Photocatalytic Degradation of Methylene Blue Dye with g-C3N4/ZnO Nanocomposite Materials Using Visible Light
Abstract
1. Introduction
2. Results and Discussion
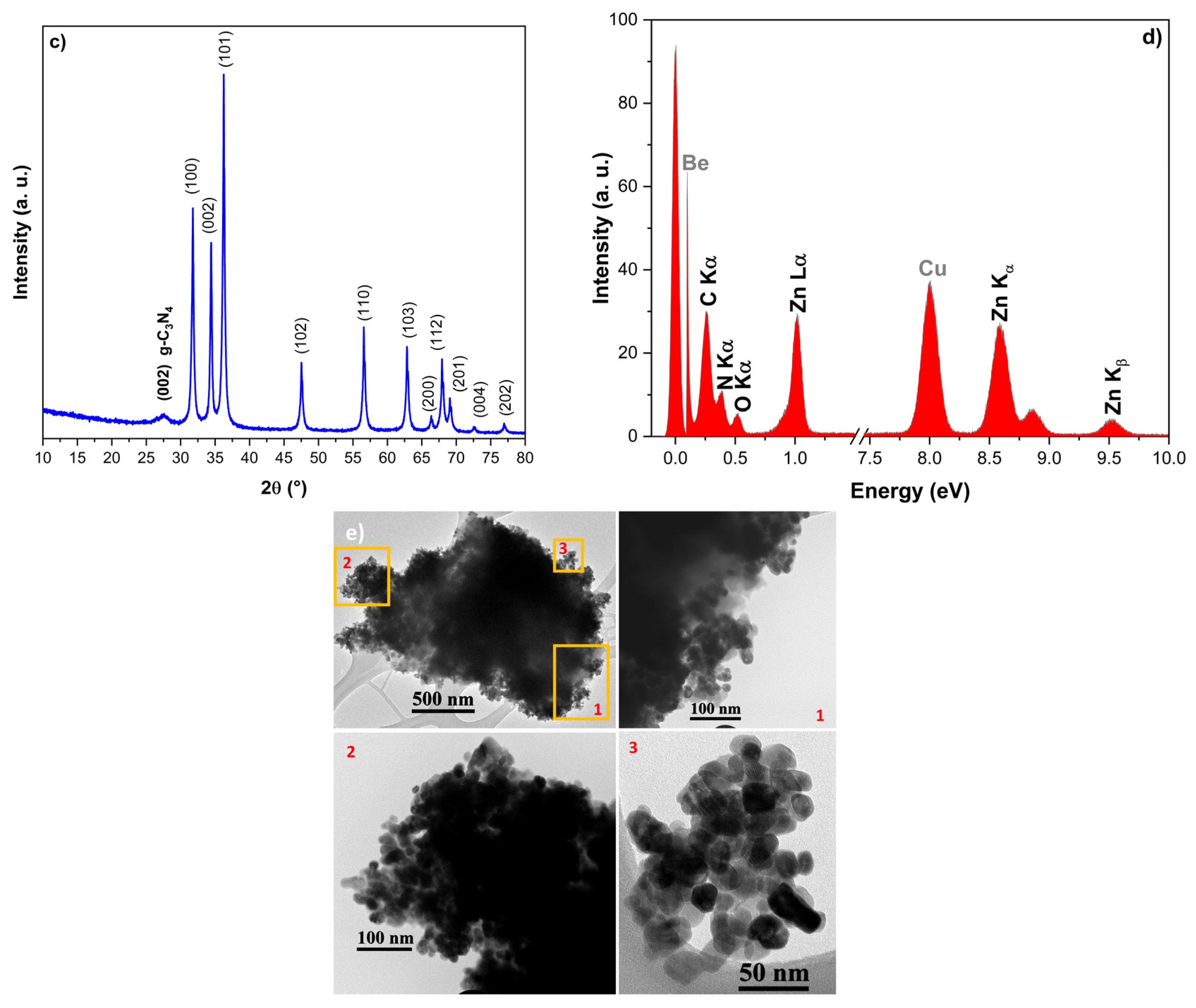
2.1. XRD
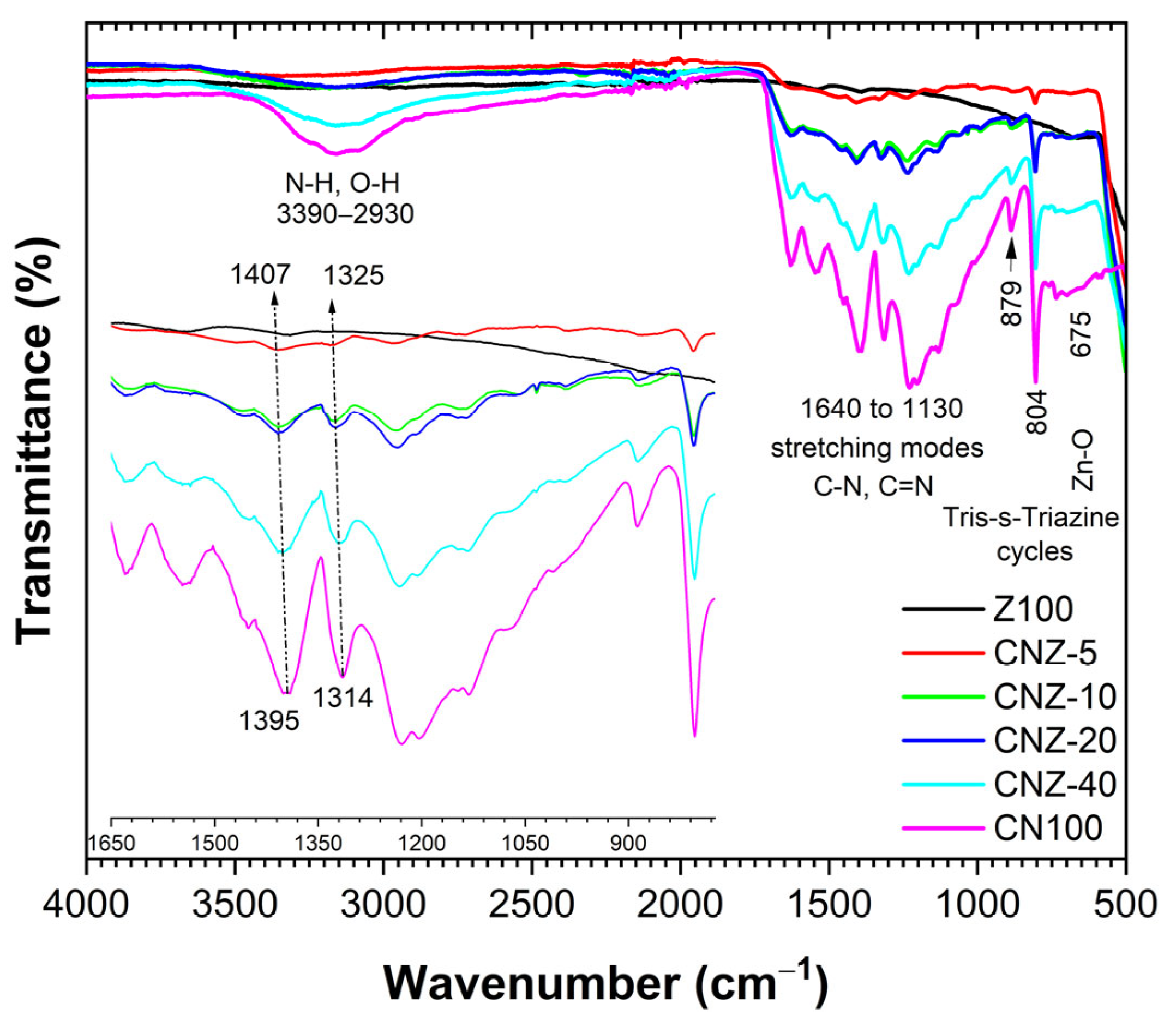
2.2. FTIR
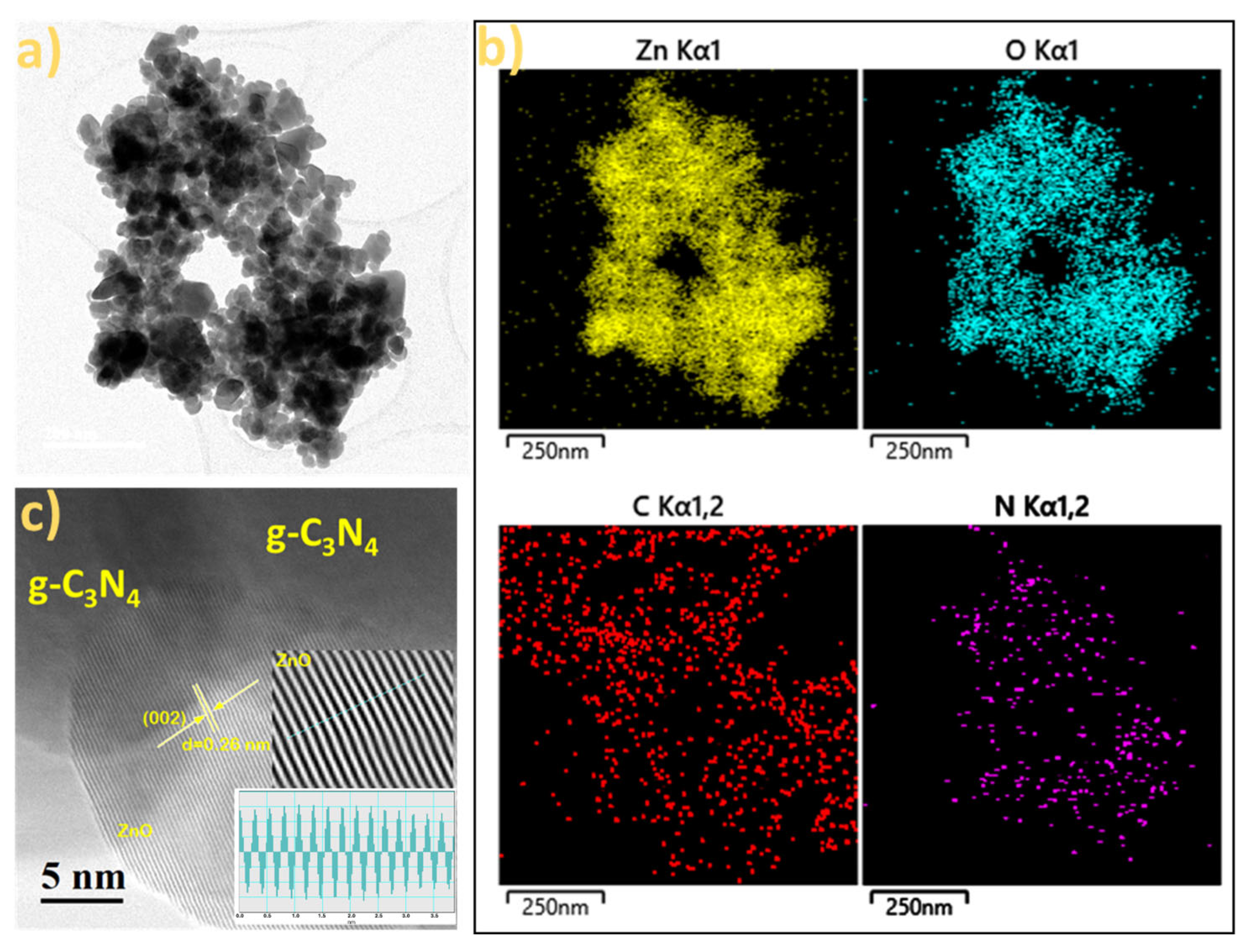
2.3. SEM and TEM
2.4. BET Analyses
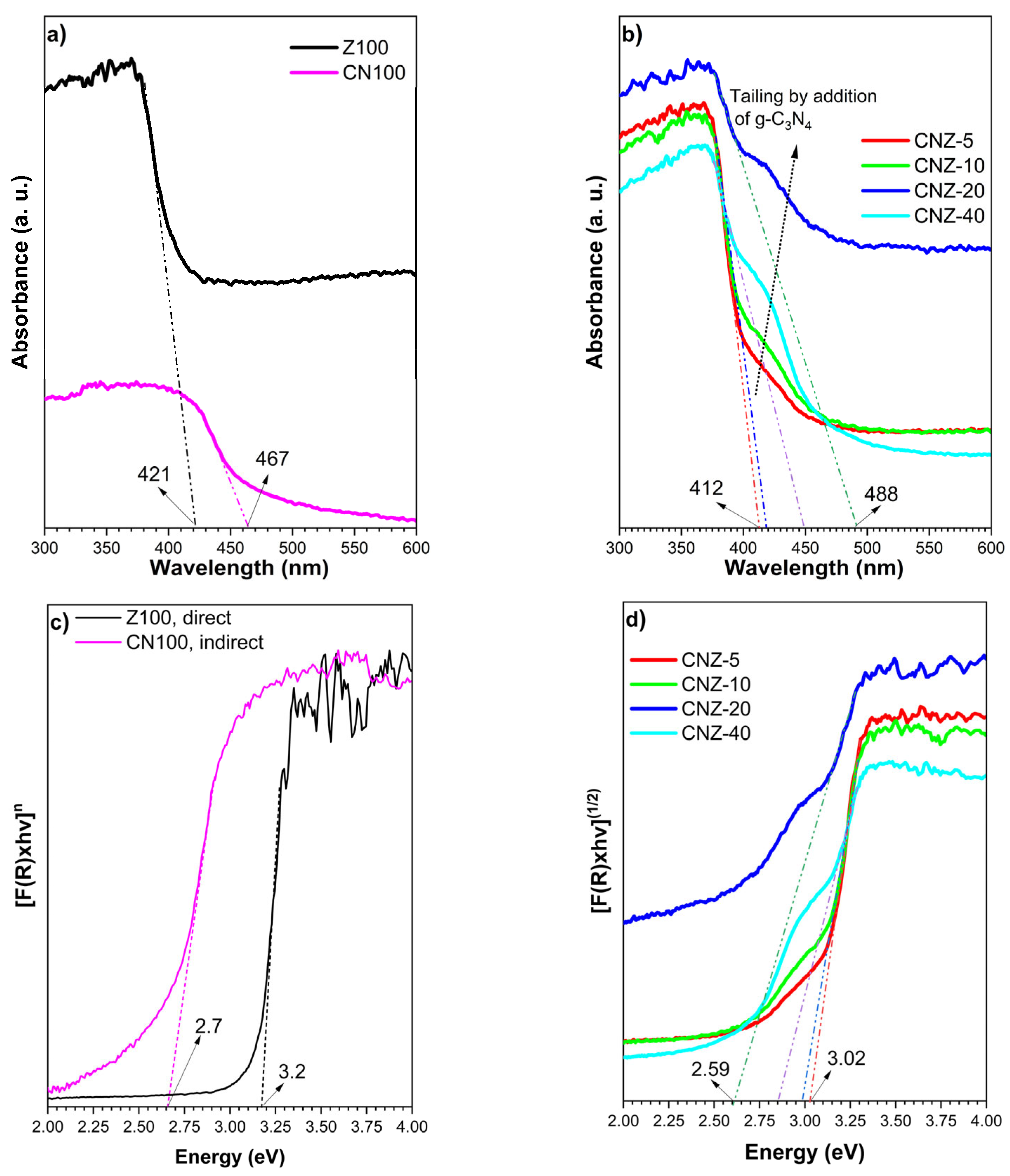
2.5. UV-Vis
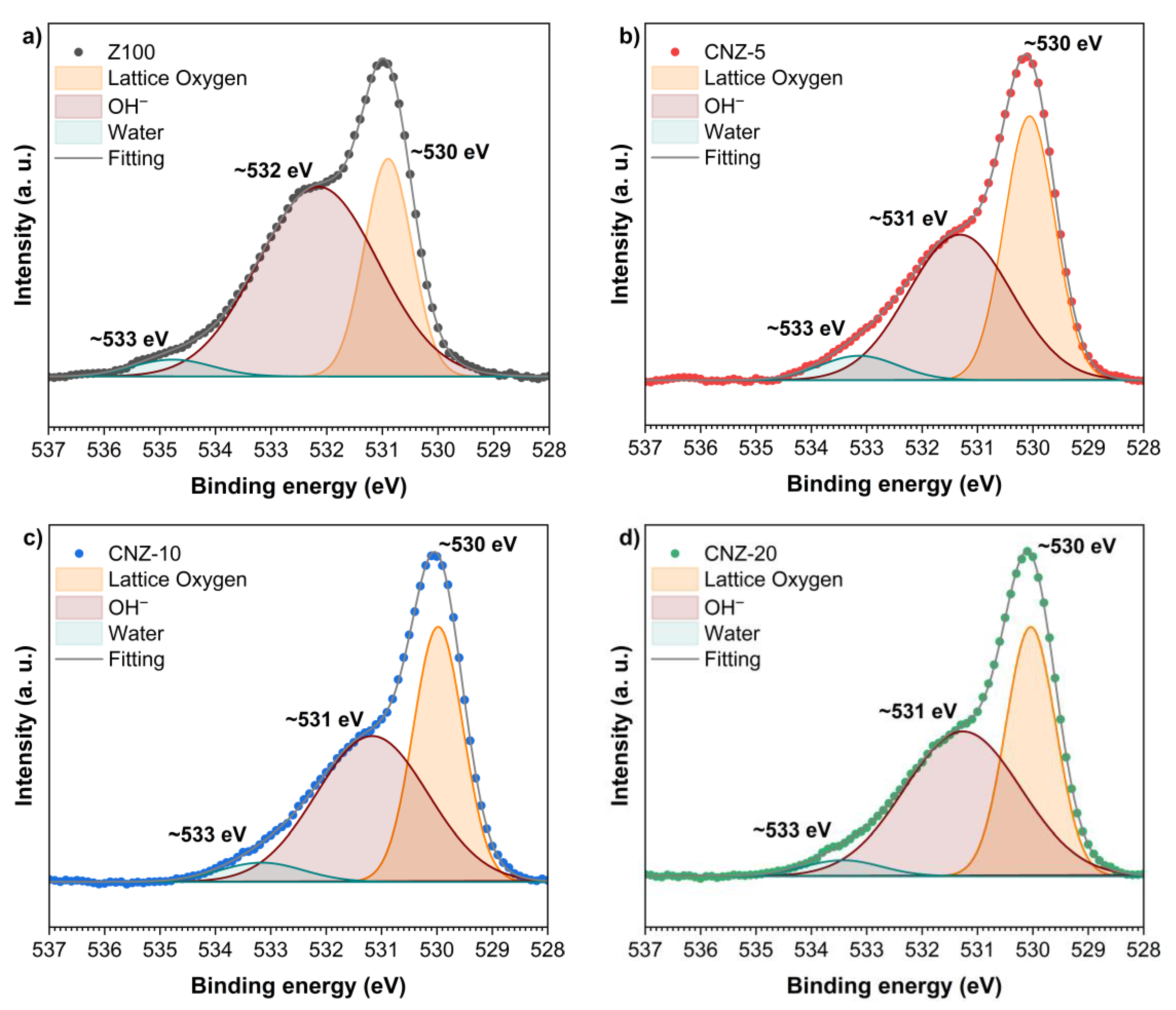
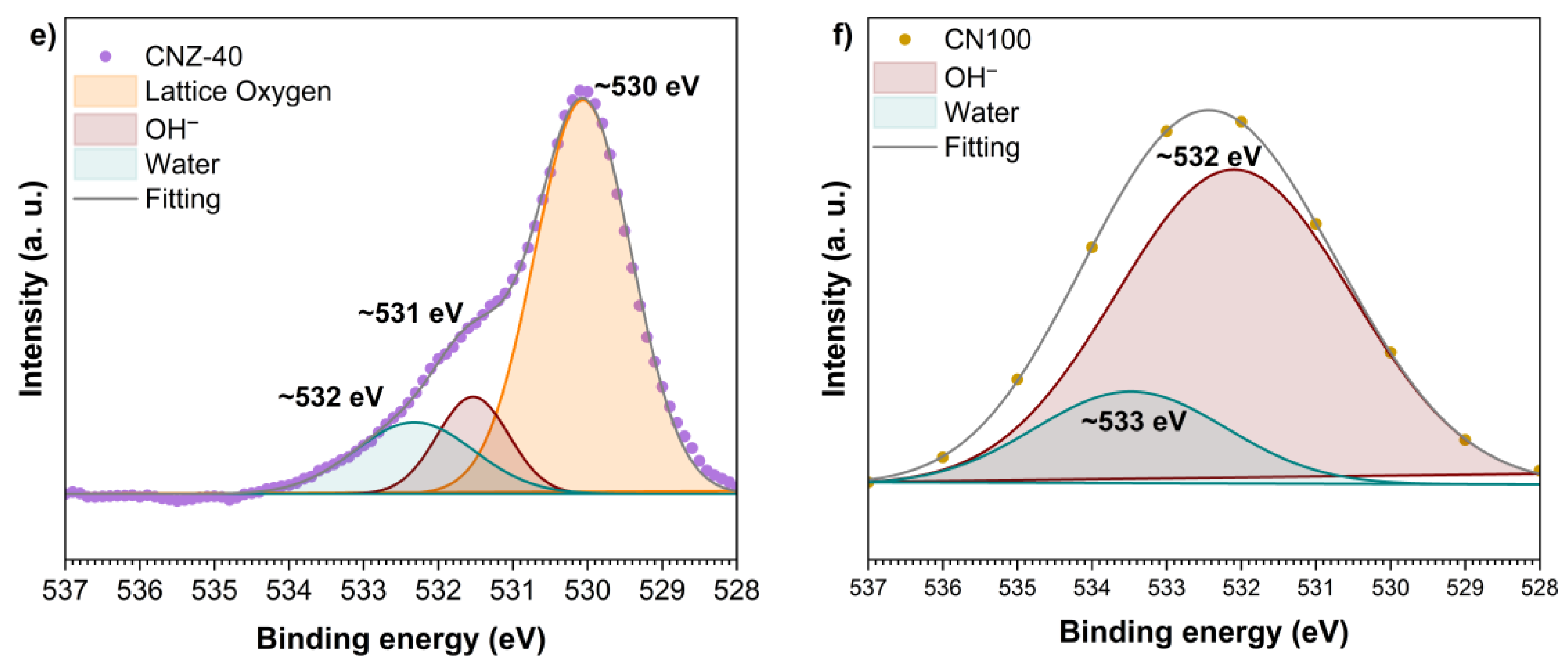
2.6. XPS
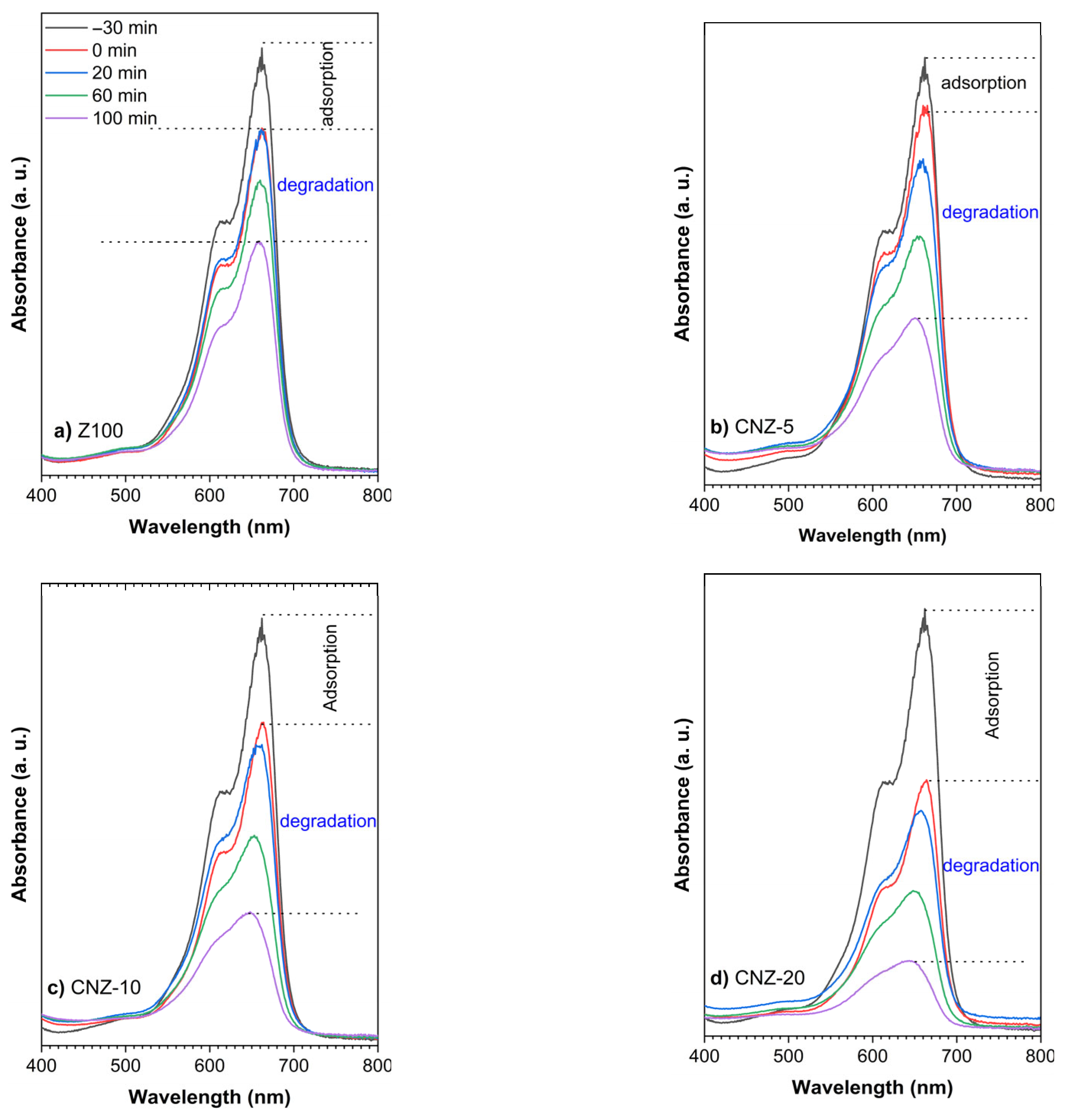
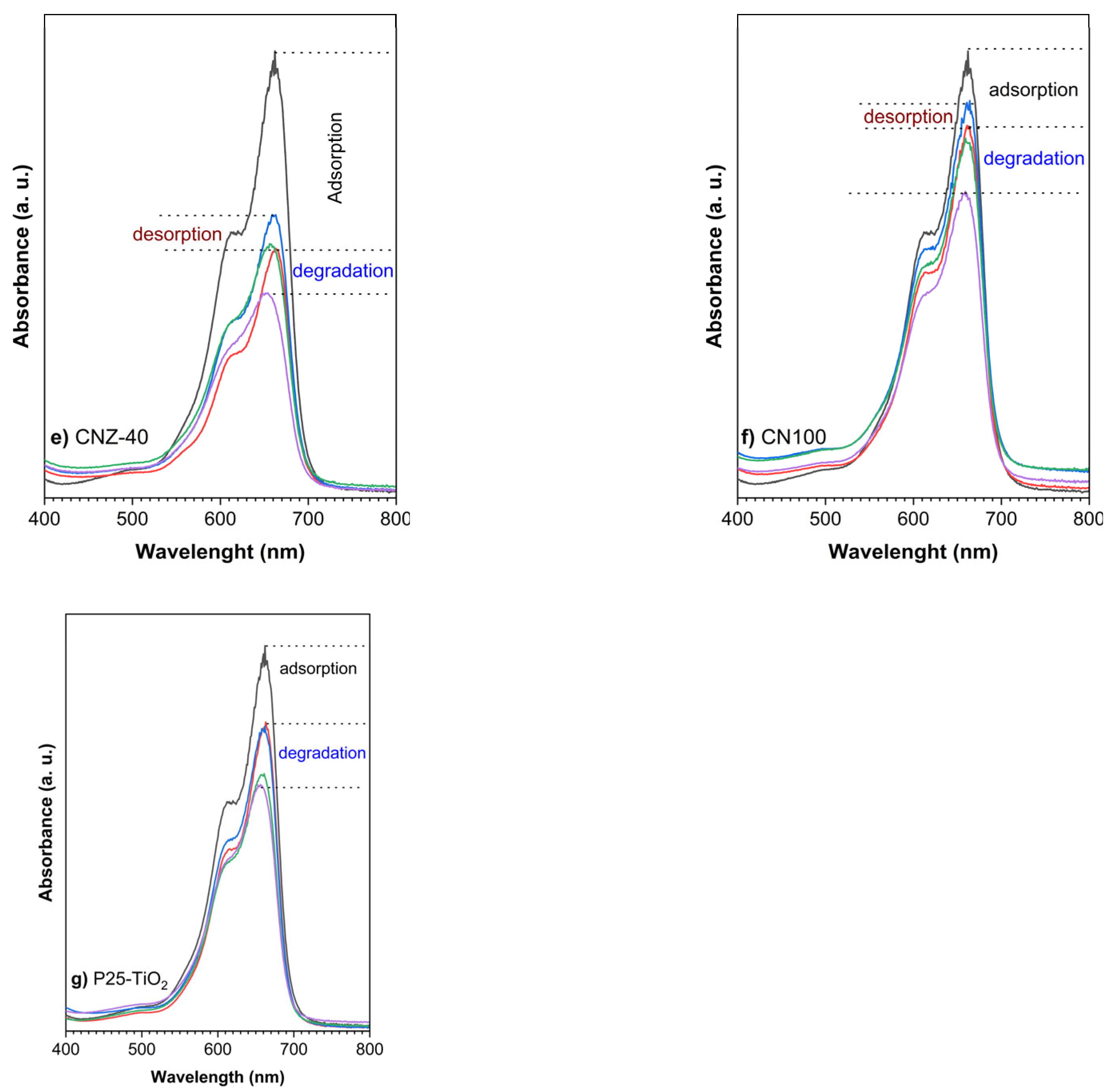
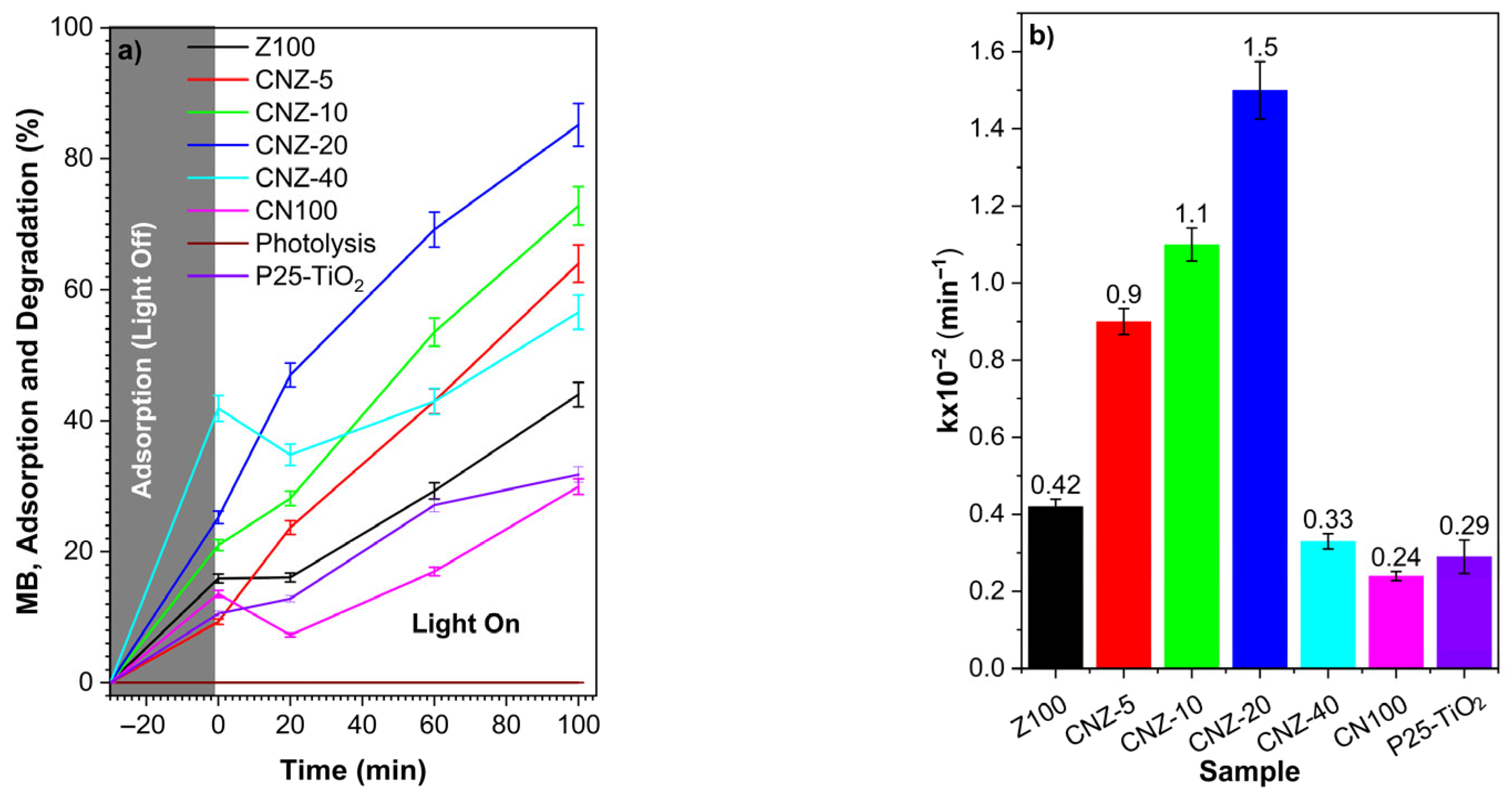
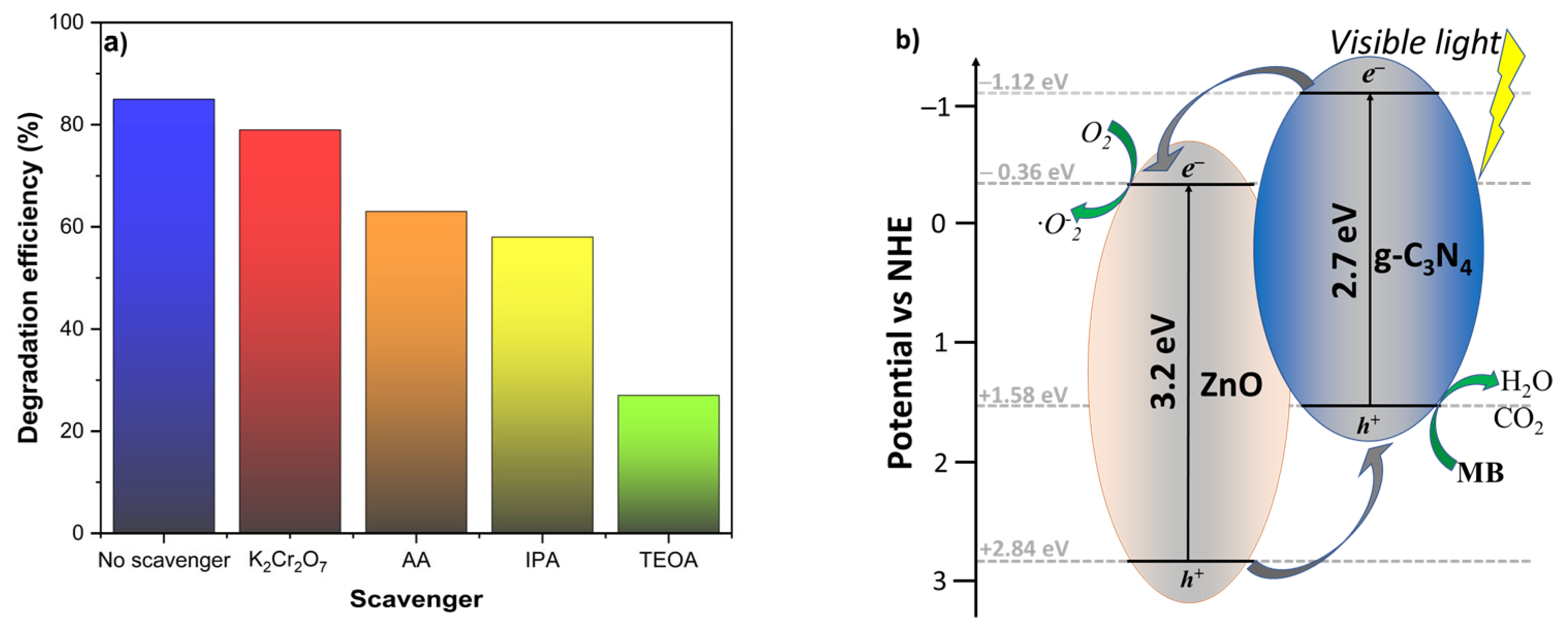
2.7. Adsorption and Photocatalytic Degradation
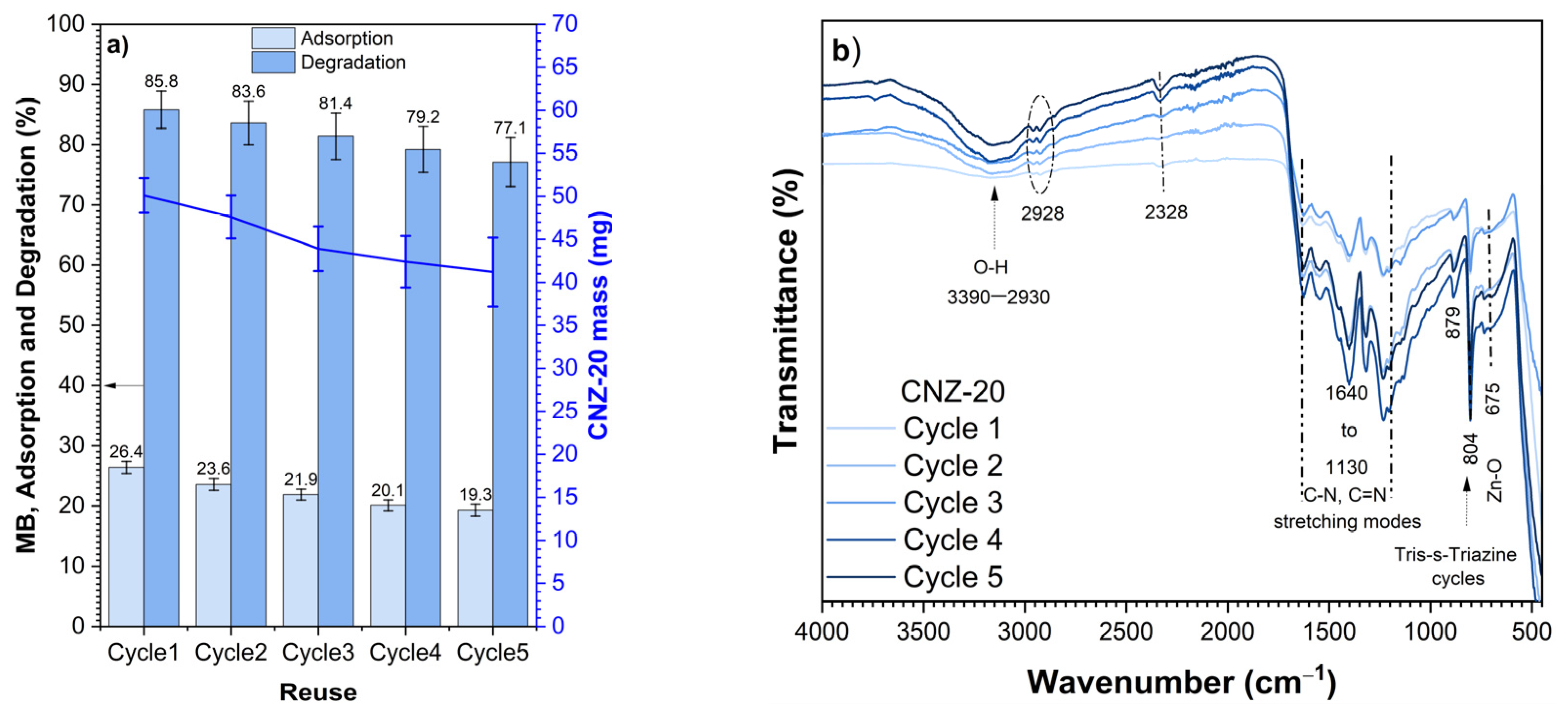
2.8. Repeatability, Reuse, and Stability Tests
3. Materials and Methods
3.1. Synthesis of g-C3N4/ZnO Nanocomposites
3.2. Characterization
3.3. Removal by Adsorption and Photocatalytic Degradation of MB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| DRS | Diffuse reflectance spectra |
| BET | Brunauer–Emmett–Teller |
| BF | Bright field |
| EDS | Energy dispersive X-ray spectroscopy |
| FTIR | Fourier transform infrared spectroscopy |
| HR | High resolution |
| k | Photocatalytic degradation constant rate (min−1) |
| MB | Methylene blue |
| p-value | Probability value |
| R2 | Coefficient of determination |
| SEM | Scanning electron microscope |
| STEM | Scanning transmission electron microscopy |
| t | Time (min) |
| t(1/2) | Half-life (min) |
| TEM | Transmission electron microscopy |
| UV-Vis | Ultraviolet–visible spectrophotometry |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
References
- Lin, L.; Yang, H.; Xu, X. Effects of Water Pollution on Human Health and Disease Heterogeneity: A Review. Front. Environ. Sci. 2022, 10, 880246. [Google Scholar] [CrossRef]
- Deokar, G.K.; Ingale, A.G. Exploring Effective Catalytic Degradation of Organic Pollutant Dyes Using Environment Benign, Green Engineered Gold Nanoparticles. Inorg. Chem. Commun. 2023, 151, 110649. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Ihaddaden, S.; Aberkane, D.; Boukerroui, A.; Robert, D. Removal of Methylene Blue (Basic Dye) by Coagulation-Flocculation with Biomaterials (Bentonite and Opuntia Ficus Indica). J. Water Process Eng. 2022, 49, 102952. [Google Scholar] [CrossRef]
- Zhu, W.; Wu, Z.; Zhao, S.; Lv, F.; Zhang, Y.; Guo, S. Selective Adsorption and Separation of Methylene Blue from Wastewater by Self-Standing Polyvinylpyrrolidone and SiO2 Electrospun Membranes. Chem. Eng. Sci. 2023, 280, 119009. [Google Scholar] [CrossRef]
- Fito, J.; Abewaa, M.; Mengistu, A.; Angassa, K.; Ambaye, A.D.; Moyo, W.; Nkambule, T. Adsorption of Methylene Blue from Textile Industrial Wastewater Using Activated Carbon Developed from Rumex Abyssinicus Plant. Sci. Rep. 2023, 13, 5427. [Google Scholar] [CrossRef] [PubMed]
- Shapira, B.; Penki, T.R.; Cohen, I.; Elias, Y.; Dalpke, R.; Beyer, A.; Gölzhäuser, A.; Avraham, E.; Aurbach, D. Combined Nanofiltration and Advanced Oxidation Processes with Bifunctional Carbon Nanomembranes. RSC Adv. 2021, 11, 14777–14786. [Google Scholar] [CrossRef] [PubMed]
