Crehuet, R. Quantum chemical reactivity: Beyond the study of small molecules. In Fundamentals of Molecular Similarity; Carbó-Dorca, R., Gironés, X., Mezey, P.G., Eds.; Springer: New York, NY, USA, 2001; pp. 125–141. [Google Scholar]
- Wester, R.; Bragg, A.E.; Davis, A.V.; Neumark, D.M. Time-resolved study of the symmetric SN2-reaction I–+CH3I. J. Chem. Phys. 2003, 119, 10032–10039. [Google Scholar] [CrossRef]
- Arnaut, L.G.; Formosinho, S.J. The rates of SN2 reactions and their relation to molecular and solvent properties. Chem. Eur. J. 2007, 13, 8018–8028. [Google Scholar] [CrossRef] [PubMed]
- Bender, M.L. Metal ion catalysis of nucleophilic organic reaction in solution. Adv. Chem. Ser. 1963, 37, 19–36. [Google Scholar]
- Pocker, Y.; Kevill, D.N. Electrophilic Catalysis in Nucleophilic Substitution and Elimination 3. Conductances of Soms Silver and Tetraethylammonium Salts in Acetonitrile and Kinetics of Reaction of 2-Octyl Bromide with Tetraethylammonium and Silver Nitrates in That Solvent. J. Am. Chem. Soc. 1965, 87, 4760–4770. [Google Scholar] [CrossRef]
- Pocker, Y.; Wong, W.H. Electrophilic Catalysis in Nucleophilic-Substitution and Elimination 7. Kinetics and Mechanism of Reaction of Neopentyl Iodide with Tetraethylammonium and Silver Nitrates and Perchlorates in Acetonitrile. J. Am. Chem. Soc. 1975, 97, 7097–7104. [Google Scholar] [CrossRef]
- Maia, A.; Landini, D.; Betti, C.; Leska, B.; Schröder, G. Catalytic activity and anion activation in SN2 reactions promoted by complexes of silicon polypodands. Comparison with traditional polyethers. New J. Chem. 2005, 29, 1195–1198. [Google Scholar] [CrossRef]
- Buncel, E.; Nagelkerke, R.; Thatcher, G.R.J. Metal ion catalysis and inhibition in nucleophilic displacement reactions at carbon, phosphorous, and sulfur centers part 10—Alkali metal ion catalysis in nucleophilic displacement by ethoxide ion on p-nitrophenyl phenylphosphonate: Evidence for multiple metal ion catalysis. Can. J. Chem. 2003, 81, 53–63. [Google Scholar]
- Takada, K.; Tanaka, S.; Nagasawa, K. Asymmetric Mannich-type reaction of aromatic α-amido sulfone with malonate using guanidine-thiourea bifunctional organocatalyst. Synlett 2009, 1643–1646. [Google Scholar] [CrossRef]
- Makosza, M.; Serafin, B. Reactions of organic anions I. Catalytic ethylation of phenylacetonitrile in aqueous medium. Rocz. Chem. 1965, 39, 1223–1230. [Google Scholar]
- Makosza, M.; Jonczyk, A. Phase-transfer alkylation of nitriles: α-phenylbutyronitrile. Org. Synth. 1976, 55, 91–95. [Google Scholar]
- Makosza, M.; Fedorynski, M. Thirty years of phase transfer catalysis. Pol. J. Chem. 1996, 70, 1093–1110. [Google Scholar]
- Makosza, M. Phase-transfer catalysis. A general green methodology in organic synthesis. Pure Appl. Chem. 2000, 72, 1399–1403. [Google Scholar] [CrossRef]
- Starks, C.M. Phase-Transfer Catalysis 1. Heterogeneous Reactions Involving Anion Transfer by Quaternary Ammonium and Phosphonium Salts. J. Am. Chem. Soc. 1971, 93, 195–199. [Google Scholar] [CrossRef]
- Starks, C.M.; Liotta, C. Phase Transfer Catalysis: Principles and Techniques; Academic Press: New York, NY, USA, 1978; p. 384. [Google Scholar]
- Herriott, A.W.; Picker, D. Phase Transfer Catalysis—Evaluation of Catalysts. J. Am. Chem. Soc. 1975, 97, 2345–2349. [Google Scholar] [CrossRef]
- Halpern, M. Phase-Transfer catalysis. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2002; pp. 495–500. [Google Scholar]
- Dehmlow, E.V.; Dehmlow, S.S. Phase Transfer Catalysis: Principles and Techniques, 2nd ed.; Verlag Chemie: Weinheim, Germany, 1983; Volume 11, p. 386. [Google Scholar]
- Makosza, M.; Fedorynski, M. Catalysis in 2-Phase Systems—Phase-Transfer and Related Phenomena. Adv. Catal. 1987, 35, 375–422. [Google Scholar]
- Yadav, G.D. Insight into green phase transfer catalysis. Top. Catal. 2004, 29, 145–161. [Google Scholar] [CrossRef]
- Shirakawa, S.; Maruoka, K. Recent Developments in Asymmetric Phase-Transfer Reactions. Angew. Chem. Int. Ed. 2013, 52, 4312–4348. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shirakawa, S.; Maruoka, K. Phase-Transfer-Catalyzed Asymmetric Conjugate Cyanation of Alkylidenemalonates with KCN in the Presence of a Brønsted Acid Additive. Org. Lett. 2013, 15, 1230–1233. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, S.; Tokuda, T.; Maruoka, K. Phase-transfer-catalyzed asymmetric desymmetrizations of cyclopentanones. Org. Chem. Front. 2015, 2, 336–339. [Google Scholar] [CrossRef]
