Tunable Optical Bandgap and Enhanced Visible Light Photocatalytic Activity of ZnFe2O3-Doped ZIF-8 Composites for Sustainable Environmental Remediation
Abstract
1. Introduction
2. Results and Discussion
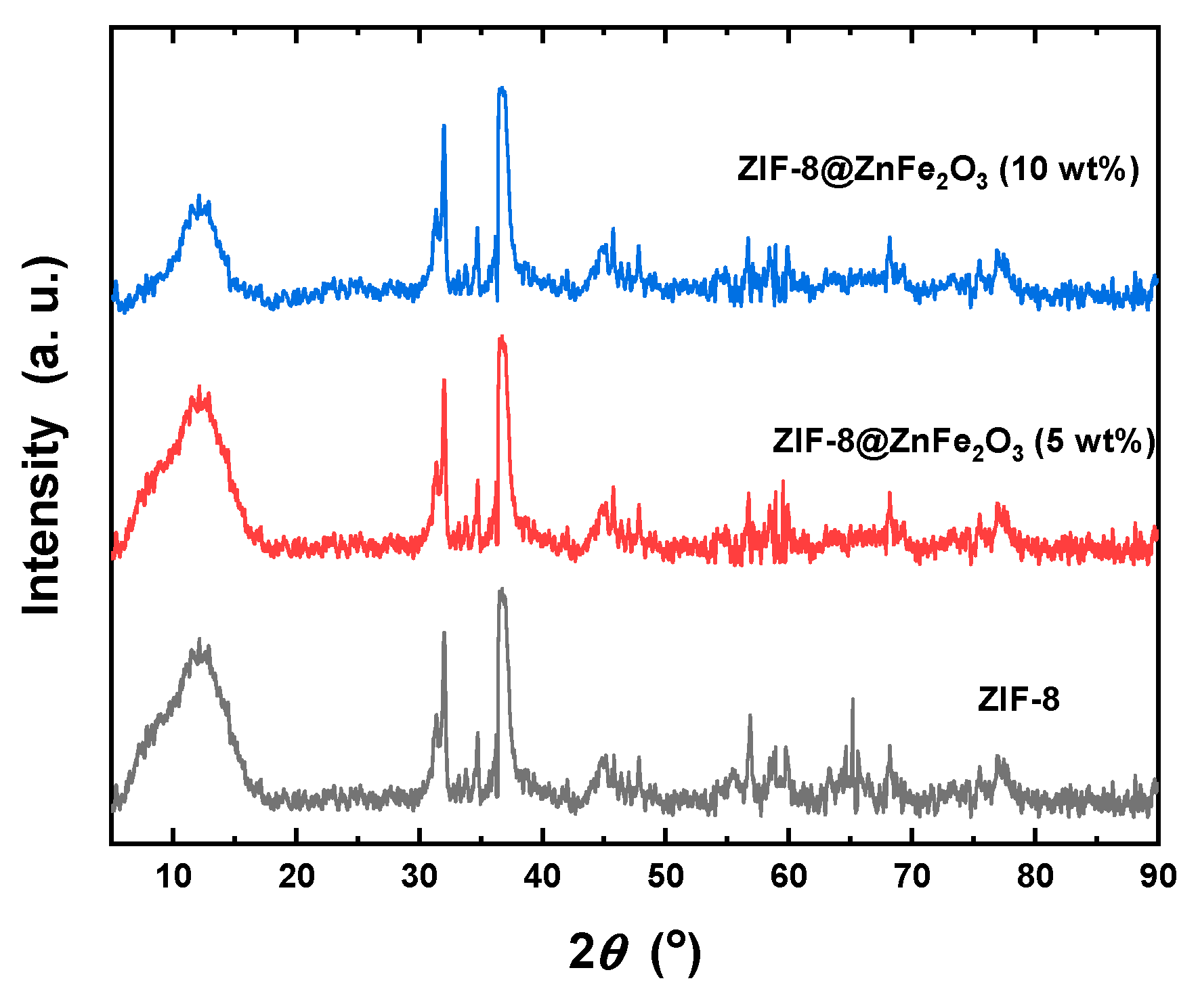
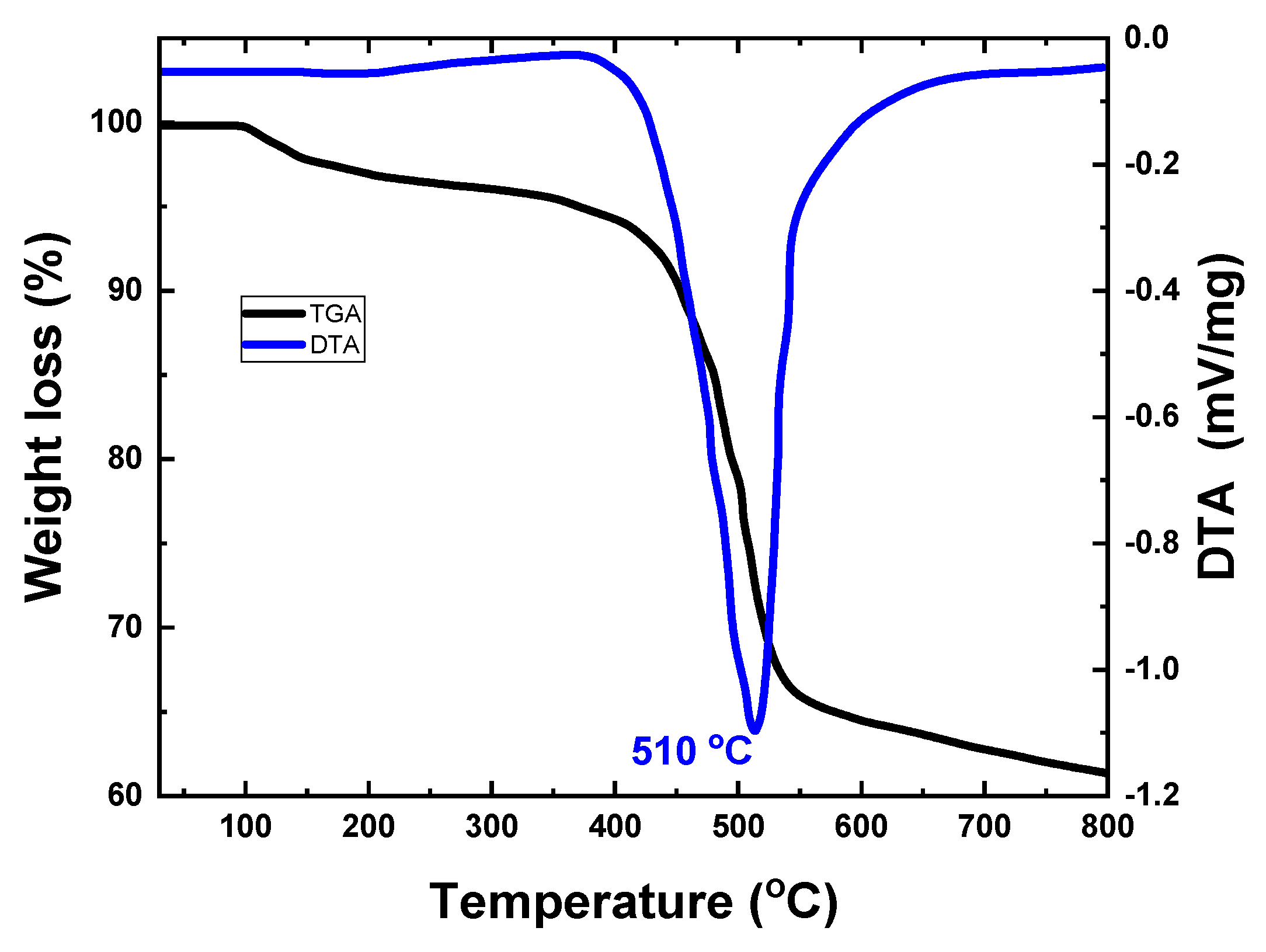
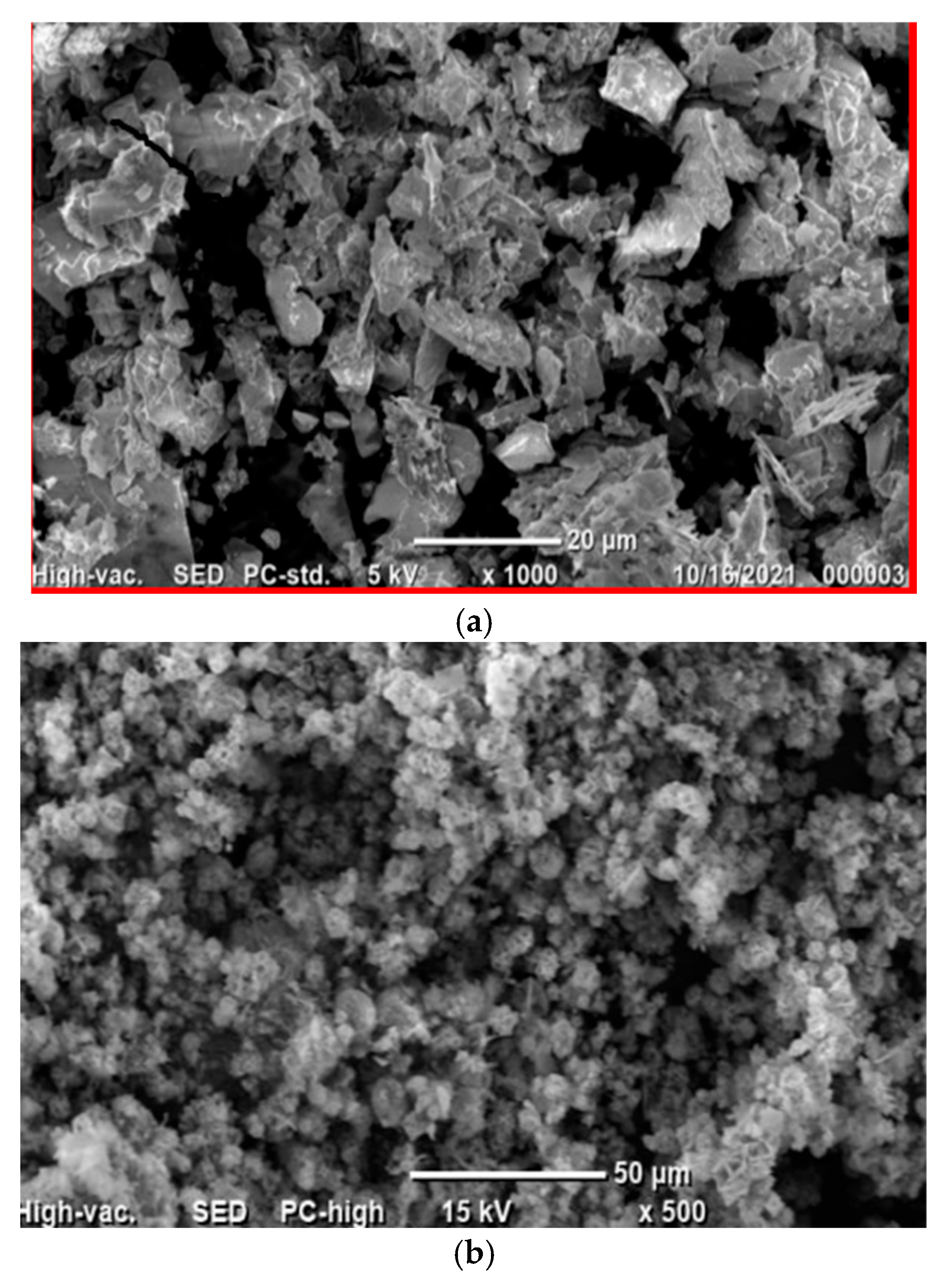
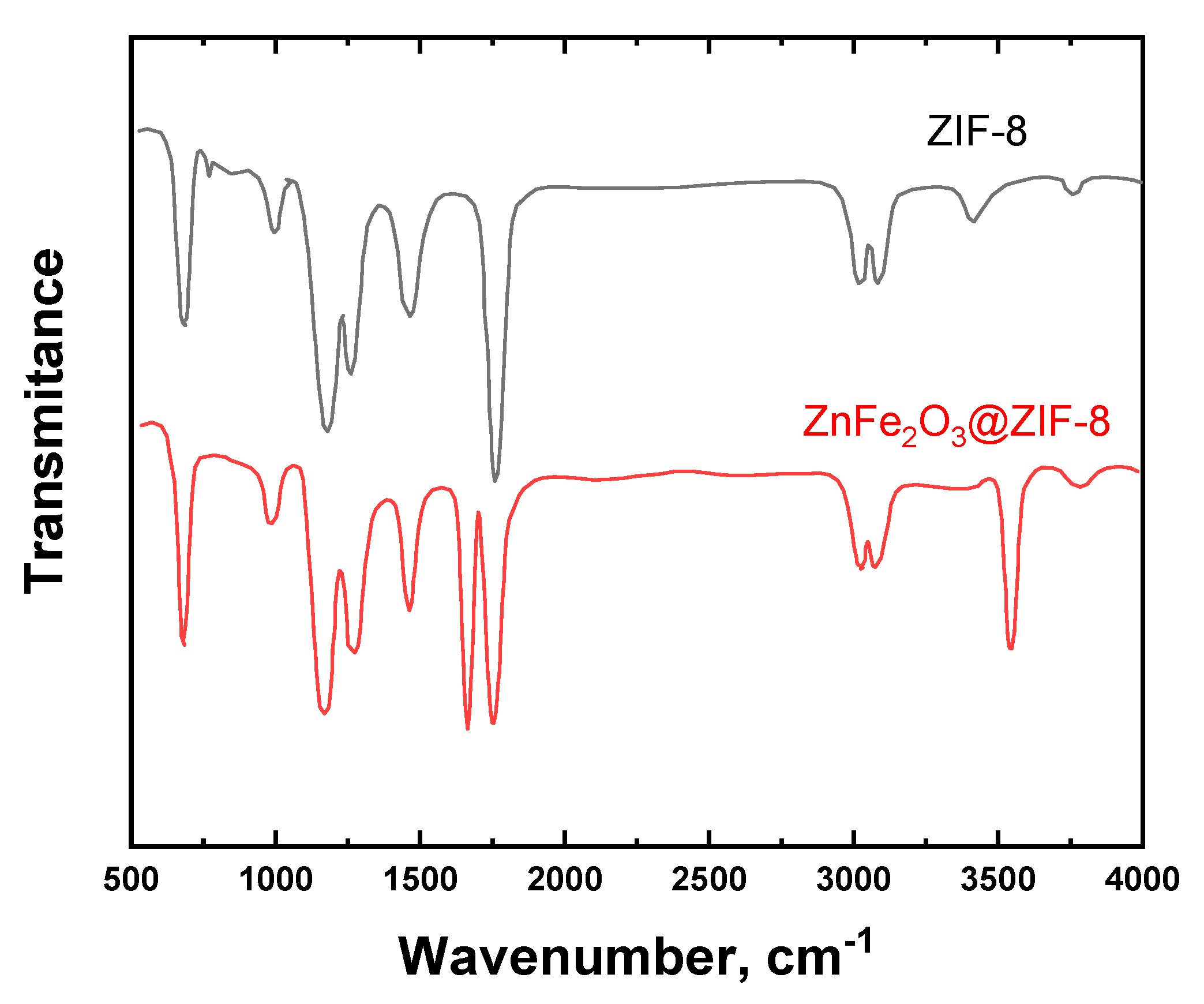
2.1. Structural Properties
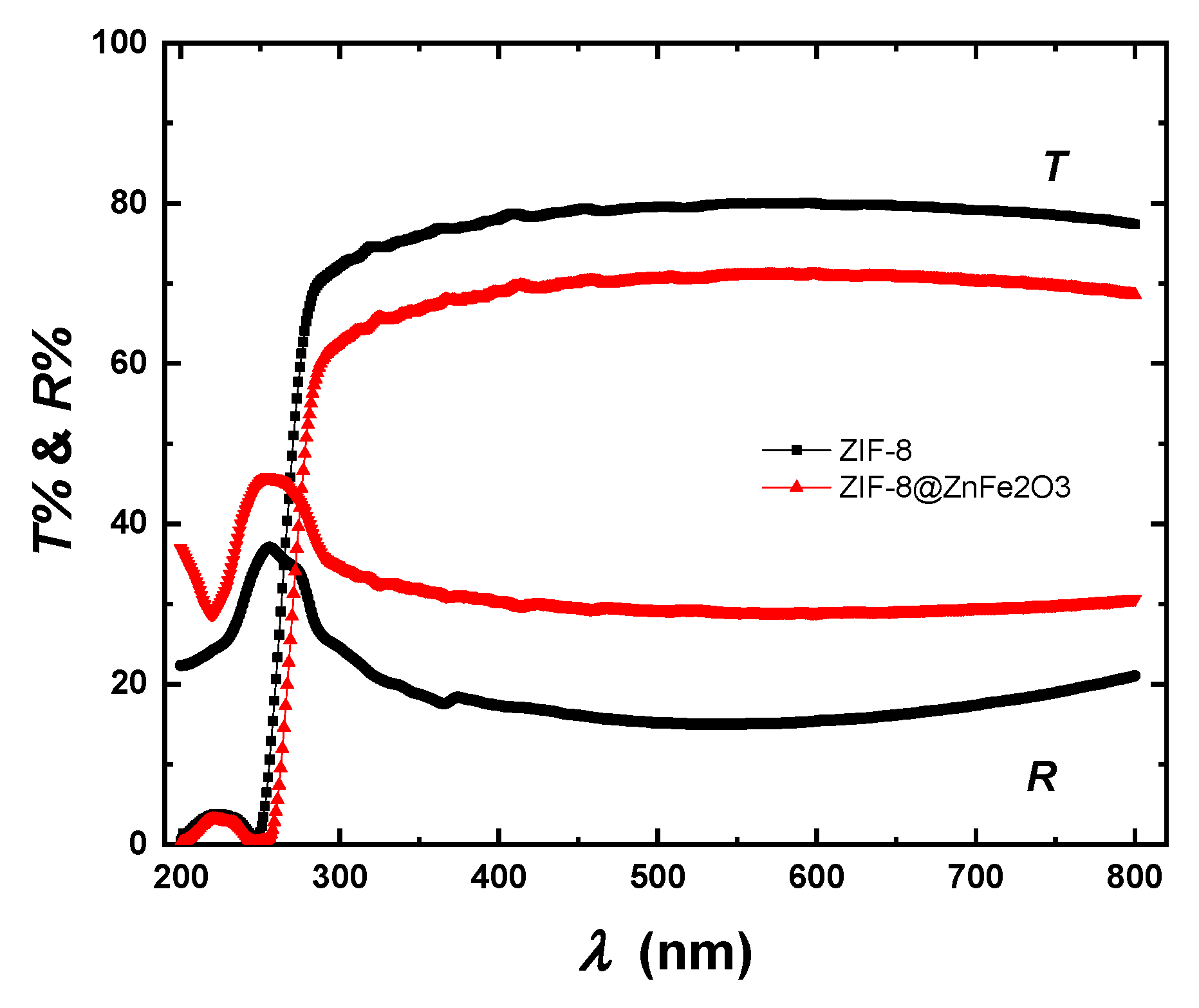
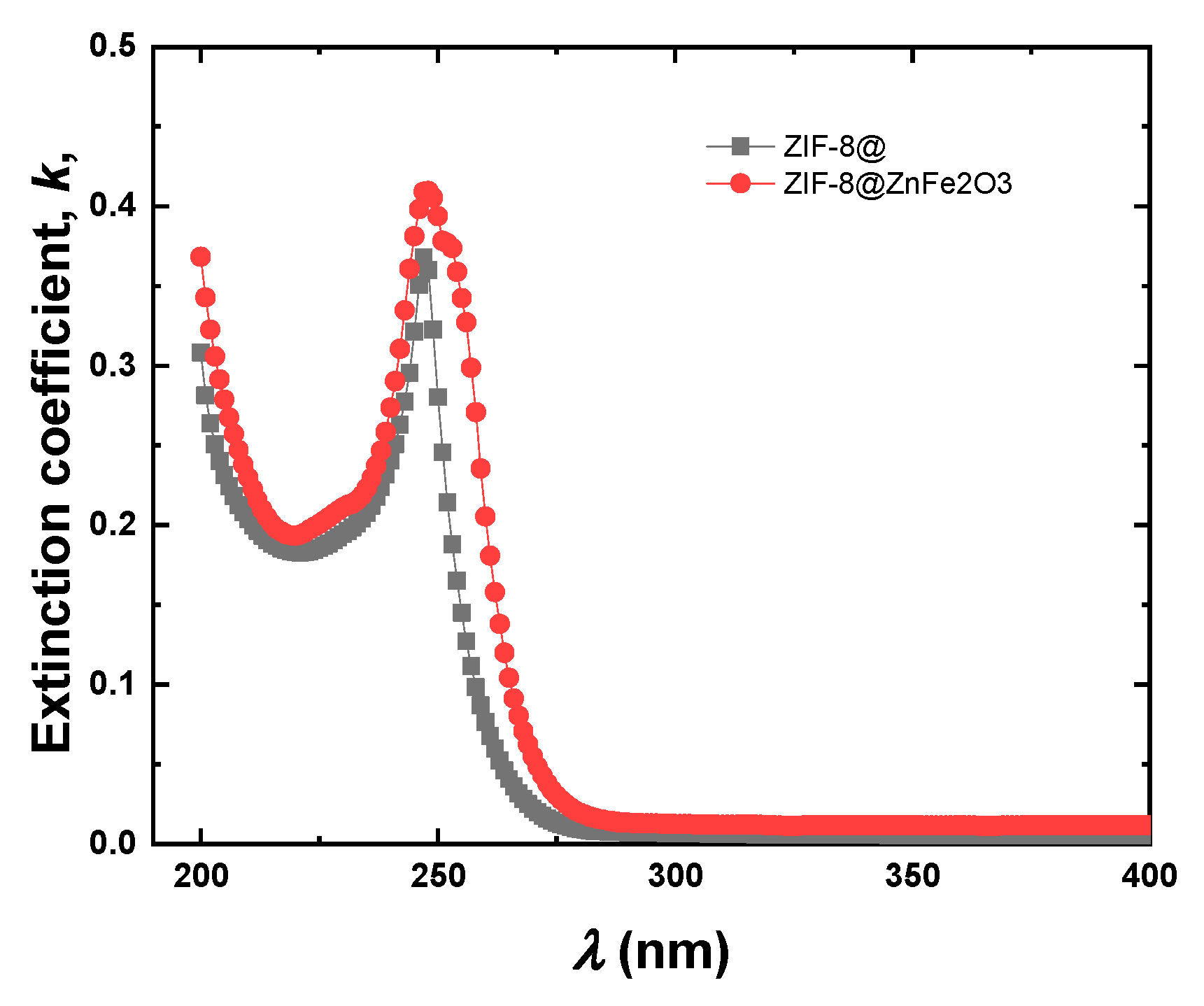
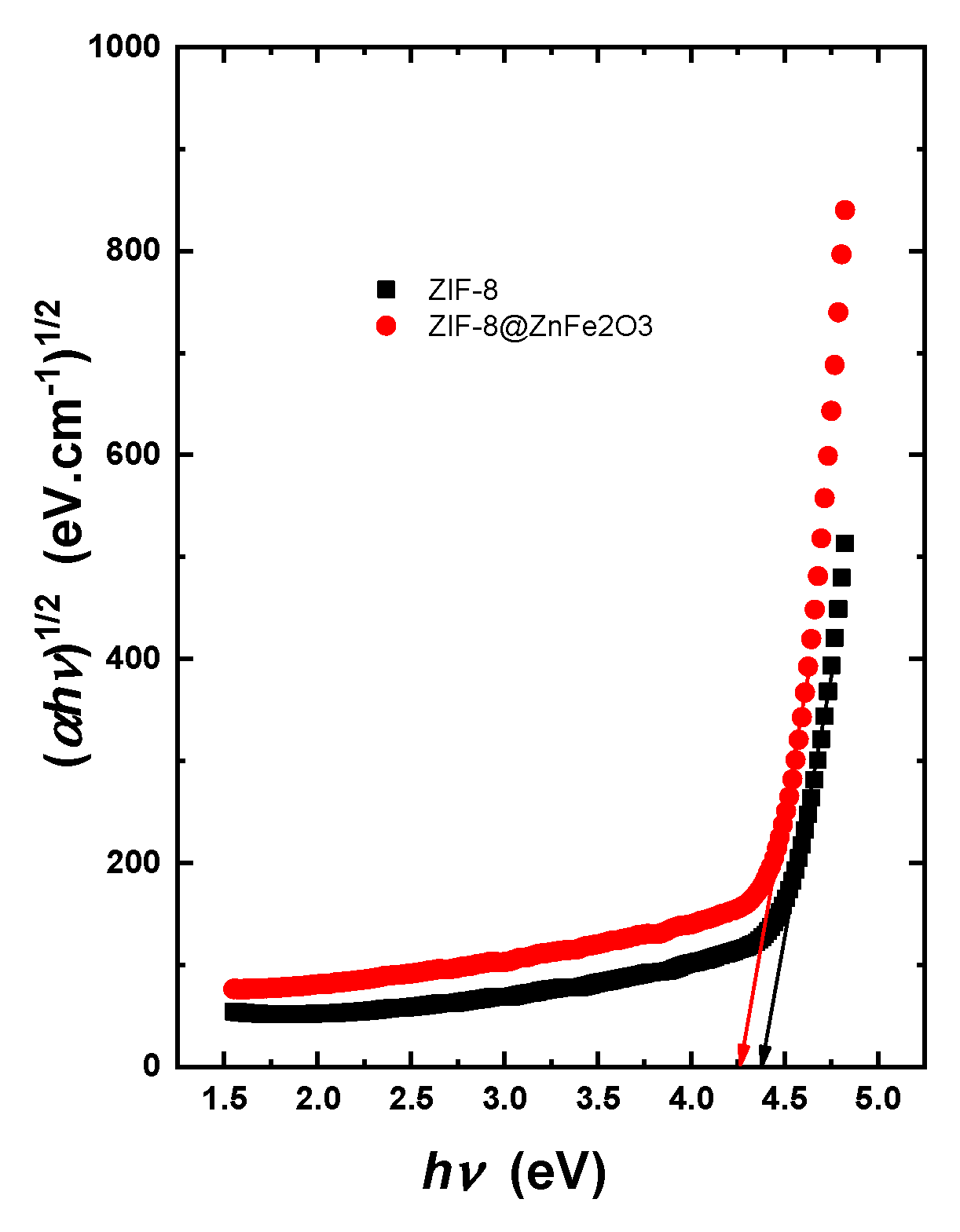
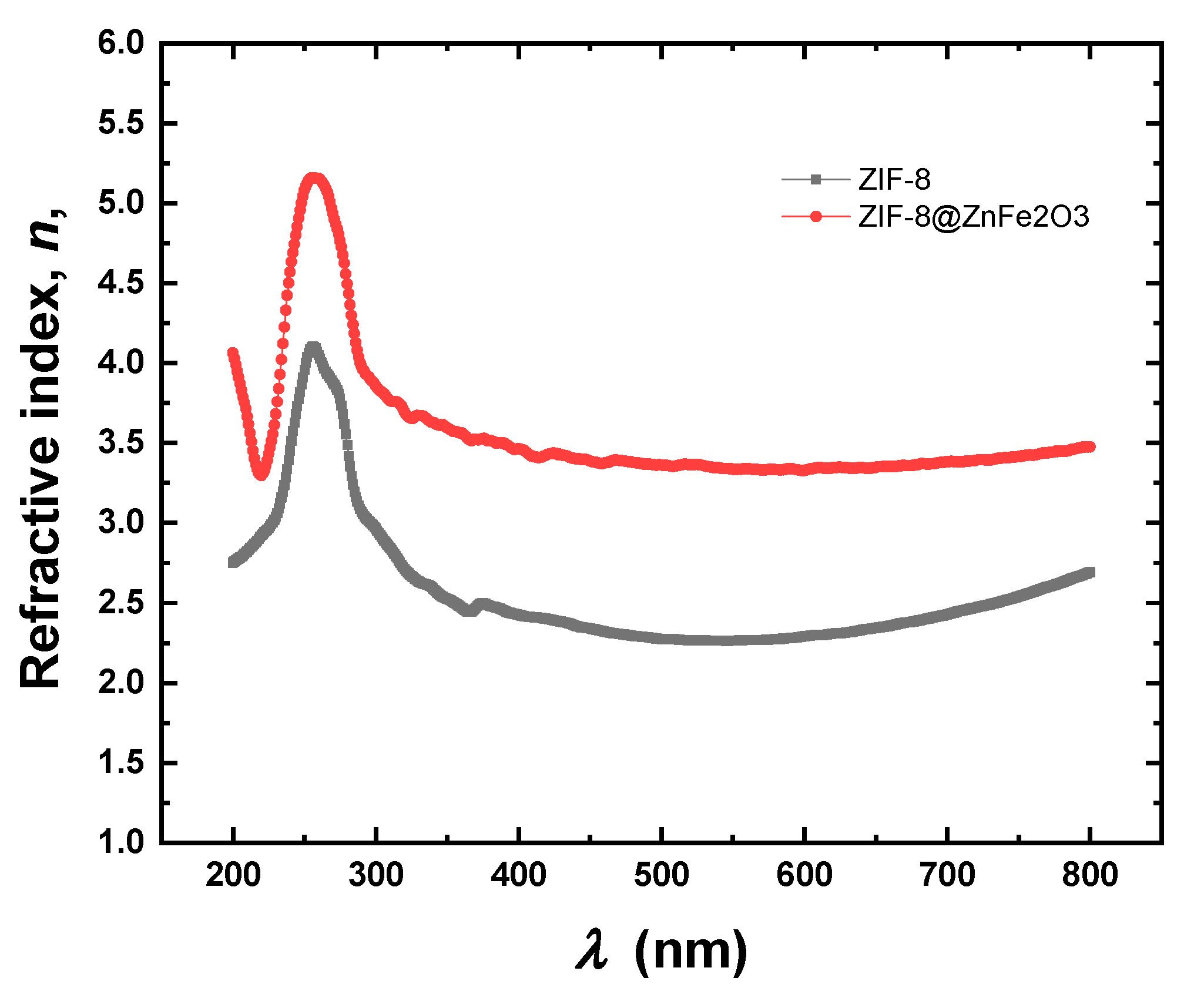
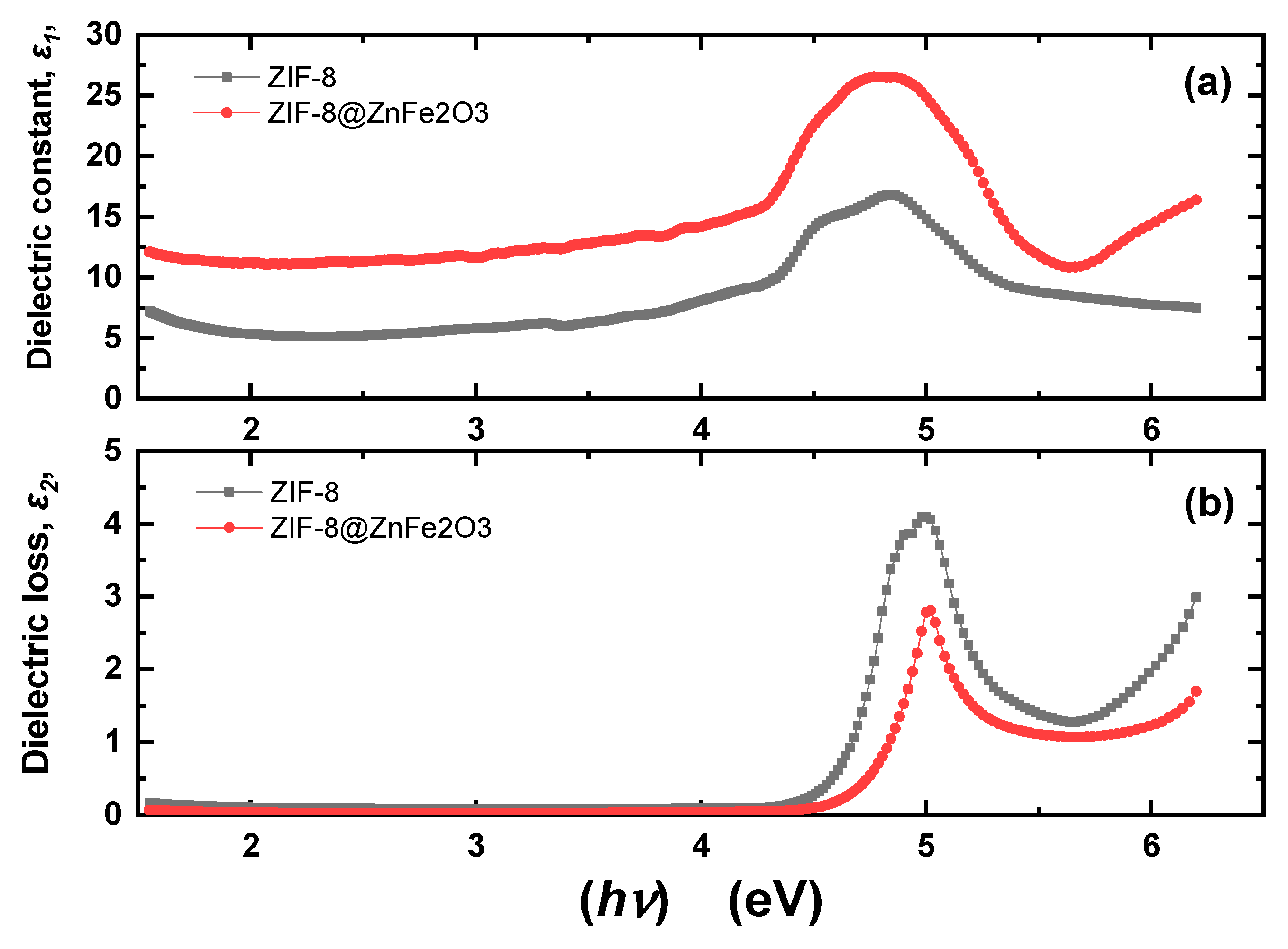
2.2. Optical Properties
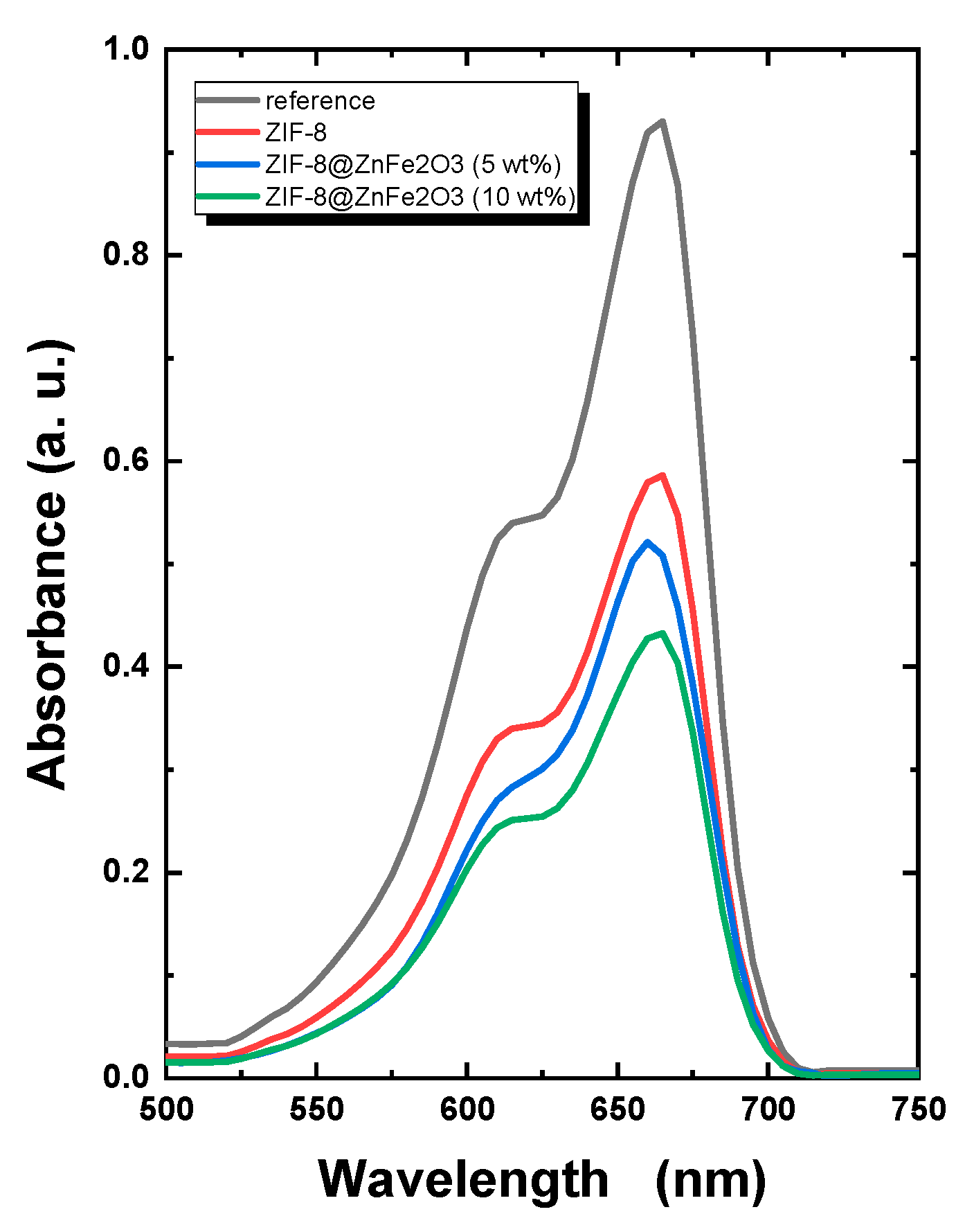
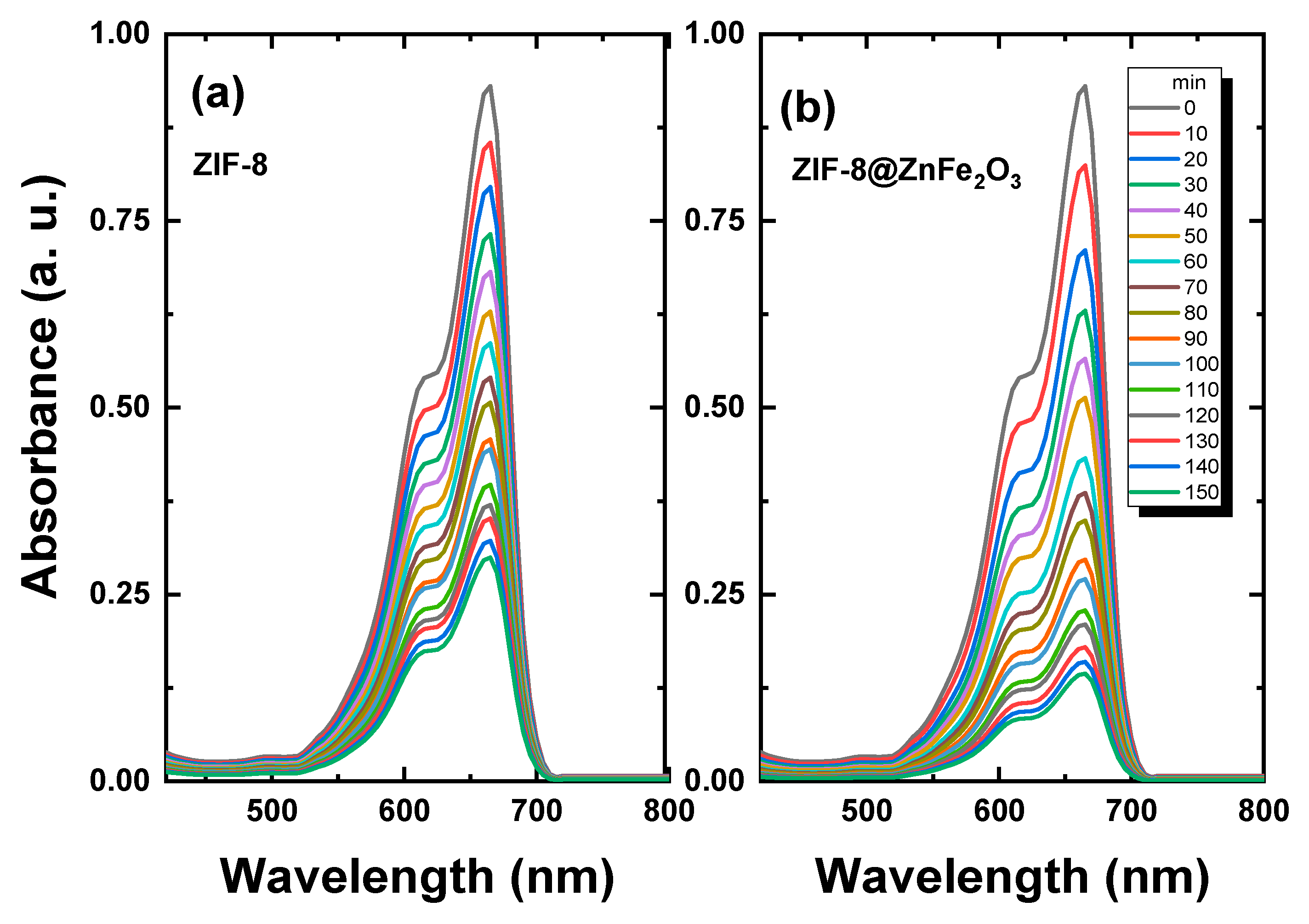
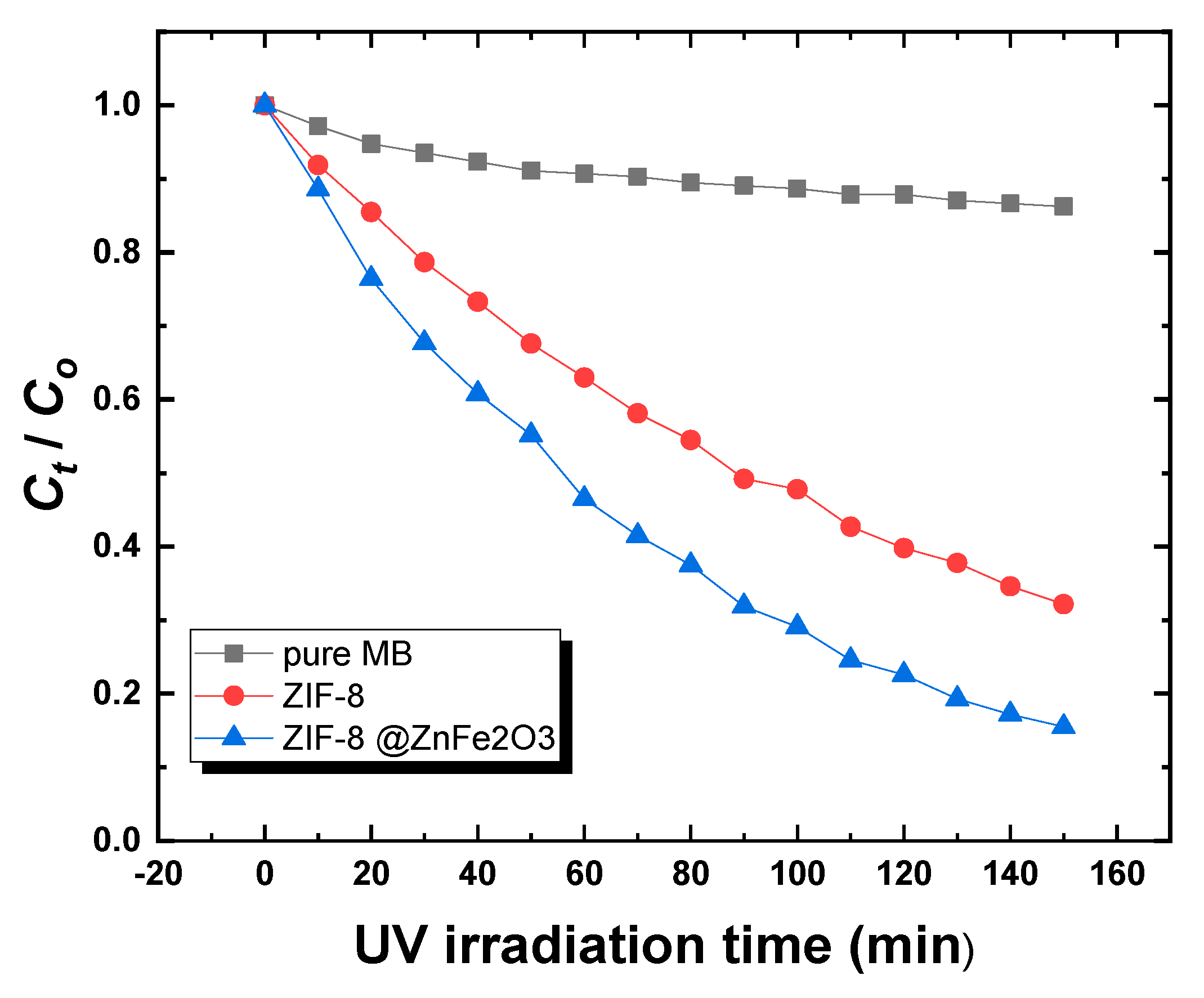
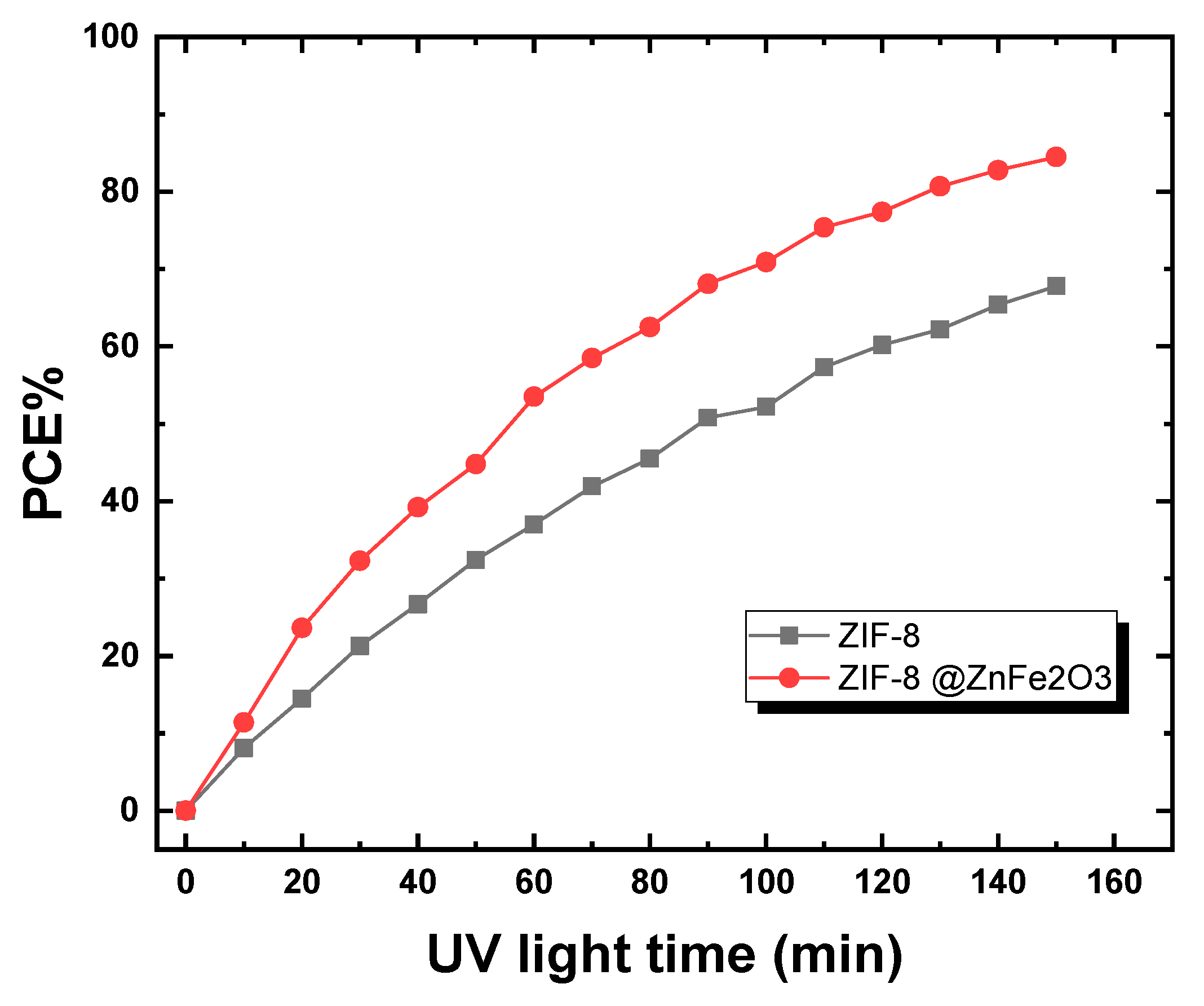
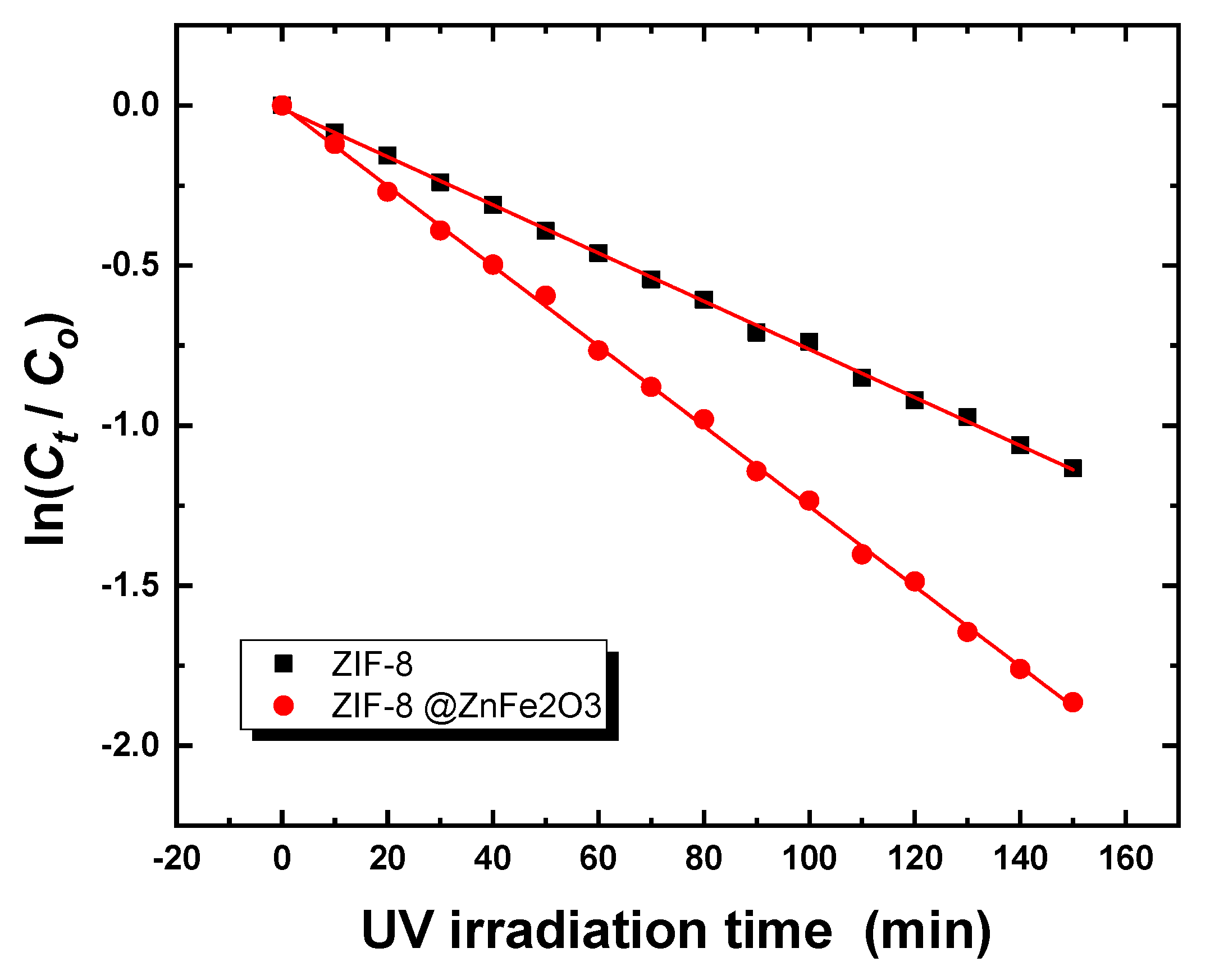
2.3. Photocatalytic Study
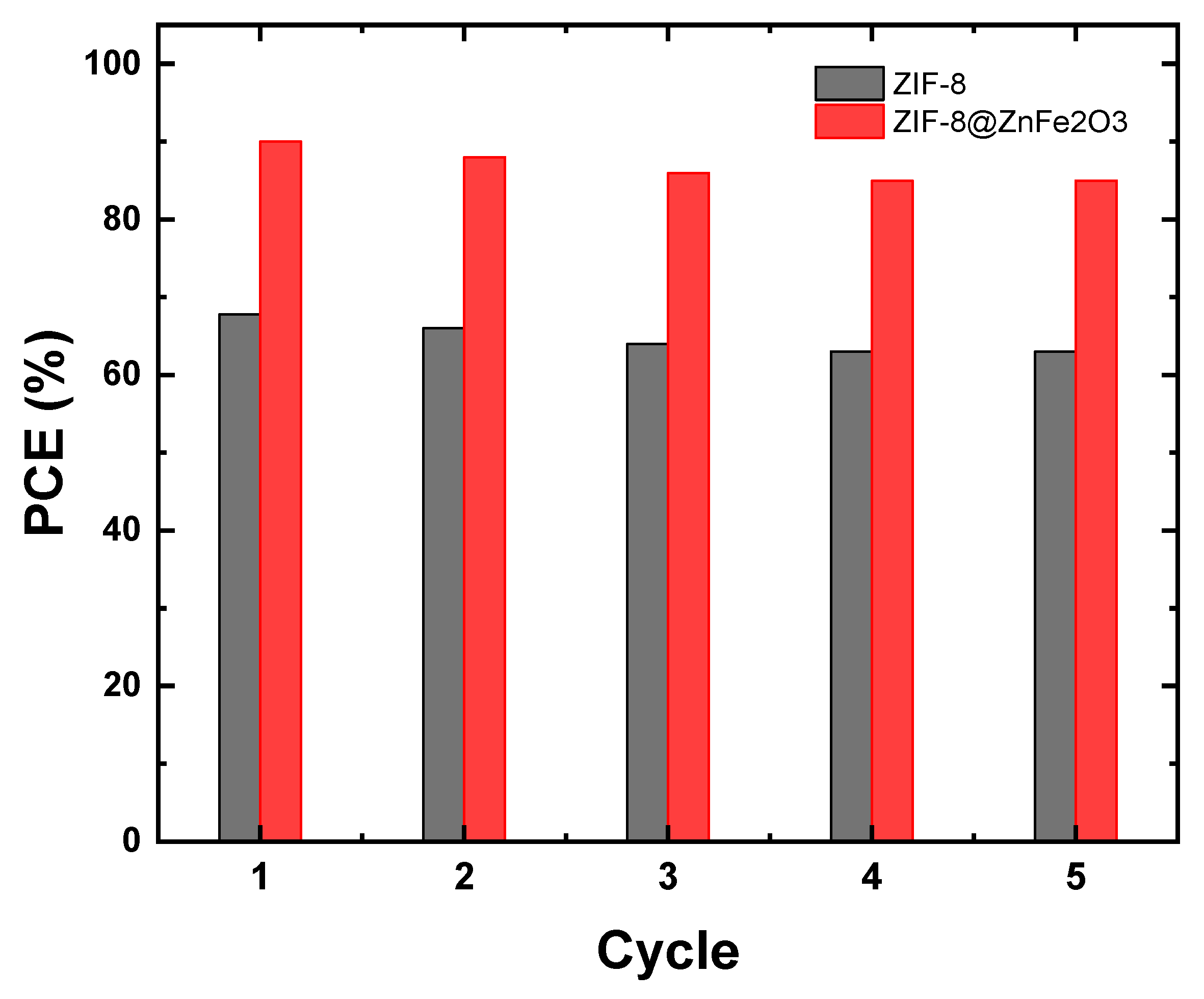
2.4. Photocatalytic Reusability and Stability
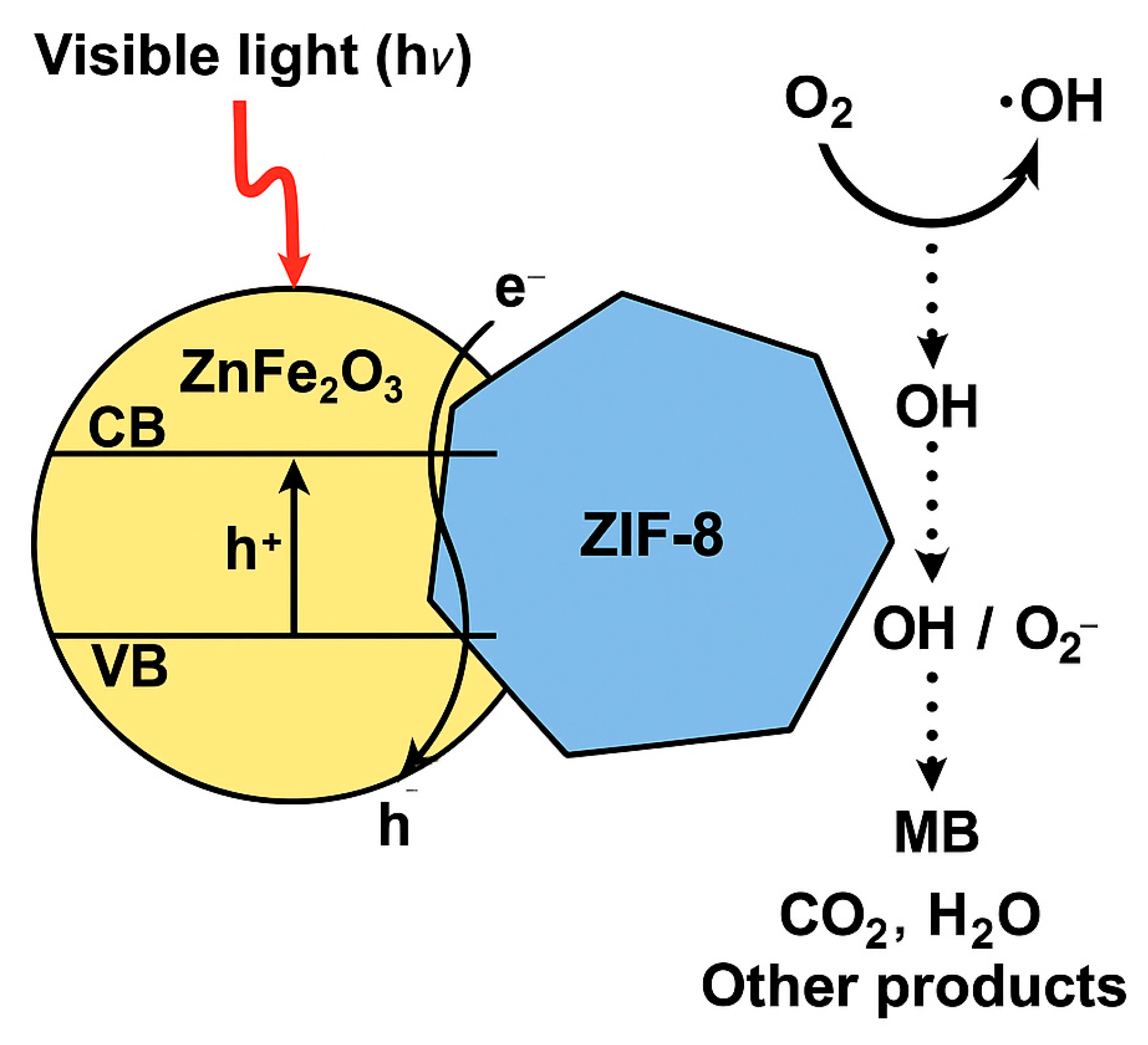
2.5. Mechanism of Photocatalytic Degradation
3. Experimental Technique
3.1. Sample Fabrication
3.2. Measurement Tools
3.3. Photocatalytic Degradation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mane, P.V.; Rego, R.M.; Yap, P.L.; Losic, D.; Kurkuri, M.D. Unveiling cutting-edge advances in high-surface-area porous materials for the efficient removal of toxic metal ions from water. Prog. Mater. Sci. 2024, 146, 101314. [Google Scholar] [CrossRef]
- Jia, T.; Gu, Y.; Li, F. Progress and potential of metal-organic frameworks (MOFs) for gas storage and separation: A review. J. Environ. Chem. Eng. 2022, 10, 108300. [Google Scholar] [CrossRef]
