Abstract
The development of heterogeneous nanocatalysts with high performance is essential for improving hydrogen production through formic acid dehydrogenation, but challenging. Herein, highly dispersed Pd nanoparticles (NPs) were successfully immobilized on porous nitrogen-doped carbon cages (PNCCs) derived from zeolitic imidazole frameworks. By virtue of the synergistic effect, the optimized Pd/PNCC nanocatalytic systems exhibit an excellent catalytic kinetics toward catalyzing FA dehydrogenation with a turnover frequency (TOF) value as high as 3174 h−1 at 323 K, which is 59 times relative to that of Pd nanoparticles. The exceptional activity may be ascribed to the PNCC solid support may induce a strong electronic metal–support interaction to optimize the electron configuration of Pd active sites and accelerate the kinetics of O-H bond cleavage, resulting in an enhanced catalytic performance toward FA dehydrogenation. This work will supply a novel strategy for the development of supported nanocatalysts with high performance for tremendous catalytic applications in the future.
1. Introduction
Replacing conventional fossil fuels with a carbon dioxide-neutral energy cycle is one of the most significant challenges in mitigating global climate change [1,2,3,4,5,6]. Hydrogen fuel, as a clean as well as sustainable energy carrier, has been deemed an ideal alternative to conventional fossil fuels [7,8,9,10,11,12,13,14]. However, the hydrogen-based energy cycle suffers from complex hydrogen storage and transportation, and the development of a convenient method associated with safe hydrogen transport and production technology is of importance for achieving a hydrogen economy [15,16]. Formic acid (FA, HCOOH), a product of biomass processing, has emerged as an ideal candidate for hydrogen storage because of its high hydrogen content, nontoxic nature, convenience, and renewability [17,18]. Under mild reaction conditions, FA can be promoted to release H2 via a dehydrogenation reaction (HCOOH → H2 + CO2) using suitable homogeneous and heterogeneous catalysts [19]. Up to now, although homogeneous catalysts, such as ruthenium (Ru) complexes, exhibit excellent catalytic performance, the complex separation process poses a significant challenge for large-scale industrial application, thus necessitating the development of heterogeneous catalysts with high performance for catalyzing FA dehydrogenation. Recent studies suggest that the noble metal palladium (Pd) nanocatalysts possess high catalytic kinetics of FA dehydrogenation [20,21]. However, Pd nanoparticles (NPs) are prone to agglomeration because of their thermodynamically unstable nature, resulting in a sluggish performance toward FA dehydrogenation.
Recently, solid-supported nanocatalysts have attracted considerable interest in inhibiting the agglomeration of metal NPs. Additionally, by virtue of the strong metal–support interaction (EMSI), the reactive kinetics can be efficiently accelerated, leading to improved catalytic activity [22,23,24,25,26,27,28,29,30]. Up to now, a variety of solid support materials, including metal oxide, porous carbon, and mesoporous silica, have been developed to immobilize the active metal NPs [31,32]. Among them, metal–organic frameworks (MOFs) and their derivates materials have attracted tremendous attention because of their unique physicochemical properties [33,34,35,36,37,38,39,40,41]. Very recently, zeolitic imidazolate frameworks (ZIFs) derived nitrogen (N) doped porous carbon have garnered considerable attention due to inheriting uniform heteroatom N decoration and porous structure of pristine MOFs, which could afford a large surface area as well as coordinated sites to anchor precursor metallic ions, achieving a well dispersion of metal NPs [13,14,42,43,44]. Deng et al. reported N-doped carbon-supported Pd nanocatalysts for FA dehydrogenation [44]. In this work, Pd is deposited on a series of N-doped carbons, which are prepared by co-carbonization of N-containing zeolite imidazole frameworks (ZIF-8) and secondary N sources (melamine, xylitol, urea, and glucose), for hydrogen generation from FA. When melamine is used as a modifier, the as-prepared Pd/CNZM catalyst displays the best catalytic performance with complete conversion of FA and reaches the theoretical gas production of 446 mL in 60 min at 50 °C. Especially, ZIF-67@ZIF-8 materials have been recognized as promising precursors. After the pyrolysis process, the gas produced from core ZIF-8 at high temperatures (Zn species will evaporate beyond 907 °C) accelerates metal ions, allowing them to move outside and generate a hollow polyhedron, while ZIF-67 could be transferred into carbon layer, yielding a core–shell structured N-doped porous carbon framework with well-developed inner channels as well as the effective carbon protection layer [45,46,47]. As a result, those containing N species can be used as affinitive proton buffers to accelerate the cleavage of O-H bonds in FA. More importantly, multi-dimensional hybrid architectures benefit the interface charge as well as mass transport, thereby leading to enhanced catalytic performance [48,49]. However, up to now, few studies have focused on porous N-doped carbon framework-supported nanocatalytic systems. Thus, it is imperative to construct a novel and cost-effective porous N-doped carbon framework-supported nanocatalytic system with high performance for hydrogen evolution from FA dehydrogenation under mild conditions.
Herein, we construct a novel hierarchical structure consisting of Pd nanoparticles supported on a porous N-doped carbon cage (PNCC) with a core–shell structure, obtained from a ZIF-8@ZIF-67 polyhedron via a facile epitaxial growth–pyrolysis strategy. A KCl template was melt-evaporated into the ZIF-8@ZIF-67 precursor and then pyrolyzed to obtain a porous N-doped carbon framework. During the carbonization process, KCl templates not only inhibit nitrogen loss and accelerate graphitization but could also be used as an “intercalator” to generate a mesoporous structure. The exposed hierarchical polyhedral will form an efficient conducting porous carbon framework, which not only supplies abundant areas to anchor nanoparticles but also maximizes the active area of the active center in contact with the FA molecule. As a result, the as-synthesized Pd/PNCC nanocatalytic system exhibits an excellent catalytic performance toward the dehydrogenation of FA, and the corresponding turnover frequency (TOF) value can achieve as high as 3174 h−1 at mild conditions, which is comparable to those of other reported heterogeneous nanocatalysts. This outstanding catalytic performance can be primarily attributed to the PNCC support providing a suitable coordinated microenvironment and efficiently inducing the synergistic interaction to reconfigure the electronic redistribution of Pd active sites, thereby achieving accelerated catalytic kinetics.
2. Results and Discussion
The synthesis procedure of Pd/PNCC is illustrated in Figure 1. Initially, a core–shell structured imidazole molecular sieve skeleton, ZIF-8@ZIF-67, was synthesized via the seed epitaxial growth method. Subsequently, the as-synthesized ZIF-8@ZIF-67 samples were dispersed in KCl solution and evaporated at 90 °C for KCl recrystallization, and encapsulation into ZIF-8@ZIF-67 precursor. Next, the composites can be transformed into porous nitrogen-doped carbon cages (PNCCs) through a controlled pyrolysis. Then, an aqueous solution of K2PdCl4 is mixed with PNCC suspension under continuous sonication. Subsequently, NaBH4 is added to the mixture as a reducing agent, and the resultant solution is continuously stirred for 90 min. Ultimately, Pd nanoparticles were successfully immobilized on the PNCC support and then washed repeatedly using deionized water and dried under vacuum (10−3 Torr) at 60 °C.
