Abstract
Photocatalytic overall water splitting (PWS) offers a green, economical, and sustainable pathway for hydrogen production. However, the efficiency is still hindered by severe charge recombination in catalysts, high energy barriers for water oxidation, and sluggish reaction kinetics. Therefore, it is crucial to address these challenges by enhancing charge separation efficiency, accelerating reaction kinetics, and lowering PWS energy barriers. In this work, we constructed donor–acceptor covalent triazine-based organic frameworks (CTFs), such as CTF-BP, CTF-DBT, and CTF-DBTS, using biphenyl (BP), benzothiophene (DBT), and benzothiophene sulfone (DBTS) as basic units, respectively. DFT calculations revealed that all three CTFs exhibit comparable bandgaps with strong visible-light absorption. Notably, strong dipole moments between donor and acceptor units were observed within these frameworks, effectively promoting in-plane charge separation. DBT and DBTS derivatives generated stronger dipole moments compared to biphenyl. Furthermore, hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) pathway analyses demonstrated that CTF-DBTS substantially reduces energy barriers for both half-reactions relative to CTF-DBT and CTF-BP, exhibiting the most promising potential for PWS. This work provides a reference for the application of DBTS-incorporated COFs in PWS systems.
1. Introduction
Photocatalytic overall water splitting (PWS) has emerged as a promising strategy for sustainable hydrogen production [1,2,3]. Despite significant progress since its initial discovery in 1972, the practical application of this technology remains limited due to challenges in efficiency, stability, and scalability under solar irradiation [4]. For instance, SrTiO3:Al-based systems modified with CoOOH and Rh/Cr2O3 co-catalysts exhibit near-unity quantum yields under ultraviolet light (350–360 nm), yet their reliance on UV photons restricts their practical utility under natural sunlight [5]. While visible-light-responsive photocatalysts address light absorption limitations, they often suffer from critical drawbacks such as rapid charge recombination, insufficient redox potentials, and high surface reaction barriers, hindering their industrial adoption [6,7,8,9].
Covalent triazine frameworks (CTFs), a subclass of covalent organic frameworks (COFs), have garnered significant attention as robust organic photocatalysts due to their tunable electronic structures, chemical stability, and inherent donor–acceptor configurations [10,11,12,13]. The strong dipole moments generated between donor and acceptor units within CTFs enhance charge separation, while their periodic microporous structures facilitate reactant adsorption and redox site isolation [14,15]. However, the predominance of light nonmetallic elements in CTFs often leads to weak interactions with small molecules like H2O, H2, and O2, resulting in high activation energy barriers for hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) [16,17]. Recent studies suggest that incorporating functional groups such as benzothiophene sulfone (DBTS) into COFs can significantly improve photocatalytic performance [18,19,20,21]. For example, DBTS-modified COFs demonstrate enhanced PWS activity, particularly when hybridized with oxidative semiconductors [22,23]. Nevertheless, the mechanistic role of DBTS in reducing reaction barriers and optimizing charge transfer dynamics remains poorly understood, underscoring the need for systematic theoretical investigations.
In this study, three structurally analogous CTFs—CTF-BP, CTF-DBT, and CTF-DBTS—were constructed using biphenyl (BP), benzothiophene (DBT), and DBTS as building blocks. These frameworks share comparable pore sizes and crystallinity, facilitating a direct comparison to elucidate the superior catalytic performance of DBTS-functionalized CTFs. Structural integrity and stability were confirmed through XRD, FTIR, and Raman spectroscopy. Electronic properties, including band structures and density of states, were analyzed to evaluate redox capabilities, while UV–vis absorption spectra were simulated to assess light-harvesting efficiency. Surface electrostatic potential mapping and dipole moment calculations provided insights into charge separation mechanisms, and HER/OER pathway analyses via Gibbs free energy diagrams identified key reaction barriers. This work not only clarifies the role of DBTS in boosting photocatalytic activity but also establishes design principles for developing efficient, visible-light-driven COFs tailored for PWS.