- Shirakawa, S.; Koga, K.; Tokuda, T.; Yamamoto, K.; Maruoka, K. Catalytic Asymmetric Synthesis of 3,3′-Diaryloxindoles as Triarylmethanes with a Chiral All-Carbon Quaternary Center: Phase-Transfer-Catalyzed SNAr Reaction. Angew. Chem. Int. Ed. 2014, 53, 6220–6223. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.J. Cyclic Polyethers and Their Complexes with Metal Salts. J. Am. Chem. Soc. 1967, 89, 7017–7036. [Google Scholar] [CrossRef]
- Pedersen, C.J. Cyclic Polyethers and Their Complexes with Metal Salts. J. Am. Chem. Soc. 1967, 89, 2495–2496. [Google Scholar] [CrossRef]
- Newcomb, M.; Gokel, G.W.; Cram, D.J. Pyridyl Unit in Host Compounds. J. Am. Chem. Soc. 1974, 96, 6810–6811. [Google Scholar] [CrossRef]
- Ooi, T.; Maruoka, K. Recent advances in asymmetric phase-transfer catalysis. Angew. Chem. Int. Ed. 2007, 46, 4222–4266. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Maruoka, K. Recent development and application of chiral phase-transfer catalysts. Chem. Rev. 2007, 107, 5656–5682. [Google Scholar] [CrossRef] [PubMed]
- Patterson, D.E.; Xie, S.; Jones, L.A.; Osterhout, M.H.; Henry, C.; Roper, T.D. Large Scale Application of Asymmetric Phase-Tansfer Catalysis for Amino Acid Synthesis. In Asymmetric Catalysis on Industrial Scale: Challenges, Approaches, and Solutions, 2nd ed.; Blaser, H.-U., Federsel, H.-J., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 473–484. [Google Scholar]
- Nicolas, C.; Lacour, J. Triazatriangulenium cations: Highly stable carbocations for phase-transfer catalysis. Org. Lett. 2006, 8, 4343–4346. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.J.; Frensdorff, H.K. Macrocyclic Polyethers and Their Complexes. Angew. Chem. Int. Ed. 1972, 11, 16–25. [Google Scholar] [CrossRef] [PubMed]
- DiBiase, S.A.; Gokel, G.W. Crown-Cation Complex Effects. 8. Reactions of Crown Ether Activated tert-Butoxide Ion. J. Org. Chem. 1978, 43, 447–452. [Google Scholar] [CrossRef]
- Gokel, G.W. Crown Ethers and Cryptands; Monographs in Supramolecular Chemistry, The Royal Society of Chemistry: London, UK, 1991; Volume 3, p. 199. [Google Scholar]
- Brachvogel, R.-C.; Maid, H.; von Delius, M. NMR Studies on Li+, Na+ and K+ complexes of orthoesters Cryptand o-Me2–1.1.1. Int. J. Mol. Sci. 2015, 16, 20641–20656. [Google Scholar] [CrossRef] [PubMed]
- Izatt, R.M.; Bradshaw, J.S.; Nielsen, S.A.; Lamb, J.D.; Christensen, J.J.; Sen, D. Thermodynamic and Kinetic Data for Cation Macrocycle Interaction. Chem. Rev. 1985, 85, 271–339. [Google Scholar] [CrossRef]
- Landini, D.; Montanari, F.; Pirisi, F.M. Crown Ethers as Phase-Transfer Catalysts in 2-Phase Reactions. J. Chem. Soc. Chem. Commun. 1974, 879–880. [Google Scholar] [CrossRef]
- Wong, K.H. Kinetics of Esterification of Potassium Para-Nitrobenzoate by Benzyl Bromide Using Dicyclohexyl-18-Crown-6 as Phase-Transfer Agent. J. Chem. Soc. Chem. Commun. 1978, 282–283. [Google Scholar] [CrossRef]
- Cinquini, M.; Montanari, F.; Tundo, P. Alkyl Substituted Aza-Macrobicyclic Polyethers—Highly Efficient Catalysts in 2-Phase Reactions. J. Chem. Soc. Chem. Commun. 1975, 393–394. [Google Scholar] [CrossRef]
- Rapi, Z.; Bako, P.; Keglevich, G.; Szoellosy, A.; Drahos, L.; Botyanszki, A.; Holczbauer, T. Asymmetric phase transfer Darzens reactions catalyzed by d-glucose- and d-mannose-based chiral crown ethers. Tetrahedron: Asymmetry 2012, 23, 489–496. [Google Scholar] [CrossRef]
- Bako, P.; Keglevich, G.; Rapi, Z.; ToKe, L. The Enantiomeric Differentiation Ability of Chiral Crown Ethers Based on Carbohydrates. Curr. Org. Chem. 2012, 16, 297–304. [Google Scholar] [CrossRef]
- Rapi, Z.; Demuth, B.; Keglevich, G.; Grun, A.; Drahos, L.; Soti, P.L.; Bako, P. Enantioselective Michael addition of malonates to aromatic nitroalkenes catalyzed by monosaccharide-based chiral crown ethers. Tetrahedron: Asymmetry 2014, 25, 141–147. [Google Scholar] [CrossRef]
- Bako, P.; Rapi, Z.; Gruen, A.; Nemcsok, T.; Hegedus, L.; Keglevich, G. Asymmetric Michael Addition of Malonates to Enones Catalyzed by an α-d-Glucopyranoside-Based Crown Ether. Synlett 2015, 26, 1847–1851. [Google Scholar] [CrossRef]
- Mann, J. Modern Methods for the Introduction of Fluorine into Organic-Molecules—An Approach to Compounds with Altered Chemical and Biological-Activities. Chem. Soc. Rev. 1987, 16, 381–436. [Google Scholar] [CrossRef]