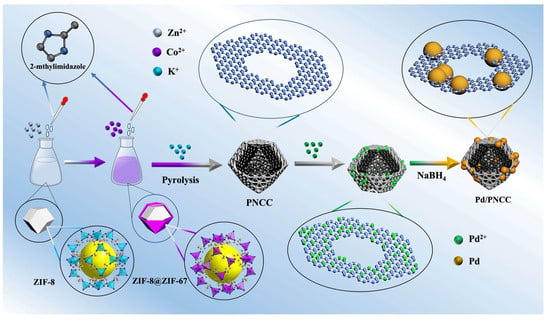
Figure 1.
Schematic illustration for the preparation of Pd/PNCC.
The morphologies as well as microstructures of the obtained samples were first explored using XRD, SEM, and TEM. As seen in Figure S1, the X-ray diffraction peaks at diffraction peaks located at 7.26, 10.37, 12.69, 14.75, 16.53, 18.13, 22.14, 24.29, and 26.82° can be observed, corresponding to the (011), (002), (112), (022), (013), (222), (114), (233), and (134) planes, respectively. No new diffraction peaks were observed relative to those of parent ZIFs, which suggests that core–shell ZIFs can inherit the intrinsic framework structure from parent ZIFs. After carbonization at 750 °C for 2 h, all diffraction peaks associated with the parent ZIFs completely disappeared. At the same time, a series of broad and small peaks appear in the range of 2θ = 10°–60°, originating from carbon species. Compared with PNCC, a new small diffraction peak is observed at 40.2° for Pd/PNCC, suggesting the formation of Pd NPs on the PNCC support. The as-made ZIF-8@ZIF-67 particles demonstrate a clear-edged rhombic dodecahedron morphology with a smooth surface combined with an average diameter of about 1 μm (Figure 2a). Upon calcination, all diffraction peaks of parent ZIFs disappear, and a broad characteristic peak at around 26.43° arises from graphitic carbon (PDF no. 23–0064), as shown in Figure S1.
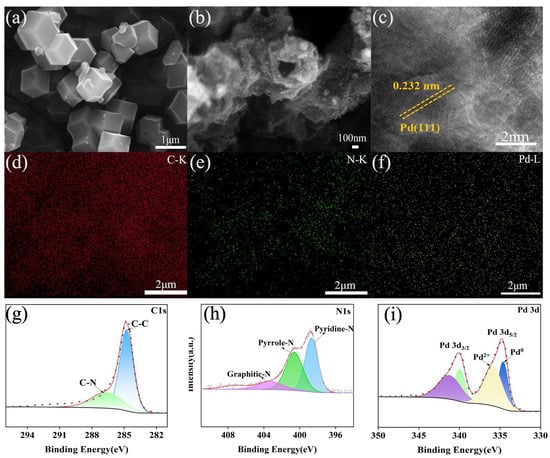
Figure 2.
(a) SEM, (b) TEM, (c) HETEM, and (d–f) mapping images of samples; (g–i) XPS spectrum of C 1s, N 1s, and Pd 3d.
The obtained PNCC inherits the morphological feature of ZIF-8@ZIF-67 precursor in Figure 2b, and a certain shrinkage (average size of approximately 600 nm) associated with a rough surface is observed for PNCC, which is attributed to precursor carbonization and the acid etching of residual metal species. After immobilization of Pd NPs, the as-synthesized Pd/PNCC still inherits the rhombic dodecahedron (Figure S2). As depicted in Figures S3–S6, the Brunauer–Emmett–Teller (BET) surface area of PNCC is measured to be 972.6 m2g−1. The pore size distribution (PSD) suggests PNCC possesses a uniform nanoporous structure (ca. 0.7 to 2.0 nm), which endows an optimized environment to anchor ultrafine metallic NPs. After immobilization of Pd NPs, the corresponding BET surface area of Pd/PNCC will decrease the 673.5 m2 g−1. Appreciable decreases in BET may be due to Pd NPs were highly dispersed in the framework of the PNCC support and occupied and/or blocked the cavities of the PNCC. Figure 2c, Figures S7 and S8 further display transmission electron microscopy (TEM) images of the as-synthesized Pd/PNCC sample, where the rough surface is distinctly observable because of the relatively uniform-sized metal nanoparticles with a size of 2.5 nm anchored on the PNCC surface. As displayed in the high-resolution transmission electron microscopy (HRTEM) image, a lattice spacing of about 0.232 nm could be clearly seen, consisting with the (110) crystal face of Pd (Figure 2c). Moreover, energy dispersive X-ray spectroscopy (EDS) elemental mapping analysis confirmed the uniform distribution of Pd, C, and N throughout the whole sample, validating the successful construction of Pd NPs on the PNCC surface (Figure 2d–f). The corresponding result proves the presence of Pd/PNCC with a mass ratio of 1:9.5, which is in good agreement with inductively coupled plasma–atomic emission spectrometry (ICP-AES) results and the original stoichiometric composition in the experimental procedure.
To perform an in-depth evaluation of the surface chemical composition as well as valence states of the as-synthesized Pd/PNCC, X-ray photoelectron spectroscopy (XPS) was further conducted. The Pd 3d, C 1s, and N 1s spectra of Pd/PNCC were analyzed in detail. As shown in Figure 2g, the high-resolution C 1s spectrum could be deconvolved into two peaks, including C-C (284.79 eV) and C-N (286.62 eV), respectively. For the N 1s spectrum in Figure 2h, three characteristic peaks at 397.2, 398.88, and 403.96 eV correspond to pyrrole-N, pyridine-N, and graphitic-N, respectively. The molar ratio of N species is estimated to be 8.9 (pyridine-N): 4.2 (pyrrole-N): 1 (graphitic-N). As displayed in the high-resolution core-level spectrum of Pd, the peaks at 334.61 and 339.95 eV correspond to metallic Pd 3d5/2 and Pd 3d3/2, respectively, while the peaks at 335.9 and 334.5 eV are recognized as Pd2+ peaks due to the surface oxidation of Pd under air exposure (Figure 2i). Intriguingly, compared with Pd NPs [50], Pd signals in Pd/PNCC displayed a negative shift, indicating increased electron transfer from the PNCC support to the Pd catalytic site due to the strong electron-donating nature of PNCC.