- El-Bery, H.M.; Saleh, M.; El-Gendy, R.A.; Saleh, M.R.; Thabet, S.M. High Adsorption Capacity of Phenol and Methylene Blue Using Activated Carbon Derived from Lignocellulosic Agriculture Wastes. Sci. Rep. 2022, 12, 5499. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, A. Advanced Oxidation Process: A Remediation Technique for Organic and Non-Biodegradable Pollutant. Results Surf. Interfaces 2023, 11, 100122. [Google Scholar] [CrossRef]
- Chakravorty, A.; Roy, S. A Review of Photocatalysis, Basic Principles, Processes, and Materials. Sustain. Chem. Environ. 2024, 8, 100155. [Google Scholar] [CrossRef]
- Di Mauro, A.; Fragalà, M.E.; Privitera, V.; Impellizzeri, G. ZnO for Application in Photocatalysis: From Thin Films to Nanostructures. Mater. Sci. Semicond. Process. 2017, 69, 44–51. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the Improvement of the Photocatalytic and Antibacterial Activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Turkten, N.; Bekbolet, M. Photocatalytic Performance of Titanium Dioxide and Zinc Oxide Binary System on Degradation of Humic Matter. J. Photochem. Photobiol. Chem. 2020, 401, 112748. [Google Scholar] [CrossRef]
- Murali, R.; Amirthavalli, C. Defect Assisted Reactive Oxygen Species Generation for Photocatalytic Degradation of Methylene Blue by Nano-Structured Zinc Oxide Synthesized in CTAB Medium. Mater. Chem. Phys. 2023, 294, 127014. [Google Scholar] [CrossRef]
- Yao, G.; Liu, Y.; Liu, J.; Xu, Y. Facile Synthesis of Porous G-C3N4 with Enhanced Visible-Light Photoactivity. Molecules 2022, 27, 1754. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; He, J.; Xiao, B.; Zhou, M.; Wang, W.; Cunha, J.; Chen, Z.; Hou, Z.; Zhang, T.; Yu, Z. Advances and Prospects of G-C3N4 in Lithium-Sulfur Batteries. Nano Res. Energy 2024, 3, e9120138. [Google Scholar] [CrossRef]
- Gu, W.; Lu, D.; Kondamareddy, K.K.; Li, J.; Cheng, P.; Ho, W.; Wang, Y.; Zhao, Z.; Wang, Z. Efficient Photocatalytic Decomposition of NO and Mechanism Insight Enabled by NaBH4-Reduced N(Ligancy-3)-Vacancy-Rich-Graphitic Carbon Nitride. Mater. Today Phys. 2024, 46, 101487. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Chen, X.; Zang, J. MoO3/g-C3N4 Heterostructure for Degradation of Organic Pollutants under Visible Light Irradiation: High Efficiency, General Degradation and Z-Scheme Degradation Mechanism. Ceram. Int. 2021, 47, 33697–33708. [Google Scholar] [CrossRef]
- Muthuganesh, A.; Rafi, S.M.; Jacob, I.D.; Soundranayagam, J.P.; Surender, S.; Elangovan, P.; Flora, X.H. Efficient Methylene Blue Dye Degradation via Visible Light-Activated g-C3N4/CuO Nanocomposites. Hybrid Adv. 2025, 9, 100420. [Google Scholar] [CrossRef]
- Gao, R.; Lin, W.; Lin, H.; He, Y.; Mai, X.; Zhang, Y. Construction of (001)-TiO2/g-C3N4 Heterojunction for Enhanced Photocatalytic Degradation of Methylene Blue. React. Kinet. Mech. Catal. 2025, 138, 455–469. [Google Scholar] [CrossRef]
- Tahir, S.; Zahid, M.; Hanif, M.A.; Bhatti, I.A.; Naqvi, S.A.R.; Bhatti, H.N.; Jilani, A.; Alshareef, S.A.; El-Sharnouby, M.; Shahid, I. The Synergistic Effect of G-C3N4/GO/CuFe2O4 for Efficient Sunlight-Driven Photocatalytic Degradation of Methylene Blue. Int. J. Environ. Sci. Technol. 2025, 22, 4829–4846. [Google Scholar] [CrossRef]