- Hu, S.; Guo, G.; Zhang, J.; Khan, M.N.; Xu, S.; Yang, F.; Schwandt, B.W.; Hu, Z.; Zou, J. Advanced porous MOF materials and technologies for high-efficiency ppm-level toxic gas separation. Mater. Sci. Eng. R 2024, 161, 100874. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, X.; Hou, Y.; Liu, C.; Xie, G.; Chen, X. Current research status of MOF materials for catalysis applications. Mol. Catal. 2024, 555, 113851. [Google Scholar] [CrossRef]
- Qu, L.; Xu, Y.; Cui, W.; Wu, L.; Feng, Y.; Gu, Y.; Pan, H. Trends in conductive MOFs for sensing: A review. Anal. Chim. Acta 2024, 1336, 343307. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, V.; Alfè, M.; Giordano, L.; Lettieri, S. Materials for Chemical Sensing: A Comprehensive Review on the Recent Advances and Outlook Using Ionic Liquids, Metal-Organic Frameworks (MOFs), and MOF-Based Composites. Chemosensors 2022, 10, 290. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Tang, Y.; Huang, X.; Pang, H. Recent advances and challenges of metal-organic framework/graphene-based composites. Compos. Part B Eng. 2022, 230, 109532. [Google Scholar] [CrossRef]
- Qi, X.; Wang, H.; Huang, B.; Yuan, X.; Zhang, J.; Zhang, J.; Jiang, L.; Xiong, T.; Zeng, G. State-of-the-art advances and challenges of iron-based metal-organic frameworks from attractive features, synthesis to multifunctional applications. Small 2019, 15, 1803088. [Google Scholar]
- Ghafari, S.; Kazemzad, M.; Naderi, N.; Eshraghi, M.J. Performance Improvement of Photodetectors Based on ZIF-8 Nanostructures on Porous Silicon Substrate. J. Electron. Mater. 2024, 53, 1577–1589. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Gafari, S.; Jamali, S.; Kazemzad, M. Concepts; fabrication, and applications of MOF thin films in optoelectronics: A review. Appl. Mater. Today 2024, 38, 102153. [Google Scholar] [CrossRef]