To investigate the catalytic kinetics, the time-dependent dehydrogenation of formic acid under mild conditions was explored. As shown in Figure 3a,b, for the parent ZIF-8@ZIF-67-supported Pd nanocatalyst, poor activity was observed. After carbonization, the obtained PNCC-supported nanocatalytic system displays an impressive catalytic performance toward catalyzing FA dehydrogenation, and the corresponding TOF with 100% conversion could reach as high as 3174 h−1, 46-fold higher than that of Pd/ZIF-8@ZIF-67 (69 h−1). Because the PNCC support itself represents no dehydrogenation kinetics of FA, the excellent catalytic activity observed is independent of the individual PNCC support but rather closely related to the strong synergistic effect induced between PNCC and metal Pd NPs. The effect of solid support on catalyzing over Pd-based nanosystems was further explored.
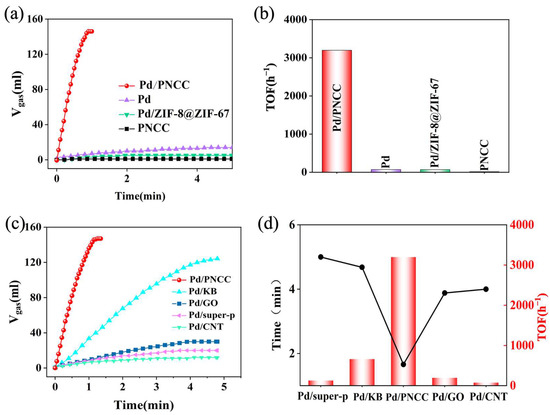
Figure 3.
(a) Catalytic performance and (b) corresponding TOF values of Pd/PNCC, Pd/ZIF-8@ZIF-67, Pd, and PNCC catalysts; (c,d) catalytic performances and TOF values of Pd anchored on various solid supports.
As displayed in Figure 3c,d, if Pd NPs were directly immobilized on carbon nanotubes (CNTs), super P, and graphene oxide (GO), the obtained supported Pd nanocatalytic systems exhibited sluggish catalytic performance. For the Pd/KB nanocatalytic system, enhanced active performance toward catalyzing FA dehydrogenation was observed. Intriguingly, the as-synthesized PNCC-supported Pd nanocatalytic system demonstrates superior active kinetics toward selectively catalyzing FA dehydrogenation, and 146 mL (H2 + CO2) can be quickly generated in only 1.15 min at 323 K. The corresponding TOF value with 100% conversion could reach as high as 3174 h−1, which is 4.80, 16.50, 25.19, and 44.08 times higher compared with that of Pd/KB (660 h−1), Pd/GO (192 h−1), Pd/super P (126 h−1), and Pd/CNT (72 h−1), respectively. As shown in Figures S7–S11, Pd NPs immobilized on different supports exhibit similar particle sizes, confirming a fair comparison of catalytic dehydrogenation of FA on different catalytic systems, excluding the effect of Pd particle size on determining catalytic property. Such impressive catalytic kinetics could be comparable with most state-of-the-art nanocatalysts reported recently [51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79], as shown in Table S1. The exceptional activity may be ascribed that PNCC solid support may induce a strong electronic metal–support interaction to adjust multiple electron transfer kinetics of Pd NPs and PNCC support. Thus, a novel electronic configuration of metallic active sites will be feasibly reconstructed and efficiently facilitate the catalytic kinetics of FA dehydrogenation [48,80].
In order to further explore the effect of hierarchical structure, we compare the catalytic performances of Pd/PNCC, Pd/PNCC-67, and Pd/PNCC-8 under the same measurement conditions. Both Pd/PNCC-67 and Pd/PNCC-8 displayed inferior active performance compared with Pd/PNCC toward the selective catalysis of FA dehydrogenation (Figure 4a,b). Such enhanced reaction kinetics is mainly attributed to the multi-dimensional hierarchical structure, which not only supplied accessible active sites but also favourably accelerates mass transfer ability and enables the FA molecule to rapidly diffuse into the catalytic sites of the Pd surface, accelerating the efficiency of FA dehydrogenation. Additionally, the addition of KCl also plays an important role in determining the catalytic activities of Pd/PNCC nanocatalytic systems.
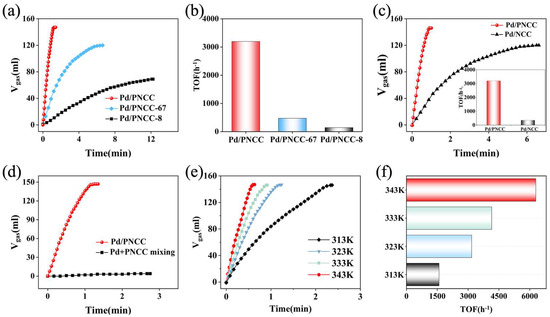
Figure 4.
(a) Catalytic performances and (b) corresponding TOF values of Pd/PNCC, Pd/PNCC-67, and Pd/PNCC-8 catalysts; (c) catalytic performances and TOF values of Pd anchored on PNCC and NCC; (d) comparison of catalytic performances of Pd/PNCC and Pd and PNCC mixture for FA dehydrogenation; (e,f) catalytic performances and TOF values of Pd/PNCC at different temperatures.
As shown in Figure 4c and Figure S12, with the initial amount of KCl increasing to 2.0 g, the Pd/PNCC nanocatalytic systems demonstrate the best performance for FA dehydrogenation with the TOF of 3174 h−1 at 323 K, 9.06 times relative to that of Pd/NCC nanocatalytic systems (350 h−1), and a further increase in KCl amount will result in a negative effect on FA dehydrogenation. KCl served as the confining template, not only preventing nitrogen overflow and promoting graphitization but also efficiently intercalating into the carbon framework to form a hierarchical porous structure. Figures S13–S15 further compare the catalytic properties of Pd NPs immobilized on PNCC calcined under different heat treatment conditions. Obviously, the kinetic rate as well as conversion over Pd-based nanocatalytic systems are efficiently accelerated by optimal PNCC support.
Under suitable calcination conditions, the PNCC-supported Pd-based nanocatalytic system demonstrates superior catalytic performance toward catalyzing FA dehydrogenation for hydrogen evolution. In addition, a comprehensive comparison was performed to determine the effect of FA molarity. As demonstrated in Figure S16, under the same reaction conditions, the optimized FA/SF molar ratio is 1:2.5. However, a further SF molarity increase will result in a slight decay, suggesting that excess SF molecules will poison the active sites and lead to a decrease in the catalysis of FA dehydrogenation. To gain insight into the synergistic effect arising from PNCC support, we further compare the catalytic activities of Pd/PNCC and the physical mixture of Pd and PNCC with the same Pd amount at 323 K. As depicted in Figure 4d and Figure S17, for a free Pd and PNCC mixture, only 4 mL of gas (H2 + CO2) is released in 2.1 min with a low TOF of 45.45 h−1. Significantly, the in situ synthesized Pd/PNCC nanocatalytic system exhibits a striking catalytic kinetics and the corresponding TOF with 100% conversion could reach up as high as 3174 h−1, 69.8-fold higher than that of a physical mixture of Pd and PNCC (45.45 h−1) at the same measured conditions. Figure 4e,f and Figure S18 further reveal the temperature-dependent kinetics activities of FA dehydrogenation compared with the Pd/PNCC nanocatalytic system. The kinetics of H2 generation exhibited a strong correlation with reaction temperature. High TOFs of 1587, 3174, 4148, and 6295 h−1 were obtained at 313, 323, 333, and 343 K, respectively, and compared in Figure 4f. Gas chromatography (GC) was further carried out to probe high-purity H2 production, and no CO was detected at the level of detection limit (Figures S19 and S20). These results emphasize the critical role of PNCC solid support, which not only benefited the in situ formation of Pd-based nanocatalysts but also induced a strong synergistic effect to accelerate the catalytic kinetics of FA dehydrogenation.