- Liang, L.; Cong, Y.; Wang, F.; Yao, L.; Shi, L. Hydrothermal Pre-Treatment Induced Cyanamide to Prepare Porous g-C3N4 with Boosted Photocatalytic Performance. Diam. Relat. Mater. 2019, 98, 107499. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.; Gan, H.; Yu, M.; Huang, Y. The Effect of Different G-C3N4 Precursor Nature on Its Structural Control and Photocatalytic Degradation Activity. Catalysts 2023, 13, 848. [Google Scholar] [CrossRef]
- Mengesha, D.N.; Shiferraw, B.T.; Kim, H. Modification of the Electronic Structure of G-C3N4 Using Urea to Enhance the Visible Light–Assisted Degradation of Organic Pollutants. Environ. Sci. Pollut. Res. 2023, 30, 102910–102926. [Google Scholar] [CrossRef]
- Müller, R.; Huber, F.; Gelme, O.; Madel, M.; Scholz, J.-P.; Minkow, A.; Herr, U.; Thonke, K. Chemical Vapor Deposition Growth of Zinc Oxide on Sapphire with Methane: Initial Crystal Formation Process. Cryst. Growth Des. 2019, 19, 4964–4969. [Google Scholar] [CrossRef]
- Davis, K.; Yarbrough, R.; Froeschle, M.; White, J.; Rathnayake, H. Band Gap Engineered Zinc Oxide Nanostructures via a Sol–Gel Synthesis of Solvent Driven Shape-Controlled Crystal Growth. RSC Adv. 2019, 9, 14638–14648. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Ghosh, S.; Gryzenhout, M.; Conradie, J. One-Pot Synthesis of Zinc Oxide Nanoparticles via Chemical Precipitation for Bromophenol Blue Adsorption and the Antifungal Activity against Filamentous Fungi. Sci. Rep. 2021, 11, 8305. [Google Scholar] [CrossRef]
- Ejsmont, A.; Goscianska, J. Hydrothermal Synthesis of ZnO Superstructures with Controlled Morphology via Temperature and pH Optimization. Materials 2023, 16, 1641. [Google Scholar] [CrossRef]
- Luwang, N.C.; Rana, D.K.; Yadav, M.K.; Sharma, H.; Kumar, A.; Kumar, S. Surbhi Synthesis of ZnO Nanostructure via CBD and Solvothermal Method Using Seed Technique. J. Sol-Gel Sci. Technol. 2024, 112, 728–737. [Google Scholar] [CrossRef]
- De Conti, M.C.M.D.; Dey, S.; Pottker, W.E.; La Porta, F.A. An Overview into Advantages and Applications of Conventional and Unconventional Hydro(Solvo)Thermal Approaches for Novel Advanced Materials Design. Mater. Today Sustain. 2023, 23, 100458. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Li, Q.; Yang, J. Enhanced Visible Light Activity on Direct Contact Z-Scheme g-C3N4-TiO2 Photocatalyst. Appl. Surf. Sci. 2017, 391, 184–193. [Google Scholar] [CrossRef]
- Deng, H.; Qin, C.; Pei, K.; Wu, G.; Wang, M.; Ni, H.; Ye, P. TiO2/Reduced Hydroxylated Graphene Nanocomposite Photocatalysts: Improved Electron–Hole Separation and Migration. Mater. Chem. Phys. 2021, 270, 124796. [Google Scholar] [CrossRef]
- Ashiegbu, D.C.; Potgieter, H.J. ZnO-Based Heterojunction Catalysts for the Photocatalytic Degradation of Methyl Orange Dye. Heliyon 2023, 9, e20674. [Google Scholar] [CrossRef]
- Wang, W.; Lv, B.; Tao, F. NiO/g-C3N4 Composite for Enhanced Photocatalytic Properties in the Wastewater Treatment. Environ. Sci. Pollut. Res. 2022, 30, 25620–25634. [Google Scholar] [CrossRef]