2. Results and Discussion
To systematically elucidate the structure-property relationships, three monolayer CTFs functionalized with distinct groups—BP, DBT, and DBTS—were constructed, denoted as CTF-BP, CTF-DBT, and CTF-DBTS, respectively. These frameworks feature alternating triazine rings and functional groups, forming highly ordered and periodic porous architectures optimized for photocatalytic activity. As illustrated in Figure 1a–f, the pore sizes sequentially decrease from 16.34 Å in CTF-BP to 15.89 Å in CTF-DBT and 14.51 Å in CTF-DBTS. This observation confirms that the integration of functional groups effectively modulates the pore dimensions, suggesting a compact and uniform incorporation of these moieties into the CTF backbone. Simulated X-ray diffraction (XRD) patterns (Figure 1g) reveal characteristic peaks in the 4–13° range, consistent with the ordered crystallinity of CTFs. Specifically, CTF-BP exhibits five distinct diffraction peaks at 4.62°, 5.89°, 7.48°, 11.79°, and 12.67°, corresponding to the (010), (001), (011), (002), and (012) crystallographic planes, respectively. Remarkably, CTF-DBT and CTF-DBTS demonstrate nearly identical peak positions and intensities, indicating negligible variation in lattice parameters upon functional group incorporation. Detailed lattice parameter analysis further validates this observation: CTF-DBT (a = 21.73 Å, b = 21.70 Å, c = 15.00 Å) and CTF-DBTS (a = 21.78 Å, b = 21.77 Å, c = 15.00 Å) retain lattice dimensions nearly identical to those of CTF-BP (a = 22.17 Å, b = 22.12 Å, c = 15.00 Å). These results underscore the structural resilience of the CTF framework, demonstrating its remarkable tolerance to functional group modifications without compromising long-range order. The preserved long-range order and minimal lattice distortion highlight the robustness and design flexibility of CTFs. Such structural consistency ensures that the introduction of functional groups does not disrupt the periodic microporous network, which is critical for maintaining efficient reactant diffusion and active site accessibility. Furthermore, the tunable pore size achieved through functional group engineering provides a strategic avenue to optimize substrate adsorption and mass transport, addressing a key limitation of conventional nonmetallic CTFs. This combination of structural stability and pore tunability shows CTFs as versatile platforms for tailoring photocatalytic performance while preserving framework integrity.
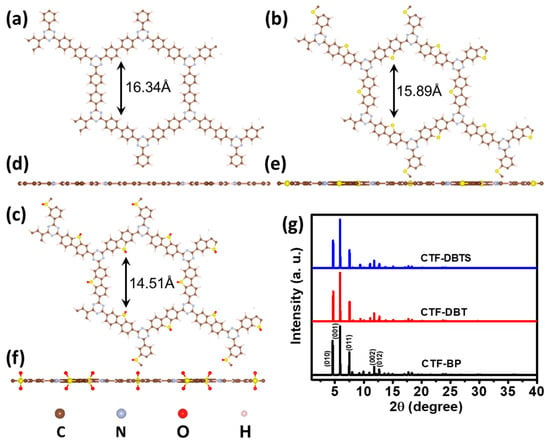
Figure 1.
Top and side displays of crystal structures for (a,d) CTF-BP, (b,e) CTF-DBT, and (c,f) CTF-DBTS. (g) Simulated XRD patterns.
To assess the photocatalytic water-splitting performance of the designed CTFs, the work functions (Wf) of the (001) crystal planes for CTF-BP, CTF-DBT, and CTF-DBTS were systematically calculated (Figure 2a–c). The Wf values were determined as 5.9, 5.6, and 6.6 eV for CTF-BP, CTF-DBT, and CTF-DBTS, respectively. A higher work function corresponds to a lower Fermi level, thereby raising the energy barrier for electrons to transition from the Fermi level to the vacuum level. This energetic barrier can enhance the redox activity of photogenerated charge carriers and improve photocatalytic efficiency. Notably, CTF-DBTS, with the highest work function among them, demonstrates superior redox capability of its photogenerated electrons and holes. To further elucidate the impact of functional group incorporation on charge distribution, dipole moment analyses were conducted (Figure 2d). Functionalization significantly enhanced the dipole moments of CTF-DBT and CTF-DBTS compared to CTF-BP. In CTF-DBT, the dipole moment components along the X and Y axes rose by approximately 6 and 4-fold, respectively, yielding a 10-fold enhancement of the total dipole moment. Similarly, CTF-DBTS exhibited 3 and 2-fold increases in X and Y-directional dipole moments, with an overall 5-fold amplification. These elevated dipole moments generate robust built-in electric fields, providing a driving force for directional charge migration and effectively suppressing electron-hole recombination. Consequently, CTF-DBT and CTF-DBTS achieve markedly improved charge separation efficiency relative to CTF-BP. The synergistic interplay between enhanced work function and amplified dipole moments in CTF-DBTS establishes an ideal electronic environment for PWS. The stronger built-in electric field not only facilitates rapid separation of photogenerated carriers but also aligns their migration pathways with redox reaction sites, thereby lowering activation barriers for both HER and OER. This dual optimization of electronic structure and charge dynamics underscores the critical role of functional group engineering in advancing CTF-based photocatalysts.