Based on the above analyses, a plausible reactive mechanism of FA dehydrogenation over Pd/PNCC is proposed. PNCC can induce a strong synergistic effect to accelerate electron transfer affinity, and more electrons were accumulated on the Pd surface, resulting in electron-rich Pd catalytic sites, as confirmed by the XPS results (Figure 2i). Such a unique electronic configuration could absorb abundant FA molecules and facilitate the O-H bond antisymmetric dissociation in reactive molecules, along with intermediate −N [H]+ groups as well as formate species bridged on the Pd surface. Subsequently, the C-H bond in the Pd-formate complex will further cleave into Pd-hydride species, accompanied by the release of CO2, which is usually regarded as the RDS in the FA dehydrogenation process. Finally, Pd-hydride species react with −N [H]+ groups to generate H2 molecules and release from the nanocatalyst surface [81,82,83].
3. Experimental Section
3.1. Synthesis of ZIF-8@ZIF-67
Typically, two solutions were prepared from 8.925 g (30 mmol) of Zn(NO3)3·6H2O in 300 mL of methanol and 9.853 g (120 mmol) of 2-methylimidazole (2-MelM) in 100 mL of methanol, respectively. Then, the two solutions were mixture and aged for 24 h. The white solid, ZIF-8, was obtained by centrifugation, washed with MeOH several times, and dried at 60 °C overnight.
As-prepared ZIF-8 (1.0 g) was transferred into 300 mL of methanol to obtain a well-mixed solution by long-term ultrasonication. Then, 900 mg (3.09 mmol) of cobalt nitrate hexahydrate (Co(NO3)2·6H2O) was subsequently poured quickly into the above solution of ZIF-8 under continuous stirring. Afterward, 100 mL of a methanol solution containing 3.28 g (40 mmol) of 2-methylimidazole (2-MelM) was then added with a rotation speed of 550 rpm. After stirring for 24 h, the light purple precipitate, ZIF-8@ZIF-67, was obtained and washed with fresh methanol, and finally dried at 60 °C under vacuum.
3.2. Synthesis of Porous N-Doped Carbon Cage (PNCC)
Porous N-doped carbon cages (PNCCs) were prepared according to the following steps: first, ZIF-8@ZIF-67 (1 g) was mixed with KCl (2 g) by adequately grinding. Subsequently, the mixture was pyrolyzed in a tube furnace under an N2 flow, which was heated from 20 °C to 750 °C at a rate of 2 °C/min and held at 750 °C for 2 h. Later, the resulting mixture was added to the aqueous solution of HF to remove metal ions. Finally, PNCC was obtained by filtration, washed with 100 mL of H2O, and dried at 100 °C for 12 h.
3.3. Characterization
Energy dispersive spectroscopy (EDS) and scanning electron microscopy (SEM) were performed, and elemental mapping images were obtained using an FEI Nova Nano SEM 450 (Thermo Fisher Scientific, Waltham, MA, USA). The powder X-ray diffraction image was obtained by a Rigaku D/MAX/2500PC powder X-ray diffractometer, using a Cu-K source (40 kV, 40 mA) (Rigaku, Tokyo, Japan). X-ray photoelectron spectroscopy (XPS) measurements were performed by the ESCALAB 250Xi spectrophotometer (Thermo Fisher Scientific, USA). A Tecnai G2 F30 S-Twin instrument with a field emission gun operating at 200 kV was used to obtain transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) images (Thermo Fisher Scientific, USA).
3.4. Catalytic Performance Testing
In general, a mixture of as-synthesized catalyst and aqueous solution was placed in a two-necked round-bottom flask (50 mL), which was placed in a water bath at a preset temperature (313–343 K). The reaction was initiated by injecting the aqueous FA solution into the mixture using a syringe. A gas burette filled with water was connected to the reaction flask to measure the volume of released gas (temperature kept constant at 298 K during measurements). The reaction started when aqueous FA solution was injected into the mixture using a syringe. The volume of the evolved gas was monitored by recording the displacement of water in the gas burette. The reaction was complete when there was no more gas generation.
The turnover frequency (TOF) in this work is the overall TOF value based on the number of Pd atoms in the catalyst, which is calculated from the following equation:
where P0 is the atmospheric pressure (101,325 Pa), V is the volume of generated hydrogen, R is the universal gas constant (8.3145 m3 Pa mol−1 K−1), T is the reaction temperature (K), nPd is the total mole number of Pd in the catalyst, and t is the overall reaction time.
TOF = P0V/(2RTnPdt)
4. Conclusions
In summary, we successfully immobilized Pd NPs on a porous N-doped carbon framework derived from ZIF-8@ZIF-67 via a controlled pyrolysis, where KCl was used as an etching agent. The as-synthesized Pd NPs were well-dispersed on PNCC because of the synergistic effect between the support and metal precursor. As a result, the obtained Pd/PNCC catalyst exhibits superior catalytic efficiency compared with other supported Pd nanocatalysts, achieving a TOF of 3174 h−1 at 50 °C. N-doped carbon frameworks could induce a strong synergistic effect to adjust the electron configuration of Pd active sites. The electron-rich Pd can promote O-H activation in the FA molecule, achieving improved catalytic kinetics of FA dehydrogenation. This work supplies a novel paradigm for the efficient hydrogen evolution from FA dehydrogenation by developing a highly active heterogeneous Pd-based nanocatalytic system.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15090852/s1, Figure S1: XRD patterns of ZIF-8@ZIF-67, PNCC and Pd/PNCC; Figure S2: SEM image of Pd/PNCC; Figure S3: N2 sorption isotherms of the as-synthesized PNCC; Figure S4: The pore size distributions of PNCC; Figure S5: N2 sorption isotherms of the as-synthesized Pd/PNCC; Figure S6: The pore size distributions of Pd/PNCC; Figure S7: TEM image of Pd/PNCC; Figure S8: Particle size distribution of Pd/PNCC; Figure S9: TEM image of Pd/KB; Figure S10: TEM image of Pd/GO; Figure S11: TEM image of Pd/super P; Figure S12: Comparison of catalytic properties for Pd/PNCC with different amounts of KCl; Figure S13: XRD patterns of PNCC calcined different temperatures; Figure S14: Comparison of catalytic properties of Pd/PNCC calcined at different temperatures; Figure S15: Comparison of catalytic properties of Pd/PNCC calcined at different times; Figure S16: Comparison of catalytic properties of Pd/PNCC calcined at different FA/SF ratios; Figure S17: Comparison of catalytic properties of the Pd/PNCC and Pd+PNCC mixtures; Figure S18: The corresponding Arrhenius curve; Figures S19 and S20: Gas chromatogram result of the released gas; Table S1: Comparison of catalytic performance for FA dehydrogenation.
