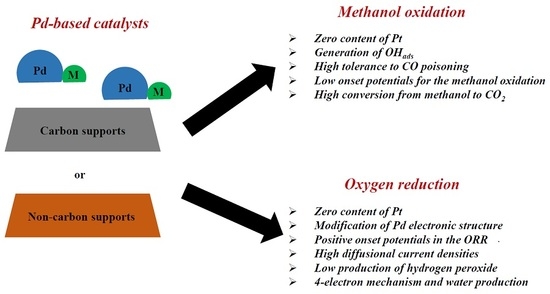
Palladium-Based Catalysts as Electrodes for Direct Methanol Fuel Cells: A Last Ten Years Review
Abstract
:1. Introduction
2. Methanol Oxidation on Pd-Based Catalysts
2.1. Carbon-Supported Pd-Alloys
2.2. Non Carbon-Supported Pd-Alloys
3. Oxygen Reduction on Pd-Based Catalysts
3.1. Carbon-Supported Pd-Alloys
3.2. Non Carbon-Supported Pd-Alloys
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DMFC | Direct Methanol Fuel Cells |
| CNT | Carbon Nanotubes |
| MWCNT | Multi-Walled Carbon Nanotubes |
| RGO | Reduced Graphene Oxide ( by sodium borohydride) |
| MOR | Methanol oxidation reaction |
| MEA | Membrane electrode assembly |
| DEMS | Differential Electrochemical Mass Spectrometry |
| ORR | Oxygen Reduction Reaction |
| VC | Vulcan Carbon |
| TKK | Tanaka Kikinzoku Kogyo® |
References
- Lee, J.B.; Park, Y.K.; Yang, O.B.; Kang, Y.K.; Jun, K.W.; Lee, Y.J.; Kim, H.Y.; Lee, K.H.; Choi, W.C. Synthesis of porous carbons having surface functional groups and their application to direct-methanol fuel cells. J. Power Sources 2006, 158, 1251–1255. [Google Scholar] [CrossRef]
- Brouzgou, A.; Song, S.Q.; Tsiakaras, P. Low and non-platinum electrocatalysts for PEMFCs: Current status, challenges and prospects. Appl. Catal. B 2012, 127, 371–388. [Google Scholar] [CrossRef]
- Fournier, J.; Faubert, G.; Tilquin, J.Y.; Cote, R.; Guay, D.; Dodelet, J.P. High-performance, low Pt content, catalysts for the electroreduction of oxygen in polymer electrolyte fuel cells. J. Electrochem. Soc. 1997, 144, 145–154. [Google Scholar] [CrossRef]
- Lefèvre, M.; Dodelet, J.P.; Bertrand, P. Molecular oxygen reduction in PEM fuel cells: Evidence for the simultaneous presence of two active sites in Fe-based catalysts. J. Phys. Chem. B 2002, 106, 8705–8713. [Google Scholar] [CrossRef]
- Matter, P.H.; Zhang, L.; Ozkan, U.S. The role of nanostructure in nitrogen-containing carbon catalysts for the oxygen reduction reaction. J. Catal. 2006, 239, 83–96. [Google Scholar] [CrossRef]
- Zaikovskii, V.I.; Nagabhushana, K.S.; Kriventsov, V.V.; Loponov, K.N.; Cherepanova, S.V.; Kvon, R.I.; Bönnemann, H.; Kochubey, D.I.; Savinova, E.R. Synthesis and structural characterization of Se-modified carbon-supported Ru nanoparticles for the oxygen reduction reaction. J. Phys. Chem. B 2006, 110, 6881–6890. [Google Scholar] [CrossRef] [PubMed]
- Serov, A.A.; Min, M.; Chai, G.; Han, S.; Kang, S.; Kwak, C. Preparation, characterization, and high performance of RuSe/C for direct methanol fuel cells. J. Power Sources 2008, 175, 175–182. [Google Scholar] [CrossRef]
- Mustain, W.E.; Prakash, J. Kinetics and mechanism for the oxygen reduction reaction on polycrystalline cobalt-palladium electrocatalysts in acid media. J. Power Sources 2007, 170, 28–37. [Google Scholar] [CrossRef]
- Assiongbon, K.A.; Roy, D. Electro-oxidation of methanol on gold in alkaline media: Adsorption characteristics of reaction intermediates studied using time resolved electro-chemical impedance and surface plasmon resonance techniques. Surf. Sci. 2005, 594, 99–119. [Google Scholar] [CrossRef]
- Hu, F.P.; Shen, P.K. Ethanol oxidation on hexagonal tungsten carbide single nanocrystal-supported Pd electrocatalyst. J. Power Sources 2007, 173, 877–881. [Google Scholar] [CrossRef]
- Li, R.S.; Wei, Z.; Huang, T.; Yu, A.S. Ultrasonic-assisted synthesis of Pd–Ni alloy catalysts supported on multi-walled carbon nanotubes for formic acid electrooxidation. Electrochimica Acta 2011, 56, 6860–6865. [Google Scholar] [CrossRef]