- Teng, D.; Zheng, W.; Wu, L.; Murtaza, G.; Meng, Z.; Qiu, L. Smart manufacturing of ZIF-8 photonic crystals: Catalytic formaldehyde degradation and colorful eco-friendly coatings. Chem. Eng. J. 2024, 495, 15352. [Google Scholar] [CrossRef]
- Karim, M.R.; Choi, C.-H.; Mohammad, A.; Yoon, T. Fabrication of high-performance supercapacitor of surface-engineered ZIF-8 for energy storage applications. J. Energy Storage 2024, 93, 112199. [Google Scholar] [CrossRef]
- Chafiq, M.; Chaouiki, A.; Al-Moubaraki, A.H.; Ko, Y.G. Recent progress in ZIF nanocomposite materials for wastewater pollutant in aqueous solution: A mini-review. Process Saf. Environ. Prot. 2024, 184, 1017–1033. [Google Scholar] [CrossRef]
- Li, K.; Miwornunyuie, N.; Chen, L.; Jingyu, H.; Amaniampong, P.S.; Koomson, D.A.; Ewusi-Mensah, D.; Xue, W.; Li, G.; Lu, H. Sustainable Application of ZIF-8 for Heavy-Metal Removal in Aqueous Solutions. Sustainability 2021, 13, 984. [Google Scholar] [CrossRef]
- Dai, H.; Yuan, X.; Jiang, L.; Wang, H.; Zhang, J.; Zhang, J.; Xiong, T. Recent advances on ZIF-8 composites for adsorption and photocatalytic wastewater pollutant removal: Fabrication, applications and perspective. Coord. Chem. Rev. 2021, 441, 213985. [Google Scholar] [CrossRef]
- Shen, B.; Wang, B.; Zhu, L.; Jiang, L. Properties of Cobalt- and Nickel-Doped Zif-8 Framework Materials and Their Application in Heavy-Metal Removal from Wastewater. Nanomaterials 2020, 10, 1636. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Moriceau, J.; Liu, M.; Yuan, Y.; Zakaria, Q.; Jiang, X.; Yu, A. Hydrothermal synthesis of ternary α-Fe2O3–ZnO–Au nanocomposites with high gas-sensing performance. Sens. Actuators B Chem. 2015, 209, 889–897. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Kim, H.-T.; Yoo, K.-O. Effect of ferric oxide on the high-temperature removal of hydrogen sulfide over ZnO-Fe2O3 mixed metal oxide Sorbent. Ind. Eng. Chem. Res. 1995, 34, 1181–1188. [Google Scholar] [CrossRef]
