Zn0.8Cd0.2S Photocatalyst Modified with Ni(OH)2 for Enhanced Photocatalytic Hydrogen Production
Abstract
1. Introduction
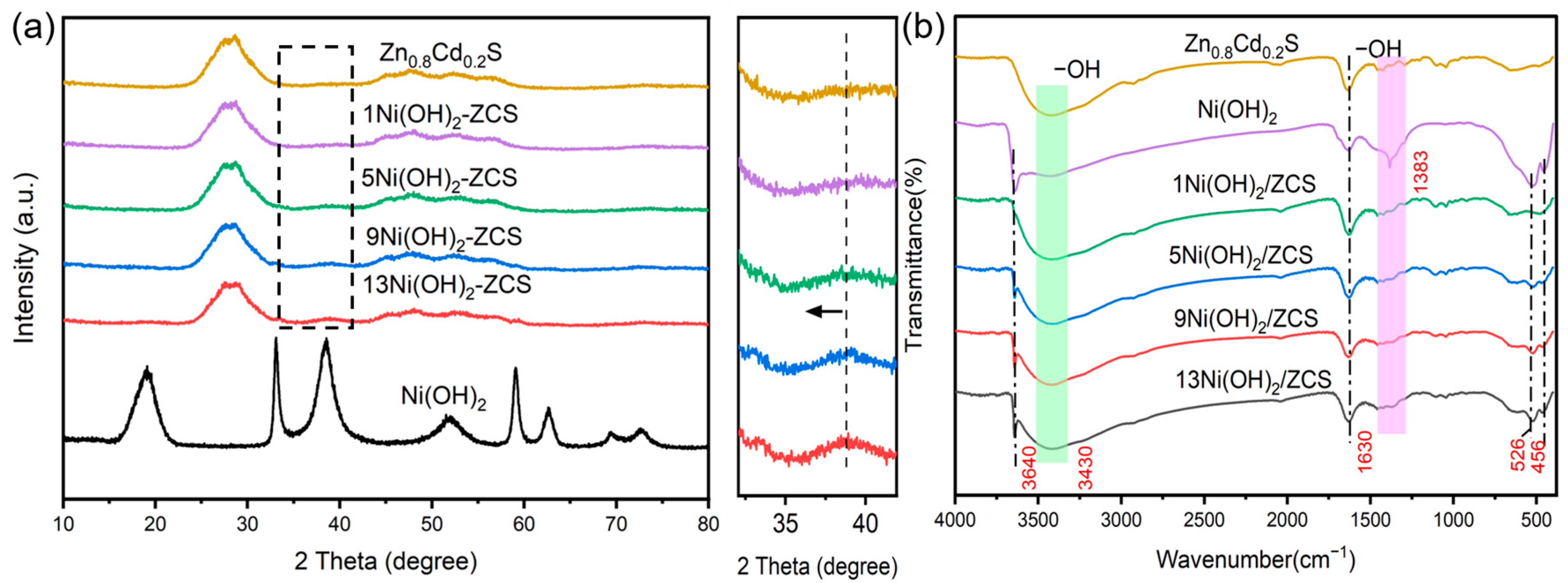
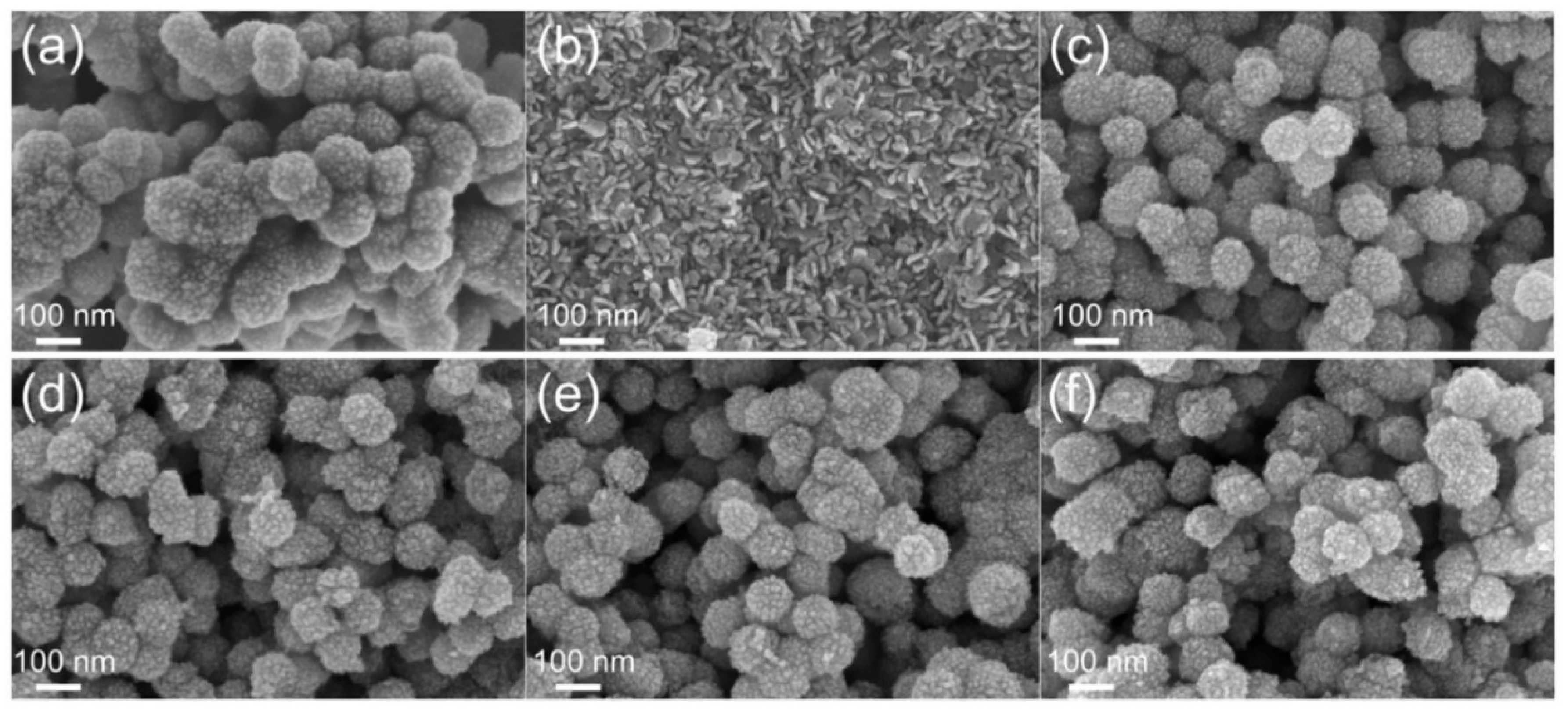
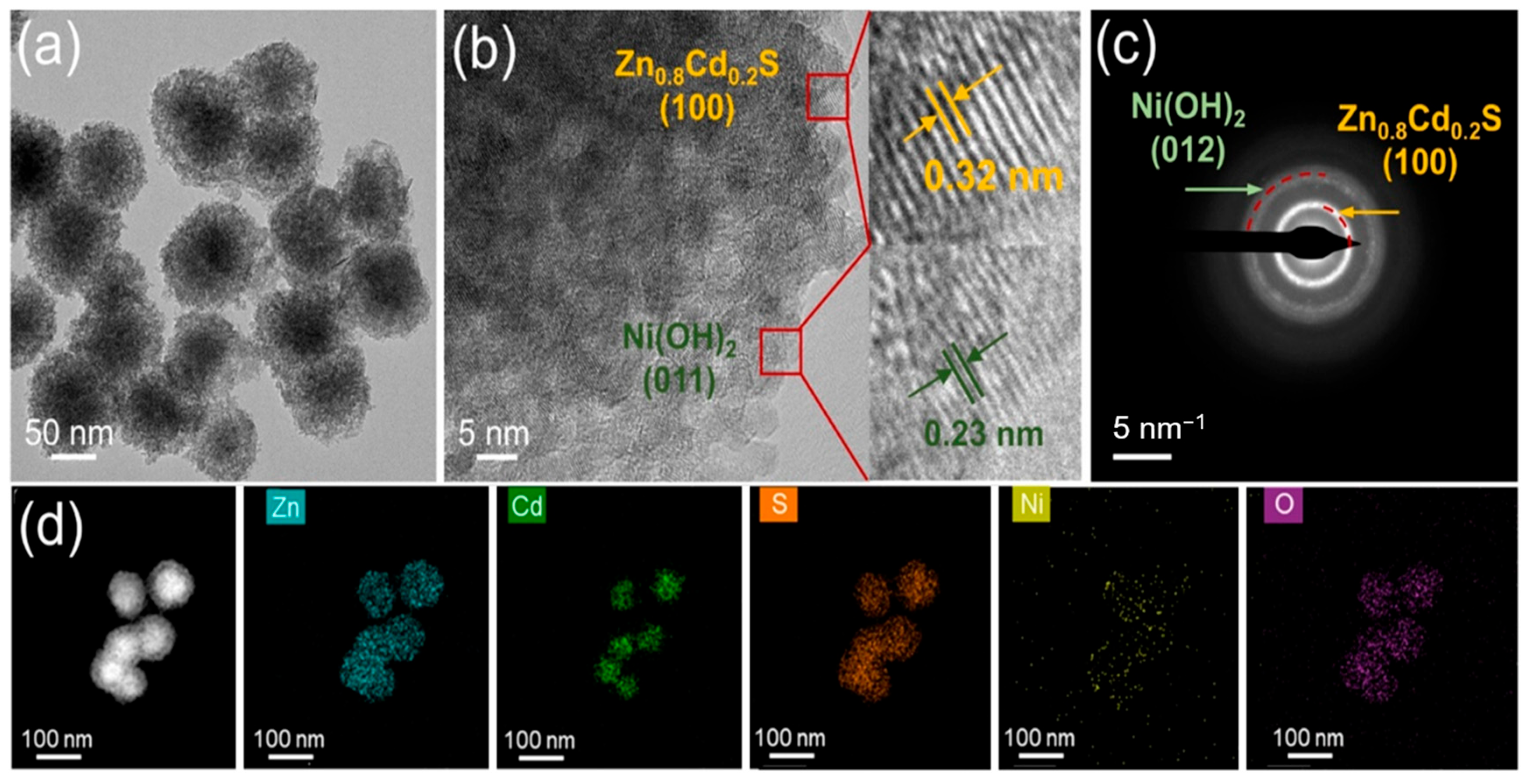
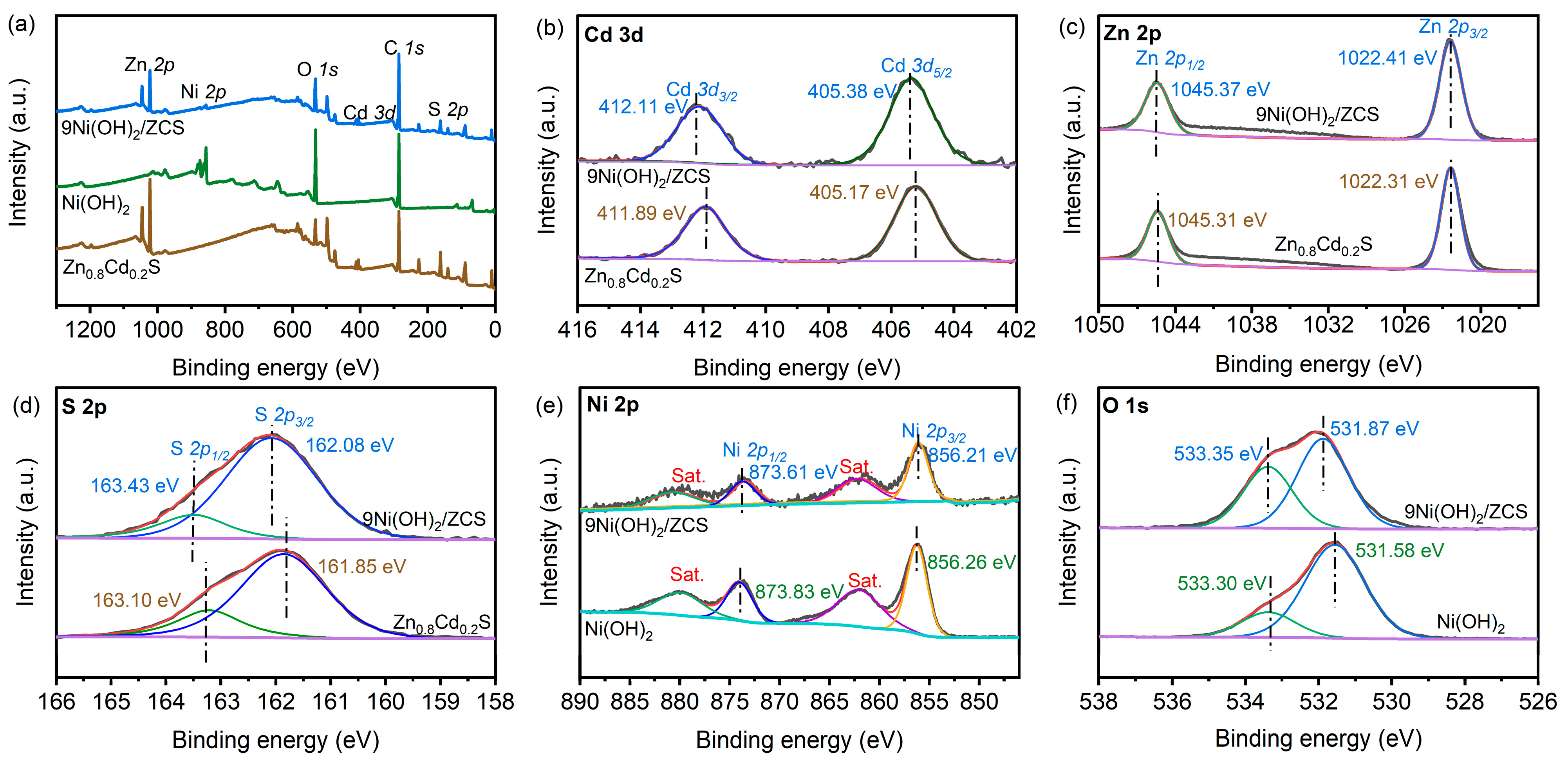
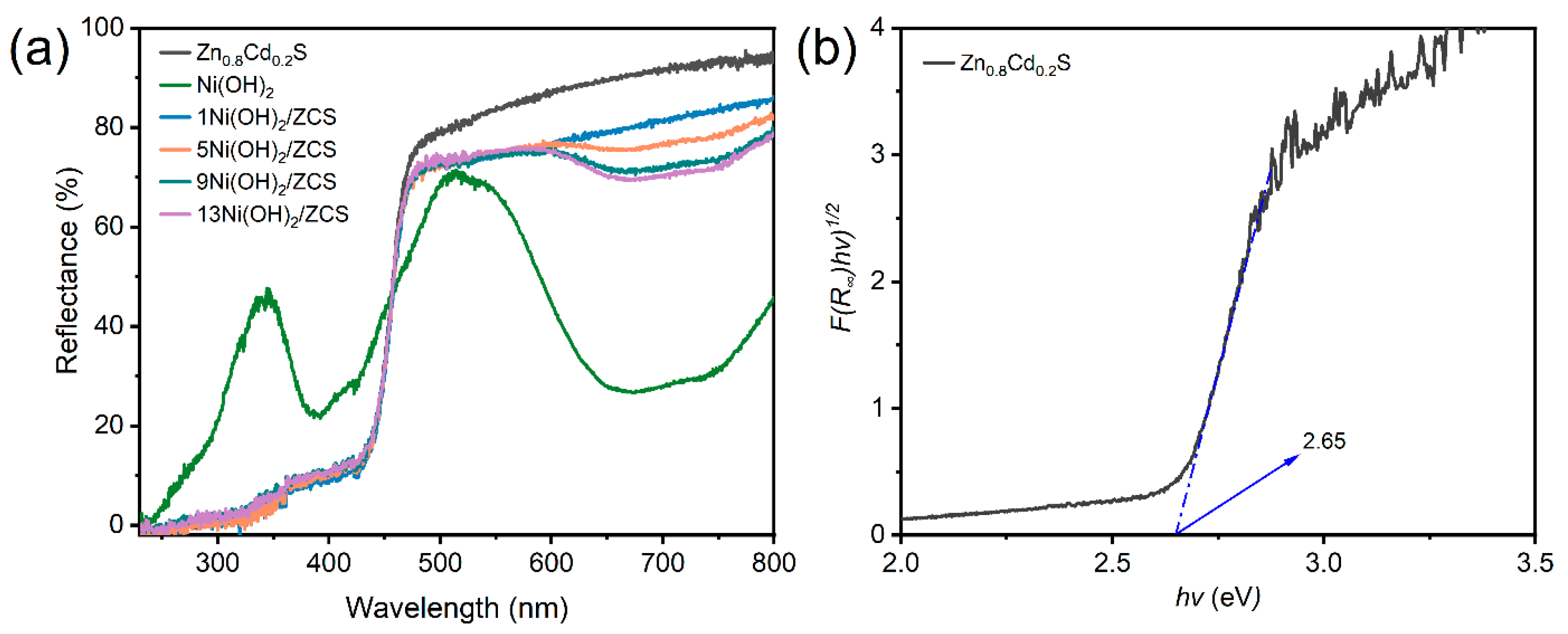
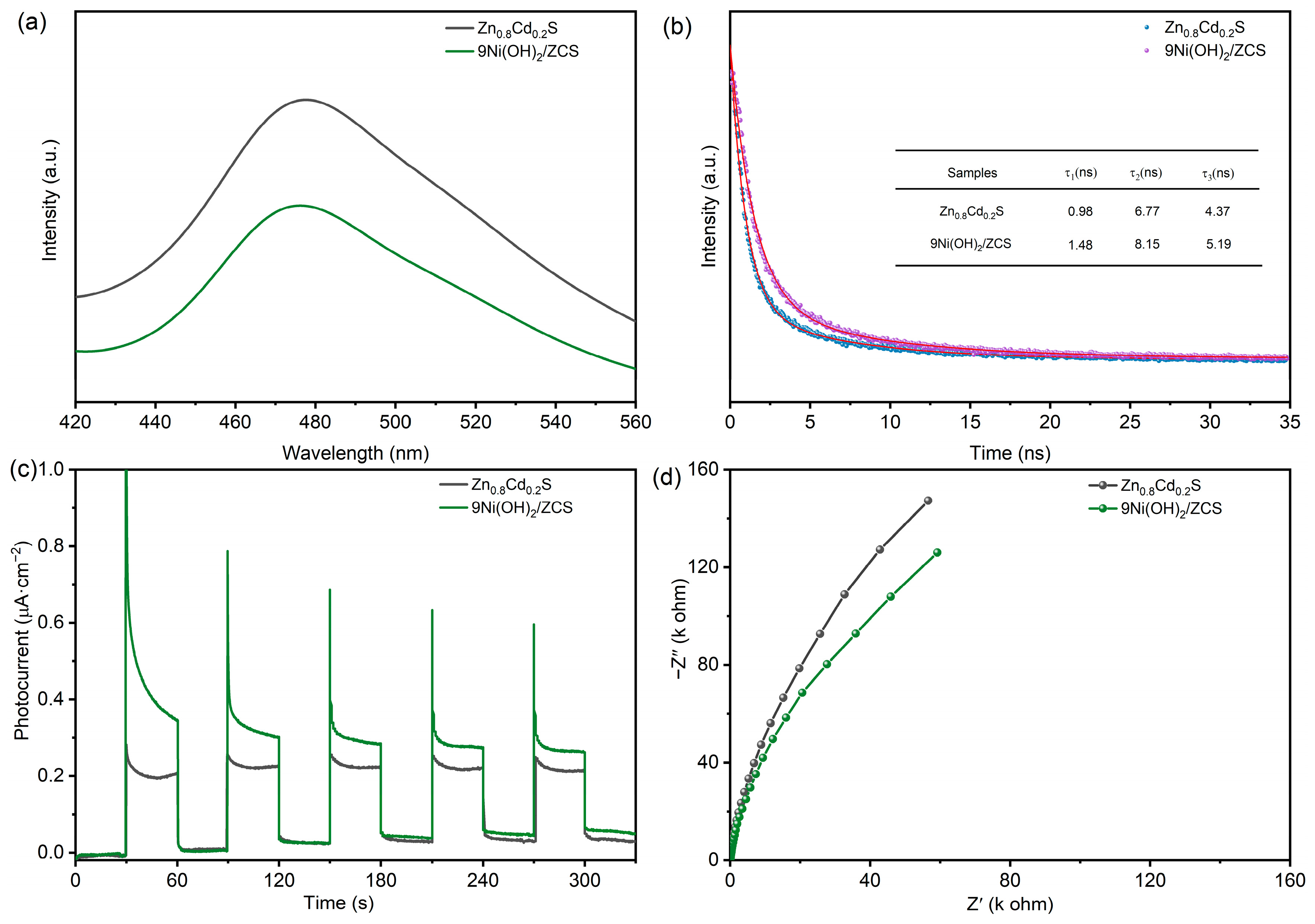
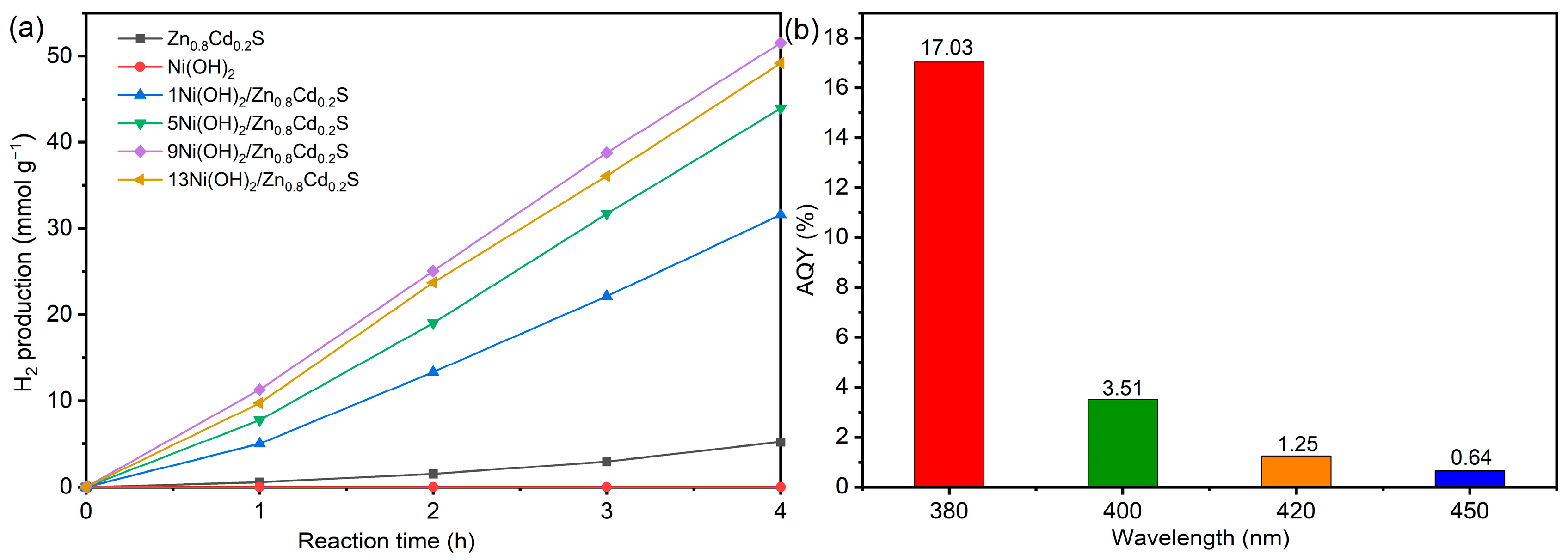
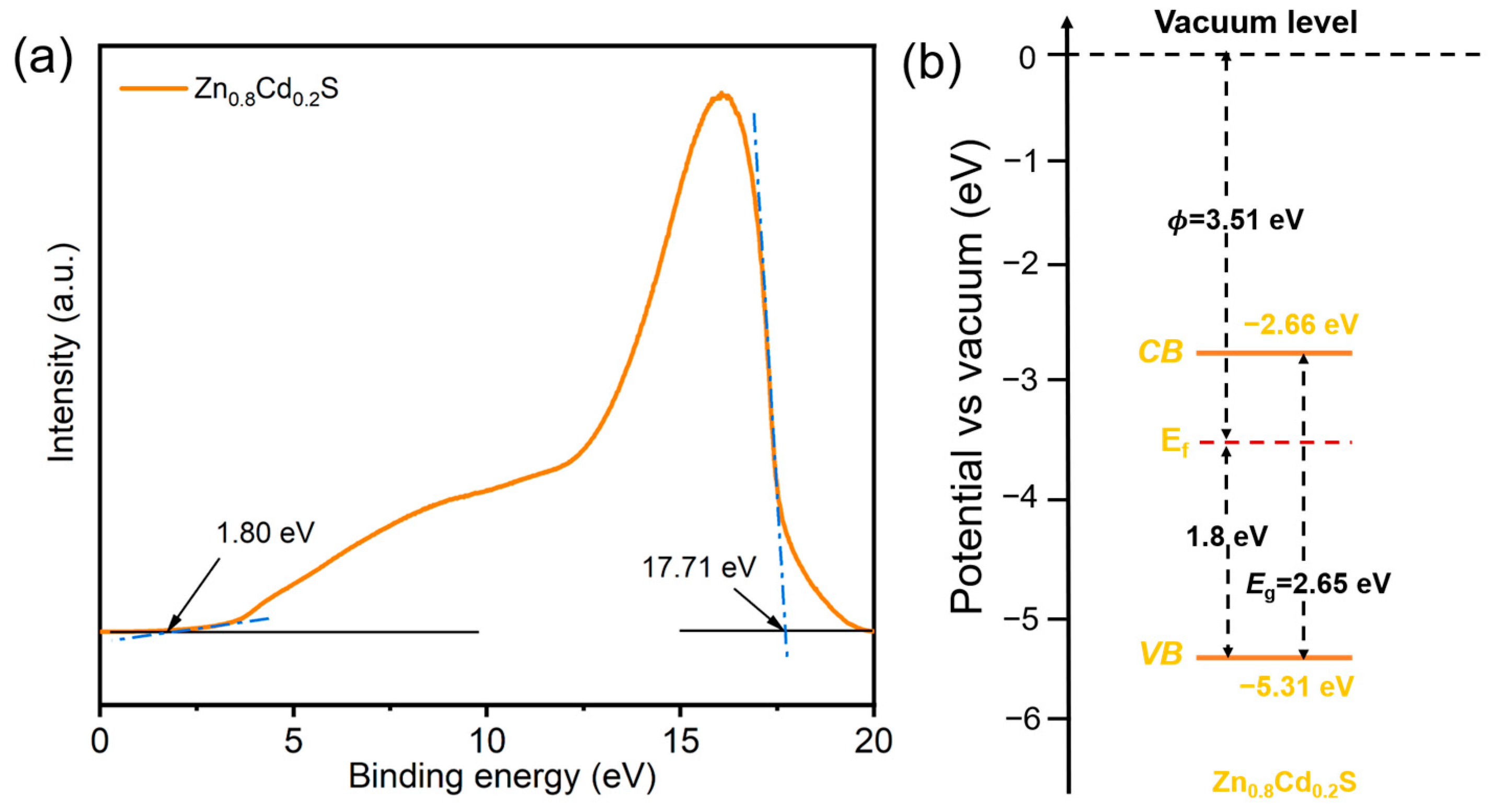
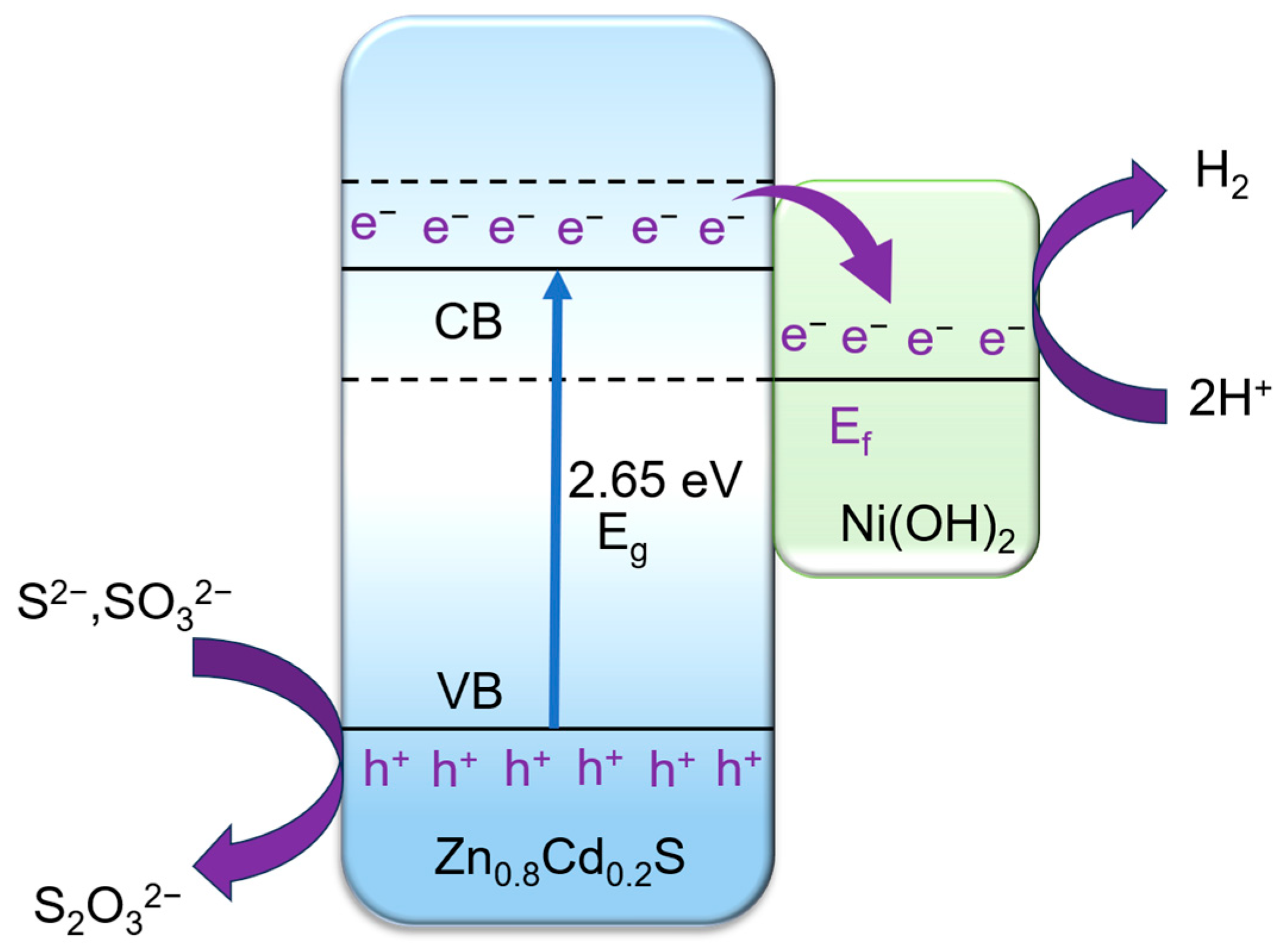
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Synthesis of Zn0.8Cd0.2S
3.3. Synthesis of Ni(OH)2
3.4. Synthesis of Ni(OH)2/ZCS
3.5. Characterization of the Catalysts
3.6. Photoelectrochemical Measurements
3.7. Photocatalytic Hydrogen Evolution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhong, W.; Xu, J.; Wang, P.; Zhu, B.; Fan, J.; Yu, H. Novel core-shell Ag@AgSe nanoparticle co-catalyst: In situ surface selenization for efficient photocatalytic H2 production of TiO2. Chin. J. Catal. 2022, 43, 1074–1083. [Google Scholar] [CrossRef]
- Wang, J.; Niu, X.; Wang, R.; Zhang, K.; Shi, X.; Yang, H.Y.; Ye, J.; Wu, Y. High-entropy alloy-enhanced ZnCdS nanostructure photocatalysts for hydrogen production. Appl. Catal. B Environ. Energy 2025, 362, 124763. [Google Scholar] [CrossRef]
- Huang, C.; Liu, N.; Zhou, Y.; Mao, H.; Qi, F.; Ouyang, X. Construction of 3D/0D Sv-ReS2/ZnCdS hierarchical structure with improved interfacial charge transfer for enhanced photocatalytic hydrogen evolution. Sep. Purif. Technol. 2025, 354, 128838. [Google Scholar] [CrossRef]
- Hiragond, C.B.; Powar, N.S.; Kim, H.; In, S.-I. Unlocking solar energy: Photocatalysts design for tuning the CO2 conversion into high-value (C2+) solar fuels. EnergyChem 2024, 6, 100130. [Google Scholar] [CrossRef]
- Lian, Z.; Qu, M.; Xiao, H.; Wang, L.; Wu, H.; Zi, J.; Wang, W.; Li, H. Direct Observation of Z-Scheme Route in Cu31S16/ZnxCd1-xS Heteronanoplates for Highly Efficient Photocatalytic Hydrogen Evolution. Small 2024, 20, 2400611. [Google Scholar] [CrossRef]
