Anti-Interference Fe-N-C/PMS System: Synergistic Radical-Nonradical Pathways Enabled by sp2 Carbon and Metal-N Coordination
Abstract
1. Introduction
2. Results and Discussion
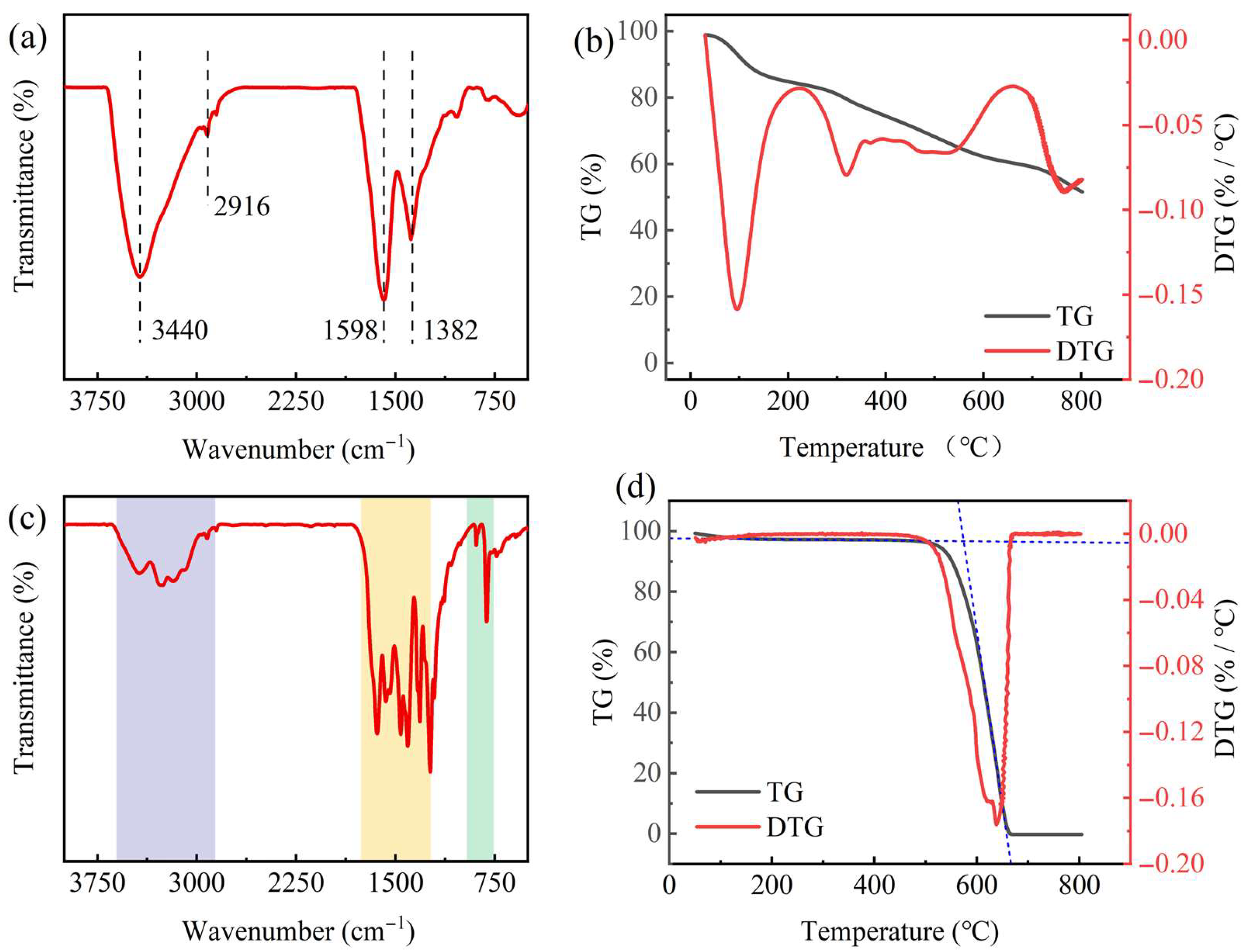
2.1. Analysis of Precursor Properties
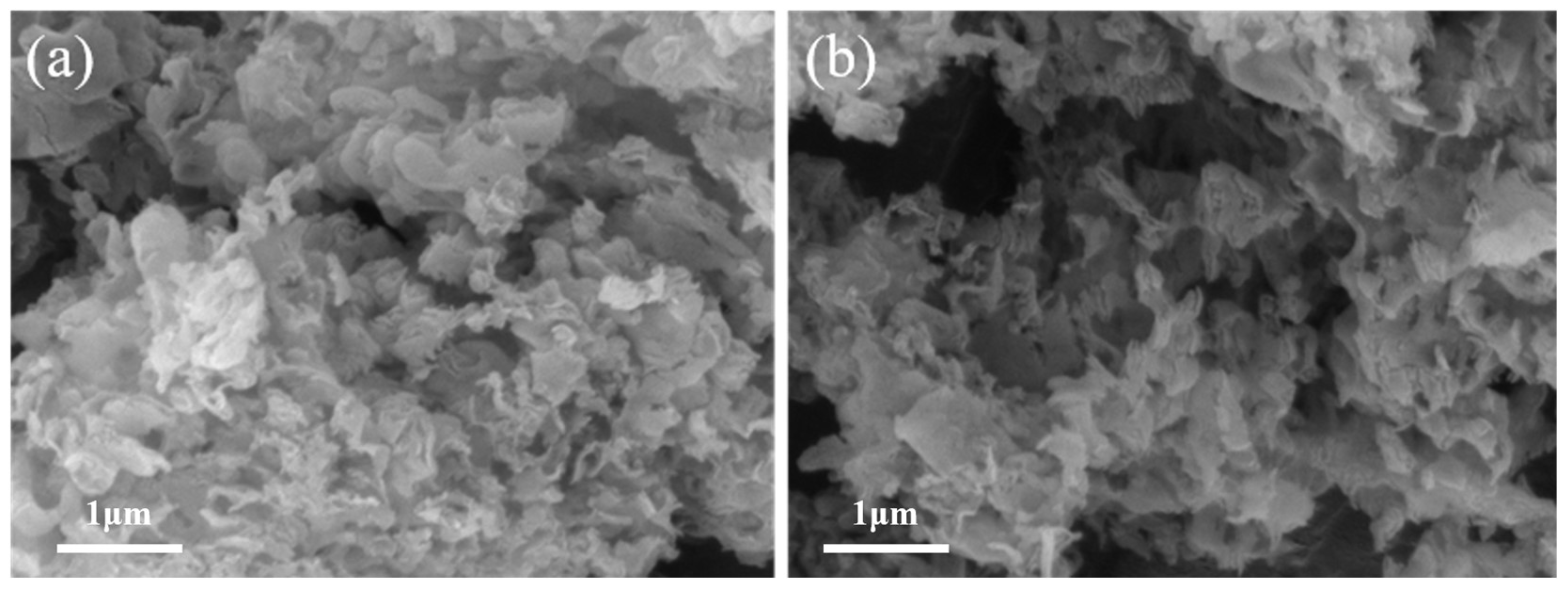
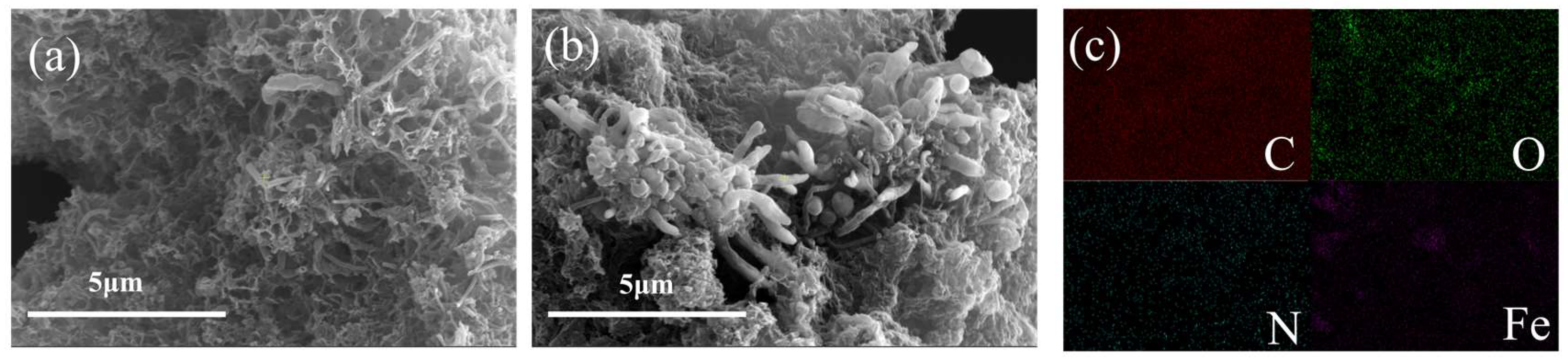
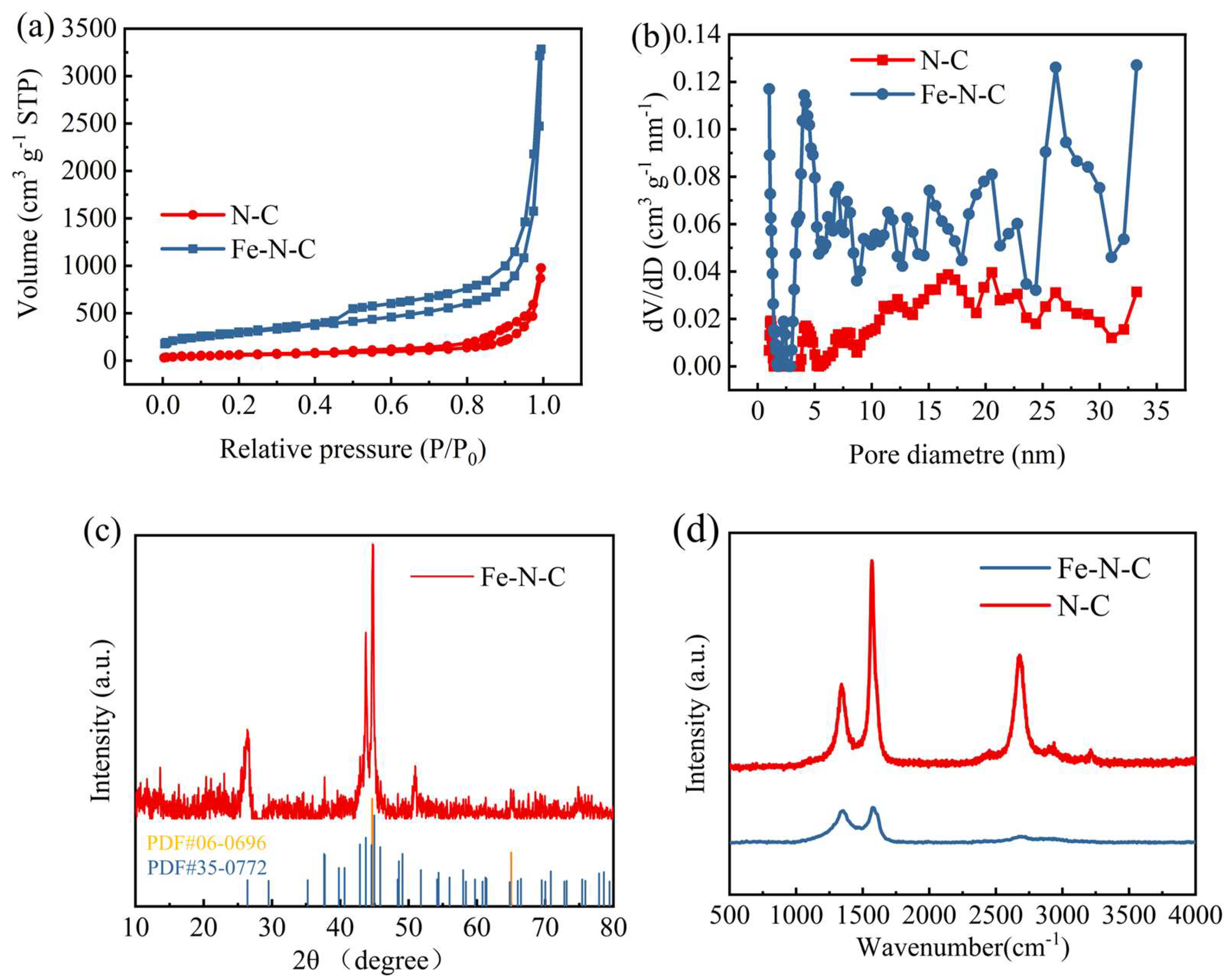
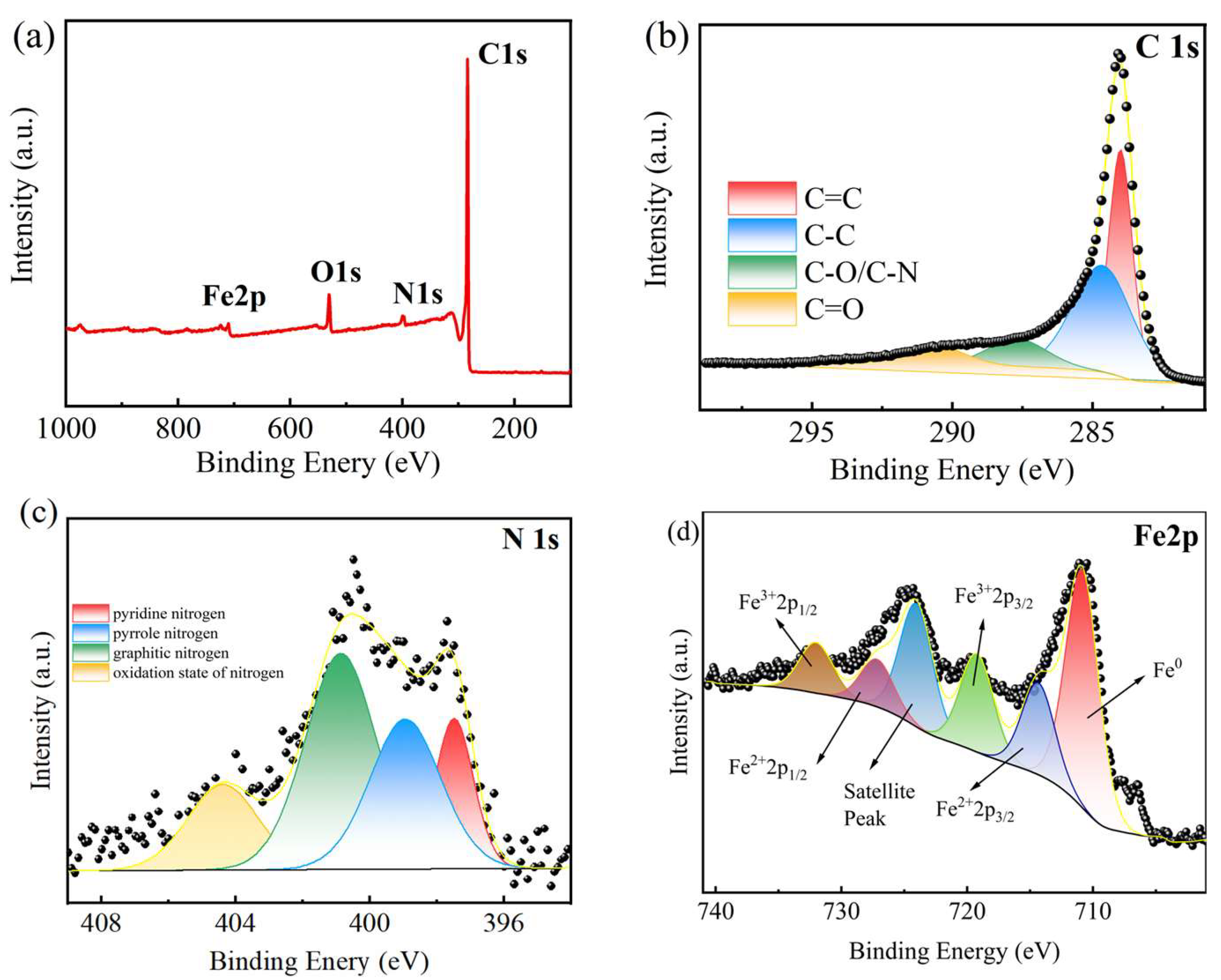
2.2. Characterization of Fe-N-C
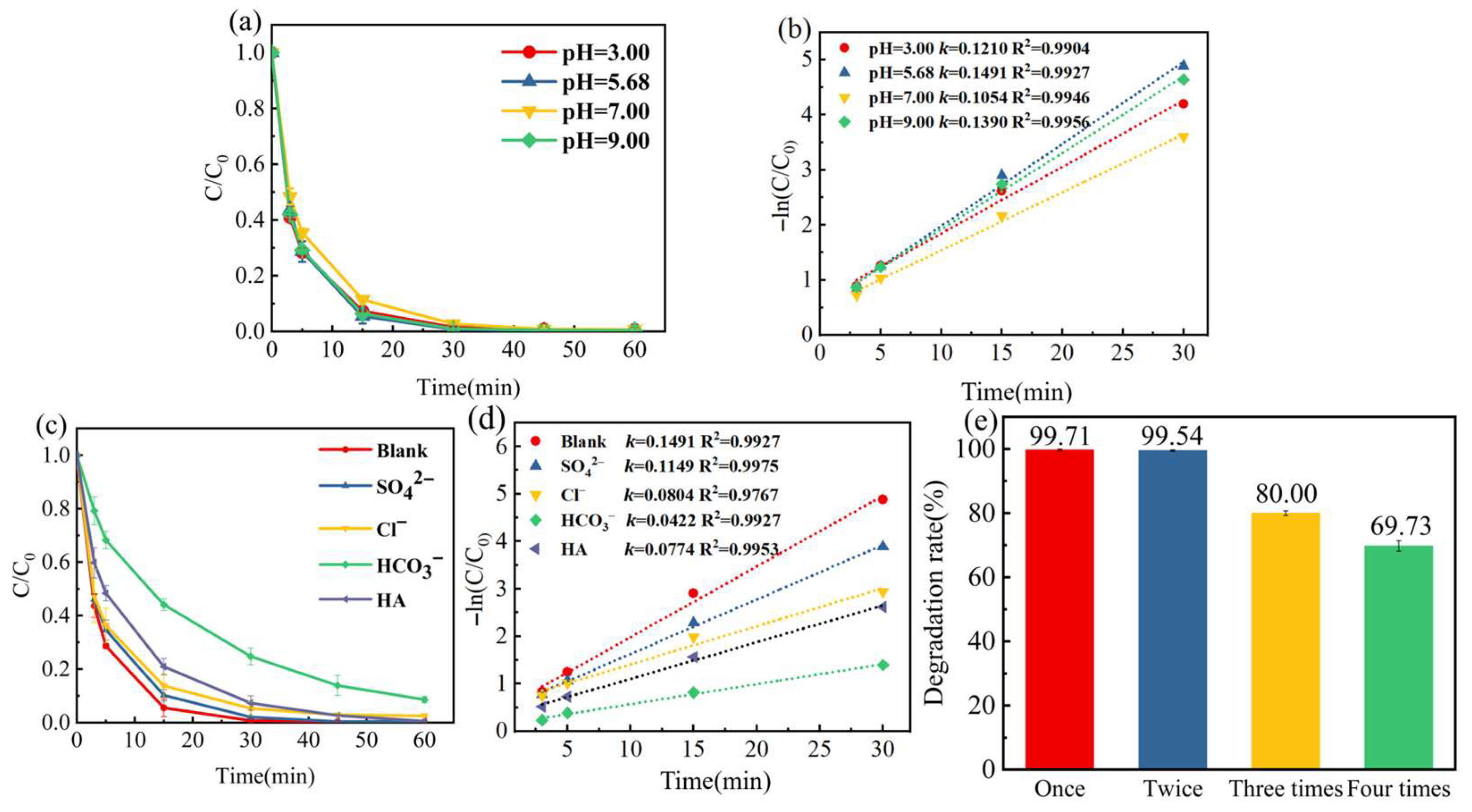
2.3. Performance Evaluation of Fe-N-C Activated PMS
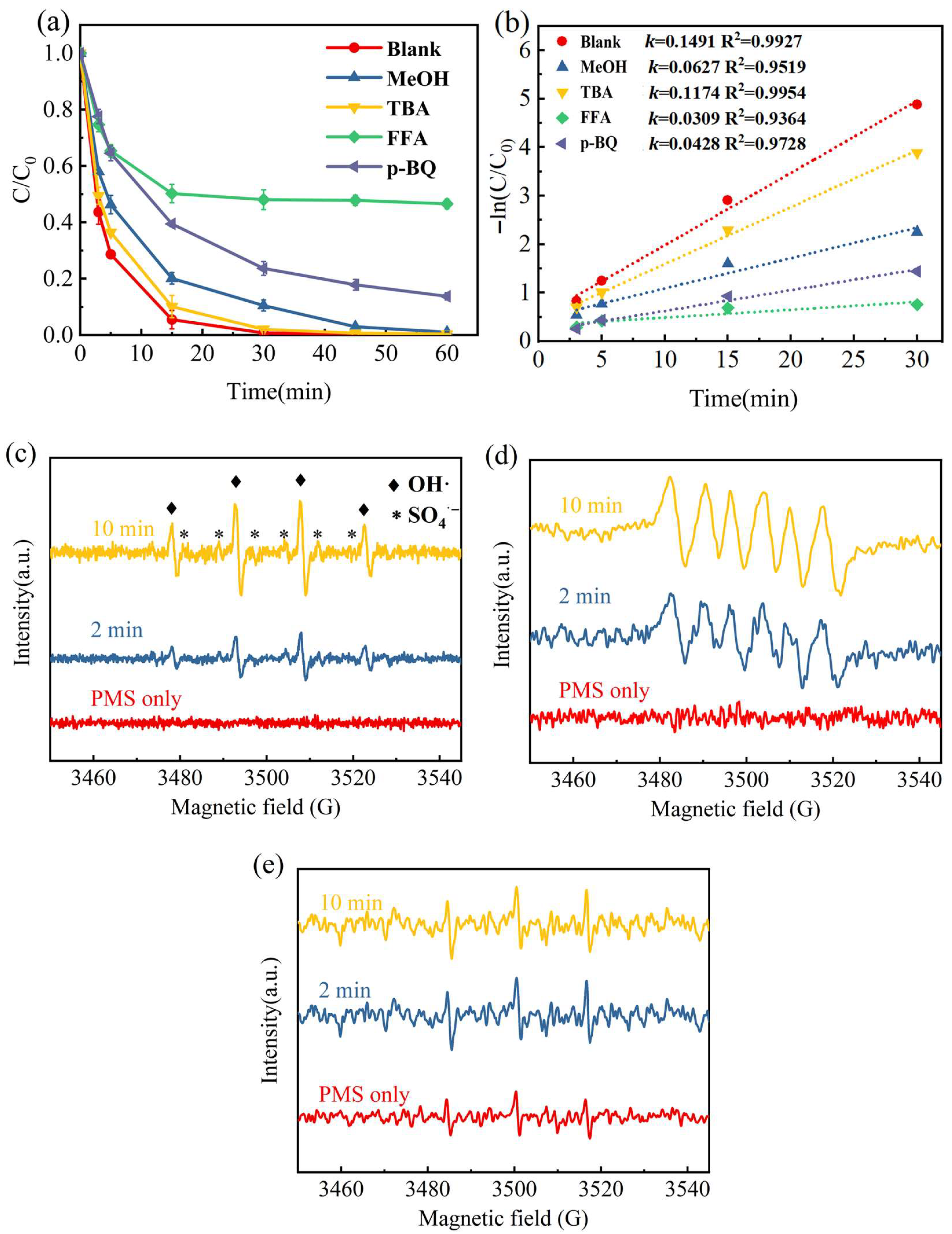
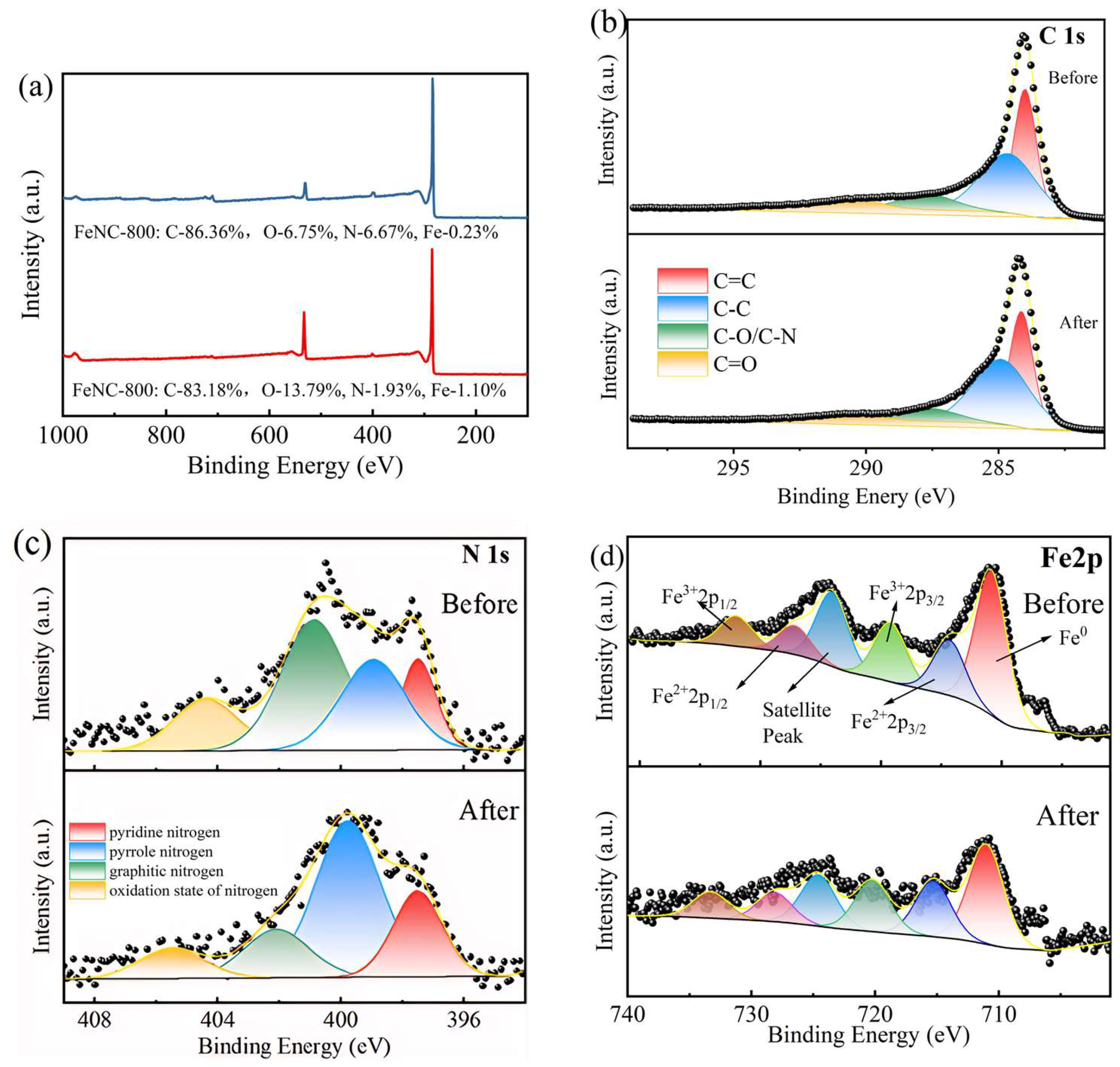
2.4. Mechanism Study of Fe-N-C Activated PMS
2.5. Material Comparison
3. Materials and Methods
3.1. Chemical Reagents
3.2. Material Preparation
3.3. Material Characterization
3.4. Material Performance Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, L.; Yin, Y.; Zhang, L.-C.; Wang, S.; Sillanpaa, M.; Sun, H. Design and engineering heterojunctions for the photoelectrochemical monitoring of environmental pollutants: A review. Appl. Catal. B-Environ. 2019, 248, 405–422. [Google Scholar] [CrossRef]
- Yen, J.-H.; Lin, K.-H.; Wang, Y.-S. Acute lethal toxicity of environmental pollutants to aquatic organisms. Ecotoxicol. Environ. Saf. 2002, 52, 113–116. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef]
- Chen, M.; He, Y.; Gu, Z. Microwave irradiation activated persulfate and hydrogen peroxide for the treatment of mature landfill leachate effluent from a membrane bioreactor. Sep. Purif. Technol. 2020, 250, 117111. [Google Scholar] [CrossRef]
- Tian, K.; Hu, L.M.; Li, L.T.; Zheng, Q.Z.; Xin, Y.J.; Zhang, G.S. Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment. Chin. Chem. Lett. 2022, 33, 4461–4477. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Lo, S.-L.; Kuo, J.; Huang, C.-P. Promoted degradation of perfluorooctanic acid by persulfate when adding activated carbon. J. Hazard. Mater. 2013, 261, 463–469. [Google Scholar] [CrossRef]