- Mikami, K.; Itoh, Y.; Yamanaka, M. Fluorinated carbonyl and olefinic compounds: Basic character and asymmetric catalytic reactions. Chem. Rev. 2004, 104, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, M. CF3-bearing aromatic and heterocyclic building blocks. Angew. Chem. Int. Ed. 2006, 45, 5432–5446. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Faeh, C.; Diederich, F. Fluorine in pharmaceuticals: Looking beyond intuition. Science 2007, 317, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Gronert, S.; Kass, S.R. Theoretical studies of eliminations 6. The regiochemistry and stereochemistry of the gas-phase reactions of 3-halocyclohexenes with fluoride. An ab initio study. J. Org. Chem. 1997, 62, 7991–8000. [Google Scholar] [CrossRef] [PubMed]
- Pliego, J.R.; Riveros, J.M. Theoretical study of the gas-phase reaction of fluoride and chloride ions with methyl formate. J. Phys. Chem. A 2002, 106, 371–378. [Google Scholar] [CrossRef]
- Mugnai, M.; Cardini, G.; Schettino, V. Substitution and elimination reaction of F– with C2H5Cl: An ab Initio molecular dynamics study. J. Phys. Chem. A 2003, 107, 2540–2547. [Google Scholar] [CrossRef]
- Wilkinson, J.A. Recent Advances in the Selective Formation of the C–F Bond. Chem. Rev. 1992, 92, 505–519. [Google Scholar] [CrossRef]
- Murray, C.B.; Sandford, G.; Korn, S.R. Ionic liquids as media for nucleophilic fluorination. J. Fluorine Chem. 2003, 123, 81–84. [Google Scholar] [CrossRef]
- Kim, D.W.; Song, C.E.; Chi, D.Y. New method of fluorination using potassium fluoride in ionic liquid: Significantly enhanced reactivity of fluoride and improved selectivity. J. Am. Chem. Soc. 2002, 124, 10278–10279. [Google Scholar] [CrossRef] [PubMed]
- Liotta, C.L.; Harris, H.P. Chemistry of Naked Anions 1. Reactions of 18-Crown-6 Complex of Potassium Fluoride with Organic Substrates in Aprotic Organic-Solvents. J. Am. Chem. Soc. 1974, 96, 2250–2252. [Google Scholar] [CrossRef]
- Cox, D.P.; Terpinski, J.; Lawrynowicz, W. Anhydrous Tetrabutylammonium Fluoride—A Mild But Highly Efficient Source of Nucleophilic Fluoride-Ion. J. Org. Chem. 1984, 49, 3216–3219. [Google Scholar] [CrossRef]
- Sun, H.R.; DiMagno, S.G. Anhydrous tetrabutylammonium fluoride. J. Am. Chem. Soc. 2005, 127, 2050–2051. [Google Scholar] [CrossRef] [PubMed]
- Landini, D.; Maia, A.; Rampoldi, A. Dramatic Effect of the Specific Solvation on the Reactivity of Quaternary Ammonium Fluorides and Poly(Hydrogen Fluorides), (HF)n•F–, in Media of Low Polarity. J. Org. Chem. 1989, 54, 328–332. [Google Scholar] [CrossRef]
- Albanese, D.; Landini, D.; Penso, M. Hydrated tetrabutylammonium fluoride as a powerful nucleophilic fluorinating agent. J. Org. Chem. 1998, 63, 9587–9589. [Google Scholar] [CrossRef]
- Pliego, J.R.; Pilo-Veloso, D. Chemoselective nucleophilic fluorination induced by selective solvation of the SN2 transition state. J. Phys. Chem. B 2007, 111, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Leadbeater, N.E.; Marco, M.; Tominack, B.J. First examples of transition-metal free Sonogashira-type couplings. Org. Lett. 2003, 5, 3919–3922. [Google Scholar] [CrossRef] [PubMed]
- Cinquini, M.; Colonna, S.; Molinari, H.; Montanari, F.; Tundo, P. Heterogeneous Phase-Transfer Catalysts: Onium Salts, Crown Ethers, and Cryptands Immobilized on Polymer Supports. J. Chem. Soc. Chem. Commun. 1976, 394–396. [Google Scholar] [CrossRef]
- He, L.N.; Yasuda, H.; Sakakura, T. New procedure for recycling homogeneous catalyst: propylene carbonate synthesis under supercritical CO2 conditions. Green Chem. 2003, 5, 92–94. [Google Scholar] [CrossRef]
- Shirakawa, S.; Tanaka, Y.; Maruoka, K. Development of a recyclable fluorous chiral phase-transfer catalyst: Application to the catalytic asymmetric synthesis of α-amino acids. Org. Lett. 2004, 6, 1429–1431. [Google Scholar] [CrossRef] [PubMed]
- Stuart, A.M.; Vidal, J.A. Perfluoroalkylated 4,13-diaza-18-crown-6 ethers: Synthesis, phase-transfer catalysis, and recycling studies. J. Org. Chem. 2007, 72, 3735–3740. [Google Scholar] [CrossRef] [PubMed]
- Brak, K.; Jacobsen, E.N. Asymmetric Ion-Pairing Catalysis. Angew. Chem. Int. Ed. 2013, 52, 534–561. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.; Fletcher, S.; Nelson, A. Towards phase-transfer catalysts with a chiral anion: Inducing asymmetry in the reactions of cations. Tetrahedron: Asymmetry 2003, 14, 1995–2004. [Google Scholar] [CrossRef]
- Hamilton, G.L.; Kanai, T.; Toste, F.D. Chiral Anion-Mediated Asymmetric Ring Opening of meso-Aziridinium and Episulfonium Ions. J. Am. Chem. Soc. 2008, 130, 14984–14986. [Google Scholar] [CrossRef] [PubMed]