- He, J.; Chen, L.; Xie, X.; Qin, Z.; Ji, H.; Su, T. Construction of ZnCoP/CdLa2S4 Schottky Heterojunctions for Enhancing Photocatalytic Hydrogen Evolution. Acta Phys. Chim. Sin. 2024, 40, 2404030. [Google Scholar] [CrossRef]
- Kim, H.; Seo, J.W.; Chung, W.; Narejo, G.M.; Koo, S.W.; Han, J.S.; Yang, J.; Kim, J.Y.; In, S.I. Thermal Effect on Photoelectrochemical Water Splitting Toward Highly Solar to Hydrogen Efficiency. ChemSusChem 2023, 16, e202202017. [Google Scholar] [CrossRef]
- HONDA, A.F.K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Han, M.; Guo, B.; Zhang, Z.; Hu, X.; Wang, Z. Hollow Bi4Ti3O12/TiO2 nanocakes for photocatalytic hydrogen generation. J. Alloys Compd. 2023, 963, 171192. [Google Scholar] [CrossRef]
- Ren, T.; Dang, Y.; Xiao, Y.; Hu, Q.; Deng, D.; Chen, J.; He, P. Depositing Ag nanoparticles on g-C3N4 by facile silver mirror reaction for enhanced photocatalytic hydrogen production. Inorg. Chem. Commun. 2021, 123, 108367. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, J.; Leng, J.; Yin, Z.; Yin, Y.; Zhang, F.; Sun, C.; Jin, S. Long-Lived Internal Charge-Separated State in Two-Dimensional Metal–Organic Frameworks Improving Photocatalytic Performance. ACS Energy Lett. 2022, 7, 2323–2330. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, D.; Shi, J.; Chen, P.; Zong, S.; Cheng, C.; Chen, K.; Chen, Y.; Ma, L. (Oxy)nitride heterojunction-strengthened separation of photogenerated carriers in g-C3N4 towards enhanced photocatalytic H2 evolution. Appl. Catal. A Gen. 2022, 643, 118746. [Google Scholar] [CrossRef]
- Yang, H.; Li, C.; Mao, M.; Sun, H.; Zhu, X.; Zhang, Y.; Li, Y.; Jiang, Z. Synthesis of Type-S Ni3S4/ZnCdS Quantum Dots via Constitution Controller l-Cysteine for Photocatalytic H2 Evolution. ACS Appl. Nano Mater. 2024, 7, 22093–22103. [Google Scholar] [CrossRef]