Author Contributions
X.D.: Investigation and formal analysis. L.X.: Visualization, validation, and conceptualization. S.T.: Investigation and formal analysis. H.Q.: Methodology and formal analysis. J.W.: Writing—original draft, methodology, formal analysis, data curation, and conceptualization. F.S.: Writing—review and editing, writing—original draft, resources, project administration, and conceptualization. M.L.: Writing—review and editing, and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data supporting the findings of this study are available within the paper and its Supplementary Information.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef]
- Boddien, A.; Mellmann, D.; Gärtner, F.; Jackstell, R.; Junge, H.; Dyson, P.J.; Laurenczy, G.; Ludwig, R.; Beller, M. Efficient dehydrogenation of formic acid using an iron catalyst. Science 2011, 333, 1733–1736. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Strmcnik, D.; Lopes, P.P.; Markovic, N.M. Energy and fuels from electrochemical interfaces. Nat. Mater. 2017, 16, 57–69. [Google Scholar] [CrossRef]
- Pan, X.; Qiu, J.; Tang, S.; Lv, Q.; Dong, J.; Jiang, N.; Liu, L.; Wan, Y.; Yang, X.; Han, J.; et al. Engineering cobalt coordination environment with dual heteroatom doping for boosting urea-assisted hydrogen evolution. Fuel 2025, 395, 135161. [Google Scholar] [CrossRef]
- Pan, S.; Zabed, H.M.; Wei, Y.; Qi, X. Technoeconomic and environmental perspectives of biofuel production from sugarcane bagasse: Current status, challenges and future outlook. Ind. Crops Prod. 2022, 188, 115684. [Google Scholar] [CrossRef]
- Holechek, J.L.; Geli, H.M.E.; Sawalhah, M.N.; Valdez, R. A global assessment: Can renewable energy replace fossil fuels by 2050? Sustainability 2022, 14, 4792. [Google Scholar] [CrossRef]
- Turner, J.A. Sustainable hydrogen production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef]
- Wang, L.; Tang, G.; Liu, S.; Dong, H.; Liu, Q.; Sun, J.; Tang, H. Interfacial active-site-rich 0D Co3O4/1D TiO2 p-n heterojunction for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 428, 131338. [Google Scholar] [CrossRef]
- Li, W.; Tian, H.; Ma, L.; Wang, Y.; Liu, X.; Gao, X. Low-temperature water electrolysis: Fundamentals, progress, and new strategies. Mater. Adv. 2022, 3, 5598–5644. [Google Scholar] [CrossRef]
- Liang, J.; Li, H.; Chen, L.; Ren, M.; Fakayode, O.A.; Han, J.; Zhou, C. Efficient hydrogen evolution reaction performance using lignin-assisted chestnut shell carbon-loaded molybdenum disulfide. Ind. Crops Prod. 2023, 193, 116214. [Google Scholar] [CrossRef]
- Prasad, C.; Tang, H.; Liu, Q.; Bahadur, I.; Karlapudi, S.; Jiang, Y. A latest overview on photocatalytic application of g-C3N4 based nanostructured materials for hydrogen production. Int. J. Hydrogen Energy 2020, 45, 337–379. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, Y.; Qian, L.; Yin, Y.; Yuan, Z.; Dai, Y.; Zhang, T.; Yang, D.; Qiu, F. Lamellar Ti3C2 MXene composite decorated with platinum-doped MoS2 nanosheets as electrochemical sensing functional platform for highly sensitive analysis of organophosphorus pesticides. Food Chem. 2024, 459, 140379. [Google Scholar] [CrossRef]
- Jiang, E.; Song, N.; Hong, S.; She, C.; Li, C.; Fang, L.; Dong, H. Zn, S, N self-doped carbon material derived from waste tires for electrocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 16544–16551. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, S.; Xu, L.; Wang, J.; Li, A.; Jing, M.; Yang, X.; Song, F. Encapsulation of ruthenium oxide nanoparticles in nitrogen-doped porous carbon polyhedral for pH-universal hydrogen evolution electrocatalysis. Int. J. Hydrogen Energy 2024, 74, 10–16. [Google Scholar] [CrossRef]
- Yao, Q.; Ding, Y.; Lu, Z.-H. Noble-metal-free nanocatalysts for hydrogen generation from boron- and nitrogen-based hydrides. Inorg. Chem. Front. 2020, 7, 3837–3874. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, Z.; Lv, Q.; Pan, X.; Dong, J.; Liu, L.; Wan, Y.; Han, J.; Song, F. Heteroatom engineering in earth-abundant cobalt electrocatalyst for energy-saving hydrogen evolution coupling with urea oxidation. ACS Appl. Mater. Interfaces 2024, 16, 66008–66017. [Google Scholar] [CrossRef]
- Yao, Q.; Zhang, X.; Lu, Z.-H.; Xu, Q. Metal-organic framework-based catalysts for hydrogen production from liquid-phase chemical hydrides. Coord. Chem. Rev. 2023, 493, 215302. [Google Scholar] [CrossRef]
- Han, J.; Zhang, Z.; Hao, Z.; Li, G.; Liu, T. Immobilization of palladium silver nanoparticles on NH2-functional metal-organic framework for fast dehydrogenation of formic acid. J. Colloid Interface Sci. 2021, 587, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Bulushev, D.A.; Yang, J.; Zhang, Q. New liquid chemical hydrogen storage technology. Energies 2022, 15, 6330. [Google Scholar] [CrossRef]
- Ding, Y.; Peng, W.; Zhang, L.; Xia, J.; Feng, G.; Lu, Z.-H. Chromic hydroxide-decorated palladium nanoparticles confined by amine-functionalized mesoporous silica for rapid dehydrogenation of formic acid. J. Colloid Interface Sci. 2023, 630, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.-L.; Xu, Q. Liquid organic and inorganic chemical hydrides for high-capacity hydrogen storage. Energy Environ. Sci. 2015, 8, 478–512. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, Q.; Wu, H.; Zhou, Y.; Zhu, M.; Lu, Z.-H. Carbon-doped mesoporous TiO2-immobilized Ni nanoparticles: Oxygen defect engineering enhances hydrogen production. Appl. Catal. B 2023, 339, 123153. [Google Scholar] [CrossRef]