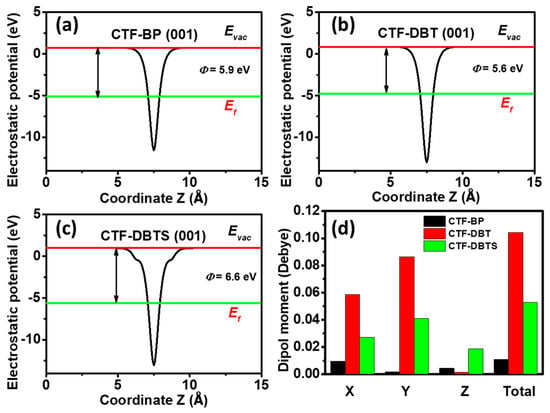
Figure 2.
Electrostatic potential profiles of (a) CTF-BP, (b) CTF-DBT, and (c) CTF-DBTS. (d) Dipole moments of CTF-BP, CTF-DBT, and CTF-DBTS along different axes.
The electrostatic potential profiles are essential to evaluate the work function and Fermi level of CTFs. As shown in Figure 3a, the infrared spectra of CTF-BP, CTF-DBT, and CTF-DBTS were theoretically simulated in the range of 500–4000 cm−1. The peaks at 1384 and 1538 cm−1 for CTF-BP, 1367 and 1549 cm−1 for CTF-DBT, and 1382 and 1550 cm−1 for CTF-DBTS are assigned to the characteristic triazine ring vibrations. The signals at 1603 cm−1 for CTF-BP, 1595 cm−1 for CTF-DBT, and 1606 cm−1 for CTF-DBTS originate from the C=C stretching modes of the biphenyl units. Additionally, CTF-DBT features a characteristic peak at 1475 cm−1, while CTF-DBTS shows a peak at 1476 cm−1, both corresponding to the C-S stretching vibrations of the DBT and DBTS functional groups. Notably, a prominent peak at 1338 cm−1 in CTF-DBTS is assigned to the S=O stretching vibration of the sulfone group in DBTS, unequivocally confirming the successful incorporation of the DBTS moiety into the framework. Raman spectral simulations (Figure 3b) further validated the structural integrity of the functional groups. The biphenyl rings in CTF-BP display five characteristic absorption peaks at 1280, 1379, 1442, 1558, and 1645 cm−1, corresponding to stretching modes of the biphenyl backbone. A C-H stretching vibration peak of CTF-BP is observed at 989 cm−1. Analogous features are observed in CTF-DBT and CTF-DBTS, albeit with slight redshifts attributable to electronic interactions induced by the functional group modifications.
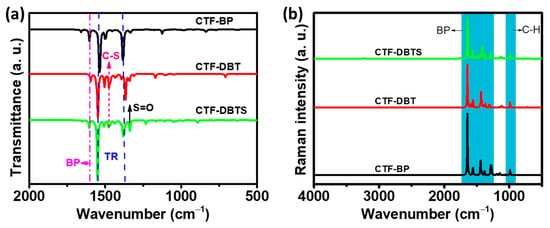
Figure 3.
Simulated (a) infrared (IR) spectra and (b) Raman spectra of CTF-BP, CTF-DBT, and CTF-DBTS.
To investigate the optical properties of the three CTF materials, electronic excitation spectra were theoretically modeled in the UV–vis range (200–800 nm), emphasizing the lowest-energy excited states. Vertical lines in Figure 4 denote the energy levels of excited states, color-coded to distinguish the three CTF variants. The results reveal that functional group modifications significantly enhance the excitation wavelengths of CTF-DBT and CTF-DBTS compared to CTF-BP in the 200–300 nm range, indicating improved utilization of light energy. For CTF-BP, the first excited state (S1) primarily originates from electron transitions between the HOMO and LUMO orbitals, corresponding to an excitation wavelength of 391 nm (energy: 3.17 eV). Introduction of the DBT functional group increases the S1 excitation wavelength to 384 nm (energy: 3.23 eV), while the DBTS modification also increases the S1 wavelength to 387 nm (energy: 3.20 eV). The minimal variations in excitation wavelengths and energies across the three CTFs suggest comparable light absorption ranges, highlighting that functional group engineering primarily fine-tunes electronic transitions rather than drastically altering the optical absorption profile.