- Yang, W.; Jiang, Z.; Hu, X.; Li, X.; Wang, H.; Xiao, R. Enhanced activation of persulfate by nitric acid/annealing modified multi-walled carbon nanotubes via non-radical process. Chemosphere 2019, 220, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Bekris, L.; Frontistis, Z.; Trakakis, G.; Sygellou, L.; Galiotis, C.; Mantzavinos, D. Graphene: A new activator of sodium persulfate for the advanced oxidation of parabens in water. Water Res. 2017, 126, 111–121. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Ao, Z.; Zhou, L.; Wang, G.; Wang, S. Unveiling the active sites of graphene-catalyzed peroxymonosulfate activation. Carbon 2016, 107, 371–378. [Google Scholar] [CrossRef]
- Liu, B.; Guo, W.; Wang, H.; Si, Q.; Zhao, Q.; Luo, H.; Ren, N. B-doped graphitic porous biochar with enhanced surface affinity and electron transfer for efficient peroxydisulfate activation. Chem. Eng. J. 2020, 396, 125119. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Chen, B.-J. Magnetic carbon-supported cobalt derived from a Prussian blue analogue as a heterogeneous catalyst to activate peroxymonosulfate for efficient degradation of caffeine in water. J. Colloid Interface Sci. 2017, 486, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhang, Q.; Wang, Z.; Gao, B.; Fan, Z.; Li, M.; Hao, H.; Wei, X.; Zhong, M. Degradation of anthraquinone dye reactive blue 19 using persulfate activated with Fe/Mn modified biochar: Radical/non-radical mechanisms and fixed-bed reactor study. Sci. Total Environ. 2021, 758, 143584. [Google Scholar] [CrossRef]
- Tian, H.; Chen, C.; Zhu, T.; Zhu, B.; Sun, Y. Characterization and degradation mechanism of bimetallic iron-based/AC activated persulfate for PAHs-contaminated soil remediation. Chemosphere 2021, 267, 128875. [Google Scholar] [CrossRef]
- Jin, Q.; Zhang, S.; Wen, T.; Wang, J.; Gu, P.; Zhao, G.; Wang, X.; Chen, Z.; Hayat, T.; Wang, X. Simultaneous adsorption and oxidative degradation of Bisphenol A by zero-valent iron/iron carbide nanoparticles encapsulated in N-doped carbon matrix. Environ. Pollut. 2018, 243, 218–227. [Google Scholar] [CrossRef]