- Wan, C.; Li, G.; Wang, J.; Xu, L.; Cheng, D.G.; Chen, F.; Asakura, Y.; Kang, Y.; Yamauchi, Y. Modulating electronic metal-support interactions to boost visible-light-driven hydrolysis of ammonia borane: Nickel-platinum nanoparticles supported on phosphorus-doped titania. Angew. Chem. Int. Ed. Engl. 2023, 62, e202305371. [Google Scholar] [CrossRef]
- Qin, H.; Tang, S.; Xu, L.; Li, A.; Lv, Q.; Dong, J.; Liu, L.; Ding, X.; Pan, X.; Yang, X.; et al. Alkaline titanium carbide (MXene) engineering ultrafine non-noble nanocatalysts toward remarkably boosting hydrogen evolution from ammonia borane hydrolysis. J. Alloys Compd. 2025, 1010, 177644. [Google Scholar] [CrossRef]
- Wu, H.; Huang, L.; Timoshenko, J.; Qi, K.; Wang, W.; Liu, J.; Zhang, Y.; Yang, S.; Petit, E.; Flaud, V.; et al. Selective and energy-efficient electrosynthesis of ethylene from CO2 by tuning the valence of Cu catalysts through aryl diazonium functionalization. Nat. Energy 2024, 9, 422–433. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Chen, L.; Yarley, O.P.N.; Zhou, C. Facile preparation of sugarcane bagasse-derived carbon supported MoS2 nanosheets for hydrogen evolution reaction. Ind. Crops Prod. 2021, 172, 114064. [Google Scholar] [CrossRef]
- Zhong, M.; Xu, M.; Ren, S.; Li, W.; Wang, C.; Gao, M.; Lu, X. Modulating the electronic structure of Ni(OH)2 by coupling with low-content Pt for boosting the urea oxidation reaction enables significantly promoted energy-saving hydrogen production. Energy Environ. Sci. 2024, 17, 1984–1996. [Google Scholar] [CrossRef]
- Yu, W.; Huang, H.; Qin, Y.; Zhang, D.; Zhang, Y.; Liu, K.; Zhang, Y.; Lai, J.; Wang, L. The synergistic effect of pyrrolic-n and pyridinic-n with pt under strong metal-support interaction to achieve high-performance alkaline hydrogen evolution. Adv. Energy Mater. 2022, 12, 2200110. [Google Scholar] [CrossRef]
- Xu, F.; Wang, Y.; Wang, C.; Huang, W.; Liu, X. Dehydrogenation of hydrous hydrazine over carbon nanosphere- supported PtNi nanoparticles for on-demand H2 release. Fuel 2023, 332, 126116. [Google Scholar] [CrossRef]
- Wen, J.; Tang, S.; Ding, X.; Yin, Y.; Song, F.; Yang, X. In situ raman study of layered double hydroxide catalysts for water oxidation to hydrogen evolution: Recent progress and future perspectives. Energies 2024, 17, 5712. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, Z.; Xu, L.; Qin, H.; Dong, J.; Lv, Q.; Han, J.; Song, F. Ultrafine nickel-rhodium nanoparticles anchored on two-dimensional vanadium carbide for high performance hydrous hydrazine decomposition at mild conditions. J. Colloid Interface Sci. 2024, 669, 228–235. [Google Scholar] [CrossRef]
- Bai, S.; Jia, A.; Song, J.; Cao, S.; Wang, N.; Liu, X. Metal-support interactions in heterogeneous catalytic hydrogen production of formic acid. Chem. Eng. J. 2023, 474, 145612. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Song, F.-Z.; Zhu, Q.-L.; Yang, X.; Zhan, W.-W.; Pachfule, P.; Tsumori, N.; Xu, Q. Metal–organic framework templated porous carbon-metal oxide/reduced graphene oxide as superior support of bimetallic nanoparticles for efficient hydrogen generation from formic acid. Adv. Energy Mater. 2018, 8, 1701416. [Google Scholar] [CrossRef]
- Yao, Y.; Wu, X.; Gutiérrez, O.Y.; Ji, J.; Jin, P.; Wang, S.; Xu, Y.; Zhao, Y.; Wang, S.; Ma, X.; et al. Corrigendum to “Roles of Cu+ and Cu0 sites in liquid-phase hydrogenation of esters on core-shell CuZnx@C catalysts” [Appl. Catal. B: Environ. 267 (2020) 118698]. Appl. Catal. B-Environ. 2020, 276, 119233. [Google Scholar] [CrossRef]
- Shi, B.; Zhang, X.; Li, W.; Liang, N.; Hu, X.; Xiao, J.; Wang, D.; Zou, X.; Shi, J. An intrinsic dual-emitting fluorescence sensing toward tetracycline with self-calibration model based on luminescent lanthanide-functionalized metal-organic frameworks. Food Chem. 2023, 400, 133995. [Google Scholar] [CrossRef]
- Thorarinsdottir, A.E.; Harris, T.D. Metal–organic framework magnets. Chem. Rev. 2020, 120, 8716–8789. [Google Scholar] [CrossRef]
- Tang, S.; Xu, L.; Ding, X.; Lv, Q.; Qin, H.; Li, A.; Yang, X.; Han, J.; Song, F. Electronic engineering induced ultrafine non-noble nanoparticles for high-performance hydrogen evolution from ammonia borane hydrolysis. Fuel 2025, 381, 133424. [Google Scholar] [CrossRef]
- Shah, S.S.A.; Jery, A.E.; Najam, T.; Nazir, M.A.; Wei, L.; Hussain, E.; Hussain, S.; Rebah, F.B.; Javed, M.S. Surface engineering of MOF-derived FeCo/NC core-shell nanostructures to enhance alkaline water-splitting. Int. J. Hydrogen Energy 2022, 47, 5036–5043. [Google Scholar] [CrossRef]
- Li, W.; Hu, X.; Li, Q.; Shi, Y.; Zhai, X.; Xu, Y.; Li, Z.; Huang, X.; Wang, X.; Shi, J.; et al. Copper nanoclusters @ nitrogen-doped carbon quantum dots-based ratiometric fluorescence probe for lead (II) ions detection in porphyra. Food Chem. 2020, 320, 126623. [Google Scholar] [CrossRef]
- Wu, S.; Shi, W.; Cui, L.; Xu, C. Enhancing contaminant rejection efficiency with ZIF-8 molecular sieving in sustainable mixed matrix membranes. Chem. Eng. J. 2024, 482, 148954. [Google Scholar] [CrossRef]
- Mu, L.; Luo, J.; Wang, C.; Liu, J.; Zou, Y.; Li, X.; Huang, Y.; Wu, P.; Ji, H.; Zhu, W. BN/ZIF-8 derived carbon hybrid materials for adsorptive desulfurization: Insights into adsorptive property and reaction kinetics. Fuel 2021, 288, 119685. [Google Scholar] [CrossRef]