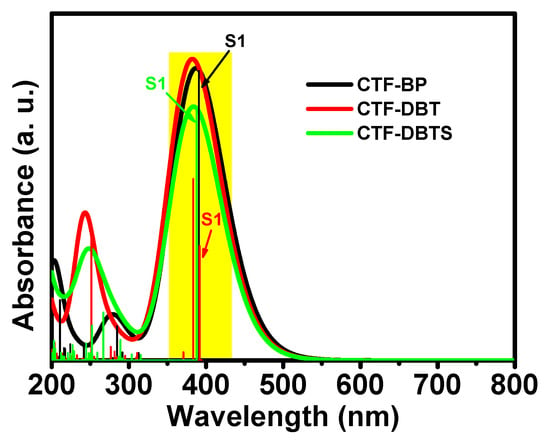
Figure 4.
Simulated UV–vis absorption spectra of CTF-BP, CTF-DBT, and CTF-DBTS.
To discern the atomic contributions to the valence band maximum (VBM) and conduction band minimum (CBM) in the three CTF materials, molecular orbital diagrams of the VBM and CBM were simulated (Figure 5a–f). For CTF-BP, the VBM originates predominantly from the N atoms in the triazine rings and C atoms in the BP units, whereas the CBM is principally characterized by the C and N atoms in the triazine rings. This indicates that BP serves as an electron donor and the triazine rings serve as electron acceptors in CTF-BP. Upon DBT functionalization, CTF-DBT exhibits a modified VBM involving N atoms in the triazine rings and C/S atoms in the DBT unit, with negligible changes to the CBM. This suggests that DBT replaces BP as the electron donor, while the triazine rings retain their acceptor role. In contrast, DBTS modification shifts the VBM of CTF-DBTS to include N atoms in the triazine rings and O atoms in the DBTS moiety, with minimal alterations to the CBM. These results demonstrate that DBTS functions as the electron donor in CTF-DBTS, complementing the triazine acceptor framework. The consistent acceptor role of triazine rings across all CTFs highlights their critical function in stabilizing charge transfer, while the modifiable donor characteristics of functional groups (BP, DBT, DBTS) enable precise modulation of electronic interactions for enhanced photocatalytic activity.
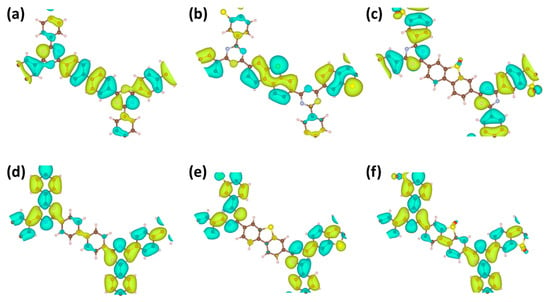
Figure 5.
Valence band maximum (VBM) orbitals for (a) CTF-BP, (b) CTF-DBT, and (c) CTF-DBTS; conduction band minimum (CBM) orbitals for (d) CTF-BP, (e) CTF-DBT, and (f) CTF-DBTS.
The calculated band gaps of CTF-BP, CTF-DBT, and CTF-DBTS are 2.43, 2.21, and 2.44 eV, respectively, as shown in Figure 6a–c. All three CTF materials exhibit band gaps within the ideal range of 1.8–3.0 eV for photocatalysts, with CTF-DBT showing a slight reduction in band gap compared to the others. Further density of states (DOS) analysis reveals distinct orbital contributions to the valence band maximum (VBM) and conduction band minimum (CBM) (Figure 6d–f). In CTF-BP, the VBM is predominantly composed of N 2p orbitals, while the CBM is dominated by C 2p orbitals. In CTF-DBT, the VBM shifts to S 3p orbitals, whereas the CBM remains consistent with CTF-BP. In CTF-DBTS, the VBM is primarily contributed by O 2p orbitals, with the CBM retaining its composition consistent with CTF-DBT. These findings align with the molecular orbital analysis, affirming that functional group modifications selectively tune the electronic structure of CTFs while preserving their fundamental photocatalytic characteristics.