- Du, S.; Chen, L.; Men, C.; Ji, H.; Su, T.; Qin, Z. Effect of surface defect states on Zn(1−x)CdxS for enhanced photocatalytic hydrogen evolution. J. Alloys Compd. 2023, 955, 170265. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, T.; Li, S.; Lu, Y.; Wang, L.; Zhang, X.; Wang, D.; Xie, T. Enhancement of photocatalytic H2 evolution on Zn0.8Cd0.2S loaded with CuS as cocatalyst and its photogenerated charge transfer properties. Dalton Trans. 2013, 42, 12998. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Yin, W.; Su, K.; He, P.; Chen, J.; Si, Y.; Xiao, Y.; Ren, T. Boosting the photocatalytic hydrogen production activity of marigold-like Zn2In2S5 by using noble-metal-free Ni2P as cocatalyst. Int. J. Hydrogen Energy 2024, 56, 596–603. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Z.; Tsubaki, N. Hollow ZnCdS and NiCoP Nanostructures for Photocatalytic Hydrogen Generation. ACS Appl. Nano Mater. 2022, 5, 14677–14688. [Google Scholar] [CrossRef]
- Li, M.; Chen, F.; Xu, Y.; Tian, M. Ni(OH)2 Nanosheet as an Efficient Cocatalyst for Improved Photocatalytic Hydrogen Evolution over Cd0.9Zn0.1S Nanorods under Visible Light. Langmuir 2024, 40, 3793–3803. [Google Scholar] [CrossRef]
- Kong, Z.; Kong, Z.; Zhang, D.; Liu, J.; Ji, X.-Y.; Cai, P.; Pu, X. Magnetic separable non-precious metal Schottky heterojunction photocatalyst toward photothermal-assisted photocatalytic hydrogen evolution. Sep. Purif. Technol. 2025, 361, 131429. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Z.; Song, Y.; Fan, J.; Sun, T.; Liu, E. S-scheme regulated Ni2P-NiS/twinned Mn0.5Cd0.5S hetero-homojunctions for efficient photocatalytic H2 evolution. J. Mater. Sci. Technol. 2024, 169, 148–157. [Google Scholar] [CrossRef]
- Ma, F.; Wen, Y.; Fu, P.; Zhang, J.; Tang, Q.; Chen, T.; Luo, W.; Zhou, Y.; Wang, J. Engineering 0D/2D Architecture of Ni(OH)2 Nanoparticles on Covalent Organic Framework Nanosheets for Selective Visible-Light-Driven CO2 Reduction. Small 2023, 20, 2305767. [Google Scholar] [CrossRef]