- Chaharlangi, N.; Molaei, P.; Yousefi, R. One-Step Fabrication of S-Scheme ZnO/g-C3N4 Composites for Enhanced Environmental Photocatalysis. J. Alloys Compd. 2024, 1010, 177289. [Google Scholar] [CrossRef]
- Naseri, A.; Samadi, M.; Pourjavadi, A.; Ramakrishna, S.; Moshfegh, A.Z. Enhanced Photocatalytic Activity of ZnO/g-C3N4 Nanofibers Constituting Carbonaceous Species under Simulated Sunlight for Organic Dye Removal. Ceram. Int. 2021, 47, 26185–26196. [Google Scholar] [CrossRef]
- Garg, R.; Gupta, R.; Bansal, A. Synthesis of G-C3N4/ZnO Nanocomposite for Photocatalytic Degradation of a Refractory Organic Endocrine Disrupter. Mater. Today Proc. 2021, 44, 855–859. [Google Scholar] [CrossRef]
- Paul, D.R.; Gautam, S.; Panchal, P.; Nehra, S.P.; Choudhary, P.; Sharma, A. ZnO-Modified g-C3N4: A Potential Photocatalyst for Environmental Application. ACS Omega 2020, 5, 3828–3838. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Jia, M.; Li, M.; Zhou, S.; Zhao, Y.; Zhong, J.; Dai, D.; Qiu, J. ZnO@g-C3N4 S-Scheme Photocatalytic Membrane with Visible-Light Response and Enhanced Water Treatment Performance. Colloids Surf. Physicochem. Eng. Asp. 2023, 667, 131259. [Google Scholar] [CrossRef]
- Meena, P.L.; Poswal, K.; Surela, A.K.; Saini, J.K. Synthesis of Graphitic Carbon Nitride/Zinc Oxide (g-C3N4/ZnO) Hybrid Nanostructures and Investigation of the Effect of ZnO on the Photodegradation Activity of g-C3N4 against the Brilliant Cresyl Blue (BCB) Dye under Visible Light Irradiation. Adv. Compos. Hybrid Mater. 2022, 6, 16. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Z.; Shi, L. Boosting Photocatalytic Activity of G-C3N4/Nano-Sized ZnO Fabricated in CO2-Saturated Solutions. Mater. Lett. 2021, 296, 129894. [Google Scholar] [CrossRef]
- Sun, H.; Zou, C.; Liao, Y.; Tang, W.; Huang, Y.; Chen, M. Modulating Charge Transport Behavior across the Interface via G-C3N4 Surface Discrete Modified BiOI and Bi2MoO6 for Efficient Photodegradation of Glyphosate. J. Alloys Compd. 2023, 935, 168208. [Google Scholar] [CrossRef]
- Roškarič, M.; Žerjav, G.; Finšgar, M.; Zavašnik, J.; Pintar, A. Influence of the Calcination Duration of G-C3N4/TiO2 “Veggie-Toast-like” Photocatalyst on the Visible-Light Triggered Photocatalytic Oxidation of Bisphenol A. J. Alloys Compd. 2023, 947, 169585. [Google Scholar] [CrossRef]
- Hajiashrafi, S.; Motakef Kazemi, N. Preparation and Evaluation of ZnO Nanoparticles by Thermal Decomposition of MOF-5. Heliyon 2019, 5, e02152. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Y.; Guo, Y.; Ren, X.-K.; Wu, L.; Liu, L.; Shi, Z.; Wang, Y. Pd Nanoparticles Confined within Triazine-Based Carbon Nitride NTs: An Efficient Catalyst for Knoevenagel Condensation-Reduction Cascade Reactions. Catal. Today 2019, 330, 124–134. [Google Scholar] [CrossRef]
- Narkbuakaew, T.; Sujaridworakun, P. Synthesis of Tri-S-Triazine Based g-C3N4 Photocatalyst for Cationic Rhodamine B Degradation under Visible Light. Top. Catal. 2020, 63, 1086–1096. [Google Scholar] [CrossRef]
- Aslam, I.; Hassan Farooq, M.; Ghani, U.; Rizwan, M.; Nabi, G.; Shahzad, W.; Boddula, R. Synthesis of Novel G-C3N4 Microrods: A Metal-Free Visible-Light-Driven Photocatalyst. Mater. Sci. Energy Technol. 2019, 2, 401–407. [Google Scholar] [CrossRef]