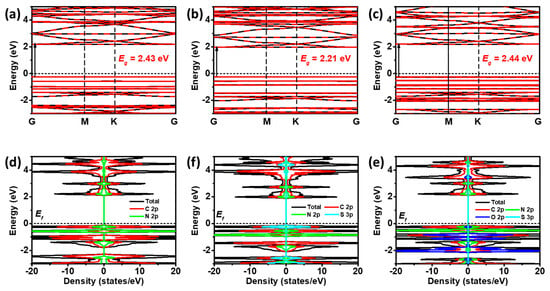
Figure 6.
Band structures of (a) CTF-BP, (b) CTF-DBT, and (c) CTF-DBTS; density of states (DOS) plots for (d) CTF-BP, (e) CTF-DBT, and (f) CTF-DBTS.
To identify the active sites in the three CTF materials, surface electrostatic potential distributions were analyzed (Figure 7a–f). Red regions denote areas of lower electrostatic potential (electron-rich zones), while blue regions correspond to higher electrostatic potential (electron-deficient zones). In CTF-BP, the N sites exhibit the lowest electrostatic potential, whereas C and H sites display higher potentials, revealing localized electron accumulation at the triazine N atoms. Under light irradiation, photoinduced electrons migrate from red (electron-rich) to blue (electron-deficient) regions, leaving holes localized primarily at the N sites. These N sites thus function as oxidation-active centers. For CTF-DBT, the introduction of S atoms minimally alters the overall electrostatic potential distribution compared to CTF-BP, maintaining N as the primary oxidation-active site. Conversely, CTF-DBTS exhibits a significantly reduced electrostatic potential at O sites within the sulfone groups, far exceeding the potential reduction observed at N sites. This distinct shift highlights O atoms as the dominant oxidation-active centers in CTF-DBTS, with a markedly enhanced oxidative capacity compared to CTF-BP. The expanded effective area of low-potential O sites in CTF-DBTS suggests stronger interactions with water molecules during photocatalytic splitting, thereby improving both oxidative driving force and overall decomposition efficiency. These findings align with the observed superior performance of CTF-DBTS in PWS.
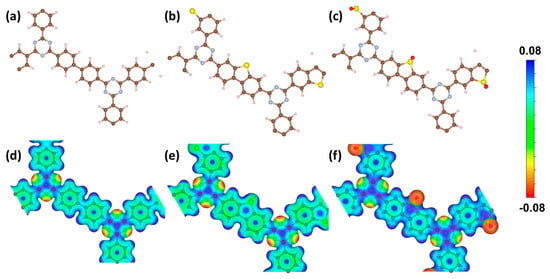
Figure 7.
(a–c) Top views of crystal structures and (d–f) surface electrostatic potential distributions for (a,d) CTF-BP, (b,e) CTF-DBT, and (c,f) CTF-DBTS.
Analysis of molecular configurations, charge distributions, and electron transfer dynamics elucidates distinct interactions between the CTFs and water molecules. As depicted in Figure 8a–c, the adsorption distances for CTF-BP, CTF-DBT, and CTF-DBTS are 2.35, 3.52, and 3.32 Å, respectively. These variations arise from differences in interfacial interactions between the CTFs and water. The calculated adsorption energies are −0.11, −0.02, and −0.11 eV for CTF-BP, CTF-DBT, and CTF-DBTS, respectively, indicating comparable adsorption strengths across the three materials. Charge transfer analysis (Figure 8d,e) reveals contrasting electron redistribution behaviors. For CTF-BP, 0.04 e is transferred from water molecules to the framework. In contrast, functionalization with DBT and DBTS induces a reversal of this trend: CTF-DBT transfers 0.078 e to water, while CTF-DBTS transfers 0.049 e (Figure 8f). This reversal highlights that DBT and DBTS-modified CTFs directly activate water molecules by facilitating electron injection into adsorbed H2O, thereby lowering the energy barrier for water dissociation. The enhanced electron transfer capacity of CTF-DBT and CTF-DBTS promotes redox reactions at the interface, enabling more efficient splitting of water into H2 and O2 compared to CTF-BP. These findings underscore the critical role of functional group-induced electronic modulation in optimizing PWS kinetics.
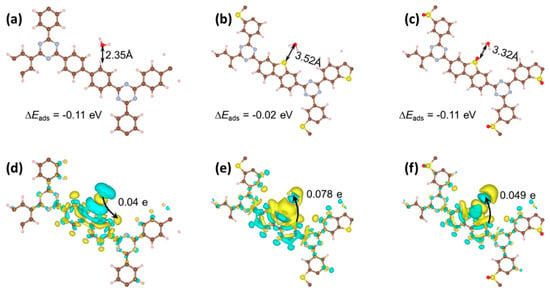
Figure 8.