- Zeng, T.; Li, S.; Hua, J.; He, Z.; Zhang, X.; Feng, H.; Song, S. Synergistically enhancing Fenton-like degradation of organics by in-situ transformation from Fe3O4 microspheres to mesoporous Fe, N-dual doped carbon. Sci. Total Environ. 2018, 645, 550–559. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, J.; Wu, G.; Wang, S.; Hu, Y.; Su, C.; Xu, T. Iron encapsulated in 3D N-doped carbon nanotube/porous carbon hybrid from waste biomass for enhanced oxidative activity. Environ. Sci. Pollut. R. 2017, 24, 7679–7692. [Google Scholar] [CrossRef]
- Zhao, X.; An, Q.-D.; Xiao, Z.-Y.; Zhai, S.-R.; Shi, Z. Seaweed-derived multifunctional nitrogen/cobalt-codoped carbonaceous beads for relatively high-efficient peroxymonosulfate activation for organic pollutants degradation. Chem. Eng. J. 2018, 353, 746–759. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Yang, Y.; Feng, Y.; Wu, D.; Mao, S. Activation of persulfate with metal-organic framework-derived nitrogen-doped porous Co@C nanoboxes for highly efficient p-Chloroaniline removal. Chem. Eng. J. 2019, 358, 408–418. [Google Scholar] [CrossRef]
- Ma, W.; Wang, N.; Fan, Y.; Tong, T.; Han, X.; Du, Y. Non-radical-dominated catalytic degradation of bisphenol A by ZIF-67 derived nitrogen-doped carbon nanotubes frameworks in the presence of peroxymonosulfate. Chem. Eng. J. 2018, 336, 721–731. [Google Scholar] [CrossRef]
- Peng, Q.; Ding, Y.; Zhu, L.; Zhang, G.; Tang, H. Fast and complete degradation of norfloxacin by using Fe/Fe3C@NG as a bifunctional catalyst for activating peroxymonosulfate. Sep. Purif. Technol. 2018, 202, 307–317. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, H.; Qin, J.; Wu, G.; Lian, C.; Zhang, J.; Wang, S. Iron encapsulated in boron and nitrogen codoped carbon nanotubes as synergistic catalysts for Fenton-like reaction. Water Res. 2016, 101, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tian, J.; Zhu, Z.; Cui, F.; Zhu, Y.-A.; Duan, X.; Wang, S. Magnetic nitrogen-doped nanocarbons for enhanced metal-free catalytic oxidation: Integrated experimental and theoretical investigations for mechanism and application. Chem. Eng. J. 2018, 354, 507–516. [Google Scholar] [CrossRef]