- Zuo, L.; Yang, N.; Xia, W.; Zeng, X.; Cao, R. Z-scheme NiS@ZnCdS heterostructures and their boosted photocatalytic H2 evolution. Appl. Surf. Sci. 2025, 689, 162447. [Google Scholar] [CrossRef]
- Lu, L.; Ma, Y.; Liu, H.; Dong, R.; Tan, P.; Yang, L.; Pan, J. Controlled preparation of hollow Zn0.3Cd0.7S nanospheres modified by NiS1.97 nanosheets for superior photocatalytic hydrogen production. J. Colloid Interface Sci. 2022, 606, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, Z.; Zhong, K.; Li, Q.; Ding, P.; Feng, Z.; Yang, J.; Du, Y.; Song, Y.; Hua, Y.; et al. Mo-O-Bi Bonds as interfacial electron transport bridges to fuel CO2 photoreduction via in-situ reconstruction of black Bi2MoO6/BiO2-x heterojunction. Chem. Eng. J. 2022, 429, 132204. [Google Scholar] [CrossRef]
- Gao, L.; Chen, Z.; Zheng, H.; Hu, J. A distinct hollow spindle-like CdIn2S4 photocatalyst for high-efficiency tetracycline removal. Mater. Today Chem. 2022, 24, 100800. [Google Scholar] [CrossRef]
- Moridon, S.N.F.; Arifin, K.; Mohamed, M.A.; Minggu, L.J.; Mohamad Yunus, R.; Kassim, M.B. Improving photoelectrochemical performance of transferred TiO2 nanotubes onto FTO substrate with Mo2C and NiS as Co-catalyst. Int. J. Hydrogen Energy 2024, 88, 1196–1206. [Google Scholar] [CrossRef]
- Bai, X.; Yang, T.; Zhao, M.; Fan, H.; Li, C.; Liu, E. Novel N-deficient g-C3N4 /Ni(OH)2 2D heterojunction design for efficient photocatalytic H2 evolution. J. Environ. Chem. Eng. 2025, 13, 115447. [Google Scholar] [CrossRef]
- Zhan, D.; Tian, J.; Fu, Q.; Liu, P.; Zhao, Y.; Liu, W.; Li, D.; Huang, Y.; Han, C. In situ photodeposition of Cu and Ni(OH)2 dual cocatalyst: Synergistic effect on enhancing g-C3N4 photocatalytic H2 evolution. Appl. Surf. Sci. 2023, 641, 158463. [Google Scholar] [CrossRef]