Interaction configurations between H2O and (a) CTF-BP, (b) CTF-DBT, (c) CTF-DBTS; charge transfer diagrams for (d) CTF-BP, (e) CTF-DBT, and (f) CTF-DBTS.
The Gibbs free energy diagrams for HER and OER of CTF-BP, CTF-DBT, and CTF-DBTS were calculated to assess their potential in PWS (Figure 9). For HER (Figure 9a), the rate-determining step (RDS) for all three CTFs is hydrogen adsorption (* + H+ + e → *H), with energy barriers of 0.59 eV (CTF-BP), 0.64 eV (CTF-DBT), and 0.54 eV (CTF-DBTS), indicating suboptimal hydrogen adsorption capabilities. In contrast, the OER pathways display distinct RDS behaviors (Figure 9b). CTF-BP and CTF-DBTS share the same RDS in the water dehydrogenation step (* + H2O + h+ → *OH + H+), while CTF-DBT exhibits a shifted RDS to the desorption of the *OOH intermediate (*OOH + h+ → * + O2 + H+), attributed to its strong adsorption of oxygen species. The calculated OER energy barriers are 2.75 eV (CTF-BP), 3.60 eV (CTF-DBT), and 2.54 eV (CTF-DBTS). Despite its higher OER barrier, CTF-DBTS demonstrates the best PWS performance due to its superior charge separation and optimized HER kinetics, while CTF-DBT’s strong adsorption of intermediates leads to inferior activity. These results highlight the critical role of functional group modifications in tuning reaction pathways and balancing HER and OER kinetics for efficient PWS.
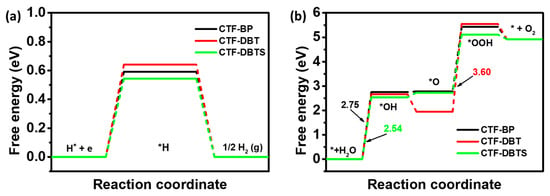
Figure 9.
Free energy diagrams for (a) HER and (b) OER of CTF-BP, CTF-DBT, and CTF-DBTS.
3. Computational Details
First-principles simulations were conducted to investigate electronic and thermodynamic properties within the framework of density functional theory (DFT). All calculations were carried out using the CP2K computational package (2024.2 release) with a hybrid Gaussian and plane-wave (GPW) scheme [24]. The Perdew–Burke–Ernzerhof (PBE) exchange–correlation functional was adopted to ensure consistency across geometric optimization and spectral analyses. Core electrons were treated via GTH pseudopotentials, while valence electrons were represented using the TZV2P-MOLOPT-GTH basis set. To ensure numerical precision, a 450 Ry energy cutoff was applied for plane-wave expansions, supplemented with stringent convergence thresholds (SCF: 1 × 10−6 Ha; atomic forces: 3 × 10−4 Ha/Bohr). Periodic boundary interactions were minimized by incorporating a 16 Å vacuum buffer between adjacent slabs. Non-covalent interactions, particularly van der Waals forces, were accounted for through the DFT-D3 dispersion correction with Grimme’s damping parameter [25]. Thermodynamic parameters were derived using the relation: ΔG = ΔEelec + ΔZPE − TΔS, where ΔEelec represents electronic energy differences, ΔZPE denotes zero-point energy corrections, and ΔS corresponds to entropy changes. Vibrational frequency analyses for ZPE and entropy contributions were computed via the Shermo toolkit (v2.6) [26], with gas-phase entropy values calibrated using standard thermodynamic tables. For excited-state characterization, Multiwfn (v3.8-dev) [27], was utilized to analyze frontier molecular orbitals and calculate UV–vis spectra.
4. Conclusions
In summary, this study systematically examined the structural, electronic, and photocatalytic properties of three donor–acceptor CTF-BP, CTF-DBT, and CTF-DBTS, respectively. The incorporation of DBTS significantly enhanced the PWS performance of CTF-DBTS compared to its counterparts. Structural simulation confirmed the preservation of ordered microporous frameworks across all CTFs, with minimal lattice distortion despite functional group modifications. The strong intrinsic dipole moments induced by the donor–acceptor architecture, particularly in CTF-DBTS, facilitated charge separation. Electronic structure analyses revealed that DBTS functionalization increased the work function and generated robust built-in electric fields, thereby enhancing redox capability. Moreover, CTF-DBTS exhibited a lower OER energy barrier of 2.54 eV and an optimized HER barrier of 0.54 eV, achieving synergistic HER/OER activity. In contrast, CTF-DBT suffered from unfavorable *O intermediate adsorption, while CTF-BP demonstrated moderate performance due to weaker dipole interactions. These findings underscore the critical role of sulfone groups in DBTS for tuning electronic properties, enhancing charge separation, and reducing PWS reaction barriers. This theoretical study provides a reference for developing visible-light-responsive CTFs with rational functional group design for PWS.