- Pourali, S.; Amrollahi, R.; Alamolhoda, S.; Masoudpanah, S.M. In Situ Synthesis of ZnO/g-C3N4 Based Composites for Photodegradation of Methylene Blue under Visible Light. Sci. Rep. 2025, 15, 462. [Google Scholar] [CrossRef]
- Malik, M.; Len, T.; Luque, R.; Osman, S.M.; Paone, E.; Khan, M.I.; Wattoo, M.A.; Jamshaid, M.; Anum, A.; Rehman, A.U. Investigation on Synthesis of Ternary G-C3N4/ZnO–W/M Nanocomposites Integrated Heterojunction II as Efficient Photocatalyst for Environmental Applications. Environ. Res. 2023, 217, 114621. [Google Scholar] [CrossRef]
- Cappelluti, M.D.; Hadzifejzovic, E.; Foord, J.S.; Gregory, D.H. Flash Microwave-Assisted Solvothermal (FMS) Synthesis of Photoactive Anatase Sub-Microspheres with Hierarchical Porosity. RSC Adv. 2020, 10, 37233–37245. [Google Scholar] [CrossRef]
- Pellegrino, F.; Pellutiè, L.; Sordello, F.; Minero, C.; Ortel, E.; Hodoroaba, V.-D.; Maurino, V. Influence of Agglomeration and Aggregation on the Photocatalytic Activity of TiO2 Nanoparticles. Appl. Catal. B Environ. 2017, 216, 80–87. [Google Scholar] [CrossRef]
- Girish, Y.R.; Udayabhanu; Byrappa, N.M.; Alnaggar, G.; Hezam, A.; Nagaraju, G.; Pramoda, K.; Byrappa, K. Rapid and Facile Synthesis of Z-Scheme ZnO/g-C3N4 Heterostructure as Efficient Visible Light-Driven Photocatalysts for Dye Degradation and Hydrogen Evolution Reaction. J. Hazard. Mater. Adv. 2023, 9, 100230. [Google Scholar] [CrossRef]
- Hutchings, G.J.; Davies, P.R.; Pattisson, S.; Davies, T.E.; Morgan, D.J.; Dlamini, M.W. Facile Synthesis of a Porous 3D G-C3N4 Photocatalyst for the Degradation of Organics in Shale Gas Brines. Catal. Commun. 2022, 169, 106480. [Google Scholar] [CrossRef]
- Hassan, F.; Backer, S.N.; Almanassra, I.W.; Ali Atieh, M.; Elbahri, M.; Shanableh, A. Solar-Matched S-Scheme ZnO/g-C3N4 for Visible Light-Driven Paracetamol Degradation. Sci. Rep. 2024, 14, 12220. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Molina, Á.; Pastrana-Martínez, L.M.; Pérez-Poyatos, L.T.; Morales-Torres, S.; Maldonado-Hódar, F.J. One-Pot Thermal Synthesis of g-C3N4/ZnO Composites for the Degradation of 5-Fluoruracil Cytostatic Drug under UV-LED Irradiation. Nanomaterials 2022, 12, 340. [Google Scholar] [CrossRef]
- Hakimi-Tehrani, M.J.; Hasanzadeh-Tabrizi, S.A.; Koupaei, N.; Saffar, A.; Rafiei, M. Synthesis of G-C3N4/ZnO/WO3 Nanocomposite as a Highly Efficient Antibacterial Adsorbent for Water Treatment. Diam. Relat. Mater. 2022, 130, 109506. [Google Scholar] [CrossRef]
- González-Suárez, B.W.; Pantoja-Espinoza, J.C.; Lardizábal-Gutierrez, D.; Flores, M.U.; Paraguay-Delgado, F. Effect of [Zn Acetate]/[KOH] Ratio on ZnO Particles Synthesis and Photocatalytic Rhodamine B Degradation. J. Mater. Res. Technol. 2024, 30, 8092–8107. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Zhang, M.; Wang, X. Polycondensation of Thiourea into Carbon Nitride Semiconductors as Visible Light Photocatalysts. J. Mater. Chem. 2012, 22, 8083. [Google Scholar] [CrossRef]
- Ievtushenko, A.; Khyzhun, O.; Shtepliuk, I.; Bykov, O.; Jakieła, R.; Tkach, S.; Kuzmenko, E.; Baturin, V.; Karpenko, O.; Olifan, O.; et al. X-Ray Photoelectron Spectroscopy Study of Highly-Doped ZnO: Al,N Films Grown at O-Rich Conditions. J. Alloys Compd. 2017, 722, 683–689. [Google Scholar] [CrossRef]