- Wang, C.; Kang, J.; Liang, P.; Zhang, H.; Sun, H.; Tade, M.O.; Wang, S. Ferric carbide nanocrystals encapsulated in nitrogen-doped carbon nanotubes as an outstanding environmental catalyst. Environ. Sci.-Nano 2017, 4, 170–179. [Google Scholar] [CrossRef]
- Pang, Y.; Luo, K.; Tang, L.; Li, X.; Song, Y.; Li, C.-y.; Wang, L.-p. Preparation and application of magnetic nitrogen-doped rGO for persulfate activation. Environ. Sci. Pollut. R. 2018, 25, 30575–30584. [Google Scholar] [CrossRef]
- Long, Y.; Huang, Y.; Wu, H.; Shi, X.; Xiao, L. Peroxymonosulfate activation for pollutants degradation by Fe-N-codoped carbonaceous catalyst: Structure-dependent performance and mechanism insight. Chem. Eng. J. 2019, 369, 542–552. [Google Scholar] [CrossRef]
- Xu, L.; Fu, B.; Sun, Y.; Jin, P.; Bai, X.; Jin, X.; Shi, X.; Wang, Y.; Nie, S. Degradation of organic pollutants by Fe/N co-doped biochar via peroxymonosulfate activation: Synthesis, performance, mechanism and its potential for practical application. Chem. Eng. J. 2020, 400, 125870. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, Z.; Zhu, Y.; Li, T.; Hu, C. New Insights into the Generation of Singlet Oxygen in the Metal-Free Peroxymonosulfate Activation Process: Important Role of Electron-Deficient Carbon Atoms. Environ. Sci. Technol. 2019, 54, 1232–1241. [Google Scholar] [CrossRef]
- Liu, J.; Li, F.; Liu, W.; Li, X. Effect of calcination temperature on the microstructure of vanadium nitride/nitrogen-doped graphene nanocomposites as anode materials in electrochemical capacitors†. Inorg. Chem. Front. 2018, 6, 164–171. [Google Scholar] [CrossRef]
- Wu, S.; Liu, H.; Yang, C.; Li, X.; Lin, Y.; Yin, K.; Sun, J.; Teng, Q.; Du, C.; Zhong, Y. High-performance porous carbon catalysts doped by iron and nitrogen for degradation of bisphenol F via peroxymonosulfate activation. Chem. Eng. J. 2020, 392, 123683. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, H.; Kharat, D.S. Adsorption of Cu(II) by low cost adsorbents and the cost analysis. Environ. Technol. Innov. 2018, 10, 91–101. [Google Scholar] [CrossRef]