Author Contributions
Writing—original draft, data curation, methodology, investigation, formal analysis, conceptualization, visualization, L.C.; validation, writing—review and editing, L.C. and S.Y.; Writing—review & editing, specifically critical review, commentary or revision, improving the English language quality through meticulous revisions of grammar, punctuation, spelling, and overall stylistic coherence, X.W.; supervision, resources, software, project administration, funding acquisition, X.W. and Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) (No. 52402116) and the Key Natural Science Research Project for Colleges and Universities of Anhui Province (2022AH050396; 2024AH051664).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Qin, F.; Kang, Y.; San, X.; Tang, Y.L.; Li, J.; Zhang, X.; Zhang, K.; Liu, G. Spontaneous Exciton Dissociation in Sc-Doped Rutile TiO2 for Photocatalytic Overall Water Splitting with an Apparent Quantum Yield of 30. J. Am. Chem. Soc. 2025, 147, 12897–12907. [Google Scholar] [CrossRef]
- Bie, C.; Wang, L.; Yu, J. Challenges for photocatalytic overall water splitting. Chem 2022, 8, 1567–1574. [Google Scholar] [CrossRef]
- Wang, Q.; Nakabayashi, M.; Hisatomi, T.; Sun, S.; Akiyama, S.; Wang, Z.; Pan, Z.; Xiao, X.; Watanabe, T.; Yamada, T.; et al. Oxysulfide photocatalyst for visible-light-driven overall water splitting. Nat. Mater. 2019, 18, 827–832. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Takata, T.; Jiang, J.; Sakata, Y.; Nakabayashi, M.; Shibata, N.; Nandal, V.; Seki, K.; Hisatomi, T.; Domen, K. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 2020, 581, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Lv, H.; Liu, Q.; Wang, Z. Modulating NH2 Lewis Basicity in CTF-NH2 through Donor-Acceptor Groups for Optimizing Photocatalytic Water Splitting. Acta Phys. Chim. Sin. 2024, 40, 2405005. [Google Scholar] [CrossRef]
- Bi, Y.; Ouyang, S.; Umezawa, N.; Cao, J.; Ye, J. Facet effect of single-crystalline Ag3PO4 sub-microcrystals on photocatalytic properties. J. Am. Chem. Soc. 2011, 133, 6490–6492. [Google Scholar] [CrossRef]
- Li, R.; Zhang, F.; Wang, D.; Yang, J.; Li, M.; Zhu, J.; Zhou, X.; Han, H.; Li, C. Spatial separation of photogenerated electrons and holes among {010} and {110} crystal facets of BiVO4. Nat. Commun. 2013, 4, 1432. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Chen, C.; Gu, X.; Wu, H.; Meng, Q.; Zhang, J.; Chen, S.; Fu, X.; Liu, D.; Lei, W. Efficient photocatalytic H2 evolution, CO2 reduction and N2 fixation coupled with organic synthesis by cocatalyst and vacancies engineering. Appl. Catal. B Environ. 2021, 285, 119789. [Google Scholar] [CrossRef]
- Naranjo, T.; Collado, L.; Gomez-Mendoza, M.; Pizarro, A.H.; Barawi, M.; Gándara, F.; Liras, M.; de la Peña O’Shea, V.A. Solar-Driven Hydrogen Production Using a BODIPY Covalent Organic Framework Hybrid Photocatalyst. ACS Catal. 2023, 14, 283–291. [Google Scholar] [CrossRef]
- Gutiérrez, L.; Martin-Diaconescu, V.; Casadevall, C.; Oropeza, F.; de la Peña O’Shea, V.A.; Meng, J.; Ortuño, M.A.; Lloret-Fillol, J. Low Oxidation State Cobalt Center Stabilized by a Covalent Organic Framework to Promote Hydroboration of Olefins. ACS Catal. 2023, 13, 3044–3054. [Google Scholar] [CrossRef]