- Shao, M.H. Palladium-based electrocatalysts for hydrogen oxidation and oxygen reduction reactions. J. Power Sources 2011, 196, 2433–2444. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Tan, D.S.L.; Lee, J.M.; Chan, S.H.; Wang, J.Y.; Wang, X. Tb promoted Pd/C catalysts for the electrooxidation of ethanol in alkaline media. Int. J. Hydrogen Energy 2011, 36, 9645–9652. [Google Scholar] [CrossRef]
- Grden, M.; Łukaszewski, M.; Jerkiewicz, G.; Czerwinski, A. Electrochemical behavior of palladium electrode: Oxidation, electrodissolution and ionic adsorption. Electrochimica Acta 2008, 53, 7583–7598. [Google Scholar] [CrossRef]
- Bianchini, C.; Shen, P.K. Palladium-based electrocatalysts for alcohol oxidation in half cells and in direct alcohol fuel cells. Chem. Rev. 2009, 109, 4183–4206. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.C.; Liu, C.W.; Wang, K.W. Improvement of oxygen reduction reaction and methanol tolerance characteristics for PdCo electrocatalysts by Au alloying and CO treatment. Chem. Commun. 2011, 47, 11927–11929. [Google Scholar] [CrossRef] [PubMed]
- Calderón, J.C.; Mahata, N.; Pereira, M.F.R.; Figueiredo, J.L.; Fernandes, V.R.; Rangel, C.M.; Calvillo, L.; Lazaro, M.J.; Pastor, E. Pt–Ru catalysts supported on carbon xerogels for PEM fuel cells. Int. J. Hydrogen Energy 2012, 37, 7200–7211. [Google Scholar]
- Yu, X.W.; Ye, S.Y. Recent advances in activity and durability enhancement of Pt/C catalytic cathode in PEMFC: Part I. Physico-chemical and electronic interaction between Pt and carbon support, and activity enhancement of Pt/C catalyst. J. Power Sources 2007, 172, 133–144. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Yao, Q.S.; Wu, X.D.; Fu, Y.S.; Deng, K.M.; Wang, X. Intimately coupled hybrid of graphitic carbon nitride nanoflakelets with reduced graphene oxide for supporting Pd nanoparticles: A stable nanocatalyst with high catalytic activity towards formic acid and methanol electrooxidation. Electrochim Acta 2016, 200, 131–141. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, J.X.; Tiwary, C.S.; Ma, Z.Y.; Huang, H.J.; Zhang, J.F.; Lu, Z.Y.; Huang, W.; Wu, Y.P. Palladium nanoparticles supported on nitrogen and sulfur dual-doped graphene as highly active electrocatalysts for formic acid and methanol oxidation. ACS Appl. Mater. Interfaces 2016, 8, 10858–10865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Huang, H.J.; Li, F.; Deng, K.M.; Wang, X. Palladium nanoparticles supported on graphitic carbon nitride-modified reduced graphene oxide as highly efficient catalysts for formic acid and methanol electrooxidation. J. Mat. Chem. A 2014, 2, 19084–19094. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, Y.N.; Chen, K.; Li, J.; Li, W.J.; Tang, P.; Zhao, H.B.; Zhu, Q.J.; Bao, X.H.; Ma, D. Monodispersed bimetallic PdAg nanoparticles with twinned structures: Formation and enhancement for the methanol oxidation. Sci. Rep. 2014, 4, 4288–4296. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sheng, Z.M.; Yang, H.B.; Jiang, S.P.; Li, C.M. Electrocatalysis of carbon black- or activated carbon nanotubes-supported Pd–Ag towards methanol oxidation in alkaline media. Int. J. Hydrogen Energy 2010, 35, 10087–10093. [Google Scholar] [CrossRef]
- Luo, B.; Liu, S.M.; Zhi, L.J. Chemical approaches toward graphene-based nanomaterials and their applications in energy-related areas. Small 2012, 8, 630–646. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Z.; Chen, M.X.; Huang, G.B.; Yang, N.; Zhang, L.; Wang, H.; Liu, Y.; Wang, W.; Gao, J.P. A green method to prepare Pd–Ag nanoparticles supported on reduced graphene oxide and their electrochemical catalysis of methanol and ethanol oxidation. J. Power Sources 2014, 263, 13–21. [Google Scholar] [CrossRef]