- Phipps, R.J.; Hamilton, G.L.; Toste, F.D. The progression of chiral anions from concepts to applications in asymmetric catalysis. Nat. Chem. 2012, 4, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, Y.M.; Drljevic, A.; Rauniyar, V.; Phipps, R.J.; Toste, F.D. A combination of directing groups and chiral anion phase-transfer catalysis for enantioselective fluorination of alkenes. Proc. Nat. Acad. Sci. USA 2013, 110, 13729–13733. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wu, T.; Phipps, R.J.; Toste, F.D. Advances in Catalytic Enantioselective Fluorination, Mono-, Di-, and Trifluoromethylation, and Trifluoromethylthiolation Reactions. Chem. Rev. 2015, 115, 826–870. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.M.; Reisberg, S.H.; Shunatona, H.P.; Patel, J.S.; Toste, F.D. Chiral Anion Phase Transfer of Aryldiazonium Cations: An Enantioselective Synthesis of C3-Diazenated Pyrroloindolines. Angew. Chem. Int. Ed. 2014, 53, 5600–5603. [Google Scholar] [CrossRef] [PubMed]
- Seebach, D. Methods of Reactivity Umpolung. Angew. Chem. Int. Ed. Engl. 1979, 18, 239–258. [Google Scholar] [CrossRef]
- Smoliakova, I.P.; Caple, R.; Gregory, D.; Smit, W.A.; Shashkov, A.S.; Chizhov, O.S. Highly Selective Formation of a β-C-Glucosidic Bond in the Reactions of Arscl-Glucal Adducts with Silicon-Containing Nucleophiles. J. Org. Chem. 1995, 60, 1221–1227. [Google Scholar] [CrossRef]
- Han, M.M.; Smoliakova, I.P.; Koikov, L.N. Stereoselective synthesis of C-[2-S-(p-tolyl)-2-thio-β-d-galactopyranosyl] compounds using the reaction of TolSCl adducts of D-galactal with C-nucleophiles. Carbohydr. Res. 2000, 323, 202–207. [Google Scholar] [CrossRef]
- Wöhler, F.; Liebig, J. Untersuchungen über das Radikal der Benzoesäure. Ann. Pharm. 1832, 3, 249–282. [Google Scholar] [CrossRef]
- Lapworth, A. Reactions involving the addition of hydrogen cyanide to carbon compounds. J. Chem. Soc. 1903, 83, 995–1005. [Google Scholar] [CrossRef]
- Wiberg, K.B. The Deuterium Isotope Effect of Some Ionic Reactions of Benzaldehyde. J. Am. Chem. Soc. 1954, 76, 5371–5375. [Google Scholar] [CrossRef]
- Johnson, J.S. Catalyzed reactions of acyl anion equivalents. Angew. Chem. Int. Ed. 2004, 43, 1326–1328. [Google Scholar] [CrossRef] [PubMed]
- Ukai, T.; Tanaka, R.; Dokawa, T. A new catalyst for acyloin condensation I. J. Pharm. Soc. Jpn. 1943, 63, 296–300. [Google Scholar]
- Kluger, R. Lessons from thiamin-watching. Pure Appl. Chem. 1997, 69, 1957–1967. [Google Scholar] [CrossRef]
- Breslow, R. On the Mechanism of Thiamine Action 4. Evidence from Studies on Model Systems. J. Am. Chem. Soc. 1958, 80, 3719–3726. [Google Scholar] [CrossRef]
- Breslow, R.; Schmuck, C. The mechanism of thiazolium catalysis. Tetrahedron Lett. 1996, 37, 8241–8242. [Google Scholar] [CrossRef]
- White, M.J.; Leeper, F.J. Kinetics of the thiazolium ion-catalyzed benzoin condensation. J. Org. Chem. 2001, 66, 5124–5131. [Google Scholar] [CrossRef] [PubMed]
- Breslow, R.; Kim, R. The Thiazolium Catalyzed Benzoin Condensation with Mild Base Does Not Involve a Dimer Intermediate. Tetrahedron Lett. 1994, 35, 699–702. [Google Scholar] [CrossRef]
- Teles, J.H.; Melder, J.P.; Ebel, K.; Schneider, R.; Gehrer, E.; Harder, W.; Brode, S.; Enders, D.; Breuer, K.; Raabe, G. The chemistry of stable carbenes 2. Benzoin-type condensations of formaldehyde catalyzed by stable carbenes. Helv. Chim. Acta 1996, 79, 61–83. [Google Scholar] [CrossRef]
- Enders, D.; Balensiefer, T. Nucleophilic carbenes in asymmetric organocatalysis. Acc. Chem. Res. 2004, 37, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Butlerow, A. Bildung einer zuckerartigen Substanz durch Synthese. Liebigs Ann. Chem. 1861, 120, 295–298. [Google Scholar] [CrossRef]
- Shigemasa, Y.; Ueda, T.; Saimoto, H. Formose Reactions 26. 1St Synthesis of DL-2-C-Hydroxymethyl-3-Pentulose in the Formose Reaction. J. Carbohydr. Res. 1989, 8, 669–673. [Google Scholar] [CrossRef]
- Tajima, H.; Niitsu, T.; Inoue, H. Polymerization of Formaldehyde by an Immobilized Thiamine Catalyst on Cation-Exchange Resin. J. Chem. Eng. Jpn. 1999, 32, 776–782. [Google Scholar] [CrossRef]
- López-Calahorra, F.; Castro, E.; Ochoa, A.; Marti, J. Further evidences about the role of bis(thiazolin-2-ylidene)s as the actual catalytic species in the generalised benzoin condensation. Tetrahedron Lett. 1996, 37, 5019–5022. [Google Scholar] [CrossRef]
- Li, G.Q.; Dai, L.X.; You, S.L. Thiazolium-derived N-heterocyclic carbene-catalyzed cross-coupling of aldehydes with unactivated imines. Chem. Commun. 2007, 852–854. [Google Scholar] [CrossRef] [PubMed]
- Lappert, M.F.; Maskell, R.K. A New Class of Benzoin Condensation Catalyst, the Bi-(1,3-Dialkylimidazolidin-2-Ylidenes). J. Chem. Soc. Chem. Commun. 1982, 580–581. [Google Scholar] [CrossRef]