- Zhang, S.; Su, C.; Ren, H.; Li, M.; Zhu, L.; Ge, S.; Wang, M.; Zhang, Z.; Li, L.; Cao, X. In-Situ Fabrication of g-C3N4/ZnO Nanocomposites for Photocatalytic Degradation of Methylene Blue: Synthesis Procedure Does Matter. Nanomaterials 2019, 9, 215. [Google Scholar] [CrossRef]
- Li, Y.; Gu, M.; Shi, T.; Cui, W.; Zhang, X.; Dong, F.; Cheng, J.; Fan, J.; Lv, K. Carbon Vacancy in C3N4 Nanotube: Electronic Structure, Photocatalysis Mechanism and Highly Enhanced Activity. Appl. Catal. B Environ. 2020, 262, 118281. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, W.; Lin, Q.; Li, Z.; Zhong, W.; Fang, B. Nitrogen Vacancies-Engineered Graphitic Carbon Nitride Nanosheets for Boosting Photocatalytic H2 Production. Appl. Surf. Sci. 2023, 640, 158386. [Google Scholar] [CrossRef]
- Meng, L.-J.; Moreira De Sá, C.P.; Dos Santos, M.P. Study of the Structural Properties of ZnO Thin Films by X-Ray Photoelectron Spectroscopy. Appl. Surf. Sci. 1994, 78, 57–61. [Google Scholar] [CrossRef]
- Panthi, G.; Park, M. Graphitic Carbon Nitride/Zinc Oxide-Based Z-Scheme and S-Scheme Heterojunction Photocatalysts for the Photodegradation of Organic Pollutants. Int. J. Mol. Sci. 2023, 24, 15021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jaroniec, M. Fundamentals of Adsorption for Photocatalysis. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 31, pp. 39–62. ISBN 978-0-08-102890-2. [Google Scholar]
- Sher, M.; Javed, M.; Shahid, S.; Hakami, O.; Qamar, M.A.; Iqbal, S.; AL-Anazy, M.M.; Baghdadi, H.B. Designing of Highly Active G-C3N4/Sn Doped ZnO Heterostructure as a Photocatalyst for the Disinfection and Degradation of the Organic Pollutants under Visible Light Irradiation. J. Photochem. Photobiol. Chem. 2021, 418, 113393. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, H. Coupled Adsorption and Photocatalysis of G-C3N4 Based Composites: Material Synthesis, Mechanism, and Environmental Applications. Chem. Eng. J. 2023, 453, 139755. [Google Scholar] [CrossRef]
- Zhu, B.; Xia, P.; Ho, W.; Yu, J. Isoelectric Point and Adsorption Activity of Porous G-C3N4. Appl. Surf. Sci. 2015, 344, 188–195. [Google Scholar] [CrossRef]
- Malebadi, K.A.; Seheri, N.H.; Ojelere, O.; Onwudiwe, D.C. ZnO Nanoparticles Modified with G-C3N4: Optical and Structural Properties. Mater. Sci. Eng. B 2024, 310, 117676. [Google Scholar] [CrossRef]
- Mihai, S.; Cursaru, D.L.; Matei, D.; Manta, A.M.; Somoghi, R.; Branoiu, G. Rutile RuxTi1-xO2 Nanobelts to Enhance Visible Light Photocatalytic Activity. Sci. Rep. 2019, 9, 18798. [Google Scholar] [CrossRef]
- Hao, R.; Wang, G.; Jiang, C.; Tang, H.; Xu, Q. In Situ Hydrothermal Synthesis of G-C3N4/TiO2 Heterojunction Photocatalysts with High Specific Surface Area for Rhodamine B Degradation. Appl. Surf. Sci. 2017, 411, 400–410. [Google Scholar] [CrossRef]
- Wagh, S.S.; Chougale, A.S.; Survase, A.A.; Patil, R.S.; Naik, N.; Naushad, M.; Pathan, H.M. Rapid Photocatalytic Dye Degradation, Enhanced Antibacterial and Antifungal Activities of Silver Stacked Zinc Oxide Garnished on Carbon Nanotubes. Sci. Rep. 2024, 14, 14045. [Google Scholar] [CrossRef]