- Francis Kurisingal, J.; Kim, H.; Hyeak Choe, J.; Seop Hong, C. Covalent organic framework-based catalysts for efficient CO2 utilization reactions. Coord. Chem. Rev. 2022, 473, 214835. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Alzamly, A. Covalent Organic Frameworks as Emerging Platforms for CO2 Photoreduction. ACS Catal. 2021, 11, 9809–9824. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, N.; Seo, J.-M.; Jeon, J.-P.; Noh, H.-J.; Kweon, D.H.; Ryu, J.; Baek, J.-B. Benzothiazole-Based Covalent Organic Frameworks with Different Symmetrical Combinations for Photocatalytic CO2 Conversion. Chem. Mater. 2021, 33, 8705–8711. [Google Scholar] [CrossRef]
- Zhu, C.; Yao, Y.; Fang, Q.; Song, S.; Chen, B.; Shen, Y. Unveiling the Dynamic Evolution of Single-Atom Co Sites in Covalent Triazine Frameworks for Enhanced H2O2 Photosynthesis. ACS Catal. 2024, 14, 2847–2858. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, X.; Wang, Z. Highly crystalline carbon nitride by covalent remedy for CO2 photoreduction. Sep. Purif. Technol. 2024, 330, 125457. [Google Scholar] [CrossRef]
- Zhao, Z.; Zheng, D.; Guo, M.; Yu, J.; Zhang, S.; Zhang, Z.; Chen, Y. Engineering Olefin-Linked Covalent Organic Frameworks for Photoenzymatic Reduction of CO2. Angew. Chem. Int. 2022, 61, e202200261. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, L.; Chong, S.Y.; Little, M.A.; Wu, Y.; Zhu, W.H.; Clowes, R.; Yan, Y.; Zwijnenburg, M.A.; Sprick, R.S.; et al. Sulfone-containing covalent organic frameworks for photocatalytic hydrogen evolution from water. Nat. Chem. 2018, 10, 1180–1189. [Google Scholar] [CrossRef]
- Sachs, M.; Sprick, R.S.; Pearce, D.; Hillman, S.A.J.; Monti, A.; Guilbert, A.A.Y.; Brownbill, N.J.; Dimitrov, S.; Shi, X.; Blanc, F.; et al. Understanding structure-activity relationships in linear polymer photocatalysts for hydrogen evolution. Nat. Commun. 2018, 9, 4968. [Google Scholar] [CrossRef]
- Sprick, R.S.; Bai, Y.; Guilbert, A.A.Y.; Zbiri, M.; Aitchison, C.M.; Wilbraham, L.; Yan, Y.; Woods, D.J.; Zwijnenburg, M.A.; Cooper, A.I. Photocatalytic Hydrogen Evolution from Water Using Fluorene and Dibenzothiophene Sulfone-Conjugated Microporous and Linear Polymers. Chem. Mater. 2018, 31, 305–313. [Google Scholar] [CrossRef]
- Lan, Z.-A.; Ren, W.; Chen, X.; Zhang, Y.; Wang, X. Conjugated donor-acceptor polymer photocatalysts with electron-output “tentacles” for efficient hydrogen evolution. Appl. Catal. B Environ. 2019, 245, 596–603. [Google Scholar] [CrossRef]
- Shu, G.; Li, Y.; Wang, Z.; Jiang, J.-X.; Wang, F. Poly (dibenzothiophene-S,S-dioxide) with visible light-induced hydrogen evolution rate up to 44.2 mmol h−1 g−1 promoted by K2HPO4. Appl. Catal. B Environ. 2020, 261, 118230. [Google Scholar] [CrossRef]
- Singh, C.; Kim, J.Y.; Park, N.-J.; Yadav, R.K.; Baeg, J.-O. Highly effective solar CO2 fixation via photocatalytic carboxylation of aromatic amines with carbon dioxide over a covalent organic framework (COF) as a photocatalyst. Catal. Sci. Technol. 2024, 14, 6670–6677. [Google Scholar] [CrossRef]
- Chen, J.W.; Zhang, Z.; Yan, H.M.; Xia, G.J.; Cao, H.; Wang, Y.G. Pseudo-adsorption and long-range redox coupling during oxygen reduction reaction on single atom electrocatalyst. Nat. Commun. 2022, 13, 1734. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, Q. Shermo: A general code for calculating molecular thermochemistry properties. Comput. Theor. Chem. 2021, 1200, 113249. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).