- Iwamoto, K.; Hamaya, M.; Hashimoto, N.; Kimura, H.; Suzuki, Y.; Sato, M. Benzoin reaction in water as an aqueous medium catalyzed by benzimidazolium salt. Tetrahedron Lett. 2006, 47, 7175–7177. [Google Scholar] [CrossRef]
- Reich, B.J.E.; Justice, A.K.; Beckstead, B.T.; Reibenspies, J.H.; Miller, S.A. Cyanide-catalyzed cyclizations via aldimine coupling. J. Org. Chem. 2004, 69, 1357–1359. [Google Scholar] [CrossRef] [PubMed]
- Breslow, R.; Kool, E. A ɣ-Cyclodextrin Thiazolium Salt Holoenzyme Mimic for the Benzoin Condensation. Tetrahedron Lett. 1988, 29, 1635–1638. [Google Scholar] [CrossRef]
- Ikeda, H.; Horimoto, Y.; Nakata, M.; Ueno, A. Artificial holoenzymes for benzoin condensation using thiazolio-appended β-cyclodextrin dimers. Tetrahedron Lett. 2000, 41, 6483–6487. [Google Scholar] [CrossRef]
- Xin, L.H.; Bausch, C.C.; Johnson, J.S. Mechanism and scope of the cyanide-catalyzed cross silyl benzoin reaction. J. Am. Chem. Soc. 2005, 127, 1833–1840. [Google Scholar]
- Brook, A.G. Some Molecular-Rearrangements of Organosilicon Compounds. Acc. Chem. Res. 1974, 7, 77–84. [Google Scholar] [CrossRef]
- Moser, W.H. The Brook rearrangement in tandem bond formation strategies. Tetrahedron 2001, 57, 2065–2084. [Google Scholar] [CrossRef]
- Bausch, C.C.; Johnson, J.S. Cyanide-catalyzed additions of acyl phosphonates to aldehydes: A new acyl donor for benzoin-type reactions. Adv. Synth. Catal. 2005, 347, 1207–1211. [Google Scholar] [CrossRef]
- Mattson, A.E.; Bharadwaj, A.R.; Zuhl, A.M.; Scheidt, K.A. Thiazolium-catalyzed additions of acylsilanes: A general strategy for acyl anion addition reactions. J. Org. Chem. 2006, 71, 5715–5724. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.S.; Biju, A.T.; Nair, V. Recent advances in N-heterocyclic carbene (NHC)-catalysed benzoin reactions. Beilstein J. Org. Chem. 2016, 12, 444–461. [Google Scholar] [CrossRef] [PubMed]
- Stetter, H.; Schreckenberg, M. Addition of aldehydes to activated double bonds. Angew. Chem. 1973, 85, 89. [Google Scholar] [CrossRef]
- Stetter, H. Catalyzed Addition of Aldehydes to Activated Double-Bonds—New Synthetic Approach. Angew. Chem. Int. Ed. Engl. 1976, 15, 639–647. [Google Scholar] [CrossRef]
- Stetter, H.; Kuhlmann, H. The catalyzed nucleophilic addition of aldehydes to electrophilic double bonds. Org. React. 1991, 40, 408–496. [Google Scholar]
- Trost, B.M.; Shuey, C.D.; Di Ninno, F. Stereocontrolled Total Synthesis of (±)-Hirsutic Acid-C. J. Am. Chem. Soc. 1979, 101, 1284–1285. [Google Scholar]
- Ciganek, E. Esters of 2,3-Dihydro-3-Oxobenzofuran-2-Acetic Acid and 3,4-Dihydro-4-Oxo-2H-1-Benzopyran-3-Acetic Acid by Intramolecular Stetter Reactions. Synthesis 1995, 1311–1314. [Google Scholar] [CrossRef]
- Zhou, Z.-Z.; Ji, F.-Q.; Cao, M.; Yang, G.-F. An Efficient Intramolecular Stetter Reaction in Room Temperature Ionic Liquids Promoted By Microwave Irradiation. Adv. Synth. Catal. 2006, 348, 1826–1830. [Google Scholar] [CrossRef]
- Enders, D.; Breuer, K.; Runsink, J.; Teles, J.H. The first asymmetric intramolecular Stetter reaction. Helv. Chim. Acta 1996, 79, 1899–1902. [Google Scholar] [CrossRef]
- Kerr, M.S.; Rovis, T. Enantioselective synthesis of quaternary stereocenters via a catalytic asymmetric Stetter reaction. J. Am. Chem. Soc. 2004, 126, 8876–8877. [Google Scholar] [CrossRef] [PubMed]
- Christmann, M. New developments in the asymmetric Stetter reaction. Angew. Chem. Int. Ed. 2005, 44, 2632–2634. [Google Scholar] [CrossRef] [PubMed]
- Mattson, A.E.; Bharadwaj, A.R.; Scheidt, K.A. The thiazolium-catalyzed sila-Stetter reaction: Conjugate addition of acylsilanes to unsaturated esters and ketones. J. Am. Chem. Soc. 2004, 126, 2314–2315. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Perreault, S.; Rovis, T. Catalytic Asymmetric Intermolecular Stetter Reaction of Glyoxamides with Alkylidenemalonates. J. Am. Chem. Soc. 2008, 130, 14066–14067. [Google Scholar] [CrossRef] [PubMed]
- DiRocco, D.A.; Oberg, K.M.; Dalton, D.M.; Rovis, T. Catalytic Asymmetric Intermolecular Stetter Reaction of Heterocyclic Aldehydes with Nitroalkenes: Backbone Fluorination Improves Selectivity. J. Am. Chem. Soc. 2009, 131, 10872–10874. [Google Scholar] [CrossRef] [PubMed]