- Jurzinsky, T.; Cremers, C.; Pinkwart, K.; Tübke, J. On the influence of Ag on Pd-based electrocatalyst for methanol oxidation in alkaline media: A comparative differential electrochemical mass spectrometry study. Electrochimica Acta 2016, 199, 270–279. [Google Scholar] [CrossRef]
- Shen, P.K.; Xu, C.W.; Zeng, R.; Liu, Y.L. Electro-oxidation of methanol on NiO-promoted Pt/C and Pd/C catalysts. Electrochem. Solid-State Lett. 2006, 9, A39–A42. [Google Scholar] [CrossRef]
- Li, G.L.; Jiang, L.H.; Jiang, Q.; Wang, S.L.; Sun, G.Q. Preparation and characterization of PdxAgy/C electrocatalysts for ethanol electrooxidation reaction in alkaline media. Electrochimica Acta 2011, 56, 7703–7711. [Google Scholar] [CrossRef]
- Qi, Z.; Geng, H.; Wang, X.; Zhao, C.; Ji, H.; Zhang, C.; Xu, J.; Zhang, Z. Novel nanocrystalline PdNi alloy catalysts for methanol and ethanol electro-oxidation in alkaline media. J. Power Sources 2011, 196, 5823–5828. [Google Scholar] [CrossRef]
- Amin, R.S.; Abdel Hameed, R.M.; El-Khatiba, K.M. Microwave heated synthesis of carbon supported Pd, Ni and Pd–Ni nanoparticles for methanol oxidation in KOH solution. Appl. Catal. B 2014, 148-149, 557–567. [Google Scholar] [CrossRef]
- Amin, R.S.; Abdel Hameed, R.M.; El-Khatib, K.M.; Elsayed Youssef, M. Electrocatalytic activity of nanostructured Ni and Pd–Ni on Vulcan XC-72R carbon black for methanol oxidation in alkaline medium. Int. J. Hydrogen Energy 2014, 39, 2026–2041. [Google Scholar] [CrossRef]
- Liu, Z.L.; Zhang, X.H.; Hong, L. Physical and electrochemical characterization of nanostructured Pd/C and PdNi/C catalysts for methanol oxidation. Electrochem. Commun. 2009, 11, 925–928. [Google Scholar] [CrossRef]
- Calderón, J.C.; Nieto-Monge, M.J.; Pérez-Rodríguez, S.; Pardo, J.I.; Moliner, R.; Lázaro, M.J. Palladium–nickel catalysts supported on different chemically-treated carbon blacks for methanol oxidation in alkaline media. Int. J. Hydrogen Energy 2016. [Google Scholar] [CrossRef]
- Singh, R.N.; Singh, A.; Anindita. Electrocatalytic activity of binary and ternary composite films of Pd, MWCNT and Ni, Part II: Methanol electrooxidation in 1 M KOH. Int. J. Hydrogen Energy 2009, 34, 2052–2057. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Zhan, L.; Tian, J.N.; Nie, S.L.; Ning, Z. MnO2 modified multi-walled carbon nanotubes supported Pd nanoparticles for methanol electro-oxidation in alkaline media. Int. J. Hydrogen Energy 2010, 35, 10522–10526. [Google Scholar] [CrossRef]
- Sasahara, A.; Tamura, H.; Tanaka, K.I. Catalytic activity of Pt-deposited Rh(110) bimetallic surface for NO + H2 reaction. J. Phys. Chem. B 1997, 101, 1186–1189. [Google Scholar] [CrossRef]
- Ross, P.N.; Kinoshita, K.; Scarpellino, A.J.; Stonehart, P. Electrocatalysis on binary alloys: I. Oxidation of molecular hydrogen on supported Pt−Rh alloys. J. Electroanal. Chem. Interfacial Electrochem. 1975, 59, 177–189. [Google Scholar] [CrossRef]
- Shen, S.Y.; Zhao, T.S.; Xu, J.B. Carbon supported PtRh catalysts for ethanol oxidation in alkaline direct ethanol fuel cell. Int. J. Hydrogen Energy 2010, 35, 12911–12917. [Google Scholar] [CrossRef]
- de Souza, J.P.I.; Queiroz, S.L.; Bergamaski, K.; Gonzalez, E.R.; Nart, F.C. Electro-oxidation of ethanol on Pt, Rh, and PtRh electrodes. A study using DEMS and in-situ FTIR techniques. J. Phys. Chem. B 2002, 106, 9825–9830. [Google Scholar] [CrossRef]
- Bergamaski, K.; Gonzalez, E.R.; Nart, F.C. Ethanol oxidation on carbon supported platinum-rhodium bimetallic catalysts. Electrochimica Acta 2008, 53, 4396–4406. [Google Scholar] [CrossRef]
- Delpeuch, A.B.; Asset, T.; Chatenet, M.; Cremers, C. Electrooxidation of ethanol at room temperature on carbon-supported Pt and Rh-containing catalysts: A DEMS study. J. Electrochem. Soc. 2014, 161, F918–F924. [Google Scholar] [CrossRef]