- Alsharif, M.A.; Darwish, A.A.A.; Alghamdi, N.; Alfadhli, S.; Khasim, S.; Ahmed, S.; Hamdalla, T.A. Synergistic Enhancements of Zn-ZIF with Nano Zinc Oxide for Hydrogen adsorption, energy storage, and photocatalytic technologies. Ceram. Int. 2024, 50, 47677–47686. [Google Scholar] [CrossRef]
- Saravanan, M.; Sabari Girisun, T.C. Enhanced nonlinear optical absorption and optical limiting properties of superparamagnetic spinel zinc ferrite-decorated reduced graphene oxide nanostructures. Appl. Surf. Sci. 2017, 392, 904–991. [Google Scholar]
- Danilo, D.; Calvete, M.J.F.; Hanack, M. Nonlinear optical materials for the smart filtering of optical radiation. Chem. Rev. 2016, 116, 13043–13233. [Google Scholar] [CrossRef]
- Saba, S.; Alsharari, A.M.; Aldaleeli, N.Y.; Aljohani, M.M.; Hamdalla, T.A. Structural, Electrochemical, and Supercapacitor Characterization of Double Metal Oxides Doped Within ZIF-8 Composites. Processes 2025, 13, 859. [Google Scholar] [CrossRef]
- Liao, W.-L.; Abdelaal, M.M.; Amirtha, R.-M.; Fang, C.-C.; Yang, C.-C.; Hung, T.-F. In Situ Construction of Nitrogen-Doped and Zinc-Confined Microporous Carbon Enabling Efficient Na+-Storage Abilities. Int. J. Mol. Sci. 2023, 24, 8777. [Google Scholar] [CrossRef]
- Cravillon, J.; Schröder, C.A.; Bux, H.; Rothkirch, A.; Caro, J.; Wiebcke, M. Formate modulated solvothermal synthesis of ZIF-8 investigated using time-resolved in situ X-ray diffraction and scanning electron microscopy. CrystEngComm 2012, 14, 492–498. [Google Scholar] [CrossRef]