- Burstein, C.; Glorius, F. Organocatalyzed conjugate umpolung of α,β-unsaturated aldehydes for the synthesis of ɣ-butyrolactones. Angew. Chem. Int. Ed. 2004, 43, 6205–6208. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.S.; Rosen, E.L.; Bode, J.W. N-Heterocyclic carbene-catalyzed generation of homoenolates: ɣ-butyrolactones by direct annulations of enals and aldehydes. J. Am. Chem. Soc. 2004, 126, 14370–14371. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, W.A. N-Heterocyclic carbenes: A new concept in organometallic catalysis. Angew. Chem. Int. Ed. 2002, 41, 1290–1309. [Google Scholar] [CrossRef]
- Burstein, C.; Tschan, S.; Xie, X.; Glorius, F. N-Heterocyclic carbene-catalyzed conjugate umpolung for the synthesis of ɣ-butyrolactones. Synthesis 2006, 2418–2439. [Google Scholar]
- Nair, V.; Vellalath, S.; Poonoth, M.; Suresh, E. N-Heterocyclic carbene-catalyzed reaction of chalcones and enals via homoenolate: An efficient synthesis of 1,3,4-trisubstituted cyclopentenes. J. Am. Chem. Soc. 2006, 128, 8736–8737. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Bode, J.W. Enantioselective, NHC-Catalyzed bicyclo-β-lactam formation via direct annulations of enals and unsaturated N-sulfonyl ketimines. J. Am. Chem. Soc. 2008, 130, 418–419. [Google Scholar] [CrossRef] [PubMed]
- Huisgen, R.; Blaschke, H.; Brunn, E. Zur 1.3-Dipolaren Cycloaddition Aromatischer Nitriloxide an Azodicarbonsaureester. Tetrahedron Lett. 1966, 405–409. [Google Scholar] [CrossRef]
- White, D.A.; Baizer, M.M. Catalysis of Michael Reaction by Tertiary Phosphines. Tetrahedron Lett. 1973, 3597–3600. [Google Scholar] [CrossRef]
- Gomez-Bengoa, E.; Cuerva, J.M.; Mateo, C.; Echavarren, A.M. Michael reaction of stabilized carbon nucleophiles catalyzed by [RuH2(PPh3)4]. J. Am. Chem. Soc. 1996, 118, 8553–8565. [Google Scholar] [CrossRef]
- Stewart, I.C.; Bergman, R.G.; Toste, F.D. Phosphine-catalyzed hydration and hydroalkoxylation of activated olefins: Use of a strong nucleophile to generate a strong base. J. Am. Chem. Soc. 2003, 125, 8696–8697. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Kazmaier, U. Internal Redox Catalyzed by Triphenylphosphine. J. Am. Chem. Soc. 1992, 114, 7933–7935. [Google Scholar] [CrossRef]
- Trost, B.M.; Li, C.J. Novel Umpolung in C–C Bond Formation Catalyzed by Triphenylphosphine. J. Am. Chem. Soc. 1994, 116, 3167–3168. [Google Scholar] [CrossRef]
- Trost, B.M.; Li, C.J. Phosphine-Catalyzed Isomerization-Addition of Oxygen Nucleophiles to 2-Alkynoates. J. Am. Chem. Soc. 1994, 116, 10819–10820. [Google Scholar] [CrossRef]
- Alvarez-Ibarra, C.; Csákÿ, A.G.; de la Oliva, C.G.; Gomez, C. Carboxylates as pronucleophiles in the phosphine-catalyzed ɣ-addition reaction. Tetrahedron Lett. 1999, 40, 8465–8467. [Google Scholar] [CrossRef]
- Trost, B.M.; Dake, G.R. Nitrogen pronucleophiles in the phosphine-catalyzed ɣ-addition reaction. J. Org. Chem. 1997, 62, 5670–5671. [Google Scholar] [CrossRef]
- Fischer, C.; Smith, S.W.; Powell, D.A.; Fu, G.C. Umpolung of Michael acceptors catalyzed by N-heterocyclic carbenes. J. Am. Chem. Soc. 2006, 128, 1472–1473. [Google Scholar] [CrossRef] [PubMed]
- Virieux, D.; Guillouzic, A.F.; Cristau, H.J. Phosphines catalyzed nucleophilic addition of azoles to allenes: synthesis of allylazoles and indolizines. Tetrahedron 2006, 62, 3710–3720. [Google Scholar] [CrossRef]
- Rauhut, M.M.; Currier, H. Dialkyl 2-Methyleneglutarates. U.S. Patent 3074999, 22 January 1963. [Google Scholar]
- McClure, J.D. Dimerization of Acrylonitrile. U.S. Patent 3225083, 21 December 1965. [Google Scholar]
- Baizer, M.M.; Anderson, J.D. Electrolytic Reductive Coupling 8. Utilization and a New Preparation of α-Methyleneglutaronitrile. J. Org. Chem. 1965, 30, 1357–1360. [Google Scholar] [CrossRef]
- Wang, L.C.; Luis, A.L.; Agapito, F.; Jang, H.Y.; Krische, M.J. Organocatalytic Michael cycloisomerization of bis(enones): The intramolecular Rauhut–Currier reaction. J. Am. Chem. Soc. 2002, 124, 2402–2403. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.A.; Mergott, D.J.; Roush, W.R. The vinylogous intramolecular Morita–Baylis–Hillman reaction: Synthesis of functionalized cyclopentenes and cyclohexenes with trialkylphosphines as nucleophilic catalysts. J. Am. Chem. Soc. 2002, 124, 2404–2405. [Google Scholar] [CrossRef] [PubMed]
- Krafft, M.E.; Haxell, T.F.N. Organomediated Morita–Baylis–Hillman cyclization reactions. J. Am. Chem. Soc. 2005, 127, 10168–10169. [Google Scholar] [CrossRef] [PubMed]
- Tran, Y.S.; Kwon, O. Phosphine-catalyzed [4+2] annulation: Synthesis of cyclohexenes. J. Am. Chem. Soc. 2007, 129, 12632–12633. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Hosseinpour, R. An Efficient Synthesis of Tetraalkyl 2,3,4,5-Thiophenetetracarboxylate Derivatives. Synthesis 2009, 1960–1962. [Google Scholar] [CrossRef]