- Tamiru Mengistu, M.; Wondimu, T.H.; Andoshe, D.M.; Kim, J.Y.; Zelekew, O.A.; Hone, F.G.; Tegene, N.A.; Gultom, N.S.; Jang, H.W. G-C3N4–Co3O4 Z-Scheme Junction with Green-Synthesized ZnO Photocatalyst for Efficient Degradation of Methylene Blue in Aqueous Solution. Bioinorg. Chem. Appl. 2023, 2023, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.G.; Kavitha, R.; Manjunatha, C. Review and Perspective on Rational Design and Interface Engineering of G-C3N4/ZnO: From Type-II to Step-Scheme Heterojunctions for Photocatalytic Applications. Energy Fuels 2023, 37, 14421–14472. [Google Scholar] [CrossRef]
- Valiyathur, M.D.F.; Sakvai, M.S.; Mithra, S.; Majeed, S.A.; Kottur, A.B.; Hameed, A.S.S.; Raza, A.A. Photocatalytic and Toxicity Assessment of Alginate Reinforced ZnO-g-C3N4 Photocatalyst for the Degradation of Methylene Blue—A Sustainable Strategy. Int. J. Biol. Macromol. 2025, 298, 139935. [Google Scholar] [CrossRef] [PubMed]
- Schott, J.A.; Do-Thanh, C.-L.; Shan, W.; Puskar, N.G.; Dai, S.; Mahurin, S.M. FTIR Investigation of the Interfacial Properties and Mechanisms of CO2 Sorption in Porous Ionic Liquids. Green Chem. Eng. 2021, 2, 392–401. [Google Scholar] [CrossRef]
- Manea, Y.K.; Khan, A.M. Enhanced Photocatalytic Degradation of Methylene Blue and Adsorption of Metal Ions by SDS-TiP Nanocomposite. SN Appl. Sci. 2019, 1, 821. [Google Scholar] [CrossRef]
- Dimitropoulos, M.; Aggelopoulos, C.A.; Sygellou, L.; Tsantis, S.T.; Koutsoukos, P.G.; Yannopoulos, S.N. Unveiling the Photocorrosion Mechanism of Zinc Oxide Photocatalyst: Interplay between Surface Corrosion and Regeneration. J. Environ. Chem. Eng. 2024, 12, 112102. [Google Scholar] [CrossRef]
- Ma, X.; Li, H.; Liu, T.; Du, S.; Qiang, Q.; Wang, Y.; Yin, S.; Sato, T. Comparison of Photocatalytic Reaction-Induced Selective Corrosion with Photocorrosion: Impact on Morphology and Stability of Ag-ZnO. Appl. Catal. B Environ. 2017, 201, 348–358. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, J.; Xue, Y.; Yu, P.; Zhang, B.; Wang, L.; Liu, R. Effect of Aspect Ratio and Surface Defects on the Photocatalytic Activity of ZnO Nanorods. Sci. Rep. 2014, 4, 4596. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, N.; Xu, Y.-J. Synthesis of Graphene–ZnO Nanorod Nanocomposites with Improved Photoactivity and Anti-Photocorrosion. CrystEngComm 2013, 15, 3022. [Google Scholar] [CrossRef]
- Zhang, H.; Zong, R.; Zhu, Y. Photocorrosion Inhibition and Photoactivity Enhancement for Zinc Oxide via Hybridization with Monolayer Polyaniline. J. Phys. Chem. C 2009, 113, 4605–4611. [Google Scholar] [CrossRef]
- Han, C.; Yang, M.-Q.; Weng, B.; Xu, Y.-J. Improving the Photocatalytic Activity and Anti-Photocorrosion of Semiconductor ZnO by Coupling with Versatile Carbon. Phys. Chem. Chem. Phys. 2014, 16, 16891. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Bai, Z.; Gao, T.; Bai, Z.; Zhu, Y. Preparation Effects on the Morphology and Photocatalytic Properties of Carbon Nitride Nanotubes. Results Phys. 2019, 13, 102254. [Google Scholar] [CrossRef]




| Sample | ZnO | g-C3N4 | ||
|---|---|---|---|---|
| ~31.83° | ~34.48° | ~36.32° | 27.35° | |