- Nair, M.M.; Iacoban, A.C.; Neaţu, F.; Florea, M.; Neaţu, Ş. A comparative overview of MXenes and metal oxides as cocatalysts in clean energy production through photocatalysis. J. Mater. Chem. A 2023, 11, 12559–12592. [Google Scholar] [CrossRef]
- Wang, H.; Shao, B.; Chi, Y.; Lv, S.; Wang, C.; Li, B.; Li, H.; Li, Y.; Yang, X. Engineering of Ni(OH)2 Modified Two-Dimensional ZnIn2S4 Heterostructure for Boosting Hydrogen Evolution under Visible Light Illumination. Nanomaterials 2022, 12, 946. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, L.; Sun, B.; Zhang, H.; Hou, D.; Qiao, X.-q.; Ma, H.; Li, D.-S. Efficient photothermal catalytic CO2 reduction over in situ construction ZnIn2S4@Ni(OH)2/NiO Z-scheme heterojunction. Chem. Eng. J. 2024, 479, 147719. [Google Scholar] [CrossRef]
- Chen, R.; Zhu, H.; Liu, W.; Zhan, D.; Fu, Q.; Tian, J.; Huang, Y.; Han, C. Facile photodeposition Ni(OH)2 anchored ZnIn2S4 as an efficient 1D/2D heterojunctions for photocatalytic H2 evolution. J. Am. Ceram. Soc. 2024, 107, 5201–5211. [Google Scholar] [CrossRef]
- Siang, T.J.; Zhang, P.; Chen, B.; Ong, W.-J. Surface defect engineering of ZnCoS in ZnCdS with twin crystal structure for visible-light-driven H2 production coupled with benzyl alcohol oxidation. Chin. J. Catal. 2025, 69, 84–98. [Google Scholar] [CrossRef]
- Guan, C.; Zhou, H.; Liao, Y.; Xiang, Q. 2D/2D g-C3N4/ZnxCd1-xS Van der Waals heterojunctions modulation: Interfacial chemical bond accelerating charge separation for enhanced photocatalytic CO2 reduction. Appl. Surf. Sci. 2023, 619, 156734. [Google Scholar] [CrossRef]
- Govindarajan, D.; Murugadoss, G.; Kirubaharan, K.; Manavalan, R.K.; Manibalan, G.; Shaikh, J.; Etesami, M.; Kheawhom, S. Improved electrochemical supercapacitive properties of CuO-Ni(OH)2 nanocomposites by eco-friendly low-temperature synthesis. J. Alloys Compd. 2023, 942, 169130. [Google Scholar] [CrossRef]