- Li, J.H.; Shen, B.A.; Hong, Z.H.; Lin, B.Z.; Gao, B.F.; Chen, Y.L. A facile approach to synthesize novel oxygen-doped g-C3N4 with superior visible-light photoreactivity. Chem. Commun. 2012, 48, 12017–12019. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Q.; Huang, J.F.; Shi, L.; Cao, L.Y.; Yu, Q.; Jie, Y.N.; Fei, J.; Ouyang, H.B.; Ye, J.H. A surface modification resultant thermally oxidized porous g-C3N4 with enhanced photocatalytic hydrogen production. Appl. Catal. B-Environ. 2017, 204, 335–345. [Google Scholar] [CrossRef]
- Huang, Z.F.; Song, J.J.; Pan, L.; Wang, Z.M.; Zhang, X.Q.; Zou, J.J.; Mi, W.B.; Zhang, X.W.; Wang, L. Carbon nitride with simultaneous porous network and O-doping for efficient solar-energy-driven hydrogen evolution. Nano Energy 2015, 12, 646–656. [Google Scholar] [CrossRef]
- Yan, Z.; Dai, C.; Lv, X.; Zhang, M.; Zhao, X.; Xie, J. Iron promoted nitrogen doped porous graphite for efficient oxygen reduction reaction in alkaline and acidic media. J. Alloys Compd. 2019, 773, 819–827. [Google Scholar] [CrossRef]
- Li, R.; Lu, X.; Yan, B.; Li, N.; Chen, G.; Cheng, Z.; Hou, L.a.; Wang, S.; Duan, X. Sludge-derived biochar toward sustainable Peroxymonosulfate Activation: Regulation of active sites and synergistic production of reaction oxygen species. Chem. Eng. J. 2022, 440, 135897. [Google Scholar] [CrossRef]
- Yu, H.J.; Shang, L.; Bian, T.; Shi, R.; Waterhouse, G.I.N.; Zhao, Y.F.; Zhou, C.; Wu, L.Z.; Tung, C.H.; Zhang, T.R. Nitrogen-Doped Porous Carbon Nanosheets Templated from g-C3N4 as Metal-Free Electrocatalysts for Efficient Oxygen Reduction Reaction. Adv. Mater. 2016, 28, 5080–5086. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Lin, C.; Zhou, Z.; He, M.; Ouyang, W. Catalytic oxidation of contaminants by Fe0 activated peroxymonosulfate process: Fe(IV) involvement, degradation intermediates and toxicity evaluation. Chem. Eng. J. 2020, 382, 123013. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, N.; Guan, J. Charge-Transfer Effects in Fe-Co and Fe-Co-Y Oxides for Electrocatalytic Water Oxidation Reaction. ACS Appl. Energy Mater. 2019, 2, 8903–8911. [Google Scholar] [CrossRef]
- de Andrade, J.R.; Vieira, M.G.A.; Carlos da Silva, M.G.; Wang, S. Oxidative degradation of pharmaceutical losartan potassium with N-doped hierarchical porous carbon and peroxymonosulfate. Chem. Eng. J. 2020, 382, 122971. [Google Scholar] [CrossRef]
- Shang, E.; Li, Y.; Niu, J.; Zhou, Y.; Wang, T.; Crittenden, J.C. Relative importance of humic and fulvic acid on ROS generation, dissolution, and toxicity of sulfide nanoparticles. Water Res. 2017, 124, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.D.; Fan, Q.F.; Yang, Y.H.; Duan, X.Y.; Jiang, K.; Wang, J. Polyaniline@g-C3N4 derived N-rich porous carbon for selective degradation of phenolic pollutants via peroxymonosulfate activation: An electron transfer mechanism. Chemosphere 2023, 311, 137022. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Duan, H.; Li, H.; Zhen, J.; Lv, W. Efficient activation of peroxymonosulfate by MoS2 intercalated MgCuFe layered double hydroxide for phenol pollutant control. J. Environ. Chem. Eng. 2023, 11, 109502. [Google Scholar] [CrossRef]
- Ding, S.; Ren, X.; Chen, R.; Yang, Z. Efficient degradation of Phenol by 1 T/2H-MoS2/CuFe2O4 activated peroxymonosulfate and mechanism research. Appl. Surf. Sci. 2023, 612, 155931. [Google Scholar] [CrossRef]
- Li, N.; Li, R.; Duan, X.; Yan, B.; Liu, W.; Cheng, Z.; Chen, G.; Hou, L.a.; Wang, S. Correlation of Active Sites to Generated Reactive Species and Degradation Routes of Organics in Peroxymonosulfate Activation by Co-Loaded Carbon. Environ. Sci. Technol. 2021, 55, 16163–16174. [Google Scholar] [CrossRef]