- Dong, X.; Liang, L.; Li, E.; Huang, Y. Highly Enantioselective Intermolecular Cross Rauhut–Currier Reaction Catalyzed by a Multifunctional Lewis Base Catalyst. Angew. Chem. Int. Ed. 2015, 54, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Suzuki, Z.; Hirose, H. A Tertiary Phosphine-Catalyzed Reaction of Acrylic Compounds with Aldehydes. Bull. Chem. Soc. Jpn. 1968, 41, 2815. [Google Scholar] [CrossRef]
- Baylis, A.B.; Hillman, M.E.D. Acrylic Compounds. Ger. Offen DE 2155113, 10 May 1972. Chem. Abstr. 1972, 77, 34174. [Google Scholar]
- Basavaiah, D.; Rao, A.J.; Satyanarayana, T. Recent advances in the Baylis–Hillman reaction and applications. Chem. Rev. 2003, 103, 811–891. [Google Scholar] [CrossRef] [PubMed]
- Langer, P. New strategies for the development of an asymmetric version of the Baylis–Hillman reaction. Angew. Chem. Int. Ed. 2000, 39, 3049–3052. [Google Scholar] [CrossRef]
- Basavaiah, D.; Kumaragurubaran, N.; Sharada, D.S. Baylis–Hillman chemistry: A novel synthesis of functionalized 1,4-pentadienes. Tetrahedron Lett. 2001, 42, 85–87. [Google Scholar] [CrossRef]
- Basavaiah, D.; Sharada, D.S.; Kumaragurubaran, N.; Reddy, R.M. The Baylis–Hillman reaction: One-pot facile synthesis of 2,4-functionalized 1,4-pentadienes. J. Org. Chem. 2002, 67, 7135–7137. [Google Scholar] [CrossRef] [PubMed]
- Koech, P.K.; Krische, M.J. Phosphine catalyzed α-arylation of enones and enals using hypervalent bismuth reagents: Regiospecific enolate arylation via nucleophilic catalysis. J. Am. Chem. Soc. 2004, 126, 5350–5351. [Google Scholar] [CrossRef] [PubMed]
- Robiette, R.; Aggarwal, V.K.; Harvey, J.N. Mechanism of the Morita–Baylis–Hillman reaction: A computational investigation. J. Am. Chem. Soc. 2007, 129, 15513–15525. [Google Scholar] [CrossRef] [PubMed]
- Plata, R.E.; Singleton, D.A. A Case Study of the Mechanism of Alcohol-Mediated Morita Baylis–Hillman Reactions. The Importance of Experimental Observations. J. Am. Chem. Soc. 2015, 137, 3811–3826. [Google Scholar] [CrossRef] [PubMed]
- Krafft, M.E.; Haxell, T.F.N.; Seibert, K.A.; Abboud, K.A. Mechanistic implications in the Morita–Baylis–Hillman alkylation: Isolation and characterization of an intermediate. J. Am. Chem. Soc. 2006, 128, 4174–4175. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.W.; Krische, M.J. Regio- and stereoselective construction of ɣ-butenolides through phosphine-catalyzed substitution of Morita–Baylis–Hillman acetates: An organocatalytic allylic alkylation. Angew. Chem. Int. Ed. 2004, 43, 6689–6691. [Google Scholar] [CrossRef] [PubMed]
- Masson, G.; Housseman, C.; Zhu, J. The enantioselective morita-baylis-hiliman reaction and its aza counterpart. Angew. Chem. Int. Ed. 2007, 46, 4614–4628. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Shi, M. Recent Advances in Organocatalytic Asymmetric Morita–Baylis–Hillman/aza-Morita–Baylis–Hillman Reactions. Chem. Rev. 2013, 113, 6659–6690. [Google Scholar] [CrossRef] [PubMed]
- Gergelitsova, I.; Tauchman, J.; Cisarova, I.; Vesely, J. Bifunctional (Thio)urea-Phosphine Organocatalysts Derived from d-Glucose and α-Amino Acids and Their Application to the Enantioselective Morita–Baylis–Hillman Reaction. Synlett 2015, 26, 2690–2696. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, Q.; Shi, M. Enantioselective Synthesis of Polycyclic Indole Derivatives Based on aza-Morita–Baylis–Hillman Reaction. ACS Catal. 2015, 5, 6608–6614. [Google Scholar] [CrossRef]
- Krishna, P.R.; Kannan, V.; Reddy, P.V.N. N-Methylprolinol catalysed asymmetric Baylis–Hillman reaction. Adv. Synth. Catal. 2004, 346, 603–606. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Wang, B.-L.; Cao, J.-J.; Chen, L.; Zhang, Y.-X.; Wang, C.; Zhou, J. Organocatalytic Asymmetric Synthesis of Substituted 3-Hydroxy-2-oxindoles via Morita–Baylis–Hillman Reaction. J. Am. Chem. Soc. 2010, 132, 15176–15178. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Chen, L.H.; Teng, W.D. Asymmetric aza-Morita–Baylis–Hillman reaction of N-sulfonated imines with methyl vinyl ketone catalyzed by chiral phosphine Lewis bases bearing perfluoroalkanes as pony tails. Adv. Synth. Catal. 2005, 347, 1781–1789. [Google Scholar] [CrossRef]