- Jurzinsky, T.; Bär, R.; Cremers, C.; Tübke, J.; Elsner, P. Highly active carbon supported palladium-rhodium PdxRh/C catalysts for methanol electrooxidation in alkaline media and their performance in anion exchange direct methanol fuel cells (AEM-DMFCs). Electrochimica Acta 2015, 176, 1191–1201. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Yu, P.Y.; Tzou, D.Y.; Hsu, J.P.; Chiu, Y.R. Bimetallic Pd–Rh nanoparticles onto reduced graphene oxide nanosheets as electrocatalysts for methanol oxidation. J. Electroanal. Chem. 2016, 761, 28–36. [Google Scholar] [CrossRef]
- Alvi, M.A.; Akhtar, M.S. An effective and low cost Pd–Ce bimetallic decorated carbon nanofibers as electrocatalyst for direct methanol fuel cells applications. J. Alloys Compd. 2016, 684, 524–529. [Google Scholar] [CrossRef]
- He, L.L.; Song, P.; Feng, J.J.; Fang, R.; Yu, D.X.; Chen, J.R.; Wang, A.J. Porous dandelion-like gold@ palladium core-shell nanocrystals in-situ growth on reduced graphene oxide with improved electrocatalytic properties. Electrochimica Acta 2016, 200, 204–213. [Google Scholar] [CrossRef]
- Zhang, S.S.; Yuan, X.Z.; Wang, H.J.; Mérida, W.; Zhu, H.; Shen, J.; Wu, S.H.; Zhang, J.J. A review of accelerated stress tests of MEA durability in PEM fuel cells. Int. J. Hydrogen Energy 2009, 34, 388–404. [Google Scholar] [CrossRef]
- Niu, X.H.; Zhao, H.L.; Lan, M.B. Palladium deposits spontaneously grown on nickel foam for electrocatalyzing methanol oxidation: Effect of precursors. J. Power Sources 2016, 306, 361–368. [Google Scholar] [CrossRef]
- Park, K.W.; Han, S.B.; Lee, J.M. Photo (UV)-enhanced performance of Pt–TiO2 nanostructure electrode for methanol oxidation. Electrochem. Commun. 2007, 9, 1578–1581. [Google Scholar] [CrossRef]
- Xue, X.D.; Gu, L.; Cao, X.B.; Song, Y.Y.; Zhu, L.W.; Chen, P. One-pot, high-yield synthesis of titanate nanotube bundles decorated by Pd (Au) clusters for stable electrooxidation of methanol. J. Solid State Chem. 2009, 182, 2912–2917. [Google Scholar] [CrossRef]
- Guo, X.; Guo, D.J.; Qiu, X.P.; Chen, L.Q.; Zhu, W.T. Excellent dispersion and electrocatalytic properties of Pt nanoparticles supported on novel porous anatase TiO2 nanorods. J. Power Sources 2009, 194, 281–285. [Google Scholar] [CrossRef]
- Dvoranova, D.; Brezova, V.; Mazur, M.; Malati, M.A. Investigations of metal-doped titanium dioxide photocatalysts. Appl. Catal. B 2002, 37, 91–105. [Google Scholar] [CrossRef]
- Wang, M.; Guo, D.J.; Li, H.L. High activity of novel Pd/TiO2 nanotube catalysts for methanol electro-oxidation. J. Solid State Chem. 2005, 178, 1996–2000. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Momeni, M.M.; Khalilpur, H. Synthesis and characterization of palladium nanoparticles immobilized on TiO2 nanotubes as a new high active electrode for methanol electro-oxidation. Int. J. Nanosci. 2012, 11, 1250016. [Google Scholar] [CrossRef]
- Ju, J.; Shi, Y.; Wu, D. TiO2 nanotube supported PdNi catalyst for methanol electro-oxidation. Powder Technol. 2012, 230, 252–256. [Google Scholar] [CrossRef]
- Cao, S.; Xu, W.; Zhu, S.; Liang, Y.; Li, Z.; Cui, Z.; Yang, X.; Inoue, A. Synthesis of TiO2 nanoparticles loaded Pd/CuO nanoporous catalysts and their catalytic performance for methanol, ethanol and formic acid electro-oxidations. J. Electrochem. Soc. 2016, 163, E263–E271. [Google Scholar] [CrossRef]