- Gao, S.; Yang, H.; Rao, D.; Wang, N.; Li, Y.; Li, J.; Rao, S.; Maouche, C.; Wu, Z.; Li, B.; et al. Supercritical CO2 assisted synthesis of highly accessible iron single atoms and clusters on nitrogen-doped carbon as efficient oxygen reduction electrocatalysts. Chem. Eng. J. 2022, 433, 134460. [Google Scholar] [CrossRef]
- Deng, M.; Yang, A.; Ma, J.; Yang, C.; Cao, T.; Yang, S.; Yao, M.; Liu, F.; Wang, X.; Cao, J. Enhanced Catalytic Performance of N-Doped Carbon SphereSupported Pd Nanoparticles by Secondary Nitrogen Source Regulation for Formic Acid Dehydrogenation. ACS Appl. Mater Interfaces 2022, 14, 18550. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, F.; Lu, H.; Hong, X.; Jiang, H.; Wu, Y.; Li, Y. Hollow Zn/Co zif particles derived from core–shell ZIF-67@ZIF-8 as selective catalyst for the semi-hydrogenation of acetylene. Angew. Chem. 2015, 127, 11039–11043. [Google Scholar] [CrossRef]
- Hassan, M.; Wang, M.; Hussain, S.; Zhang, X.; Lei, S.; Jin, M.; Qiao, G.; Liu, G. Synchronized integration of iron/cobalt dual-metal in nitrogen-doped carbon hollow spheres for enriched supercapacitive and oxygen reduction reaction performances. J. Energy Storage 2022, 56, 105895. [Google Scholar] [CrossRef]
- Yi, Y.; Zhao, W.; Zeng, Z.; Wei, C.; Lu, C.; Shao, Y.; Guo, W.; Dou, S.; Sun, J. ZIF-8@ZIF-67-derived nitrogen-doped porous carbon confined cop polyhedron targeting superior potassium-ion storage. Small 2020, 16, 1906566. [Google Scholar] [CrossRef]
- Zhang, A.; Xia, J.; Yao, Q.; Lu, Z.-H. Pd–WOx heterostructures immobilized by MOFs-derived carbon cage for formic acid dehydrogenation. Appl. Catal. B-Environ. 2022, 309, 121278. [Google Scholar] [CrossRef]
- Saliba, D.; Ammar, M.; Rammal, M.; Al-Ghoul, M.; Hmadeh, M. Crystal Growth of ZIF-8, ZIF-67, and Their Mixed-Metal Derivatives. J. Am. Chem. Soc. 2018, 140, 1812–1823. [Google Scholar] [CrossRef]
- Kim, E.H.; Lee, M.H.; Kim, J.; Ra, E.C.; Lee, J.H.; Lee, J.S. Synergy between single atoms and nanoclusters of Pd/g-C3N4 catalysts for efficient base-free CO2 hydrogenation to formic acid. Chin. J. Catal. 2023, 47, 214–221. [Google Scholar] [CrossRef]
- Xu, L.; Jin, B.; Zhang, J.; Cheng, D.-g.; Chen, F.; An, Y.; Cui, P.; Wan, C. Efficient hydrogen generation from formic acid using AgPd nanoparticles immobilized on carbon nitride-functionalized SBA-15. RSC Advances 2016, 6, 46908–46914. [Google Scholar] [CrossRef]
- Xu, L.; Yao, F.; Luo, J.; Wan, C.; Ye, M.; Cui, P.; An, Y. Facile synthesis of amine-functionalized SBA-15-supported bimetallic Au–Pd nanoparticles as an efficient catalyst for hydrogen generation from formic acid. RSC Adv. 2017, 7, 4746–4752. [Google Scholar] [CrossRef]
- Bi, Q.-Y.; Du, X.-L.; Liu, Y.-M.; Cao, Y.; He, H.-Y.; Fan, K.-N. Efficient subnanometric gold-catalyzed hydrogen generation via formic acid decomposition under ambient conditions. J. Am. Chem. Soc. 2012, 134, 8926–8933. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, D.H. Hydrogen production from formic acid dehydrogenation over a Pd supported on N-doped mesoporous carbon catalyst: A role of nitrogen dopant. Appl. Catal. A. Gen. 2020, 608, 117887. [Google Scholar] [CrossRef]
- Yin, B.; Zhao, E.; Hua, X.; Wang, K.; Wang, W.; Li, G.; Liu, T. Ultrafine PdAg nanoparticles immobilized on nitrogen-doped carbon/cerium oxide for superior dehydrogenation of formic acid. New J. Chem. 2020, 44, 2011–2015. [Google Scholar] [CrossRef]
- Zhang, C.; Leng, Y.; Jiang, P.; Li, J.; Du, S. Immobilizing palladium nanoparticles on nitrogen-doped carbon for promotion of formic acid dehydrogenation and alkene hydrogenation. ChemistrySelect 2017, 2, 5469–5474. [Google Scholar] [CrossRef]
- Deng, Q.-F.; Xin, J.-J.; Ma, S.-K.; Cui, F.-J.; Zhao, Z.-L.; Jia, L.-H. Hydrogen production from the decomposition of formic acid over carbon nitride-supported agpd alloy nanoparticles. Energy Techno. 2018, 6, 2374–2379. [Google Scholar] [CrossRef]
- Tang, C.; Surkus, A.-E.; Chen, F.; Pohl, M.-M.; Agostini, G.; Schneider, M.; Junge, H.; Beller, M. A stable nanocobalt catalyst with highly dispersed con active sites for the selective dehydrogenation of formic acid. Angew. Chem. Int. Ed. Engl. 2017, 56, 16616–16620. [Google Scholar] [CrossRef]
- Wang, Q.; Tsumori, N.; Kitta, M.; Xu, Q. Fast dehydrogenation of formic acid over palladium nanoparticles immobilized in nitrogen-doped hierarchically porous carbon. ACS Catal. 2018, 8, 12041–12045. [Google Scholar] [CrossRef]
- Bi, Q.-Y.; Lin, J.-D.; Liu, Y.-M.; He, H.-Y.; Huang, F.-Q.; Cao, Y. Dehydrogenation of formic acid at room temperature: Boosting palladium nanoparticle efficiency by coupling with pyridinic-nitrogen-doped carbon. Angew. Chem. Int. Ed. Engl. 2016, 55, 11849–11853. [Google Scholar] [CrossRef]
- Wang, X.; Meng, Q.; Gao, L.; Liu, J.; Ge, J.; Liu, C.; Xing, W. Metal organic framework derived nitrogen-doped carbon anchored palladium nanoparticles for ambient temperature formic acid decomposition. Int. J. Hydrogen Energy 2019, 44, 28402–28408. [Google Scholar] [CrossRef]
- Zhu, Q.-L.; Tsumori, N.; Xu, Q. Sodium hydroxide-assisted growth of uniform Pd nanoparticles on nanoporous carbon MSC-30 for efficient and complete dehydrogenation of formic acid under ambient conditions. Chem. Sci. 2014, 5, 195–199. [Google Scholar] [CrossRef]