- Zappa, D.; Galstyan, V.; Kaur, N.; Arachchige, H.M.M.M.; Sisman, O.; Comini, E. “Metal oxide-based heterostructures for gas sensors”—A review. Anal. Chim. Acta 2018, 1039, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, H.; Li, X.; Chen, X.; Li, W.; Li, X.; Zhang, C. MOF-on-MOF-derived hollow tubular ZnFe2O4/Er-ZnO heterojunctions for the efficient degradation of tetracycline by visible light photocatalysis. Appl. Surf. Sci. 2025, 684, 161634. [Google Scholar] [CrossRef]
- Dutta, T.; Kim, T.; Vellingiri, K.; Tsang, D.C.W.; Shon, J.R.; Kim, K.-H.; Kumar, S. Recycling and regeneration of carbonaceous and porous materials through thermal or solvent treatment. Chem. Eng. J. 2019, 364, 514–529. [Google Scholar] [CrossRef]
- Lei, Y.; Xiao, Y.; Chen, X.; Zhang, W.; Yang, X.; Yang, H.; Fang, D. Research Progress on CO2 Capture and Catalytic Conversion of Metal-Organic Frameworks Materials. Catalysts 2025, 15, 421. [Google Scholar] [CrossRef]
- Chen, P.; Wang, S.; Peng, H.; Wang, S.; Wang, J.; Zhao, X.; Chen, C.; Zhou, H. Preparation of ZIF-8/Fe3O4 modified halloysite nanotubes blended ultrafiltration membranes via magnetic field regulation: Enhanced performance and efficient filler distribution. Sep. Purif. Technol. 2025, 362, 131833. [Google Scholar] [CrossRef]
- Jiao, L.; Seow, J.Y.; Skinner, W.S.; Wang, Z.U.; Jiang, H.L. Metal-organic frameworks: Structures and functional applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; Zeyada, H.M.; Abd-El-Rahman, K.F.; Farag, A.A.M.; Darwish, A.A.A. Fourier-transform infrared and optical absorption spectra of 4-tricyanovinyl-N,N-diethylaniline thin films. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 69, 205–210. [Google Scholar] [CrossRef]
- Guan, D.; Xu, H.; Huang, Y.-C.; Jing, C.; Tsujimoto, Y.; Xu, X.; Lin, Z.; Tang, J.; Wang, Z.; Sun, X.; et al. Operando Studies Redirect Spatiotemporal Restructuration of Model Coordinated Oxides in Electrochemical Oxidation. Adv. Mater. 2025, 37, 2413073. [Google Scholar] [CrossRef]