- Sekar, S.; Lee, S.; Kim, D.Y.; Preethi, V.; Kalirajan, K.M.; Sutha, S.; Saravanan, S.; Therli, A.; Roy, M.; Jagannathan, K. Biomass activated carbon-decorated spherical β-Ni(OH)2 nanoparticles for enhanced hydrogen production from sulphide wastewater. J. Water Process Eng. 2020, 38, 101669. [Google Scholar] [CrossRef]
- Li, D.; Shen, C.; Lu, Q.; Yan, R.; Xiao, B.; Zi, B.; Zhang, J.; Lu, Q.; Liu, Q. Excellent performance supercapacitors with the compounding of Ni(OH)2 and ZIF-67 derived Co–C–N nanosheets as flexible electrode materials. Nanoscale Adv. 2022, 4, 4381–4390. [Google Scholar] [CrossRef]
- Yang, D.; Li, Z.-G.; Lang, F.; Zhang, X.; Li, H.; Zhang, J.; Bu, X.-H. Molecular Adsorption─An Effective Modification for a ZnCdS Photocatalyst in a Hydrogen Production Reaction. ACS Mater. Lett. 2024, 6, 2837–2845. [Google Scholar] [CrossRef]
- Parvin, S.; Aransiola, E.; Ammar, M.; Lee, S.; Zhang, L.; Weber, J.; Baltrusaitis, J. Tailored Ni(OH)2/CuCo/Ni(OH)2 Composite Interfaces for Efficient and Durable Urea Oxidation Reaction. ACS Appl. Mater. Interfaces 2024, 16, 67715–67729. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Z.; Han, D.; Wang, T.; Ma, C.; Huo, P.; Yan, Y. Interface engineered 2D/2D Ni(OH)2/Bi4Ti3O12 nanocomposites with higher charge transfer towards improving photocatalytic activity. J. Alloys Compd. 2020, 816, 152530. [Google Scholar] [CrossRef]
- Hu, Q.; Chen, L.; Xie, X.; Qin, Z.; Ji, H.; Su, T. Construction of Electron Bridge and Activation of MoS2 Inert Basal Planes by Ni Doping for Enhancing Photocatalytic Hydrogen Evolution. Acta Phys.-Chim. Sin. 2024, 40, 2406024. [Google Scholar] [CrossRef]
- Liu, S.; Wang, W.; Shi, S.; Liao, S.; Zhong, M.; Xiao, W.; Wang, S.; Wang, X.; Chen, C. Hydrogen-bonded organic framework derived ultra-fine ZnCdS/ZnS heterojunction with high-porosity for efficient photocatalytic hydrogen production. Appl. Surf. Sci. 2024, 657, 159795. [Google Scholar] [CrossRef]