- Fernandez, J.L.; Walsh, D.A.; Bard, A.J. Thermodynamic guidelines for the design of bimetallic catalysts for oxygen electroreduction and rapid screening by scanning electrochemical microscopy. M–Co (M: Pd, Ag, Au). J. Am. Chem. Soc. 2005, 127, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.K.; Raman, R.K. Methanol-resistant oxygen-reduction catalysts for direct methanol fuel cells. Annu. Rev. Mater. Res. 2003, 33, 155–168. [Google Scholar] [CrossRef]
- Baranton, S.; Coutanceau, C.; Roux, C.; Hahn, F.; Leger, J.M. Oxygen reduction reaction in acid medium at iron phthalocyanine dispersed on high surface area carbon substrate: tolerance to methanol, stability and kinetics. J. Electroanal. Chem. 2005, 577, 223–234. [Google Scholar] [CrossRef]
- Wagner, A.J.; Wolfe, G.M.; Fairbrother, D.H. Reactivity of vapor-deposited metal atoms with nitrogen-containing polymers and organic surfaces studied by in situ XPS. Appl. Surf. Sci. 2003, 219, 317–328. [Google Scholar] [CrossRef]
- Matter, P.H.; Zhang, L.; Ozkan, U.S. The role of nanostructure in nitrogen-containing carbon catalysts for the oxygen reduction reaction. J. Catal. 2006, 239, 83–96. [Google Scholar] [CrossRef]
- Mustain, W.E.; Kepler, K.; Prakash, J. Investigations of carbon-supported CoPd3 catalysts as oxygen cathodes in PEM fuel cells. Electrochem. Commun. 2006, 8, 406–410. [Google Scholar] [CrossRef]
- Serov, A.A.; Cho, S.Y.; Han, S.; Min, M.; Chai, G.; Nam, K.H.; Kwak, C. Modification of palladium-based catalysts by chalcogenes for direct methanol fuel cells. Electrochem. Commun. 2007, 9, 2041–2044. [Google Scholar] [CrossRef]
- Zheng, J.S.; Zhang, X.; Li, P.; Zhu, J.; Zhou, X.G.; Yuan, W.K. Effect of carbon nanofiber microstructure on oxygen reduction activity of supported palladium electrocatalyst. Electrochem. Commun. 2007, 9, 895–900. [Google Scholar] [CrossRef]
- Chakraborty, S.; Retna Raj, C. Electrocatalytic performance of carbon nanotube-supported palladium particles in the oxidation of formic acid and the reduction of oxygen. Carbon 2010, 48, 3242–3249. [Google Scholar] [CrossRef]
- Lüsi, M.; Erikson, H.; Sarapuu, A.; Tammeveski, K.; Solla-Gullón, J.; Feliu, J.M. Oxygen reduction reaction on carbon-supported palladium nanocubes in alkaline media. Electrochem. Commun. 2016, 64, 9–13. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, H.; House, S.D.; Jin, R.; Yang, J.C.; Jin, R. Ultrasmall palladium nanoclusters as effective catalyst for oxygen reduction reaction. ChemElectroChem. 2016, 3, 1225. [Google Scholar] [CrossRef]
- Jukk, K.; Alexeyeva, N.; Johans, C.; Kontturi, K.; Tammeveski, K. Oxygen reduction on Pd nanoparticle/multi-walled carbon nanotube composites. J. Electroanal. Chem. 2012, 666, 67–75. [Google Scholar] [CrossRef]
- Li, B.; Prakash, J. Oxygen reduction reaction on carbon supported palladium-nickel alloys in alkaline media. Electrochem. Commun. 2009, 11, 1162–1165. [Google Scholar] [CrossRef]
- Ramos-Sanchez, G.; Santana-Salinas, A.; Vazquez-Huerta, G.; Solorza-Feria, O. Electrochemical impedance study and performance of PdNi nanoparticles as cathode catalyst in a polymer electrolyte membrane fuel cell. J. New Mat. Electrochem. Syst. 2010, 13, 213–217. [Google Scholar]
- Ramos-Sánchez, G.; Yee-Madeira, H.; Solorza-Feria, O. PdNi electrocatalyst for oxygen reduction in acid media. Int. J. Hydrogen Energy 2008, 33, 3596–3600. [Google Scholar] [CrossRef]
- Calderón, J.C.; Celorrio, V.; Nieto-Monge, M.J.; Fermín, D.J.; Pardo, J.I.; Moliner, R.; Lázaro, M.J. Palladium-nickel materials as cathode electrocatalysts for alkaline fuel cells. Int. J. Hydrogen Energy 2016. accepted for publication. [Google Scholar]
- Arroyo-Ramírez, L.; Montano-Serrano, R.; Luna-Pineda, T.; Román, F.R.; Raptis, R.G.; Cabrera, C.R. Synthesis and characterization of palladium and palladium-cobalt nanoparticles on Vulcan XC-72R for the oxygen reduction reaction. ACS Appl. Mater. Interfaces 2013, 5, 11603–11612. [Google Scholar] [CrossRef] [PubMed]