- El-Zaidia, E.F.M.; Ali, H.A.M.; Hamdalla, T.A.; Darwish, A.A.A.; Hanafy, T.A. Optical linearity and bandgap analysis of Erythrosine B doped in polyvinyl alcohol films. Opt. Mater. 2020, 100, 109661. [Google Scholar] [CrossRef]
- Nafee, S.S.; Hamdalla, T.A.; Darwish, A.A.A. Studies of the morphology and optical properties of nano erbium oxide embedded in PMMA matrix. Opt. Laser Technol. 2020, 129, 106282. [Google Scholar] [CrossRef]
- Yang, X.; Wen, Z.; Wud, Z.; Luo, X. Synthesis of ZnO/ZIF-8 hybrid photocatalysts derived from ZIF-8 with enhanced photocatalytic activity. Inorg. Chem. Front. 2018, 5, 687. [Google Scholar] [CrossRef]
- Sen, P.; Bhattacharya, P.; Mukherjee, G.; Ganguly, J.; Marik, B.; Thapliyal, D.; Verma, S.; Verros, G.D.; Chauhan, M.S.; Arya, R.K. Advancements in Doping Strategies for Enhanced Photocatalysts and Adsorbents in Environmental Remediation. Technologies 2023, 11, 144. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, Y.; Duan, T.; Song, L. Interfacial engineering of heterogeneous catalysts for electrocatalysis. Mater. Today 2021, 48, 115–134. [Google Scholar] [CrossRef]
- Lv, Y.; Tian, J.; Chen, Z.; Wang, J.; Ma, L.-A.; Zhang, L.; Chen, S.; Wang, Q.; Ye, X. MXene/bimetallic CoNi-MOF-derived magnetic-dielectric balanced composites with multiple heterogeneous interfaces for excellent microwave absorption. Chem. Eng. J. 2023, 478, 147413. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Leong, S.; Lin, X.; Kong, J.W.B.; Xu, Y.; Low, Z.-X.; Yao, J.; Wang, H. Rapid Construction of ZnO@ZIF-8 Heterostructures with Size-Selective Photocatalysis Properties. ACS Appl. Mater. Interfaces 2016, 8, 9080–9087. [Google Scholar] [CrossRef] [PubMed]