- Zhu, K.; Bin, Q.; Shen, Y.; Huang, J.; He, D.; Chen, W. In-situ formed N-doped bamboo-like carbon nanotubes encapsulated with Fe nanoparticles supported by biochar as highly efficient catalyst for activation of persulfate (PS) toward degradation of organic pollutants. Chem. Eng. J. 2020, 402, 126090. [Google Scholar] [CrossRef]
- Ye, S.; Zeng, G.; Tan, X.; Wu, H.; Liang, J.; Song, B.; Tang, N.; Zhang, P.; Yang, Y.; Chen, Q.; et al. Nitrogen-doped biochar fiber with graphitization from Boehmeria nivea for promoted peroxymonosulfate activation and non-radical degradation pathways with enhancing electron transfer. Appl. Catal. B-Environ. 2020, 269, 118850. [Google Scholar] [CrossRef]
- Chen, J.; Zhan, Z.; Chen, Z.; Wang, Y.; Li, X.; Du, M.; Teng, D.; Lv, D.; Da, C.; Xu, M. Ultrasound synergistic iron-nitrogen biocarbon activated persulfate to ofloxacin degradation. J. Environ. Chem. Eng. 2024, 12, 112759. [Google Scholar] [CrossRef]
- Luo, J.; Yi, Y.; Ying, G.; Fang, Z.; Zhang, Y. Activation of persulfate for highly efficient degradation of metronidazole using Fe(II)-rich potassium doped magnetic biochar. Sci. Total Environ. 2022, 819, 152089. [Google Scholar] [CrossRef]
- Mady, A.H.; Baynosa, M.L.; Tuma, D.; Shim, J.-J. Heterogeneous activation of peroxymonosulfate by a novel magnetic 3D γ-MnO2@ZnFe2O4/rGO nanohybrid as a robust catalyst for phenol degradation. Appl. Catal. B-Environ. 2019, 244, 946–956. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Duan, X.; Ang, H.M.; Tade, M.O.; Wang, S. A new magnetic nano zero-valent iron encapsulated in carbon spheres for oxidative degradation of phenol. Appl. Catal. B-Environ. 2015, 172, 73–81. [Google Scholar] [CrossRef]
- Wang, S.; Dai, Y.; Wang, H.; Miao, S.; Deng, Z.; Yin, L. Activation of peroxymonosulfate by Fe based metal organic framework for selective degradation of phenol in water. J. Water Process Eng. 2024, 68, 106454. [Google Scholar] [CrossRef]
| Sample (wt. %) | Cad | Had | Nad | Sad | Oad |
|---|---|---|---|---|---|
| HA | 46.04 | 2.77 | 1.09 | 0.46 | 49.64 |
| Morphological Changes of C | C=C | C-C | C-O/C-N | C=O |
|---|---|---|---|---|
| Before | 36.92% | 43.62% | 11.48% | 7.98% |