| Z100 | 15 | 17 | 15 | - |
| CNZ-5 | 22 | 24 | 20 | Not identified |
| CNZ-10 | 14 | 21 | 23 | 20 |
| CNZ-20 | 10 | 21 | 23 | 20 |
| CNZ-40 | 6 | 21 | 23 | 20 |
| CN100 | - | - | - | 4 |
| Sample | BET Area (m2/g) | Pore Size (nm) |
|---|---|---|
| Z100 | 18 | 15 |
| CNZ-5 | 20 | 3 |
| CNZ-10 | 21 | 3 |
| CNZ-20 | 25 | 3 |
| CNZ-40 | 19 | 3 |
| CN100 | 12 | 11 |
| Sample | Absorption (nm) | Band Gap (eV) |
|---|---|---|
| Z100 | 416 | 3.20 |
| CNZ-5 | 413 | 3.02 |
| CNZ-10 | 418 | 2.99 |
| CNZ-20 | 488 | 2.59 |
| CNZ-40 | 449 | 2.55 |
| CN100 | 467 | 2.66 |
| Sample | k × 10−2 (min−1) | t(1/2) | R2 |
|---|---|---|---|
| Z100 | 0.43 | 161 | 0.9501 |
| CNZ-5 | 0.91 | 76 | 0.9888 |
| CNZ-10 | 1.08 | 64 | 0.9821 |
| CNZ-20 | 1.45 | 48 | 0.9848 |
| CNZ-40 | 0.33 | 210 | 0.7003 |
| CN100 | 0.24 | 289 | 0.7713 |
| P25-TiO2 | 0.29 | 235 | 0.9368 |
| Synthesis Method | Experimental Conditions | Photocatalytic Evaluation | ||||||
|---|---|---|---|---|---|---|---|---|
| Heating | g-C3N4/ZnO (mg) | MB (mg/L) | Time (min) | Irradiation Source | Degradation (%) | Kinetic Constant (k, min−1) | Ref. | |
| Precursors mixing | Conventional furnace 550 °C 2 h | 10 | 10 | 120 | 200 W tungsten lamp with 420 nm filter | 90 | Not reported | [38] |
| Solid-state | Electric furnace 550 °C 2 h | 50 | 10 | 120 | Direct sunlight | 92 | Not reported | [35] |
| Electrospinning technique | Tubular furnace under N2 atmosphere, 460 °C, 15 min | 10 | 1 × 10−5 M | 120 | 300 W ceramic Xe lamp | 91.8 | Not reported | [36] |
| In situ growing crystals | 130 °C for 2 h | 10.75 cm2 membranes | 5 | 150 | 300 W Xe lamp with a 420 nm filter | 94.4 | Not reported | [39] |
| Combustion thermal condensation | Conventional furnace 550 °C 2 h | 100 | 5 | 180 | Two 100 W xenon lamps (UV-visible regions) | 95 | 0.022 | [48] |
| Co-precipitation | Conventional furnace 300 °C 2 h | 300 | 1 × 10−5 M | 60 | 300 W tungsten halogen lamp | 78 | Not reported | [75] |
| Solvothermal | Microwave, 160 °C, 30 min | 50 | 10 | 100 | 23 W fluorescent visible lamp, 400–1050 nm, 10 W/m2 | 85 | 0.0145 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantoja-Espinoza, J.C.; DelaCruz-Alderete, G.A.; Paraguay-Delgado, F. Photocatalytic Degradation of Methylene Blue Dye with g-C3N4/ZnO Nanocomposite Materials Using Visible Light. Catalysts 2025, 15, 851. https://doi.org/10.3390/catal15090851
Pantoja-Espinoza JC, DelaCruz-Alderete GA, Paraguay-Delgado F. Photocatalytic Degradation of Methylene Blue Dye with g-C3N4/ZnO Nanocomposite Materials Using Visible Light. Catalysts. 2025; 15(9):851. https://doi.org/10.3390/catal15090851
Chicago/Turabian StylePantoja-Espinoza, Juan C., Gema A. DelaCruz-Alderete, and Francisco Paraguay-Delgado. 2025. "Photocatalytic Degradation of Methylene Blue Dye with g-C3N4/ZnO Nanocomposite Materials Using Visible Light" Catalysts 15, no. 9: 851. https://doi.org/10.3390/catal15090851
APA StylePantoja-Espinoza, J. C., DelaCruz-Alderete, G. A., & Paraguay-Delgado, F. (2025). Photocatalytic Degradation of Methylene Blue Dye with g-C3N4/ZnO Nanocomposite Materials Using Visible Light. Catalysts, 15(9), 851. https://doi.org/10.3390/catal15090851