- Elaouni, A.; El Ouardi, M.; Zbair, M.; BaQais, A.; Saadi, M.; Ahsaine, H.A. ZIF-8 metal-organic framework materials as a superb platform for the removal and photocatalytic degradation of organic pollutants: A review. RSC Adv. 2022, 12, 31801–31817. [Google Scholar] [CrossRef]
- Mittal, H.; Ivaturi, A. MoSe2-modified ZIF-8 novel nanocomposite for photocatalytic remediation of textile dye and antibiotic-contaminated wastewater. Environ. Sci. Pollut. Res. 2023, 30, 4151–4165. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.-P.; Wang, C.-C.; Zhang, Y.-W.; Wang, P.; Li, R. Photocatalytic degradation of methylene blue in ZIF-8. RSC Adv. 2014, 4, 54454–54462. [Google Scholar] [CrossRef]
- Rashad, M.; Hamdalla, T.A.; Darwish, A.A.A.; Seleim, S.M. High-performance efficiency of water purification using Cr2O3 nanoparticles synthesized by thermal combustion technique. Mater. Res. Express 2019, 6, 065048. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Mkhalid, I.A.; Baeissa, E.S.; Al-Rayyani, M.A. Photocatalytic Degradation of Methylene Blue by Fe/ZnO/SiO2 Nanoparticles under visible light. J. Nanotechnol. 2012, 2012, 329082. [Google Scholar] [CrossRef]
- Orasugh, J.T.; Temane, L.T.; Ray, S.S. Application of MXenes in Water Purification, CO2 Capture and Conversion. In Two-Dimensional Materials for Environmental Applications; Springer International Publishing: Cham, Switzerland, 2023; pp. 17–74. [Google Scholar]
- Rahman, I.A.; Ayob, M.T.M.; Radiman, S. Enhanced Photocatalytic Performance of NiO-Decorated ZnO Nanowhiskers for Methylene Blue Degradation. J. Nanotechnol. 2014, 2014, 212694. [Google Scholar] [CrossRef]