- Golmohammadi, F.; Gharibi, H.; Sadeghi, S. Synthesis and electrochemical characterization of binary carbon supported Pd3Co nanocatalyst for oxygen reduction reaction in direct methanol fuel cells. Int. J. Hydrogen Energy 2016, 41, 7373–7387. [Google Scholar] [CrossRef]
- Wu, J.; Shan, S.; Luo, J.; Joseph, P.; Petkov, V.; Zhong, C.J. PdCu nanoalloy electrocatalysts in oxygen reduction reaction: role of composition and phase state in catalytic synergy. ACS Appl. Mater. Interfaces 2015, 7, 25906–25913. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, S.; Wang, J.; Lin, R.; Kawasaki, M.; Rus, E.; Silberstein, K.E.; Lowe, M.A.; Lin, F.; Nordlund, D.; et al. Spontaneous incorporation of gold in palladium-based ternary nanoparticles makes durable electrocatalysts for oxygen reduction reaction. Nat. Commun. 2016. [Google Scholar] [CrossRef] [PubMed]
- Neergat, M.; Gunasekar, V.; Rahul, R. Carbon-supported Pd–Fe electrocatalysts for oxygen reduction reaction (ORR) and their methanol tolerance. J. Electroanal. Chem. 2011, 658, 25–32. [Google Scholar] [CrossRef]
- Abo Zeid, E.F.; Kim, Y.T. Effect of heat treatment on nanoparticle size and oxygen reduction reaction activity for carbon-supported Pd–Fe alloy electrocatalysts. Am. J. Nano. Res. Appl. 2015, 3, 71–77. [Google Scholar]
- Rivera Gavidia, L.M.; García, G.; Anaya, D.; Querejeta, A.; Alcaide, F.; Pastor, E. Carbon-supported Pt-free catalysts with high specificity and activity toward the oxygen reduction reaction in acidic medium. Appl. Catal. B 2016, 184, 12–19. [Google Scholar] [CrossRef]
- Dai, Y.; Yu, P.; Huang, Q.; Sun, K. Pd-W alloy electrocatalysts and their catalytic property for oxygen reduction. Fuel Cells 2016, 16, 165–169. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Chen, Y.; Yu, J.; Xu, X.; Su, C.; Tadé, M.O.; Shao, Z. Boosting oxygen reduction reaction activity of palladium by stabilizing Its unusual oxidation states in perovskite. Chem. Mater. 2015, 27, 3048–3054. [Google Scholar] [CrossRef]
- Lo Vecchio, C.; Alegre, C.; Sebastián, D.; Stassi, A.; Aricò, A.S.; Baglio, V. Investigation of supported Pd-based electrocatalysts for the oxygen reduction reaction: performance, durability and methanol tolerance. Materials 2015, 8, 7997–8008. [Google Scholar] [CrossRef]
- Ko, A.R.; Lee, Y.W.; Moon, J.S.; Han, S.B.; Cao, G.Z.; Park, K.W. Ordered mesoporous tungsten carbide nanoplates as non-Pt catalysts for oxygen reduction reaction. Appl. Catal. A 2014, 477, 102–108. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, M.; Xie, J.; Zhu, J.; Shen, P.K. A bimetallic carbide Fe2MoC promoted Pd electrocatalyst with performance superior to Pt/C towards the oxygen reduction reaction in acidic media. Appl. Catal. B 2015, 165, 636–641. [Google Scholar] [CrossRef]
- Zuo, L.X.; Jiang, L.P.; Zhu, J.J. A facile sonochemical route for the synthesis of MoS2/Pd composites for highly efficient oxygen reduction reaction. Ultrason. Sonochem. 2016. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Si, C.; Wang, Y.; Ding, Y.; Zhang, Z. Multicomponent platinum-free nanoporous Pd-based alloy as an active and methanol-tolerant electrocatalyst for the oxygen reduction reaction. Nano Res. 2016, 9, 1831–1843. [Google Scholar] [CrossRef]
- Xu, C.; Liu, Y.; Hao, Q.; Duan, H. Nanoporous PdNi alloys as highly active and methanol tolerant electrocatalysts towards oxygen reduction reaction. J. Mater. Chem. A 2013, 1, 13542–13548. [Google Scholar] [CrossRef]