- Serpone, N.; Borgarello, E.; Barbeni, M.; Pelizzetti, E. Effect of CdS Preparation on the Photo-catalyzed Decomposition of Hydrogen. Inorganica Chim. Acta 1984, 90, 191–196. [Google Scholar]
- Li, Z.; Liu, F.; Yan, X.; Wang, Y.; Lu, K.; Liu, J.; Qiao, Y.; Liu, M. Photoreforming lignocellulose to hydrogen over noble-metal-free Ni(OH)2/Cd0.5Zn0.5S nanotwins. Int. J. Hydrogen Energy 2024, 69, 234–241. [Google Scholar] [CrossRef]
- Gao, X.; Zeng, D.; Yang, J.; Ong, W.-J.; Fujita, T.; He, X.; Liu, J.; Wei, Y. Ultrathin Ni(OH)2 nanosheets decorated with Zn0.5Cd0.5S nanoparticles as 2D/0D heterojunctions for highly enhanced visible light-driven photocatalytic hydrogen evolution. Chin. J. Catal. 2021, 42, 1137–1146. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, W.; Liang, S.; Bi, J.; Wu, L.; Wang, X. Engineering a highly dispersed co-catalyst on a few-layered catalyst for efficient photocatalytic H2 evolution: A case study of Ni(OH)2/HNb3O8 nanocomposites. Catal. Sci. Technol. 2017, 7, 5662–5669. [Google Scholar] [CrossRef]
- Wang, C.; Xiong, J.; Wen, Z.; Cheng, G. Integrated Ni(OH)2-TiO2-Cu2O Hybrids with a Synergic Impact of the p–n Heterojunction/Cocatalyst for Enhanced Photocatalytic Hydrogen Production. Ind. Eng. Chem. Res. 2023, 62, 11402–11413. [Google Scholar] [CrossRef]
- Liu, H.; Su, P.; Jin, Z.; Ma, Q. Enhanced Hydrogen Evolution over Sea-Urchin-Structure NiCoP Decorated ZnCdS Photocatalyst. Catal. Lett. 2020, 150, 2937–2950. [Google Scholar] [CrossRef]
- Li, Q.; Jin, F.; Liu, J.; Wang, P.; Yang, B.; Jin, Z. Construction of S-scheme CoMn2O4/ZnCdS p–n heterojunction for enhanced photocatalytic hydrogen production. J. Mater. Chem. C 2025, 13, 7380–7392. [Google Scholar] [CrossRef]
- Chen, C.; Xia, Q.; Li, Z.; Wang, Y. Microwave synthesis and photocatalytic H2 production performance of SiO2 aerogel/ZnCdS solid solution catalyst. Inorg. Chem. Commun. 2024, 168, 112874. [Google Scholar] [CrossRef]