- Chen, M.L.; Cho, K.Y.; Oh, W.C. Synthesis and photocatalytic behaviors of Cr2O3–CNT/TiO2 composite materials under visible light. J. Mater. Sci. 2010, 45, 6611–6616. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
| Catalyst | Light Source | PCE (%) | kₐₚₚ (min−1) | Reference |
|---|---|---|---|---|
| ZIF-8 | Visible | 67.8 | 7.52 × 10−3 | Present work |
| ZIF-8@ZnFe2O | Visible | 90 | 12.51 × 10−3 | Present work |
| Fe/ZnO/SiO2 | Visible | 100 | Not reported | [44] |
| MXene | Visible | 90.2 | 30 × 10−3 | [45] |
| NiO-ZnONCs | UV | 72 | 1.50 × 10–2 | [46] |
| Cr2O3-CNT NPs | Visible | 68 | 9.80 × 10–4 | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, F.; Hamdalla, T.; Al-Ghamdi, H.; Albarzan, B.; Darwish, A. Tunable Optical Bandgap and Enhanced Visible Light Photocatalytic Activity of ZnFe2O3-Doped ZIF-8 Composites for Sustainable Environmental Remediation. Catalysts 2025, 15, 720. https://doi.org/10.3390/catal15080720
Alharbi F, Hamdalla T, Al-Ghamdi H, Albarzan B, Darwish A. Tunable Optical Bandgap and Enhanced Visible Light Photocatalytic Activity of ZnFe2O3-Doped ZIF-8 Composites for Sustainable Environmental Remediation. Catalysts. 2025; 15(8):720. https://doi.org/10.3390/catal15080720
Chicago/Turabian StyleAlharbi, Fatma, Taymour Hamdalla, Hanan Al-Ghamdi, Badriah Albarzan, and Ahmed Darwish. 2025. "Tunable Optical Bandgap and Enhanced Visible Light Photocatalytic Activity of ZnFe2O3-Doped ZIF-8 Composites for Sustainable Environmental Remediation" Catalysts 15, no. 8: 720. https://doi.org/10.3390/catal15080720
APA StyleAlharbi, F., Hamdalla, T., Al-Ghamdi, H., Albarzan, B., & Darwish, A. (2025). Tunable Optical Bandgap and Enhanced Visible Light Photocatalytic Activity of ZnFe2O3-Doped ZIF-8 Composites for Sustainable Environmental Remediation. Catalysts, 15(8), 720. https://doi.org/10.3390/catal15080720