- Chen, L.; Guo, H.; Fujita, T.; Hirata, A.; Zhang, W.; Inoue, A.; Chen, M. Nanoporous PdNi bimetallic catalyst with enhanced electrocatalytic performances for electro-oxidation and oxygen reduction reactions. Adv. Funct. Mater. 2011, 21, 4364–4370. [Google Scholar] [CrossRef]
- Xiong, L.; Huang, Y.X.; Liu, X.W.; Sheng, G.P.; Li, W.W.; Yu, H.Q. Three-dimensional bimetallic Pd–Cu nanodendrites with superior electrochemical performance for oxygen reduction reaction. Electrochimica Acta 2013, 89, 24–28. [Google Scholar] [CrossRef]
| Catalyst | Electrochemical Parameter | Reference | ||
| Onset Potential (V vs. RHE) | Anodic Peak Potential (V vs. RHE) | Anodic Peak Current (mA mg−1 Pd) | ||
| Pd/CNNF-G | 0.420 | 0.800 | 1780 | [19] |
| Pd/C3N4-RGO | 0.450 | 0.800 | 1550 | |
| Pd/RGO | 0.570 | 0.810 | 860 | |
| Pd/CNT | 0.570 | 0.850 | 700 | |
| Pd/AC | 0.580 | 0.870 | 550 | |
| Pd/C3N4 | 0.780 | 0.870 | 80 | |
| Catalyst | Onset Potential (V vs. RHE) | Anodic Peak Potential (V vs. Hg/HgO) | Anodic Peak Current (mA cm−2) | Reference |
| Pd/NS-G | 0.472 | 0.880 | 12.5 | [20] |
| Pd/G | 0.600 | 0.880 | 7 | |
| Pd/C | 0.520 | 0.890 | 5.6 | |
| Catalyst | Electrochemical Parameter | Reference | ||
| Onset Potential (V vs. RHE) | Anodic Peak Potential (V vs. RHE) | Anodic Peak Current (mA mg−1 Pd or Pt) | ||
| Pd/C | 0.545 | 0.915 | 210.5 | [22] |
| Pd80Ag20/C | 0.475 | 0.904 | 691.6 | |
| Pd65Ag35/C | 0.435 | 0.865 | 629.6 | |
| Pd46Ag54/C | 0.475 | 0.855 | 453.4 | |
| Pt/C TKK | 0.475 | 0.925 | 689.3 | |
| Catalyst | Onset Potential (V vs. RHE) | Anodic Peak Potential (V vs. RHE) | Anodic Peak Current (mA cm−2) | Reference |
| Pd/C | 0.536 | 0.928 | 0.557 | [23] |
| Pd-Ag(2:1)/C | 0.446 | 0.886 | 0.635 | |
| Pd-Ag(1:1)/C | 0.436 | 0.856 | 0.678 | |
| Pd-Ag(1:1)/CNTs | 0.436 | 0.886 | 0.950 | |
| Pd-Ag(1:1.5)/C | 0.446 | 0.856 | 0.707 | |
| Catalyst | Anodic Peak Potential (V vs. RHE) | Anodic Peak Current (mA mg−1 Pd) | Ratio Between Forward and Backward Anodic Currents | Reference |
| Pd/C | 0.915 | 311 | 1.41 | [25] |
| Pd-Ag(1:1)/GO | 0.865 | 225 | 1.50 | |
| Pd-Ag(1.5:1)/RGO | 0.915 | 334 | 1.42 | |
| Pd-Ag(1:1)/RGO | 0.875 | 630 | 3.15 | |
| Pd-Ag(1:1.5)/RGO | 0.895 | 585 | 6.55 | |
| Pd-Ag(1:1)/RGO-SB | 0.870 | 545 | 1.48 | |
| Catalyst | Electrochemical Parameter | Reference | ||
| Onset Potential (V vs. RHE) | Anodic Peak Current (mA cm−2) | – | ||
| Pd/C | 0.555 | 14 | – | [27] |
| Pd-NiO(8:1)/C a | – | 51 | – | |
| Pd-NiO(6:1)/C a | – | 61 | – | |
| Pd-NiO(4:1)/C a | – | 74 | – | |
| Pd-NiO(2:1)/C | 0.535 | 63 | – | |
| Pt/C | 0.525 | 18 | – | |
| Catalyst | Anodic Peak Potential (V vs. RHE) | Anodic Peak Current (mA cm−2) | – | Reference |
| Pd-Ni(1:1)/C | 0.914 | 7,64 | – | [30] |
| Pd-Ni(1–5wt %)/MWCNTs | 0.969 | 341,68 | – | [34] |
| Catalyst | Onset Potential (V vs. RHE) | Anodic Peak Potential (V vs. RHE) | Anodic Peak Current (mA cm−2) | Reference |
| Pd/C | 0.611 | 1.006 | 1.41 | [32] |
| Pd-Ni(1:1)/C | 0.421 | 0.941 | 1.50 | |
| Pt/C | 0.441 | 1.006 | 1.48 | |
| Catalyst | Onset Potential (V vs. RHE) | Anodic Peak Current (mA cm−2) | Reference | |
| Pd-Ni/CB 1:1 | 0.452 | 0.912 | [33] | |
| Pd-Ni/CBO 1:1 | 0.556 | 0.397 | ||
| Pd-Ni/CBN 1:1 | 0.511 | 0.536 | ||
| Pd-Ni/CB 1:2 | 0.498 | 1.100 | ||
| Pd-Ni/CBO 1:2 | 0.458 | 1.126 | ||
| Pd-Ni/CBN 1:2 | 0.551 | 0.815 | ||
| Pd/C 1:1 | 0.600 | 0.310 | ||
| Catalyst | Electrochemical Parameter | Reference | |
| Onset Potential (V vs. RHE) | Anodic Peak Current (mA mg−1 Pd) | ||
| Pt/C (HiSPECTM 3000) | 0.475 | 669.9 | [42] |
| Pd/C | 0.585 | 543.8 | |
| Rh/C | 0.504 | 177.6 | |
| PdRh3/C | 0.500 | 369.2 | |
| PdRh/C | 0.445 | 933.9 | |
| Pd3Rh/C | 0.497 | 955.7 | |
| Catalyst | Onset Potential (V vs. RHE) | Anodic Peak Current (mA mg−1 Pd) | Reference |