- Zou, Y.; Guo, C.; Cao, X.; Chen, T.; Kou, Y.; Zhang, L.; Wang, T.; Akram, N.; Wang, J. Photocatalytic performance and mechanism of hydrogen evolution from water over ZnCdS/Co@CoO in sacrificial agent-free system. Int. J. Hydrogen Energy 2022, 47, 25289–25299. [Google Scholar] [CrossRef]
- Li, L.; Su, J.; Qiu, Y.; Gao, Y.; Li, N.; Ge, L. Design and fabrication of ternary Au/Co3O4/ZnCdS spherical composite photocatalyst for facilitating efficient photocatalytic hydrogen production. Chin. J. Struct. Chem. 2024, 43, 100472. [Google Scholar] [CrossRef]
- Bai, T.; Shi, X.; Liu, M.; Huang, H.; Zhang, J.; Bu, X.-H. g-C3N4/ZnCdS heterojunction for efficient visible light-driven photocatalytic hydrogen production. RSC Adv. 2021, 11, 38120–38125. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Yang, T.; Guo, X.; Jin, Z. S-scheme heterojunction constructed by ZnCdS and CoWO4 nano-ions promotes photocatalytic hydrogen production. Surf. Interfaces 2023, 43, 103577. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Q.; Yu, X.; Peng, J.; Du, S.; Chen, L.; Xu, X.; Su, T. Zn0.8Cd0.2S Photocatalyst Modified with Ni(OH)2 for Enhanced Photocatalytic Hydrogen Production. Catalysts 2025, 15, 886. https://doi.org/10.3390/catal15090886
Feng Q, Yu X, Peng J, Du S, Chen L, Xu X, Su T. Zn0.8Cd0.2S Photocatalyst Modified with Ni(OH)2 for Enhanced Photocatalytic Hydrogen Production. Catalysts. 2025; 15(9):886. https://doi.org/10.3390/catal15090886
Chicago/Turabian StyleFeng, Qianran, Xiaoting Yu, Jinlian Peng, Siying Du, Liuyun Chen, Xinyuan Xu, and Tongming Su. 2025. "Zn0.8Cd0.2S Photocatalyst Modified with Ni(OH)2 for Enhanced Photocatalytic Hydrogen Production" Catalysts 15, no. 9: 886. https://doi.org/10.3390/catal15090886
APA StyleFeng, Q., Yu, X., Peng, J., Du, S., Chen, L., Xu, X., & Su, T. (2025). Zn0.8Cd0.2S Photocatalyst Modified with Ni(OH)2 for Enhanced Photocatalytic Hydrogen Production. Catalysts, 15(9), 886. https://doi.org/10.3390/catal15090886