| After | 35.99% | 48.78% | 9.63% | 5.60% |
| Morphological Changes of N | Pyridinic N | Pyrrolic N | Graphitic N | Oxidized N |
|---|---|---|---|---|
| Before | 15.22% | 28.10% | 40.53% | 16.15% |
| After | 21.46% | 52.10% | 16.25% | 10.20% |
| Morphological Changes of Fe | Fe0 | Fe2+ | Fe3+ |
| Before | 56.01% | 23.39% | 20.60% |
| After | 47.81% | 27.25% | 24.95% |
| Materials | Reaction Conditions | Degradation Effect | Reference |
|---|---|---|---|
| Fe3C-N-CNT | [phenol] = 20 mg/L; [catalyst] = 200 mg/L; [PMS] = 3 mM | 100% 20 min | [24] |
| γ-MnO2-ZnFe2O4-rGO | [phenol] = 20 mg/L; [catalyst] = 200 mg/L; [PMS] = 3 mM | 100% 30 min | [50] |
| Fe0-Fe3C-CS | [phenol] = 10 mg/L; [catalyst] = 20 mg/L; [PMS] = 3 mM | 100% 10 min | [51] |
| Fe-MOF | [phenol] = 20 mg/L; [catalyst] = 200 mg/L; [PMS] = 3 mM | 100% 60 min | [52] |
| Fe-N-C | [phenol] = 50 mg/L; [catalyst] = 150 mg/L; [PMS] = 1.5 mM | 100% 60 min | This thesis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Wu, X.; Ma, P.; Song, Z.; Wu, X.; Gao, R.; Miao, Z. Anti-Interference Fe-N-C/PMS System: Synergistic Radical-Nonradical Pathways Enabled by sp2 Carbon and Metal-N Coordination. Catalysts 2025, 15, 850. https://doi.org/10.3390/catal15090850
He Q, Wu X, Ma P, Song Z, Wu X, Gao R, Miao Z. Anti-Interference Fe-N-C/PMS System: Synergistic Radical-Nonradical Pathways Enabled by sp2 Carbon and Metal-N Coordination. Catalysts. 2025; 15(9):850. https://doi.org/10.3390/catal15090850
Chicago/Turabian StyleHe, Qiongqiong, Xuewen Wu, Ping Ma, Zhaoyang Song, Xiaoqi Wu, Ruize Gao, and Zhenyong Miao. 2025. "Anti-Interference Fe-N-C/PMS System: Synergistic Radical-Nonradical Pathways Enabled by sp2 Carbon and Metal-N Coordination" Catalysts 15, no. 9: 850. https://doi.org/10.3390/catal15090850
APA StyleHe, Q., Wu, X., Ma, P., Song, Z., Wu, X., Gao, R., & Miao, Z. (2025). Anti-Interference Fe-N-C/PMS System: Synergistic Radical-Nonradical Pathways Enabled by sp2 Carbon and Metal-N Coordination. Catalysts, 15(9), 850. https://doi.org/10.3390/catal15090850