- Shi, M.; Chen, L.H.; Li, C.Q. Chiral phosphine Lewis bases catalyzed asymmetric aza-Baylis-Hiliman reaction of N-sulfonated imines with activated olefins. J. Am. Chem. Soc. 2005, 127, 3790–3800. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-L.; Yuan, K.; Wu, X.-Y. Chiral phosphine-squaramides as enantioselective catalysts for the intramolecular Morita-Baylis-Hillman reaction. Chem. Commun. 2011, 47, 1012–1014. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Takizawa, S.; Sasai, H. Bifunctional organocatalysts for enantioselective aza-Morita–Baylis–Hillman reaction. J. Am. Chem. Soc. 2005, 127, 3680–3681. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, H.; Yu, X.H.; Zu, L.S.; Wang, W. Chiral binaphthyl-derived amine-thiourea organocatalyst-promoted asymmetric Morita–Baylis–Hillman reaction. Org. Lett. 2005, 7, 4293–4296. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, S.; Rémond, E.; Arteaga Arteaga, F.; Yoshida, Y.; Sridharan, V.; Bayardon, J.; Jugé, S.; Sasai, H. P-chirogenic organocatalysts: Application to the aza-Morita–Baylis–Hillman (aza-MBH) reaction of ketimines. Chem. Commun. 2013, 49, 8392–8394. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Lan, J.; Kwon, O. An expedient phosphine-catalyzed [4+2] annulation: Synthesis of highly functionalized tetrahydropyridines. J. Am. Chem. Soc. 2003, 125, 4716–4717. [Google Scholar] [CrossRef] [PubMed]
- Sunden, H.; Ibrahem, I.; Eriksson, L.; Cordova, A. Direct catalytic enantioselective aza-Diels–Alder reactions. Angew. Chem. Int. Ed. 2005, 44, 4877–4880. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Komiyama, S.; Ishitani, H. The first enantioselective aza-Diels–Alder reactions of imino dienophiles on use of a chiral zirconium catalyst. Angew. Chem. Int. Ed. 1998, 37, 979–981. [Google Scholar] [CrossRef]
- Yao, S.L.; Saaby, S.; Hazell, R.G.; Jørgensen, K.A. Catalytic enantioselective aza-Diels–Alder reactions of imines—An approach to optically active nonproteinogenic α-amino acids. Chem. Eur. J. 2000, 6, 2435–2448. [Google Scholar] [CrossRef]
- Itoh, J.; Fuchibe, K.; Akiyama, T. Chiral Bronsted acid catalyzed enantioselective aza-Diels–Alder reaction of Brassard’s diene with imines. Angew. Chem. Int. Ed. 2006, 45, 4796–4798. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.; Xin, J.; Liu, Y.; Zhou, X.; Liu, X.; Feng, X. Enantioselective aza-Diels–Alder reaction of aldimines with Danishefsky-Type Diene catalyzed by chiral scandium(III)-N,N’-dioxide complexes. J. Org. Chem. 2008, 73, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Jurcik, V.; Arai, K.; Salter, M.; Yamashita, Y.; Kobayashi, S. Niobium-catalyzed highly enantioselective aza-Diels–Alder reactions. Adv. Synth. Catal. 2008, 350, 647–651. [Google Scholar] [CrossRef]
- He, M.; Struble, J.R.; Bode, J.W. Highly enantioselective azadiene Diels–Alder reactions catalyzed by chiral N-heterocyclic carbenes. J. Am. Chem. Soc. 2006, 128, 8418–8420. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ruhl, K.E.; Rovis, T. N-Heterocyclic-Carbene-Catalyzed Asymmetric Oxidative Hetero-Diels–Alder Reactions with Simple Aliphatic Aldehydes. Angew. Chem. Int. Ed. 2012, 51, 12330–12333. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Ni, Q.; Zhu, C.; Raabe, G.; Enders, D. Asymmetric N-Heterocyclic Carbene Catalyzed Annulation of 2-Alkenylbenzothiazoles with α-Chloro Aldehydes. Synthesis 2015, 47, 421–428. [Google Scholar] [CrossRef]
- France, S.; Weatherwax, A.; Taggi, A.E.; Lectka, T. Advances in the catalytic, asymmetric synthesis of β-lactams. Acc. Chem. Res. 2004, 37, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.R.; He, L.; Wu, X.; Shao, P.L.; Ye, S. Chiral N-heterocyclic carbene catalyzed Staudinger reaction of ketenes with imines: Highly enantioselective synthesis of N-Boc β-lactams. Org. Lett. 2008, 10, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.M.; Lu, X.Y. Phosphine-Catalyzed Cycloaddition of 2,3-Butadienoates Or 2-Butynoates with Electron-Deficient Olefins—A Novel [3+2]-Annulation Approach to Cyclopentenes. J. Org. Chem. 1995, 60, 2906–2908. [Google Scholar] [CrossRef]
- Pham, T.Q.; Pyne, S.G.; Skelton, B.W.; Whitet, A.H. Synthesis of carbocyclic hydantocidins via regioselective and diastereoselective phosphine-catalyzed [3+2]-cycloadditions to 5-methylenehydantoins. J. Org. Chem. 2005, 70, 6369–6377. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.R.; Williams, M.C.; Pyne, S.G.; Ung, A.T.; Skelton, B.W.; White, A.H.; Turner, P. Synthesis of 2-azaspiro[4.4]nonan-1-ones via phosphine-catalysed [3+2]-cycloadditions. Tetrahedron 2005, 61, 8120–8129. [Google Scholar] [CrossRef]
- Shao, P.L.; Chen, X.Y.; Ye, S. Formal [3+2] Cycloaddition of Ketenes and Oxaziridines Catalyzed by Chiral Lewis Bases: Enantioselective Synthesis of Oxazolin-4-ones. Angew. Chem. Int. Ed. 2010, 49, 8412–8416. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).