- Bulut, A.; Yurderi, M.; Karatas, Y.; Say, Z.; Kivrak, H.; Kaya, M.; Gulcan, M.; Ozensoy, E.; Zahmakiran, M. MnOx-promoted pdag alloy nanoparticles for the additive-free dehydrogenation of formic acid at room temperature. ACS Catal. 2015, 5, 6099–6110. [Google Scholar] [CrossRef]
- Yan, J.-M.; Wang, Z.-L.; Gu, L.; Li, S.-J.; Wang, H.-L.; Zheng, W.-T.; Jiang, Q. AuPd–MnOx/MOF–graphene: An efficient catalyst for hydrogen production from formic acid at room temperature. Adv. Energy Mater. 2015, 5, 1500107. [Google Scholar] [CrossRef]
- Lee, J.H.; Ryu, J.; Kim, J.Y.; Nam, S.-W.; Han, J.H.; Lim, T.-H.; Gautam, S.; Chae, K.H.; Yoon, C.W. Carbon dioxide mediated, reversible chemical hydrogen storage using a Pd nanocatalyst supported on mesoporous graphitic carbon nitride. J. Mater. Chem. A 2014, 2, 9490–9495. [Google Scholar] [CrossRef]
- Feng, C.; Hao, Y.; Zhang, L.; Shang, N.; Gao, S.; Wang, Z.; Wang, C. AgPd nanoparticles supported on zeolitic imidazolate framework derived N-doped porous carbon as an efficient catalyst for formic acid dehydrogenation. RSC Adv. 2015, 5, 39878–39883. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Kobayashi, T.; Suenobu, T. unusually large tunneling effect on highly efficient generation of hydrogen and hydrogen isotopes in ph-selective decomposition of formic acid catalyzed by a heterodinuclear iridium−ruthenium complex in water. J. Am. Chem. Soc. 2010, 132, 1496–1497. [Google Scholar] [CrossRef]
- Gu, X.; Lu, Z.-H.; Jiang, H.-L.; Akita, T.; Xu, Q. Synergistic catalysis of metal–organic framework-immobilized Au–Pd nanoparticles in dehydrogenation of formic acid for chemical hydrogen storage. J. Am. Chem. Soc. 2011, 133, 11822–11825. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hua, X.; Su, J.; Luo, W.; Chen, S.; Cheng, G. Highly efficient hydrogen generation from formic acid-sodium formate over monodisperse AgPd nanoparticles at room temperature. Appl. Catal. B-Environ. 2015, 168-169, 423–428. [Google Scholar] [CrossRef]
- Dai, H.; Xia, B.; Wen, L.; Du, C.; Su, J.; Luo, W.; Cheng, G. Synergistic catalysis of AgPd@ZIF-8 on dehydrogenation of formic acid. Appl. Catal. B-Environ. 2015, 165, 57–62. [Google Scholar] [CrossRef]
- Cheng, J.; Gu, X.; Liu, P.; Wang, T.; Su, H. Controlling catalytic dehydrogenation of formic acid over low-cost transition metal-substituted AuPd nanoparticles immobilized by functionalized metal–organic frameworks at room temperature. J. Mater. Chem. A 2016, 4, 16645–16652. [Google Scholar] [CrossRef]
- Tedsree, K.; Li, T.; Jones, S.; Chan, C.W.A.; Yu, K.M.K.; Bagot, P.A.J.; Marquis, E.A.; Smith, G.D.W.; Tsang, S.C.E. Hydrogen production from formic acid decomposition at room temperature using a Ag–Pd core–shell nanocatalyst. Nat. Nanotechnol. 2011, 6, 302–307. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, L.; Tang, S.-Y.; Lai, D.-M.; Liao, B.; Fu, Y.; Guo, Q.-X. Selective decomposition of formic acid over immobilized catalysts. Energy Fuels 2011, 25, 3693–3697. [Google Scholar] [CrossRef]
- Jiang, K.; Xu, K.; Zou, S.; Cai, W.-B. B-doped Pd catalyst: Boosting room-temperature hydrogen production from formic acid–formate solutions. J. Am. Chem. Soc. 2014, 136, 4861–4864. [Google Scholar] [CrossRef]
- Zhang, S.; Metin, Ö.; Su, D.; Sun, S. Monodisperse AgPd alloy nanoparticles and their superior catalysis for the dehydrogenation of formic acid. Angew. Chem. Int. Ed. Engl. 2013, 52, 3681–3684. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Q.-L.; Tsumori, N.; Xu, Q. Immobilizing highly catalytically active noble metal nanoparticles on reduced graphene oxide: A non-noble metal sacrificial approach. J. Am. Chem. Soc. 2015, 137, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, L.; Wu, H.; Zu, H.; Cui, P.; Guo, J.; Guo, R.; Ye, J.; Zhu, J.; Zheng, X.; et al. Anchoring Pt single atoms on te nanowires for plasmon-enhanced dehydrogenation of formic acid at room temperature. Adv. Sci. 2019, 6, 1900006. [Google Scholar] [CrossRef]
- Duan, J.; Xiang, Z.; Zhang, H.; Zhang, B.; Xiang, X. Pd-Co2P nanoparticles supported on N-doped biomass-based carbon microsheet with excellent catalytic performance for hydrogen evolution from formic acid. Appl. Surf. Sci. 2020, 530, 147191. [Google Scholar] [CrossRef]
- Himeda, Y. Highly efficient hydrogen evolution by decomposition of formic acid using an iridium catalyst with 4,4′-dihydroxy-2,2′-bipyridine. Green Chem. 2009, 11, 2018–2022. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Q.; Jiang, H.-L. Metal–organic frameworks meet metal nanoparticles: Synergistic effect for enhanced catalysis. Chem. Soc. Rev. 2017, 46, 4774–4808. [Google Scholar] [CrossRef]
- Zhang, A.; Yang, F.; Luo, Y.; Shen, X.; Yao, Q.; Wang, K.; Xia, J.; Lu, Z.-H. Microenvironmental regulation of PdCo nanoclusters in mesoporous carbon enhances hydrogen production from formic acid. Appl. Catal. B-Environ. 2025, 373, 125360. [Google Scholar] [CrossRef]
- Zhang, Z.; He, D.; Wang, Z.; Wu, S.; Liu, T. Bimetallic palladium chromium nanoparticles anchored on amine-functionalized titanium carbides for remarkably catalytic dehydrogenation of formic acid at mild conditions. J. Catal. 2022, 410, 121–127. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, G.; Yao, Q.; Li, H.; Feng, G.; Lu, Z.-H. Amine-functionalized carbon bowl-supported Pd-La(OH)3 for formic acid dehydrogenation. Inorg. Chem. 2022, 61, 18102–18111. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).