| Pd/GO | 0.260 | 73 | [43] |
| Pd75Rh25/GO | 0.280 | 100 | |
| Pd50Rh50/GO | 0.370 | 35 | |
| Pd25Rh75/GO | 0.310 | 31 | |
| Catalyst | Onset Potential (V vs. RHE) | Anodic Peak Current (mA mg−1 Pd) | Reference |
| Pd/C | 0.510 | 190 | [35] |
| Pd/MWCNTs | 0.510 | 285 | |
| Pd-MnO2/MWCNTs | 0.460 | 420 | |
| Catalyst | Onset Potential (V vs. RHE) | Anodic Peak Current (mA cm−2) | Reference |
| Au@Pd/RGO | 0.500 | 28 | [45] |
| Pd/RGO | 0.700 | 4 | |
| Pd/C | 0.640 | 10 | |
| Catalyst | Electrochemical Parameter | Reference | |
| Onset Potential (V vs. RHE) | Anodic Peak Current (A) | ||
| Pd (pure) | 0.22 | 1.0 | [52] |
| Pd/TiO2 (nanoparticles) | 0.22 | 4.5 | |
| Pd/TiO2 (nanotubes) | 0.23 | 9.0 | |
| Catalyst | Onset Potential (V vs. RHE) | Anodic Peak Current Density (mA cm−2) | Reference |
| np-Pd/CuO/80TiO2 | 0.55 | 1.5 | [55] |
| np-Pd/CuO/160TiO2 | 0.51 | 2.8 | |
| np-Pd/CuO/240TiO2 | 0.55 | 1.4 | |
| np-Pd/CuO | 0.60 | 0.8 | |
| np-Pd | 0.55 | 0.7 | |
| Catalyst | Onset Potential (V vs. RHE) | Reference |
|---|---|---|
| Pd/AC | 0.584 | [63] |
| Pd/CNFfisbone | 0.624 | |
| Pd/CNFplatelet | 0.764 | |
| Pd/MWCNTs | 1.014 | [64] |
| Pd/MWCNTs-Nafion composite | 0.900 | [67] |
| Pd/MWCNTs-PVP composite | 0.870 | |
| Pdnanocubes/Vulcan XC-72R | 1.000 | [65] |
| Pdnanoclusters/XC-72R (Ligand on) | 0.897 | [66] |
| Pdnanoclusters/XC-72R (Ligand off) | 1.017 | |
| Pt/XC-72R | 0.987 | |
| Pd/C | 1.085 | [68] |
| Pd-Ni(3:1)/C | 1.085 | |
| Pd-Ni(1:1)/C | 1.105 | |
| Pd-Ni(1:3)/C | 1.005 | |
| Pd/C E-TEK | 1.050 | [71] |
| Pd-Ni/CB 1:2 | 0.960 | |
| Pd-Ni/CNF 1:2 | 0.955 | |
| Pd-Ni/CNFO 1:2 | 0.960 | |
| Pd-Ni/CNFN 1:2 | 0.940 | |
| Pd/C (commercial) | 0.728 | [72] |
| Pd2Co/C | 0.735 | |
| PdCo2/C | 0.731 | |
| PtCo/C (commercial) | 0.836 | |
| Pt/C (commercial) | 0.844 | |
| Pd-Cu(36:64)/C | 0.799 | [74] |
| Pd-Cu(54:46)/C | 0.919 | |
| Pd-Cu(75:25)/C | 0.799 | |
| Pd/C (commercial) | 0.879 | |
| Pd-Fe/C (non-heat treated) | 0.655 | [77] |
| Pd-Fe/C (300 °C) | 0.865 | |
| Pd-Fe/C (500 °C) | 0.815 | |
| Pd-Fe/C (700 °C) | 0.805 | |
| Pd/C | 0.940 | [79] |
| Pd19W/C | 0.950 | |
| Pd9W/C | 0.950 | |
| Pd3W/C | 0.950 | |
| JM Pt/C | 0.950 |
| Catalyst | Onset Potential (V vs. RHE) | Reference |
|---|---|---|
| Pd/LF | 0.662 | [80] |
| Pd/LFP0.05 | 0.792 | |
| Pd/LFP0.05-R | 0.722 | |
| Pd/LFP0.05-RO | 0.772 | |
| Pd/WC-700-m | 0.692 | [82] |
| Pd/WC-800-m | 0.812 | |
| Pd/WC-900-m | 0.872 | |
| Pt/C | 1.08 | [83] |
| Pd/C | 0.92 | |
| Pd/C-MoC | 0.95 | |
| Pd/C-Fe2MoC | 1.08 | |
| Pt/C (commercial) | 1.04 | [86] |
| PdNi dealloyed | 1.04 | |
| Pd dealloyed | 0.90 | |
| Pt/C (commercial) | 0.90 | [88] |
| Pd-Cu(nanodendrites) | 1.00 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón Gómez, J.C.; Moliner, R.; Lázaro, M.J. Palladium-Based Catalysts as Electrodes for Direct Methanol Fuel Cells: A Last Ten Years Review. Catalysts 2016, 6, 130. https://doi.org/10.3390/catal6090130
Calderón Gómez JC, Moliner R, Lázaro MJ. Palladium-Based Catalysts as Electrodes for Direct Methanol Fuel Cells: A Last Ten Years Review. Catalysts. 2016; 6(9):130. https://doi.org/10.3390/catal6090130
Chicago/Turabian StyleCalderón Gómez, Juan Carlos, Rafael Moliner, and Maria Jesus Lázaro. 2016. "Palladium-Based Catalysts as Electrodes for Direct Methanol Fuel Cells: A Last Ten Years Review" Catalysts 6, no. 9: 130. https://doi.org/10.3390/catal6090130
APA StyleCalderón Gómez, J. C., Moliner, R., & Lázaro, M. J. (2016). Palladium-Based Catalysts as Electrodes for Direct Methanol Fuel Cells: A Last Ten Years Review. Catalysts, 6(9), 130. https://doi.org/10.3390/catal6090130